Abstract
The effect of Cr6+ on Allium cepa root length was studied using both clean and polluted river waters. Seven series of Cr6+-doped polluted and non-polluted river waters were used to grow onions. Chromium concentration (Cr6+) of 4.2 mg L−1(EC50 value), doped in clean river water caused a 50% reduction of root length, while in organically polluted samples similar root growth inhibition occurred at 12.0 mg Cr6+ L−1. The results suggested that there was a dislocation to higher values in toxic chromium concentration in polluted river water due to the eutrophization level of river water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In natural aquatic ecosystems, metallic compounds can occur in low concentrations such as nanogram or microgram per liter. Metals can usually come from natural sources or can be introduced via several anthropogenic activities, which in the last decades caused an increasing concern. The trivalent chromium appears in the form of colloidal hydrous oxides and at low concentration plays an important role in the living organisms’ metabolism, while hexavalent chromium has a toxic effect on them above 0.05 mg L−1 (Richard and Bourg 1991). In the last decades, to evaluate the quality of underground and surface waters, and effluents, the Allium test has been applied. Several parameters have been studied such as root growth inhibition, metaphase and anaphase cellular aberrations, and cellular division inhibition (Fiskesjö 1989; Kumar and Sinha 1989; Ruiz et al. 1992; Vesna et al. 1996).
The aim of the present study was to estimate the Allium cepa L. sensitivity to hexavalent chromium in two river waters with different quality. Further, to check the effect of river water eutrophization level on the onion roots inhibition response. The results could reflect on a direction of the environmental educational projects where A. cepa L. could be used as a test organism for toxicity assessment in polluted river waters.
Materials and Methods
Commercial variety bulbs of the common onion (A. cepa L.) were purchased locally. Only onion bulbs in a good condition (3.5–4.0 cm diameter) were chosen to grow up in each liquid test. Germinating onions have been discarded. The bulbs were cleaned by cutting their dry roots off, washed, rinsed and then stored in a refrigerator at 4°C. For sampling, 20 L polyethylene recipients were subjected to a cleaning process in accordance with the Standard Methods for the Examination of Water and Wastewater (APHA, AWWA, WEF 1999). A 300 L total volume of river water was collected in 1 day at two sites in the Toledo River, Brazilian Paraná state. The first collection site was at the beginning, and the second one was at the end of Toledo River. It can be noticed, that there were strong oscillations in the river water quality at the second collection site because of the low effectiveness of the industrial and domestic effluent treatment plants installed downstream of Toledo city. All used chemicals were of analytical grade. Stock solution of 1.0 g L−1 of Cr6+ was prepared in volumetric glass flasks, by dissolving the chromic anhydride (CrO3, Merck; CAS# 1333-82-0) into deionized water. Six kinds of Cr6+-doped river waters containing from 0.2 to 40 mg Cr6+ L−1 were prepared by adding aliquots of 1.0 g Cr6+ L−1 into clean or polluted river waters, and preparations were stored in 2 L acid-washed volumetric flasks. As blank controls river waters from both collection sites with no addition of chromium solution were used. Each quality of river water was previously characterized through the following physical–chemical parameters: pH, total phosphate (TP), total Kjeldahl nitrogen (TKN), chemical oxygen demand (COD), and biological oxygen demand (BOD). These physical–chemical parameters were measured according to the water and waste water methodologies on the Standard Methods 20th edition (APHA, AWWA, WEF 1999).
A set of 250 mL conical polyethylene cups (8 cm height) and their respective 7.0 cm diameter lids, washed previously with diluted nitric acid (10%) were used. As a bulb support in the central and upper part of the cup, the lids were perforated (3.0 cm hole) allowing the upright bulb position to have a direct contact with the tested liquid. Seven hexavalent chromium-doped test solutions were used for each river water quality, including the control river water. For each river water quality, seven series of ten cups were filled with 200 mL Cr-doped river waters and controls. In each cup, one clean onion bulb was grown for 72 h in laboratory conditions. The tests were performed at room temperature (about 20°C) with a natural light-dark regime, and were protected from direct contact with the sunlight. At the end of growth experiment, Cr-doped and control river waters were collected for metal analysis. From each series of ten cups, onions with the shortest and the longest roots were excluded, considering that only the remaining eight replicates of both onions and test liquids have represented the series. The average root length was estimated for each series. Finally, the mean root length determined from eight onions of each series and its standard deviation were determined. In order to separate all the suspended and precipitated matter from the liquid phase, a mixture of eight replicates test liquids from each series was made and an aliquot of 50 mL was filtered with a Millipore filtration system by using cellulose acetate membranes (47 mm of diameter and 0.45 μm of pore size).
For metal analysis, all samples were prepared by addition of 20 μL Ga (1.0 g L−1 Ga standard solution in 2% HNO3, Acros® organics) as an internal standard to 2 mL of test liquid-filtered aliquots. The accuracy of quantitative analysis was checked with a multi-element standard reference material containing Cr, As, Se, Ag, Cd, Ba and Pb. For a standard preparation, 500 μL of drinking water pollutants (Aldrich) standard was mixed with 50 μL Ga (102.5 mg L−1). The calculated data were compared with the certified values. An aliquot of 5 μL of each sample was deposited on a pre-cleaned acrylic disk (ϕ 30 mm, 3 mm thick) and dried at room temperature to form thin layer of dry residues. Further, the metal amounts of the samples were analyzed by Synchrotron radiation total reflection X-ray fluorescence (SR-TXRF) technique. The SR-TXRF measurements were carried out by using a polychromatic X-ray beam (maximum energy of 20 keV of D09-XRF beam line) at the Brazilian Light Synchrotron Laboratory (LNLS), in São Paulo state. Each reflector disk containing the sample was positioned for the total reflection condition, and the acquisition time was set up at 200 s. A HP-Ge detector with 160 eV of FWHM@Mn-Kα line was used.
Results and Discussion
In Table 1, the physicochemical parameter values for both sample points of river waters are summarized. The chemical and biological oxygen demand (COD and BOD) were frequently used as indicators of the water source quality. These parameters can be used as well to determine the dynamics of oxygen uptake by the organism. According to the Brazilian Environmental Legislation (BEL) (Brazil 2005), the water quality of the first sample point in Toledo river was assigned as good one and called as clean water (CW), while the second sampling site at downstream Toledo city was corresponded to a very poor water quality and was labeled as polluted water (PW).
The SR-TXRF spectra were analyzed by using the AXIL program (Van Espen et al. 1986). SR-TXRF elemental sensitive curve for the K X-ray series was previously performed by applying five well-known concentration values containing multi-elemental standards such as P, K, V, Co, Cu, Ga, Se and Zr (1.0 g L−1 standard solution for AAS, Acros® organics). An exponential-type function (see Eq. 1.) was chosen to describe the relative-to-Gallium sensitivity experimental data. The obtained results have shown a good statistical fitting (r 2 = 0.9974 and χ 2 = 0.0070).
where Z- is the atomic number and it can range from 14 to 40.
The total chromium concentrations were determined according to Eq. 2 by using the relative-to-gallium fluorescent sensitivity curve. This evaluation was considered for the SR-TXRF system and Cr-Kα fluorescent intensity. The detection limit (LD) for chromium is directly related to the background intensity (I BG), in agreement with the Eq. 3.
where I Cr and I Ga represent the X-ray Kα fluorescent intensities of chromium and gallium (internal standard), respectively; S r(24) is the Cr relative-to-gallium fluorescent sensitivity; C Cr and C Ga represent the Cr and Ga concentrations.
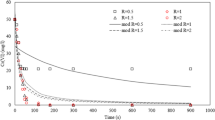
In Table 2 are summarized the measured and certified values for the elements Cr, As and Se. Measured values showed relative standard deviation (RSD) lower than 10% for all elements in the analyzed standards. A detection limit for chromium around 0.001 mg L−1 was calculated based on the Eq. 3 estimations. In Table 3 are summarized the mean lengths of the roots and dissolved chromium concentrations measured by SR-TXRF for both clean (CW) and polluted river waters (PW). Boltzmann-type function (see Eq. 4) was applied to fit the experimental data and to analyze the quantitative relation between the water pollution levels of test liquids and the amount of hexavalent chromium in Cr treatments (where, CW and PW quality points were used). The program was coded in software Origin® version 8.0. The obtained results and experimental data are shown in Fig. 1.
where R stands for the mean root length; C is the Cr concentration; A 1, A 2, C 0 and ΔC are the Boltzmann-type function parameters.
In Table 4 are summarized the Boltzmann-type function parameter values and the statistical parameters for both quality checking points of river waters. The non- chromium-doped clean and polluted river waters have approximately the same amount of initial total chromium concentration, which was less than 0.1 mg L−1. At low Cr6+ ions concentration (for CW and PW points) such as 0.5 and 1.0 mg L−1, the mean root length is close to constant within a standard deviation (Fig. 1). When the hexavalent chromium concentrations in CW point were below 0.55 mg L−1 there was no root growth inhibition, and this river water was classified as II type according to the BEL on water quality (Brazil 2005). Above 1.0 mg L−1 of Cr6+ ions concentration it was evident that the root growth inhibition at CW and PW points took place. In PW, at low chromium concentration, the mean root length was about 3.7 cm, while in CW point this value was 3.2 cm, most probably because of the low amount of organic compounds or limiting nutrients in CW region. On the other hand, at high concentrations of Cr6+ in Cr6+-doped clean and polluted river waters, such as 3.0 and 4.0 mg L−1, root length was reduced with 20% and 30%, respectively, which was close to the C 0-Boltzmann parameters for CW and PW, respectively. The EC50 values can be determined for each fitting curve (EC50 = effective concentration permitting 50% growth in relation to control growth). In CW point and according to the fitting data, the EC50 has been estimated to occur at 4.2 mg L−1 of Cr6+ as shown in Table 4. In Cr6+-doped polluted water treatments, the same phenomenon was observed for concentration equals to 12.0 mg L−1. In PW point it was observed that the chromium concentration should be decreased approximately 2.9 times in order EC50 to reach the same inhibition effect on the root length as in CW point. Based on physical–chemical parameters values, the correlation between the root length inhibition and river water quality can be considered similar for CW and PW points.
In our work, the obtained results in the Allium test by using hexavalent chromium-doped river waters have indicated a dislocation factor for EC50 value to higher chromium concentrations, when the toxic effects of Cr-doped PW and CW were compared. It can be noticed that for Cr6+-doped river water treatments, the higher difference between CW and PW was observed in the water quality, because PW contained higher amount of organic and inorganic compounds reflecting in higher BOD values than ones observed in CW point. Thus, in the polluted waters, the roots have naturally created a blockade mechanism to prevent the chromium toxicity resulting in dislocation of the EC50 value to higher Cr6+ concentration. Hence, this phenomenon should be taken into account when the river water quality is estimated by using a root length-based comparative method and Allium as a bio-indicator. Moreover, for PW treatments, the ΔC Boltzmann-type function parameter value was higher than CW one (see Table 4) indicating that its value strongly depended on chemical and biological oxygen demand in the river water. Hence, this model parameter can be used as a sensitive model-based indicator of river water quality. Reviewing the results of other authors can be notice that this phenomenon was observed in an early work of Palácio et al. 2005, where copper and lead ions were used as inhibitor metals on Allium root lengths. In order to obtain the same root growth inhibition effect in CW point, Palácio et al. (2005) have suggested that in PW point an increase of copper and lead concentrations by a factor of 7.3 and 6.0, respectively can be achieved.
In conclusion, the Allium test can be considered more sensitive when Cr+6 concentration is higher than 1.0 mg L−1 for both clean and polluted river waters. The lack of nutrients or organic pollutants in CW point reflected on chromium toxicity in lower hexavalent chromium concentration, but on water body when high organic pollutant took place, the EC50 value is dislocated to higher hexavalent chromium concentration. In polluted water, growth inhibition effects showed a 2.9-concentration dislocating factor for EC50 value, due to the higher chemical and biological oxygen demand. A. cepa L. exhibits different sensitivities, depending on the river water quality (CW and PW) and the applied metal. Finally, the authors believe that further studies have to be performed in order to reveal the correlation between the EC50 values and physical–chemical parameters of tested liquids. The Allium test is promising method for quality control of polluted river waters and must be developed by using complex scientific approaches.
References
APHA, AWWA, WEF (1999) Standard methods for examination of water and wastewater. In: Clescerl LS, Greenberg AE, Eaton AD (eds) 20th edn, Washington, DC
Brazil (2005) Brazilian government environmental norm – CONAMA 357. In: Water quality conditions and standards (in portuguese), on March 17th 2005, pp 5–12. Available in http://www.mma.gov.br/port/conama/res/res05/res35705.pdf. Accessed 15 Jun 2008
Fiskesjö G (1989) The Allium test—an alternative in environmental studies: the relative toxicity of metal ions. Mutat Res 197:243–260. doi:10.1016/0027-5107(88)90096-6
Kumar D, Sinha SP (1989) Threshold dose of cytogenetic toxicity of Lindane, Malthion and Metacid in Allium cepa root-tip cells. Cytologia 54:547–552
Palácio SM, Espinoza-Quiñones FR, Galante RM, Zenatti DC, Seolatto AA, Lorenz EK, Zacarkim CE, Rossi N, Rizzutto MA, Tabacniks MH (2005) Correlation between heavy metal ions (Copper, Zinc, Lead) concentrations and root length of Allium cepa L. in polluted river water. Braz Arch Biol Technol 48:191–196
Richard FC, Bourg AC (1991) Aqueous geochemistry of chromium: a review. Water Res 25:807–816. doi:10.1016/0043-1354(91)90160-R
Ruiz EF, Rabago VME, Lecona SV, Perez AB, Ma TH (1992) Tradescantia-micronucleus (trad-MCN) bioassay on clastogeniticity of wastewater and in situ monitoring. Mutat Res 270:45–53. doi:10.1016/0027-5107(92)90100-G
Van Espen P, Janssens K, Swenters I (1986) AXIL X-ray analysis software. Canberra Packard, Benelux
Vesna S, Stegnar P, Lovka M, Toman MJ (1996) The evaluation of waste, surface and ground water quality using the Allium test procedure. Mutat Res 368:171–179. doi:10.1016/S0165-1218(96)90059-2
Acknowledgments
We gratefully acknowledge the Brazilian Light Synchrotron Laboratory for partial financing of this study through the 4654 project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Espinoza-Quiñones, F.R., Szymanski, N., Palácio, S.M. et al. Inhibition Effect on the Allium cepa L. Root Growth When Using Hexavalent Chromium-Doped River Waters. Bull Environ Contam Toxicol 82, 767–771 (2009). https://doi.org/10.1007/s00128-009-9682-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-009-9682-z