Abstract
In this research, the Cu phytoremediation capacity of common mullein (Verbascum thapsus L.) was evaluated concerning plant growth, antioxidant enzymes, and photosynthetic activities. Plants were subjected to five Cu concentrations (0, 125, 250, 375, and 500 mg/L) under the hydroponic conditions for 2 weeks. The results showed that at 125 mg/L, root and shoot biomass and chlorophylls remained the same as that of the control and then declined with increasing concentrations of Cu, when compared with control. The carotenoid contents remained unchanged up to 250 mg/L compared with control and then dropped with raising Cu dose. The raising of antioxidant enzymes activity reflected the occurrence of stress due to Cu exposure as manifested by increased MDA and ion leakage level. However, increased antioxidant enzymes may be associated with the tolerance capacity of V. thapsus to protect the plant from oxidative damage. Except for the highest concentration (500 mg/L), Cu accumulation in the roots and shoots all increased significantly with increasing Cu concentration, and the Cu accumulation in shoots was greater than roots. The Cu accumulation reached its maximum level at 375 mg/L Cu concentration, with 492.8 and 447.3 mg/kg DW in shoots and roots, respectively, which is highly greater than the threshold value for a Cu (hyper)accumulator plant. The extraction coefficient (EC) close to 1, and translocation factor (TF) > 1 from 125 to 375 mg/L Cu, suggested that V. thapsus could be used as a viable plant species for Cu phytoextraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While the demands for crop products continue to increase, global crop production has undergone sever variations in current years and it is severely restricted by different abiotic stresses such as drought, salinity, and heavy metals (Soares et al. 2019). Among these stresses, contamination of soils by heavy metals is a considerable environmental problem worldwide over the past several decades due to different human activities, including the use of fertilizers and pesticides, mining and smelting operations, industrial effluents, use of fossil fuels, and wastewater (Bokhari et al. 2016; Zhou et al. 2019). It may result in a critical threat to animal and human health as well as production capability and food quality (Jan et al. 2015). Although some heavy metals like copper (Cu), boron (B), zinc (Zn), molybdenum (Mo), manganese (Mn), and iron (Fe) are essential micronutrient, many other metals such as mercury (Hg), silver (Ag), cadmium (Cd), and lead (Pb) have no biological role and they are non-essential elements (Adrees et al. 2015). Essential micronutrients are needed in little amounts for healthy plant growth and development throughout life but are toxic to plants at higher levels (Dalcorso et al. 2014). Copper is an essential micronutrient used in several electron transport reactions in mitochondria and chloroplasts (Contreras et al. 2018). It also participates in hormone signaling, protein trafficking machinery, cell wall metabolism, iron mobilization, and transcription signaling (Ducic and Polle 2005). However, at high levels, copper is strongly phytotoxic and induce the increase of reactive oxygen species (ROS) levels within subcellular compartments and might, therefore, cause oxidative damage such as protein oxidation, lipid peroxidation, and nucleic acid cleavage (Martins and Mourato 2006). Several techniques are available to remedy soils that are contaminated by heavy metals (Nouri et al. 2009). Cleaning up of metal-contaminated soils using traditional remediation methods is not economical, and in some instances, they can degrade the biodiversity and result in deterioration of soil structure and fertility (Ghazaryan et al. 2019). Phytoremediation is an emerging sustainable, cost-effective, and eco-friendly (green) technique that uses the ability of hyperaccumulator species to immobilize, remove, or degrade pollutants from contaminated soils (Goswami and Das 2016). There are many techniques in phytoremediation such as phytoextraction, phytodesalination, rhizofiltration, phytovolatilization, phytostabilization, and phytofiltration (Palanivel et al. 2020). However, phytoextraction, phytostabilization, and rhizofiltration are commercially more viable than other methods (Thakur et al. 2016). The phytoextraction is the process of using hyperaccumulator plants to eradicate heavy metals from the surroundings by taking them from roots to shoots and consequently, eliminating the elements from the polluted environment (Reeves 2003). Hyperaccumulators can uptake high quantities of one or more heavy metals from the growth medium. Furthermore, the heavy metals are not maintained in the roots but are transferred to the shoots and gathered in the aerial parts, particularly leaves, at concentrations 100–1000-fold greater than those found in non-hyperaccumulators, without showing toxicity symptoms (Muszyńska and Hanus-Fajerska 2015). Generally, 5–30 mg/kg of Cu in crop tissues are considered as sufficient (Ballabio et al. 2018). (Hyper)accumulation of Cu was first defined as > 1000 mg/kg foliar Cu (Malaisse et al. 1978), but recently revised downwards to 300 mg/kg (van der Ent et al. 2013; Reeves et al. 2018). The fast and efficacious translocation of heavy metals from roots to shoots, high biomass yield, and capability to withstand heavy metal stress and metal toxicity is prominent characteristics of hyperaccumulators (van der Ent et al. 2013; Tiwari and Lata 2018). The excessive Cu can catalyze the generation of the ROS and free radicals in Fenton-type reactions, which induce oxidation of macromolecules like carbohydrates, proteins, lipids, and nucleic acids and causing oxidative stress in cells (Fatnassi et al. 2015). The detoxification of ROS compounds in hyperaccumulator plants is required to protect the plant cells against the deleterious consequence of ROS and free radicals (Rascio and Navari-Izzo 2011). The toxic effects resulting from the oxidative stress may be alleviated by enzymatic defense systems including superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT), and nonenzymatic protection systems like proline, phenolic compounds, ascorbate, and glutathione (Flora 2009). Therefore, examining the antioxidant defense system (ADS) in such a plant may furnish valuable data about its phytoremediation ability (Goswami and Das 2016). The genus Verbascum L., relates to the family Scrophulariaceae and Iran, Pakistan, and Turkey, are the foremost centers of its diversity. It contains 360 species all over the world and 42 species in Iran (Karamian and Ghasemlou 2014). The V. thapsus (common mullein) is a biennial plant that is widely distributed throughout Asia, Europe, North Africa, and North America (Zhao et al. 2011). In Iran, it is grown mainly in the Northern, North-Western, and Central provinces (Morteza-Semnani et al. 2012). The species tolerates a wide range of environmental conditions and is adapted to the various habitats and different regions of Iran, including roadsides, open forests, rocky mountains, the margins of the rivers, and heavy metal polluted environments (Karamian and Ghasemlou 2014). However, very few studies have explored the potential of V. thapsus to remediate Cu-polluted environments (Morina et al. 2016). The objectives of the current study are to (1) observe the phytotoxicity of Cu on morphological and biochemical parameters of V. thapsus, (2) to determine the Cu accumulation efficiency in below and above ground biomass of V. thapsus growing on a Cu enriched medium, (3) to analyze the role of the antioxidant defense system in the Cu tolerance mechanism of V. thapsus, and (4) to investigate the possibility of using V. thapsus for Cu phytoremediation. Findings from the present study will add to our knowledge about the mechanism of Cu tolerance and accumulation in V. thapsus.
Materials and methods
Plant material, growth condition, and treatment
The seeds of common mullein were prepared from the Pakan Bazr Company (Isfahan, Iran). The seeds were sterilized with 5% NaOCl for 5 min and then rinsed three times with ultrapure water. The seeds were placed on water-moistened filter paper in 9.5-Cm Petri dishes and germinated in the dark condition at 20 °C. After 10 days of germination, the seedlings were transferred to small plastic beakers (20 × 15 × 10 cm) filled with one-tenth (v/v)-strength Hoagland nutrient medium (600 mL) and transferred to a growth chamber (Noor Sanat Ferdows, Iran) with a 16-h light/8-h dark photoperiod and 15 °C/25 °C day/night temperature regime. Plants were irradiated by white fluorescent 36-W tubes that provided irradiance (400–700 nm) of ca. 80 μmol photons/m2/s1. The nutrient medium was renewed every second day, and the concentration of nutrients was increased by 10% every week. After 8 weeks, the uniform plants bearing 8-leaves were selected and exposed to different levels of Cu (0 (control), 125, 250, 375, and 500 mg/L) in 80% Hoagland nutrient solution. Copper was supplied as CuSO4. 5H2O (Merck, KG, Darmstadt, Germany). Plants were harvested after 14 days of Cu treatment without damaging the roots. Roots were washed twice in 10 mM Na2EDTA (pH 7.0) and subsequently rinsed with distilled water to remove adsorbed metal ions. The plant separated into shoots and roots and stored at − 70 °C after freezing in liquid nitrogen for further analysis.
Biomass determination
The dry biomass of shoots and roots was determined 14 days after the initiation of the experiment. The plant samples were then dried in an oven for 48 h at 80 °C, and their dry biomass was measured (Morina et al. 2016).
The capacity of copper uptake in plant organs
Copper concentration in common mullein shoots and roots was analyzed after digesting the dried samples in an acidic mixture of nitric and perchloric acid (HNO3: HClO4), according to the APHA method (1992) using an Atomic Absorption Spectrometer (Vario6, Analytik Jena, Germany). The phytoremediation ability of V. thapsus plants was evaluated by the translocation factor (TF) and the extraction coefficient (EC) using the following formula (Goswami and Das 2015):
Measurement of the photosynthetic pigments
For the estimation of the photosynthetic pigments, 0.25 g of shoot samples were extracted with 80% acetone. The extract was then centrifuged at 3000 g, and the absorbances of the extract were taken at 646.8, 663.2, and 470 nm with a UV/Vis spectrophotometer (Specord 200 Plus, Analytik Jena, Germany) based on the Lichtenthaler (1987) method.
Assessment of lipid peroxidation and electrolyte leakage
The level of lipid peroxidation was estimated using the measurement of the malondialdehyde (MDA) content based on the Sun et al. (2010). The fresh sample (0.5 g) was homogenized in 10% trichloroacetic acid (TCA) and centrifuged at 12,000 g for 10 min. Then, 2 mL of extract was added to 2 mL of reaction solution containing 10% TCA and 0.5% thiobarbituric acid (TBA). The mixture was heated in boiled water for 30 min and then cooled immediately on an ice bath. The mixture was centrifuged at 10,000 g for 15 min, and the absorbance was read at 450, 532, and 600 nm. The concentration of MDA was measured as 6.45 (A532-A600) − 0.56 A450.
Electrolyte leakage was determined according to Dionisio-Sese and Tobita (1998). Root sample (100 mg) was cut into 5-mm length and placed in closed tubes containing 10 mL of deionized water. The tubes were covered with rubber caps and put in a water bath maintained at 32 °C. After 2 h, the initial electrical conductivity (EC1) of the medium was determined using a conductivity meter (SG3-FK2 SevenGoTM, Mettler-Toledo, USA). The samples were then autoclaved at 120 °C for 30 min, cooled down to 25 °C, and their final electrical conductivity (EC2) was measured. The electrolyte leakage (EL) was calculated from EL = (EC1/EC2) × 100 (%). The experiment was performed with three replicates for each treatment.
Antioxidant enzyme extraction and assays
Shoot and root samples (0.1 g) were extracted in 2 mL of 50 mM sodium phosphate buffer (pH 7.0) including 1 mM EDTANa2 and 0.5% polyvinylpyrrolidone. The homogenate was centrifuged for 30 min at 13,000 g, and the extract was applied for enzymatic assays. Total protein content was determined according to Bradford (1976) assay with the use of bovine serum albumin as the standard solution. The SOD activity was measured spectrophotometrically based on its ability to inhibit the photochemical reduction of nitroblue tetrazolium, adopting the method of Dhindsa et al. (1981). The CAT activity was determined based on the method of Beers and Sizer (1952), by measuring the H2O2 absorbance reduction at 240 nm. The APX activity was assayed following the ascorbate oxidation by the decrement in absorption at 290 nm according to the method of Nakano and Asada (1981).
Statistical analysis
In all experiments (except for the ion leakage experiment which was performed in three replicates), five replicates were considered for each treatment. The data analysis was performed with one-way ANOVA followed by Duncan’s multiple range tests (p = 0.05) for the comparison of mean values using SAS 9.1.
Results and discussion
Influence of Cu on biomass and photosynthetic pigments
The presence of Cu in the growth medium resulted in a gradual reduction in the shoot and root biomass of V. thapsus plants, and the highest growth retardation was found in plants treated with 500 mg/L Cu. Data on the biomass production indicated that V. thapsus plants could tolerate 125 mg/L Cu for 14 days without a significant decrease in shoot and root biomass compared with control. With increasing Cu levels in the nutrient medium, the growth of V. thapsus plants slowed. For 250, 375, and 500 mg/L Cu levels, the shoot biomass decreased by 17%, 33%, and 45%, and the root biomass declined by 30%, 47%, and 63%, respectively, compared with the control (Table 1). Moreover, excess copper was progressively resulted in significant increase of leaf chlorosis and a concomitant decrease in the percentage of healthy green leaves (Fig. 1).
The results presented in Table 2 designated that in comparison with control, no significant variation was found in terms of chlorophylls (Chlorophyll a, Chlorophyll b, and total chlorophyll) at 125 mg/L Cu level. Increasing the Cu concentration in the nutrient solution significantly reduced the chlorophylls in the leaves of V. thapsus plants. A considerable decrement in total chlorophyll contents (15%, 40.7%, and 54.1% for 250, 375, and 500 mg/L, respectively, compared with the control) was registered. The chlorophyll a/b ratio was slightly decreased with elevating the Cu concentration. However, there were significant differences only at 375 and 500 mg/L Cu levels. Other results indicated that Cu had no punctual impact on carotenoid content in V. thapsus leaves up to 250 mg/L. Indeed, although the content of carotenoid had a decreasing trend with raising Cu concentration, the influence was not statistically significant (p < 0.05). However, when the Cu level in the nutrient solution was exceeded 250 mg/L, the carotenoid content significantly declined, and at 500 mg/L, Cu was 41% lower than the control.
Cu stress significantly declined V. thapsus biomass in a dose-dependent manner. However, at 125 mg/L Cu, no significant changes in shoot and root biomass were found. The reduction of biomass and yield is a noticeable effect of exposure to excess Cu in plants, which was also detected in this study (Adrees et al. 2015). Various studies have demonstrated the adverse impact of excessive amounts of Cu on the biomass and yield of plants grown in soil conditions and hydroponics (Fidalgo et al. 2013; Li et al. 2018; Saleem et al. 2020a). An overall reduction of plant biomass and photosynthesis rate, inhibition of root growth, altered nutrient uptake, membrane disruption, chlorosis, and necrosis are the typical reported symptoms of a Cu excess (Lange et al. 2017; Morina et al. 2016). Photosynthesis is one of the principal constituents determining plant biomass (Aggarwal et al. 2011). Chlorophylls are the essential photosynthetic pigments and can be regarded as a fundamental biochemical index of the photosynthetic potential of the plant in response to different constraints (Ashraf and Harris 2013). The reduction of photosynthetic pigments is amongst the common detrimental effects of excessive Cu concentration in the plant's culture medium (Llagostera et al. 2016). It has been suggested that Cu at toxic doses blocks the enzymes acting in chlorophyll biosynthesis and stimulates the chlorophyll degradation by chlorophyllase activity (Shiyab 2018). High Cu concentrations can also induce degradation of the internal and structural constituents of chloroplasts and may even replace the Mg ion needed for chlorophyll biosynthesis, which impairs its synthesis (Marques et al. 2018). Our results indicated that Cu stress significantly dwindled the photosynthetic pigment in plants, which eventually caused a reduction in plant biomass. Diminution of chlorophyll content owing to higher doses of Cu was also reported in Chinese cabbage (Shahbaz et al. 2010), sunflower (Kolbas et al. 2014), rapeseed and Indian mustard (Feigl et al. 2015), Calendula officinalis L. (Goswami and Das 2016), and ramie (Boehmeria nivea L.) (Rehman et al. 2019). An overall reduction in carotenoid content was observed with increasing Cu concentrations; however, no significant changes were observed in leaf carotenoid contents for 125 and 250 mg/L Cu levels. Carotenoids play a crucial role in the quenching of singlet oxygen and detoxifying of other ROS compounds that are generated upon exposure to various constraints, including heavy metals (Bose et al. 2014). In addition to their activity as subsidiary pigments, carotenoids exhibit antioxidant characteristics (Strzalka et al. 2003). Therefore, the lack of significant changes in the carotenoid contents of the V. thapsus at 125 and 250 mg/L Cu levels maybe due to this role. However, when the Cu level was raised, a significant reduction in leaf carotenoid contents was observed. Diminution in carotenoid contents in higher Cu concentrations might be a result of Cu-induced oxidative stress, which manifested as MDA accumulation. This indicates that carotenoids as antioxidants are not capable of inhibiting the interference of Cu ions and the production of the ROS compounds that damage photosynthetic pigments.
Influence of Cu on lipid peroxidation, electrolyte leakage, and protein contents
In our study, The MDA levels, which portend oxidative damage, were increased in the shoots and roots of V. thapsus plants at all the Cu levels compared with the control (Table 3). In 125, 250, 375, and 500 mg/L Cu levels, the shoot MDA contents increased by 2.79-, 4.56-, 8.2-, and 12.59-fold, whereas the root MDA contents increased by 2.2-, 3.64-, 7.07-, and 10.72-fold, respectively, compared with the control. Electrolyte leakage tended to increase significantly in the roots of V. thapsus plants with increasing Cu concentration. At higher Cu concentration (500 mg/L), the electrolyte leakage increased more than 3 times compared with the control (Table 3). Although Cu as a metal cofactor participates in the structure of the SOD enzyme, it can directly induce the production of ROS compounds via Fenton and Haber–Weiss reactions (Küpper et al. 2009). It has been revealed that additional Cu stimulates the production of free radicals and the ROS compounds and can induce lipid peroxidation in plants (Fatnassi et al. 2015). Lipid peroxidation due to excess copper interrupts the membrane organization, which may inactivate membrane-bound enzymes and receptors, increasing the permeability of the cell membranes and promote the leakage of electrolyte and subsequent loss of cell turgor pressure (Panda 2008). The MDA is the end product of membrane lipid peroxidation and accumulates in plants after exposure to oxidative stress. Electrolyte leakage showed the same trend with MDA contents in the root of V. thapsus plants. This finding suggests that Cu-induced MDA accumulation could cause damage to the cell membranes, resulting in increased electrolyte leakage. Similar results were reported in Hibiscus cannabinus L. (Saleem et al. 2020b), Verbascum olympicum Boiss. (Akpinar et al. 2016), Alternanthera bettzickiana L. (Khalid et al. 2020), etc. Accordingly, the level of MDA and electrolyte leakage are commonly applied as an indicator to assess the sensibility of the plant to oxidative stress under different constraints including heavy metal stress (Juknys et al. 2012).
The total soluble protein content of shoots was progressively enhanced with the elevated concentrations of Cu, reach its highest in 375 mg/L Cu level, and after that decreased at 500 mg/L Cu concentration. However, no statistical differences were found among 125, 250, and 375 mg/L Cu Levels. Alteration in the protein metabolism of plants is one of the harmful effects of heavy metal-induced oxidative stress. Accordingly, the content of soluble protein is employed as an essential index of the physiological status of plants (Doğanlar 2013). The increment in total soluble protein content of V. thapsus plants under Cu stress may be due to inducing the biosynthesis of stress-related proteins, including metal chelator polypeptides (metallothionein and phytochelatin) and other proteins involved in metal detoxification or homeostasis for adaptation to excess Cu level (Parmar et al. 2013). The increase in total soluble protein content in response to heavy metals stress was expressed by other researchers (Heiss et al. 2003; Rolli et al. 2010; Doğanlar 2013; Zoufan et al. 2018). With increasing the Cu concentration in the nutrient solution, the content of total soluble protein remarkably declined. The reduction in protein content under the highest Cu level can be related to inhibition of protein synthesis or an increase in protein degradation due to ROS accumulation (Hasan et al. 2017).
Different letters indicate significant differences at p < 0.05 according to the Duncan’s test
Effect of Cu on antioxidant enzymes activity
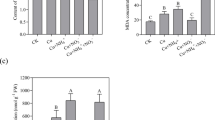
The antioxidant enzymes activity in shoots and roots of V. thapsus plants exposed to Cu for 14 days were shown in Fig. 2. The SOD activity in both the shoots and roots was progressively enhanced proportionally to the increasing Cu levels in the growth medium. The highest SOD activity was determined in the shoots and roots of plants exposed to 375 mg/L Cu. Further application of Cu at 500 mg/L slightly decreased the enzyme activity in both of the shoots and roots. However, the difference was not statistically significant (Fig. 2a). In plants exposed to Cu, the CAT activity in both the shoots and roots gradually increased and the highest value for CAT activity was observed at Cu Level of 375 mg/L and then was declined significantly at 500 mg/L Cu treatment (Fig. 2b). Our results demonstrated that 125 mg/L Cu had no significant effect on the APX activity of the roots and shoots. With enhancing Cu levels, the activity of APX was significantly increased. Similar to SOD and CAT enzymes activity, the highest APX activity was observed in the shoots and roots of plants exposed to 375 mg/L Cu for 14 days and then was significantly diminished at 500 mg/L Cu level (Fig. 2c). Different heavy metals, including copper, stimulate oxidative stress due to the overproduction of the ROS compounds in plant cells (Shahid et al. 2014). To refrain from the detrimental effects of the ROS on cellular components, plants have formed a highly organized and powerful antioxidant system that comprises enzymatic and non-enzymatic antioxidants that are ordinarily effective in limiting adverse effects of the ROS (Das and Roychoudhury 2014). Antioxidant enzymes are substantial constituents in hindering the oxidative stress in plants by detoxifying free radicals (Caverzan et al. 2016). Many investigations have stated that increases in activity of the antioxidant enzymes in plants are correlated to the level of tolerance against Cu toxicity (Thounaojam et al. 2013; Fatnassi et al. 2015; Goswami and Das 2016; Liu et al. 2018). In this study, the efficiency of enzymatic antioxidants was assessed to specify more accurately the strategies of Cu tolerance in V. thapsus plants. The SOD is a metalloenzyme and most potent antioxidant that constitutes the front line of protection versus ROS by accelerating the dismutation of superoxide (O2•–) to hydrogen peroxide (H2O2) in the cytosol, chloroplast, and mitochondria of plant cells (Gupta et al. 2018). Because SOD functions as a first-line enzyme in defense against oxidative stress, the alterations found in its activity confirm that exposure to excess Cu, produce superoxide radicals in the shoots and roots of V. thapsus plants, and induce the stress responses. The hydrogen peroxide, which is formed by the SOD activity, is yet toxic and requires to be eliminated in the succeeding reactions by conversion to H2O (Das and Roychoudhury 2014). Different enzymes are responsible for the elimination and adjusting of the intracellular levels of H2O2 in higher plants, but the most prominently characterized enzymes are CAT and APX (Caverzan et al. 2016). The CAT is one of the most vital antioxidant enzymes implicated in the elimination of toxic peroxides by decomposing H2O2 into water and molecular oxygen (Sharma and Ahmad 2014). The CAT activity was raised in all Cu concentrations except 500 mg/L, which can be regarded as inferred evidence for the role of CAT in scavenging of H2O2 that was induced under Cu stress in V. thapsus plants. The ascorbate-glutathione cycle is another significant hydrogen peroxide scavenging system in plant cells, in which APXs perform a pivotal function by catalyzing the transformation of H2O2 into H2O, applying ascorbate as a particular electron donor (Caverzan et al. 2012). These results indicate that the ascorbate-glutathione cycle probably has a significant role in the ROS detoxification in V. thapsus exposed to copper. Our result showed that high Cu concentration is detrimental for the antioxidative ability of V. thapsus and resulted in antioxidant enzyme activities reduction, which is in agreement with the finding in Vicia faba L. (Fatnassi et al. 2015), watercress (Nasturtium officinale R. Br.) (Ercan et al. 2018), Verbascum olympicum Boiss. (Akpinar et al. 2016), and B. nivea (Rehman et al. 2019). Detoxification of ROS can be attained by antioxidant enzymes such as SOD, CAT, and APX. Thus, the remarkable increase in activity of these enzymes, as found in our study, may be regarded as evidence for the capability of the plant to retain an equilibrium between the creation and detoxification of ROS. The elevated biosynthesis of important antioxidant enzymes in hyperaccumulator plants functions as a mechanism to augment the antioxidant defense system of the cell and confront the hazards of ROS increment as a result of heavy metal stress (Goswami and Das 2016). Therefore, it can be concluded that the production of SOD, CAT, and APX can serve as valuable biomarkers for Cu tolerance in V. thapsus.
The copper concentration in shoots and roots and indexes
The copper concentration in shoots and roots of V. thapsus plants exposed to various treatments of Cu are given in Table 4. The Cu concentration progressively was elevated in both tissues up to 375 mg/L Cu, but at higher doses, the copper accumulation began to decline. Excluding for the highest concentrations of copper (500 mg/L), the accumulation of Cu in shoots was higher than in roots. The results indicate that at 500 mg/L Cu, as an exclusion strategy, the plant limited the transport of Cu to the aerial parts to avoid phytotoxicity. In this research, V. thapsus plants gathered Cu in the range 92.5–492.8 mg/kg, that is greatly exceeding from the standard (5–25 mg/kg) and noxious (20–100 mg/kg) levels of copper in plants (Ballabio et al. 2018). Arslan et al. (2010) reported that Verbascum bombyciferum Boiss. has the capability of Cd2+, Cr3+, Cu2+, Ni2+, Pb2+, and Zn2+ accumulation, especially in aboveground parts. Morina et al. (2016) showed that metal tolerance can occur in Verbascum populations after a relatively short time of exposure to metal-contaminated soil. Plants Cu tolerance varies with species and cultivar, but in general, plants are Cu-excluders and uncommon are Cu accumulator plants (Baker and Brooks 1989; Reeves 2003). However, the phytoextraction of copper by some plant species including Calendula officinalis L. (Goswami and Das 2016), Cyamopsis tetragonoloba L. (Amin et al. 2018), B. nivea (Rehman et al. 2019), and Indian mustard (Napoli et al. 2019) have been reported. The heavy metal uptake by the plant includes the movement of metal ions through the plasma membrane (PM) of the root cells, xylem loading, and translocation to shoots and, ultimately detoxification, and sequestration of metal ions at the whole plant, cellular, and subcellular levels (Cheng 2003). A characteristic feature of a suitable hyperaccumulator is its ability to gather metal ions principally in their shoots at different concentrations of exogenous metal ions (Peer et al. 2005). The prerequisite for the accumulation of metal in the plant is metal tolerance (Muszyńska and Hanus-Fajerska 2015). To depreciate the harmful effects of heavy metal exposure and their accumulation, plants have developed a variety of detoxification mechanisms. Such mechanisms principally relied on metal chelation and compartmentalization within the vacuole (Yruela 2009; Yadav 2010). Plants employ the conserved CTR/COPT family of high-affinity Cu transport proteins at the PM to facilitate cellular Cu acquisition (Sanz et al. 2018). Copper enters the cytoplasm in Cu+ form. The Cu ions are bound by chaperons and chelators. Chelators, including phytochelatins, metallothioneins, amino acids, and organic acids, are involved in the detoxification of metals, while chaperons mainly distribute metal ions to metal-requiring protein and organelles (Clemens 2001; Ducic and Polle 2005). The plant cells store amazing kinds of toxic compounds in their vacuoles, and there is plentiful evidence for vacuolar sequestration of metal ions in plant species (Clemens 2001). The vacuolar compartmentalization relies on two vacuolar pumps (V-PPase and V-ATPase) and some transporters on tonoplast, which are directly driven by proton motive force, and primal ATP-dependent pumps (Sharma et al. 2016). In the current research, the Cu concentrations in shoots were higher than in roots, suggesting the effective transfer of Cu from roots to shoots. The highest accumulation of Cu was 492.8 ± 20.143 mg/kg DW which was seen in the shoots at 375 mg/L treatment. The Phytoremediation efficiency for V. thapsus was quantified by evaluating the extraction coefficient (EC) and translocation factor (TF) values (Table 4). There was an increment in the EC index with raising the Cu levels. The maximum EC was 0.9856 at 375 mg/L. As the concentration of copper in the medium increased, the EC index declined. The TF is the index that shows the potential of the plant to translocate the Cu from roots to shoots. Except at 500 mg/L, which demonstrated TF of 0.869, the translocation of Cu from root to shoot at other concentrations, was > 1, which is the specified limit for an optimum hyperaccumulator (Muszyńska and Hanus-Fajerska 2015). The decrement in the TF index with raising Cu dose implicates an exclusion mechanism (Goswami and Das 2016). Lower shoot metal concentration and higher root metal concentration represent the confinement of Cu translocation from the roots towards upper parts through the xylem sap stream at the higher levels (phytostabilization).
Conclusions
The current research demonstrated that V. thapsus showed excellent Cu tolerance and can accumulate phytotoxic quantities of Cu in its shoots. The species can also be recommended for its capacity for Cu phytoremediation objectives due to its extensive root system for metal uptake, and elevated production of biomass in the presence of a high level of heavy metal. With inducible and efficient antioxidant enzymes, high accumulation of metal in organs, EC close to 1, and TF > 1, V. thapsus can be suggested for broad-scale utilization to amend Cu polluted soils. Although the results of the current research are promising, additional investigation is required to appraise the usefulness of V. thapsus under field conditions for remediation purposes and in the amendment of soils polluted with various metals.
Data availability
Not applicable
References
Adrees M, Ali S, Rizwan M, Ibrahim M, Abbas F, Farid M, Zia-ur-Rehman M, Irshad MK, Bharwana SA (2015) The effect of excess copper on growth and physiology of important food crops: a review. Environ Sci Pollut Res 22:8148–8162. https://doi.org/10.1007/s11356-015-4496-5
Aggarwal A, Sharma I, Tripathi B, Munjal AK, Baunthiyal M, Sharma V (2011) Metal toxicity and photosynthesis. In: Itoh S, Mohanty P, Guruprasad KN (eds) Photosynthesis: overviews on recent progress and future perspectives. IK International, New Delhi, pp 229–236
Akpinar AU, Arsalan H, Guleryuz G, Kirmizi S (2016) Antioxidative defense mechanism of the ruderal Verbascum olympicum Boiss. against copper (Cu)-induced stress. Open Life Sci 11:10–20. https://doi.org/10.1515/biol-2016-0002
Amin H, Arain BA, Abbasi MS, Jahangir TM, Amin F (2018) Potential for phytoextraction of Cu by Sesamum indicum L. and Cyamopsis tetragonoloba L.: a green solution to decontaminate soil. Earth Syst Environ 2:133–143. https://doi.org/10.1007/s41748-018-0038-x
APHA (1992) Standard methods for the examination of water and wastewater, 14th edn. American public health association–AWWA–WPCF, Washington (DC)
Arslan H, Güleryüz G, Leblebici Z, Kırmızı S, Aksoy A (2010) Verbascum bombyciferum Boiss. (Scrophulariaceae) as possible bio-indicator for the assessment of heavy metals in the environment of Bursa, Turkey. Environ Monit Assess 163:105–113. https://doi.org/10.1007/s10661-009-0820-1
Ashraf M, Harris PJC (2013) Photosynthesis under stressful environments: an overview. Photosynthetica 51:163–190. https://doi.org/10.1007/s11099-013-0021-6
Baker AJM, Brooks R (1989) Terrestrial higher plants which hyperaccumulate metallic elements. A review of their distribution, ecology and phytochemistry. Biorecovery 1:81e126. https://doi.org/10.1080/01904168109362867
Ballabio C, Panagos P, Lugato E, Huang JH, Orgiazzi A, Jones A, Fernández-Ugalde O, Borrelli P, Montanarella L (2018) Copper distribution in European topsoils: an assessment based on LUCAS soil survey. Sci Total Environ 636:282–298. https://doi.org/10.1016/j.scitotenv.2018.04.268
Beers RF, Sizer IW (1952) A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–140
Bokhari SH, Ahmad I, Mahmood-Ul-Hassan M, Mohammad A (2016) Phytoremediation potential of Lemna minor L. for heavy metals. Int J Phytoremediation 218(1):25–32. https://doi.org/10.1080/15226514.2015.1058331
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257. https://doi.org/10.1093/jxb/ert430
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Caverzan A, Passaia G, Barcellos Rosa S, Werner Ribeiro C, Lazzarotto F, Margis-Pinheiro M (2012) Plant responses to stresses: role of ascorbate peroxidase in the antioxidant protection. Genet Mol Biol 35:1011–1019. https://doi.org/10.1590/s1415-47572012000600016
Caverzan A, Casassola A, Brammer SP (2016) Reactive oxygen species and antioxidant enzymes involved in plant tolerance to stress. In: Shanker A (ed) Abiotic and biotic stress in plants. Intech Open, London, pp 463–480. https://doi.org/10.5772/61368
Cheng S (2003) Heavy metals in plants and phytoremediation. Environ Sci Pollut Res 10(5):335–340. https://doi.org/10.1065/espr2002.11.141.3
Clemens S (2001) Molecular mechanism of plant metal tolerance and homeostasis. Planta 212:475–486. https://doi.org/10.1007/s004250000458
Contreras RA, Pizarro M, Köhler H, Sáez CA, Zúñiga GE (2018) Copper stress induces antioxidant responses and accumulation of sugars and phytochelatins in Antarctic Colobanthus quitensis (Kunth) Bartl. Biol Res 51:48. https://doi.org/10.1186/s40659-018-0197-0
Dalcorso G, Manara A, Piasentin S, Furini A (2014) Nutrient metal elements in plants. Metallomics 26:1770–1788. https://doi.org/10.1039/c4mt00173g
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Plant Sci 2:53. https://doi.org/10.3389/fenvs.2014.00053
Dhindsa RS, Plumb-Dhindsa P, Throne TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–110. https://doi.org/10.1093/jxb/32.1.93
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135(1):1–9. https://doi.org/10.1016/S0168-9452(98)00025-9
Doğanlar ZB (2013) Metal accumulation and physiological responses induced by copper and cadmium in Lemna gibba, L. minor and Spirodela polyrhiza. Chem Speciat Bioavailab 25(2):79–88. https://doi.org/10.3184/095422913X13706128469701
Ducic T, Polle A (2005) Transport and detoxification of manganese and copper in plants. Braz J Plant Physiol 17:103–112. https://doi.org/10.1590/S1677-04202005000100009
Ercan FS, Ercan N, Yilmaz DD (2018) Effect of heavy metal stress on antioxidant enzymes and DNA damage in Nasturtium officinale R.Br. (watercress). Toxin Rev 38(4):1–10. https://doi.org/10.1080/15569543.2018.1471091
Fatnassi IC, Chiboub M, Saadani O, Jebara M, Jebara SH (2015) Impact of dual inoculation with Rhizobium and PGPR on growth and antioxidant status of Vicia faba L. under copper stress. C R Biol 338:241e254–241e254. https://doi.org/10.1016/j.crvi.2015.02.001
Feigl G, Kumar D, Lehotai N, Molnar A, Raca E, Ordog A, Erdei L, Kolbert Z, Laskay G (2015) Comparing the effects of excess copper in the leaves of Brassica juncea (L. Czern) and Brassica napus (L.) seedlings: Growth inhibition, oxidative stress and photosynthetic damage. Acta Biol Hung 66(2):205–221. https://doi.org/10.1556/018.66.2015.2.7
Fidalgo F, Azenha M, Silva AF, de Sousa A, Santiago A, Ferraz P, Teixeira J (2013) Copper-induced stress in Solanum nigrum L. and antioxidant defense system responses. Food Energy Secur 2:70–80. https://doi.org/10.1002/fes3.20
Flora SJS (2009) Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxidative Med Cell Longev 2(4):191–206. https://doi.org/10.4161/oxim.2.4.9112
Ghazaryan K, Movsesyan H, Ghazaryan N, Watts BA (2019) Copper phytoremediation potential of wild plant species growing in the mine polluted areas of Armenia. Environ Pollut 249:491–501. https://doi.org/10.1016/j.envpol.2019.03.070
Goswami S, Das S (2015) A Study on cadmium phytoremediation potential of Indian mustard, Brassica juncea. Int J Phytoremediation 17:583–588. https://doi.org/10.1080/15226514.2014.935289
Goswami S, Das S (2016) Copper phytoremediation potential of Calendula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotoxicol Environ Saf 126:211e218–211e218. https://doi.org/10.1016/j.ecoenv.2015.12.030
Gupta DK, Palma JM, Corpas FJ (2018) Antioxidants and antioxidant enzymes in higher plants, 1st edn. Springer International Publishing AG, Gewerbestrasse. https://doi.org/10.1007/978-3-319-75088-0
Hasan MK, Cheng Y, Kanwar MK, Chu XY, Ahammed GJ, Qi ZY (2017) Responses of Plant Proteins to Heavy Metal Stress-A Review. Front Plant Sci 8:1492. https://doi.org/10.3389/fpls.2017.01492
Heiss S, Wachter A, Bogs J, Cobbet C, Rausch T (2003) Phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after prolonged Cd exposure. J Exp Bot 54:1833–1839. https://doi.org/10.1093/jxb/erg205
Jan AT, Azam M, Siddiqui K, Ali A, Choi I, Rizwanul Haq QM (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16(12):29592–29630. https://doi.org/10.3390/ijms161226183
Juknys R, Vitkauskaite G, Račaite M, Vencloviene J (2012) The impacts of heavy metals on oxidative stress and growth of spring barley. Cent Eur J Biol 7(2):299–306. https://doi.org/10.2478/s11535-012-0012-9
Karamian R, Ghasemlou F (2014) Plant Regeneration via Somatic Embryogenesis and Organogenesis in Verbascum speciosum Schard. Acta Biol Cracov Ser Bot 56(1):97–103. https://doi.org/10.2478/abcsb-2014-0010
Khalid A, Farid M, Zubair M, Rizwan M, Iftikhar U, Ishaq HK, Farid S, Latif U, Hina K, Ali S (2020) Efficacy of Alternanthera bettzickiana to Remediate Copper and Cobalt Contaminated Soil Physiological and Biochemical Alterations. Int J Environ Res 14:243–255. https://doi.org/10.1007/s41742-020-00251-8
Kolbas A, Mench M, Marchand L, Herzig R, Nehnevajova E (2014) Phenotypic seedling responses of a metal-tolerant mutant line of sunflower growing on a Cu-contaminated soil series. Plant Soil 376:377–397. https://doi.org/10.1007/s11356-018-1837-1
Küpper H, Götz B, Mijovilovich A, Küpper FC, Meyer-Klaucke W (2009) Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol 151:702–714. https://doi.org/10.1104/pp.109.139717
Lange B, van der Ent A, Baker AJM, Echevarria G, Mahy G, Malaisse F, Meerts P, Pourret O, Verbruggen N, Faucon MP (2017) Copper and cobalt accumulation in plants: a critical assessment of the current state of knowledge. New Phytol 213(2):537–551. https://doi.org/10.1111/nph.14175
Li L, Zhang K, Gill RA, Islam F, Farooq MA, Wang J, Zhou W (2018) Ecotoxicological and interactive effects of copper and chromium on physiochemical, ultrastructural, and molecular profiling in Brassica napus L. Biomed Res Int 16:9248123–9248117. https://doi.org/10.1155/2018/9248123
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382. https://doi.org/10.1016/0076-6879(87)48036-1
Liu J, Wang J, Lee S, Wen R (2018) Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS One 13:603–612. https://doi.org/10.1371/journal.pone.0203612
Llagostera I, Cervantes D, Sanmartý N, Romero J, Pérez M (2016) Effects of copper exposure on photosynthesis and growth of the Seagrass Cymodocea nodosa: an experimental assessment. Bull Environ Contam Toxicol 97:374–379. https://doi.org/10.1007/s00128-016-1863-y
Malaisse F, Gregoire J, Brooks RR, Morrison RS, Reeves RD (1978) Aeolanthus biformifolius: a hyperaccumulator of copper from Zaeıre. Science 199:887–888
Marques D, Veroneze-Júnior V, Silva A, Ricardo Mantovani J, César Magalhães P, Souza T (2018) Copper toxicity on photosynthetic responses and root morphology of Hymenaea courbaril L. (Caesalpinioideae). Water Air Soil Pollut 229(5):138. https://doi.org/10.1007/s11270-018-3769-2
Martins LL, Mourato MP (2006) Effect of excess copper on tomato plants: growth parameters, enzyme activities, chlorophyll, and mineral content. J Plant Nutr 29:2179–2198. https://doi.org/10.1080/01904160600972845
Morina F, Jovanović L, Prokić L, Veljović-Jovanović S (2016) Physiological basis of differential zinc and copper tolerance of Verbascum populations from metal-contaminated and uncontaminated areas. Environ Sci Pollut Res 23:10005–10020. https://doi.org/10.1007/s11356-016-6177-4
Morteza-Semnani K, Saeedi M, Akbarzadeh M (2012) Chemical composition and antimicrobial activity of the essential oil of Verbascum thapsus L. J Essent Oil Bearing Plants 15(3):373–379. https://doi.org/10.1080/0972060X.2012.10644063
Muszyńska E, Hanus-Fajerska E (2015) why are heavy metal hyperaccumulating plants so amazing? BioTechnologia 96(4):265–271. https://doi.org/10.5114/bta.2015.57730
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Napoli M, Cecchi S, Grassi C, Baldi A, Zanchi CA, Orlandini S (2019) Phytoextraction of copper from a contaminated soil using arable and vegetable crops. Chemosphere 219:122–129. https://doi.org/10.1016/j.chemosphere.2018.12.017
Nouri J, Khorasani N, Lorestani B, Karami M, Hassani AH, Yousefi N (2009) Accumulation of heavy metals in soil and uptake by plant species with phytoremediation potential. Environ Earth Sci 59(2):315–323. https://doi.org/10.1007/s12665-009-0028-2
Palanivel TM, Pracejus B, Victor R (2020) Phytoremediation potential of castor (Ricinus communis L.) in the soils of the abandoned copper mine in Northern Oman: implications for arid regions. Environ Sci Pollut Res 27:17359–17369. https://doi.org/10.1007/s11356-020-08319-w
Panda SK (2008) Impact of copper on reactive oxygen species, lipid peroxidation and antioxidants in Lemna minor. Biol Plant 52:561–564. https://doi.org/10.1007/s10535-008-0111-7
Parmar P, Dave B, Sudhir A, Panchal K, Subramanian RB (2013) Physiological, biochemical and molecular response of plants against heavy metal stress. Int J Curr Res 5:80–89
Peer WA, Baxter IR, Richards EL, Freeman JL, Murphy AS (2005) Phytoremediation and hyperaccumulator plants. In: Thomas MJ, Martinoia E (eds) Molecular biology of metal homeostasis and detoxification. Springer, Berlin/Heidelberg, pp 299–340. https://doi.org/10.1007/4735_100
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci 180:169–181. https://doi.org/10.1016/j.plantsci.2010.08.016
Reeves RD (2003) Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant Soil 249:57–65. https://doi.org/10.1023/A:1022572517197
Reeves RD, Baker AJM, Jaffré T, Erskine PD, Echevarria G, van der Ent A (2018) A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol 218(2):407–411. https://doi.org/10.1111/nph.14907
Rehman M, Maqbool Z, Peng D, Liu L (2019) Morpho-physiological traits, antioxidant capacity and phytoextraction of copper by ramie (Boehmeria nivea L.) grown as fodder in copper-contaminated soil. Environ Sci Pollut Res 26:5851–5861. https://doi.org/10.1007/s11356-018-4015-6
Rolli NM, Suvarnakhandi SS, Mulgund GS, Ratageri RH, Taranath TC (2010) Biochemical responses and accumulation of cadmium in Spirodela polyrhiza. J Environ Biol 31:529–532
Saleem MH, Kamran M, Zhou Y, Parveen A, Rehman M, Ahmar S, Malik Z, Mustafa A, Anjum RMA, Wang B, Liu L (2020a) Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J Environ Manag 257:109994. https://doi.org/10.1016/j.jenvman.2019.109994
Saleem MH, Fahad S, Rehman M, Saud S, Jamal Y, Khan S, Liu L (2020b) Morpho-physiological traits, biochemical response and phytoextraction potential of short-term copper stress on kenaf (Hibiscus cannabinus L.) seedlings. Peer J 8:e8321. https://doi.org/10.7717/peerj.8321
Sanz A, Pike S, Khan MA, Carrió-Seguí A, Mendoza-Cózatl DG, Peñarrubia L, Gassmann W (2018) Copper uptake mechanism of Arabidopsis thaliana high-affinity COPT transporters. Protoplasma 256:161–170. https://doi.org/10.1007/s00709-018-1286-1
Shahbaz M, Tseng MH, Stuiver CEE, Koralewska A, Posthumus FS, Venema JH, Parmar S, Schat H, Hawkesford MJ, De Kok LJ (2010) Copper exposure interferes with the regulation of the uptake, distribution and metabolism of sulfate in Chinese cabbage. J Plant Physiol 167:438–446. https://doi.org/10.1016/j.jplph.2009.10.016
Shahid M, Pourrut B, Dumat C, Nadeem M, Aslam M, Pinelli E (2014) Heavy-metal-induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev Environ Contam Toxicol 232:1–44. https://doi.org/10.1007/978-3-319-06746-9_1
Sharma I, Ahmad P (2014) Catalase: a versatile antioxidant in plants. In: Ahmad P (ed) Oxidative damage to plants. Academic Press, San Diego, pp 131–148. https://doi.org/10.1016/B978-0-12-799963-0.00004-6
Sharma SS, Dietz KJ, Mimura T (2016) Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ 39:1112–1126. https://doi.org/10.1111/pce.12706
Shiyab S (2018) Phytoaccumulation of copper from irrigation water and its effect on the internal structure of lettuce. Agriculture 8:29. https://doi.org/10.3390/agriculture8020029
Soares JC, Santos CS, Carvalho SM, Pintado MM, Vasconcelos MW (2019) Preserving the nutritional quality of crop plants under a changing climate: importance and strategies. Plant Soil 443:1–26. https://doi.org/10.1007/s11104-019-04229-0
Strzalka A, Kostecka-Gugala A, Latowski D (2003) Carotenoids and environmental stress in plants: significance of carotenoid-mediated modulation of membrane physical properties. Russ J Plant Physiol 50:168–172. https://doi.org/10.1023/A:1022960828050
Sun CQ, Chen FD, Teng NJ, Liu ZL, Fang WM, Hou XL (2010) Interspecific hybrids between Chrysanthemum grandiflorum (Ramat.) Kitamura and C. indicum (L.) Des Moul. and their drought tolerance evaluation. Euphytica 174:51–60. https://doi.org/10.1007/s10681-009-0117-z
Thakur S, Singh L, Ab Wahid Z et al (2016) Plant-driven removal of heavy metals from soil: uptake, translocation, tolerance mechanism, challenges, and future perspectives. Environ Monit Assess 188:206. https://doi.org/10.1007/s10661-016-5211-9
Thounaojam CT, Panda P, Mazumdar P, Kumar D, Sharma DG, Sahoo L, Sanjib P (2013) Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol Biochem 53:33–39. https://doi.org/10.1016/j.plaphy.2012.01.006
Tiwari S, Lata C (2018) Heavy metal stress, signaling, and tolerance due to plant-associated microbes: an overview. Front Plant Sci 9:452. https://doi.org/10.3389/fpls.2018.00452
van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362:319–333. https://doi.org/10.1007/s11104-012-1287-3
Yadav SK (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179. https://doi.org/10.1016/j.sajb.2009.10.007
Yruela I (2009) Copper in plants: acquisition, transport and interactions. Funct Plant Biol 36:409–430. https://doi.org/10.1071/FP08288
Zhao YL, Wang SF, Li Y, He QX, Liu KC, Yang YP, Li XL (2011) Isolation of chemical constituents from the aerial parts of Verbascum thapsus and their antiangiogenic and antiproliferative activities. Arch Pharm Res 34(5):703–707. https://doi.org/10.1007/s12272-011-0501-9
Zhou J, Cheng K, Zheng J, Liu Z, Shen W, Fan H, Jin Z (2019) Physiological and Biochemical Characteristics of Cinnamomum camphora in Response to Cu- and Cd-Contaminated Soil. Water Air Soil Pollut 230:15. https://doi.org/10.1007/s11270-018-4048-y
Zoufan P, Jalali R, Hassibi P, Neisi E, Rastegarzadeh S (2018) Evaluation of antioxidant bioindicators and growth responses in Malva parviflora L. exposed to cadmium. Physiol Mol Biol Plants 24(6):1005–1016. https://doi.org/10.1007/s12298-018-0596-2
Funding
The authors received financial support from the Shahid Bahonar University of Kerman, Kerman, Iran.
Author information
Authors and Affiliations
Contributions
Karimi MR performed most of the experiments. Kavousi HR supervised the experimental design and wrote the manuscript. Ghorbanzadeh M did some of the experimentation and provided reagents and materials. All authors reviewed and approved the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Responsible Editor: Elena Maestri
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kavousi, H.R., Karimi, M.R. & Neghab, M.G. Assessment the copper-induced changes in antioxidant defense mechanisms and copper phytoremediation potential of common mullein (Verbascum thapsus L.). Environ Sci Pollut Res 28, 18070–18080 (2021). https://doi.org/10.1007/s11356-020-11903-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-11903-9