Abstract
A study was performed for phyto-genotoxic assay of chromium (Cr) and arsenic (As) through Allium cepa. Various concentrations (0, 1, 3, 6 and 12 mg L−1) of Cr and As for 48 and 168 h time points exposed to A. cepa. The phytotoxic effects of metal(loid) were evident through inhibited root length and root protein. Metal(loid) toxicity also lead to genotoxic effects, which included depression of mitotic index and increased frequency of chromosomes aberrations like break, fragments, c-metaphase, multipolar arrangements etc. Genotoxic endpoint as progressive frequency of micronuclei in interphase of root meristem cells in treated plants was also observed. This genotoxic endpoint revealed carcinogenic nature of both aforementioned metal(loid). Along with inhibition in root length and protein content, depression in mitotic index as well as stimulation of various abnormality in mitotic cell division indicated that both metal(loid) are hazardous in nature and causing harmful effect on the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Heavy metals are one of major causes of environmental contamination worldwide, culminates in deterioration of agricultural yields as well as human health via food chain. Primarily, the sources of increasing concentrations of heavy metals are anthropogenic activities (Siddiqui 2015). Chromium (Cr) and arsenic (As) are two major environmental hazards whose contamination in water and soils is widespread due to a variety of industrial applications or owing to natural processes induced by human intervention. Chromium is utilized in various industries like tanning of leather, mining, paint, petroleum, manufacturing of textile, fungicide and wood preservative. (Mishra and Bhargava 2016). It has different oxidation states in nature with two different stable oxidation states, CrIII and CrVI. Both Cr species differ in terms of mobility in the environment and bioavailability, uptake and toxicity in plants (Panda and Choudhory 2005; da Conceição and Gomes 2017; Stambulska et al. 2018). Both forms of Cr are toxic, but the solubility and toxicity of the CrIII form is less than that of CrIV (Nematshahi et al. 2012). WHO (2004) recommended the permissible limit of Cr in drinking waters as 0.05 mg L−1. Deleterious effects of Cr on several biological activities are well correlated with its increasing concentrations, which include disruption of several physiological and cytological processes in cells.
Arsenic is a ubiquitously present toxic metalloid, which is released from several industrial processes, such as mining, coal combustion, semiconductors, pesticides etc. as well as through natural geogenic activities. The level of As in drinking waters of many areas has increased beyond WHO standard drinking water limits (0.01 mg L−1). The phyto-toxicity of As is affected considerably by its chemical form. Arsenite (AsIII) is generally considered to be more toxic than arsenate (AsV). Arsenite binds to sulfydryl groups of proteins affecting their structures or catalytic functions (Li et al. 2016). It also induces an upregulation of enzymes participating in the antioxidant responses (Kumar et al. 2014a; Singh et al. 2017). Besides these effects, it also alters DNA repair mechanism (Gebel 2001; Wu et al. 2010). It also generates reactive oxygen species (ROS) leading to lipid peroxidation, DNA and oxidative base damage in plant tissues (Kumar et al. 2014b, 2016; Abbas et al. 2018).
For the last few years, the bio-assays have been continually used for assessment of chemical toxicity at whole organism (toxicity) level as well as molecular (genotoxicity) levels. In this context, plants have been shown to be promising because chemicals cause identical chromosome abnormalities in plant cells as well as in cultured animal cells (Ditika and Anila 2013). The sensitivity of the root of Allium towards chemical is probably due to large size of chromosome along with higher number of metacentric chromosomes (Ditika and Anila 2013). Considering the lack of knowledge in this area, this study was planned to evaluate the phyo-genotoxic effects in response to Cr and As in Allium cepa.
Materials and Methods
Bulbs of onion (A. cepa) were exposed to different concentrations of CrVI and AsIII (0, 1, 3, 6 and 12 mg L−1) using potassium dichromate (K2Cr2O7) and sodium arsenite (NaAsO2) respectively. Dry roots on the disc of the bulb were cut and the outer scales of the bulb were removed. Subsequently, the bulbs were washed with milli Q water and placed over glass jars containing clean water and left for formation of roots. A set of plants grown in water served as control. Roots (1–2 cm long) were treated with Cr and As solutions for 48 and 168 h duration. The root length was measured in centimeter (cm).
Protein was estimated by following Lowry et al. (1951) method. Leaf samples were homogenized in 0.1 M phosphate buffer (pH 6.8). After adding required reagent optical density was recorded at 750 nm and concentration was calculated using a calibration curve standard (Bovine serum albumin) made with BSA.
Cytological studies of Cr and As treated A. cepa were done by root tip squash method. 1–2 cm of root tips were cut off and fixed in fixative of acetic acid and ethanol. Roots are then put into 1 N HCl for 10 min. Then root were washed and keep into mordant (Iron Alum) and left for 45 min. Again the root tips are washed and stained with Hematoxylin for 40 min (Fiskejo 1985). Then they are washed with distilled water and put on to a microscope slide. Root tips are cut in to 4–5 mm size and gently macerated with a needle. Digital images were obtained using a digital camera (Nikon P-5100) linked to an optical microscope (Nikon E-200) with 10× ocular and 40× objectives. This squashed root tip is used for recording mitotic index. The dividing cells (about 1000 cells) which are regular in shape are counted for various mitotic phases. The observations also included analysis of cytological abnormalities like fragments, bridges, micronuclei (MN), multi polarity and vagrants indicating various clastogenic and physiological disturbances. The data obtained are recorded and tabulated.
Estimation of total Cr and As, 0.5 g oven dried tissue were analyzed using Inductively Coupled Plasma Mass Spectrophotometry (ICP-MS 7500cx, Agilent Technologies, Japan). Multi-element calibration standard 2A (Agilent, Part # 8500-6940) and 3 (Agilent, Part # 8500-6948) were used for calibration and quality assurance for each analytical batch. The protocol detailed earlier in Dwivedi et al. (2010) and Kumar et al. (2014a, b) was followed. The detection limit of Cr and As and nutrient elements was 1 µg L−1. The result of the analysis was validated by digesting and analyzing Standard Reference Materials. Analysis of variance (ANOVA), Duncan’s multiple range test (DMRT) and t-test analysis were performed to determine the significant difference between treatments by using SPSS 17.0 software.
Result and Discussion
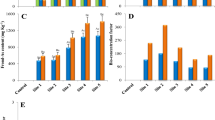
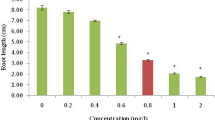
The results show that with increasing the concentration of Cr and As, root length decreased significantly establishing negatively correlation between root length and treatments of Cr and As. The protein content in both stressed plants was found to be significantly declined in increase in treatments (Table 1). The maximum inhibition of 77% and 69% protein content was recorded in higher exposure of Cr and As treated plants respectively. The reduction of protein content could be attributed to increased activity of catalytic enzymes like proteases or to fragmentation of proteins caused by ROS, which are known to be induced upon exposure to metal(loids) (Kumar et al. 2014a; Gupta et al. 2012, 2016).
The accumulation of Cr and As was a dose dependent response in both root and shoot (Table 1) (p > 0.05). However, accumulation of both Cr and As was more in roots in comparison to shoot. Chromium (permissible limit 2.3 µg g−1) and As (permissible limit 0.43 µg g−1) concentration of onions bulbs from the study area was found to be more than the WHO/FAO safe limit of these metal(loid)s for edible vegetables (JECFA 2010). The Cr content was ranged from 18.09 to 33.28 µg g−1 dw in roots and 3.42–7.26 µg g−1 dw in shoot while As content ranged from 4.4 to 8.5 µg g–1 dw in root and 0.77–1.97 µg g−1 dw in shoot of A. cepa.
These results are consistent with the earlier results reported various plants like wheat, corn, cabbage and black bean (Nematshahi et al. 2012) along with A. cepa (Espinoza-Quiñones et al. 2009; Gupta et al. 2012). The higher accumulation of Cr in roots of the plants could be due to immobilization of Cr in the vacuoles present in root cells (Dube et al. 2003; Datta et al. 2011). Accumulation of As in plant parts were also showed agreement with previous reported (Kumar et al. 2016).
Dose and duration dependent statistically significant reduction in mitotic index was recorded. The minimum mitotic index of 5.24% and 6.47% was estimated at 12 mg L−1 of Cr and As treated root meristem respectively at 168 h (Fig. 1). Reduction in the mitotic activity increased remarkably with enhancement of treatment duration. Glińska et al. (2007) suggested that the inhibition of mitotic index in roots exposed to metal could be due to disturbed cell cycle or dysfunction of chromatin induced by interaction of metal and DNA. Mito depressive action of Cr in A. cepa roots leading to reduction in root length. This inhibition could also be due to blockage of G1 stage and suppressing DNA synthesis or inhibition of DNA synthesis at the S stage. Chromium salts easily penetrate the cell membrane and modified to trivalent species which directly react with DNA unlike other metals, causing damage of DNA viz. modification of base, breaks of single-strand and double-strand and various adducts like Cr-DNA, DNA-Cr-DNA and protein-Cr-DNA adducts. By this Cr acts directly on DNA causing genotoxicity (Santos and Rodriguez 2012).
The present investigation showed a significant increase in the abnormal cells frequency with chromosome aberrations in root meristematic cells with Cr and As treatments (Table 2; Fig. 2). The maximum chromosome aberration of 17.51% and 12.65% was evident at highest dose and exposure time of both metals. Various treatments could strongly induce genotoxic logical effect, leading to physiological (c-mitosis, laggard and sticky chromosomes) as well as clastogenic (bridges and fragments) aberrations. The present study showed that Cr significantly stimulated the MN formation in root cells which evident its ability to directly react with DNA and to cause clastogenic and physiological aberrations in chromosomes. Results investigation revealed that treatment of roots with As compounds resulted in dose dependent decrease in mitotic index and increase in abnormal cells and micro nucleated cells. The reason of dose and duration dependent retardation in the mitotic activity could be due to inhibition of DNA synthesis (Schneiderman et al. 1971; Sudhakar et al. 2001) or a blockage of the G2 phase of the cell cycle resulting prevention of cell from entering mitosis (Van’t Hoff 1968). Arsenic has also been shown to enhance the clastogenicity and mutagenicity of other DNA damaging agents. Treatment with As and Cr compounds induced similar types of chromosomal and mitotic abnormalities in root tip cells which revealed that both these compounds are clastogenic and also act as spindle poison.
Chromosomal abnormalities along with normal cell division observed in root meristem of A. cepa due to treatments with various doses of As and Cr at 48 and 168 h exposure. a bridge, b1 normal anaphase, b2 normal telophase, c stickiness in anaphase, d C-metaphase, e normal metaphase, f stickiness in metaphase, g break in anaphase, h micronuclei
Micronucleus (MN) and chromosomal aberration (CA) assays in the root tips of A. cepa have been employed extensively in recent years for detection of potential DNA damaging properties of environmental contaminants (Silveira et al. 2017). Major causes of the most frequently detected CA types are dysfunction of chromatin in form of stickiness, bridges and fragments or spindle failure viz. c-mitosis and laggard chromosomes. Result from breakage and fusion of chromosome and/or chromatid leads to Chromosome bridges, however, laggard chromosomes from weak c-mitosis, increasing the risk for aneuploidy (Leme and Marin-Morales 2009). An irreversible stickiness is considered as common sign of highly toxic effects on chromosomes, type presumably leads to cell death. Micronucleus often resulting from the acentric fragments or laggard chromosomes which are unable to incorporate in to the telophasic daughter nuclei causing primary genes deletion which may leads to cellular death (Kirsch et al. 2011). Present investigations are well collaborated with previous results depicting the effect of pollutants including heavy metals on the plants (Gupta et al. 2012; Hemachandra and Pathiratne 2015). The reactivity of As is likewise to phosphorus in soil where AsV is taken up via phosphate transport systems, and subsequently acts in the cell as a phosphate analog to disrupt phosphate metabolism, inhibit DNA repair and induce MN formation (Wu et al. 2010).
In the present study, it could be concluded that both metal(loids) impart phytotoxic effects on A. cepa by inhibiting root growth and reduction in root protein content. Due to genotoxic characteristics, theses metal(loids) also reduced miototic index as well as enhanced various types of chromosomal aberrations in root meristem. Hence, the present research depicted that Cr and As are mutagenic and carcinogenic in this test system.
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Pub Health 15(1):59
da Conceição Gomes MA, Hauser-Davis RA (2017) Plant chromium uptake and transport, physiological effects and recent advances in molecular investigations. Ecotoxicol Environ Saf 140:55–64
Datta JK, Bandhyopadhyay A, Banerjee A, Mondal NK (2011) Phytotoxic effect of chromium on the germination, seedling growth of some wheat (Triticum aestivum L.) cultivars under laboratory condition. J Agric Technol 7(2):395–402
Ditika K, Anila M (2013) Assessment of cytotoxic and genotoxic potency of Cr(vi)-doped river water of Nen-shkodra lowland, albania, on Allium cepa L. J Environ Res Dev 7(4):1322–1332
Dube BK, Tewari K, Chatterjee J, Chatterjee C (2003) Excess chromium alters uptake and translocation of certain nutrients in citrullus. Chemosphere 53(9):1147–1153
Dwivedi S, Srivastava S, Mishra S, Kumar A et al (2010) Characterization of native microalgal strains for their chromium bioaccumulation potential: phytoplankton response in polluted habitats. J Hazard Mater 173:95–101
Espinoza-Quiñones FR, Szymanski N, Palácio SM, Módenes AN, Rizzutto MDA, Silva FG, Oliveira AP, Oro ACP, Martin N (2009) Inhibition effect on the Allium cepa L. root growth when using hexavalent chromium-doped river waters. Bull Environ Contam Toxicol 82(6):767–771
Fiskejo G (1985) The allium test as a standard in environmental monitoring. Hereditas 102:92–112
Gebel TW (2001) Genotoxicity of arsenical compounds. Int J Hyg Environ Health 203:249–262
Glińska S, Bartczak M, Oleksiak S, Wolska A et al (2007) Effects of anthocyanin-rich extract from red cabbage leaves on meristematic cells of Allium cepa L. roots treated with heavy metals. Ecotoxicol Environ Saf 68(3):343–350
Gupta K (2016) Role of Eichhorniacrassipes for evaluation of quality of water polluting Ganga river. Res Environ life Sci 9(10):1266–1269
Gupta K, Gaumat S, Mishra K (2012) Studies on phyto-genotoxic assessment of tannery effluent on Allium cepa. J Environ Biol 33:557–563
Hemachandra CK, Pathiratne A (2015) Assessing toxicity of Co, Cd and Cr levels relevant to discharge limits of industrial effluents into inland surface waters using common onion, Allium cepa bioassay. Bull Environ Contamin Toxicol 94:199–203
JECFA (2010) Evaluation of certain food additives and contaminants. 73th Report of the Joint FAO/WHO Expert Committee on Food Additive. WHO Technical Report Series 960
Kirsch VM, Plas G, Elhajouji A, Lukamowicz M, Gonzalez L, Vande Loock K (2011) The in vitro MN assay in 2011: origin and fate, biological significance, protocols, high throughput methodologies and toxicological relevance. Arch Toxicol 85(8):873–899
Kumar A, Singh RP et al (2014a) Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice. Ecotoxicology 23:1153–1163
Kumar A, Tripathi RD, Singh RP, Dwivedi S, Chakrabarty D, Mallick S et al (2014b) Evaluation of amino acid profile in contrasting arsenic accumulating rice (Oryza sativa L.) genotypes under arsenic stress grown in hydroponic condition. Biol Plant 58(4):733–742
Kumar A, Dixit G, Singh AP, Srivastava S, Mishra K, Tripathi RD (2016) Selenate mitigates arsenite toxicity in rice (Oryza sativa L.) by reducing arsenic uptake and ameliorates amino acid content and thiol metabolism. Ecotoxicol Environ Saf 133:350–359
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res Rev 682(1):71–81
Li N, Wang J, Song WY (2016) Arsenic uptake and translocation in plants. Plant Cell Physiol 57(1):4–13
Lowry OH, Rasebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin-phenol reagent. J Biol Chem 193:265–275
Mishra S, Bhargava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health 34:1–32
Nematshahi N, Mehrdad Lahouti M, Ganjeali A (2012) Accumulation of chromium and its effect on growth of (Allium cepa cv. Hybrid). Eur J Exp Biol 2(4):969–974
Panda SK, Choudhory S (2005) Chromium stress in plants. Braz Plant Physiol 17(1):95–102
Santos C, Rodriguez E (2012). Review on some emerging endpoints of chromium (VI) and lead phytotoxicity. In: Mworia JK (ed) Botany, InTech, Rijeka
Schneiderman MH, Dewey WC, Highfield DP (1971) Inhibition of DNA synthesis in synchronized Chinese hamster cells treated in G1 with cycloheximide. Exp Cell Res 67:147–155
Siddiqui S (2015) DNA damage in Cicer plant grown on soil polluted with heavy metal. J King Saud Univ Sci 27(3):217–223
Silveira MA, Datsch DL, Ribeiro et al (2017) Direct and indirect anthropogenic contamination in water sources: evaluation of chromosomal stability and cytotoxicity using the Allium cepa test. Bull Environ Contamin Toxicol 100:1–5
Singh AP, Dixit G, Kumar A, Mishra S, Kumar N, Dixit S, Singh PK, Dwivedi S et al (2017) A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice(Oryza sativa L.). Plant Physiol Biochem 115:163–173
Stambulska UY, Bayliak MM, Lushchak VI (2018). Chromium (VI) toxicity in legume plants: modulation effects of rhizobial symbiosis. Bio Med Res Int. https://doi.org/10.1155/2018/8031213
Sudhakar R, Gowda N, Venu G (2001) Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia 66:235–239
Van’t Hoff J (1968) The action of IAA and kinetin on the mitotic cycle of proliferative and stationary phase excised root meristem. Exp Cell Res 51:167
World Health Organization (2004). Guidelines for drinking-water quality: recommendations vol 1, WHO, Geneva
Wu L, Yi H, Yi M (2010) Assessment of arsenic toxicity using Allium/Vicia root tip micronucleus assays. J Hazard Mater 176:952–956
Acknowledgements
The authors are thankful to Department of Botany, University of Lucknow, Lucknow for the facilities. Kiran Gupta is thankful to University Grant Commission, New Delhi, India, for the award of the Post-doctoral fellowship for women. Amit Kumar is acknowledging the DSKPDF Cell, Pune, India, and University Grant Commission, New Delhi, India, for the award of the D.S. Kothari Postdoctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, K., Mishra, K., Srivastava, S. et al. Cytotoxic Assessment of Chromium and Arsenic Using Chromosomal Behavior of Root Meristem in Allium cepa L.. Bull Environ Contam Toxicol 100, 803–808 (2018). https://doi.org/10.1007/s00128-018-2344-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-018-2344-2