Abstract
Rice (Oryza sativa L.) seedlings were treated with different concentrations of copper (Cu) either in presence or absence of zinc (Zn), and different events were investigated to evaluate the ameliorative effect of Zn on Cu stress. In presence of high Cu concentration, growth of both root and shoots were considerably reduced. Decline in elongation and fresh mass was observed in root and shoot. Zn alone did not show any considerable difference as compared to control, but when supplemented along with high concentration Cu, it prompted the growth of both root and shoot. After 7 days, root growth was 9.36 and 9.59 cm, respectively, at 200 and 500 μM of Cu alone as compared to 10.59 and 12.26 cm at similar Cu concentrations, respectively, in presence of Zn. Cu accumulation was considerably high after 7 days of treatment. In absence of Zn, significant accumulation of Cu was observed. Zn supplementation ameliorated the toxic impact of Cu and minimized its accumulation. Cu treatment for 1 and 7 days resulted in a dose-dependent increase in hydrogen peroxide (H2O2). When Cu was added in presence of Zn, the H2O2 production in root and shoot was reduced significantly. The increase in H2O2 production under Cu stress was accompanied by augmentation of lipid peroxidation. In absence of Zn, Cu alone enhanced the malondialdehyde (MDA) production in both root and shoot after 1 and 7 days of treatment. The MDA content drastically reduced in root and shoot as when Zn was added during Cu treatment. The activities of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX) were elevated under Cu stress both in root and shoot. Addition of Zn further stimulated the activities of these enzymes. Both ascorbate (AsA) and glutathione (GSH) contents were high under Cu stress either in presence or absence of Zn. The results suggests that Zn supplementation improves plant survival capacity under high Cu stress by modulating oxidative stress through stimulation of antioxidant mechanisms and restricts the accumulation of toxic concentrations of Cu.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper (Cu) is a transition metal and an important micronutrient required by the plants for vital metabolic process. Regardless of the fact that Cu has a significant role in cellular metabolism and growth in plants, its presence beyond this requirement imposes severe threat to plants (Mazhoudi et al. 1997; Teisseir et al. 1998). Toxic concentrations of Cu directly affect root plasma membrane and hinder important physiological processes in plants (Quartacci et al. 2001; Irtelli et al. 2009). Photosynthetic reactions involving photosystem II (PSII) are the main target of Cu toxicity (Küpper et al. 2009). Cu toxicity in plants is also associated with production of reactive oxygen species (ROS), leading to oxidative stress in plants (Panda et al. 2006; Brewer 2010; Andre et al. 2010; Choudhury et al. 2013). Cu intensifies the production of ROS entities like superoxide radical (O2 •−) and hydrogen peroxide (H2O2) in plants, which is considered as the main cause of membrane lipid peroxidation and ion leakage from cells along with oxidative damage to proteins and nucleic acids (Cuypers et al. 2000; Demirevska-Kepova et al. 2004; Panda 2007, 2008; Upadhyay and Panda 2009; Thounaojam et al. 2012). Like Cu, zinc (Zn) is also an important micronutrient that is required by plants for growth and metabolism. It plays an important function in several metabolic processes. Zn activates enzymes that are actively involved in protein synthesis, carbohydrate, lipid, and nucleic acid metabolism (Bonnet et al. 2000). Presence of Zn in plants above threshold concentration induced oxidative damage, lipid peroxidation, and degradation of antioxidant enzymes (Luna et al. 1994; Kampfenkel et al. 1995; Bonnet et al. 2000; Panda and Choudhury 2005). Studies have shown that regulations of gene expression under various environmental stresses are dependent on Zn (Cakmak 2000).
The increased production of ROS in plants under heavy metal stress is a frequent phenomenon that can disrupt normal metabolism through oxidative damage to cells (Choudhury et al. 2013). Cu is a redox active metal, which presence in excess induces oxidative damage in plants (Kampfenkel et al. 1995; Bonnet et al. 2000). Plants have developed antioxidant defense mechanisms to eradicate the deleterious effects of ROS. These include array of antioxidant enzymes like catalase (CAT 1.11.1.6), superoxide dismutase (SOD 1.15.1.1), ascorbate peroxidase (APX 1.11.1.11), etc. along with other metabolites like ascorbate (AA), glutathione (GSH), α-tocopherol, etc. (Choudhury et al. 2013). Antioxidants act in a coordinated manner to detoxify the ROS and reduce the oxidative stress load in plants (Choudhury et al. 2013). SOD helps to convert O2 •− to H2O2, at the same time as APX, CAT, and GPX detoxifies H2O2 (Apel and Hirt 2004). AA acts as a substrate for APX for reduction of H2O2 to H2O, while GPX uses GSH as a reducing agent (Gapper and Dolan 2006; Suzuki et al. 2012; Choudhury et al. 2013). The normal redox state under abiotic stress is primarily regulated by the antioxidant network and any imbalance in cellular redox homeostasis leads to oxidative stress.
Cu and Zn interact in diverse ways in plants. Zinc plays a key role in abiotic stress resistance in plants. It was reported that Zn supplementation can reduce Cu absorption, thereby reducing the toxic impact of Cu and enhancing uptake of Zn itself (Kausar et al. 1976; Hafeez et al. 2013). Studies have also shown that Zn has a key role in maintaining cell membrane integrity and restraining the free radical-induced cellular damage in root cells (Cakmak 2000). Reports also suggests that Zn can ameliorate cadmium (Cd)-induced oxidative stress in plants (Hassan et al. 2005; Zhao et al. 2011). Thus, the aim of this study was to investigate the ameliorative effect of Zn on Cu-induced oxidative stress in developing rice seedlings. This was studied by observing changes in growth pattern, Cu accumulation, ROS production, oxidative damage, and antioxidant metabolism in root and shoot after 1 and 7 days of Cu treatment. The objective was focused to understand the effect of Zn supplementation on oxidative stress responses and probable regulation of antioxidant defense system in rice under Cu stress.
Materials and methods
Plant growth and treatments
Viable seeds of rice (Oryza sativa L. cv MSE-9) were procured from the Regional Agricultural Research Station, Akbarpur, Karimganj, India. The seeds were surface sterilized with 0.01 % mercuric chloride (HgCl2) and set for germination at 30 °C in dark. Uniformly germinated seeds were selected and transferred to plastic pots containing Hoagland nutrient solution. Plants were grown for a period of 5 days in a growth camber under white light with photon flux density of 52 μmol s−1 −2 (PAR) with 16-h photoperiod. On 5 days, plants were treated with different concentrations of Cu (0, 200, and 500 μM) and Zn supplementation was added at 50 μM concentration either alone or in combination with Cu. Cu and Zn were given in the form of CuCl2 and ZnCl2, respectively. After 1 and 7 days of treatment, root and shoot were excised out for analysis.
Growth analysis and Cu uptake
Growth was measured in terms of elongation (centimeters) and fresh mass (grams) of root and shoot after 1 and 7 days of treatment. For determination of Cu content, the root and shoot were oven dried at 80 °C for 72 h. Dried plant material (0.1 g) was acid digested with 5 ml of nitric acid (HNO3) at 120 °C until complete digestion was achieved. The final volume was adjusted to 20 ml with deionized water and filter. The total Cu content was measured using an atomic absorption spectrometer (AAnalyst 200, PerkinElmer, USA).
Estimation of H2O2
The total H2O2 content was measured as per the method of Sagisaka (1979). Briefly, 0.2 g of plant tissue was homogenized with 5 % (w/v) trichloroacetic acid (TCA) and the homogenate was centrifuged at 12,500 rpm for 10 min. The reaction mixture contained 1.6 ml of supernatant tissue extract, 0.4 ml of TCA (50 %), 0.4 ml of ferrous ammonium sulfate, and 0.2 ml of potassium thiocyanate (KSCN). The absorbance was recorded at 480 nm.
Lipid peroxidation assay
The rate of lipid peroxidation was measured in terms of malondialdehyde (MDA) content determined by thiobarbituric acid (TBA) reaction (Heath and Packer 1968). Plant tissue (0.2 g) was homogenized with 2 ml 1 % (w/v) TCA and centrifuged at 12,000 rpm at 4 °C for 20 min. To 1 ml of the supernatant, 1 ml of 20 % TCA containing 0.5 % TBA and 1 μl of butylated hydroxyltoluene (BHT, a 4 % solution in ethanol) were added. The mixture was heated at 95 °C for 30 min and centrifuged at 10,000 rpm for 15 min. The absorbance was recorded at 532 nm and corrected by measurement at 600 nm.
Antioxidant enzymes
For extraction of antioxidant enzymes, viz. superoxide dismutase (SOD), catalase (CAT), and guaiacol peroxidase (GPX), 0.5 g of plant tissue was homogenized in 0.1 M of sodium phosphate (Na-PO4) buffer (pH 6.8) in a prechilled mortar and pestle. The homogenate was centrifuged at 16,000 rpm for 15 min at 4 °C to collect supernatant for estimation of SOD, CAT, and GPX. The SOD (EC. 1.15.1.1) activity was measured as per the method of Giannopolitis and Ries (1977). The assay mixture was comprised of 79.2 mM of tris–HCl buffer (pH 6.8) containing 0.12 mM of EDTA and 10.8 mM of tetraethylenediamine to which 0.0033 % bovine serum albumin (BSA), 6 mM of nitro blue tetrazolium (NBT) salt, 600 mM of riboflavin in 5 mM KOH, and 0.2 ml of supernatant extract were added. The reaction was initiated by placing the glass tubes between two fluorescent lamps (Philips 20 W, India). By switching the light on and off, the reaction was started and terminated, respectively. The increase in absorbance due to formazan formation was recorded at 560 nm. The GPX (EC 1.11.1.7) activity was assessed according to the method of Chance and Maehly (1955). The reaction mixture for GPX contained 2.1 ml (0.1 M) of phosphate buffer, 0.3 ml of 1 % (v/v) guaiacol, 0.3 ml of 1 % H2O2, and 0.3 ml of supernatant enzyme extract. The reaction mixture was incubated for 5 min at room temperature and absorbance was recorded at 470 nm (extinction coefficient, ε = 26.6). The CAT (EC 1.11.1.6) activity was estimated as per the method of Chance and Maehly (1955). The reaction mixture contained 2 ml (0.1 M) of phosphate buffer (pH 6.8), 0.5 ml of 30-mM H2O2, and 0.5 ml of enzyme extract. The mixture was incubated for 1 min. The absorbance was recorded at 240 nm (extinction coefficient, ε = 43.6). The activities of all the antioxidant enzymes were expressed as units per minute per gram from weight.
Estimation of AA and GSH contents
Tissue sample (0.2 g) was homogenized in 5 % (w/v) sulfosalicylic acid and centrifuged at 17,000 rpm for 15 min. For estimation of AA, the reaction mixture contained 2 % (w/v) sodium molubdate, 0.1 N of H2SO4, 1.5 N of Na2PO4, and supernatant plant extract. The mixture was incubated at 60 °C for 40 min followed by centrifugation at 3,000 rpm for 10 min. The absorbance was recorded at 660 nm (Oser and Hawks 1985). For estimation of GSH, the assay mixture contained tissue extract neutralized with 0.5 ml of 0.5 M potassium phosphate buffer (pH 7.5) with EDTA, 0.2 ml of 6 mM 5,5′-dithiobis nitrobenzoic acid (DTNB), 0.1 ml of 2 mM NADPH, and 1 U yeast GR type III. The absorbance was recorded at 412 nm (Grifith 1980).
Statistical analysis
All the experiments were repeated three times and the data represents ±standard error (SE). The results were subjected to ANOVA and LSD test was used to compare significant differences between pairs of treatment either using Microsoft Excel 2010 or SPSS 12.1.
Results
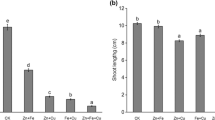
Rice seedlings treated with Cu in presence or absence of Zn for 1 day showed no significant changes in root and shoot elongation as compared to control (Fig. 1a–b and Fig. 2a–b). Cu alone did not have an effect on root elongation after 1 day of treatment, but considerably reduced the root growth after 7 days. Zn alone did not exert any major difference as compared to control, but when supplemented along with Cu prompted better root and shoot growth after 1 and 7 days. The root length was increased by 20 and 28 % in plants treated with 200 and 500 μM Cu in presence of 50 μM Zn as compared to those under Cu alone. In shoots, 13 and 8 % increases were observed after 7 days for Cu-treated plants in presence of Zn (Fig. 1a–b; Fig. 2a–b). The decline in fresh mass was recuperated by 50 μM Zn as compared to control and Cu alone (Fig. 2c–d). Dose- and time-dependent accumulation of Cu was observed in root and shoot in Cu-treated plants in absence of Zn, while Cu uptake was considerably minimized under the influence of Zn. About 25 and 30 % reduction in Cu uptake were observed in plants at 200 and 500 μM Cu in presence of Zn (Fig. 1e–f).
Changes in elongation (a–b), fresh mass (c–d), and Cu uptake (e–f) in root and shoot, respectively, under Cu treatment in presence or absence of 50 μM of Zn. All data show mean ± SE of five replicates of three independent experiments. Significance levels were calculated in comparison to control and among the treatment groups. a represents p < 0.05 as compared to control; b represents p < 0.001 as compared to Zn alone and *, **, and *** represent significance levels at p < 0.05, p < 0.01, and p < 0.001, respectively, among the Cu-treated plants in presence and absence of Zn
The changes in H2O2 production in rice seedlings after 1 and 7 days of treatment is shown in Fig. 3a–b). Cu stress to rice seedlings in presence or absence of Zn did not affect the H2O2 production in roots (Fig. 3b). As compared to control, the increase in H2O2 production was significant but did not show any change within the treated plants. In presence of Zn H2O2, production was controlled in roots after 1 day of Cu treatment but increased significantly after 7 days. In roots, H2O2 content was reduced by 30 and 18 % after Cu treatments of 200 and 500 μM in presence of Zn after 7 days (Fig. 3b) in comparison to those under Cu alone. In case of shoot, the reduction in H2O2 levels were 17 and 10 %, respectively, after 200 and 500 μM of Cu in presence of Zn. This reduction was statistically significant after 7 days of treatment as compared to those under Cu treatment alone. Cu treatment for a period of 1 and 7 days initiated the process of lipid peroxidation in root and shoot of rice seedlings (Fig. 4a–b). The increase in malondialdehyde (MDA) content increased under Cu treatment as compared to control in both shoot and root after 1 and 7 days of treatment (p < 0.05). Cu treatment in presence of Zn considerably reduced the MDA content in both root and shoot, with more prominent effect noticed after 7 days in roots (Fig. 4b). The increase was distinctly dose and time dependent; however, Zn, when applied along with Cu, showed its ameliorative effect in declining and, to an extent, normalizing the MDA levels in both root and shoot.
H2O2 production in leaf (a) and root (b) of rice plants subjected to 200 and 500 μM of Cu or Zn alone or in combination. All the data show mean ± SE of five replicates of three independent experiments. Significance levels were calculated in comparison to control or among the treatments. a represents significance level at p < 0.05 as compared to control; b represents significance level at p < 0.001 among the treatments as compared to Zn alone and ** and *** represent significance levels at p < 0.01 and p < 0.001, respectively, among the Cu-treated plants in presence and absence of Zn
Changes in MDA content in root (a) and shoot (b) of rice seedlings after Cu treatment in presence or absence of 50 μM Cu. Significance levels were calculated in comparison to control or among the treatments. a represents significance level at p < 0.05 as compared to control; b represents significance level at p < 0.001 among the treatments as compared to Zn alone and ** represents significance level at p < 0.01 among the Cu-treated plants in presence and absence of Zn
The changes in ASA content in shoot and root rice seedlings under Cu treatment in presence or absence of Zn is shown in Fig. 5a–b. In shoots, the ASA content increased considerably after Cu treatment for 1 and 7 days (Fig. 5a). Zn supplementation also increased the ASA content in shoots but was not significantly different as compared to those under Cu treatment alone. In roots, Cu alone did not alter the ASA content as compared to control; major increase was recorded after 1 and 7 days in presence of Zn. The GSH content in shoot increased after 7 days of treatment (Fig. 5c). This increase was not important as compared to control. When Cu treatment was given in presence of Zn, the GSH level declined after 7 days. After 1 day of treatment, difference was observed in shoots treated with 200 μM of Cu in presence of Zn as compared to Cu alone. At 500 μM, the difference in GSH level was not significant. In roots, GSH was not affected by Cu at 200 μM, though it increased at 500 μM (Fig. 5d). Zn supplementation along with 200 and 500 μM Cu showed a minor increase after 1 day of treatment. On 7 days, Zn supplementation increased the GSH content at 200 μM of Cu only.
Changes in AA content (a–b) and GSH content (c–d) in shoot and root, respectively, of rice seedlings after Cu treatment in presence or absence of 50 μM Cu. Significance levels were calculated in comparison to control or among the treatments. a represents significance level at p < 0.05 as compared to control; b represents significance level at p < 0.001 among the treatments as compared to Zn alone and c represents significance level at p < 0.01 among the Cu-treated plants in presence and absence of Zn
The activities of antioxidant enzymes, viz. SOD, CAT, and GPX, in rice seedlings under Cu stress in presence or absence of Zn are shown in Fig. 6a–f. All these enzymes showed increase in activity after 1 and 7 days of treatment. The SOD was high in both root and shoot after 1 and 7 days (Fig. 6a–b). The activity pattern of SOD remained uniform and increase was dependent on dose and duration of treatment. As compared to control, Cu alone resulted in increase in SOD, which further increased after Zn supplementation. When the SOD activity was measured in roots and compared among the treatment groups, it was clearly observed that Zn supplementation improved the SOD activity considerably. CAT also showed progressive increase in its activity after 1 and 7 days (Fig. 6c–d). In shoot, Cu alone has no significant change in CAT activity after 1 and 7 days (Fig. 6c). When Cu treatment was given in presence of Zn, enhancement in CAT activity was observed. In roots, CAT activity increased in a dose-dependent manner initially after 1 day under Cu treatment alone (Fig. 5d). The GPX activity in roots increased considerably after Cu treatment along with 50 μM of Zn, where 82 and 59 % increases were recorded at 200 and 500 μM Cu + 50 μM Zn as compared to Cu alone. In shoots, a uniform increase in GPX activity was observed after 7 days of treatment. These results collectively suggest that Zn exerts ameliorative impact in plants by reducing Cu-induced oxidative damage. Presence of Zn may modulate the ROS metabolism by regulating the antioxidant defense system.
Changes in activities of SOD (a–b), CAT (c–d), and GPX (e–f) in shoot and root, respectively, of rice seedlings after Cu treatment in presence or absence of 50 μM Cu. Significance levels were calculated in comparison to control or among the treatments. a represents significance level at p < 0.05 as compared to control; b represents significance level at p < 0.001 among the treatments as compared to Zn alone and c represents significance level at p < 0.01 among the Cu-treated plants in presence and absence of Zn
Discussion
Cu is an essential micronutrient for plants and acts as cofactor for large number of proteins concerned with vital physiological processes (Andre et al. 2010; Choudhary et al. 2012). In the past few decades, Cu has turned up to be an important environmental pollutant that severely affects plant growth and agricultural productivity. The importance of Cu in plant growth and development is well recognized; however, excess of Cu can lead to phytotoxic symptoms and oxidative stress. In this study, we investigate the impact of excess Cu in inducing oxidative damage vis-à-vis the ameliorative effect of Zn in regulating Cu stress in developing rice seedlings. Inhibition of plant growth accompanied by high accumulation of Cu indicated a high and toxic impact of Cu. Supplementation of 50 μM Zn ameliorated the toxic effects of Cu. Zn is an important component of several enzymes and acts as a stabilizer/protector of proteins (Arvind and Prasad 2005). Growth inhibition and biomass reduction is a common indicator of heavy metal toxicity in plants (Woolhouse 1985). In hydrophonic cultures, Zn supplementation supported adequate plant growth under Cu stress. High concentration of Cu at 200 and 500 μM exerted its significant effect; however, upon addition of Zn, the growth rate enhanced. Accumulation pattern of Cu was also affected under the influence of Zn. It was observed that Cu uptake was considerably reduced and the same pattern was observed in shoots. This indicated that Zn interferes with Cu uptake and limits high accumulation of the metal, which seems to be vital for diminishing oxidative stress induced by it. The decline in Cu uptake and absorption was reported earlier in plants under the influence of Zn (Kausar et al. 1976; Hafeez et al. 2013). Low uptake of Cu in presence of Zn can be fundamental to increase photosynthetic efficiency and low ROS production in rice seedlings.
The increase in H2O2 content in root and shoot clearly indicated Cu-induced ROS production. Elevated production of H2O2 under Cu stress was previously reported in Lemna minor, Polytrichum commune, and O. sativa (Panda and Choudhury 2005; Panda 2007, 2008). Cu being a redox-active metal facilitates the production of ROS and this increase imposes severe threat to plants. Zn supplementation along with Cu resulted in a significant reduction in H2O2 content. This might be possible due to interference of Zn during Cu absorption that hinder H2O2 production via reduction of O2 •− or through stimulation of antioxidant defense system to scavenge ROS (Arvind and Prasad 2005; Upadhyay and Panda 2009).
Lipid peroxidation is considered as an index of oxidative damage under abiotic stress (Matewally et al. 2005). Cu treatment prompted lipid peroxidation suggesting oxidative damage in both root and shoot. High rate of lipid peroxidation in Cu-treated plants could be due to high ROS production, which attacks membrane lipid leading to ion leakage and membrane deterioration. The effect was completely dose dependently. The increase in MDA content directly correlated with high ROS production and growth inhibition, indicating that Cu-induced ROS production is the primary cause of lipid peroxidation. Addition of 50 μM Zn reduced the Cu-induced oxidative damage, where rate of lipid peroxidation was also reduced significantly. The ameliorative impact of Zn was further substantiated by changes in the pattern of antioxidants. It was observed that Cu alone resulted in a significant increment of SOD, CAT, and GPX after 1 and 7 days. This indicated that upon Cu exposure, the ROS accumulation was high and these enzymes played a vital role in ROS detoxification. Cu-induced changes in antioxidant activity were previously reported in plants (Panda and Choudhury 2005; Matewally et al. 2005; Panda 2008; Singh et al. 2012). SOD catalyzes the reduction of O2 •− to H2O2, which is further scavenged by CAT or GPX (Choudhury et al. 2013). These results indicate that high SOD activity is due to detoxification of O2 •−, which further accumulated high concentrations of H2O2 in both root and shoot. Both CAT and GPX acted in a coordinated manner to detoxify H2O2. Zn supplementation increased the antioxidant efficiency of rice seedlings under Cu stress. The results are found to be in agreement with those reported in aquatic plants under Zn supplementation (Arvind and Prasad 2005; Upadhyay and Panda 2009).
AA and GSH have a significant role in plants for ROS detoxification (Choudhury et al. 2013). Cu exposure for 1 and 7 days showed key changes. In roots, the AA content declined at 500 μM of Cu alone, though not much change could be observed for other concentrations. A similar trend was observed in the case of GSH. Supplementation of Zn improved both AA and GSH levels after 1 and 7 days of treatment. It might be assumed that though antioxidant enzymes are active in ROS detoxification, these nonenzymic antioxidants simultaneously helped in reducing the oxidative load. In all cases, Zn, when added in absence of Cu, did not exert any toxic symptoms. The alleviation of Cu stress upon Zn supplementation indicated that Zn played an important role by modulating the uptake of Cu and antioxidant defense system to reduce the oxidative damage.
In this study, the ameliorative impact of Zn on Cu-induced oxidative stress was clearly observed. The change in H2O2 levels and MDA content in both root and shoot evidently indicate that Zn can amend Cu toxicity. It limits Cu accumulation and also supported growth under toxic concentrations of Cu. Moreover, the antioxidant response under Cu stress also revealed that addition of Zn resulted in the increase in antioxidant efficiency under Cu stress. Thus, these events are indicative of a direct ameliorative effect of Zn on Cu-induced oxidative stress in developing rice seedlings.
References
Andre CM, Larondelle Y, Evers D (2010) Dietary antioxidants and oxidative stress from a human and plant perspective: a review. Curr Nutr Food Sci 6:2–12
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Ann Rev Plant Biol 55:373–399
Arvind P, Prasad MNV (2005) Modulation of cadmium-induced oxidative stress in Ceratophyllum demersum by zinc involves ascorbate-glutathione cycle and glutathione metabolism. Plant Physiol Biochem 43:107–116
Bonnet M, Camares O, Veisseire P (2000) Effects of zinc and influence of Acremonium lolii on growth parameters, chlorophyll a fluorescence and antioxidant enzyme activities of ryegrass (Lolium perene L. cv Apollo). J Exp Bot 51:945–953
Brewer GJ (2010) Copper toxicity in general population. Clin Neurophysiol 121:459–460
Cakmak I (2000) Possible role of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol 146:185–205
Chance B, Maehly AC (1955) Assay of catalase and peroxidases. Method Enzymol 2:764–778
Choudhary SP, Oral HV, Bhardwaj R, Yu JQ, Tran LSP (2012) Interaction of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. J Exp Bot 63:5659–5675
Choudhury S, Panda P, Sahoo L, Panda SK (2013) Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav 8:e23681. doi:10.4161/psb.23681
Cuypers A, Vangronsveld J, Clijsters H (2000) Biphasic effect of copper on ascorbate glutathione pathway in primary leaves of Phaseolus vulgaris seedlings during early stages of metal assimilation. Physiol Plant 110:512–517
Demirevska-Kepova K, Simova-Stiliova L, Stoyanova Z, Hölzer R, Feller U (2004) Biochemical changes in barley plants after excessive supply of copper and manganese. Enviorn Exp Bot 52:253–266
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Giannopolitis CN, Ries SK (1977) Superoxide dismutase: I. Occurrence in higher plants. Plant
Grifith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–221
Hafeez B, Khanif YM, Saleem M (2013) Role of zinc in plant nutrition—a review. Amm J Exp Agril 3:374–391
Hassan MJ, Zhang G, Wu F, Wei K, Chen Z (2005) Zinc alleviates growth inhibition and oxidative stress caused by cadmium in rice. J Plant Nutr Soil Sci 168:255–261
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. I. Kinetics and stiochiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Irtelli B, Petrucci WA, Navari-Izzo F (2009) Nicotianamine and histidine/proline are, respectively, the most important copper chelators in xylem sap of Brassica carinata under conditions of copper deficiency and excess. J Exp Bot 60:269–277
Kampfenkel K, Van Montagu M, Inźe D (1995) Effect of iron excess on Nicotiana plumbaginifolia plants. Plant Physiol 107:725–735
Kausar MA, Chaudry FM, Rashid A, Latif A, Alam SM (1976) Micronutrient availability to cereals from calcareous soil. I. Comparative Zn and Cu deficiency and their mutual interaction in rice and wheat. Plant Soil 45:397–410
Küpper H, Götz B, Mijovilovich A, Küpper FC, Meyer-Klaucke W (2009) Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol 151:702–714
Luna CM, Gonzalez CA, Trippi VA (1994) Oxidative stress caused by excess copper in oat leaves. Plant Cell Physiol 35:11–15
Matewally A, Safronova VI, Belimov AA, Dietz KJ (2005) Genotypic variation of the response to cadmium toxicity in Pisum sativum L. J Exp Bot 56:167–178
Mazhoudi S, Chaoui A, Ghorbal MH, El Ferjani E (1997) Responses of antioxidant enzymes to excess copper in tomato (Lycopersicon esculentum Mill.). Plant Sci 127:129–137
Oser B, Hawks L (1985) Physiological chemistry. McGraw-Hill, New York
Panda SK (2007) Chromium-mediated oxidative stress and ultrastructural changes in root cells of developing rice seedlings. J Plant Physiol 164:1419–1428
Panda SK (2008) Impact of copper on reactive oxygen species, lipid peroxidation and antioxidants in Lemna minor. Biol Plant 52:561–564
Panda SK, Choudhury S (2005) Changes in nitrate reductase activity and oxidative stress response in moss Polytrichum commune subjected to chromium, copper and zinc phytotoxicity. Braz J Plant Physiol 17(2):191–197
Panda SK, Choudhury S, Matsumoto H (2006) Molecular physiology of heavy metal stress in Physiol 59: 309–314 plants. In: Hemantaranjan A (ed) Advances in plant physiology, vol 9. Scientific, India, pp 169–192
Quartacci MF, Cosi E, Navari-Izzo F (2001) Lipids and NADPH-dependent superoxide production in plasma membrane vesicle from roots of wheat grown under copper deficiency or excess. J Exp Bot 52:77–84
Sagisaka S (1979) The occurrence of peroxide in perennial plant Populus glerica. Plant Physiol 57:308–309
Singh VP, Srivastava PK, Prasad SM (2012) Differential effect of UV-B radiation on growth, oxidative stress and ascorbate-glutathione in two cyanobacteria under copper stress. Plant Physiol Biochem 61:61–70
Suzuki N, Koussevitzky S, Mittler R, Miller G (2012) ROS and redox signaling in the response of plants under abiotic stress. Plant Cell Env 35:259–270
Teisseire H, Couderchet M, Vernet G (1998) Toxic responses and catalase activity of Lemna minor L. exposed to folpet, copper, and their combination. Ecotoxcol Env Saf 40(3):194–200
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Panda SK (2012) Excess copper induced oxidative stress and responses of antioxidants in rice. Plant Physiol Biochem 53:33–39
Upadhyay R, Panda SK (2009) Zinc reduced copper toxicity induced oxidative stress by promoting antioxidant defense in freshly grown duckweed Spirodela polyrhiza. J Hazard Mat 175:1081–1084
Woolhouse HW (1985) Toxicity and tolerance in the response of plants to metals. In: Lange OL, Nobel PS, Osmond CB et al (eds) Encyclopedia of plant physiology—physiological plant ecology III. Springer, Berlin, pp 245–300
Zhao AQ, Tian XH, Lu WH, Gale WJ, Lu XC, Cao YX (2011) Effect of zinc on cadmium toxicity in winter wheat. J Plant Nutr 34:1372–1385
Acknowledgments
We sincerely thank Regional Agricultural Research Station, Akbarpur (Karimganj), India, for providing rice seeds. We thank Central Instrumentation Laboratory, Assam University, Silchar, India, for providing Atomic Absorption Spectroscopy facility.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bhumi Nath Tripathi
Rights and permissions
About this article
Cite this article
Thounaojam, T.C., Panda, P., Choudhury, S. et al. Zinc ameliorates copper-induced oxidative stress in developing rice (Oryza sativa L.) seedlings. Protoplasma 251, 61–69 (2014). https://doi.org/10.1007/s00709-013-0525-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-013-0525-8