Abstract
Nitric oxide (NO) and glutathione (GSH) regulate a variety of physiological processes and stress responses; however, their involvement in mitigating Cu toxicity in plants has not been extensively studied. This study investigated the interactive effect of exogenous sodium nitroprusside (SNP) and GSH on Cu homeostasis and Cu-induced oxidative damage in rice seedlings. Hydroponically grown 12-day-old seedlings were subjected to 100 μM CuSO4 alone and in combination with 200 μM SNP (an NO donor) and 200 μM GSH. Cu exposure for 48 h resulted in toxicity symptoms such as stunted growth, chlorosis, and rolling in leaves. Cu toxicity was also manifested by a sharp increase in lipoxygenase (LOX) activity, lipid peroxidation (MDA), hydrogen peroxide (H2O2), proline (Pro) content, and rapid reductions in biomass, chlorophyll (Chl), and relative water content (RWC). Cu-caused oxidative stress was evident by overaccumulation of reactive oxygen species (ROS; superoxide (O2 •–) and H2O2). Ascorbate (AsA) content decreased while GSH and phytochelatin (PC) content increased significantly in Cu-stressed seedlings. Exogenous SNP, GSH, or SNP + GSH decreased toxicity symptoms and diminished a Cu-induced increase in LOX activity, O2 •–, H2O2, MDA, and Pro content. They also counteracted a Cu-induced increase in superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), and glyoxalase I and glyoxalase II activities, which paralleled changes in ROS and MDA levels. These seedlings also showed a significant increase in catalase (CAT), glutathione peroxidase (GPX), dehydroascorbate reductase (DHAR), glutathione S-transferase (GST) activities, and AsA and PC content compared with the seedlings stressed with Cu alone. Cu analysis revealed that SNP and GSH restricted the accumulation of Cu in the roots and leaves of Cu-stressed seedlings. Our results suggest that Cu exposure provoked an oxidative burden while reduced Cu uptake and modulating the antioxidant defense and glyoxalase systems by adding SNP and GSH play an important role in alleviating Cu toxicity. Furthermore, the protective action of GSH and SNP + GSH was more efficient than SNP alone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modern agricultural practices, rapid urbanization and the industrial revolution intensified heavy metal pollution, which eventually turned into a significant global concern for the growth and productivity of crops worldwide (Nagajyoti et al. 2010). Among heavy metals, copper (Cu) is essential for the normal growth and development of all living organisms, including plants. Being an integral part of numerous metalloproteins such as plastocyanin (photosynthetic system), cytochrome oxidase (respiratory electron transport chain), laccases, monoamine oxidase, uricase, Cu/Zn-superoxide dismutase (SOD) and ascorbate oxidase (antioxidative defense), Cu plays an important role in the cellular metabolism of plants (Burkhead et al. 2009). Like most micronutrients, plants require Cu in trace amounts for normal growth. Typically, the average concentration of Cu in leaves is 10 μg g−1 dry weight (Yruela 2009), and the critical toxicity level for most crop species is above 20–30 μg g−1 dry weight (Marschner 2012). Susceptibility to Cu also varies with plant species. For instance, alfalfa and barley are more tolerant to Cu stress than rice and potato (Jones 1998). In addition, rice is more susceptible to Cu toxicity than to other heavy metals, such as Ni, Co, and Zn (Chino 1981).
At elevated levels (>20–30 μg g−1 dry weight), Cu can induce toxicity by disturbing key cellular processes, including photosynthesis, electron transport, cell wall metabolism, nitrogen assimilation, and senescence, which may lead to reduced growth and even death of plants (Yruela 2005). Cu is highly reactive to thiols and can possibly displace other essential metals in proteins causing disruption of their structure and functions (Lippard and Berg 1994). Excess Cu induces oxidative stress by generating harmful reactive oxygen species (ROS) such as singlet oxygen (1O2), superoxide (O2 .–), hydrogen peroxide (H2O2) and hydroxyl radicals (OH.), all of which can damage biological molecules and membranes by lipid peroxidation (Demirevska-Kepova et al. 2004; Contreras et al. 2009). In addition to ROS, methylglyoxal (MG), a cytotoxic compound, has been found to accumulate during abiotic stresses including heavy metal stress (Singla-Pareek et al. 2006; Hossain et al. 2009). Excess MG can cause cell death by interacting with major biomolecules such as proteins, lipids, carbohydrates, and nucleic acids, and also by inactivating the antioxidant defense system (Yadav et al. 2005). Moreover, MG can produce ROS and stimulate oxidative stress generation in plants (Saito et al. 2011; Hoque et al. 2012).
Plants have evolved an array of homeostatic mechanisms to avoid metal toxicity such as metal exclusion, retention in the roots, immobilization in the cell wall, chelation by phytochelatins (PC), metallothioneins, organic acids, or heat shock proteins (Hossain et al. 2012). These mechanisms depend on the concentration of metal supplied, plant species, and duration of metal exposure. Moreover, plants employ antioxidant defense and glyoxalase systems to combat oxidative injury induced by ROS and MG, respectively, under heavy metal stress. The antioxidant system includes several ROS scavenging enzymes such as SOD, catalases (CAT), glutathione peroxidases (GPX), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), glutathione S-transferases (GST), and low molecular mass antioxidants such as ascorbate (AsA) and glutathione (GSH). Likewise, plant cells possess an MG detoxifying glyoxalase system comprising two enzymes, glyoxalase I (Gly I) and glyoxalase II (Gly II), which reduce MG to d-lactate by employing and regenerating GSH. The efficient inducing of the antioxidative and glyoxalase systems often correlates to the elevated level of GSH and high GSH/GSSG ratio which ensure enhanced tolerance of abiotic stresses (Mostofa et al. 2013; Mostofa and Fujita 2013; Hossain et al. 2013a, b).
Nitric oxide (NO), a ubiquitous bioactive molecule, plays multifunctional roles in plant growth, development, and cellular responses to environmental stresses (Siddiqui et al. 2011). NO-mediated adaptive responses to biotic and abiotic stresses are mainly attributed to its signaling role that triggers a number of redox-regulated and defense-related genes (Wendehenne et al. 2005). NO can alleviate oxidative stress by directly scavenging ROS, such as O2 .–, to form peroxynitrite (ONOO−) (Beligni and Lamattina 2002), which is less toxic than peroxides, and also by enhancing antioxidant enzymes (Singh et al. 2013). However, the protective and harmful effects of NO depend on the concentration and location of NO in the plant cells (Siddiqui et al. 2011). At low concentrations, sodium nitroprusside (SNP; an NO donor) was found to be effective in managing abiotic stresses including metal toxicity such as Cu (detached rice leaves, tomato plants), Cd (wheat roots, sunflower leaves, rice leaves), Al (wheat plants), and As (rice roots) (Yu et al. 2005; Wang et al. 2010; Singh et al. 2008, 2009; Laspina et al. 2005; Panda et al. 2011; Zhang et al. 2008). However, little information is available on the effect of SNP in modulating the antioxidant defense and glyoxalase systems in growing rice seedlings under Cu stress.
Reduced GSH (γ-Glu–Cys–Gly) is water-soluble and a ubiquitously distributed low molecular weight antioxidant present in all cellular compartments (Noctor et al. 2002). GSH plays a central role in several physiological processes, including sulfate transport, signal transduction, conjugation of metabolites, detoxification of xenobiotics, redox balance, expression of stress responsive genes, and scavenging of ROS produced during cellular metabolism or oxidative stress (Anjum et al. 2012). GSH detoxifies H2O2 and MG via the ascorbate–glutathione cycle and glyoxalase pathway, respectively. It also protects cell membranes by maintaining AsA, α-tocopherol, and zeaxanthin in the reduced state (Gill and Tuteja 2010). Previous studies revealed that intracellular GSH plays a protective role against metal toxicity by altering the rates of metal uptake and elimination (Kang and Enger 1987), and by chelating metal ions in cells (Jozefczak et al. 2012). Directly applying GSH with Cu may alleviate Cu toxicity as observed in rice and barley plants upon Cd toxicity (Cai et al. 2010; Chen et al. 2010a). However, so far no study has been conducted on the effect of extracellular GSH on cellular responses to Cu stress in plants. Therefore, further study is needed to elucidate the regulatory or antioxidant intervention strategy of external GSH in preventing oxidative stress in response to Cu stress.
Rice (Oryza sativa L.) is one of the important crops grown across the world and also considered as a staple food, especially in Bangladesh, China and India. Nowadays, due to rapid environmental changes, rice plants experience different types of abiotic stresses including heavy metal toxicity. In Bangladesh, cultivated lands adjacent to industrialized areas are highly contaminated with heavy metals including Cu (Bhuiyan et al. 2010). With this overall background in mind, we conducted a hydroponic experiment using rice to investigate the effects of exogenous SNP and GSH on Cu-induced modulation of O2 .−, H2O2, lipid peroxidation, proline, biomass, relative water content (RWC), total Chl, ascorbate, GSH, PC content, and on the activities of the enzymes involved in ascorbate–glutathione and glyoxalase systems supplementing CAT, GPX, and GST. Additionally, SNP and GSH were applied together to evaluate their combined versus individual roles in mitigating oxidative stress. In this study, we report that SNP and GSH induce Cu tolerance through modulating Cu homeostasis, antioxidant defense and glyoxalase systems in which GSH seems to be more efficient than SNP.
Materials and methods
Plant materials, growth condition, and treatments
Rice (Oryza sativa L. cv. BR 11) seeds were surface sterilized with 1 % (v/v) sodium hypochlorite solution for 20 min, washed with distilled water and imbibed for 24 h. The seeds were sown on plastic nets floating on distilled water in 250 ml plastic beakers and kept in the dark at 28 ± 2°C for germination. After 48 h, uniformly germinated seeds were transferred to a growth chamber and grown in a commercial hydroponics solution (Hyponex, Japan) diluted according to the manufacturer’s instructions. The nutrient solution consisted of 8 % N, 6.43 % P, 20.94 % K, 11.8 % Ca, 3.08 % Mg, 0.07 % B, 0.24 % Fe, 0.03 % Mn, 0.0014 % Mo, 0.008 % Zn, and 0.003 % Cu. The seedlings were grown under controlled conditions (photon density: 100 μmol m−2 s−1, temperature: 26 ± 2°C, RH: 65–70 %). Each plastic beaker contained about 50 rice seedlings. The nutrient solution (pH 5.5) was renewed every 3 days. At 12 days, seedlings were subjected to stress with CuSO4 at a concentration of 100 μM either with or without 200 μM SNP or 200 μM GSH or 200 μM SNP + 200 μM GSH in the hyponex solution. Therefore, the treatments were as follows: control (Con), 200 μM SNP (SNP), 200 μM GSH (GSH), 200 μM SNP + 200 μM GSH (SNP + GSH), 100 μM Cu (Cu), 100 μM Cu + 200 μM SNP (Cu + SNP), 100 μM Cu + 200 μM GSH (Cu + GSH), and 100 μM Cu + 200 μM SNP + 200 μM GSH (Cu + SNP + GSH). Sodium ferrocyanide (SF; an analogue of SNP that does not release NO; 200 μM) was also applied as an additional control of SNP. After 48 h of growth under the above conditions, the second leaf of rice seedlings was harvested to determine various physiological and biochemical parameters. Each treatment was replicated three times under the same experimental conditions.
Determination of Cu content by atomic absorption spectrophotometer
To determine Cu content, the root and leaf samples were harvested separately and the roots were washed thoroughly with distilled water to remove excess Cu from the root surface. The root and leaf samples were oven dried at 80°C for 48 h. Dried samples (0.1 g) were ground and acid digested with HNO3/HClO4 (5:1 v/v) mixture for 24 h at 80°C followed by Cu estimation using an atomic absorption spectrophotometer (Hitachi Z-5000, Japan).
Determination of biomass, total chlorophyll, and relative water content
To determine fresh biomass, seedlings were separated from the culture medium and the roots were washed thoroughly with distilled water followed by blotting with tissue paper. After separating the adherent seeds, ten seedlings from each treatment were weighed to determine fresh biomass (g seedling−1). To estimate total chlorophyll (Chl) content, leaves (0.5 g) were extracted in 80 % chilled acetone and Chl was estimated according to the method of Arnon (1949). To estimate RWC, 20 leaf segments (4–5 cm) were weighed separately to determine fresh weight (FW). Leaf segments were then placed between two layers of filter paper and immersed in deionized water. When the leaf segments became fully turgid, they were gently dried with tissue paper and turgid weight (TW) was measured. Dry weight (DW) was measured after oven drying at 80°C for 48 h. RWC was calculated using the following formula: RWC (%) = 100 X (FW − DW)/(TW − DW).
Determination of lipid peroxidation, hydrogen peroxide, and proline content
Lipid peroxidation of the second leaves was measured by estimating malondialdehyde (MDA) according to the method of Heath and Packer (1968). Fresh leaf samples (0.5 g) were homogenized with 5 % (w/v) trichloroacetic acid (TCA) and centrifuged at 11,500×g for 12 min. The supernatant was mixed with 20 % TCA containing 0.5 % of TBA and heated at 95°C for 30 min. MDA content was calculated by the difference in absorbance at 532 and 600 nm using an extinction coefficient of 155 mM−1 cm−1. Hydrogen peroxide (H2O2) was extracted by homogenizing 0.5 g of fresh leaf samples with 50 mM K-phosphate buffer (pH 6.5) and the content was determined after reaction with 0.1 % TiCl4 in 20 % H2SO4 following the method of Hossain et al. (2010). Proline (Pro) content was determined according to the method of Bates et al. (1973). Fresh leaf samples (0.5 g) were homogenized with 5 ml of 3 % aqueous sulfosalicylic acid and the homogenate was centrifuged at 10,000×g for 12 min. Supernatant (2 ml) was mixed with 2 ml of glacial acetic acid and 2 ml of acid ninhydrin solution. The resultant mixture was boiled at 100°C for 1 h and then transferred to ice to stop the reaction. The developed red color was extracted with 2 ml toluene and absorption of the chromophore was measured at 520 nm. Pro concentration was calculated using a calibration curve developed with Pro standards.
Histochemical detection of superoxide and H2O2
Superoxide (O2 .–) and H2O2 were detected in rice leaves according to the method of Chen et al. (2010a) with modifications. In brief, the second leaves were stained in 0.1 % nitroblue tetrazolium (NBT) or 1 % 3,3-diaminobenzidine (DAB) solution for 12 h under dark and light, respectively. Incubated leaves were then decolorized by immersing in boiling ethanol to detect blue insoluble formazan (for O2 .–) or deep brown polymerization product (for H2O2). After cooling, glycerol was used to open the leaves and photographs were taken by placing the leaves between two glass plates.
Estimation of non-enzymatic antioxidants
Fresh leaves (0.5 g) were homogenized in 3 ml of ice-cold 5 % meta-phosphoric acid containing 1 mM EDTA and centrifuged at 11,500×g for 12 min. Reduced and total AsA content were determined following the method of Dutilleul et al. (2003) with minor modifications. To estimate total AsA, the oxidized fraction was reduced by 0.1 M dithiothreitol. Reduced and total AsA content were assayed at 265 nm in 100 mM K-phosphate buffer (pH 7.0) with 1.0 U of ascorbate oxidase (AO). Oxidized ascorbate (DHA) = total AsA − reduced AsA. Based on enzymatic recycling, reduced GSH, oxidized GSH (GSSG), and total GSH (GSH + GSSG) were determined according to the method of Griffiths (1980). GSSG was determined after removal of GSH by 2-vinylpyridine derivatization. GSH was measured after subtracting the value of GSSG from total GSH.
Non-protein thiol extraction and determination of phytochelatin content
Non-protein thiol extraction and determination of PC content were carried out following the method of De Vos et al. (1992). In brief, non-protein thiols (NP-SH) were extracted by grinding 0.5 g of fresh leaf in 3 ml of 3 % (w/v) sulfosalicylic acid. The homogenate was centrifuged at 10,000×g for 15 min at 4°C. The clear supernatants were collected and immediately used for NP-SH determination. The supernatant was mixed with Ellman’s reaction mixture (5 mM EDTA and 0.6 mM 5,5 0-dithiobis (2-nitrobenzoic acid) in 120 mM phosphate buffer, pH 7.5) and the absorbance was recorded spectrophotometrically after 5 min at 412 nm to determine the level of NP-SH. The level of PC was calculated by subtracting the amount of GSH from the amount of total NP-SH.
Extraction and assay of enzymes
To extract enzymes, fresh leaf samples (0.5 g) were homogenized separately with a reaction mixture containing 50 mM K-phosphate buffer (pH 7.0), 100 mM KCl, 1 mM AsA, 5 mM β-mercaptoethanol, and 10 % (w/v) glycerol in pre-chilled mortars and pestles. The homogenate was centrifuged at 12,500×g for 15 min and the resultant supernatants were collected for analysis of enzyme activities and protein content. All procedures were performed at 0–4°C.
Lipoxygenase (LOX; EC 1.13.11.12) activity was estimated according to the method of Doderer et al. (1992). The substrate solution was prepared by adding 35 μl linoleic acid to 5 ml distilled water containing 50 μl Tween-20. After adjusting the pH to 6.5, 0.1 M phosphate buffer (pH 6.5) was added to make a total volume of 100 ml. LOX activity was determined by adding 10 μl of enzyme extract to 590 μl substrate. The absorbance was recorded at 234 nm and the activity was calculated using the extinction coefficient of 25 mM−1 cm−1. SOD (EC 1.15.1.1) activity was estimated according to the method of El-Shabrawi et al. (2010), which is based on a xanthine–xanthine oxidase system. The reaction mixture contained K-phosphate buffer (50 mM), NBT (2.24 mM), CAT (0.1 units), xanthine oxidase (0.1 units), xanthine (2.36 mM), and enzyme extract. CAT was added to avoid the possible H2O2-mediated inactivation of Cu/Zn-SOD. SOD activity was expressed as units (i.e., amount of enzyme required to inhibit NBT reduction by 50 %) min−1 mg−1 protein.
CAT (EC 1.11.1.6) activity was measured according to the method of Hossain et al. (2010) by monitoring the decrease of absorbance at 240 nm for 1 min. The reaction mixture contained 50 mM K-phosphate buffer (pH 7.0), 15 mM H2O2 and enzyme solution in a final volume of 0.7 ml. The reaction was initiated with enzyme extract and the activity was calculated using the extinction coefficient of 39.4 M−1 cm−1.
APX (EC 1.11.1.11) activity was determined following the method of Nakano and Asada (1981). The final reaction solution contained 50 mM K-phosphate buffer (pH 7.0), 0.5 mM AsA, 0.1 mM H2O2, 0.1 mM EDTA, and enzyme extract in a final volume of 0.7 ml. The reaction was initiated by adding H2O2 and activity was measured by observing the decrease in absorbance at 290 nm for 1 min using the extinction coefficient of 2.8 mM−1 cm−1.
MDHAR (EC 1.6.5.4) activity was determined according to the method of Hossain et al. (1984). The reaction mixture contained 50 mM Tris–HCl buffer (pH 7.5), 0.2 mM NADPH, 2.5 mM AsA, 1.0 U of AO and enzyme solution in a final volume of 0.7 ml. The activity was calculated from the change in ascorbate at 340 nm for 1 min using the extinction coefficient of 6.2 mM−1 cm−1.
DHAR (EC 1.8.5.1) activity was measured by monitoring the formation of AsA from DHA at 265 nm using GSH (Nakano and Asada 1981). The reaction buffer contained 50 mM K-phosphate buffer (pH 7.0), 2.5 mM GSH, and 0.1 mM DHA. The activity was calculated from the change in absorbance at 265 nm for 1 min using the extinction coefficient of 14 mM−1 cm−1.
GR (EC 1.6.4.2) activity was measured by monitoring the decrease in the absorbance of NADPH at 340 nm for GSSG-dependent oxidation of NADPH, as described by Foyer and Halliwell (1976). The reaction mixture contained 0.1 M K-phosphate buffer (pH 7.8), 1 mM EDTA, 1 mM GSSG, 0.2 mM NADPH, and enzyme solution in a final volume of 1 ml. The activity was calculated using the extinction coefficient of 6.2 mM−1 cm−1.
GST (EC 2.5.1.18) was measured as described by Hossain et al. (2010). The reaction mixture contained 100 mM Tris–HCl buffer (pH 6.5), 1.5 mM GSHs, 1 mM 1-chloro-2,4-dinitrobenzene (CDNB), and enzyme solution in a final volume of 0.7 ml. The enzyme reaction was initiated by adding CDNB and the increase in absorbance was recorded at 340 nm for 1 min. The activity was calculated using the extinction coefficient of 9.6 mM−1 cm−1.
GPX (EC: 1.11.1.9) activity was measured as described by Elia et al. (2003). The reaction mixture consisted of 100 mM Na-phosphate buffer (pH 7.5), 1 mM EDTA, 1 mM NaN3, 0.12 mM NADPH, 2 mM GSH, 1.0 U GR, 0.6 mM H2O2 and 20 μl of enzyme extract. The oxidation of NADPH was recorded at 340 nm for 1 min and the activity was calculated using the extinction coefficient of 6.62 mM−1 cm−1.
Glyoxalase I (Gly I, EC 4.4.1.5) assay was carried out according to the method of Hossain et al. (2009). The assay mixture contained 100 mM K-phosphate buffer (pH 7.0), 15 mM magnesium sulphate, 1.7 mM GSH and 3.5 mM MG in a final volume of 0.7 ml. The increase in absorbance was recorded at 240 nm for 1 min and the activity was calculated using the extinction coefficient of 3.37 mM−1 cm−1.
Glyoxalase II (Gly II, EC 3.1.2.6) activity was determined according to the method of Hossain et al. (2010). The reaction mixture contained 100 mM Tris–HCl buffer (pH 7.2), 0.2 mM DTNB and 1 mM S-d-lactoylglutathione (SLG) in a final volume of 1 ml. The reaction was started by adding SLG and the activity was calculated using the extinction coefficient of 13.6 mM−1 cm−1.
Protein content was determined following the method of Bradford (1976) using bovine serum albumin (BSA) as a standard.
Statistical analysis
The data were subjected to one-way analysis of variance (ANOVA) and different letters indicate significant differences between treatments at p < 0.05, according to Duncan’s multiple range test (DMRT) using IRRISTAT version 3 (International Rice Research Institute, Biometrics Unit, Manila, Philippines). Data represented in the table and figures are means ± standard deviations (SD) of three replicates for each treatment.
Results
Effect on visual toxicity symptoms in rice seedlings
Cu toxicity was phenotypically observed in the leaves of rice seedlings after 48 h treatment. The presence of Cu in the nutrient medium caused visible toxicity symptoms like stunted growth, chlorosis, and leaf rolling (Fig. S1). Adding SNP, GSH, or SNP + GSH relatively decreased the toxic effects of Cu, but GSH and SNP + GSH were more effective than the SNP alone. No differences were observed between control and SNP-, GSH-, or SNP + GSH-treated seedlings without Cu.
SF is an analogue of SNP but does not release NO upon light decomposition. Adding SF to Cu-stressed seedlings also caused visible toxicity symptoms as it was with the seedlings stressed with Cu alone (Fig. S2). The control seedlings treated with SF alone did not show any toxic symptoms.
Effect on fresh biomass, total Chl, and RWC in rice seedlings
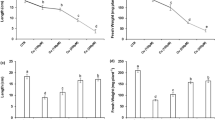
Cu treatment sharply reduced the fresh biomass of the rice seedlings compared with control (Table 1). SNP-, GSH-, and SNP + GSH-supplemented seedlings without Cu stress did not affect biomass compared with control seedlings. However, adding SNP, GSH, or SNP + GSH markedly inhibited biomass reduction and alleviated Cu-induced growth inhibition. Moreover, GSH- and SNP + GSH-supplemented Cu-stressed seedlings had better growth compared with SNP-supplemented Cu-stressed seedlings. Cu treatment caused a significant decrease in RWC and Chl content of the second leaves after 48 h of stress treatment (Table 1). No differences, with respect to the controls, were observed in SNP-, GSH-, or SNP + GSH-treated seedlings without Cu. Exogenously applying SNP, GSH, and SNP + GSH significantly alleviated the inhibitory effects of Cu on RWC and Chl content (Table 1). However, adding GSH resulted in greater protection of Chl and RWC than applying SNP to Cu-stressed seedlings. In addition, SF-treated Cu-stressed seedlings also showed a significant decrease in the level of total Chl compared with the control seedlings (Table S1). However, total Chl increased significantly in the control seedlings treated with SF alone compared with the control seedlings.
Effect on Cu uptake in rice seedlings
In Cu-treated seedlings, Cu accumulated significantly in the roots and leaves but the content was significantly higher in the roots compared with leaves (Table 2). Adding SNP or GSH, especially SNP + GSH, to the Cu-stressed seedlings significantly reduced the accumulation of Cu in the roots and leaves compared with the seedlings treated with Cu alone.
Effect on MDA, H2O2, and Pro content in rice seedlings
MDA content in Cu-treated rice seedlings increased significantly compared with the control seedlings (Table 3), whereas adding SNP, GSH, or SNP + GSH reduced the level significantly. GSH-treated seedlings without stress also showed a decreased level of MDA compared with the control seedlings. GSH-supplemented Cu-stressed seedlings also showed a low MDA level compared with SNP-treated Cu-stressed seedlings (Table 3). In addition, SF-treated Cu-stressed seedlings also showed a significant increase in the level of MDA compared with the control seedlings (Table S1).
Cu treatment led to higher H2O2 content in leaves, i.e., 56 % higher than those of control seedlings (Table 3). GSH-treated seedlings without Cu stress also showed a lower level of H2O2 than the control seedlings. Applying SNP, GSH, or SNP + GSH significantly reduced H2O2 accumulation in Cu-stressed seedlings. Moreover, GSH-treated Cu-stressed seedlings showed a lower level of H2O2 than those of SNP- or SNP + GSH-treated seedlings (Table 3).
Pro content increased significantly in response to Cu stress and it was 18 times higher than that of control (Table 3). SNP or GSH, especially SNP + GSH, decreased proline content significantly in Cu-stressed seedlings, but the levels were still significantly higher than the control level. In non-stress conditions, applying SNP, GSH, or SNP + GSH did not affect Pro content (Table 3).
Effect on the levels of ROS (O2 •– and H2O2) in rice seedlings
In our experiment, both O2 •– and H2O2 accumulated markedly in Cu-stressed rice seedlings (Fig. 1a, b). Cu-induced accumulation of O2 •– and H2O2 was confirmed by histochemical staining with NBT and DAB, respectively. Distinctive amounts of O2 •– and H2O2 were observed as scattered dark blue spots and as brown polymerization compounds, respectively, in the second leaf plates of Cu-stressed seedlings (Fig. 1a, b). However, adding SNP, GSH, or SNP + GSH markedly diminished ROS accumulation in the leaves of Cu-stressed rice seedlings (Fig. 1a, b). In particular, adding GSH reduced the ROS level close to that of control.
Effect on the levels of non-enzymatic antioxidants (AsA, GSH) in rice seedlings
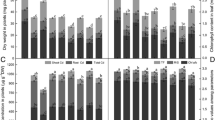
Reduced ascorbic acid (AsA) decreased by 45 % whereas oxidized ascorbic acid (DHA) increased by 189 % in Cu-stressed seedlings (Fig. 2a, b). Consequently, the AsA/DHA ratio in Cu-stressed seedlings decreased significantly (Fig. 2c). Upon applying SNP, GSH, or SNP + GSH to Cu-stressed seedlings, AsA content and the AsA/DHA ratio increased significantly compared with Cu stress only (Fig. 2a, c). Compared with Cu stress and other treatments, high levels of AsA and AsA/DHA ratio were found in GSH-supplemented Cu-stressed seedlings.
Effect of exogenous SNP, GSH, and SNP + GSH on AsA (a), DHA (b), AsA/DHA ratio (c), GSH (d), GSSG (e), and GSH/GSSG ratio (f) in Cu-treated rice seedlings. Vertical bars represent standard deviation of the mean (n = 3). Different letters indicate significant differences between treatments at p < 0.05, according to DMRT
Upon adding Cu, both GSH and GSSG levels increased by 191 % and 576 %, respectively, whereas the GSSH/GSSG ratio decreased by 59 % compared with control (Fig. 2d–f). Adding SNP, GSH, and SNP + GSH to Cu-stressed seedlings significantly decreased the Cu-induced increase in both GSH and GSSG levels and also brought the levels toward or above that in the control seedlings (Fig. 2d, e). Adding SNP maintained a higher level of GSH in Cu-stressed seedlings than those in GSH- or SNP + GSH-supplemented rice seedlings. In non-Cu conditions, adding GSH and SNP + GSH also resulted in an increase in GSH content compared with the control seedlings. Moreover, Cu-stressed seedlings treated with GSH showed a significantly higher GSH/GSSG ratio than those treated with SNP and SNP + GSH (Fig. 2f).
Effect on phytochelatin content in rice seedlings
The amount of PC was measured as the difference between total non-protein thiol (NP-SH) content and GSH level. Treatment with Cu resulted in an increase in PC level by 183 % with respect to control (Fig. 3). PC synthesis was also observed in control seedlings as a means of constitutive synthesis. External SNP, GSH, and SNP + GSH again boosted the level of PC in Cu-treated rice seedlings. However, exogenous GSH was more effective in inducing the synthesis of PC compared with Cu stress and other combinations of protectants with Cu.
Effect on LOX, SOD, and CAT activities in rice seedlings
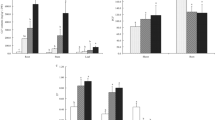
In Cu-treated seedlings, LOX activity increased by 95 % compared with control (Fig. 4a). In the presence of SNP or GSH, especially SNP + GSH, LOX activity decreased significantly in Cu-stressed seedlings. SNP- and SNP + GSH-treated control rice seedlings also showed a 28 % and 24 % increase in LOX activity, respectively, compared with control (Fig. 4a).
SOD activity increased significantly upon Cu exposure and the induction level was 23 % higher than the control value (Fig. 4b). However, upon adding SNP, GSH, and SNP + GSH, the induction level of SOD was significantly lower than in Cu-stressed seedlings. GSH supplementation reduced SOD activity more than SNP and SNP + GSH with Cu stress (Fig. 4b). CAT activity decreased significantly in Cu-stressed rice seedlings compared with control (Fig. 4c). In contrast, CAT activity increased significantly over the Cu-stressed seedlings upon adding SNP, GSH, and SNP + GSH. In non-stress conditions, SNP or SNP + GSH did not inhibit CAT activity (Fig. 4c).
Effect on the enzyme activities of ascorbate–glutathione cycle
APX activity increased by 80 % in Cu-stressed seedlings compared with the control seedlings (Fig. 5a). Although adding SNP, GSH, and SNP + GSH to Cu-stressed seedlings decreased APX activity compared with the seedlings stressed with Cu alone, it remained significantly higher than control (60 %, 32 %, and 31 %, respectively). Furthermore, SNP-treated Cu-stressed seedlings showed significantly higher APX activity than those treated with GSH or SNP + GSH. Cu stress increased MDHAR activity by 78 % over the control level (Fig. 5b). Adding SNP, GSH, or SNP + GSH to Cu-stressed seedlings counteracted the increase in MDHAR activity and brought it down towards the control level. Similar to MDHAR, DHAR activity increased by 91 % in Cu-stressed seedlings compared with the control seedlings (Fig. 5c). Although adding SNP and SNP + GSH to Cu-stressed seedlings maintained DHAR activity as it was with the seedlings stressed with Cu alone, applying GSH further boosted DHAR activity which was significantly higher compared with the seedlings stressed with Cu alone. GR activity increased by 60 % in Cu-stressed seedlings compared with the control seedlings (Fig. 5d). However, when Cu-stressed seedlings were supplemented with SNP, GSH, or SNP + GSH, the increase in GR activity diminished and brought it down towards the control level.
Effect of exogenous SNP and GSH on the activities of ascorbate-glutathione cycle enzymes, APX (a), MDHAR (b), DHAR (c), and GR (d), in Cu-treated rice seedlings. Vertical bars represent standard deviation of the mean (n = 3). Different letters indicate significant differences between treatments at p < 0.05, according to DMRT
Effect on glutathione metabolizing enzymes in rice seedlings
GPX activity (Fig. 6a) increased by 44 % over the control level in response to Cu stress. However, adding SNP, GSH, or SNP + GSH to Cu-stressed seedlings increased GPX activity significantly compared with the seedlings stressed with Cu alone. GST activity (Fig. 6b) decreased by 50 % in response to Cu stress compared with control. Adding SNP, GSH, or SNP + GSH to Cu-stressed seedlings increased GST activity significantly compared with the seedlings stressed with Cu alone. There was no significant change in GST activity between control and SNP-, GSH-, or SNP + GSH-supplemented seedlings without Cu stress.
Effect of exogenous SNP, GSH, and SNP + GSH on the activities of glutathione metabolizing enzymes: GPX (a) and GST (b) in Cu-treated rice seedlings. Vertical bars represent standard deviation of the mean (n = 3). Different letters indicate significant differences between treatments at p < 0.05, according to DMRT
Effect on the enzyme activities of the glyoxalase cycle in rice seedlings
A significant increase in Gly I activity (Fig. 7a) was observed in response to Cu stress. SNP-, GSH-, or SNP + GSH-supplemented Cu-stressed seedlings showed a significant increase in Gly I activity compared with control seedlings but was lower than the seedlings stressed with Cu alone. However, Cu + SNP-treated seedlings had higher Gly I activity than those of Cu + GSH and Cu + SNP + GSH. Gly II activity increased significantly in response to Cu stress compared with the control level (Fig. 7b). However, SNP-, GSH-, or SNP + GSH-supplemented Cu-stressed seedlings brought Gly II activity down towards the control level. GSH-supplemented control seedlings also showed a significant increase in Gly II activity compared with the control seedlings.
Effect of exogenous SNP, GSH, and SNP + GSH on the activities of glyoxalase cycle enzymes, Gly I (a) and Gly II (b), in Cu-treated rice seedlings. Vertical bars represent standard deviation of the mean (n = 3). Different letters indicate significant differences between treatments at p < 0.05, according to DMRT
Discussion
The inherent redox active nature of Cu accelerates production of free radicals, which are the major cause of oxidative stress in plant tissues. Oxidative stress is a key damaging factor and plants resist oxidative damage by inducing and modulating antioxidant systems. Recent studies also revealed that MG contributes to eliciting oxidative stress under various stressful conditions (Saito et al. 2011; Hoque et al. 2012). In this study, we aimed to investigate the effects of NO and GSH on Cu homeostasis, oxidative stress, and responses of the antioxidative and MG detoxification systems in hydroponically grown rice seedlings.
Inhibited growth and reduced biomass are frequently observed in plants exposed to excess Cu (Thounaojam et al. 2012, 2013; Choudhary et al. 2012), which was also found in this study (Table 1). A significant decrease in plant biomass suggested Cu-induced toxicity in these seedlings. Cu content in the roots and leaves indicated an altered pattern of Cu accumulation in the rice seedlings. In our study, roots were in direct contact with Cu solution where it was readily absorbed by the roots with reduced accumulation in the leaves (Table 2). Previous studies with various plant species also reported a correlation to Cu tolerance and its greater accumulation in roots with poor translocation to shoots (Thounaojam et al. 2012; Choudhary et al. 2012; Mostofa and Fujita 2013). However, we observed a considerable decrease in Cu uptake and its accumulation in leaves upon adding SNP and GSH, which is consistent with the previous findings in Luffa (Singh et al. 2013) and rice (Chen et al. 2010a) seedlings under arsenic and Cd stress, respectively. This indicates that SNP and GSH interfered with Cu uptake and limited further accumulation, which seems to be vital in diminishing oxidative stress induced by Cu. This pattern of Cu homeostasis constitutes an important mechanism allowing plant Cu tolerance, consequently protecting plants from phytotoxicity and enabling normal growth.
It is well documented that Cu toxicity perturbs water relations and induces Chl loss (Wang et al. 2010; Kholodova et al. 2011; Mostofa and Fujita 2013), which we also observed in this experiment. Excess Cu can interfere with water balance by damaging plasma membranes, decreasing water transporters, and affecting transpiration rates of plant tissues (Kholodova et al. 2011). It has been proposed that excess Cu interferes with Fe and Mg homeostasis, activities of the enzymes of chlorophyll biosynthesis, and protein composition of photosynthetic membranes (Yruela 2009). However, Cu-induced loss of RWC and total Chl content were almost completely reversed when Cu-stressed seedlings were supplemented with SNP, GSH, or SNP + GSH (Table 1). External SNP and GSH are also known to protect water loss and Chl degradation in various plant species under heavy metal stress (Wang et al. 2010; Cai et al. 2010). In our experiment, GSH was found to be more effective in protecting these parameters compared with SNP, indicating a membrane protecting role for GSH.
Exposure of plants to heavy metals generates ROS and thus oxidative stress, which is thought to be primarily responsible for oxygen toxicity in the cell (Xiong et al. 2010). Oxidative stress causes disturbance of metabolic pathways and damage to macromolecules. In this study, Cu-stressed seedlings resulted in an overaccumulation of O2 •– and H2O2 (Table 3, Fig. 1), which clearly explains Cu-mediated oxidative burst. However, applying SNP, GSH, or SNP + GSH effectively eliminated the high levels of O2 •– and H2O2, and thus played an important role in mitigating oxidative stress induced by Cu. These findings also demonstrate the direct ROS quenching role of NO and GSH in rice leaves under Cu stress. The increased concentrations of ROS led to lipid peroxidation causing membrane damage, electrolyte leakage, and malfunctioning of membrane proteins and ion channels. MDA, a lipid peroxidation product, serves as an indicator of the extent of oxidative stress under stress conditions. In parallel with ROS, the MDA level increased substantially (Table 3), which indicates severe membrane damage in Cu-exposed seedlings. Our observation is in good agreement with previous reports (Thounaojam et al. 2013; Mostofa and Fujita 2013) and suggests that Cu-induced ROS production is the primary cause of lipid peroxidation. Cu-induced MDA was greatly diminished by exogenously supplying SNP, GSH, and SNP + GSH to Cu-stressed seedlings (Table 3). NO- and GSH-mediated reduction of oxidative stress might be achieved through their antioxidant nature in direct quenching of ROS leading to lower production of MDA, interfering with Cu uptake or by modulating the antioxidant system involved in eliminating ROS.
Pro is known to accumulate in many plants grown under abiotic stresses and is regarded as a stress metabolite (Metwally et al. 2003; Mostofa and Fujita 2013). Heavy metal-induced plant–water imbalance may trigger the accumulation of Pro, for example, in response to Cu in rice (Chen et al. 2004). In this experiment, the reason for an increased Pro level seems to be the water stress generated by Cu as evident from decreased RWC in the leaves (Tables 1 and 3). In contrast, adding SNP, GSH, and SNP + GSH to Cu-treated rice seedlings decreased Cu-induced Pro accumulation, which indicates partial relief in these plants from Cu stress. Reduced Pro content by SNP has also been reported in canola plants under Ni stress (Kazemi et al. 2010). Importantly, GSH- and SNP + GSH-supplemented seedlings showed greater reduction in Pro content than with the SNP alone, indicating these seedlings suffered from less stress.
To evaluate the NO-dependent role of SNP in the alleviation of Cu toxicity, we carried out a control experiment with SF. Light decomposition of SNP releases residual products such as sodium cyanide, ferrocyanide, and iron (Fe). In particular, Fe released from SNP might have a significant effect on total Fe content and leaf chlorosis. Sun et al. (2007) observed that adding SNP mitigated Fe deficiency-induced oxidative stress in maize plants. There have been other reports indicating that NO plays a key role in the regulatory mechanisms of iron uptake and homeostasis in plants (Chen et al. 2010b; Graziano and Lamattina 2007). Our results reveal that adding SF instead of SNP to a Cu solution did not alleviate oxidative stress as evident by higher levels of MDA and Chl loss (Table S1). Visual toxicity symptoms in SF-treated Cu-stressed seedlings also indicated that none of the residual products of SNP decomposition significantly alleviated Cu toxicity (Fig. S2). By comparing these results, we can conclude that SNP alleviating the oxidative stress induced by Cu stress was attributed to its releasing NO.
LOX is an iron-containing oxidative enzyme involved in the propagation of lipid peroxidation in the membranes. In our experiment, we also found a marked increase in the activity of LOX, which is also thought to contribute to higher MDA content. LOX activity was found to increase in rice plants under Cu, Pb, and Hg stresses (Mishra and Choudhuri 1999; Mostofa and Fujita 2013). However, SNP- and GSH-mediated reduction in LOX activity demonstrated their role in minimizing lipid peroxidation as observed by the lower amount of MDA in the respective rice seedlings.
AsA and GSH are two major non-enzymatic antioxidants that play multifaceted roles in plant metabolism under both normal and stress conditions. They scavenge ROS directly and also constitute the vital components of an antioxidant defense system. It is well established that not only AsA and GSH concentration, but also their redox ratios are important determinants of plant survival under stress conditions (Gill and Tuteja 2010). In this experiment, Cu treatment led to a substantial decline in AsA, and an increase in DHA, GSH, and GSSG content, whereas the AsA/DHA and GSH/GSSG ratios concomitantly decreased (Fig. 2a–f). This resultant decrease in the AsA content and decrease in the AsA and GSH redox ratios interferes with many defense reactions including the ascorbate–glutathione cycle, which decreases antioxidant capacity and thereby culminates in oxidative stress. Cu-induced decrease in AsA content and elevation in GSH content has also been observed in Brassica (Li et al. 2009) and rice (Thounaojam et al. 2012), respectively. However, SNP and GSH supplementation amended the AsA level, and AsA/DHA and GSH/GSSG ratios, which might have led to a better antioxidant system to combat Cu phytotoxicity. On the other hand, SNP, GSH, and SNP + GSH effectively counteracted the Cu-induced increase in GSH content (Fig. 2d), which can partially be supported by (1) the antioxidant nature of both protectants scavenging ROS directly, (2) inhibited Cu uptake, both of which allowed the seedlings to suffer less stress and hence less GSH was produced, and (3) enhanced PC synthesis, and GPX, GST, Gly I, and DHAR activities, which all consume GSH. Similar to our findings, exogenous NO and GSH were also found to counteract a Cd-induced increase in GSH content in rice cultivars (Cai et al. 2010; Panda et al. 2011).
PCs, which are cysteine-rich small polypeptides, are regarded as biomarkers of metal toxicity. PCs have been shown to play an important role in Cu toxicity by forming stable complexes with Cu and their subsequent transportation into the vacuoles (Cobbett and Goldsbrough 2002). In the present study, PC content significantly increased in response to Cu stress (Fig. 3), which is corroborated by the finding in Silene cucubalus (De Vos et al. 1992). PC synthesis increased without a decrease in GSH levels, which might be the result of significant inducing of GSH, because it did not decrease much lower when PC synthesis consumed GSH as a substrate. It is also noticeable that adding SNP, GSH, or SNP + GSH to Cu-stressed plants resulted in a marked increase in PC content, which suggests that they might have some regulatory roles in the integrated mechanism of Cu homeostasis and detoxification through PC. What is more notable, GSH-supplemented Cu-stressed plants showed a higher level of PCs compared with other treatments, which indicates the role of GSH as the direct precursor for the synthesis of PC. Cai et al. (2010) also observed that external adding of GSH to Cd treatments further markedly increased PC production in Cd-sensitive (Xiushui 63) and tolerant (Bing 97252) rice genotypes.
In the case of oxidative stress, the activity of antioxidant enzymes like SOD, CAT, and POD generally increases in plants and this elevated activity correlates with increased stress tolerance (Gill and Tuteja 2010). However, a proper balance between the activities of ROS scavenging enzymes is crucial in preventing cellular damage. In the current study, Cu exposure significantly up-regulated some of the antioxidant enzymes, indicating higher inductions were associated with a strategy to overcome Cu-induced stress. However, a decline in AsA content and redox ratios (Fig. 2a,c,f) along with decreased activities of CAT, GPX, and GST (Figs. 4c and 6a, b) led to enforced severe oxidative stress. On the other hand, supplying SNP, GSH, or SNP + GSH to the Cu-stressed seedlings counteracted the Cu-caused dramatic increases in SOD, APX, GR, and MDHAR activities but elevated the depressed CAT and GST activities. Moreover, these protectants also augmented the upregulation of the activities of GPX and DHAR. The observed trend in changes in antioxidant enzymes upon adding SNP, GSH, and SNP + GSH paralleled the changes in ROS (O2 •– and H2O2) and MDA content (Table 3, Fig. 1). In particular, the decreased activity of SOD and lower content of O2 •– and H2O2 upon adding SNP, GSH, and SNP + GSH indicates the direct ROS quenching roles of NO and GSH. Moreover, antioxidative enzyme activity seems to be related to the oxidative stress intensity. The direct ROS quenching role of NO and GSH could have resulted in the plants suffering less stress and thereby contributed to less induction of antioxidative enzymes. In addition, SNP and GSH induced elevated activities of CAT, GPX, and GST further boosted the antioxidant capacity of the rice seedlings. Therefore, NO and GSH not only act as a directly preformed antioxidant but also function indirectly in modifying the redox balance by pro- or co-activating such antioxidant responses to alleviate Cu toxicity. The present work also corroborated earlier works where SNP supplementation decreased the activities of ROS scavenging enzymes that were enhanced in Cassia tora roots under Al stress (Wang and Yang 2005), in sunflower leaves under Cd stress (Laspina et al. 2005), in rice roots under As stress (Singh et al. 2009), and in wheat roots under Cd stress (Singh et al. 2008). Cai et al. (2010) and Chen et al. (2010a) also demonstrated that adding GSH counteracted the Cd-induced elevation of antioxidant enzymes like DHAR, MDHAR, GR, CAT, POD, and SOD in rice and barley genotypes.
MG is an inevitable product of environmental stresses which can aggravate oxidative stress (Hoque et al. 2012). Therefore, detoxification of this toxic compound is also a priority to confer higher tolerance to oxidative stress. In addition to MG detoxification, the physiological significance of the glyoxalase system is to maintain redox homeostasis by regenerating GSH. In this study, Gly I and Gly II activities significantly increased upon Cu exposure (Fig. 7a–b), which is consistent with our previous findings (Mostofa and Fujita 2013). Upregulation of their activities also correlated with the higher accumulation of GSH and also indicates their participation in mitigating MG-mediated damage in rice plants under Cu stress. However, SNP, GSH, and SNP + GSH did not show any stimulating effect on these enzymes, suggesting that they were less able to enhance GSH regeneration via the glyoxalase system. Gly I activity in SNP-, GSH-, or SNP + GSH-supplemented Cu-stressed seedlings was significantly higher than in control, which indicates that this increased level of Gly I consumed GSH and eliminated MG to some extent. Yadav et al. (2005) also observed that exogenous GSH lowers the MG level and optimal levels of both GSH and Gly I are needed for efficient detoxification of MG.
Conclusion
The findings of this study reveal that adding SNP and GSH had significant beneficial effects against Cu toxicity, which was reflected in the growth, photosynthesis, and vigor of the Cu-exposed seedlings. Exogenous SNP and GSH successfully decreased Cu-induced ROS and MDA levels, providing strong evidence that SNP and GSH substantially protects against oxidative stress. Here, reduced Cu uptake, elevated levels of AsA, redox ratios, PC, and higher activities of CAT, GPX, GST, and DHAR play an important role, at least in part, in SNP- and GSH-mediated alleviation of Cu-induced oxidative stress. We added SNP and GSH to a Cu solution to evaluate their combined versus individual effect. No extraordinary influence was observed for SNP + GSH treatment; however, considering the ROS level, induction level of enzyme activities and visual toxicity, SNP + GSH was shown to be more effective than SNP alone in the amelioration of Cu-induced oxidative damage. This study was carried out under laboratory conditions and the responses of SNP and GSH may vary from plant species to plant species under Cu stress. Therefore, it is necessary to conduct further experiments under field conditions with other plant varieties, which would be important research for augmenting sustainable crop production in heavy metal polluted areas.
Conflict of interest
The authors declare that they have no conflict of interest.
References
Anjum NA, Ahmad I, Mohmood I, Pacheco M, Duarte AC, Pereira E, Umar S, Ahmad A, Khan NA, Iqbal M, Prasad MNV (2012) Modulation of glutathione and its related enzymes in plants’ responses to toxic metals and metalloids — a review. Environ Exp Bot 75:307–324
Arnon DT (1949) Copper enzymes in isolated Chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beligni MV, Lamattina L (2002) Nitric oxide interferes with plant photooxidative stress by detoxifying reactive oxygen species. Plant Cell Environ 25:737–748
Bhuiyan MAH, Islam MA, Dampare SB, Parvez L, Suzuki S (2010) Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J Hazard Mater 179:1065–1077
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burkhead JL, Reynolds KAG, Abdel-Ghany SE, Cohu CM, Pilon M (2009) Copper homeostasis. New Phytol 182:799–816
Cai Y, Cao F, Cheng W, Zhang G, Wu F (2010) Modulation of exogenous glutathione in phytochelatins and photosynthetic performance against Cd stress in the two rice genotypes differing in Cd tolerance. Biol Trace Elem Res 143:1159–1173
Chen CT, Chen TH, Lo KF, Chiu CY (2004) Effects of proline on copper transport in rice seedlings under excess copper stress. Plant Sci 166:103–111
Chen F, Wang F, Wu FB, Mao WH, Zhang GP, Zhou M (2010a) Modulation of exogenous GSH on antioxidant defense system against Cd stress in two barley genotypes differing in Cd tolerance. Plant Physiol Biochem 48:663–672
Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, Zheng SJ (2010b) Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol 154:810–819
Chino M (1981) Metal stress in rice plants. In: Kitagishi K, Yamane I (eds) Heavy metal pollution in soils of Japan. Japan Scientific Societies Press, Tokyo, pp 65–80
Choudhary SP, Oral HV, Bhardwaj R, Yu JQ, Tran LSP (2012) Interaction of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. J Exp Bot 63:5659–5675
Cobbett CS, Goldsbrough P (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53:159–182
Contreras L, Mella D, Moenne A, Correa JA (2009) Differential responses to copper-induced oxidative stress in the marine macroalgae Lessonia nigrescens and Scytosiphon lomentaria (Phaeophyceae). Aquat Toxicol 94:94–102
De Vos RCH, Vonk MJ, Vooijs R, Schat H (1992) Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98l:853–858
Demirevska-Kepova K, Simova-Stoilova L, Stoyanova Z, Hölzer R, Feller U (2004) Biochemical changes in barley plants after excessive supply of copper and manganese. Environ Exp Bot 52:253–266
Doderer A, Kokkelink I, van der Veen S, Valk B, Schram A, Douma A (1992) Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta 112:97–104
Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G (2003) Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol 131:264–275
El-Shabrawi H, Kumar B, Kaul T, Reddy MK, Singla-Pareek SL, Sopory SK (2010) Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245:85–96
Elia AC, Galarini R, Taticchi MI, Dorr AJM, Mantilacci L (2003) Antioxidant responses and bioaccumulation in Ictalurus melas under mercury exposure. Ecotoxicol Environ Saf 55:162–167
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Graziano M, Lamattina L (2007) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52:949–960
Griffiths OW (1980) Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stochiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hoque TS et al (2012) Methylglyoxal-induced stomatal closure accompanied by peroxidase-mediated ROS production in Arabidopsis. J Plant Physiol 169:979–986
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plant 26:259–272
Hossain MA, Hossain MZ, Fujita M (2009) Stress induced changes of methylglyoxal level and Glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust J Crop Sci 3:53–64
Hossain MA, Mostofa MG, Fujita M (2013a) Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. Mol Plant Breed 4:50–70
Hossain MA, Mostofa MG, Fujita M (2013b) Heat-shock positively modulates oxidative protection of salt and drought-stressed mustard (Brassica campestris L.) seedlings. J Plant Sci Mol Breed 2:1–14
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hossain MA, Piyatida P, da Silva JAT, Fujita M (2012) Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J Bot 2012:1–37
Jones JB (1998) The micronutrients. In: Jones JB Jr (ed) Plant nutrition manual. CRC Press, Boca Raton, pp 55–76
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Kang YJ, Enger MD (1987) Effect of cellular glutathione depletion on cadmium induced cytotoxicity in human lung carcinoma cells. Cell Biol Toxicol 3:347–360
Kazemi N, Khavari-Nejad RA, Fahimi H, Saadatmand S, Nejad-Sattari T (2010) Effects of exogenous salicylic acid and nitric oxide on lipid peroxidation and antioxidant enzyme activities in leaves of Brassica napus L. under nickel stress. Sci Hortic 126:402–407
Kholodova V, Volkov K, Abdeyeva A, Kuznetsov V (2011) Water status in Mesembryanthemum crystallinum under heavy metal stress. Environ Exp Bot 71:382–389
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Li Y, Song YP, Shi GJ, Wang JJ, Hou XL (2009) Response of antioxidant activity to excess copper in two cultivars of Brassica campestris ssp. chinensis Makino. Acta Physiol Plant 31:55–162
Lippard SJ, Berg JM (1994) Principles of bioinorganic chemistry. University Science Books, Mill Valley
Marschner P (2012) Marschner’s mineral nutrition of higher plants, 3rd edn. Academic Press, Elsevier, London
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Mishra A, Choudhuri MA (1999) Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol Plant 42:409–415
Mostofa MG, Fujita M (2013) Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22:959–973
Mostofa MG, Yoshida N, Fujita M (2013) Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. doi:10.1007/s10725-013-9865-9
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Noctor G, Gomez L, Vanacker H, Foyer CH (2002) Interactions between biosynthesis, compartmentation, and transport in the control of glutathione homeostasis and signaling. J Exp Bot 53:1283–1304
Panda P, Nath S, Chanu TT, Sharma GD, Panda SK (2011) Cadmium stress-induced oxidative stress and role of nitric oxide in rice (Oryza sativa L.). Acta Physiol Plant 33:1737–1747
Saito R, Yamamoto H, Makino A, Sugimoto T, Miyake C (2011) Methylglyoxal functions as Hill oxidant and stimulates the photoreduction of O2 at photosystem I: a symptom of plant diabetes. Plant Cell Environ 34:1454–1464
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455
Singh HP, Batish DR, Kaur G, Arora K, Kohli RK (2008) Nitric oxide (as sodium nitroprusside) supplementation ameliorates Cd toxicity in hydroponically grown wheat roots. Environ Exp Bot 63:158–167
Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 20:289–297
Singh VP, Srivastava PK, Prasad SM (2013) Nitric oxide alleviates arsenic-induced toxic effects in ridged Luffa seedlings. Plant Physiol Biochem 71:155–163
Singla-Pareek SL, Yadav SK, Pareek A, Reddy MK, Sopory SK (2006) Transgenic tobacco overexpressing Glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol 140:613–623
Sun B, Jing Y, Chen K, Song L, Chen F, Zhang L (2007) Protective effect of nitric oxide on iron deficiency induced oxidative stress in maize (Zea mays). J Plant Physiol 164:536–543
Thounaojam TC, Panda P, Mazumdar P, Kumar D, Sharma GD, Sahoo L, Panda SK (2012) Excess copper induced oxidative stress and responses of antioxidants in rice. Plant Physiol Biochem 53:33–39
Thounaojam TC, Panda P, Choudhury S, Patra HK, Panda SK (2013) Zinc ameliorates copper-induced oxidative stress in developing rice (Oryza sativa L.) seedlings. Protoplasma. doi:10.1007/s00709-013-0525-8
Wang L, Yang L, Yang F, Li X, Song Y, Wang X, Hu X (2010) Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to Cu toxicity. J Plant Physiol 167:1298–1306
Wang YS, Yang ZM (2005) Nitric oxide reduces aluminum toxicity by preventing oxidative stress in the roots of Cassia tora L. Plant Cell Physiol 46:1915–1923
Wendehenne D, Gould K, Lamotte O, Durner J, Vandelle E, Lecourieux D, Courtois C, Barnavon L, Bentejac M, Pugin A (2005) NO signaling functions in the biotic and abiotic stress responses. BMC Plant Biol 5 (Suppl. 1) S35, doi:10.1186/1471-2229-5-S1-S35
Xiong J, Fu G, Tao L, Zhu C (2010) Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys 497:13–20
Yadav SK, Singla-Pareek SL, Reddy MK, Sopory SK (2005) Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants 11:1–11
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430
Yu CC, Hung KT, Kao CH (2005) Nitric oxide reduces Cu toxicity and Cu-induced NH4 + accumulation in rice leaves. J Plant Physiol 162:319–330
Zhang H, Li YH, Hu LY, Wang SH, Zhang FQ, Hu KD (2008) Effects of exogenous nitric oxide donor on antioxidant metabolism in wheat leaves under aluminum stress. Russ J Plant Physiol 55:469–474
Acknowledgements
M. G. Mostofa thankfully acknowledges the financial support from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Néstor Carrillo
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplementary material table T1 Effect of exogenous 0.2 mM sodium ferrocyanide (SF) and 0.2 mM sodium nitroprusside (SNP) on visual appearances in 0.1 mM copper (Cu) -treated rice seedlings. Supplementary material Fig. S1 Effect of exogenous SNP (A), GSH (B), and SNP+GSH (C) on visual appearances in Cu-treated rice seedlings. Supplementary material Fig. S2 Effect of exogenous 0.2 mM sodium ferrocyanide (SF) and 0.2 mM sodium nitroprusside (SNP) on visual appearances in 0.1 mM copper (Cu) -treated rice seedlings. (PDF 522 kb)
Rights and permissions
About this article
Cite this article
Mostofa, M.G., Seraj, Z.I. & Fujita, M. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251, 1373–1386 (2014). https://doi.org/10.1007/s00709-014-0639-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0639-7