Abstract
Cotton, the most important economic crop in the world, displays strong hybrid vigor, and has long been a subject for hybrid cultivar breeding. Here, advances in the theoretical and applied research in cotton heterosis along with its hybrid cultivar development by hand-emasculation and pollination (HEP), cytoplasmic (CMS) and genic male sterile lines (GMS) mainly in China during the past few decades are presented in this review. Three types of hybrids produced by HEP, CMS and GMS facilitating hybrid seed production with hand-pollination have been developed and are being planted simultaneously in cotton production. However, most hybrids commercially planted in production are produced by HEP, therefore, F2 seeds are being extensively planted due to the high cost to produce F1 seed. F2 generations of these combinations exceed the check cultivars in yield usually up to 5~15%. GMS genes (ms2 and ms5ms6) used in hybrid seed production and casual mitochondrial genes for G. harknessii CMS have been cloned. Challenges and opportunities in cotton heterosis and future hybrid cultivar development in cotton are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exploiting the hybrid vigor phenomenon or heterosis is important for improving crop yield (Schnable and Springer 2013). The use of heterosis in rice, rape, sorghum and other major crops depends on male sterile lines (Atlin 1995, Lambright 2019, Westhues et al. 2017, Xie et al. 2019). Cotton (Gossypium sp.) is a major fiber and oil crop that is widely cultivated in the world as an essential industrial resource for human and social development. Like other crops, heterosis has been exploited as an important way to improve lint yield and fiber quality in cotton. In cotton production practices, the superior F1 exhibits significant advantage of early maturity and high yield, fiber quality, biotic and abiotic stress tolerances over its parents (Zhang & Pan 1999, Munir et al. 2016, Tian et al. 2019, Zaidi et al. 2020). Therefore, breeding for cotton hybrids is an important way to achieve the breeding goal. Most F1 hybrids commercially planted in cotton production are produced by hand-emasculation and pollination (HEP). Although F2 seeds are not strictly considered as hybrids, F2 seeds are being extensively planted in China due to high cost to produce F1 seeds. Compared with F1 plants, F2 plants may result in yield reduction. In the 1990s, F2 seeds were planted in more than 80% of hybrid cotton growing area in China. Since the beginning of 21st centry, with the successful planting of hybrids containing transgenic Bt trait, the utilization of cotton hybrids has been accelerated and resulted in covering whole cotton growing region in Yangtze (YaRCGR), and most of the Yellow River (YeRCGR) and partly in the Northwestern Inland Cotton Growing Region (NICGR) including Xinjiang.
Male sterility had been found in 617 species belonging to 162 genera in 43 families, including major crops such as rice, maize, wheat, cotton (Shen et al. 2017, Wan et al. 2019, Yang et al. 2021). The application of a male sterility line can facilitate large-scale production of crop hybrids. The male sterility system is one of the important means for hybrid breeding and production in cotton (Zhang & Pan 1999, Budar & Pelletier 2001). Both cytoplasmic (CMS) and genic male sterile (GMS) hybrids have been developed and planted commercially in China and India. GMS genes (ms2 and ms5ms6) and casual mitochondrial genes for G. harknessii CMS used commercially in hybrid seed production have been cloned (Wu et al. 2022, Ma et al. 2022, Mao et al. 2022, Xuan et al. 2022).
During the past few decades, more than 100 hybrid cultivars, most were transgenic Bt bollworm-resistant cultivars, have been developed and extensively planted in China. The extension and application of such hybrids played an important role in cotton production in China. Almost all cotton growing areas were planted with transgenic Bt hybrids produced by HEP, CMS and GMS in the YaRCGR, and mostly in YeRCGR. Most of the hybrids grown now are produced by hand-emasculation and pollination in cotton because this type of hybrid is easy to develop due to its wide parent selection and some of them can be used in F2. In this article, advances in cotton hybrid cultivar development by HEP, CMS and GMS are reviewed. Insight into future development and commercial production of cotton hybrid is provided.
Hybrid breeding and production by hand-emasculation and pollination
Hybrid cultivar development by hand-emasculation and pollination
Loden and Richmond (1951), Meyer (1969), Davis (1978), Meredith (1984, 1990) and Zhang & Pan (1999) made comprehensive reviews concerning the general situation of heterosis utilization at different times. Interspecific heterosis between G. hirsutum L. and G. barbadense L. was first published in cotton in 1894 (Mell 1894). Consequently, Ball (1908), Hua et al. (1963), etc. further proved the prominent interspecific heterosis in agronomic and fiber characteristics (see Zhang & Pan’s review 1999). This kind of interspecific F1 hybrid combinations such as Varalaxmi, JKHyl1, DCH32, HB224, NHB12, and TCHB213 developed in India had been cultivated for the first time in production (Paroda & Basu 1993).
Kime & Tilley (1947) reported this intervarietal hybrid yield heterosis performance of F1, and F2 as well, and further revealed for the first time that the possibility of producing super F1 hybrid with large heterosis was greater in crosses between two relatively distant parents. Simpson (1948) not only confirmed the exact existence of hybrid vigor, but also the consecutive presence of obvious hybrid vigor in F2 generation. However, the heterosis in cotton has not yet been utilized effectively in USA, and other cotton growing countries.
The exploration of hybrid cotton with high yields and excellent fiber qualities had been achieved for the first time by Dr. Patel and his colleagues in India. The successful Hybrid 4 was bred by crossing a commercial Gujarat variety G-67 with ‘American Nectariless’ introduced from USA. This hybrid cotton was characterized by very strong boll setting capability and good fiber quality spinning potential. This was thus the first commercial hybrid released not only in India, but also in the world. Not long after the development of Hybrid 4, another distinguished interspecific hybrid Varalaxmi (G. hirsutum × G. barbadense), was released in the Karnataka. Varalaxmi had the same yield potential of Hybrid 4, but better fiber qualities. Since then, many interspecific and intervarietal hybrids have been bred, released, and planted in a large scale in place of conventional varieties. It was considered as one of the main factors for increasing yield and improved fiber qualities during the 1970s in India. Hybrids occupied 28% of total cotton area, while the total yield from hybrid cotton accounted for about 40% of total cotton production in the whole India in 1993 (Paroda & Basu 1993).
Almost at the same time, scientists in Sichuan, China, discovered a male sterile plant in the cultivated Dongting 1 which was pedigree-selected from Deltapine 15 in 1972. Hybrid cotton development using this naturally-occurring GMS mutant designated as Dong-A was initiated and more than 20 hybrids have been developed in this province (Huang et al. 1982, Zhang et al. 1992, Zhang 1995, Zhang & Jing 1997, Zhang & Pan 1999, Huang 2007, Xing et al. 2017). Scientists in other provinces such as Henan, Hunan also focused their efforts toward developing cotton hybrids by HMP. Several hybrids such as Xiangzamian 2 (XZM 2), Wanza 40, Jimian 16 were developed; however, their F1 seeds were not planted commercially due to too high cost to produce F1 hybrid seeds by HMP. Therefore, their F2 seeds were extensively planted instead.
Since the beginning of 21st century, with the successful introduction and utilization of transgenic Bacillus thuringiensis (Bt) cotton inbreds containing a toxin-producing gene, a lot of transgenic Bt hybrid cultivars have been developed and the utilization of cotton hybrids has been accelerated. F1 hybrid cottons are being planted in almost entire YaRCGR and most of the YeRCGR. Many transgenic Bt hybrids such as Nankang 3 and Sikang 3 released in Jiangsu, Lumianyan 25 in Shangdong, Biaoza A1 in Hebei, Cikangza 3 in Zhejiang, Huakangmian 1 in Hubei, Xiangzamian (XZM) 8 in Hunan have been developed and commercially planted on a large scale in China. In India, hybrids containing Cry1Ac from Monsanto were developed and commercially planted by Indian seed companies (Jayaraman 2005). Two largest cotton growing countries in the world have developed and grown many transgenic insect-resistant hybrids in cotton.
F2 Heterosis and its utilization
Heterosis existing in F2 hybrids was reported early and commanded certain interests in USA (Simpson 1948, Meredith et al. 1970, Meredith 1990, Dever & Gannway 1992, Tang et al. 1993a, 1993b). In China, almost at the same time of identification and utilization of Dong-A GMS line in the mid seventies last centry, the hybrid vigor in F2 in cotton production has been testified and found to exist usually 6 ~ 10% higher yield than the control cultivars (Xing et al. 1987, Huang 2007). From the theoretical point of view, F1 hybrid is heterozygous genetically and may express relatively prominent heterosis, while F2 generation segregates into homozygous and heterozygous individuals. Cotton indefinite growth is characterized by its long duration of flowering, boll-setting and harvesting; therefore, there will be no segregation in maturing stage in F2. It had been well known early that there is exactly hybrid vigor in F2 generation (Kime & Tilley 1947, Simpson 1948). Although yield reduction in F2 was usually observed compared to its F1, F2 as a less expensive alternate to F1hybrid has been used quite often in China. Accordingly, hybrid vigor in F2 should decrease due to the decrease in heterozygous plants. However, there may still exist some amount of heterozygous individuals and heterosis as well. Since the late eighties last century, seven hybrids such as Zhongmiansuo (ZMS) 28 (Jing et al. 1995), Jimian 18 (Cui et al. 1994), XZM 1 and 2 (Li et al. 1997), and Suzha 16 (Qian et al. 1997) specifically for F2 generation have been developed and released at different times in China (Huang 2007). Their yield performance and growing area are given in Table 1. In New Cotton Cultivar Trial Test organized by Hunan province from 1994 to 1996, its lint yield of XZM 2 averagely increased by 19.30% in F1, and 7.4% in F2. Our QTL mapping by using immortalized population of XZM 2 revealed that dominance played an important role in the genetic basis of heterosis for yield and its components (Liu et al. 2012). However, additive genetic variance was predominantly responsible for genetic variability in fiber quality traits, therefore, low level of heterosis existed in XZM2 for this trait (Wang et al. 2007). This hybrid cultivar was released in Hunan in 1997 and further expanded to theYaRCGR in 2001. It was planted in more than 1.5 mil. ha. Another well-known hybrid cultivar, Wanza 40, increased lint yield by 16.48% for F1 in 1996, and 11.40% for F2 in 1997, in New Cotton Cultivar Trial Test organized by Anhui province, China. Wanza 40 was planted in more than 1.3 mil. ha. It is interesting that these two hybrid cultivars developed by Cotton Research Institutes in Hunan and Anhui, respectively, have indicative markers, yellow pollen for male parent in XZM 2, and female parent for glandless in Wanza 40, therefore, it is easy to distinguish their F1 from F2 through scoring seed or field performance of the indicative character, no segregation for F1 but obvious segregation in F2. Generally, F2 hybrids can be expected to have approximately half the heterosis effect of F1. However, cost to produce hybrid seeds is greatly reduced more than 10 times. In Chinese working experience, if one ha field is used to produce F1 hybrid seeds, approximate 100 ha fields can be grown for F1 generation next year and 10,000 ha for F2 for the third year. A transgenic Bt hybrid, ZMS29, released by CRI/CAAS, may be one of the most successful hybrid that commanded the largest planting acreage in China.
Heterosis exploitation through utilization of GMS lines
Genic male sterile lines
Male sterility can be used as an important tool for hybrid breeding and heterosis utilization of various crops, especially for heterosis utilization in self-pollinated crops and less frequent in cross-pollinated crops. Male sterility refers to the dysplasia of male organs, loss of reproductive function, resulting in infertility, which is common in the plant kingdom (Chen & Liu 2014b). In majority of the cases, this phenomenon appears as a result of spontaneous mutation yielding male-sterile mutants. Male sterile lines can be divided into genic (GMS) and cytoplasmic (CMS) ones based on their mode of inheritance.
Many male sterile lines showing either complete or partial sterility have been identified in Gossypium. GMS line is generally simple in inheritance and controlled by one or two dominant or recessive genes. Since Justus and Leinweber (1960) first identified a GMS line named as ms1 in 1960, a total of 19 GMS genes in 17 GMS lines including ms1, ms2, ms3, Ms4, ms5, ms6, Ms7, ms8, ms9, Ms10, Ms11, Ms12, ms13, ms14, ms15, ms16, Ms17, Ms18 and Ms19 have been identified successively. Among them, ms1, ms2, ms3, ms13, ms14, ms15 and ms16 are inherited by single gene, while ms5ms6 and ms8ms9 by a pair of recessive genes. Except for Ms11, Ms12, ms13, Ms18, and Ms19 found in G. barbadense, all the others are found in G. hirsutum (see review by Zhang & Pan 1999). Scientists in Sichuan province, China, discovered a male sterile plant in the cultivated Dongting 1 in 1972 (Zhang & Jing 1997). Genetic analysis indicated that it was a naturally-occurring GMS mutation designated as Dong-A and controlled by one recessive male sterile gene (Huang et al. 1982, Zhang et al. 1992, Zhang 1995). Now only completely GMS lines such as ms2 (1355A), ms14 (Dong-A) and ms5ms6 lines are being utilized to produce hybrid seed in China and India.
Genomics and molecular genetics of GMS in cotton
Early studies on cotton GMS lines mainly focused on their collection and identification. With linkage tests, the ms8ms9 sterile genes were mapped on chr. 12 and 26 or chr. A12 and D12 (Rhyne 1991), ms3 on chr. D07 (Kohel et al. 1984), and Ms11 on chr. A12 (Turcotte and Feaster 1979). Wang et al. (2007b) mapped ms2 on chr. D02 using ‘1335A’ GMS line. By using ‘Zhongkang-A’ (ZK-A), Chen et al. (2009) anchored ms5 on chr. A12 between SSR markers NAU3561 and NAU2176 with a genetic distance of 3.2 cM, and ms6 on chr. D12 between markers BNL1227 and NAU460 at 3.1 cM genetic distance, respectively. Furthrmore, they also located ms15 on chr. A12 at a 2.7 cM genetic distance between NAU2176 and NAU1278 (Chen et al. 2009).
Using the cDNA-AFLP to screen the differentially expressed genes between the fertile and sterile plants of the “Dong-A”, Hou et al. (2002) identified three differentially expressed genes GHA27, GHA28 and GHA47 in the mononuclear stage. The expression of many key genes required for anther development was suppressed at the meiotic and uninucleate microspore stages in the “Dong-A” (Wei et al. 2013). These genes were mainly associated with hormone synthesis, sucrose and starch metabolism, pentose phosphate pathway, glycolysis, flavonoid metabolism, and histone synthesis. Differentially expressed genes were involved in related processes such as cell wall development in pollen of duplicated ms5ms6 line (Ma et al. 2007). A total of 2446 differentially expressed genes enriched in pollen wall and anther development were identified between the male fertile and sterile plants in different anther development stages in 1355A (ms2) through RNA-seq analysis (Wu et al. 2015).
With release of cotton genome sequences (Zhang et al. 2015, Hu et al. 2019), the progress in genetic and genomic research including the cloning of male sterile genes has been accelerated in cotton. The ms2 and ms5ms6 GMS genes have been cloned (Ma et al. 2022, Wu et al. 2022, Mao et al. 2022). Wu et al. (2022) fine-maped and cloned the ‘1355A’ male sterile gene ms2, which encodes polygalacturonase protein (GhNSP). The Ghnsp mutant exhibits abundant of de-esterified homogalacturonan in the tapetum and exine, which leads to defects in pollen exine formation and finally male sterility. The duplicate mutations of GhCYP450 genes encoding a cytochrome P450 protein essential for pollen exine formation and pollen development resulted in producing male sterility for the duplicate ms5ms6 line. GhCYP450 acts as the hydroxylation monooxygenases to catalyze hydroxylation of fatty acids (C12) for producing 7-OH-lauric acid required for the precursor synthesis of sporopollenin. Compared to the fertile wild-type TM-1, GhCYP450-At (ms5) appeared to be premature termination of GhCYP450 translation, while GhCYP450-Dt (ms6) had a 7 bp (GGAAAAA) insertion in the promoter domain in addition to three amino acid changes (D98E, E168K, G198R) in the coding region. Investigating the mechanisms of ms5ms6 male sterility will deepen our understanding of the development and utilization of heterosis (Mao et al. 2022).
In higher plants, tapetum development and pollen formation, are unique events required for development of the male gamete, and any error will lead to the abnormal pollen grain development (Zhang et al. 2016). Meanwhile, the fatty acid metabolism is involved in the synthesis of cuticle and sporopollenin which are important components of pollen exine and wall (Chacon et al. 2013). For example, GhCYP450 acts as the hydroxylation monooxygenases to catalyze hydroxylation of fatty acids required for sporopollenin precursor synthesis. And GhNSP can function as the pectin-degrading enzymes, which lead to the degradation of de-esterified pectin/ homogalacturonan. Interestingly, the GhNSP mutant (Ghnsp) accumulates abundantly de-esterified homogalacturonan in tapetum and pollen exine, generates the thicker nexine and fails to form spines on the pollen wall surface and lacks intine, and finally causes male sterility (Wu et al. 2022).
Development of hybrid cotton cultivars using GMS lines
In Sichuan province, Dong-A and its sibling lines have been utilized in breeding more than various types of twenty hybrids including high yield, super fiber quality and transgenic Bt cottons, such as Chuanzamian (CZM) 4, CZM 27, CZM 32, CZM 35, CZM 40 which generally surpassed local cultivars over 10% in yield, indicating prominent heterosis, high yielding capability and superior fiber qualities (Huang 2007, Xing et al. 2017). Among these hybrids, CZM 1, 2 and 4 were the first cotton GMS hybrids developed and released in China in 1985. CZM 4 was the first Fusarium wilt resistant hybrid and therefore planted on a large scale (Huang & Shi 1988).
In India, ms5ms6 GMS line, Greg, was imported from USA in 1970. A first GMS hybrid named as ‘Suguna’ was developed and released, but it was not extensively grown (Paroda & Basu 1993, Raja et al. 2018). This double recessive GMS line is also widely used in cotton hybrid seed production in China. Several hybrid cultivars have been bred using this ms5ms6 sterile lines, including good fiber quality cultivars such as NAU 98–4, transgenic Bt high yield cultivars such as NAU 6, and planted extensively in YaRCGR (Zhang & Zhu 2004, 2005). By transferring the Bt and ms5ms6 GMS genes into the commercial cultivar ZMS 12 (Xing et al. 2017), the successful cultivar commanding largest planting acreage in China, ZK-A GMS line was bred and released by CRI/CAAS (Xing et al. 1999). Using ZK-A as parent, transgenic Bt ZMS38 and ZMS54 have been developed and planted extensively in YeRCGR (Xing et al. 2017).
Production of GMS hybrid seed by hand-pollination
How to produce GMS hybrid seed using one line with two proposed procedures by hand-pollination including identification and roguing the male fertile plants and conducting hand-pollination was reviewed previously (Zhang & Pan 1999). Multiplication of GMS line such as ms5ms6 and the production of hybrid seed are illustrated in Fig. 1. As a duplicate male sterile line, when heterozygous fertile plants such as Ms5ms5ms6ms6 or ms5ms5Ms6ms6 are bred and crossed as male parent with male sterile plants (ms5ms5ms6ms6), their progeny will segregate into male sterile and fertile plants in the ratio of 1:1. They may be used as the single recessive male sterile line in producing hybrid seeds. In hybrid seed production, there need to be three isolation regions for propagating GMS line, parental restorer line, and producing hybrid seeds, respectively. Hybrid seeds are produced on a huge area entirely depending upon hand-pollination plant to plant with restorer cultivar. This is one of the important characteristics of GMS lines.
In production of F1 hybrid seeds, one has to wait until the flowering stage to recognize and eliminate the fertile plants. The procedure is tedious, and time-consuming and increases the cost of hybrid seed production.Therefore, it should be helpful to develop a GMS line with indicative characters appearing in seed or seedling stage. Although such GMS lines have been identified, a leaf abnormality for the identification of the ms2 male sterility (Quisenberry & Kohel 1968), a virescent marker completely associated with ms16 sterility (81A) (Feng 1988, Zhang & Pan 1990, Zhang et al. 1992), no such hybrid cultivars have been developed using this GMS lines.
Being different from the ordinary “one line with two purpose” procedures illustrated in Fig. 1, hybrid seeds produced using the duplicate GMS line can be used for F1 and potential F2 so that the cost for producing hybrid seeds can be reduced further. Although approximately 6% of male sterile plants will be segregated out in this F2 population, they may be plucked off at budding or flowering stage in the field without too much effect on the total yield. Moreover, in the region with high percentage of natural crossing, it is even not necessary to pluck off such sort of male sterile plants (Weaver 1987, Jing et al. 1994).
Heterosis exploitation through utilization of CMS lines
Cytoplasmic male sterile lines
Many types of CMS lines had been developed in cotton (see review by Zhang & Pan 1999). Since 1965, CMS lines with cytoplasms from G. arboreum L. (A2), G. anomalum Wawr. & Peyr (B1) (Meyer & Meyer 1965), G. harknessii Brandg. (D2-2) (Meyer 1975), and G. trilobum (DC.) Skov. (D8) (Stewart 1992) have been developed in the USA. One new type of CMS line, 104-7A, selected from the progeny of a cross between G. hirsutum cv. Shiduan 5 and G. barbadense cv. Junhaimian was developed in China (Jia 1990). A CMS line “Xiangyuan A” was also cultivated by crossing G. thurberi with Dong-A GMS line and backcrossed with G. hirsutum as a recurrent parent (Zhu et al. 2013). What is relationship among these newly identified CMS lines remains to be explored. The male sterility of CMS lines in G. arboreum and G. anomalum cytoplasm is not stable, prominently influenced by environmental factors, and without any practical utility value (Meyer & Meyer 1965). Only G. harknessii CMS lines (CMS-D2-2) and 104-7A have been used to produce hybrid cotton in India and China (Xing et al. 2017), respectively.
CMS can be further divided into sporophytic and gametophytic sterile types according to the process of male pollen abortion. The sporophyte sterility is controlled by sporophyte genotype, and the hybrid F1 of CMS line crossed with restorer line has full ability of pollen fertility. G. harknessii CMS-D2-2 as well as 104-7A belong to sporophyte CMS type. However, G. trilotum CMS-D8 is identified as gametophytic one, and its fertility is controlled by gametophyte genotype, only half pollen of the hybrid F1 have their fertility recovery.
Genetics and genomics of CMS fertility restoration
Two different dominant genes, Rf1 and Rf2, control the fertility restoration to two main CMS systems, CMS-D2-2 and CMS-D8, respectively. The Rf1 gene from D2-2 can restore fertility to both CMS-D2-2 and CMS-D8 lines, but the Rf2 gene from D8 can only restore fertility to the CMS-D8 lines (Zhang & Stewart 2001). Two independent genes Rf1 and Rf2 with a distance of 0.93 cM contribute to the fertility restoration (Zhang & Stewart 2004). So, Rf1 possesses great potential for heterosis exploitation.
Both restorer genes Rf1 and Rf2 have been fine-mapped. Guo et al. (1998) first reported a random amplified polymorphic DNA (RAPD) marker OPV-15300 linked to Rf1 gene. Combined bulked segregant analysis (BSA) and near isogenic line (NIL), this laboratory further anchored Rf1 gene with three more SSR and two RAPD markers (Liu et al. 2003). A high-resolution genetic map of Rf1 containing 13 markers in a genetic distance of 0.9 cM was constructed and used to screen a bacterial artificial chromosome (BAC) library from a restorer line 0–613-2R containing Rf1 gene. Based on sequences of 50 BAC ends of single positive clones screened, two new sequence-tagged site (STS) markers tightly linked to Rf1 gene had been tagged and integrated into this map. The physical map for the Rf1 gene was constructed by fingerprinting the positive clones digested with the HindIII enzyme. The location of the Rf1 gene was further delimited to a minimum of two BAC clones spanning an interval of approximately 100 kb between two clones designated 081-05 K and 052-01N (Yin et al. 2006). By sequenceing these two BACs, five clustered and very high similar pentatricopeptide repeat protein (PPR) protein genes were deduced as Rf1 candidate for CMS-D2-2 restoration (Yang et al. 2010), consistent to most reports in which restorer fertility (Rf) genes encode PPR protein (Kim & Zhang 2018). However, it is difficult to distinguish which one (or likely several) is responsible for Rf1 or even Rf2.
Owing to its important in heterosis utilization, many markers such as RAPD, SSR, STS and InDEL linked to Rf1 have been developed and used in molecular marker assisted-selection breeding (Zhang & Stewart 2004; Feng et al. 2005).
A RAPD marker, UBC188-500, closely linked to Rf2 was first reported (Zhang & Stewart 2001, 2004). Rf1 and Rf2 as fertility restoration for CMS-D2 and CMS-D8, respectively, were anchored within a genetic distance of 1.4 cM (Wang et al. 2007a, Wang et al. 2009b). PPR genes were clustered on the Rf1 loci (Wu et al. 2014, Wu et al. 2017, Zhang et al. 2018). Integrated BSA-seq, high-throughput SNP genotyping and InDel, Rf2 locus was anchorred on interval of 1.48 Mb containing 8 PPR genes on chr. 5D (Feng et al. 2021). Using homocap-seq technology, Gao et al. (2022) reported extensive differences within the D05_PPR cluster in restorer line, inferring that D05_PPR cluster was associated with fertility recovery. The cloning of restorer genes can facilitate their molecular exploration in cotton heterosis and improve the efficiency of three-line hybrid seeding system.
Molecular mechanism of CMS in cotton
Mitochondrial, as a semi-autonomous organelle, has a large quantity of repeat sequences, which mediate gene rearrangement to generate new chimeric genes that are closely associated with cytoplasmic male sterility (Chase 2007, Hu et al. 2014). Until now, 31 CMS genes located in mitochondrial genome have been identified in 14 crop species, such as rice, maize, radish, soybean and wheat ( Jing et al. 2012, Luo et al. 2013, Iwabuchi et al. 1999,, Yamagishi et al. 2019, Melonek et al. 2021, Jiang et al. 2022, Yang et al. 2022). Wang et al. (1998) analyzed the mitochondrial DNA from multiple CMS lines using RAPD markers and determined the main driver of CMS in cotton is the aberrant mitochondrial DNA. Feng et al. (2000) identified significant differences between the mitochondrial genomes of G. harknessii CMS line and the normal fertile G. hirsutum by RFLP. Through comparative studies of the mitochondrial proteins and DNAs between G. harknessii CMS A-line and its corresponding B-line, a 31 KDa polypeptide is found missing in the CMS mitochondrial at abortion stage (Wang 2000). Using four mitochondrial genes (rrn26S, atp9, atp6, coxII) as probes, the CMS line is found lacking a 1.9 Kb fragment that is homology with the coxII gene, the mutation in coxII gene may lead to mitochondrial dysfunction and result in production of male sterility (Wang 2000, Wang et al. 2009a). Li et al. (2018) assembled the mitochondrial genomes of 2074A, 2074S as well as their B-line and restorer line, four specifically transcribed ORFs in 2074A are found. An aberrant transcription of cox3 was found in CMS line H276A (Khan et al. 2022a). Methylated genes mainly related with starch, sucrose and galactose metabolism pathways are differentially expressed and five key genes associated with CMS are identified (You et al. 2022). Some differential expression miRNAs may be the regulators of CMS occurrence (Li et al. 2021).
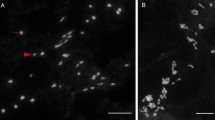
Sequenced mitochondrial genome information for three-line hybrid system in cotton is given in Table 2. By comparative analysis of the mitochondrial genome and transcriptome, four specific ORFs are identified in CMS-D2 line Simian 3A, of them, orf606a, a homologous to orf610a, highly expressed in CMS-D2 line, is supposed as CMS-D2 candidate gene (Xuan et al. 2022). At the same time, the complete sequence of mitochondrial (mt) genome for CMS-D2 line “ZBA” is also assembled as a single circular molecule with 634,036 bp in length. Of 194 annonated ORFs, 36 protein-coding genes, six rRNAs, and 24 tRNAs, a previously unknown chimeric gene, orf610a, which is composed of atp1 and a 485-bp downstream sequence of unknown nature, is identified. The orf610a expresses specifically in CMS-D2 line. Ectopic expression of orf610a in A. thaliana fused with a mitochondrial targeting peptide displays partial male sterility. Interaction between ORF610a and the nuclear-encoded protein RD22 indicated an association between ORF610a and pollen abortion, suggesting that the mt CMS gene, orf610a, may account for CMS-D2 male sterility (Zhang et al. 2022). The abortion process of CMS line is accompanied with programmed cell death and accumulation of reactive oxygen species (Fig. 2). The key problem that needs to be solved by combining the functional Rf genes to elucidate the molecular mechanism of mutual regulation in CMS cytoplasm and nuclear restore genes.
Development and production of CMS hybrid cultivars
CMS-based hybrid breeding system employs a three-line hybrid system, CMS (A-line), maintainer (B-line) and restorer line (R-line). Of them, the selection of R-line is the most important.
Development of CMS R-lines
Selection of restorer line is generally based on cross hybridizing, backcross breeding and test-cross screening. By Agrobacterium-mediated introduction of glutathione S-transferase (GST) into the restorer line, Wang et al. (2003) developed a strong restoring R-line named as “Zhedaqianghui,” with which Zheza 2 CMS hybrid was developed (Zhu et al. 2006). By distant hybridization between G. hirsutum and G. anomalum, a restorer line introgressed Rf gene from G. anomalum was produced, which had strong restoring ability and 100% restoration rate for CMS-D2-2 line (Hua et al. 2003). Excellent CMS A- and R-lines have been selected during the past decades, which lead to dominant contributions to cotton heterosis.
Development of CMS hybrid cultivars
In 2005, the first two three-line CMS hybrids named as Yinmian 2 and Zheza 2 were developed by Biotchnology Institute/CAAS and Zhejiang University, respectively. Yinmian 2 is a transgenic Bt insect-resistance hybrid cultivar (Zhu et al. 2006, Guo et al. 2007). It is characterized by its high yield for Yinmian 2, 21.1% higher than the control ZMS 41. Subsequently, transgenic Bt CMS hybrids named as ZMS83 and ZMS 99 developed by CRI/CAAS in 2011 and 2016, respectively, and Luza 2138 by Shangdong Cotton Research Center, China in 2017, have been released and planted in China (Xing et al. 2017). However, due to high cost to produce hybrid seeds by CMS with hand-pollination, these hybrids are not extensively planted in production.
Some interspecific CMS-D2-2 hybrids such as Xinluzhong 24 and 43 have been developed in Xinjiang. Xinluzhong 43 crossed between G. hirsutum acc. H-268A and G. barbadense acc. 75R was released in Xinjiang in 2009. Its lint yield (2217.9 kg/ha) is the same as the control (2185.1 kg/ha), but its fiber quality much better, fiber length 35.0 mm, strength 36.46 cN/tex and micronaire 3.53.
It is reported that the cytoplasm of G. harknessii may render F1 hybrid some detrimental effects on yield. Partial female sterility is the reason that results in high rate of abortive seeds which reduce the seed-cotton and lint yield (Weaver 1986, Wei et al. 1995, Wang et al. 1997). Nevertheless, an excellent hybrid might be selected through wide testcrossing and selection overcoming the negative effects.
Producing hybrid seeds with male sterile lines pollinated by insects
Cotton pollen bearing large spheroid and spinate pollen grains is not wind-disseminated, and insects are the natural agents for the pollen transfer. Therefore, much attention has been paid to the exploitation of such a way of producing hybrid seeds with male sterile lines pollinated by insects as reviewed before (Zhang & Pan 1999). It is concluded that honeybee (Apis mellifera L.) is the main vector for pollination and the transfer efficiency depends upon the number and distribution of the colonies. If bee source for hybrid seed production can fundamentally be met, or visiting bees exceeding 0.5–1.0 for 100 flower is necessary for good pollen dispersal, honey bee pollination is feasible to produce hybrid seeds via male sterile line (Waller et al. 1985, Feng 1990). However, in areas where natural crossing rate is high, the average seed-cotton yield in male sterile plant could not be comparable to the fertile one, not to mention in the locations where natural crossing rate is low and yield decreases more significantly (Gururajan & Srinivasan 1975).
Honeybee is extensively raised artificially and easily available, therefore, it is considered to be the most effective vector to produce hybrid seeds. At blooming stage, 22 kinds of insects belong to Hymenoptera, and 5 to Diptera, were identified to be associated with cotton pollination from the cotton growing area in Sichuan, China (Department of Biology/Nanchong Normal College 1978). Among Hymenoptera insects, honey bee, bumble bee (Bumbus spp), and leaf cutting bee (Megachile conjunctifomis) were the main vectors for cotton pollination. Two peak periods of honeybee visiting the cotton field, 11 o’clock in the morning and 3 o’clock in the afternoon were observed in Zu county of Hebei, China. The insect activity was tremendously influenced by environmental conditions, especially, spraying insecticides would cause severest effects at full blooming stage. Unfavorable weather such as great storm, thunder and showers also adversely affected the pollination activities, particularly the honeybee (Feng 1990).
In 1999, hybrid seed production of transgenic Bt bollworm-resistant combination (ZK-A × Bollgard 33B) was conducted on net room by hand- (1000 M2) and honeybee-pollination (600 M2), respectively. Each net room had one honeybee colony having around 6000–7000 bees for pollinating the ms5ms6-line. It was workable for this hybrid seed yield (1110.5 kg/ha) under 1 row of Ms-line (Bollgard 33B, Ms5Ms6) and 3 rows of ms-line (ZK-A, ms5ms6) by honeybee pollination considering seed cost although it was still significantly lower (1291.6 kg/ha) by −16.31% by hand-pollination (Xing et al. 2002). The seed yield reduction mainly resulted from its decreasing in bolls per plant (10.9 vs 13.5) by 23.85% and boll weight (4.9 g vs 5.2 g) by 6.12% respectively for bee- and hand-pollination. No spraying insecticide to control bollworm helps increasing seed yield at full blooming stage in insect-resistent cottons.
A survey experiment in producing hybrid seeds in large field planted in both YeRCGR and YaRCGR was further conducted for three years with an alternated 3 ms:1Ms, 4 ms:1Ms, 5 ms:1Ms, 6 ms:1Ms row pattern, respectively (Xing et al. 2005). 15 colonies per hm2 were placed along the sides of three fields. Each colony had around 8000 ~ 10,000 bees. In this system of honeybee pollination producing cotton hybrids, the parent plant proportion, bee varieties, parent plant patterns, honeybee behavior and weather influence were presented. Average hybrid seed of two locations is 1220.4 kg/hm2 for 1:3, 1242.1 kg/hm2 for 1:4, 998.8 kg/hm2 for 1:5, and 639.2 kg/hm2 for 1:6 Ms-line vs ms-line, respectively. It is concluded that 1:4 proportion of parents is ideal, honey bee is better than bear bee in pollination effect, and there is no remarkable difference between mixing plant patterns and alternating plant patterns (Table 3). Weather has obviously effect on honeybee pollination, which leads to direct hybrid yield loss.
Challenges and opportunities
Core parental development for heterosis utilization.
Germplasm enhancement is the foundation of hybrid breeding work. ZMS-12, a largest Upland cotton cultivar grown in China with high yield, superior fiber quality and disease resistance, is characterized by its wide adaption and high combining ability. ZMS 12 and its pedigree-derived lines were used to develop elite hybrids, including ZMS-28, ZMS-29, XZM 2 and Jimian18 (Table 1). Due to its good combining ability of ZMS-12, 84 cultivars had been directly bred from ZMS -12, seven hybrids and six transgenic Bt pest-resistant cotton cultivars before 2002 (Guo et al. 2002). Among them, ZMS-12, ZMS-28 and Jimian18 are widely cultivated in the YeRCGR, and ZMS-29 and XZM-2 extensively planted in the YaRCGR (Huang 2007, Xing et al. 2017) (Table 1). By transferring the Bt and ms5ms6 GMS genes into the ZMS 12, a GMS line in ZMS 12 background named as Zhongkang-A (ZK-A) has been bred (Xing et al. 2017). With this ZK-A, super hybrids are easily identified due to its high combility ability and ZMS38 and ZMS54 have been developed in CRI/CAAS. Therefore, ZMS 12 as a core parent plays a very important role in Chinese cotton breeding including heterosis utilization in China. The pedigrees and utilization in hybrid cultivar improvement as a foundation parent for ZMS-12 as well as Shiyuan 321 are presented in Fig. 3. From the development and utilization ZMS 12, a great attention shall be paid to core parent development.
F2 heterosis utilization in cotton
As continuous rising of labor cost, the simplified, high effective and low cost cotton planting has become an inevitable trend, which bring a challenge in cotton heterosis utilization. The efficient and controllable pollination process is a prerequisite for the mass commercial production of hybrid seeds. F2 hybrid does exist heterosis, usually, 6% ~ 10% higher yield than the control cultivars, and has being used extensively in China for many years. F2 heterosis utilization at least in China is still a way in future cotton breeding. Now, the Xinjiang Uygur autonomous region has gradually become one of the largest bases of cotton production worldwide. In 2021, cotton in Xinjiang was planted over a span of more than 2.50 million hectares and produced 5.39 million tons of cotton lint, accounting for approximately 82.8% of the planting area and 89.5% of production in cotton in China (http://www.stats.gov.cn), which in turn constitutes almost one fifth of the world’s cotton production; thus, Xinjiang occupies a unique position in the global cotton industry. Some breeders have developed such hybrids such as Xinluzhong 32, Xinluzao 14, 67, and 69 specifically for F2 generation in this region.
Challenges developing F2 hybrid which can be used in production is given the following:
-
(1)
In selecting parental lines, no big difference in fiber length for two parents (within 1.5 mm) and growth stage, otherwise, the uniformity of lint length in F2 cannot meet the demands of yarn spinning.
-
(2)
As a gametophytic CMS, hybrid (s) developed using G. trilotum CMS-D8 can be used in F2 because their all F2 plants have a complete fertility recovery although only half pollens of the hybrid F1 have their fertility recovery.
-
(3)
Selection and testification of high heterosis in F2 because some F2 does not show heterosis although high heterosis exists in F1.
-
(4)
Heterosis does exist in yield, but not in fiber quality in F2.
-
(5)
Using chemical gametocide. Hybrid cultivar development by hand-emasculation is easy to make crosses with convenience and freedom. Some chemical gametocides for killing stamens, but without any damage to the pistil and normal capability of fertilization has been screened out in cotton. Without hand-emasculation, hybrid seed production can be further reduced.
Exploration and enhancement of environment‑sensitive genic male sterility
The most desirable method to produce hybrid seeds is using the GMS or CMS line combined with insect pollination if there is adequate pollen pollinated by honey bee, A- and B-lines can produce equal seed-cotton yields in field. The discovery and enhancement of the environment‑sensitive genic male sterility (EGMS) induced by environmental factors such as light and temperature has enabled some GMS traits to be used for hybrid crop breeding. The EGMS line can be used as a sterile line and a maintainer line as well by controlling the appropriate environment, and realizing cross-breeding of two lines. Since the 1970s, EGMS has been continuously found in major crops such as rice, wheat and soybean. According to environmental dominant factors, EGMS can be divided into three categories: photoperiod-sensitive (PGMS), temperature-sensitive GMS (TGMS) and photo-thermosensitive GMS (PTGMS) (Chen et al. 2019). In Upland cotton, a PGMS mutant CCRI9106 derived from CCRI040029 using Space mutation breeding technology is found. The mutated CCRI9106 becomes male sterile under long day conditions and is genetically controlled by one recessive gene named as ys-1 anchored on chr. D12 (Liu et al. 2014, Zhang et al. 2020). The photoperiod- and thermo-sensitive GMS in rice is caused by a point mutation in a novel noncoding RNA that produces a small RNA(Zhou et al. 2012), However, what is mutation mechanism for ys-1 and how to use this PGMS in heterosis utilization remains to be explored to solve the high cost of pollination problem for hybrid seed production effectively. Producing hybrid seeds with EGMS lines pollinated by insects remains to further study.
References
Atlin GN (1995) Cytoplasmic male-sterile synthetics: a new approach to the exploitation of heterosis in rape. Theor Appl Genet 91:1173–1176
Balls WL (1908) Mendelian studies in Egyptian cotton. J Agric Sci 2:346–379
Bohra A, Jha UC, Adhimoolam P, Bisht D, Singh NP (2016) Cytoplasmic male sterility (CMS) in hybrid breeding in field crops. Plant Cell Rep 35:967–993
Budar F, Pelletier G (2001) Male sterility in plants: occurrence, determinism, significance and use. C R Acad Sci III 324:543–550
Chacón MG, Fournier AE, Tran F, Dittrich-Domergue F, Pulsifer IP, Domergue F, Rowland O (2013) Identification of amino acids conferring chain length substrate specificities on fatty alcohol-forming reductases FAR5 and FAR8 from Arabidopsis thaliana. J Biol Chem 288(42):30345–30355
Chase CD (2007) Cytoplasmic male sterility: a window to the world of plant mitochondrial-nuclear interactions. Trends Genet 23:81–90
Chen L, Liu YG (2014) Male sterility and fertility restoration in crops. Ann Rev Plant Biol 65:579–606
Chen DY, Ding YZ, Guo WZ, Zhang TZ (2009) Molecular mapping of genic male-sterile genes ms15, ms5 and ms6 in tetraploid cotton. Plant Breed 128:193–198
Cui LM, Fu HQ, Zhang XY (1994) Development of cotton hybrid, Jimian 18. China Cottons 21(8):24
Davis DD (1978) Hybrid cotton: specific problems and potential. Adv Agron 30:129–157
Department of Biology, Nanchong Normal University (1978) Studies on insect pollination in cotton. China Cottons 5(3):23–27
Dever JK, Gannaway JR (1992) Relative fiber uniformity between parent and F1 and F2 generations in cotton. Crop Sci 32(6):1402–1408
Dewey RE, Timothy DH, Levings CS (1991) Chimeric mitochondrial genes expressed in the C male-sterile cytoplasm of maize. Curr Genet 20:475–482
Feng FZ (1988) An introduction to a male sterile germplasm in cotton. China Cottons 15(3):15–16
Feng FZ (1990) Primary studies on insect pollination male sterile line to produce hybrid seeds. China Cottons 17(5):16
Feng CD, Stewart JM, Zhang JF (2005) STS markers linked to the Rf1 fertility restorer gene of cotton. Theor Appl Genet 110:237–243
Feng J, Zhang X, Zhang M, Guo L, Qi T, Tang H, Zhu H, Wang H, Qiao X, Xing C, Wu J (2021) Physical mapping and InDel marker development for the restorer gene Rf2 in cytoplasmic male sterile CMS-D8 cotton. BMC Genom 22:24
Feng C, Guo J, Nie Y, Wu Z, Zhang X, Zhang J, Stewart JM (2000) Cytoplasmic-nuclear male sterility in cotton: comparative RFLP analysis of mitochondrial DNA. In: Proceedings Beltwide Cotton Production Research Conference, San Antonio, USA, pp. 511–512
Gao B, Ren G, Wen T, Li H, Zhang X, Lin Z (2022) A super PPR cluster for restoring fertility revealed by genetic mapping, homocap-seq and de novo assembly in cotton. Theor Appl Genet 135:637–652
Guo WZ, Zhang TZ, Pan JJ, Kohel RJ (1998) Identification of RAPD marker linked with fertility-restoring gene of cytoplasmic male sterile lines in upland cotton. Ch Sci Bull 43:52–54
Guo XM, Tan LW, Liu ZD (2002) Evaluating germplasm resource value of Zhongmian 12. China Cotton 29:12–14
Guo SD, Zhang R, Wang Y (2007) Research on genetic basis of three-line cotton. Bull Agric Sci Technol 12:11–12
Gururajan KN, Srinivasan K (1975) Estimation of natural cross-pollination in male sterile upland cotton. Ind J Agric Sci 45:352–355
Horn R, Gupta KJ, Colombo N (2014) Mitochondrion role in molecular basis of cytoplasmic male sterility. Mitochondrion 19:198–205
Hou L, Xiao Y, Li X, Wang W, Luo X, Pei Y (2002) The cDNA-AFLP differential display in developing anthers between cotton male sterile and fertile line of “Dong A.” J Genet Genom 29:359–363
Hu J, Huang W, Huang Q, Qin X, Yu C, Wang L, Li S, Zhu R, Zhu Y (2014) Mitochondria and cytoplasmic male sterility in plants. Mitochondrion 19:282–288
Hu Y, Chen J, Fang L, Zhang Z, Ma W, Niu Y, Ju L, Deng J, Zhao T, Lian J, Baruch K, Fang D, Liu X, Ruan Y-L, Rahman M-U, Han J, Wang K, Wang Q, Wu H, Mei G, Zang Y, Han Z, Xu C, Shen W, Yang D, Si Z, Dai F, Zou L, Huang F, Bai Y, Zhang Y, Brodt A, Ben-Hamo H, Zhu X, Zhou B, Guan X, Zhu S, Chen X, Zhang TZ (2019) Gossypium barbadense and Gossypium hirsutum genomes provide insights into the origin and evolution of allotetraploid cotton. Nat Genet 51:739–748
Hua JP, Zhang C, Yi XD, Zhang SX, Chen DF (2003) Cultivation and application of distant nucleoplasm hybrids in cotton. Hubei Agric Sci 4:25–28
Hua XN, Zhou X, Huang JQ, Zhu SL, Yu SJ, Zhang LS, Liu XM (1963) Studies on heterosis exploitation of F1 hybrids between G. barbadense and G. hirsutum L. Acta Agron Sin 2(1):1–27
Huang ZK (2007) Cotton varieties and their genealogy in China. China Agric Sci Press, Beijing
Huang GW, Shi XZ (1988) Chuanzha 4, a cotton hybrid by genic male sterile line. China Cottons 15(3):19
Huang GW, Gou YG, Zhang DM, Jiang W, Zhang XQ (1982) Genetic analysis of several genic male sterile lines in Upland cotton in China. J Sichuan Agric Sci Technol 2:1–4
Iwabuchi M, Koizuka N, Fujimoto H, Sakai T, Imamura J (1999) Plant Mol Biol 39:183–188
Jayaraman KS (2005) Indian Bt gene monoculture, potential time bomb. Nat Biotechnol 23:158
Jia ZC (1990) Selection of a male-sterile line 104–7A of cotton and its complete set of three lines. China Cottons 6:11
Jiang H, Lu Q, Qiu S, Yu H, Wang Z, Yu Z, Lu Y, Wang L, Xia F, Wu Y, Li F, Zhang Q, Liu G, Song D, Ma C, Ding Q, Zhang X, Zhang L, Zhang X, Li X, Zhang J, Xiao J, Li X, Wang N, Ouyang Y, Zhou F, Zhang Q (2022) Fujian cytoplasmic male sterility and the fertility restorer gene OsRf19 provide a promising breeding system for hybrid rice. Proc Nat Acad Sci, USA 119(34):e2208759119
Jing SR, Xing ZZ, Yuan JL, Liu SL, Wang HL (1995) Development of Zhongzha 028 hybrid and its cultivation. China Cottons 22(12):22–23
Jing B, Heng S, Tong D, Wan Z, Fu T, Tu J, Ma C, Yi B, Wen J, Shen J (2012) A male sterility-associated cytotoxic protein ORF288 in Brassica juncea causes aborted pollen development. J Exp Bot 63:1285–1295
Jing SR, Liu SL, Yuan YL, Xing CZ (1994) Studies of utilization of genetic double recessive male sterile G. hirsutum L. Acta Gossypii Sin 6:28–30
Justus N, Leinweber L (1960) A heritable partially male-sterile character in cotton. J Hered 51:192–192
Khan A, Kong X, Liao X, Zheng J, You J, Li M, Hussain RM, Raza H, Zhou R (2022) Mitochondrial gene expression analysis reveals aberrant transcription of cox3 in Gossypium barbadense CMS line H276A. Dev Genes Evol 232:15–23
Kim YJ, Zhang D (2018) Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci 23:53–65
Kime PH, Tilley RH (1947) Hybrid vigor in Upland cotton-effect on yield and quality. J Am Soc Agron 39:308–317
Kohel RJ, Lewis CF (1984) Cotton. American Society of Agronomy, Madison, Wisconsin
Lambright L (2019) Hybrid sorghum product development and production. Methods Mol Biol 1931:3–9
Li YQ, Zhen ZY, Jing L, Yang FQ (1997) An introduction to Xiangzamian 2. China Cottons 24(10):28–29
Li S, Chen Z, Zhao N, Wang Y, Nie H, Hua J (2018) The comparison of four mitochondrial genomes reveals cytoplasmic male sterility candidate genes in cotton. BMC Genom 19(1):1–15
Li M, Chen L, Khan A, Kong X, Khan MR, Rao MJ, Wang J, Wang L, Zhou R (2021) Transcriptome and MiRNA omics analyses identify genes associated with cytoplasmic male sterility in cotton (Gossypium hirsutum L). Int J Mol Sci 22(9):4684
Liu LW, Guo WZ, Zhu XF, Zhang TZ (2003) Inheritance and fine mapping of fertility restoration for cytoplasmic male sterility in Gossypium hirsutum L. Theor Appl Genet 106:461–469
Liu RZ, Wang BH, Guo WZ, Qin YS, Wang LG, Zhang YM, Zhang TZ (2012) Quantitative trait loci mapping for yield and its components by using two immortalized populations of a heterotic hybrid in Gossypium hirsutum L. Mol Breed 29:297–311
Liu J, Pang C, Wei H, Song M, Meng Y, Fan S, Yu S (2014) Proteomic analysis of anthers from wild-type and photosensitive genetic male sterile mutant cotton (Gossypium hirsutum L.). BMC Plant Biol 14:390
Loden HD, Richmond TR (1951) Hybrid vigor in cotton: cytogenetic aspects and potential application. Econ Bot 5:387–408
Luo D, Xu H, Liu Z, Guo J, Li H, Chen L, Fang C, Zhang Q, Bai M, Yao N, Wu H, Wu H, Ji C, Zheng H, Chen Y, Ye S, Li X, Zhao X, Li R, Liu YG (2013) A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat Genet 45:573–577
Ma X, Xing C, Guo L, Gong Y, Wang H, Zhao Y, Wu J (2007) Analysis of differentially expressed genes in genic male sterility cotton (Gossypium hirsutum L.) using cDNA-AFLP. J Genet Genom 34:536–543
Ma H, Wu Y, Lv R, Chi H, Zhao Y, Li Y, Liu H, Ma Y, Zhu L, Guo X, Kong J, Wu J, Xing C, Zhang X, Min L (2022) Cytochrome P450 mono-oxygenase CYP703A2 plays a central role in sporopollenin formation and ms5ms6 fertility in cotton. J Integr Plant Biol 64:2009–2025
Mao Y, Dai F, Si ZF, Fang L, Zhang TZ (2022) Duplicate mutations of GhCYP450 lead to the production of ms5m6 male sterile line in cotton. Theor Appl Genet (accepted)
Mell PH (1894) Experiments in crossing for the purpose of improving the cotton fibers. Alabama Agric Exp Stat Bull 56
Melonek J, Duarte J, Martin J, Beuf L, Murigneux A, Varenne P, Comadran J, Specel S, Levadoux S, Bernath-Levin K, Torney F, Pichon JP, Perez P, Small I (2021) The genetic basis of cytoplasmic male sterility and fertility restoration in wheat. Nat Commun 12:1036
Meredith WR Jr (1990) Yield and fiber-quality potential for second-generation cotton hybrids. Crop Sci 30:1045–1048
Meredith WR Jr, Bridge RR, Chism JF (1970) Relative performance of F1 and F2 hybrids from doubled haploids and their parent varieties in Upland cotton, Gossypium hirsutum L. Crop Sci 10:295–298
Meredith WR (1984) Quantitative genetics. In: Lewis CF, Kohel RJ (eds) Cotton. SSSA Inc Publishers, Madison, pp 131–150
Meyer VV (1969) Some aspects of genes, cytoplasm and environment on male sterility of cotton(Gossypium). Crop Sci 9:237–242
Meyer VG (1975) Male-sterility from Gossypium-harknessii. J Hered 66:23–27
Meyer VG, Meyer JR (1965) Cytoplasmically controlled male sterility in cotton. Crop Sci 5:444–448
Munir S, Hussain SB, Manzoor H, Quereshi MK, Zubair M, Nouman W, Shehzad AN, Rasul S, Manzoor SA (2016) Heterosis and correlation in interspecific and intraspecific hybrids of cotton. Genet Mol Res 15(2):15028083
Paroda RS, Basu AK (1993) Cotton scenario with particular reference to hybrid cotton. In: Proceedings FAO-ICAR regional expert consultation on hybrid cotton. Oct. 22-25, 1990. CICR, Nagpur, India, pp. 21-46
Qian DS, Zhang XG, Zhu Y, Xie QL, Xu NN, Yuan ZK, Duan MX, Zhang JQ, Jing CL, Ma JF (1997) High yield hybrid resistant to Fusarium in Upland cotton-Suzha16. China Cottons 24(10):30
Quisenberry JE, Kohel RJ (1968) A leaf abnormality for the identification of a genetic male sterile in Gossypium hirsutum L. Crop Sci 8:369–370
Raja D, Kumar MS, Devi PR, Loganathan S, Ramya K, Kannan N, Subramanian V (2018) Identification of molecular markers associated with genic male sterility in tetraploid cotton (Gossypium hirsutum L.) through bulk segregant analysis using a cotton SNP 63K array. Czech J Genet Plant Breed 54:154–160
Rhyne CL (1991) Male-steriles ms5ms5ms6ms6 and ms8ms8ms9ms9. In: Proceedings Beltwide cotton production research conference, USA, pp. 532-533
Sarfraz Z, Iqbal MS, Pan Z, Jia Y, He S, Wang Q, Qin H, Liu J, Liu H, Yang J, Ma Z, Xu D, Yang J, Zhang J, Gong W, Geng X, Li Z, Cai Z, Zhang X, Zhang X, Huang A, Yi X, Zhou G, Li L, Zhu H, Qu Y, Pang B, Wang L, Iqbal MS, Jamshed M, Sun J, Du XM (2018) Integration of conventional and advanced molecular tools to track footprints of heterosis in cotton. BMC Genom 19(1):776
Schnable PS, Springer NM (2013) Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol 64:71–88
Shen R, Wang L, Liu X, Wu J, Jin W, Zhao X, Xie X, Zhu Q, Tang H, Li Q, Chen L, Liu YG (2017) Genomic structural variation-mediated allelic suppression causes hybrid male sterility in rice. Nat Commun 8:1310
Simpson DM (1948) Hybrid vigor from natural crossing for improving cotton production. J Am Soc Agron 40:970–979
Stewart JM (1992) A new cytoplasmic male sterile and restorer for cotton. In: Proceedings Beltwide cotton production research conference, National Cotton Council, Memphis TN, p 610
Tang B, Jenkins JN, McCarty JC, Watson CE (1993) F2 hybrids of host plant germplasm and cotton cultivars: I. Heterosis and combining ability for lint yield and yield components. Crop Sci 33(4):700–705
Tang B, Jenkins JN, McCarty JC, Watson CE (1993) F2 hybrids of host plant germplasm and cotton cultivars: II. Heterosis and combining ability for fiber properties. Crop Sci 33(4):706–710
Tian SH, Xu X, Zhu XF, Wang F, Song XL, Zhang TZ (2019) Overdominance is the major genetic basis of lint yield heterosis in interspecific hybrids between G. hirsutum and G. barbadense. Heredity 123:384–394
Turcotte EL and CV Feaster (1979) Linkage tests in American Pima cotton. Crop Sci 19:119–120
Waller GD, Moffet JO, Loper GM, Martin JH (1985) An evaluation of honey bee foraging activity and pollination efficacy for male sterile cotton. Crop Sci 25:211–214
Wan X, Wu S, Li Z, Dong Z, An X, Ma B, Tian Y, Li J (2019) Maize genic male-sterility genes and their applications in hybrid breeding: progress and perspectives. Mol Plant 12:321–342
Wang XD (2000) Analyses of mitochondrial protein and DNA from cytoplasmic male sterile cotton. Acta Agron Sin 26:35–39
Wang XD, Zhang TZ, Pan JJ (1997) Cytoplasmic effects of cytopplasmic male sterile Upland cotton. Acta Agron Sin 23:393–399
Wang XD, Zhang TZ, Pan JJ (1998) Cytological observation of microsporogenesis and RAPD analysis of mitochondrial DNAs for cytoplasmic male sterile cotton lines. Sci Agric Sin 31:70–75
Wang XD, Zhu YG, Zhao PO, Ni XY (2003) Relationship between glutathione S-transferase activity of restorer anthers and pollen fertility of F1 hybrid in Upland cotton. Acta Agron Sin 29:693–696
Wang BH, Wu YT, Guo WZ, Zhu XF, Huang NT, Zhang TZ (2007a) QTL analysis and epistasis effects dissection of fiber qualities in an elite cotton hybrid grown in second generation. Crop Sci 47:1384–1392
Wang F, Stewart JM, Zhang J (2007b) Molecular markers linked to the Rf2 fertility restorer gene in cotton. Genome 50:818–824
Wang F, Feng C, O’Connell MA, Stewart JM, Zhang JF (2009a) RFLP analysis of mitochondrial DNA in two cytoplasmic male sterility systems (CMS-D2 and CMS-D8) of cotton. Euphytica 172:93–99
Wang F, Yue B, Hu J, Stewart JM, Zhang JF (2009b) A target region amplified polymorphism marker for fertility restorer gene Rf1 and chromosomal localization of Rf1 and Rf2 in cotton. Crop Sci 49:1602–1608
Wang GY (2007b) Sterile stability of four genetic male sterile lines and genetic male sterile gene localization in 1355A. Dissertation, Huazhong Agricultural University
Weaver JB Jr (1986). Performance of open pollinated cultivars, F2 and CMS Upland x Upland restorer strain. In: Proceedings Beltwide cotton production research conference, pp. 98–100
Weaver JB Jr (1987a) Performance of ms5–6 genetic male sterile vs sib-fertile plants. In: Proceedings Beltwide cotton production research conference, pp. 123–124
Weaver JB Jr (1987b) Performance of F2 hybrids produced through natural crossing with a dominant naked seed strain of cotton. In: Proceedings Beltwide cotton production research conference, pp. 118–119
Wei ZG, Li ZY, Hua JP, Yi XD (1995) Studies on effects of the male sterile cytoplasm of G. harknessii. Acta Gossypii Sin 7:78–82
Wei M, Song M, Fan S, Yu S (2013) Transcriptomic analysis of differentially expressed genes during anther development in genetic male sterile and wild type cotton by digital gene-expression profiling. BMC Genom 14:97
Westhues M, Schrag TA, Heuer C, Thaller G, Utz HF, Schipprack W, Thiemann A, Seifert F, Ehret A, Schlereth A, Stitt M, Nikoloski Z, Willmitzer L, Schön CC, Scholten S, Melchinger AE (2017) Omics-based hybrid prediction in maize. Theor Appl Genet 130:1927–1939
Wu YT, Yin JM, Guo WZ, Zhu XF, Zhang TZ (2004) Heterosis performance of yield and fiber quality in F1 and F2 hybrids in Upland cotton. Plant Breed 123(3):285–289
Wu J, Cao X, Guo L, Qi T, Wang H, Tang H, Zhang J, Xing C (2014) Development of a candidate gene marker for Rf1 based on a PPR gene in cytoplasmic male sterile CMS-D2 Upland cotton. Mol Breed 34:231–240
Wu Y, Min L, Wu Z, Yang L, Zhu L, Yang X, Yuan D, Guo X, Zhang XL (2015) Defective pollen wall contributes to male sterility in the male sterile line 1355A of cotton. Sci Rep 5:9608
Wu J, Zhang M, Zhang X, Guo L, Qi T, Wang H, Tang H, Zhang J, Xing C (2017) Development of InDel markers for the restorer gene Rf1 and assessment of their utility for marker-assisted selection in cotton. Euphytica 213(11):1–8
Wu Y, Li X, Li Y, Ma H, Chi H, Ma Y, Yang J, Xie S, Zhang R, Liu L, Su X, Lv R, Khan AH, Kong J, Guo X, Lindsey K, Min L, Zhang XL (2022) Degradation of de-esterified pctin/homogalacturonan by the polygalacturonase GhNSP is necessary for pollen exine formation and male fertility in cotton. Plant Biotechnol J 20:1054–1068
Xie Y, Shen R, Chen L, Liu YG (2019) Molecular mechanisms of hybrid sterility in rice. Sci China Life Sci 62:737–743
Xing YH, Jing SR, Zhang XH, Qiu J (1987) Studies on performance of F2 hybrids in cotton. China Cottons 14(2):12–15
Xing CZ, Jing SL, Guo LP, Yuan YL, Liu SL, Wang HL (1999) Nuclear sterile ms5ms6 of Upland cotton resistant to cotton bollworm-Zhongkang A. China Cotton 26(6):27
Xing CZ, Guo LP, Jing SL, Wang HL (2002) Study on heterosis and insect pollination of nuclear sterile of Upland cotton resistant to cotton bollworm-Zhongkang A. Acta Agron Sin 28:574–576
Xing CZ, Guo LP, Miao CD, Wang HL, Lou BQ (2005) Study on effects of producing cotton hybrids by bees pollination. Cotton Sci 17:207–210
Xing CZ, Guo LP, Li W, Wu JY, Yang DG, Qi TT, Ma XF, Zhang XX (2017) Ten-year achievements and future development of cotton heterosis utilization. Cotton Sci 29:28–36
Xuan LS, Qi GA, Li X, Yan S, Cao Y, Huang C, He L, Zhang TZ, Shang H, Hu Y (2022) Comparison of mitochondrial genomes between a cytoplasmic male-sterile line and its restorer line for identifying candidate CMS genes in Gossypium hirsutum. Int J Mol Sci 23(16):9198
Yamagishi H, Tanaka Y, Shiiba S, Hashimoto A, Fukunaga A, Terachi T (2019) Mitochondrial orf463 causing male sterility in radish is possessed by cultivars belonging to the ‘Niger’ group. Euphytica 215(6):1–8
Yang LM, Zhu HY, Guo WZ, Zhang TZ (2010) Molecular cloning and characterization of five genes encoding pentatricopeptide repeat proteins from Upland cotton (Gossypium hirsutum L.). Mol Biol Rep 37:801–808
Yang W, Li Y, Sun L, Shoaib M, Sun J, Wang D, Li X, Liu D, Zhan K, Zhang A (2021) Genetic mapping of ms1s, a recessive gene for male sterility in common wheat. Int J Mol Sci 22(16):8541
Yang H, Xue Y, Li B, Lin Y, Li H, Guo Z, Li W, Fu Z, Ding D, Tang J (2022) The chimeric gene atp6c confers cytoplasmic male sterility in maize by impairing the assembly of the mitochondrial ATP synthase complex. Mol Plant 15:872–886
Yin JM, Guo WZ, Yang LM, Liu LW, Zhang TZ (2006) Physical mapping of the Rf1 fertility-restoring gene to a 100 kb region in cotton. Theor Appl Genet 112:1318–1325
You J, Li M, Li H, Bai Y, Zhu X, Kong X, Chen X, Zhou R (2022) Integrated methylome and transcriptome analysis widen the knowledge of cytoplasmic male sterility in cotton (Gossypium barbadense L.). Front Plant Sci 13:770098
Zaidi SS, Naqvi RZ, Asif M, Strickler S, Shakir S, Shafiq M, Khan AM, Amin I, Mishra B, Mukhtar MS, Scheffler BE, Scheffler JA, Mueller LA, Mansoor S (2020) Molecular insight into cotton leaf curl geminivirus disease resistance in cultivated cotton (Gossypium hirsutum). Plant Biotechnol J 18:691–706
Zhang TZ (1995) A discussion on the inheritance of Dong-A male sterility and its fertility-maintaining line(Mb) in Upland cotton. Hereditas (Beijing) 17(6):30–33
Zhang TZ, Jing SL (1997) Theory and practice of hybrid cotton development via male sterile lines in cotton. China Agricultural Press, Beijing
Zhang TZ, Pan JJ (1990) A genetic male sterile line with virescent marker character in Upland cotton. Euphytica 48:233–237
Zhang JF, Stewart JM (2001) Inheritance and genetic relationships of the D8 and D2–2 restorer genes for cotton cytoplasmic male sterility. Crop Sci 41:289–294
Zhang J, Stewart JM (2004) Identification of molecular markers linked to the fertility restorer genes for CMS-D8 in cotton. Crop Sci 44:1209–1217
Zhang TZ, Zhu XF (2004) Breeding and cultivation technology of Nannong6 (NAU6). China Cotton 31(8):18–19
Zhang TZ, Zhu XF (2005) Breeding and cultivation technology of Nannong9 (NAU9). China Cotton 32(8):19
Zhang TZ, Feng YJ, Pan JJ (1992) Genetic analysis of four genic male sterile line in Upland cotton. Acta Gossypii Sinica 4:1–8
Zhang TZ, Hu Y, Jiang W, Fang L, Guan X, Chen J, Zhang J, Saski CA, Scheffler BE, Stelly DM, Hulse-Kemp AM, Wan Q, Liu B, Liu C, Wang S, Pan M, Wang Y, Wang D, Ye W, Chang L, Zhang W, Song Q, Kirkbride RC, Chen X, Dennis E, Llewellyn DJ, Peterson DG, Thaxton P, Jones DC, Wang Q, Xu X, Zhang H, Wu H, Zhou L, Mei G, Chen S, Tian Y, Xiang D, Li X, Ding J, Zuo Q, Tao L, Liu Y, Li J, Lin Y, Hui Y, Cao Z, Cai C, Zhu X, Jiang Z, Zhou B, Guo W, Li R, Chen ZJ (2015) Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat Biotechnol 33:531–537
Zhang D, Shi J, Yang X (2016) Role of lipid metabolism in plant pollen exine development. Subcell Biochem 86:315–337
Zhang B, Zhang X, Guo L, Qi T, Wang H, Tang H, Qiao X, Shahzad K, Xing C, Wu J (2018) Genome-wide analysis of Rf-PPR-like (RFL) genes and a new InDel marker development for Rf1 gene in cytoplasmic male sterile CMS-D2 Upland cotton. J Cotton Res 1(1):1–11
Zhang M, Liu J, Ma Q, Qin Y, Wang H, Chen P, Ma L, Fu X, Zhu L, Wei H, Yu S (2020) Deficiencies in the formation and regulation of anther cuticle and tryphine contribute to male sterility in cotton PGMS line. BMC Genom 21:825
Zhang Y, Han Y, Zhang M, Zhang X, Guo L, Qi T, Li Y, Feng J, Wang H, Tang H, Qiao X, Chen L, Song X, Xing C, Wu J (2022) The cotton mitochondrial chimeric gene orf610a causes male sterility by disturbing the dynamic balance of ATP synthesis and ROS burst. Crop J 10:1683–1694
Zhang TZ, Pan JJ (1999) Hybrid seed production in cotton. In: Basra RS (ed) Hybrid seed production in crops. Food Production Press, New York, USA, pp 149–184
Zhou H, Liu Q, Li J, Jiang D, Zhou L, Wu P, Lu S, Li F, Zhu L, Liu Z, Chen L, Liu Y-G, Zhuang C (2012) Photoperiod- and thermo-sensitive genic male sterility in rice are caused by a point mutation in a novel noncoding RNA that produces a small RNA. Cell Res 22:649–660
Zhu XF, Wang XD, Sun J, Zhang TZ, Pan JJ (1998) Assessment of cytoplasmic effects of cytoplasmic male-sterile lines in upland cotton. Plant Breed 117:549–552
Zhu SJ, Tong XH, Hong CX, Ji DF, Wu W, Wang RH (2006) A new combination of three-line hybrid cotton “Zheza 2.” China Cottons 5:12–13
Zhu CS, Wu JT, Zhou DG, Li Y, Peng FJ, Gong YC, Zhou SX (2013) Breeding and utilization of cotton cytoplasmic sterile line “Xiangyuan A.” China Cotton Association, Beijing, pp 192–194
Acknowledgements
We would like express our gratitude to the late Prof. Pan Jiaju. Prof. Pan, the mentor of the corresponding author TZZ, first introduced cotton hetrosis research to the author. TZZ also thanks his many graduate students such as Feng YJ, Yuan YL, Wu YD, Liu LW, Wang BH, Zhu XX, Liu RZ, Yin JM, Yang LM, Chen DY for their contributions to this work during their scholastic research in Nanjing Agricultural University (NAU), and to his collegues at NAU: Associate Prof. Zhu XF, for his assistance to develop a series of NAU hybrids. The authors apologize to those whose work was not cited owing to space constraints.
Funding
This study is financially supported in part by grants from the NSFC (32130075), the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (2019R01002), and the Fundamental Research Funds for the Central Universities (226-2022-00100).
Author information
Authors and Affiliations
Contributions
TZ conceived and designed the research. TZ, LX, YM, and YH wrote and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The corresponding author, Tianzhen Zhang, is a member of the journal’s editorial board.
Additional information
Communicated by David D Fang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, T., Xuan, L., Mao, Y. et al. Cotton heterosis and hybrid cultivar development. Theor Appl Genet 136, 89 (2023). https://doi.org/10.1007/s00122-023-04334-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04334-w