Abstract
Elevated levels of many metals are toxic to plant roots, but their modes of action are not well understood. We investigated the toxicities of aluminium (Al), copper (Cu), and lanthanum (La) in solution on the growth and external morphology of 3-d-old cowpea (Vigna unguiculata L.) roots for periods of up to 48 h. Root elongation rate decreased by 50% at ca. 30 µM Al, 0.3 µM Cu, or 2.0 µM La, accompanied by a decrease in the distance from the root tip to the proximal lateral root. Kinks developed in some roots 2.0 ± 0.4 mm from the root apex on exposure to Al or La (but not Cu). Light and scanning electron microscopy showed that soluble Al, Cu, or La caused similar transverse ruptures to develop > 1 mm from the root apex through the breaking and separation of the rhizodermis and outer cortex from inner-layers. The metals differed, however, in the range in concentration at which they had this effect; developing in solutions containing 54 to‑600 µM Al, but only from 0.85 to 1.8 µM Cu or 2.0 to 5.5 µM La. These findings suggest that Al, Cu, and La bind to the walls of cells, causing increased cell wall rigidity and eventual cell rupturing of the rhizodermis and outer cortex in the elongating zone. We propose that this is a major toxic effect of Al, and that Cu and La also have additional toxic effects.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soluble aluminium (Al) is a major factor limiting the growth of plants on acidic soils (Foy 1984). However, despite decades of intensive research into Al toxicity, the exact mechanism causing root growth reduction remains unclear. Indeed, there is still debate as to whether the primary site of Al toxicity (and that of other metals) is symplastic (i.e. inside the cell) or apoplastic (i.e. in the cell wall). The most familiar symptom of Al toxicity is the production of short, stubby root systems (see Rout et al. (2001) as an example). Besides being short, Al-intoxicated roots also develop lateral roots close to the tip of the taproot, as in soybean (Glycine max (L.) Merr.) for example (Blamey 2001). A number of studies have reported that the root’s rhizodermis and cortex may rupture when exposed to Al (Yamamoto et al. 2001; Blamey et al. 2004; Jones et al. 2006).
Interestingly, a number of other metals have been reported to affect plant roots in a similar manner to Al. Clarkson (1965) found that the results of experiments with Ga, In, and La on onion (Allium cepa L.) “were similar in every respect” to those with Al. Diatloff (1997) found that ruptures, markedly similar to those caused by Al, form when mungbean roots are exposed to La (which is also a cation channel blocker (Jorge and Menossi 2005)). Wheeler et al. (1993) reported that the visual symptoms of Cu toxicity are similar to those of Al toxicity, although the symptoms were not described. Ouzounidou et al. (1995) also reported that high Cu causes damage to the rhizodermal cells of maize (Zea mays L.). Subsequent light microscopy studies have revealed that ruptures also form in roots of Rhodes grass (Chloris gayana Kunth.) when exposed to excess Cu (Sheldon and Menzies 2005). Further, Kinraide et al. (1992) found that monovalent and divalent cations had similar ameliorative effects on Al and La rhizotoxicity in wheat (Triticum aestivum L.) and Kinraide and Sweeney (2003) reported that Al, Cu and La had less toxic effects on rhizobia at low pH.
Although ruptures have been reported in Al-, Cu-, and La-intoxicated roots, no studies have examined these ruptures in detail. Other than the work of Diatloff (1997) (which was not published in the freely-available literature), studies referring to ruptures have generally used light microscopy though electron microscopy has been used in some instances (for example, Hecht-Buchholz et al. (1990)). Further, the observation of ruptures has represented a side-line or minor issue; the majority of studies cited above simply reported observing ruptures without describing them (or the conditions in which they formed) in any detail. Therefore, knowledge regarding the formation of these ruptures under stress caused by elevated levels of various metals is limited, particularly with regard to: (1) the morphology of the ruptures, (2) the metal concentrations that reduce root elongation and cause rupturing, (3) the length of time required for the ruptures to form, and (4) the location on the root where the ruptures form. These shortcomings limit our understanding of why many metals are toxic; as a corollary, detailed examination of the symptoms should help elucidate the reasons for metal toxicities.
The objective of the current work was to examine the effects of Al, Cu, and La on the growth and external morphology of cowpea (Vigna unguiculata L.) roots when exposed to these metals. Roots were observed using both light microscopy and scanning electron microscopy (SEM). Care was taken to ensure that the concentrations of Al, Cu, and La used are environmentally relevant, being similar to those likely to be found in acidic soils or contaminated sites.
Materials and methods
Experimental procedures
Two solution culture experiments with Al, Cu, and La were conducted in a laboratory maintained at ca. 24°C at The University of Queensland, St Lucia, Australia. These experiments followed several preliminary trials in the laboratory and one glasshouse trial in which seedlings were grown for 7 days in complete nutrient solution as described by Kopittke & Menzies (2006). Cowpea (cv. Caloona) seeds were germinated in a rolled paper towel suspended vertically with the bottom 20 mm of the paper towel in tap water. After ca. 3 days, seven seedlings with ca. 30-mm-long, straight taproots were transplanted into 2 mm diameter holes in 10 mm wide Perspex strips placed on the top of glass beakers filled with solution. Glass beakers (600 ml) were filled to the brim with 650 ml basal solution with 1 mM Ca and 5 µM B (pH 5.6). In some treatments, solution pH was lowered to pH 4.5 with ca. 0.225 ml of 0.1 M HCl.
Immediately after transplanting and at set times up until 48 h thereafter, each Perspex strip was placed horizontally 300 mm beneath a digital camera mounted on a tripod, a digital image captured, and the strip replaced on the beaker. This took ca. 30 s ensuring minimal disruption to root growth. The length of each root was determined using the image processing and analysis software, UTHSCSA ImageTool Ver. 3.0 (which is available free of charge at http://ddsdx.uthscsa.edu/dig/itdesc.html). When calculating the rate of root elongation, the length of time that the root had been growing was calculated to the nearest minute using the digital time-stamp attached to each image.
Samples of the solutions were taken from the beakers at the end of the growth period, filtered to 0.22 µm (Millipore GSWP), acidified to pH <2.0 using 20 µL of concentrated HCl, and refrigerated (3.5°C) before analysis. Inductively coupled plasma-optical emission spectrometry (ICP-OES) was used for Al, Ca, and Cu, and inductively coupled plasma-mass spectrometry (ICP-MS) for La (ICP-OES could not be used for measuring La). For Experiment 1 (see below), the measured Ca concentration ranged between 950 and 1,000 µM; corresponding values for Experiment 2 were 920 and 970 µM. All concentrations of Al, Cu, and La mentioned subsequently are those measured by ICP analysis.
Metal concentrations decreasing root growth and causing rupturing
Experiment 1 examined the concentrations of Al, Cu, or La that reduce elongation and cause the formation of ruptures in the roots of cowpea. A total of 17 treatments was established using the 600 ml beakers, consisting of: control (pH 5.6), control (pH 4.5), Al (21, 54, 110, 220, 600 µM at pH 4.5), Cu (0.40, 0.85, 1.3, 1.8, 2.2 µM at pH 5.6), and La (0.6, 2.0, 3.6, 5.5, 7.3 µM at pH 5.6). The treatments were arranged in a completely randomized block design replicated twice over time, with each experimental unit containing seven seedlings (the average of these forming one replicate). Digital images were captured (for determination of root length) 0, 24, and 48 h after transplanting. After 48 h growth, roots were stained using 0.5% crystal violet and examined using a light microscope to determine whether or not ruptures had formed.
Microscopic examination of ruptures
Experiment 2 was conducted to: (1) confirm the effects of Al, Cu, or La on root elongation, (2) determine the timing and location of rupture formation, and (3) examine the morphology of the ruptures using light microscopy and high resolution SEM. Based upon the results of preliminary trials and Experiment 1, five combinations of metal and pH were established, being: control (pH 4.5), control (pH 5.6), 40 µM Al (pH 4.5), 1.1 µM Cu (pH 5.6), and 1.6 µM La (pH 5.6). Five Perspex strips were placed across the tops of the four beakers to which Ca, B, and the metals had been added, six strips placed on the control (pH 5.6) beaker, and seven strips placed on the control (pH 4.5) beaker. Six 3-d-old seedlings were transplanted into each Perspex strip, resulting in a total of 30 plants in the three metal-treatment beakers, 36 plants in the control (pH 5.6) beaker, and 42 plants in the control (pH 4.5) beaker. Immediately thereafter, digital images of all roots were captured. One strip was removed from each of the two control treatments, photographed again, and the roots prepared for SEM (see below). The strips were then progressively removed (and roots processed) from each of the five beakers as follows: after 2, 4, 12, 24, 36, and 48 h for the pH 4.5 control and Al treatment, and after 4, 12, 24, 36, and 48 h for the pH 5.6 control, Cu, and La treatments. In addition, at the 36 and 48 h harvest, some of the roots that were not frozen in liquid nitrogen (see below) were stained with 0.5% crystal violet and examined under a dissecting light microscope. These observations confirmed that the symptoms that were observed later on the highly-processed SEM samples were present also in unprocessed roots.
After being photographed to determine root elongation rate, four of the six root tips (ca. 20 mm) were cut off and pinned to a polystyrene block. The roots were freeze-substituted according to the method of Wharton (1991), with the roots frozen in liquid nitrogen for ca. 10 s and transferred to a solution of 3% glutaraldehyde in methanol at −20°C for 24 h. Roots were then transferred to methanol (without glutaraldehyde) at −20°C for a further 24 h, removed from the freezer, allowed to warm to room temperature, and dried using a Balzers critical point drier. Thereafter, roots were coated with ca. 15 nm of Pt using an Eiko IB-5 sputter coater. The roots were examined using a field emission SEM (JEOL JSM 6400F) at 10 kV and 39 mm working distance.
Results
Experiment 1
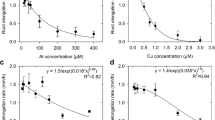
Soluble Al, Cu, or La markedly decreased the rate of root elongation (Fig. 1a, b, c). There was a 50% reduction in root elongation rate at ca. 30 µM Al, 0.3 µM Cu and 2 µM La. This confirms the findings of many studies that plant root growth is decreased by these trace metals. Indeed, the toxicity of Al and La was markedly similar to their effects on wheat (cv. Atlas) (Kinraide et al. 1992), but in contrast to other results which used considerably higher concentrations of metals. For example, Ouzounidou et al. (1995) used up to 80 µM Cu and demonstrated a 50% decrease in maize root growth with 7 µM Cu added to solution. Root elongation rate decreased by 95% at 600 µM Al, 92% at 2.2 µM Cu, and 91% at 7.3 µM La (the highest concentrations tested).
Effects of soluble Al, Cu or La on (a, b, c) the rate of root elongation, and (d, e, f) the distance from the root apex to the proximal lateral root, for cowpea grown for 48 h in a solution containing 1 mM Ca and 5 µM B at the pH values stated. Hollow symbols (a, b, c) represent treatments where no morphological damage was observed, with solid symbols representing treatments with ruptures to the rhizodermis and outer cortex. Results are the arithmetic mean of two replicates each of seven roots, with the bars representing the standard deviations
Besides the reduction in root elongation rate, the distance between the root apex and the proximal (i.e. closest to the root tip) lateral root decreased with increasing concentrations of all three trace metals (Fig. 1d, e, f). Indeed, the distance to the proximal lateral root decreased from 83 mm in the control treatments to 24 mm at 600 µM Al, 27 mm at 2.2 µM Cu, and 31 mm at 7.3 µM La.
There were marked similarities in the nature of the ruptures formed on exposure of roots for 48 h to Al, Cu, or La (Fig. 2). These trace metals caused transverse cracking of the outer layers of the root, as demonstrated for Al by Yamamoto et al. (2001) and Jones et al. (2006), Cu by Sheldon and Menzies (2005), and La by Diatloff (1997). No transverse ruptures were evident closer than ca. 1 mm from the root apex, an observation in keeping with those of Yamamoto et al. (2001) and Jones et al. (2006) regarding Al toxicity. After 48 h growth, ruptures were observed in roots growing in treatments where the rate of root elongation had been reduced by ≥ ca. 50% for La, and by ≥ ca. 75% for Al or Cu (Fig. 1). It is noteworthy that no ruptures were evident at 2.2 µM Cu or 7.3 µM La where root elongation had been reduced by > 90% (Fig. 1). This was in contrast to Al, where ruptures formed in roots grown at 600 µM Al in which elongation was reduced by 95% (Fig. 1). These results confirmed preliminary trials in the laboratory and glasshouse which had also shown that, unlike Al, the addition of Cu or La at concentrations sufficiently high to cause an almost complete cessation of root elongation did not cause the roots to rupture (data not presented).
As with the digital light microscopy studies of Blamey et al. (2004) with Al, some of the roots in the higher Al and La (but not Cu) treatments developed kinks an average of 2.0 ± 0.4 mm behind the root apex. Whilst no kinks were observed in roots exposed to 21 or 54 µM Al, kinks developed in 20% of the roots at 110 µM Al, 50% at 220 µM Al, and 80% at 600 µM Al. Similarly, whilst no kinks were observed at 0.6, 2.0 or 3.6 µM La, ca. 40% of the roots at 5.5 and 7.3 µM La developed similar kinks. By 48 h, these kinks had deformed many of the roots to such an extent that the tips had bent at right angles.
Experiment 2
Root growth in the two control treatments at pH 4.5 and 5.6 during the 48 h experimental period was good, with root length increasing by 60 mm. Soluble Al, Cu, or La reduced root growth, with average root length over 48 h only increasing by ca. 13 mm at 40 µM Al, 8 mm at 1.1 µM Cu, and 34 mm at 1.6 µM La. Besides these effects, the kinetics of the reduction in root elongation rate depended upon the treatment (Fig. 3). Root elongation rate decreased by ca. 50% within 2 h of exposure to 40 µM Al, within 4 h exposure to 1.1 µM Cu, or after 24 h exposure to 1.6 µM La (Fig. 3).
Effects of Al, Cu, or La (at the concentrations indicated) on the rate of elongation of cowpea roots grown for up to 48 h in a solution containing 1 mM Ca and 5 µM B at the pH values stated, compared with the two control treatments without trace metals added. Hollow symbols represent treatments where no morphological damage was observed, with solid symbols representing treatments with ruptures to the rhizodermis and outer cortex. Values represent the arithmetic mean of six measurements, with the bars representing the standard deviations
Of particular importance to this study (and as observed in Experiment 1 (Fig. 2)), soluble Al, Cu, or La caused the formation of transverse ruptures in the rhizodermal and outer-cortical cells, as illustrated for Al (Fig. 4). The severity of the rupturing increased with time of exposure to Al, Cu, or La, with Al-induced ruptures increasing in width between 4 and 12 h, and often several hundred micrometers wide after 24 h exposure (Fig. 4). In some instances, the rhizodermis (or rhizodermal and outer-cortical layers) appeared to have become detached from the inner tissues (Fig. 4c).
The time taken for these ruptures to form was dependent upon the treatment; ruptures forming in Al-treated roots after 4 h, Cu-treated roots after 12 h, and La-treated roots after 24 h. The ruptures were evident > 1 mm from the root apex (as evident also in Fig. 2). Indeed, ruptures were first observed at an average of 2.0 mm from the apex for Al, 1.6 mm for Cu, and 4.8 mm for La (Fig. 5). The distance between the apex and the first (i.e. closest to the root tip) rupture decreased with increased time of exposure to the metal, decreasing from 1.6 to 1.0 mm for Cu and from 4.8 to 3.1 mm for La (Fig. 5). For Al, however, after an initial decrease from 2.0 mm (at 4 h) to 0.7 mm (at 24 h), the distance from the apex to the first rupture increased to 2.3 mm after 48 h (Fig. 5). This subsequent increase in distance for Al-treated roots is consistent with preliminary work which had shown that after 7 d growth at 68 µM Al in a complete dilute nutrient solution, the first rupture was present at an average of 2.9 mm from the apex.
Closer examination of roots using higher magnification SEM required the use of root samples with ruptures of similar magnitude (Fig. 6), but at different times (viz. 12, 24, or 48 h for Al, Cu, or La) to allow the ruptures to form (Fig. 3). In all cases, the transverse ruptures formed in the rhizodermis and outer cortex (Fig. 6). Importantly, the ruptures formed not by the separation between intact cells, but by the breaking and tearing of individual cells (Fig. 7). It is pertinent that in all instances the rupture of the rhizodermal layer was wider than that between the cortical layers below, and that (at least initially) the rhizodermal cells between the ruptures were undamaged. Indeed, in some individual cells, it was clearly evident that the length of the rupture was greater on the exterior-surface of the cell than on the interior-surface of the cell (see arrows in Fig. 7b).
Scanning electron micrographs illustrating the effect of a Al at pH 4.5, b Cu at pH 5.6, or c La at pH 5.6 on cowpea roots grown in a solution containing 1 mM Ca and 5 µM B for the times indicated. Note the tearing of the cells in the rhizodermis and outer cortex resulting in the formation of transverse ruptures
Scanning electron micrographs of ruptures in the rhizodermis and outer cortex of a cowpea root grown a for 4 h in a solution containing 1 mM Ca, 5 µM B, and 40 µM Al for 4 h, or (b) and c for 7 days in a complete nutrient solution containing 68 µM Al. Note that the ruptures are formed by the breaking and separation of the cells in the outer-portions of the root whilst the underlying cells remain intact. The dotted box in (b) represents the approximate area shown in c. The white arrows indicate the distance of a rupture at the interior- and exterior-surface of an individual cell
Discussion
The results of the present study have confirmed that soluble Al, Cu, and La are toxic to plant roots, reducing their rate of elongation (Figs. 1 and 3) and causing external damage (Figs. 2 and 6). Further, we have provided information on the concentrations of Al, Cu, or La that reduce root elongation, and have used microscopy to provide details of the ruptures, including the timing and location of their formation.
All three metals caused a similar breaking and tearing of cells in the rhizodermis and outer cortex of the root resulting in the formation of ruptures ≥ 1 mm behind the apex (Figs. 2, 6 and 7). Measurements on the location of the ruptures suggest that they formed within the zone of elongation as found by Ryan et al. (1993) (typically located ca. 1.5–2.0 mm to 5–8 mm behind the apex (Ishikawa and Evans 1993)). A concentration of 40 µM Al initially caused the formation of ruptures at a distance of 2.0 mm from the apex, 1.1 µM Cu at 1.6 mm, and 1.6 µM La at 4.8 mm (Fig. 5). It is not clear if the Al- and Cu-induced ruptures initially formed in the transition or elongation zones (Fig. 5); a similar uncertainty was evident with mungbean roots exposed to 50 µM Al (Blamey et al. 2004). However, the initial ruptures in roots exposed to La formed at a distance of 4.8 mm from the apex (Fig. 5) – almost certainly beyond the transition zone. Furthermore, preliminary experiments identified that after 7 days growth in a dilute nutrient solution containing 68 µM Al, the rupture closest to the apex was at an average distance of 2.9 mm (data not presented). However, it is unclear why the distance between the ruptures and the root apex decreases with increased time of exposure to the metals (Fig. 5). It is possible that as the time of metal-exposure increased, the length of the transition zone decreased, thereby resulting in a decrease in the distance between the zone of cell elongation (where the ruptures formed) and the root apex.
Reduced cowpea root growth was evident at lower concentrations of metals than those which induced ruptures (Fig. 1). Indeed, even though no ruptures were observed, 21 µM Al reduced root elongation rate by 44%, 0.4 µM Cu by 57%, and 0.6 µM La by 22%. This decrease in root growth may involve increased cell wall rigidity but not to such an extent as to cause ruptures. With a > 11-fold increase in Al concentration (from 54 to 600 µM), there was both a further reduction in root elongation (of up to 95%) and the formation of ruptures. This decrease in root growth and rupture formation only occurred from 0.85 to 1.8 µM Cu and 2.0 to 5.5 µM La (Fig. 1), a < 3-fold increase in Cu or La in solution. The absence of ruptures at 2.2 µM Cu and 7.3 µM La is noteworthy in that these concentrations caused a near-complete inhibition of root elongation, a finding in contrast to Al where severe ruptures formed even in roots where the rate of root elongation had been reduced by 95% (Fig. 1).
Given the results of this and previous studies along with the above discussion, we propose that both the decrease in root growth and the formation of ruptures result through the binding of Al, Cu, or La to the walls of cells in the rhizodermis and outer cortex (along with a concomitant desorption of Ca). This may involve outer cells of the transition zone which has been implicated as the target for Al toxicity (Sivaguru and Horst 1998) or cells of the elongation zone. We suggest that the presence of Al, Cu, or La in the wall increases the rigidity of the cell wall and reduces its ability to elongate. Indeed, cells within the zone of elongation only expand when the pressure inside the cell exceeds the forces present within the cell wall. Factors that loosen the walls of cells in the elongation zone, an example being low pH (Winch and Pritchard 1999; Blamey et al. 2004), increase root growth in the short term. Proton alleviation of Al, Cu, and La toxicity has also been observed in rhizobia (Kinraide and Sweeney 2003) while a decrease in pH decreases Al and La rhizotoxicity in wheat. The opposite would also apply, viz. factors that make cell walls more rigid would decrease root growth. Given that the Al, Cu and La concentrations within the cell wall are higher in the rhizodermis and outer cortex than in the inner-portions of the roots (as shown for Al by Ma et al. (2004), Marienfeld et al. (2000), and Stass et al. (2006)), it follows that the walls of cells in the outer-portions of the root would have been more rigid (and hence expanding slower) than those cells in the inner-root. Further, there is an accumulation of callose (an early sign of cell wounding) in the rhizodermal and outer-cortical cells of Al-intoxicated roots (Wissemeier et al. 1987; Yamamoto et al. 2001; Stass et al. 2006). Thus, we hypothesize that the ruptures formed due to the presence of rigid (slowly expanding) outer cells overlying cells of the stele and inner cortex that are expanding at a faster rate. Further research is needed in this regard, however, given the indication that Al acts principally on cells of the root interior (Kinraide et al. 2005).
As outlined above, the results indicate that Al, Cu, and La reduce root growth, at least in part, due to the binding of these metals to the walls of epidermal and outer cortical cells. This supports the findings of Jones et al. (2006) who concluded that Al reduced root growth of maize by increasing cell wall rigidity in the epidermis. Furthermore, both the increase in rupture width (Fig. 4) and the observation that the length of the rupture was greater on the exterior than on the interior surface of the cell (Fig. 7b) suggests that the ruptures do not result from an increase in osmotic pressure within cells of the outer layers, but rather to an increase in cell wall rigidity in the outer layers while cells of the inner layers continue to elongate. Our results also suggest that cell wall rigidification represents a major toxic effect of Al, whilst Cu and La have additional toxic effects also (for example, accumulation and toxicity in the symplasm). This conclusion arises from the observations that (1) Al causes ruptures over a ≥ 11-fold concentration range compared with a < 3-fold range for Cu or La (Fig. 1), and (2), in contrast to Cu and La, Al causes roots to rupture even at a concentration which causes a near-complete inhibition of root elongation (Fig. 1). However, further work is required to test this hypothesis.
The kinks that formed ca. 2 mm from the root apex (Fig. 4b) were particularly prevalent in solutions containing ≥ 220 µM Al or ≥ 5.5 µM La, although some kinks were also present at lower concentrations. It is unclear why these kinks form in some metal-intoxicated roots and not others. This is confounded further by findings that (1) no kinks were evident in any of the roots grown in any of the Cu treatments despite the presence of ruptures with 0.85 to‑1.8 µM Cu in solution, and (2) kinks were evident in roots in the 7.3 µM La treatment in which no ruptures were observed (Fig. 1).
The final effect of Al, Cu, or La on root morphology evident in the present study was the marked reduction in the distance between the apex and the proximal lateral root (Fig. 1d,e,f). This may be through earlier maturation of pericycle cells or a disruption of root apical dominance by decreased symplastic and apoplastic transport of auxin.
We propose that further research is warranted to investigate the possibility that other cations reduce root elongation and cause ruptures similar to those observed in the current study, as suggested by Clarkson (1965) with Ga and In. However, experiments conducted within our laboratory have shown that Pb does not cause ruptures (data not presented). Further investigation is also required into rhizotoxic effects on other plant species differing in tolerance among various metals and into environmental factors (for example, solution pH which affects metal rhizotoxicity (Kinraide et al. 1992)) which may influence the formation of these ruptures.
In conclusion, all cowpea roots exposed to Al, Cu, or La had reduced growth along with reduced distance between the apex and the proximal lateral root. Roots grown at 54 to‑600 µM Al, 0.85 to 1.8 µM Cu, or 2.0‑to 5.5 µM La had transverse ruptures in the zone of elongation caused by the breaking and separation of the rhizodermal and outer-cortical cells, but the metals differed in the time taken for these ruptures to form. Also, only some roots at high Al or La (but not Cu) developed a kink 2.0 mm from the apex. These findings suggest that Al, Cu, and La displace Ca from the negatively charged sites of the cell walls, thereby increasing their rigidity and reducing the cells’ rate of elongation. Given that there would be higher concentrations of Al, Cu, or La in the cell walls in the outer- than in the inner-layers of the root, the cells in the rhizodermis and outer cortex would elongate at a slower rate than those in the stele and inner cortex, resulting in the tearing of the outer-cell walls and the formation of ruptures.
Abbreviations
- ICP-OES/MS:
-
Inductively coupled plasma-optical emission spectrometry/mass spectroscopy
- SEM:
-
scanning electron microscopy
References
Blamey FPC (2001) The role of the root cell wall in aluminum toxicity. In: Ae N, Arihara J, Okada K, Srinivasan A (eds) Plant nutrient acquisition – new perspectives. Springer-Verlag, Tokyo, pp 201–227
Blamey FPC, Nishizawa NK, Yoshimura E (2004) Timing, magnitude, and location of initial soluble aluminium injuries to mungbean roots. Soil Sci Plant Nutr 50:67–76
Clarkson DT (1965) Effect of aluminium and some other trivalent metal cations on cell division in root apices of Allium cepa. Ann Bot 29:309–315
Diatloff E (1997) Effects of the rare earth elements, lanthanum and cerium, on the growth and nutrition of corn and mungbean. In Department of Agriculture. The University of Queensland, St Lucia, pp 141
Foy CD (1984) Physiological effects of hydrogen, aluminum, and manganese toxicities in acid soil. In: Adams F (ed) Soil acidity and liming. American Society of Agronomy, Madison, Wisconsin, pp 57–97
Hecht-Buchholz CH, Brady DJ, Asher CJ, Edwards DG (1990) Effects of low activities of aluminium on soybean (Glycine max). II. Root cell structure and root hair development. In: van Beusichem ML (ed) Plant nutrition – physiology and applications. Kluwer Academic, Dordrecht, pp 335–343
Ishikawa H, Evans ML (1993) The role of the distal elongation zone in the response of maize roots to auxin and gravity. Plant Physiol 102:1203–1210
Jones DL, Blancaflor EB, Kochian LV, Gilroy S (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29:1309–1318
Jorge RA, Menossi M (2005) Effect of anion channel antagonists and La3+ on citrate release, Al content and Al resistance in maize roots. J Inorg Biochem 99:2039–2045
Kinraide TB, Sweeney BK (2003) Proton alleviation of growth inhibition by toxic metals (Al, La, Cu) in rhizobia. Soil Biol Biochem 35:199–205
Kinraide TB, Ryan PR, Kochian LV (1992) Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol 99:1461–1468
Kinraide TB, Parker DR, Zobel RW (2005) Organic acid secretion as a mechanism of aluminium resistance: a model incorporating the root cortex, epidermis, and the external unstirred layer. J Exp Bot 56:1853–1865
Kopittke PM, Menzies NW (2006) Effect of Cu toxicity on the growth of cowpea (Vigna unguiculata). Plant Soil 279:287–296
Ma JF, Shen R, Nagao S, Tanimoto E (2004) Aluminum targets elongating cells by reducing cell wall extensibility in wheat roots. Plant Cell Physiol 45:583–589
Marienfeld S, Schmohl N, Klein M, Schroder WH, Kuhn AJ, Horst WJ (2000) Localisation of aluminium in root tips of Zea mays and Vicia faba. J Plant Physiol 156:666–671
Ouzounidou G, Ciamporova M, Moustakas M, Karataglis S (1995) Responses of maize (Zea mays L.) plants to copper stress – I. Growth, mineral content and ultrastructure of roots. Environ Exp Bot 35:167–176
Rout GR, Samantaray S, Das P (2001) Aluminium toxicity in plants: a review. Agronomie 21:3–21
Ryan PR, DiTomaso JM, Kochian LV (1993) Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot 44:437–446
Sheldon AR, Menzies NW (2005) The effect of copper toxicity on the growth and root morphology of Rhodes grass (Chloris gayana Knuth.) in resin buffered solution culture. Plant Soil 278:341–349
Sivaguru M, Horst WJ (1998) The distal part of the transition zone is the most aluminum-sensitive apical root zone of maize. Plant Physiol 116:155–163
Stass A, Wang Y, Eticha D, Horst W (2006) Aluminium rhizotoxicity in maize grown in solutions with Al3+ or \({\text{Al}}\left( {{\text{OH}}} \right)_4^ - \) as predominant solution Al species. J Exp Bot 57:4033–4042
Wharton DA (1991) Freeze-substitution techniques for preparing nematodes for scanning electron microscopy. J Microsc-Oxford 164:187–196
Wheeler DM, Power IL, Edmeades DC (1993) Effect of various metal ions on growth of two wheat lines known to differ in aluminium tolerance. Plant Soil 155/156:489–492
Winch S, Pritchard J (1999) Acid-induced wall loosening is confined to the accelerating region of the root growing zone. J Exp Bot 50:1481–1487
Wissemeier AH, Klotz F, Horst WJ (1987) Aluminum induced callose synthesis in roots of soybean (Glycine max L.). J Plant Physiol 129:487–492
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Acknowledgments
The authors thank Dr Kim Sewell, Rick Webb, Robyn Webb, and Rob Gould from the Centre for Microscopy and Microanalysis at The University of Queensland for their assistance with the electron microscopy. This research was funded through the Cooperative Research Centre for Contamination Assessment and Remediation of the Environment (CRC-CARE) Project 3-3-01-05/6 and through the Australian Research Council’s Discovery funding scheme (DP0665467).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Thomas B. Kinraide
Rights and permissions
About this article
Cite this article
Kopittke, P.M., Blamey, F.P.C. & Menzies, N.W. Toxicities of soluble Al, Cu, and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil 303, 217–227 (2008). https://doi.org/10.1007/s11104-007-9500-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9500-5