Abstract
Arsenic (As) contamination of rice is a major problem for South-East Asia. In the present study, the effect of selenium (Se) on rice (Oryza sativa L.) plants exposed to As was studied in hydroponic culture. Arsenic accumulation, plant growth, thiolic ligands and antioxidative enzyme activities were assayed after single (As and Se) and simultaneous supplementations (As + Se). The results indicated that the presence of Se (25 µM) decreased As accumulation by threefold in roots and twofold in shoots as compared to single As (25 µM) exposed plants. Arsenic induced oxidative stress in roots and shoots was significantly ameliorated by Se supplementation. The observed positive response was found associated with the increased activities of ascorbate peroxidase (APX; EC 1.11.1.11), catalase (CAT; EC 1.11.1.6) and glutathione peroxidase (GPx; EC 1.11.1.9) and induced levels of non-protein thiols (NPTs), glutathione (GSH) and phytochelatins (PCs) in As + Se exposed plants as compared to single As treatment. Selenium supplementation modulated the thiol metabolism enzymes viz., γ-glutamylcysteine synthetase (γ-ECS; EC 6.3.2.2), glutathione-S-transferase (GST; EC 2.5.1.18) and phytochelatin synthase (PCS; EC 2.3.2.15). Gene expression analysis of several metalloid responsive genes (LOX, SOD and MATE) showed upregulation during As stress, however, significant downregulation during As + Se exposure as compared to single As treatment. Gene expressions of enzymes of antioxidant and GSH and PC biosynthetic systems, such as APX, CAT, GPx, γ-ECS and PCS were found to be significantly positively correlated with their enzyme activities. The findings suggested that Se supplementation could be an effective strategy to reduce As accumulation and toxicity in rice plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) contamination in groundwater naturally occurs in many countries particularly in South and South-East Asia. The As contaminated groundwater is commonly used for irrigating crops (Moore et al. 2010) and thus finds way to contaminate food crops including rice (Meharg et al. 2004). Rice is the staple food of As epidemic areas like Bangladesh and West Bengal, India (Rahman et al. 2008).
Two main inorganic As (iAs) species viz, arsenate (AsV) and arsenite (AsIII) predominantly occur in nature and enter into the plants through different transporter. AsV is taken up through phosphate transporter, while AsIII enters via the NIP superfamily of aquaporins in rice (Zhao et al. 2009). As exposure to plants leads to growth inhibition, physiological damage and even cell death (Stoeva and Bineva 2003). iAs species generate reactive oxygen species (ROS) such as superoxide radicals and hydrogen peroxide (H2O2) in the plant, which damage the membranes and various macromolecules in the cell (Meharg and Hartley-Whitaker 2002). To know the gross effect of different As species, various physiological and biochemical parameters of different plants species have been evaluated earlier (Srivastava et al. 2009; Rai et al. 2011b; Tripathi et al. 2012; Dave et al. 2013). A hydroponic study on the expression of genes, cognate to antioxidants and thiol metabolism interpreted that some of the genes such as phytochelatin synthase (PCS), glutathione-S-transferase (GST) and γ-glutamylcysteine synthetase (γ-ECS) were up-regulated and down-regulated in different rice cultivars during AsV and AsIII stress (Rai et al. 2011a).
Selenium (Se) is an essential trace element for humans (Schwarz and Foltz 1957) and plays a critical role in maintaining healthy immune system and reduces cancer risk (Stadtman et al. 1996; Zeng et al. 2002). Selenium acts as a natural antidote to As by accelerating As excretion, and acting as an antioxidant component of the enzyme glutathione peroxidase, that may counteract the cancer (Spallholz et al. 2004). Global survey on rice Se content showed that Asian rice is good Se accumulator (Williams et al. 2009). Selenium exists predominantly as selenite (SeIV) (Elrashidi et al. 1987) and is taken up through silicon influx transporter Lsi1 (OsNIP2;1) in rice (Zhao et al. 2010). Selenium is also known to affect As accumulation in plants (Srivastava et al. 2009; Malik et al. 2012, Hu et al. 2013).
In the recent past, various studies (Bluemlein et al. 2009; Malik et al. 2012; Kumar et al. 2013) have shown the role of Se supplementation on As uptake and As induced oxidative damage. These findings indicated that As induced toxicity increased or decreased by Se supplementation in plants. In As contaminated soil, both As and Se coexist (Dwivedi et al. 2010b) and may affect physiology of the plant antagonistically or synergistically (Malik et al. 2012). Selenium either lowers the As accumulation in plants such as Pteris vittata (Feng et al. 2009), Phaseolus aureus (Malik et al. 2012) and O. sativa (Williams et al. 2009; Dwivedi et al. 2010b; Kumar et al. 2013; Hu et al. 2013), or increases the As uptake and toxicity in Thunbergia alata (Bluemlein et al. 2009) and P. vittata (Srivastava et al. 2009). Above contrasting findings made the background for the study to know the detailed impact involving physiological and molecular changes related to As induced oxidative stress and antioxidant system during Se supplementation. Thus, mechanistic details of interactive effects of As and Se remain still elusive and warrant further investigations particularly with respect to metalloid detoxification pathways in rice plants. We investigated here the role of Se supplementation on As uptake related to physiological and molecular changes during As and Se interaction in rice plants. It is hypothesized that Se supplementation will result in reduced As induced phytotoxicity through amelioration of oxidative stress involving of enhanced As detoxification system and lowering of As uptake.
Materials and methods
Plant growth, biomass and photosynthetic pigments
Rice seeds of BRG-12 were obtained from Rice Research Station, Chinsurah, West Bengal. Seeds were surface sterilized in 0.1 % HgCl2 solution for 30 s, followed by washing with deionized water and soaking in milli-Q for 24 h. Then, uniform germinated seeds were selected and transplanted to tray having fixed PVC cups (4 cm diameter and 5 cm high, ten plants per pot) and grown in modified Hewitt medium (Liu et al. 2004) for 10 days before treatment and then exposed to different concentrations of AsIII (0.0 and 25 µM) using sodium arsenite (Na2HAsO2) supplemented with SeIV (0.0, 5, 10, 25 and 50 µM) using sodium selenite (Na2O3Se) for 7 days. The experiment was carried out in a controlled environment growth chamber with a 14-h light period (260–350μE m2 s−1) and temperatures of 28 °C day and 20 °C night with 70 % relative humidity maintained by humidifier. All nutrient solutions were changed twice per week, and pH was adjusted to 5.5 using 0.1 KOH or HCl. All experiments were conducted twice comprising different AsIII supplemented with SeIV with three replicates. Plants were then harvested, washed with milli-Q, separated into roots and shoots, blotted and used for the study of various parameters. All the samples were ground in liquid N2 and stored at −80 °C till further use. Roots and shoots length were measured by metric scale. Total fresh weight of As exposed and control plants was also noted.
Arsenic and selenium quantification and quality control
Estimation of As and Se, 0.5 g oven dried tissue was taken and digested in 3 ml of HNO3 as detailed in supplementary materials (Dwivedi et al. 2010a). The As and Se was quantified with the help of inductively coupled plasma mass spectrometer (ICP-MS, Agilent 7500 cx). The standard solution material of As and Se (Agilent, Part # 8500–6940) was used for the calibration and quality assurance for each analytical batch. Recovery of Se was found to be more than 93.5 % as determined by spiking samples with a known amount of Se while, for As, rice flour NIST 1568a was used as a reference material with known spiked samples and recovery of total As were 95.3 % (±2.8; n = 5) and 92.5 % (±3.1; n = 5) respectively. The detection limit of As was 1 µg L−1.
Assay of lipid peroxidation, ion leakage, hydrogen peroxide and lipoxygenases activity
Lipid peroxidation was determined by estimation of the malondialdehyde (MDA), ion leakage was measured in terms of electrical conductivity (EC) and H2O2 was assayed according to Tripathi et al. (2012a). Lipoxygenase (LOX, EC 1.3.11.12) activity was measured by Surry (1964). The protein concentration of the enzyme solution was determined by Bradford (1976). The detailed methodologies were given in the supplementary material.
Assay of antioxidant enzymes
Plant material was homogenized in buffer containing specific for each enzyme under chilled conditions. Homogenate was centrifuged at 12,000×g for 15 min at 4 °C. The activity of superoxide dismutase (SOD, EC 1.15.1.1), catalase (CAT, EC 1.11.1.6) and ascorbate peroxidase (APX, EC 1.11.1.11) was assayed according to Rai et al. (2011a). Glutathione peroxidase (GPx, EC 1.11.1.9) activity was measured according to Drotar et al. (1985). The detailed methodologies were given in the supplementary material.
Assay of thiols and enzymes of thiolic metabolism
The estimation of cysteine, non-protein thiol (NPT), glutathione reduced GSH and oxidised GSSG was done according to Tripathi et al. (2013a). Assay of γ-glutamyl cysteine synthase (γ-ECS; EC 6.3.2.2), glutathione-S-transferase (GST; EC 2.5.1.18) and phytochelatin synthase (PCS; EC 2.3.2.15) was done according to Tripathi et al. (2013a) as detailed previously (Mishra et al. 2008). The concentration of phytochelatins (PCs) was calculated as PCs = NPT − (GSH + GSSG) (Duan et al. 2011).
Gene expression (RT-qPCR) studies
Total RNA was isolated from rice roots using SpectrumTM plant RNA Kit (SIGMA Life Science). First cDNA strand were prepared using 5 µg total RNA using RevertAid First StrandcDNA synthesis Kit (Thermo Scientific Molecular Biology). qRT-PCR was carried out using the primer pairs listed in Supplementary Table S1 which were designed by using Primique online software http://cgi-www.daimi.au.dk/cgi-chili/primique/front.py. qRT-PCR reactions were carried out in a 7500 Fast Real-Time PCR System (Applied Biosystems, USA) as described by Wu et al. (2011). To normalize the total amount of cDNA present in each reaction, the actin was co-amplified as an endogenous control for calibration of relative expression. The comparative Ct method (ΔΔCT method) of relative gene quantification recommended by Applied Biosystems (CA, USA) was used to calculate the expression levels of different treatments.
Statistical analysis
Analysis of variance (ANOVA), Duncan’s multiple range test (DMRT) and correlation analysis were performed to determine the significant difference between treatments by using SPSS 17.0 software.
Results and discussion
Growth parameter and photosynthetic pigment
A marked decrease in growth parameters and biomass was observed in As (25 µM) exposed rice plants after 7 days exposure (Table S2). Arsenic treatment significantly (p < 0.05) reduced the length and biomass of rice roots and shoots indicating its toxic nature, which is in concurrence with the pervious findings on contrasting As responsive rice genotypes (Tripathi et al. 2012, 2013a, b; Dave et al. 2013; Kumar et al. 2014). Selenium supplementation (25 µM) to As resulted in improved growth and increased biomass indicating an antagonistic interaction with As. The possible cause behind improved growth of rice in As + Se treatment might be due to lower As accumulation due to competition for uptake (Malik et al. 2011) and greater ability to tolerate As-induced toxicity due to increased antioxidant potential (Malik et al. 2012). Several studies observed that supplementation of Se exerts beneficial effects on plant growth by alleviating both biotic and abiotic stresses and nutrient imbalance (Filek et al. 2008; Kumar et al. 2013; Elguera et al. 2013). The As exposure significantly negatively altered the photosynthetic pigments, the effect was more pronounced on the chlorophyll a (33 %) than chlorophyll b (22 %). Similar results were reported in rice plants (Tripathi et al. 2013b) and duckweed (Duman et al. 2010). However, Se amended plants possessed higher amount of photosynthetic pigments over the As exposed plant (Fig. S1). Similarly Se induced plant growth and higher level of photosynthetic pigments in mungbean (P. aureus Roxb.) during As stress (Malik et al. 2012).
Effects of selenium on arsenic uptake in rice
In As exposed plants, the maximum accumulation of As was observed in roots (625 mg kg−1 dw) followed by shoots (228 mg kg−1 dw) at 25 µM As (Table 1). However, Se supplemented plants showed significantly (p < 0.05) reduced As accumulation, maximum reduction being threefold in roots (213 mg kg−1 dw) and twofold in shoots (102 mg kg−1 dw) as compared to AsIII treated plants. In general, reduced As accumulation was observed at all doses of Se supplementation, which was more prominent at 25 µM Se addition. The Se accumulation was found to be 62.35 and 17.76 mg kg−1 dw in roots and shoots, respectively at 25 µM Se exposure. Interestingly, Se accumulation was more in As + Se (roots; 86.20 and shoots; 25.45 mg kg−1 dw) as compared to single Se exposed plants. In agreement to the obtained results, Kumar et al. (2013) observed that the presence of SeIV decreases the shoot and root As uptake in rice. Whereas, significant reduction of As uptake was observed with the increase in Se concentration. This can be attributed to the competition for uptake between AsIII and SeIV across the same type of transporter such as nodulin 26-like intrinsic proteins (NIP) (Ma et al. 2008). Selenium has also been observed to reduce As uptake in mungbean (Malik et al. 2012) and also the toxicity of other metals like cadmium (Cd) and antimony (Sb) in Brassica napus, Lepidium sativum and rice plant (Filek et al. 2008; Feng et al. 2011; Elguera et al. 2013). Thus, addition of Se in As contaminated soil may be helpful in reducing human health risk associated with intake of As tainted rice.
Lipid peroxidation, ion leakage, hydrogen peroxide and lipoxygenase activity
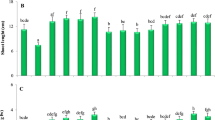
The oxidative stress was analyzed in terms of MDA, EC, H2O2 and LOX activity (Fig. 1a–d). The positive correlation was observed between As accumulation and MDA (r = 0.988**; r = 0.982*), EC (r = 0.946*; r = 0.987**), H2O2 (r = 0.998***; r = 0.972*) and activity of LOX (r = 0.892NS; r = 0.975*) in roots and shoots respectively. The induction in MDA, EC, H2O2 and LOX activity was 99, 56, 122 and 86 % in roots respectively, while 76, 47, 87 and 48 % in shoots upon As exposure, as compared to control. Earlier studies demonstrated enhancement in oxidative stress parameters because As generated oxidative stress, causing lipid peroxidation and degradation of various biomolecules (Patra et al. 2004; Tripathi et al. 2012; Hasanuzzaman and Fujita 2013). Conversely, As + Se treatment significantly (p < 0.05) reduced the level of MDA (31 %), EC (13 %), H2O2 (37 %) and activity of LOX (39 %) in roots in comparison to single As treated plants. Similar response were observed for shoots during As + Se treatment and decrease of 29, 16, 31 and 23 % for these respective parameters when compared to As exposure. The decrease in the oxidative stress during Se supplementation might be attributed to direct or indirect regulation of antioxidant system. It is well documented that Se counteracts the detrimental effects of diverse environmental stresses, including As and Cd toxicity (Filek et al. 2008; Feng et al. 2011; Kumar et al. 2012; Malik et al. 2012), drought (Hasanuzzaman and Fujita 2011), and senescence (Hartikainen et al. 2000). In agreement with the present study, supplementation of Se is known to reduce the MDA and H2O2 content in many other plants (Filek et al. 2008; Feng et al. 2011; Malik et al. 2012) during As, Cd or Sb stress. In addition, Se may itself act as an antioxidant (Feng et al. 2013) that might have contributed in reducing oxidative stress. Besides, Se supplementation might be also prevented the As induced oxidative damage in plants and may play important role for sustainable production of rice.
Changes in the level of MDA (a), EC (b), H2O2 (c), LOX (d) SOD (e), CAT (f), APX (g) and GPx (h) of the rice plant. All the values are means of 4 replicate (n = 4) ± S.D. ANOVA significant at p < 0.01. Different letters for the same tissue indicate significantly different values between treatments (DMRT, p < 0.05)
Antioxidant enzymes activities
The activities of various antioxidant enzymes were altered in As and As + Se treatment and also single Se treatment. The SOD activity was found to increase in both As and As + Se treatments, the increase being higher in As treatment (Fig. 1e). Arsenite exposure significantly increased the SOD activity by 21 % in roots and 30 % in shoots as compared to control. The activity of CAT, APX and GPx was found to significantly decrease upon As exposure, the level of decline being variable for roots and shoots (Fig. 1f–h). Upon Se supplementation, activity of these enzymes was found to increase significantly to even higher levels as compared to control. The CAT activity was significantly hampered (root −26 % and shoot −32 %) in rice cultivar. The APX and GPx activity declined more in roots (−37 and −38 %) and shoots (−26 and −41 %) respectively during As exposure, however, As + Se exposure significantly increased the APX (root 15 % and shoot 10 %) and GPx (root 15 % and shoot 26 %) activity. Selenium addition also raised the activity of GPx by 44 % in roots and 62 % in shoots, which was maximum in all the corresponding treatments. Overall GPx showed maximum increase upon Se supplementation which is in accordance with Takeda et al. (1997), who observed that GPx activity was more enhanced with Se supplementation than APX and CAT in the green alga (Chlamydomonas reinhardtii). The addition of Se to the medium considerably increased the activity of these antioxidants suggesting that Se may play a preventive role against As induce toxicity (Hartikainen et al. 2000). Earlier, Malik et al. (2012) also reported that Se antagonizes the toxic effect of As by enhancing the antioxidative capacity of mungbean and restricting the As uptake. Filek et al. (2008) also found that protective role of Se increases antioxidant enzyme activities (GPx, APX and CAT) except SOD in B. napus during Cd stress.
Thiol compounds and enzymes of thiolic metabolism
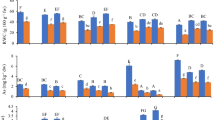
The thiolic compounds were measured in terms of Cys, NPT, GSH and GSSG (Fig. 2a–d). Thiols play a vital role for detoxification of As toxicity and provide tolerance to plant (Mishra et al. 2008; Tripathi et al. 2013a). Results indicated that As exposure significantly (p < 0.05) elevated the level of Cys in roots (32 %) and shoots (45 %) as compared to control. Se treatment slightly enhanced the Cys level 14 and 19 %, however, As + Se combination showed maximum Cys levels, 45 and 63 % higher in roots and shoots, respectively as compared to control. Norton et al. (2008) reported upregulation of several genes involved in GSH synthesis, metabolism and transport during transcriptomic analysis of rice under As stress. The NPTs and GSH content were found decreased in both roots and shoots under single As treatment, however, single Se and As + Se treatments enhanced the NPT and GSH contents when compared to control. The GSH content was negatively correlated with As accumulation in root (r = −0.947*) and shoot (r = −0.893NS). The induction of 21 and 31 % was observed in GSSG content of single As and As + Se treated shoots, however, in roots about 25 % increase was found at single As treatment. Further, GSH/GSSG ratio (Fig. 2e) was negatively correlated with As accumulation in root (r = −0.996**) and shoot (r = −0.983*). Besides higher GSH/GSSG ratio in roots during Se and As +Se treatment indicate protective role of Se in heavy metal toxicity (Kumar et al. 2012). The PCs level was found to be increased by 29 and 20 % in roots and shoots respectively during As exposure (Fig. 2f). However, the level of PCs induced maximum at As + Se exposure in roots (37 %) followed by shoots (33 %).
Change in the activities of cysteine (a), NPSH (b), GSH (c), GSSG (d), GSH/GSSG ratio (e) and PCs (f) of the rice plant. All the values are means of 4 replicate (n = 4) ± S.D. ANOVA significant at p < 0.01. Different letters for the same tissue indicate significantly different values between treatments (DMRT, p < 0.05)
NPTs represent PCs as major constituents (Scheller et al. 1987). In the present study As + Se always gave higher level of GSH and NPTs than single As treatment, showing higher metal(loid) detoxification. Arsenic and Se both have been found to induce PCs (major components of NPTs) and varying thiolic parameters such as Cys, GSH and NPTs. The higher level of PCs in roots than shoots demonstrated higher detoxification potential in roots than shoots insuring lesser mobility of As in shoot, lowering risk of food-chain contamination. Arsenic is better inducer of PCs than Se (Grill et al. 1987), As + Se could induce PCs to higher levels resulting in better metalloid detoxification, leading to reduced level of phytotoxicity in the present study. Similarly, in the red algae (Gracilaria dura), Cd + Se treatment resulted in more NPTs and PCs as compared to single Se and single Cd (Kumar et al. 2012). Malik et al. (2012) reported that Se significantly induced the level of GSH in mungbean. Similarly, Srivastva et al. (2009) observed that addition of Se increased the NPTs and GSH content in P. vittata during As stress. NPTs have both metal detoxification and antioxidant properties as it contains both GSH and PCs. Thus, it seems likely that Se singly or in combination with As (As + Se) is playing a role as antioxidant including its role in involving GSH level in the present study.
GST, γ-ECS and PCS activities (Fig. 3a–c) were positively correlated with As accumulation in roots and shoots. GST activity was observed to increase 19 % in roots and 36 % in shoots during As exposure. Similarly, As + Se exposure significantly (p < 0.05) enhanced the GST activity about 56 and 50 % comparison to As treated plant. γ-ECS is the key enzyme of thiol metabolism and increase in the activity may enhance the metalloid detoxification capability of the plant (Srivastava et al. 2009; Dave et al. 2013; Tripathi et al. 2013a). The PCS activity was positively correlated with PCs content in roots (r = 0.972*) and shoots (r = 0.973*). The PCS activity increased by 107 and 125 % at As exposure, however As + Se exposure, induction of 190 and 155 % in root and shoot was observed respectively. Increased activity of GST in the present observation might have contributed to detoxification of As induced ROS to combat metalloid generated stress in plant (Mokgalaka-Matlala et al. 2009). In the present study As + Se combination increased the level of GST, γ-ECS and PCS in rice. Similarly, Malik et al. (2012) reported that As treated plants supplemented with Se increased the thiols and GST activity, compared to control plants, suggesting Se amendments improve the detoxification ability of the cell against As toxicity.
Expression analysis of target genes
The expression pattern of genes associated with antioxidant and metalloid detoxification were also studied in roots of rice plants supplemented with Se during As exposure (Fig 4a–j). Transcript levels of LOX (Os08g39850) was significantly (p < 0.05) upregulated during As exposure as compared to As + Se exposure. Previous studies on As + Se interrelation manifests that Se minimizes toxicity of As, thus it increases growth of plants and helps in defense activity (Feng et al. 2009). ROS are controlled by the scavenging system, mainly dominated by antioxidant like SOD, CAT, APX and GPx. Interestingly, this study found that SOD (Os03g11960) was highly upregulated in roots of As exposed rice plants in comparison to As + Se, meanwhile the expression of CAT (Os03g03910), APX (Os03g17690) and GPx (Os04g46960) was found significantly upregulated during Se exposure, which were also in accordance with their corresponding enzyme activities. This strengthened prior observation that Se improves plant growth by promoting scavenging system (Malik et al. 2011). Couples of studies have suggested the role of Se in the regulation of ROS and antioxidants (Filek et al. 2008; Feng et al. 2013). Thiolic metabolism enzymes like GST (Os10g22310), γ-ECS (Os07g27790) and PCS (Os05g34290) were significantly (p < 0.05) upregulated during As exposure belong to metal detoxification machinery showing enhanced transcripts levels. On the other hand transcripts of GST were expressed relatively low in As + Se in comparison to single Se, additionally γ-ECS and PCS expression were comparatively greater in As + Se than As exposure. Higher expression of γ-ECS and PCS in As + Se exposure indicated role of Se in detoxification capacity of plants to resists against As stress. A multidrug efflux transporter, multidrug and toxic compound extrusion (MATE; Os03g08900) activity was upregulated during As stress and it was strikingly downregulated in As + Se. MATE transporters are involved in efflux of xenobiotic compounds and chemicals into vacuole or to xylem. An inverse regulation of this transporter suggests that this might be playing an important role in As transport from root to shoot, which needs to be studied in further experiments. It is to be noted that this transporters was found to be As responsive in rice in earlier transcriptomic experiments of Chakrabarty et al. (2009). Glutaredoxins acts as crucial enzyme that converts AsV into AsIII (Martin et al. 2001; Thomas et al. 2010). While, expression of glutaredoxin (Os03g40500) displayed differential expression pattern as it was more upregulated in Se treatment. Out of all studied genes, MATE and two enzymatic genes LOX and SOD were upregulated in As stress and stringently down regulated in As + Se stress, showing alleviation of As toxicity during As + Se stress.
a–j Quantitative real-time PCR analysis to study the expression gene pattern of the rice plant. Y-axis represents relative mRNA level in stressed or treated samples as compared to control samples. Actin expression was used as internal control each time. All the values are means of 3 replicate (n = 3) ± S.D. ANOVA significant at p < 0.01. Different letters indicate significantly different values between treatments (DMRT, p < 0.05)
Conclusion
The study demonstrated positive influence of Se supplementation in As-exposed rice plants, which is evident by improved growth and biomass. It is interesting to note reduction in As accumulation and increase in Se accumulation in As + Se treatment. Further, plants’ potential for combating oxidative stress and detoxifying As was enhanced due to increase in antioxidant and thiolic components. The present study concluded that As induced phytotoxicity can be reduced through supplementation of Se as evident by enhanced plant growth, thiolic ligands, antioxidant capability and lowering the As accumulation in the rice cultivar. Expression of three important genes belonging to multidrug efflux transporter, oxidative stress and antioxidant activity such as MATE, LOX and SOD were upregulated during As stress but down regulated during As + Se exposure. Thus, Se fertilization may have potential to minimize As accumulation and toxicity in rice during field experiments.
References
Bluemlein K, Raab A, Feldmann J (2009) Stability of arsenic peptides in plant extracts: off line versus on-line parallel elemental and molecular mass spectrometric detection for liquid chromatographic separation. Anal Bioanal Chem 393:357–366
Bradford MM (1976) A rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chakrabarty D, Trivedi PK, Misra P, Tiwari P, Shri M, Shukla D, Kumar S, Rai A, Pandey A, Nigam D, Tripathi RD, Tuli R (2009) Comparative transcriptome analysis of arsenate and arsenite stresses in rice seedlings. Chemosphere 74:688–702
Dave R, Singh PK, Tripathi P, Shri M, Dixit G, Dwivedi S, Chakrabarty D, Trivedi PK, Sharma YK, Dhankher OP, Corpas FJ, Barroso JB, Tripathi RD (2013) Arsenite tolerance is related to proportional thiolic metabolite synthesis in rice (Oryza sativa L.). Arch Environ Contam Toxicol 64:235–242
Drotar A, Phelps P, Fall R (1985) Evidence for glutathione peroxidise activities in cultured plant cell. Plant Sci 42:35–40
Duan GL, Hu Y, Liu WJ, Kneer R, Zhao FJ, Zhu YG (2011) Evidence for a role of phytochelatins in regulating arsenic accumulation in rice grain. Environ Exp Bot 71:416–421
Duman F, Ozturk F, Aydin Z (2010) Biological responses of duckweed (Lemna minor L.) exposed to the inorganic arsenic species As(III) and As(V): effects of concentration and duration of exposure. Ecotoxicology 19:983–993
Dwivedi S, Tripathi RD, Srivastava S, Singh R, Kumar A, Tripathi P, Dave R, Rai UN, Chakrabarty D, Trivedi PK, Tuli R, Adhikari B, Bag MK (2010a) Arsenic affects mineral nutrients in grains of various Indian rice (Oryza sativa L.) genotypes grown under arsenic-contaminated soils of West Bengal. Protoplasma 245:113–124
Dwivedi S, Tripathi RD, Tripathi P, Kumar A, Dave R, Mishra S, Singh R, Sharma D, Rai UN, Chakrabarty D, Trivedi PK, Adhikari B, Bag MK, Dhankher OP, Tuli R (2010b) Arsenate exposure affects amino acids, mineral nutrient status and antioxidant in rice (Oryza sativa L.) genotypes. Environ Sci Technol 44:9542–9549
Elguera JCT, Barrientos EY, Wrobel K, Wrobel K (2013) Effect of cadmium (Cd(II)), selenium (Se(IV)) and their mixtures on phenolic compounds and antioxidant capacity in Lepidium sativum. Acta Physiol Plant 35:431–441
Elrashidi MA, Adriano DC, Workman SM, Lindsay WL (1987) Chemical-equilibria of selenium in soils a theoretical development. Soil Sci 144:141–152
Feng R, Wei CY, Tu SX, Sun X (2009) Interactive effects of selenium and arsenic on their uptake by Pteris vittata L. under hydroponic conditions. Environ Exp Bot 65:363–368
Feng R, Wei CY, Tu SX, Tang T, Wu F (2011) Detoxification of antimony by selenium and their interaction in paddy rice under hydroponic conditions. Microchem J 97:57–61
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 65:363–368
Filek M, Keskinen R, Hartikainen H, Szarejko I, Janiak A, Miszalski Z, Golda A (2008) The protective role of selenium in rice seedlings subjected to cadmium stress. J Plant Physiol 165:833–844
Grill E, Winnacker EL, Zenk MH (1987) Phytochelatins, a class of heavy metal binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA 84:439–443
Hartikainen H, Xue T, Piironen V (2000) Selenium as an antioxidant and prooxidant in ryegrass. Plant Soil 225:193–200
Hasanuzzaman M, Fujita M (2011) Selenium pretreatment upregulates the antioxidant defense and methylglyoxal detoxification system and confers enhanced tolerance to drought stress in rapeseed seedlings. Biol Trace Elem Res 143:1758–1776
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22:584–596
Hu Y, Duan GL, Huang YZ, Liu YX, Sun GX (2013) Interactive effects of different inorganic As and Se species on their uptake and translocation by rice (Oryza sativa L.) seedlings. Environ Sci Pollut Res 21:3955–3962
Kumar M, Bijo AJ, Baghel RS, Reddy CRK, Jha B (2012) Selenium and Spermine alleviates cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidant system and DNA methylation. Plant Physiol Biochem 51:129–138
Kumar N, Mallick S, Yadava RN, Singh AP, Sinha S (2013) Co-application of selenite and phosphate reduces arsenite uptake in hydroponically grown rice seedlings: toxicity and defence mechanism. Ecotoxicol Environ Saf 91:171–179
Kumar A, Tripathi RD, Singh RP, Dwivedi S, Chakrabarty D, Mallick S, Trivedi PK, Adhikari B (2014) Evaluation of amino acid profile in contrasting arsenic accumulating rice (Oryza sativa L.) genotypes under arsenic stress grown in hydroponic condition. Biol Plant (in press)
Liu WJ, Zhu YG, Smith FA, Smith SE (2004) Do phosphorus nutrition and iron plaque alter arsenate uptake by rice seedlings in hydroponic culture? New Phytol 162:481–488
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935
Malik JA, Kumar S, Thakur P, Sharma S, Kaur N, Kaur R, Pathania D, Bhandhari K, Kaushal N, Singh K, Srivastava A, Nayyar H (2011) Promotion of growth in mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol Trace Elem Res 143:530–539
Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H (2012) Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot 77:242–248
Martin P, DeMel S, Shi J, Gladysheva T, Gatti DL, Rosen BP, Edwards BFP (2001) Insights into the structure, solvation and mechanism of ArsC arsenate reductase, novel arsenic detoxification enzyme. Structure 9:1071–1081
Meharg AA (2004) Arsenic in rice-understanding a new disaster for South-East Asia. Trends Plant Sci 9:415–417
Meharg AA, Hartley-Whitaker J (2002) Arsenic uptake and metabolism in arsenic resistant and nonresistant plant species. New Phytol 154:29–43
Mishra S, Srivastava S, Tripathi RD, Trivedi PK (2008) Thiol metabolism and antioxidant system complement each other during arsenate detoxification in Ceratophyllum demersum L. Aquat Toxicol 86:205–215
Mokgalaka-Matlala NS, Flores-Tavizón E, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL (2009) Arsenic tolerance in mesquite (Prosopis sp.): low molecular weight thiols synthesis and glutathione activity in response to arsenic. Plant Physiol Biochem 47:822–826
Moore KL, Schroder M, Lombi E, Zhao FJ, McGrath SP, Hawkesford MJ, Shewry PR, Grovenor CRM (2010) NanoSIMS analysis of arsenic and selenium in cereal grain. New Phytol 185:434–445
Norton GJ, Lou-Hing DE, Meharg AA, Price AH (2008) Rice-arsenate interactions in hydroponics: whole genome transcriptional analysis. J Exp Bot 59:2267–2276
Patra M, Bhowmik N, Bandopadhyay B, Sharma A (2004) Comparison of mercury, lead and arsenic with respect to genotoxic effects on plant systems and the development of genetic tolerance. Environ Exp Bot 52:199–223
Rahman MA, Hasegawa H, Rahman MM, Miah MAM, Tasmin A (2008) Arsenic accumulation in rice (Oryza sativa L.): human exposure through food chain. Ecotoxicol Environ Saf 69:317–324
Rai A, Tripathi P, Dwivedi S, Dubey S, Shri M, Kumar S, Tripathi PK, Dave R, Kumar A, Singh R, Adhikari B, Bag M, Tripathi RD, Trivedi PK, Chakrabarty D, Tuli R (2011a) Arsenic tolerances in rice (Oryza sativa) have a predominant role in transcriptional regulation of a set of genes including sulphur assimilation pathway and antioxidant system. Chemosphere 82:986–995
Rai R, Pandey S, Rai SP (2011b) Arsenic-induced changes in morphological, physiological, and biochemical attributes and artemisinin biosynthesis in Artemisia annua, an antimalarial plant. Ecotoxicology 20:1900–1913
Scheller HV, Huang B, Hatch E, Goldsbrough PB (1987) Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol 85:1031-1035
Schwarz K, Foltz CM (1957) Selenium as an integral part of factor against dietary necrotic liver degeneration. J Am Chem Soc 79:3292–3293
Spallholz JE, Mallory BL, Rahman MM (2004) Environmental hypothesis: is poor dietary selenium intake an underlying factor for arsenicosis and cancer in Bangladesh and West Bengal, India. Sci Total Environ 323:21–32
Srivastava M, Ma LQ, Rathinasabapathi B, Srivastava P (2009) Effect of selenium on arsenic uptake in arsenic hyperacumulator Pteris vittata L. Bioresour Technol 100:1115–1121
Stadtman TC (1996) Selenocysteine. Annu Rev Biochem 65:83–100
Stoeva N, Bineva T (2003) Oxidative changes and photosynthesis in oat plants grown in As-contaminated soil. Bul J Plant Physiol 29:87–95
Surry K (1964) Spectrophotometric method for determination of lipoxidase activity. Plant Physiol 39:65–70
Takeda T, Ishikawa T, Shigeoka S (1997) Metabolism of hydrogen peroxide by the scavenging system in Chlamydomonas reinhardtii. Physiol Plant 99:49–55
Thomas DJ (2010) Arsenolysis and thiol-dependent arsenate reduction. Toxicol Sci 117:249–252
Tripathi P, Mishra A, Dwivedi S, Chakrabarty D, Trivedi PK, Singh RP, Tripathi RD (2012) Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotoxicol Environ Saf 79:189–198
Tripathi P, Tripathi RD, Singh RP, Dwivedi S, Goutam D, Shri M, Trivedi PK, Chakrabarty D (2013a) Silicon mediates arsenic tolerance in rice (Oryza sativa L.) through lowering of arsenic uptake and improved antioxidant defence system. Ecol Eng 52:96–103
Tripathi P, Tripathi RD, Singh RP, Dwivedi S, Chakrabarty D, Trivedi PK, Adhikari B (2013b) Arsenite tolerance in rice (Oryza sativa L.) involves coordinated role of metabolic pathways of thiols and amino acids. Environ Sci Pollut Res 20:884–896
Williams P, Lombi N, Sun G, Scheckel K, Zhu Y, Feng X, Zhu J, Carey A, Adomako E, Lawgali Y, Deacon C, Meharg AA (2009) Selenium characterization in the global rice supply chain. Environ Sci Technol 43:6024–6030
Wu J, Wang F, Cheng L, Kong F, Peng Z, Liu S, Yu X, Lu G (2011) Identification, isolation and expression analysis of auxin response factor (ARF) genes in Solanum lycopersicum. Plant Cell Rep 30:2059–2207
Zeng H (2002) Selenite and selenomethionine promote HL-60 cell cycle progression. J Nutr 132:674–679
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Zhao XQ, Mitani N, Yamaji N, Shen RF, Ma JF (2010) Involvement of silicon influx transporter OsNIP2,1 in selenite uptake in rice. Plant Physiol 153:1871–1887
Acknowledgments
The authors are thankful to Director, CSIR-National Botanical Research Institute, Lucknow for the providing the institutional facilities. Authors are also thankful to Council of Scientist and Industrial Research (CSIR), New Delhi, India for financial support in the from of network projects (BSC-0111) and award of Senior Research Fellowship to Amit Kumar.
Conflict of interest
Corresponding author and all the co-authors of the MS (No. ECTX-D-14-00062R1) entitled “Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.)” have no conflict of interest pertaining to this work being submitted in the journal “Ecotoxicology”.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, A., Singh, R.P., Singh, P.K. et al. Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.). Ecotoxicology 23, 1153–1163 (2014). https://doi.org/10.1007/s10646-014-1257-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-014-1257-z