Abstract
The present study was designed to evaluate the genotoxicity of sodium arsenite by employing the Vicia faba root chromosomal aberration assay. The seedlings were treated with different concentrations (0.2, 0.4, 0.6, 0.8, and 1 mg/l) of sodium arsenite. In addition to the cytogenetic assay, effect of arsenic on biochemical parameters such as total protein content and antioxidative enzymes [Sodium dismutase (SOD) and Guaiacol peroxidase (POD)] were also studied in 7-day old Vicia faba seedlings. Results demonstrated that metal stress significantly induced chromosomal aberrations and exhibited cytotoxicity by lowering the mitotic index from 9.37 ± 0.05 to 3.73 ± 0.01. Biochemical analysis showed a significant decrease in total protein content and increased activity of antioxidative enzymes under heavy metal stress as compared to control. The present investigations not only provide insights into arsenic genotoxicity but also support the use of various arsenic induced genotoxic characteristics as toxicity indicators in plants.
Similar content being viewed by others

Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is a harmful metalloid which can exist in soil and water in different organic and inorganic forms is one of the major environmental pollutants contaminating water and soil through industrial applications such as mining, coal combustion, agricultural practices, semiconductors, etc. and natural processes [2, 35]. An inorganic form of arsenic consists of arsenite and arsenate. The arsenite form is considered to be the most toxic form [43]. Arsenic is an element which is not needed for plant growth. As enters plants and interferes with their metabolic activities, causing physiological and morphological defects as well as plant growth suppression [24,25,26, 30]. The augmented production of reactive oxygen species (ROS) is considered to be the main metabolic effect of As toxicity [27, 40]. ROS damage essential biomolecules such as lipids, proteins, carbohydrates, and DNA [38, 39]. DNA damage from genotoxic substances can cause chromosomal aberrations (CA), micronuclei, and DNA breaks, all of which can be used as genotoxic markers [31]. Arsenic has been demonstrated to be genotoxic in a wide variety of experiments and induces genetic abnormalities both in vitro and in vivo systems [3, 17, 19, 34].
Higher plants provide an effective genetic system for evaluating the damaging effects of chemicals. A variety of plant systems, including Allium cepa, Vicia faba, Arabidopsis thaliana, and Hordeum vulgare have been investigated for analyzing the mutagenic activity of chemicals [37]. The plant bioassays are easy and economical and can be used in clastogenic screening as the plants can sense genotoxicity more efficiently and promptly than animal assays [44]. Root tip chromosomal aberration assay has been extensively used for studying chemical induced chromosomal aberrations as contains a high percentage of actively dividing cells. Vicia faba has the benefit of having six pairs of relatively large chromosomes with stable karyotype. These chromosomal features of Vicia faba helps in easy identification of mitotic stages and chromosomal alterations. Genotoxic end points consist of upsurge and decline in mitotic index as compared to control [4]. The material is readily available throughout the year; it is low-cost and simple to cultivate and handle. It also does not require any sterile conditions to grow [21]. The roots may be prime subjects and first defense organs against heavy metal stress [42]. In this study, Vica faba seedlings were used as test material. The objective of the current study was to investigate the genotoxic effects in roots of Vicia faba seedlings grown in different concentrations of sodium arsenite and to further screen the defense responses in the roots against arsenic toxicity.
Materials and methods
Experimental material
In the present study, Vicia faba was used as a plant system to check the genotoxicity of sodium arsenite (CAS no. 7784-46-5) using Vicia faba root chromosomal aberration assay. The certified and disease free seeds of Vicia faba L. (Vikrant - VH-82-1) were procured from Department of Plant Breeding, Hisar, Haryana.
Morphological parameters
Root growth inhibition test
Root growth inhibition test is simple, reliable, and easy to perform and provides a better idea about the toxicity of the experimental samples. This test is generally used to calculate the EC50 on the basis of root length of the plants treated with different concentrations of the test samples. Vicia faba seedlings were soaked in tap water for 24 h and were then allowed to germinate in different concentrations of sodium arsenite (0, 0.2, 0.4, 0.6, 0.8, 1.0 and 2.0 mg/l) for 96 h. Tap water used as a negative control. The test was performed in 3 replicates. The test solutions were replaced with fresh solutions after every 24 h. The three seedlings with longest roots were selected from each replicate and the root length was measured. EC50 (the effective concentration of a chemical producing 50% of the total effect in comparison to control) was calculated for the test samples.
Genotoxicity investigations
Vicia faba seed germination
The seeds of V. Faba were surface sterilized and washed thoroughly in distilled water for three times. About 25–30 seeds were soaked in distilled water for 24 h. Seed coat was removed carefully and seeds were allowed to germinate in autoclaved petri-plates containing moist cotton for 4 days at 23 ± 1. When newly emerged primary roots reached 3.0–5.0 cm in length, the root tip (5 mm) was cut off. The seedlings were then incubated in aerated tap water at 22 ± 2 °C to allow the development of lateral roots. After 4 days, the lateral roots (1–2 cm) were used for treatment with sodium arsenite.
Treatment with sodium arsenite
A stock solution of sodium arsenite (2 mg/l) was prepared just before use. The germinated seedlings of V. faba were treated with various concentrations of sodium arsenite viz. 0.2, 0.4, 0.6, 0.8 and 1 mg/l for 3 h at 22 ± 2 °C in the dark.
Fixation of root tips and staining
After the treatment, the roots were cleaned thoroughly with tap water, harvested and fixed in Farmer’s fluid (glacial acetic acid: ethyl alcohol in the ratio of 1:3) for 24 h. These were then shifted to 70% ethanol and stored at 4 °C. The slides were prepared by hydrolyzing the root tips in 1N HCl with intermittent heating for 1 min and then treated with 1N HCl and aceto-orcein stain in ratio of 1:9. The root tips were then warmed for 2–3 min with intermittent heating, covered and then kept for 30 min. The root tips were placed on slide and squashed in 45% (v/v) glacial acetic acid and tapping the coverslip with a matchstick and mounted with DPX.
Scoring of slides
The slides were observed for various types of chromosomal aberrations. About 750 dividing cells from 7–8 root tips (~ 100 cells/root tip) were scored. Photomicrographs were taken with the help of a digital camera fixed on microscope (Olympus) connected to a computer to transfer images. The physiological type of chromosomal aberrations scored were C-mitosis, delayed anaphase/s, stickiness and vagrant/s. The clastogenic chromosomal aberrations scored were chromatin bridge/s, chromosomal break/s and ring chromosome.
Calculations
The mitotic index (MI) was calculated as the number of dividing cells per 100 cells for each treatment. Chromosomal aberrations were scored and the results were tabulated.
Percent aberrant cells were calculated by:
Biochemical investigations
Preparation of Vicia faba extracts
1 g of fresh roots of germinated seedlings of Vicia faba treated with various concentrations of sodium arsenite (0.2, 0.4, 0.6, and 0.8 mg/l) were crushed and homogenized using pestle and mortar in 3 ml of pre-chilled potassium phosphate buffer (100 mM, pH 7.0) under ice-cold conditions. The homogenate was centrifuged at 10,000g at 4 °C for 20 min. The supernatant was collected for total protein and antioxidant enzyme analyses.
Estimation of protein content
Total protein content of the seedlings was quantified by the method of Bradford [5] using bovine serum albumin as standard. 2 ml of Bradford reagent was added in 150 µl distilled water and 50 µl sample extract in each test tube and the absorbance was taken at 595 nm using a spectrophotometer (Systronics 2202 UV–Vis spectrophotometer, Gujarat, India). A graph of absorbance versus concentration for standard solution of protein was plotted and the amount of protein was calculated from the graph. The amount of protein was expressed as mg/g f.w.
Estimation of antioxidative enzymes
Superoxide dismutase (SOD, EC 1.15.1.1)
Superoxide Dismutase activity was assayed according to the methodology of Kono [23] with slight modifications. For total SOD assay, 3.0 ml reaction mixture contained 50 mM sodium carbonate buffer (pH 10.2), 96 µM NBT, 20 mM hydroxylamine hydrochloride, 0.6% Triton X-100 and 70 µl of the enzyme extract. The absorbance was recorded spectrophotometrically (Systronics 2202 UV–Vis spectrophotometer, Gujarat, India) at 540 nm for 2 min.
Guaiacol peroxidase (POD, EC 1.11.1.7)
Guaiacol Peroxidase was assayed according to the method given by Putter [36]. The activity of the peroxidase can be determined by the decrease of H2O2, or the hydrogen donor or the formation of oxidized compound. The reaction mixture (3.0 ml) contained 50 mM phosphate buffer (pH 7.0), 20 mM guaiacol solution, 12.3 mM H2O2 and 100 µl enzyme extract. The POD activity was assayed by measuring the absorbance at 436 nm and using an extinction coefficient of 26.6 mM−1 cm−1.
Statistical analysis
Each experiment was carried out in triplicates. The data was subjected to one way ANOVA and expressed as the mean ± standard error. The significance level was checked p ≤ 0.05.
Results
Morphological parameters
Root growth inhibition test
The estimated EC50 (the effective concentration of a chemical that produces 50% of the total effect when compared to the control) of Vicia faba seedlings exposed to sodium arsenite was 0.8 mg/l. The highest concentration of sodium arsenite (2 mg/l) showed 21.70% root growth as compared to control (tap water) whereas minimum tested concentration (0.2 mg/l) showed 95.73% root length as compared to control. The root length decreased as the concentration of sodium arsenite increased. One way Anova analysis showed F-ratio = 396.72 and HSD = 0.629 (Fig. 1).
Genotoxicity
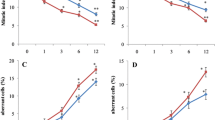
The mitotic index (MI) was found to decrease significantly as compared to control. NaAsO2 significantly induced chromosomal aberrations and exhibited cytotoxicity by lowering the mitotic index from 9.37 ± 0.05 to 3.73 ± 0.01. NaAsO2 induced more physiological aberrations (20.93%) at 0.8 mg/l as compared to clastogenic aberrations (9.87%). Physiological aberrations included c-mitosis, delayed anaphase/s, vagrant/s and stickiness while clastogenic aberrations such as chromosomal break/s, chromatin bridge/s and ring chromosome were observed (Table 1, Fig. 2). One way Anova analysis showed F-ratio = 33.683 and HSD = 6.712.
Total protein content
The total protein content decreased in the seedlings treated with arsenic metal solution as compared to untreated seedlings (Fig. 3). Protein content was decreased from 107.2 (mg/g f.w.) in control seedlings to 38.83 (mg/g f.w.) in seedlings treated with 0.8 mg/l arsenic. The protein content decreased with increasing concentration of sodium arsenite. One way Anova analysis showed F-ratio = 10.352 and HSD = 40.134.
Antioxidant enzyme activities
Specific activity of superoxide dismutase was observed to increase considerably under metal stress with increasing concentrations of sodium arsenite. Maximum value of SOD (0.306 unit activity/mg protein) was observed at 0.8 mg/l arsenic as compared to untreated control seedlings (0.069 ± 0.007 unit activity/mg protein) (Fig. 4). One way Anova analysis showed F-ratio = 6.213 and HSD = 0.177 for SOD activity. The activity of POD increased with increasing concentration of sodium arsenite. The activity of POD was more at 0.8 mg/l arsenic (0.014 unit activity/mg protein) as compared to control (0.002 unit activity/mg protein) (Fig. 5). For POD, One way Anova analysis showed F-ratio = 6.286; HSD = 0.0085. Results were found to be statistically significant at the 0.8 mg/l treatment for both enzyme activities.
Discussion
Large regions are exposed to heavy metal pollution due urbanization and industrialization [33]. Although many heavy metals when present in trace amounts are essential for various metabolic processes in organisms but at high concentration, they tend to create physiological stress due to generation of free radicals [18]. Oxidative damage is caused by an imbalance between free radical generation and the antioxidant defence system [9]. Among different heavy metals, arsenic is possibly the most abundant pollutant having complex metabolism and has been classified as a potent human carcinogen. Exposure to arsenic has been linked with augmented risk of various types of cancers, skin diseases, ischemic heart diseases, neurological effects and teratogenicity [12]. Vogt and Rossman [41] reported that As (III) can induce genetic effects such as chromosomal aberrations, micronuclei formation etc. and also recognized to inhibit DNA repair. Arsenicals can cause damage to DNA by inhibiting the enzyme activity involved in DNA repair. Genetic abnormality without effective DNA repair causes certain types of mutations and eventually initiates carcinogenesis.
As shown in Table 1, sodium arsenite significantly induced chromosomal aberrations and exhibited cytotoxicity by lowering the mitotic index from 9.37 ± 0.05 to 3.73 ± 0.01. Sodium arsenite induced physiological aberrations viz. c-mitosis, delayed anaphase/s, vagrant/s and stickiness and clastogenic aberrations viz. chromosomal break/s, chromatin bridge/s and ring chromosome. Sodium arsenite induced more physiological aberrations (20.93%) at 0.8 mg/l as compared to clastogenic aberrations (9.87%). The different types of chromosomal alterations could be induced either by disruption of spindle, C-mitosis, stickiness, by disorientation and multipolarity at anaphase [15]. Similar results were obtained in a study conducted by Bandyopadhyay [1] in Allium cepa treated with sodium arsenate and sodium arsenite. It was found that the upsurge in chromosomal aberrations and reduction in the mitotic index was directly related to the concentration of the mutagen and duration of exposure. Pandey and Upadhyay [33] also reported the clastogenic effect of arsenic on root meristem cells of Vicia faba L. The mitotic index declined gradually as the concentration of arsenic metal increased.
Heavy metals have an aneugenic impact on C-mitosis, inhibiting spindle formation and increasing chromosome adhesion owing to aberrant chromosomal proteins [33]. Sticky nature of chromosomes might be due to the disturbance in nucleic acid metabolism of the cell [6]. Stickiness might also be caused by improper folding of chromosomal fibres into a single chromatid, resulting in fibre intermingling and chromosomes becoming linked to one other via sub-chromatid bridges [22]. Delayed anaphase occurs when two anaphasic groups of chromosomes lie close to each other near equatorial plate. Fiskesjo [11] also reported the occurrence of delayed anaphase in Allium cepa after treatment with mercury and selenium. The start of vagrant chromosomes leads in the division of an uneven number of chromosomes in the daughter cells, resulting in the creation of unequally sized or irregularly shaped nuclei during interphase [8]. Chromosomal breakage was most prominent clastogenic aberration observed in the present study. The chromosomal breakage is thought to involve the DNA responsible for the chromosomes' linear continuity and may be caused by incomplete or incorrect DNA repair [10]. Gormurgen [14] attributed the formation of chromosomal bridges to the failure of free anaphase separation, uneven translocation or inversion of chromosome fragments and concluded that bridge formation causes structural chromosome mutations. Yildiz et al. [45] accredited the induction of bridges to chromosome break, stickiness, breakage and reunification of the broken ends. Distal breaking of the short and long arm of a chromosome causes rejoining at the ends leading to the formation of ring chromosomes [13].
Proteins are a critical component of the cell that is readily affected under environmental stress situations. As a result, any changes in these might be regarded as a key sign of oxidative stress in plants. The total protein concentration was found to decrease with increasing metal treatment. The decrease in total protein content caused by heavy metal stress might be ascribed to increased protease activity, which speeds up the protein breakdown process [32]. Our results are in concordance with Latif [1] who also reported a significant decrease in total protein content with increasing concentration of nickel sulphate in Raphanus sativus L. Hamid et al. [16] also reported decline in total protein content in Phaseolus vulgaris with different treatments of lead. Arsenic is believed to have caused lipid peroxidation and protein fragmentation as a result of the harmful effects of reactive oxygen species, resulting in lower protein content [20].
Arsenic toxicity can result in an increase in the generation of ROS such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide. Cells can defend themselves from oxidative damage by using enzymatic and non-enzymatic systems such as SOD, CAT, POD, or glutathione [7]. SOD and POD are significant antioxidant enzymes that check the buildup of various ROS in the aerobic cells [21]. The observations in the present study revealed that application of arsenic metal solution to Vicia faba resulted in increased activity of antioxidative enzymes viz. Superoxide dismutase (SOD) and Guaiacol peroxidase (POD), which is consistent with Lin et al. [29] who reported the enhanced SOD and POD activity with increasing arsenate concentrations in leaves of Vicia faba. The increase in SOD activity suggested that arsenate exposure caused the production of O2.−, which increased the biosynthesis of SOD while increase in the activity of POD specifies the buildup of H2O2 in plant tissues which may have stirred the biosynthesis of POD. The increased in activity of SOD and POD was a part of a damage response to stress induced by sodium arsenite.
Conclusion
Arsenic compounds can cause damage to cells through generation of free radicals and ROS such as superoxide, hydroxyl radicals, hydrogen peroxide during their metabolism in the cells and also induces chromosome aberrations. The present results showed that sodium arsenite is a clastogenic and genotoxic agent in V. faba root tip chromosomal aberration assay. These findings clearly reveal that application of sodium arsenite reduced total protein concentration while increasing the activity of antioxidative enzymes (SOD and POD).
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Bandyopadhyay B. Comparison of clastogenic effects of two arsenic salts on plant system in vivo. J CytolGenet. 1995;30:35–9.
Basu S, Datta AK, Pramanik A, Gupta S, Das D, Karmakar R, Ghosh B. Assessment of cytotoxicity induced by heavy metal arsenic trioxide and azo-dye metanil yellow in Allium cepa assay and aqueous plant extracts mediated amelioration. Cytologia. 2019;84(3):263–9.
Basu A, Mahata J, Gupta S, Giri AK. Genetic toxicology of a paradoxical human carcinogen, arsenic: a review. Mutat Res. 2001;488:171–94.
Basu S, Tripura K. Differential sensitivity of Allium cepa L. and Vicia faba L. to aqueous extracts of Cascabela thevetia (L.) Lippold. S Afr J Bot. 2021;139:67–78.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–54.
Chidambaram AL, Murugan A, Sankar Ganesh K, Sundaramoorthy P. Effect of chromium on growth and cell division of blackgram (Vigna munga L.). Plant Arch. 2006;6:763–6.
Dazy M, Masfarand JF, Fererd JF. Induction of oxidative stress biomarkers associated with heavy metal stress in FontinalisantipyreticaHedw. Chemosphere. 2009;75:297–302.
El-Ghamery AA, EL-Kholy MA, EL-Yousser A. Evaluation of cytological effects of Zn2+ in relation to germination and root growth of Nigella sativa L. and Tritium sativum L. Mutat Res. 2003;2003(537):29–41.
Elstner EF. Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol. 1982;33:73–96.
Evans HS. Molecular mechanism in the induction of chromosome aberrations. In: Scott D, Bridges BA, Sobells FH, editors. Progress in genetic toxicology. Amsterdam: Elsevier North Holland and Biochemical Press; 1977. p. 57.
Fiskesjo G. Mercury and Selenium in a modified Allium test. Hereditas. 1979;91:169–78.
Gamble MV, Liu X, Ahsan H, Pilsner R, Ilievski V, Slavkovich V, Parvez F, Levy D, Factor-Litvak P, Graziano JH. Folate, homocysteine, and arsenic metabolism in arsenic-exposed individuals in Bangladesh. Environ Health Perspect. 2005;113(12):1683–8.
Gardener RJ, Sutherland M, Grant R. Chromosome abnormalities and genetic counseling. 3rd ed. Oxford: Oxford University Press; 2004.
Gormurgen AN. Cytological effect of the potassium metabisulphite and potassium nitrate food preservative on root tips of Allium cepa L. Cytologia. 2005;70:119–28.
Gupta K, Srivastava A, Srivastava S, Kumar A. Phyto-genotoxicity of arsenic contaminated soil from Lakhimpur Kheri, India on Vicia faba L. Chemosphere. 2020;1(241):125063.
Hamid N, Bukhari N, Jawaid F. Physiological responses of Phaseolus vulgaris to different lead concentrations. Pak J Bot. 2010;42(1):239–46.
Hartmann A, Speit G. Comparative investigations of the genotoxic effects of metals in the single cell gel assay and the sister-chromatid exchange test. Environ Mol Mutagen. 1994;23:299–305.
Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. InterdiscipToxicol. 2014;7(2):60–72.
Jha AN, Noditi M, Nilsson R, Natarajan AT. Genotoxic effects of sodium arsenite on human cells. Mutat Res. 1992;284:215–21.
John R, Ahmad P, Gadgil K, Sharma S. Effect of cadmium and lead on growth, biochemical parameters and uptake in Lemnapolyrrhiza L. Plant Soil Environ. 2008;54(6):262–70.
Kanaya N, Gill BS, Grover IS, Murin A, Osiecka R, Sandhu SS, Andersson HC. Vicia faba chromosomal aberration assay. Mutation Res/Fund Mol Mech Mutagenesis. 1994;310(2):231–47.
Klasterska I, Natrajan AT, Ramel C. An interception of the origin of subchromatid aberrations and chromosome stickiness as a category of chromatid aberration. Hereditas. 1976;83:153–62.
Kono Y. Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95.
Kumar A, Dixit G, Singh AP, Dwivedi S, Srivastava S, Mishra K, Tripathi RD. Selenate mitigates arsenite toxicity in rice (Oryza sativa L.) by reducing arsenic uptake and ameliorates amino acid content and thiol metabolism. Ecotoxicol Environ Saf. 2016;133:350–9.
Kumar A, Dwivedi S, Singh RP, Chakrabarty D, Mallick S, Trivedi PK, Adhikari B, Tripathi RD. Evaluation of amino acid profile in contrasting arsenic accumulating rice genotypes under arsenic stress. Biol Plant. 2014;58(4):733–42.
Kumar A, Singh RP, Singh PK, Awasthi S, Chakrabarty D, Trivedi PK, Tripathi RD. Selenium ameliorates arsenic induced oxidative stress through modulation of antioxidant enzymes and thiols in rice (Oryza sativa L.). Ecotoxicology. 2014;23(7):1153–63.
Kumar Srivastava P, Singh PC, Gupta M, Sinha A, Vaish A, Shukla A, Singh N, Krishna TS. Influence of earthworm culture on fertilization potential and biological activities of vermicomposts prepared from different plant wastes. J Plant Nutr Soil Sci. 2011;174(3):420–9.
Latif HH. The influence of nickel sulphate on some physiological aspects of two cultivars of Raphanussativus L. Arch Biol Sci. 2010;62(3):685–93.
Lin A, Zhang X, Zhung X, Zhu YG, Zhao FJ. Arsenate-induced toxicity effects on antioxidative enzymes and DNA damage in Vicia faba. Plant Physiol Biochem. 2008;27(2):413–9.
Mokgalaka-Matlala NS, Flores-Tavizon E, Castillo-Michel H, Peralta-Videa JR, Gardea-Torresdey JL. Toxicity of arsenic (III) and (V) on plant growth, element uptake, and total amylolytic activity of mesquite (Prosopis juliflora x P. velutina). Int J Phytoremed. 2008;10(1):47–60.
Van der Oost R, Beyer J, Vermeulen NP. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol. 2003;13(2):57–149.
Palma JM, Sandalio LM, Corpas FJ, Romero-Puertas MC, McCarthy I, del Rio LA. Plant proteases, protein degradation and oxidative stress: role of peroxisomes. Plant Physiol Biochem. 2002;40:521–30.
Pandey RM, Upadhya SK. Cytological effect of heavy metals on root meristem cells of Vicia faba L. Toxicol Environ Chem. 2010;92:89–96.
Patlolla A, Tchounwou PB. Cytogenetic evaluation of arsenic trioxide toxicity in Sprague-Dawley rats. Mutat Res Gen Tox Environ Mutagen. 2005;587(1–2):126–33.
Pramanik A, Datta AK, Gupta S, Basu S, Das D, Ghosh B. Cytotoxicity assessment of heavy metal arsenic (arsenic trioxide) using Nigella sativa L. (black cumin) as test system. Cytologia. 2019;84(3):215–9.
Putter J. Peroxidase. In: Bergemeyer HU, editor. Methods of enzymatic analysis, vol. II. London: Academic Press; 1974. p. 685–90.
Sang N, Li G. Genotoxicity of municipal landfill leachate on root tips of Vicia faba. Mutation Res/Genet Toxicol Environ Mutagenesis. 2004;560(2):159–65.
Singh AP, Dixit G, Mishra S, Dwivedi S, Tiwari M, Mallick S, Pandey V, Trivedi PK, Chakrabarty D, Tripathi RD. Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front Plant Sci. 2015;6:340.
Singh KP, Kumari R, Treas J, DuMond JW. Chronic exposure to arsenic causes increased cell survival, DNA damage, and increased expression of mitochondrial transcription factor A (mtTFA) in human prostate epithelial cells. Chem Res Toxicol. 2011;24(3):340–9.
Srivastava S, Suprasanna P, D’Souza SF. Redox state and energetic equilibrium determine the magnitude of stress in Hydrilla verticillata upon exposure to arsenate. Protoplasma. 2011;248(4):805–15.
Vogt BL, Rossmann TG. Effects of arsenite on p53, p21 and cyclin D expression in normal human fibroblasts- a possible mechanism for arsenite’scomutagenecity. Mutat Res. 2001;478:159.
Wang C, Tian Y, Wang X, Geng J, Jiang J, Yu H, Wang C. Lead-contaminated soil induced oxidative stress, defense response and its indicative biomarkers in roots of Vicia faba seedlings. Ecotoxicology. 2010;19(6):1130–9.
WHO (World Health Organization) Environmental health criteria-arsenic, Geneva. 1981
Yi H, Meng Z. Genotoxicity of hydrated sulfur dioxide on root tips of Allium sativum and Vicia faba. Mutat Res. 2003;537(1):109–14.
Yildiz M, Cigerci IH, Konuk M, Fidan AF, Terzi H. Determination of genotoxic effects of Copper Sulphate and cobalt chloride in Allium cepa root cells by chromosome aberration and comet assays. Chemosphere. 2009;75:934–8.
Acknowledgements
Authors are thankful to Department of Botanical and Environmental Sciences Guru Nanak Dev University, Amritsar for providing necessary facilities to carry out this work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TK and MK carried out experiments, wrote the manuscript and analyze the data; SK designed and checked the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
All the Authors provide their consent for publication.
Ethics approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Editor: Anita Mukherjee; Reviewer: Puneet Kumar
Rights and permissions
About this article
Cite this article
Kaur, T., Kumar, M. & Kaur, S. Genotoxicity of sodium arsenite on Vicia faba root meristematic cells. Nucleus 65, 215–222 (2022). https://doi.org/10.1007/s13237-022-00385-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-022-00385-4