Abstract
The growth inhibition, biomass production and water translocation in roots and shoots of mustard (Sinapis alba L.) was evaluated in experiments with Cr(III), Cr(VI) and Ni. On the basis of IC (IC25, IC50, IC75) values for growth inhibition the following rank orders were arranged: for roots: Cr(VI) ≥ Ni(II) > > Cr(III); for shoots: Ni(II) > Cr(VI) > > Cr(III). All metals tested reduced more root than shoot growth. When the relationship between dry (DM) and fresh mass (FM) was determined, FM production was reduced more than that of DM, and root FM was reduced more strongly than that of shoots. This indicates a reduction in water uptake and problems with water translocation through the plant. For genotoxicity study, simultaneous phytotoxicity and mutagenicity assay with Vicia sativa L. var. Klára was used. For phytotoxicity, the following rank orders of growth inhibition could be arranged: for roots: Ni(II) > Cr(VI) > Cr(III); for shoots: Ni(II) > Cr(VI) ≥ Cr (III). For mutagenicity assay root tips of V. sativa were used and chromosome aberrations were determined at least in 500-anatelophases. All tested metals exerted in V. sativa a significant increase of chromosomal aberration rate in applied concentrations. Maximum of aberrations invoked Cr(VI) and the rank order of aberrations fall was: Cr(VI) > Ni(II) > Cr(III). Genotoxic effects of metals were also determined by analysis of micronuclei frequency in the pollen tetrads of Tradescantia plants. None of the tested metals significantly stimulated micronuclei frequency. Genotoxic effect decreased in order: Cr(VI) ≥ Ni(II) > Cr(III).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Over the last few years, heavy metals had received considerable attention as a consequence of increased environmental pollution from industrial, agricultural, energetic and municipal sources. They function in the soil as a stress factor causing in plants physiological disorders after having been absorbed by the root system, which results in decreased vigor of a plant and retardation of its growth. Physiological responses of plants to a toxic metal treatment are not only growth inhibition, but also changes in various biochemical and physiological characteristics (Vassilev et al. 1998). Many metals have the ability to deteriorate genetic information and chromosome structure. The results of such genotoxic effects can be lethality, generation of mutations, chromosomal aberrations and carcinogenesis (Mišík et al. 2006, 2007). Vascular plants have been found to be highly effective for recognizing and predicting metal stress in the environment (growth inhibition, reduction of biomass production, changes in water absorption and translocation (Chatterjee and Chatterjee 2000; Prasad et al. 2001; Shanker et al. 2005; Szárazová et al. 2008). For genotoxicity studies, plants are highly responsible and sensitive. Their beneficial interest is that seeds and pollen grains can be easily stored and they offer cheap, and relatively easy and accurate toxicological assessment (Kristen 1997). By their ability to accumulate toxic substances, they indicate metal presence in the environment even in very low concentration (Chandra et al. 2004).
2 Material and Methods
Mustard (Sinapis alba L.) seeds were germinated in Petri dishes (17 cm diameter, filter paper with plastic net on the bottom). Tested samples were exposed to 10 varying concentrations (Ni(II) from 1 to 50 mg/L, Cr(III) and Cr(VI) from 5 to 225 mg/L) and tap water (80 mg/L Ca, 27 mg/L Mg; pH = 7.3 ± 0.05) was used for their dilution. In each Petri dish, 50 healthy seeds of similar size were spread on the filter paper covered with a plastic net, and overflowed with 50 mL of treatment solutions or normal tap water as control. The covered Petri dishes were placed in a thermostat (darkness, t = 25 °C; air humidity 80 %). After 72 h, root and shoot lengths were measured.
Basically, the same procedure utilized for growth inhibition was used to determine the dry mass (DM) and water content (WC). After 72 h, Petri dishes with germinated seeds were transferred from the thermostat into a laboratory box with a day-light cycle of 16/8 h and a constant temperature of 23 ± 1 °C. The dishes were shielded from direct sunlight, and cultivation lasted for 7 days. The shoots were not in direct contact with tested solutions of metals. After 10 days growth (3 + 7), the plants were divided into roots and shoots, and fresh mass (FM) was immediately weighed. The plant material was then oven-dried (t = 80 °C) to constant weight. The water content of the plants was determined on the basis of fresh and dry mass by using Drazic and Mihailovic’s equation (Drazic and Mihailovic 2005).
(WC—water content; FM—fresh mass, DM—dry mass; in g/g DM).
Simultaneous phytotoxicity and mutagenicity assay was carried out on plant species Vicia sativa L. var. Klára according to Miadoková et al. (2001, 2005). After 24 h of soaking at 25 °C in distilled water or solution with metal concentration equal to IC50 value, the seeds of V. sativa were allowed to germinate in Petri dishes (diameter = 18.5 cm) with filter paper soaked with the same concentration of tested metal as that used for soaking. Phytotoxicity was assayed after 72 h of the dark cultivation in the thermostat at 25 °C by the same way as for S. alba. The seedling roots and shoots of V. sativa were measured and percent growth inhibition was assessed. The seedling roots used for chromosome and genome mutability evaluation were fixed and permanent slides were prepared by the Feulgen method. Chromosome aberrations were determined at least in 500-anatelophases. For statistic analysis the Student’s t-test was used.
The procedures for maintaining the Tradescantia plants and for analyzing micronuclei frequency in the tetrads have been described by Mišík et al. (2006, 2007). Tradescantia paludosa clone 03 was cultivated at the Department of Botany, Faculty of Natural Sciences, Comenius University in Bratislava, Slovakia. Inflorescences were harvested at the 8–10-bud stage and immersed into 500 mL of tested metal solutions (100 mg/L CrO3 and NiCl2, 1,000 mg/L Cr(NO3)3) for 12 h. As control tap-water was used. The 24 h reconvalescence, during which inflorescence peduncles were dipped in 500 mL of tap-water, succeeded to 12 h exposure. Then the buds were fixed for 24 h in ethanol: acetic acid (3:1). The fixed material was stored in 70 % ethanol. Slides were prepared from the fixed material using the aceto-carmine squash technique. Micronuclei were scored in the early tetrad stages of pollen mother cells. In the present study, 15–20 inflorescences were in a sample. Three hundred tetrads were scored from each of five slides prepared from a treatment sample for a total of 1,500 tetrads per plot. Data were recorded as the number of micronuclei (MCN) per 100 tetrads. A change of frequency of MCN/100 tetrads was considered statistically significant (at P < 0.05) if the difference between the mean of the control population and the mean of the treated population was at least twice as large as the standard error of the difference between the two means (Mišík et al. 2007; Ma et al. 1994).
The salts of tested metals, NiCl2.6H2O, Cr(NO3)3.9H2O and CrO3 of analytical grade p.a., were obtained from Lachema, Brno, Czech Republic.
All experiments were set up in a completely randomized design with three replicates. Chronic toxicity was assessed as inhibition of root and shoot growth and the results were evaluated by the Gryck-Haustein method and IC25, IC50 and IC75 concentrations were determined. The results were statistically evaluated by using the Toxicity program. For statistical evaluation of biomass production, statistical program STATISTICA 8.1 was used.
3 Results and Discussion
3.1 Determination of Phytotoxicity
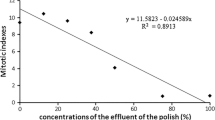
The first part of the study was carried out to determine the adverse effects of chromium and nickel on S. alba seedlings. The deleterious effect was expressed as root and shoot growth inhibition using regression analysis which yielded IC25, IC50 and IC75 values (Fig. 4.1). On the basis of these values, and their statistical evaluation, metals can be arranged in the following rank orders of inhibition: for roots: Cr(VI) ≥ Ni(II) > > Cr(III); for shoots: Ni(II) > Cr(VI) > > Cr(III). Both root and shoot prolongation was most inhibited by Cr(VI) and Ni(II). All metals tested reduced more root than shoot growth.
The presence of Cr and Ni in the external environment leads to changes in the growth and development pattern of plants, and both these metals are reported to be very toxic for root and shoot growth (Fargašová 1994, 1998). Ni in the presence of 0.1 µM NiCl2 inhibited root and shoot elongation of canola and tomato seedlings, and the roots appeared more sensitive than the shoots (Burd et al. 1998). Prasad et al. (2001) reported that the root length in Salix viminalis was affected more by Cr than by Cd and Pb; and Fargašová (1994) stated that the adverse effect of Cr on S. alba root growth was equal to that of Hg, and stronger than that of Cd and Pb, while Ni reduced root length less than that by Cr (Fargašová 1998). In accordance with our results, Burd et al. (1998) and Chatterjee and Chaterjee (2000) also reported that root growth was as a more sensitive indicator of metal toxicity than shoot growth. Here-in, this was significantly confirmed mainly for chromium. The general response of decreased root growth due to Cr and other metal toxicity may be evoked by the inhibition of root cell division/root elongation or by the extension of the cell cycle in the roots. Under high concentrations of Cr, Ni and many other heavy metals, the reduction of root growth may be due to the direct contact of seedling roots with a metal present in the medium, causing collapse, and subsequently inability of roots to absorb water from the medium (Barcelo et al. 1986). Adverse effects of Cr, Ni and other metals on plant height and shoot growth were reported by Rout et al. (2000) and Barton et al. (2000). The significant reduction in plant height of S. alba observed in this present study was also reported for this plant by Hanus and Tomas (1993) in soil with Cr concentrations of 200 or 400 mg/kg. The reduction in plant height may be due mainly to the reduced root growth and consequent lesser nutrient and water transport to the shoots. Additionally, Cr and Ni transport to the aerial parts of the plant can have a direct impact on the cellular metabolism of shoots, thus contributing to the reduction in plant height (Shanker et al. 2005).
The overall adverse effects of Cr and Ni on growth and development of plants may be a serious impairment of mineral nutrients and water uptake, which leads to deficiency in the shoots. Wilting of various crops and plant species due to Cr toxicity has been reported (Turner and Rust 1971), but little information is available on the exact effects of Cr and Ni on water relations in higher plants. When the relationship between dry (DM) and fresh mass (FM) was determined herein (Fig. 4.2), the DM fraction was increased parallel to increased Cr and Ni concentrations, while for FM fraction the growth trend was opposite. The effect of tested metals was stronger on FM than on DM production, and root FM was reduced more strongly than that of shoots. This indicates a reduction in water uptake (Fig. 4.3). Water content in both plant parts was reduced very rapidly in comparison to that in control seedlings, and varied significantly with the tested concentrations. Because the water content in the shoots was significantly reduced in the presence of tested metals, it can be concluded that Cr and Ni inhibited not only water absorption by the roots, but also water tranport into the upper seedling parts. These results disagree with Chatterjee and Chatterjee (2000) conclusion, that excess Cr decreases the water potential and transpiration rates and increases diffusive resistance and relative water content in the leaves of cauliflower. However, Barcelo et al. (1986) observed a decrease in leaf water potential in a Cr-treated bean plant. Decreased turgor and plasmolysis was also observed in the epidermal and cortical cells of bush bean plants exposed to Cr, because toxic levels of Cr decreased tracheary diameter in vessel-bearing plants, thereby reducing longitudinal water movement (Vazquez et al. 1987).
3.2 Genotoxicity Study
Toxic effects of heavy metals, mainly during chronic exposure, are not visible immediately. Hence, eco-toxicological studies suggest the assessment of genotoxicity. Genotoxicity effect is developed as early as the concentration is lower than that for phytotoxicity effect (Mičieta and Murín 1998). For phytotoxicity and clastogenicity study, V. sativa seedlings were used. Phytotoxicity was determined through IC25, IC50 and IC75 values and for roots and shoots the strongest inhibitory effect, pursuant to S. alba, had Ni(II) (Fig. 4.4). However, Cr(VI) inhibited V. sativa root growth less than that of S. alba. No significant differences were confirmed between the Cr(III) and Cr(VI) adverse effects on V. sativa shoot growth. On the basis of these values, and their statistical evaluation, metals can be arranged in the following rank orders of inhibition: for roots: Ni(II) > Cr(VI) > Cr(III); for shoots: Ni(II) > Cr(VI) ≥ Cr (III).
For mutagenicity assay, root tips of V. sativa were used and chromosome aberrations were determined at least in 500-anatelophases. All tested metals exerted a significant increase of chromosomal aberration rate in V. sativa due to applied concentrations (Table 4.1). All tested metals Cr(VI) invoked maximum of aberrations in anatelophase cells. The rank order of aberrations occurred was: Cr(VI) > Ni(II) > Cr(III). Genetic variation in susceptibility to environmental agents and metals can be considered as differences in metabolism of these agents in various organisms (Omenn 1991). In addition, DNA target size and DNA content are also important in determining genotoxic hazards of metals. According to Kovalchuk et al. (1998) and Chauhan et al. (1998) genotoxicity can be obtained as a result of multipolar anaphase and c-mitosis or damage of protein synthesis in the presence of DNA toxicant. Simultaneous toxicity and clastogenity of wastes with Cr and Ni contents was also confirmed for V. sativa by Miadoková et al. (1999) and for V. faba and Allium cepa by Chandra et al. (2004, 2005). Chromosomal fragments and bridges created in the presence of Cr(VI) indicated that CrO3 affected DNA structure and conformation (Quian 2004).
For determination the genotoxic effects of Cr and Ni, analysis of micronuclei frequency in the pollen tetrads of Tradescantia plants was done. As it is evident from Table 4.2, none of tested metals significantly stimulated micronuclei frequency in comparison with the control and genotoxic effect decreased in order: Cr(VI) ≥ Ni(II) > Cr(III). Tradescantia micronucleus test (Trad-MCN) in combination with Allium cepa L. and Vicia faba L. root tips tests are most frequently used genotoxicity tests in plants (Majer et al. 2005) and it is very popular now for in situ bio-monitoring of air pollution (Mišík et al. 2006, 2007). The results obtained during our genotoxicity tests are in good agreement with those reported by Knasmüller et al. (1998) when CrO3, CrCl3 and NiCl2 up to concentration 10 mM did not evoke genotoxic effects. The same conclusion was also drawn by Majer et al. (2005) for Cr(III). Higher genotoxicity of Cr(VI) than Cr(III) determined during our experiments also is in agreement with Němeček et al. (2002). Rossman (1995) who reported that molecular mechanism of DNA damage by Cr(VI) involves induction of DNA-DNA and DNA-protein cross-links and genotoxic effect can be also increased by reactive oxygen species produced during intracellular reduction. For Ni(II), no genotoxic effects were confirmed for bacteria. Rossman (1995) and Patierno and Costa (1987) reported that mutations after Ni applications are also the result of DNA damage and DNA-protein cross-links formation.
4 Conclusion
Obtained results confirmed chromium and nickel adverse effects on terrestrial plants. Both metals reduced plants’ growth and impaired their genetic material. Routinely phytotoxicity used test with S. alba and V. sativa seedlings confirmed for root and shoot growth the highest toxicity of Ni and Cr(VI) toxicity was several times higher than that of Cr(III). Cr and Ni also reduced dry (DM) and fresh (FM) mass production of S. alba and their adverse effect was stronger on FM than on DM production. The root FM was reduced, similar as growth, more strongly than that of shoots. This indicates a reduction in water uptake. Because tested metals significantly reduced mainly water content in the shoots, it can be concluded that Cr and Ni inhibited not only water absorption by the roots but also water transport into the upper seedlings parts. Genotoxicity of Cr and Ni was determined as number of chromosomal aberrations in V. sativa root tips and micronuclei frequency in the pollen tetrades of Tradescantia paludosa. All tested metals activated a significant increase in chromosomal aberration rate at applied IC50 concentrations. The maximum of aberrations in anatelophase cells invoked Cr(VI). None of tested metals significantly stimulated, in comparison with the control, micronuclei frequency in pollen tetrades of T. paludosa and genotoxicity effect decreased in the same order as for majority of observed parameters: Cr(VI) ≥ Ni(II) > Cr(III).
References
Barcelo J, Poschenrieder C, Gunse B (1986) Water relations of chromium VI treated bush bean plants (Phaseolus vulgaris L. cv. Contender) under both normal and water stress conditions. J Exp Bot 37:178–187
Barton LL, Johnson GV, O’Nan AG, Wagener BM (2000) Inhibition of ferric chelate reductase in alfalfa roots by cobalt, nickel, chromium, and copper. J Plant Nutr 23:1833–1845
Burd GI, Dixon DG, Glick BR (1998) A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl Environ Microbiol 64:3663–3668
Chandra S, Chauhan LKS, Pande PN, Gupta SK (2004) Cytogenetic effects of leachates from tannery solid waste on the somatic cells of Vicia faba. Environ Toxicol 19:129–133
Chandra S, Chauhan LKS, Murthy RC, Saxena PN, Pande PN, Gupta SK (2005) Comparative bio-monitoring of leachates from hazardous solid waste of two industries using Allium test. Sci Total Environ 347:46–52
Chatterjee J, Chatterjee C (2000) Phytotoxicity of cobalt, chromium and copper in cauliflower. Environ Pollut 109:69–74
Chauhan LKS, Saxena PN, Sundararaman V, Gupta SK (1998) Diuron-induced cytological and ultrastructural alterations in the root meristem cells of Allium cepa. Pestic Biochem Physiol 62:152–163
Drazic G, Mihailovic N (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci 168:511–517
Fargašová A (1994) Effect of Pb, Cd, Hg, As, and Cr on germination and root growth of Sinapis alba seeds. Bull Environ Contam Toxicol 52:452–456
Fargašová A (1998) Root growth inhibition, photosynthetic pigments production, and metal accumulation in Sinapis alba as the parameters for trace metals effect determination. Bull Environ Contam Toxicol 61:762–769
Hanus J, Tomas J (1993) An investigation of chromium content and its uptake from soil in white mustard. Acta Phytotech 48:39–47
Knasmüller S, Gottmann E, Steinkellner H, Fomin A, Pickl C, Paschke A, God R, Kundi M (1998) Detection of genotoxic effects of heavy metal contaminated soils with plant bioassay. Mutat Res 420:37–48
Kovalchuk O, Kovalchuk I, Arkhipov A, Telyuk P, Hohn B, Kovalchuk L (1998) The Allium cepa chromosome aberration test reliably measures genotoxicity of soils of inhabited areas in the Ukraine contaminated by the Chernobyl accident. Mutat Res 415:47–57
Kristen U (1997) Use of higher plants as screens for toxicity assessment. Toxicol In Vitro 11:181–191
Ma TH, Cabrera GL, Chen R, Gill BS, Sandhu SS, Vanderberg AL, Salamone MF (1994) Tradescantia micronucleus bioassay. Environ Health Perspect 95:157–189
Majer BJ, Grummt T, Uhl M, Knasmüller S (2005) Use of plant bioassays for the detection of genotoxins in the aquatic environment. Acta Hydrochim Hydrobiol 33:45–55
Miadoková E, Dúhová V, Vlčková V, Sládková L, Suchá V, Vlček D (1999) Genetic risk assessment of acid waste water containing heavy metals. Gen Physiol Biophys 18:92–98
Miadoková E, Vlčková V, Dúhová M, Trebatická M, Grolmus J, Fislová T, Danková A, Kečkešová Z, Baborová I (2001) Potential genotoxicity assessment of a new environment-friendly repellent preparation. Biologia (Bratislava) 56:703–707
Miadoková E, Svidová S, Vlčková V, Dúhová V, Pražmáriová E, Tothová K, Naďová S, Kogan G, Rauko P (2005) The role of natural biopolymers in genotoxicity of mutagens/carcinogens elimination. Biomed Pap Med Fac Univ Palacky Olomouc Czech Rep 149:493–496
Mičieta K, Murín G (1998) Tree species of genus Pinus suitable as bio-indicators of polluted environment. Water Air Soil Pollut 104:413–422
Mišík M, Solenská M, Mičieta K, Mišíková K, Knasmüller S (2006) In situ monitoring of clastogenicity of ambient air in Bratislava, Slovakia using the Tradescantia micronucleus assay and pollen abortion assays. Mutat Res/Genet Toxicol Environ Mutagen 605:1–6
Mišík M, Mičieta K, Solenská M, Mišíková K, Pisarčíková H, Knasmüller S (2007) In situ bio-monitoring of the genotoxic effects of mixed industrial emissions using the Tradescantia micronucleus and pollen abortion tests with wild life plants: demonstration of the efficacy of emission controls in an eastern European city. Environ Pollut 145:459–466
Němeček J, Podlešáková E, Vácha R (2002) Transfer of trace elements with low soil mobility into plants. Rostl Vyroba 48:45–50
Omenn GS (1991) Future research direction in cancer ecogenetics. Mutat Res 247:283–291
Patierno SR, Costa M (1987) Effect of nickel II on nuclear protein binding in intact mammalian cells. Cancer Biochem Biophys 9:113–126
Prasad MNV, Greger M, Landberg T (2001) Acacia nilotica L. bark removes toxic elements from solution: corroboration from toxicity bioassay using Salix viminalis L. in hydroponic system. Int J Phytoremediat 3:289–300
Quian X (2004) Mutagenic effects of chromium trioxide on root tip cells of Vicia faba. J Zhejiang Univ Sci 5:1570–1576
Rossman TG (1995) Metal mutagenesis. In: Goyer RA, Cherian GC (eds) Toxicology of metals. Springer, New York, pp 373–430
Rout GR, Sanghamitra S, Das P (2000) Effects of chromium and nickel on germination and growth in tolerant and non-tolerant populations of Echinochloa colona (L.). Chemosphere 40:855–859
Shanker AK, Cervantes C, Loza-Tavera H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Szárazová K, Fargašová A, Hiller E, Velická Z, Pastierová J (2008) Phytotoxic effects and translocation of Cr and Ni in washing wastewaters from cutlery production line to mustard (Sinapis alba L.) seedlings. Fresenius Environ Bull 17:58–65
Turner MA, Rust RH (1971) Effects of chromium on growth and mineral nutrition of soybeans. Soil Sci Soc Am Proc 35:755–758
Vassilev A, Berowa M, Zlatev Z (1998) Influence of Cd2+ on growth, chlorophyll content, and water relations in young barley plants. Biol Plant 41:601–606
Vazquez MD, Poschenrieder Ch, Barcelo J (1987) Chromium (VI) induced structural changes in bush bean plants. Ann Bot 59:427–438
Acknowledgements
This study was supported by the Grant of Ministry of Education, Science, Research and Sport of the Slovak Republic VEGA 1/0098/14.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Fargašová, A., Markert, B., Mičieta, K. (2015). Chromium and Nickel Phytotoxicity and Genotoxicity. In: Öztürk, M., Ashraf, M., Aksoy, A., Ahmad, M. (eds) Phytoremediation for Green Energy. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7887-0_4
Download citation
DOI: https://doi.org/10.1007/978-94-007-7887-0_4
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7886-3
Online ISBN: 978-94-007-7887-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)