Abstract
Key message
The review outlines past failures, present status and future prospects of hybrid wheat, and includes information on CMS/CHA/transgenic approaches for male sterility, heterotic groups and cost-effective hybrid seed production.
Abstract
Hybrid varieties give increased yield and improved grain quality in both cross- and self-pollinated crops. However, hybrid varieties in self-pollinated crops (particularly cereals) have not been very successful, except for hybrid rice in China. In case of hybrid wheat, despite the earlier failures, renewed efforts in recent years have been made and hybrid varieties with desirable attributes have been produced and marketed in some European countries. This review builds upon previous reviews, with a new outlook and improved knowledge base, not covered in earlier reviews. New technologies have been described, which include the Hordeum chilense-based CMS–fertility restorer system, chromosomal XYZ-4E-ms system and the following transgenic technologies: (1) conditional male sterility involving use of tapetum-specific expression of a gene that converts a pro-toxin into a phytotoxin causing male sterility; (2) barnase-barstar SeedLink system of Bayer CropScience; (3) split-barnase system that obviates the need of a barstar-based male restorer line; and (4) seed production technology of DuPont-Pioneer that makes use of transgenes in production of male-sterile lines, but gives hybrid seed with no transgenes. This review also includes a brief account of studies for discovery of heterotic QTL, genomic prediction of hybrid vigour and the development of heterotic groups/patterns and their importance in hybrid wheat production. The problem of high cost of hybrid seed due to required high seed rate in wheat relative to hybrid rice has also been addressed. The review concludes with a brief account of the current efforts and future possibilities in making hybrid wheat a commercial success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A hybrid variety is derived from a specific cross between two good combiners. The superiority of hybrid varieties is attributed to the phenomenon of hybrid vigour or heterosis, which is often expressed in the form of higher yield and other desirable attributes (Koemel et al. 2004). The feasibility of the development of hybrid varieties in crops, where these have not been commercially exploited earlier, has been an attractive area of research for plant breeders. Wheat hybrids produced so far exhibited the following desirable attributes: an average yield advantage of up to 2.05 tons ha−1 (10–20%) over the highest yielding pure line, better quality (in terms of both grain and straw), better response to fertilizers, better root penetrance and better rate/duration of grain filling (Kempe and Gils 2011; Gowda et al. 2012; Longin et al. 2013). The hybrids also exhibit higher stability relative to pure lines, so that the hybrid varieties may be suitable for a wider range of environments (Gowda et al. 2010; Mühleisen et al. 2014). In winter wheats, heterosis has also been reported for tolerance against abiotic and biotic stresses such as frost and diseases like leaf rust, stripe rust, Septoria tritici blotch and powdery mildew (Longin et al. 2013). Some other advantages include lodging tolerance and uniform plant establishment. Therefore, for hybrid wheat, it may be desirable to pyramid multiple partially dominant alleles of most favourable genes/QTL including those involved in epistatic interactions (Gowda et al. 2013; Zhao et al. 2013; Jiang et al. 2017). Studies conducted in India also demonstrated that higher returns from hybrid wheat can be achieved by small-holding farmers relative to farmers with large-holdings (Matuschke and Qaim 2006). Despite these widely known merits of hybrids, the hybrid wheat kept on eluding plant breeders for almost half a century, after its apparent initial promise in the early 1960s. These initial unsuccessful programmes of hybrid wheat were largely based on cytoplasmic male sterility (CMS), which could not be exploited for production of commercial hybrid wheat for a variety of reasons. However, fresh efforts for utilization of T. timopheevi-based CMS and restorer systems are being made at CIMMYT, and those involving Hordeum chilense-based CMS system (msH1) are being evaluated in Europe; however, details of the recent progress using msH1 are not available.
Chemical hybridization agents (CHAs) have been successfully utilized in recent years for inducing male sterility in the female parents to be used for commercial hybrid wheat production (see later for details). In recent years, some wheat hybrids derived using CHA have been reported to give 10–20% yield advantage over the best available pure lines (Gowda et al. 2012; Longin 2016). A number of promising transgenic technologies have also been developed (see later), which may be utilized for hybrid wheat production, as and when the embargo on GMO is lifted.
During the last two decades, the global area under wheat hybrids seems to be gradually increasing, although at a slow pace, approaching the current level of ~ 0.2% (Longin et al. 2012, 2013). Competitive research programmes with ambitious objectives are also in progress, suggesting a likelihood of further expansion for hybrid wheat in Europe (particularly in France and Germany), UK and USA over the next few years. The plant breeders from private companies in four European countries (France, Germany, Poland and Austria) have also established a new European alliance called HYBALLIANCE to intensify research on hybrid wheat and to develop efficient hybrid seed production systems (https://www.ig-pflanzenzucht.de/en/news/hyballiance-first-european-hybrid-wheat-breeding-alliance).
Success and popularity of hybrid wheat that has recently been witnessed in parts of Western Europe and elsewhere is also evident from the following: (1) promising results from the winter wheat variety ‘Hystar’ from Portugal and Germany (https://www.saaten-union.com/index.cfm/action/varieties/cul/296/v/1501.html); (2) successful trials of hybrid wheats in Turkey; (3) possible entry of hybrid wheat in Ukraine, as evident from the recent interest of Saaten Union Recherché (renamed as ASUR PLANT BREEDING); (4) Syngenta’s ambitious program for developing hybrid wheat for several countries including India (https://www.thehindubusinessline.com/economy/agri-business/hybrid-wheat-on-syngentas-pipeline-for-indian-market/article8480449.ece); and (5) CROPCO’s program in UK for developing hybrid wheat (http://www.cropco.co.uk).
Several reviews on hybrid wheat have already been written (Virmani and Edwards 1983; Pickett 1993; Mahajan and Nagarjan 1998; Cisar and Cooper 2002; Zhou et al. 2006; Singh et al. 2010; Kempe and Gils 2011; Longin et al. 2012; Whitford et al. 2013; Singh et al. 2015; Mette et al. 2015). These earlier reviews on hybrid wheat are mainly devoted to mechanism of heterosis, pollination control, CMS-restorer systems and reproductive biology. Except the brief review by Mette et al. (2015), who discussed briefly the commercial success of hybrid wheat, these earlier reviews do not adequately address the recent success in commercialization of hybrid wheat, since much of this information became available rather recently and largely appeared in magazines, bulletins, annual reports of seed companies and other media.
The present review builds upon previous reviews, with a new outlook and improved knowledge base that was not covered in earlier reviews. An effort has been made in this review to collect information from research papers published in journals and also from other reliable sources (bulletins, magazines, new items, etc.) to provide an up-to-date information on recent success and future prospects of hybrid wheat production and its commercialization, albeit with a word of caution. A brief account of the genetics of heterosis/heterotic QTL, progress in genomic prediction of superior hybrids, heterotic groups/patterns and their importance in hybrid seed production has also been included. New technologies involving the use of transgenic approach (including conditional male sterility, barnase-barstar SeedLink system of Bayer CropScience, split-barnase system and DuPont’s Seed Production Technology) are also covered with the hope that one or more of these technologies may be utilized for commercial hybrid wheat production in not too-distant a future, when the regulatory system for GM technology no longer remains a constraint. However, the mechanism of heterosis, floral architecture, etc., will not be covered in this review, since these have been adequately covered in earlier reviews (Virmani and Edwards 1983; Pickett 1993; Singh et al. 2010; Whitford et al. 2013).
Hybrid wheat: a recent comeback
During the late 1990s and later, hybrid wheat made a comeback. This became possible with the marketing authorization of CHAs in the USA and Europe, and also due to renewed interest in CMS in different parts of the world including India, China and Mexico (CIMMYT).
Hybrid wheat in Europe and USA
Starting in the 1990s, > 60 hybrid wheat varieties have been marketed in Europe and USA, with majority of varieties released in Europe (for more details see later). According to some reports, the area under hybrid wheat in Europe increased from ~ 100,000 ha in 2002 to 560,000 ha in the year 2017–2018 (Fig. 2; (http://www.bcwagric.co.uk/hybrid_wheat; actual area occupied by hybrid wheat in 2018–2019 was not available).
In the USA, three companies (BASF, AgriPro Syngenta and DuPont-Pioneer) are focusing on development of hybrid wheat (http://www.westernfarmerstockman.com/wheat/march-hybrid-wheat). In the public sector, wheat breeders from Texas A&M AgriLife Research and the University of Nebraska-Lincoln in the USA are making joint efforts to develop hybrid wheat varieties. The project aims at making integrated use of CHA, breeding, phenotyping, genomic selection, QTL mapping and establishment of heterotic pools to help generate a knowledge base and germplasm resources for successfully launching of hybrid wheat industry in USA (https://today.agrilife.org/2016/12/19/hybrid-wheat-breeding-gets-almost-1-million-usda-investment/).
Hybrid wheat in India
In 2009, ICAR in India initiated a network project on hybrid wheat using CMS approach, but no hybrid varieties could be developed (Singh et al. 2010). Among some private companies, Mahyco (a Maharashtra-based Hybrid seed company) released two wheat hybrids (Pratham 7070 and Pratham 7272) in 2002 using CMS system for cultivation under low-input conditions of central and peninsular India. Although these hybrid varieties occupied > 60,000 acres in six states by 2004 (Zehr 2001; Matuschke and Qaim 2006), they could not compete with newly developed high-yielding pure-line cultivars and were therefore discontinued.
The current focus in India is on the development of hybrid wheat for favourable environments of North-Western Gangetic plains with the hope of getting higher yields. Some private companies like Syngenta are also making investments for development of hybrid wheat varieties for the main wheat growing and marginal areas. Syngenta claims that they should be able to release their first hybrid wheat variety in India as early as 2020 or little later (https://www.thehindubusinessline.com/economy/agri-business/hybrid-wheat-on-syngentas-pipeline-for-indian-market/article8480449.ece), although nothing is visible on the surface to suggest that it will be possible. Therefore, it may take much longer than anticipated for hybrid wheat to reach the commercial level in India, particularly keeping in view the slow progress witnessed even in the commercialization of hybrid rice, for which major success has been witnessed in the neighbouring country China.
Hybrid wheat in China
In China, efforts for developing hybrid wheat cultivars started during the late 1980s and several experimental hybrids with significant heterosis were developed using CMS and CHA systems (Aimin et al. 2001). So far > 50 wheat hybrids with 10–20% gain in yield have been developed; of these, the following seven hybrid varieties were also approved through national and provincial yield trials: Jingmai 6, Jingmai 7, Mianyang 32, Mianzamai 168, Yunza 3, Yunza 5 and Yunza 6. During 2009–2012, hybrid wheat varieties in China were grown on ~ 66,700 mha for demonstration with an average yield improvement of 15.7% in 11 provinces. Encouraged by this success of hybrid wheat breeding, ‘Beijing Engineering Research Centre for Hybrid Wheat’ under the ‘Beijing Academy of Agriculture and Forestry Sciences’ (BAAFS) and ‘China Seed’ established a hybrid wheat company in 2011 and forged cooperation with Pakistan, Uruguay and the Netherlands (http://www.sinochem.com/en/s/1569-5518-18020.html). Two hybrid varieties (Beijing Wheat nos. 6 and 7), developed by the BAAFS, were also successfully tested on a pilot scale in 2012 in Pakistan with the expectation of the potential to increase local wheat production by 50% (https://nation.com.pk/22-Feb-2012/china-s-hybrid-wheat-to-bloom-in-pakistan). Using PGTMS system, China and Pakistan are also apparently cooperating for development of water-efficient and drought-tolerant hybrid wheat varieties (https://www.researchgate.net/project/Development-of-water-efficient-hybrid-wheat-for-Pakistan-and-China). However, these reports could not be substantiated by independent enquires and the targets appear to be too ambitious to be met (personal communication of AK Joshi with wheat breeders in China and Pakistan).
Hybrid wheat at CIMMYT
During the 1990s, hybrid wheat program was reinitiated through a CIMMYT-Monsanto joint collaboration effort, where ‘Genesis’ was used as a gametocide in 1996 (earlier programme involving T. timopheevii CMS females and restorer males that was started in 1962, was discontinued prematurely). During 2002–2004, there was a revival of interest in CMS/Rf system; this interest also did not survive long enough to develop any product.
During the present decade (starting 2010), with the renewed interest in hybrid wheat among global wheat community, CIMMYT decided to take up hybrid wheat programme once again as one of the strategic research priorities. A new collaboration on hybrid wheat research was announced in 2011 between CIMMYT and Syngenta in order to investigate the extent of heterosis in CIMMYT spring wheat germplasm. This was facilitated by the introduction of Australian wheat hybrids developed and marketed during the 1990s. As a result of this effort, more than 5000 CHA-based hybrid combinations were produced and evaluated in Mexico and also in India. With the progress in restorer breeding and CMS-line development, CIMMYT also started testing CMS hybrids once again (for details, see below).
Male sterility and restoration of fertility
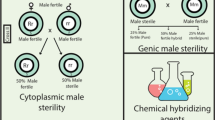
For hybrid seed production programme in any crop, female parent has to be male sterile, unless manual emasculation is economical (as in some vegetable crops). This is mainly achieved through any of the following approaches: (1) cytoplasmic/genetic male sterility (including YA-type CMS); (2) artificial induction through chemical hybridization agent (CHA) or photoperiod/temperature treatment; (3) genetic male sterility; (4) chromosomal sterility. Considerable research has gone into the study of these approaches; the progress made so far will be briefly reviewed in this section.
CMS and fertility restoration (three-line A-B-R system)
A three-line hybrid wheat seed production program consists of the following three lines: (1) line A, the female parent, which should be male sterile; (2) R line, the male parent carrying one or more fertility restorer genes (Rf genes); and (3) line B, the maintainer, which is fertile, but genetically similar to CMS female (A-line). Details of different aspects of CMS-based hybrid production system are available in several earlier reviews (Castillo et al. 2014; Singh et al. 2015); only a brief account is included here.
CMS systems derived from alien species
At present, as many as > 70 different cytoplasms are known, which induce cytoplasmic male sterility (CMS) in wheat (see Singh et al. 2010). In recent studies, four new alien male-sterile cytoplasms (Ae. kotschyi, Ae. uniaristata, Ae. mutica and H. chilense) were discovered (Martin et al. 2008, 2010; Tsunewaki 2015; Hohn and Lukaszewski 2016; Lukaszewski 2017). These CMS systems have been placed in eight different types (Table 1). Of these CMS systems, T. timopheevii (T-type) and Hordeum chilense (msH) systems are the focus of current attention. YA-type CMS systems is another newly discovered system, whose potential is yet to be recognized. Therefore, these three systems, which may be used in future for economical hybrid seed production without the use of chemicals, will be briefly described.
T. timopheevii CMS system. T. timopheevii CMS system for hybrid seed production in wheat is considered to be the best among the available CMS systems. Therefore, it has been most widely used for production of hybrid wheat and hybrid triticale (Mukai and Tsunewaki 1979; Adugna et al. 2004; Singh et al. 2010; Würschum et al. 2017a, b). But fertility restoration in lines carrying timopheevii cytoplasms has often been partial, so that pyramiding of two or more Rf genes in the same male parent was considered a necessity. This became possible through the use of associated molecular markers (see below). CIMMYT in collaboration with Syngenta also initiated a programme of testing hybrids produced using T. timopheevii CMS system. They produced multiple restorer lines, each with up to 2–4 Rf genes using linked molecular markers, which are proprietary to Syngenta. Some of these restorer lines gave 100% fertility restoration in F1 hybrids across multiple environments, including Mexico and India. Recent data (2017–2018) from CIMMYT, Mexico, showed that the F1 hybrid wheat seed production involving timopheevii-based CMS system [involving 45 improved restorers (R-lines) and 5 CMS-A lines] was superior than the chemical-hybridizing agent (CHA)-based system (involving 5 improved restorer males and 100 females) (Fig. 1). This preference for CMS system is sometimes also attributed to CHA-phytotoxicity that was witnessed in the female reproductive organs. Therefore, T. timopheevii CMS system may eventually be used at commercial scale for producing cheap and good quality hybrid seed relative to that produced using CHA.
Hordeum chilense CMS (msH1) system. For hybrid wheat, a novel CMS system (msH1) derived from Hordeum chilense and the corresponding restorer line in the form of a Robertsonian translocation (6Hch.6DL) also became available (Martin et al. 2008, 2010). This msH1 system has the following unique and attractive characteristics: (1) stable and complete male sterility, (2) effective in wide range of varieties, (3) normal flower morphology, (4) some delay in flowering and height reduction.
A major effort in Europe was started in order to exploit the above H. chilense CMS-restoration system for developing an effective and commercially viable hybrid wheat production system. For this purpose, three private companies (Agrasys SL, Spain, Saaten Union Biotech GmbH and affiliates, Germany, Saaten Union Recherche SAS, France), and two public institutions [CSIC Instituto de Agricultura Sostenible (IAS), Spain, and University of Tras-os-Montes and Alto Douro, Portugal] had started an industry-led alliance, namely HY-WHEAT Plant KBBE III. The project initially included the following activities: (1) characterization of msH1 CMS lines under different environments; (2) characterization of Rf lines in different adapted genetic backgrounds in different locations; (3) reduction in the size of 6Hch chromosome segment containing Rf gene; (4) development of molecular markers for monitoring the CMS and Rf genes during breeding. However, no details of the recent progress of this project are available.
YA-type CMS. The YA-type CMS system is based on a cytoplasmic mutant derived from the common Chinese wheat variety ‘CA805’ and was discovered and developed at The Institute of Genetics and Developmental Biology in Beijing, China. This CMS system was later transferred to several Chinese wheat cultivars. Another genotype GR1 that was initially used as the restorer carried two restorer genes (YARf1, YARf2) along with some minor genes. The restoration system was later transferred to as many as 26 different cultivars to be used in hybrid wheat programme. The YA-type CMS was initially shown to have potential for hybrid wheat breeding (Liu et al. 2006), although during the last more than one decade, there is no report on the progress towards utilization of this system for hybrid wheat production.
Genetics of CMS-restoration system (Rf genes)
Eight Rf genes (Rf1-Rf8) for timopheevii-based CMS systems are known and are located on the following wheat chromosomes: 1A, 7D, 1B, 6B, 6D, 5D, 7B and 2D (Schmidt et al. 1962; Robertson and Curtis 1967; Yen et al. 1969; Bahl and Maan 1973; Maan et al. 1984; Tahir and Tsunewaki 1969; Mukai and Tsunewaki 1979; Du et al. 1991; Zhou et al. 2005; Sinha et al. 2013). Five of these Rf genes (Rf1, Rf2, R4, Rf5 and Rf7) had their origin in T. timopheevii (Livers 1964; Bahl and Maan 1973; Ma and Sorrells 1995; Yen et al. 1969). Out of the remaining three Rf genes, Rf3 was discovered in T. spelta var. duhamelianum (Kihara and Tsunewaki 1966) and Rf6 and Rf8 were discovered in wheat (Bahl and Maan 1973; Sinha et al. 2013). So the Rf genes for timopheevi-based CMS are not only available in T. timopheevii but are also available in other related species.
Rfmultiand 1BL.1RS translocation. In addition to the above eight Rf genes, another Rf gene called Rfmulti was also discovered, when it was found that the male sterility due to cytoplasms from Ae. kotschyi, Ae. mutica and Ae. uniaristata was manifested in only those wheats, which lacked the arm 1BS, as is the case of wheat cultivars carrying 1BL.1RS translocation. Sometimes, even the gene Rf3 failed to restore the fertility of male-sterile lines carrying the cytoplasm of any of the above three Aegilops species. This suggested that perhaps 1BS carries a fertility restorer gene, which is effective against multiple cytoplasms; it was therefore named Rfmulti. This gene was assigned to a 2.9-cM segment on 1BS through the use of a number of 1BS/1RS recombinant lines (Tsunewaki 2015). Later Hohn and Lukaszewski (2016) created a chromosome 1B1:6, with ~ 2.8-cM-long insert from rye arm 1RS that replaced a 1BS segment carrying Rfmulti locus. This product with 1B1:6 gave complete male sterility, suggesting that the gene Rfmulti was interacting with the cytoplasm of all the four species including that from T. timopheevii. The segment carrying Rfmulti was later narrowed down to a single marker. Lukaszewski (2017) later created additional recombinant 1B chromosomes (1B25:6 and 1B35:6) carrying a very small 1R segment replacing Rmulti, and 1R chromosomes with Rmulti, to study further the segment carrying Rfmulti.
Interaction among Rf genes. Interactions among Rf genes have also been reported. For instance, an interaction between Rf1 (1BS) and Rf4 (6B) was demonstrated by Geyer et al. (2018). In some cases, individual major Rf genes along with modifier genes have also been shown to restore complete fertility (Kihara and Tsunewaki 1967; Bahl and Maan 1973; Kojima et al. 1997; Geyer et al. 2018). A major gene Rfv1 (in heterozygous condition) on 1BS and some minor genes from Chinese Spring (CS) and other common wheat genotypes restored complete fertility of Ae. kotschyi and Ae. variabilis cytoplasms; this was considered to be an advantage of these two cytoplasms over the T. timopheevii cytoplasm in hybrid wheat breeding (Mukai and Tsunewaki 1979). In addition to the above, two independent major genes (D2Rf1, D2Rf2) and some minor genes were reported to control the fertility of D2-CMS lines containing cytoplasm of Ae. crassa (Liu et al. 2002). Candidate genes for Rf1 and Rf3 have also been validated in collaboration with IPK, Gatersleben, Germany (Manuel Geyer, personal communication). However, a common feature among all Rf genes is that each Rf gene seems to encode a protein carrying a common degenerate motif called a pentatricopeptide repeat (PPR) (for details, see Whitford et al. 2013).
Molecular markers for Rf genes
Restoration of fertility in CMS lines due to individual Rf genes may be partial and is also influenced by the environmental factors (Ma and Sorrells 1995). Therefore, for efficient transfer of individual Rf genes and their pyramiding, molecular markers associated with several Rf genes have been developed using linkage/QTL analysis; some of these markers are tightly linked with a genetic distance as low as 0.4 cM (Table 2).
Among all Rf genes, Rf3 received the major attention. Initially, it was shown to be associated with the SNP marker IWB72107 (Geyer et al. 2016), which was later converted into a cleaved amplified polymorphic sequence (CAPS) marker CAPS_IWB72107. Using this CAPS marker, Rf3 was mapped in four populations involving different wheat and spelt restorers (PR143, Badenkrone, Badenstern and Schwabenspelz). The distance between Rf3 and the marker ranged from 0.4 to 2.3 cM, suggesting tight linkage, indicating its suitability for use in marker-assisted selection for pyramiding of Rf3 gene on to other Rf genes. Closely linked markers for other Rf genes (including Rf2, Rf5, Rf6 and Rf7) may also be developed in future. Currently, CIMMYT, Mexico, is developing new molecular markers for different Rf genes, and the linked markers will be available in public domain in the near future. Molecular markers for Rf genes for CMS systems involving male-sterile cytoplasms belonging to Ae. crassa, Ae. kotschyi, Ae. mutica and Ae. uniaristata have also been developed, although these systems are not considered as important as timopheevii-based CMS system for hybrid seed production.
Limitations of CMS systems in hybrid seed production
The cytoplasms of T. timopheevii, Ae. variabilis, Ae. kotschyi and Ae. crassa are known to have some limitations (Table 3). In particular, the CMS lines developed in wheat cultivars carrying 1BL/1RS translocation have poor fertility restoration and also produce haploids. These limitations have largely been overcome through selection against 1BL/1RS translocation (Xiyue et al. 2002). The Rf genes have also been reported to show low penetrance and expressivity in an array of genetic backgrounds. Also, the CMS-based hybrid seed suffers from the problems of kernel shrivelling/low test weight and low germination (Geyer et al. 2018).
Despite the above limitations, there are several scattered reports of commercial cultivation of CMS-based hybrid wheats. A notable example is the production of hybrid wheats in China using T. Timopheevii- and Ae. kotschyi-based CMS, albeit on a very small scale (Longin et al. 2012; Tsunewaki 2015); in this report also, T. timopheevii-based CMS has been found to be more promising and has been utilized more often for producing successful hybrids.
Induced MS and two-line system, using CHA, photoperiod and temperature
As is widely known, the following four two-line systems are available for inducing male sterility for the purpose of hybrid seed production, although not all of them have been utilized in wheat: (1) chemical-hybridizing agent (CHA)-induced male sterility; (2) photoperiod-sensitive cytoplasmic male sterility (PCMS); (3) thermo-sensitive recessive male sterility (TGMS); (4) photoperiod and temperature-sensitive genic male sterility (PTGMS). A brief account of each of these systems is included here.
CHA-induced male sterility
In general, a good CHA should have the attributes listed in Table 4 (Liable 1974; Pickett 1993; Virmani and Edwards 1983; Kempe and Gils 2011). The different CHAs that have been tried for production of hybrid wheat are listed in Table 5. The first generation of chemical compounds tested as CHA included Ethaphon, gibberellin and RH531, which were associated with problems of inadequate level of male sterility associated with female sterility and high level of phytotoxicity; these negative attributes did not allow their use at any measurable scale. The second generation of CHA compounds included RH-0007 (Hybrex) as the most important CHA, which produced 95–100% male sterility in a wide spectrum of winter and spring wheats, so that it was widely used in commercial hybrid wheat production both in Europe and USA. However, its association with reduction in hybrid seed quality discouraged its use for any long-term basis. During the 1990s, two novel CHAs, namely ‘Genesis™’ (marketed by HybriTech-Monsanto) registered as Clofencet by the US Environmental Protection Agency (EPA) (Parodi and de los Angeles Gaju 2009) and ‘Croiser® 100’ (marketed by DuPont-Hybrinova, Saaten Union, Germany), were authorized and are currently used in Europe; Croiser® 100’ is currently the most widely used CHA. These CHAs fulfil some of the attributes of a good CHA listed in Table 4.
Using CHAs, a number of commercially viable hybrid wheat cultivars were developed and released for commercial cultivation during the 1990s and thereafter (www.hybridwheat.net). In 1994, the first hybrid ‘Hpo-Precia’ was released in France by Hybrinova (then of DuPont), and ‘Domino’ was released in the USA by HybriTech-Monsanto (for more hybrid varieties, see Table 6). In 1999, the seed company Nordsaat Saatzucht GmbH released ‘Hybnos1’ as its first hybrid wheat in Germany. However, in 2003–2004, the interest in hybrid wheat picked up again, and sales of hybrid wheat seed were re-launched in several European countries including France, Germany, UK, Poland, Czech Republic, Hungary, Portugal, etc. In the meantime, marketing of ‘Croiser® 100’ and ‘Sintofen’ (the active ingredient of Croiser® 100) was also authorized in Europe. As a result, the area occupied by hybrid wheat in Europe steadily increased and approached 400,000 ha in 2016 (Fig. 2), which increased to 565,000 ha (1,400,000 acres) in 2018 (http://www.bcwagric.co.uk/hybrid_wheat).
Photoperiod-sensitive cytoplasmic male sterility (PCMS)
The cytoplasm of Ae. crassa is sensitive to a long photoperiod (≥ 15 h), which causes male sterility, and was therefore considered useful for hybrid wheat programmes (Murai and Tsunewaki 1993). In this system, the genotype to be used as female parent will be fertile under short-day (SD), i.e. < 14.5 h or less, but male sterile under LD conditions, so that female line will have to be maintained through self-pollination under SD conditions and hybrid seeds will be produced under LD conditions using a suitable pollinator (Murai 2002; Murai et al. 2002, 2016). The PCMS system is based on two kinds of fertility restorer systems, one involving a number of Rf genes and second controlled by a single dominant major Rf gene (rfd1). PCMS system in Chinese Spring has been shown to be associated with at least 20 modifiers (Murai and Tsunewaki 1993).
Thermo-sensitive recessive male sterility (TGMS)
Some wheat varieties are known to carry genes that render them male sterile under conditions of reduced temperature (Qian et al. 1986; reviewed in Sun et al. 2001). A spontaneous TGMS mutant BS20-T was recovered in a commercially grown wheat variety BS20 in China. The TGMS line (B20-T) is completely sterile when temperatures are < 10 °C and completely fertile in temperatures > 13 °C. The TGMS is controlled by a recessive gene tmsBS20T located on chromosome 2BL flanked by SSR loci Xgwm403 and Xgwm374 at genetic distances of 2.2 and 4.5 cM, respectively (Ru et al. 2015). Apart from the above, the recessive TGMS gene wtms1 on 2BL from line 337S is also known (Xing et al. 2003). This gene is located within an interval of 11.3 cM from the SSR marker Xgwm374 and is associated with an AFLP marker E:AAG/M:CTA163. However, there is no report of successful application of TGMS for hybrid wheat breeding.
Photoperiod and temperature-sensitive genic male sterility (PTGMS)
PTGMS in wheat was also reported from China for use in winter wheat hybrid breeding programmes (Changping 2013). The PTGMS is controlled by two major genes, which exhibit interaction with the genetic background. Close association between the expression of PTGMS and the small RNA was also demonstrated. About 5000 PTGMS germplasm sources of BS series (Beijing, PTMS) and CS 49S series (Chongqing, Sichuan and Yunnan) have been reported. These lines are 100% sterile and were grown for seed production on 5000 acres. They produce up to 3.75 tons/ha hybrid seed and were successfully used for development of seven PTGMS hybrids that were approved by national and provincial governments in China (https://www.cimmyt.org/news/visitors-discuss-hybrid-wheat-in-china). Around 1000 PTGMS genotypes are also available with full intellectual property rights (Changping 2013). Despite this encouraging performance of PTGMS, no commercial wheat hybrid has so far been produced using this system in any country other than China.
Genetic male sterility (GMS)
Genetic male sterility has never been exploited for commercial hybrid wheat production, although at least five nuclear genes for male sterility are known in wheat; three of these are dominant genes, namely Ms2 (4DS), Ms3 (5AS) and Ms4 (4DS) (McIntosh et al. 2013; http://www.shigen.nig.ac.jp/wheat/komugi/genes/symbol ClassList.jsp). The remaining two genes, ms1 (4BS) and ms5 (3AL), are recessive, with six allelic mutants of ms1 (reviewed in Singh et al. 2015). The maintenance of male-sterile parent is achieved through a heterozygous male fertile population (Hermsen 1965), but this is not a desirable option since the female parent must not segregate for fertility in the hybrid seed production field. The problem becomes difficult since hand-roguing of fertile segregants is uneconomical and not suitable for commercial production of hybrid seed.
Among the above five genes, Ms2 is a spontaneous mutation in common wheat ‘233’ (Liu and Deng 1986), which was discovered in Taigu County in China. Details of using Taigu wheat in China are well documented in a Chinese book titled “Use of Taigu Male-Sterile Wheat in Wheat Breeding”, published in 1995 (He et al. 1995). The wheat lines carrying Ms2 gene are 100% male sterile and are called as ‘Taigu genic male-sterile wheat’. Ms2 gene was recently cloned using map-based cloning and transcriptome approaches in China. The gene encodes for an orphan protein that confers male sterility in grass species (Ni et al. 2017). The gene Ms2 was exploited in the past for recurrent selection schemes during conventional wheat breeding programmes in China (Yang et al. 2009). Ni et al. (2017) proposed the potential of this gene for a high-throughput hybrid seed production involving the following steps: (1) transformation of a desirable wheat line with a cassette composed of two linked genes (Ms2 plus an aleurone-specific gene for pigmented seeds) which will result into a male-sterile line (A) with pigmented kernels. (2) The A-line will be maintained by inter-crossing with the non-transgenic parental line originally used for transformation; the derived seeds will be subjected to optical sorting to separate pigmented seeds (50% male sterile) and the regular seeds (50% male fertile). (3) The progeny of the pigmented seeds will be used as male-sterile female parent and crossed with an elite male parent. The F1 seed will be subjected to optical sorting, so that pigmented seeds (male sterile) will be used as feedstock and the regular F1 seed will be used for raising a wheat hybrid.
Another potential use of genetic male sterility in hybrid seed production in wheat was proposed by Tucker et al. (2017). They cloned the gene Ms1 through map-based cloning. The gene encodes lipid transfer protein (glycosylphosphatidylinositol) necessary for the development of pollen exine. The gene Ms1 complements with the EMS-induced msd1 male-sterile mutant and completely restores the fertility. It was thus proposed that Ms1 gene may be employed in a hybridization platform similar to the maize SPT where non-transgenic F1 hybrid is produced using a transgenic genetic male sterility system (see later).
Chromosomal male sterility (ChMS): XYZ-4E-ms system
This system involves the use of three lines (X, Y and Z) for production of a male-sterile line to be used as female parent in hybrid seed production; the male parent could be any elite wheat cultivar and will have nothing to do with XYZ system. All the three lines are homozygous (ms1/ms1) for the recessive allele for male sterility, located on 4BS chromosome. Additionally, an alien chromosome 4E from Agropyron elongatum, carrying dominant allele Ms1 (suppressing the activity of ms1), is also involved. In XYZ-4E system, X line has two doses (Ms1/Ms1), Y has one dose (Ms1/–) and Z having 0 dose (–/–) of the alien chromosome (4E) as an addition to the normal complement (Zhou et al. 2006). Due to the presence of Ms1 on chromosome 4E, X and Y lines are fertile, but the Z is male sterile (ms1/ms1) due to the absence of chromosome 4E (Whitford et al. 2013) (Supplementary Fig. 1). X line will be true breeding, while Y line on selfing will segregate into three types [3% fertile disomic 4E addition X line (line X), 33% monosomic 4E addition line (line Y) and 64% male sterile (line Z carrying no 4E)]. Seed that is monosomic for 4E (Ms1) (Y line) is identifiable by light blue aleurone (Ba), which is used (selfed) again for the production of male-sterile Z line, whereas disomic seed (X line) is identifiable by a dark blue colour and is discarded. Alien chromosome 4E is poorly transmitted through the male germ line, when plants monosomic for 4E are selfed, resulting in 64% white-seeded male-sterile progeny (Z line), which is used as male-sterile female line in the hybrid seed production field. A good combiner elite wheat line is used as the male parent. In this manner, XYZ system is also a convenient source of pure genetic male-sterile line, which is otherwise difficult to produce using genetic male sterility system (GMS). In future, the ChMS system may be used for commercial hybrid seed production.
Although the 4E-ms system was initially reported to be successful in enabling the production of a male-sterile seed, but later it was observed that there existed some residual pollen transmissibility of chromosome 4E that allowed selfing and seed set within the male-sterile plants; as a result, the seed harvested from the female parent would be a mixture of selfed and hybrid seed. Based on the cloning and characterization of Ms1 and majority of its mutants, it is now believed that it will be possible to bulk a male-sterile female-inbred seed (ms1/ms1), as is the case for SPT in maize. If this becomes possible, it will be a major cost-saving for hybrid seed production and can overcome the seed purity issues inherent to the 4E-ms system. The development of SPT for wheat could become possible by combination of three technologies: the availability of a functional α-amylase gene for wheat pollen disruption, a seed-selectable marker and the ability of the TaMs1 gene sequence to completely restore viable pollen production in ms1 plants (Tucker et al. 2017).
In ChMS system, the male parent need not contain fertility restoration system, so that no fertility restoration problem is encountered, which is generally associated with CMS. For ChMS, male parents for hybrid seed production can be designed to carry resistance genes for different diseases. These resistance genes may also be carried in the form of translocated alien segments bearing genes for disease resistance.
Although ChMS system looks attractive, following are some of the limitations of ChMS system: (1) the female parent will have to be ms1/ms1, so that ms1 allele for male sterility will have to be transferred to the desired female parent; (2) the desired male parent will have to be converted into monosomic alien addition line carrying alien chromosome 4E with dominant allele Ms1. These limitations will certainly discourage the use of ChMS system on a large scale involving use of diverse genotypes for hybrid seed production in wheat.
Transgenic technology for hybrid wheat
Commercial hybrid seed production using CHAs suffers from the following two major limitations leading to financial losses: (1) CHA treatment of the female parent may compromise seed production (Adugna et al. 2004); (2) CHA treatment needs to be given within a narrow window due to environmental factors, such as rain, wind and heat (Pickett 1993; Cisar and Cooper 2002). Several transgenic technologies that have been developed to overcome these limitations are described in this section, although there are following limitations associated with transgenic approach also. (1) Transgenic technologies demand additional tools, such as seed sorting using a marker (e.g. seed colour). (2) The product has to go through the passage of difficult regulatory system for GM crops, so that the acceptance of transgenic technology is itself questionable in many countries. Efforts may be made in future to examine the possible commercial use of these transgenic technologies, although the above difficult regulatory system may discourage both private and public sectors to make investments in this area. The three transgenic approaches are briefly described.
Conditional male sterility (use of a protoxin)
Conditional male sterility refers to a male-sterile condition, which appears in normal fertile plants on external application of a protoxin. This is generally achieved by transgenic approach and is often used to obviate the need of a maintainer for production of male-sterile female line, along with production of hybrid seed. The transgene is made to express in a tissue-specific manner, so that it will facilitate conversion of a protoxin (supplied from outside) into a phytotoxin (on application of protoxin) in a tapetum-specific manner (Supplementary Fig. 2a; Kempe and Gils 2011).
There are at least three examples, where conditional male sterility was successfully achieved by using genes, which are conditionally expressed in the tapetum; in each case, a protoxin is used for spray. First, in tobacco, a transgene (CYP105A1 from Streptomyces griseolus) was used, which encoded cytochrome P450 that metabolises the proherbicide R7402 to a phytotoxic metabolite. A treatment of transgenic plants with sulfonylurea proherbicide (R7402) led to dramatic reduction in pollen viability (O’Keefe et al. 1994). Second, in tobacco, a transgene argE derived from E. coli was used. It encodes N-acetyl-L-ornithine deacetylase, which is made to express in a tapetum-specific manner. When plants are sprayed with N-acetyl-L-phosphinothricin (a protoxin), the latter is deacetylated to produce the toxic L-phosphinothricin (glufosinate, a potent herbicide) causing, pollen abortion (Supplementary Fig. 2a; Kriete et al. 1996). Third, in Arabidopsis, the transgene PehA was used, which encodes phosphonate monoester hydrolase derived from Burkholderia caryophilli. The transgene converts glyceryl glyphosate (a protoxin) into glyphosate in a tapetum-specific manner. When fertile transgenic plants were treated with glyceryl glyphosate, the latter was converted into the herbicide glyphosate leading to pollen abortion (Dotson et al. 1996).
Commercial seed companies have also tried conditional male sterility to assess the utility of this approach in commercial hybrid seed production. Following are some examples: (1) Syngenta used tobacco plants expressing a modified form of d-amino acid oxidase (DAAO) (Hawkes et al. 2011). When plants carrying the transgene are sprayed with D-glufosinate (a prototoxin, which is an enantiomer of the herbicide glufosinate), the latter is oxidized to 2-oxo-4-(methylphosphinyl)-butanoic acid, which is subsequently converted to phytotoxic L-glufosinate causing death of tapetal cells, leading to male sterility (Supplementary Fig. 2b). (2) Monsanto used transgene for miRNA that inhibits 5-enolpyruvylshikimate 3-phosphate synthase (EPSPS), which confers herbicide tolerance. In the transgenic plants, EPSPS is specifically inhibited in tapetal cells (Conner et al. 2002; Allen et al. 2007), so that herbicide treatment will kill these unprotected tapetal cells, when treated with glyphosate, thus causing male sterility. In this case, ‘true mixed-planting’ of males and females can be undertaken in such a manner that the male plants are tolerant to the herbicide, and the tapetal cells of the female plants are not, so that the female line will become male sterile (Kempe and Gils 2011). However, the requirement for large-scale spraying of chemicals in seed production plots is a disadvantage, which results in an increase in cost of the production of hybrid seed. Another disadvantage (in common with the CHA approach) is the narrow application windows for application of herbicide.
Barnase-barstar system (SeedLink hybridization system)
Barnase-barstar system (SeedLink System of Bayer) for hybrid seed production has already been utilized by Bayer CropScience for the production of SeedLink oilseed rape, also known as canola (Whitford et al. 2013; Supplementary Fig. 3). As is widely known, the barnase-barstar system makes use of two genes, barnase gene encoding a ribonuclease and barstar gene encoding a ribonuclease inhibitor (first discovered in Bacillus amyloliquefaciens), which are made to express only in anther tapetum in a tissue-specific manner. In the female parent, barnase gene is expressed in the tapetum and interferes with pollen development causing male sterility. The male parent carries gene encoding barstar, which is expressed in F1 hybrid plants inhibiting the activity of barstar, thus giving full seed setting in F1 plants (Mariani et al. 1990, 1992). The male-sterile line is maintained by backcrossing with a non-transgenic line. The back-cross would give a mixture of seed, of which 50% seed will produce male-sterile plants. A gene encoding glufosinate resistance (LibertyLink) in close linkage with barnase is used, so that only male-sterile plants survive when female lines are treated with glufosinate herbicide. Although this method is being used successfully for hybrid canola production, its commercial utility in other crops including wheat has yet to be realized.
Split-barnase hybridization system
The split-barnase system makes use of non-overlapping fragments of barnase gene (Bar-N, Bar-C), so that in the female line, the expressed fragments will be non-functional, unless fused together through intein-mediated ligation after translation. The fusion product is the functional barnase. The split barnase system consists of the following two vectors carried by two different transgenic plants: (1) an expression vector called provector (A) carrying two non-overlapping barnase gene fragments (Bar-N and Bar-C), fused with the N- and C-terminal sequences of an intein (Int-N; Int-C); these intein sequences belong to the DnaB helicase gene of Synechocystis sp; thus, the split sequences are Bar-N-IntN and Bar-C-S-InC; the provector also contains sequences attp and attB, which are the targets of recombinase enzyme, for which the gene is present in the other vector, carried by another plant carrying the integrase vector and (2) the integrase expression vector pICH13130 (B) containing gene pHiC31 for site-specific recombinase enzyme. The details of two vectors and the steps involved in the production of male-sterile line are shown in Supplementary Fig. 4a (Kempe et al. 2014).
Following three operations are needed for hybrid seed production using the split-barnase system, as shown in Supplementary Fig. 4b (Kempe et al. 2014): (1) The transgenic male-sterile plant carrying the provector A is crossed with the plant carrying integrase expression vector, thus eventually producing a line that is heterozygous for A1 and A2 (shown in A, Supplementary Figure 4b); this will produce male sterility, when Bar-C and Bar-N protein fragments are fused together after translation; (2) maintenance of male-sterile line (shown in B, Figure 4b); (3) hybrid seed production (shown in C, Figure 4b). Thus, the male-sterile line would be used in maintainer plots for its maintenance in hybrid seed production field as male-sterile female parent. The maintainer will carry either A1 or A2 in homozygous condition, so that the F1 will segregate to give 50% male-sterile female plants, which can be selected using a herbicide (Supplementary Fig. 4b; as in barnase-barstar system). This split barnase system obviates the need for the presence of barstar in the male parent to restore fertility, because the F1 hybrid seed will be fertile due to the absence of complete barnase gene, and the presence of only one of the two fragments encoding barnase gene (for details, consult Kempe et al. 2014).
Pioneer-DuPont seed production technology (SPT)
The hybrid seed production technology (SPT) of Pioneer-DuPont involves the production of the following two lines: (1) a transgenic male fertile maintainer line and (2) the non-transgenic male-sterile (ms-45/ms-45) female line. The male fertile plants of the maintainer line is homozygous for male sterility (ms-45/ms-45) and contains a transgene cassette with three genes [Ms-45 for fertility despite the presence of ms-45 in homozygous condition; zn-aa1 for α-amylase causing breakdown of starch, rendering the pollen sterile; DsRed2 (AltI) for red coloured grains]. No abortion will take place in female gametes of the maintainer, due to anther-specific expression of α-amylase gene zn-aa1. (It will not express in female gametes.)
Each of the three genes in the SPT maintainer is associated with the following three sequences: (1) a transit peptide brittle-1 (zm-bt1) from Z. mays for targeting expression to the amyloplast (where starch synthesis takes place, so that zn-aa1 may block starch synthesis), (2) a promoter (PG47) for pollen-specific expression of each gene and (3) the In2-1 terminator from maize (Keil et al. 1986; An et al. 1989; Hershey and Stoner 1991).
The first step involves growing maintainer line [with the cassette Ms-45, zn-aa1, DsRed2 (AltI) in hemizygous condition and ms-45/ms-45 in homozygous condition] and ms-45/ms-45 male-sterile line in alternate rows, so that seed can be harvested from both. Only the non-transgenic pollen (lacking zn-aa1 and carrying ms-45) from the maintainer is viable and used for self-pollination of the maintainer and also for cross-pollination of the ms-45/ms-45 male-sterile rows. The selfed seed from the maintainer segregates into 50% red colour seed giving fertile maintainer plants and 50% yellow seed (homozygous for ms-45; discarded); the red colour seed can be sorted out using fluorescent illumination and used again as maintainer.
In the second step, the ms-45/ms-45 seed harvested from the non-transgenic (male sterile) female lines in the first step (due to crossing) is used for growing female rows in the hybrid seed production field, with an elite good combiner planted as the male parent. The hybrid seed produced thus is commercialized. Since the male-sterile line carries no transgene and is crossed with a non-transgenic elite wheat cultivar, the hybrid seed will carry no transgene (Supplementary Fig. 5). The transgene cassette is used in the maintainer only for the production of male-sterile line. The technology has already been successfully used in maize by Pioneer-DuPont.
Development of heterotic groups
The concept of heterotic groups and heterotic patterns is fundamental to heterosis breeding. A heterotic group comprises related or unrelated genotypes (inbred/pure lines) from the same or different populations and display similar hybrid performance, when crossed with any genotype from another genetically distinct heterotic group (Melchinger and Gumber 1998). A heterotic pattern, on the other hand, is defined as a specific pair of two heterotic groups, which exhibit high heterosis when crossed. The ratio between variances due to specific combining ability (SCA) and general combining ability (GCA) is generally low in genetically divergent heterotic groups (Reif et al. 2005; Fischer et al. 2008). In other words, a low σ2 SCA: σ2GCA ratio occurs in inter-group than in intra-group crosses, indicating that the concept of heterotic patterns effectively supports the selection of superior hybrids. It has also been shown that predominance of GCA is useful for high genetic gains in recurrent selection and also for ease in identification of promising hybrids based on GCA prediction (Reif et al. 2007).
The information on heterotic groups/patterns in any crop simplifies and increases the efficiency of all subsequent operations for hybrid seed production. This concept has been utilized very effectively in temperate maize, where heterotic patterns became known early, so that the breeders followed it very systematically. In tropical maize, however, the use of these heterotic patterns could not be effectively utilized due to complex population structure of the germplasm (Xia et al. 2004). In rice also, efforts were made for the development of heterotic groups. In one such study, SSR genotyping data and field trials were used for establishing two heterotic groups and four heterotic patterns in IRRI hybrid rice germplasm to develop hybrid rice in the tropics (Xie et al. 2014).
In order to establish heterotic groups in wheat in particular and autogamous crops in general, Boeven et al. (2016a) also proposed a unified framework HyBFrame, which involves the following steps: (1) selection of individual high-yielding lines based on per se performance and its correlation with GCA; (2) selection of male and female lines permitting good cross-pollination; (3) testing lines for GCA in factorial crosses; (4) lines with high GCA to serve as starting material for male and female pools; (5) improvement of combining ability of lines through reciprocal recurrent selection leading to divergence of the two groups; and finally (6) addition of new germplasm with high GCA that could enter the respective pools to improve the intra-group diversity.
In wheat, several attempts have been made to study the heterotic patterns and to establish heterotic groups (Zhao et al. 2013, 2014, 2015; Boeven et al. 2016a). In a very exhaustive study, Zhao et al. (2015) used a diverse set of 135 elite wheat lines from Central Europe for making 1604 single-cross hybrids; phenotypic, metabolic, and genomic data were also recorded. A number of conclusions were made. First, the predictions based on additive effect are more accurate than those based on dominance effect, and the prediction accuracy did not improve by combining metabolomics data with genomic data. Second, the grain yield is the most important trait for the search of heterotic patterns. Following three-step strategy was considered promising for the search of heterotic pattern: (1) preparation of a hybrid performance matrix using genomic prediction data, (2) search of high-yielding heterotic pattern using simulated annealing algorithm, and (3) assessment of long-term success of the identified heterotic pattern. It was demonstrated that hybrid wheat breeding based on the identified heterotic patterns can boost grain yield through the exploitation of heterosis and can enhance recurrent selection gain (Boeven et al. 2016a).
The potential of spelt wheat (T. aestivum ssp. spelta) germplasm from Central Europe was also examined for its use as a separate heterotic group in hybrid wheat breeding (Akel et al. 2018a). This work was motivated by the fact that wheat cultivars from Central Europe have certain degree of relatedness due to frequent exchange and use of germplasm across countries (Nielsen et al. 2014; Würschum et al. 2015; Boeven et al. 2016a), so that heterotic patterns among wheat cultivars from Central Europe may not be very useful for hybrid seed production programme. In contrast, spelt wheats exhibit genetic diversity (Bertin et al. 2004; Würschum et al. 2017a, b) and are genetically distinct from bread wheat (Siedler et al. 1994; Bertin et al. 2001; Müller et al. 2018). Hence, their potential for hybrid wheat was tested. For this purpose, two large experimental trials were conducted at multiple locations in Germany. In these trials, 43 spelt lines, 14 winter wheat lines and 273 winter wheat–spelt hybrids were evaluated for a number of traits including quality and disease resistance. Contrary to expectations, wheat–spelt hybrids showed lower yield heterosis than the hybrids made among elite wheat lines; heterosis for grain quality was also negative. It was, therefore, concluded that spelt wheats have no potential for their use as a separate heterotic group in hybrid wheat breeding.
However, it has been suggested that initial size of 15 lines per heterotic group may be optimum for short-term and long-term success of hybrid wheat breeding. Also, the connectivity of heterotic patterns across the climatic zones such as Central European pattern vs Asian patterns and long-term gene flow through exchange of lines across heterotic patterns has been emphasized (Zhao et al. 2015).
Prediction of hybrid wheat performance (genetics and genomics of heterosis)
For a successful hybrid wheat breeding programme, it is necessary to identify parental combinations, which will produce the best hybrids. However, as the number of inbred lines (n) increases, the number of possible single-cross hybrids would also increase [n (n − 1)/2], making it difficult to conduct phenotypic evaluation of such a large number of single-cross hybrids. In order to overcome this problem, the following two recent approaches have been followed, in place of evaluation all possible hybrids: (1) QTL analysis for identification of QTL that may be responsible for heterosis and (2) heterosis prediction models that have been proposed and evaluated (Bernardo 1994).
Genetics of heterosis and heterotic QTLs
In the past, heterosis was often treated as a qualitative trait and was explained on the basis of dominance, over-dominance and epistasis. However, a consensus later emerged in favour of over-dominance (East 1936; Hull 1945; Crow 1948) and epistasis (Richey 1942; Powers 1944; Jinks and Jones 1958). More recently, heterosis has been treated as a quantitative trait and epistatic QTLs for heterosis have been identified in several crops including maize and rice (Melchinger et al. 2007; Garcia et al. 2008). In a study in winter wheat involving 1604 F1 hybrids (derived from 120 females and 15 males) also, up to 50% of the total genetic variance for heterosis in grain yield was found to be due to additive-by-additive epistatic interactions; a mere 16% was due to dominance effect (Jiang et al. 2017). In addition, 9 small-effect heterotic QTL for grain yield were also identified (each explaining on average only 3.3% phenotypic variance).
Immortalized F2 (IF2) populations that are particularly suitable for study of heterotic QTLs were also used for heterotic QTL analysis in wheat. (Many more such studies were conducted in rice.) In these studies in wheat, Zhuo-Kun et al. (2010) reported 20 heterotic QTLs for plant height and Yuan et al. (2012) reported 17 QTL for gain number per spike; the average PVE of QTLs ranged from 6.63 to 10%. In agreement with these studies, mostly small-effect QTLs for heterosis for a variety of traits were reported using a variety of populations in rice (IF2, test cross populations involving RILs/introgression lines, etc.) (Xiao et al. 1995; Abdelkhalik et al. 2005; Xin et al. 2011; Chu et al. 2012; Qu et al. 2012). Therefore, as reiterated earlier, for hybrid wheat, it may be desirable to pyramid multiple partially dominant alleles of most favourable genes/QTL and those involved in epistatic interactions for development of superior hybrid wheat (Gowda et al. 2013; Zhao et al. 2013; Jiang et al. 2017). However, many more studies on heterotic QTLs in wheat need to be conducted to identify desirable QTLs for heterosis using suitably designed populations.
Genomic prediction of hybrid performance
A prerequisite for any hybrid programme is the selection of suitable parents based on prediction of heterosis. In conventional hybrid breeding programmes, we make all possible hybrids through manual crossing and compare their performance, or estimate general and specific combining abilities. In recent years, with the availability of molecular marker-based genomic selection (GS) as a new approach for plant breeding, it has become possible to predict the performance of a hybrid through estimation of genomic estimated breeding values (GEBVs) involving all mapped markers in the crop of interest. However, prediction accuracy still remains an issue in GS, although significant progress has been made in improving it through continuous efforts for improvement in approaches used for developing one or more suitable training populations, validation schemes and prediction models. This approach has also been used in selection of parents for hybrids.
In maize, prediction models have already been proposed and used for a reliable estimate of the hybrid performance based on DNA-based markers (Bernardo 1994). It has been argued that with moderate-to-high prediction accuracies, genomic-based predictions may provide reliable estimates of hybrid performance from an unlimited cross at a low cost and in a short period (Basnet et al. 2018). During the last few years, studies on the prediction of hybrid performance for disease resistance, grain yield and other agronomic traits have been undertaken for hybrid wheat (Table 7).
Based on simulation study, Longin et al. (2015) argued that in cases with genomic prediction accuracies of > 0.5, GS approach allows maximum annual gains compared to other breeding methods. While dealing with quality traits (mostly controlled by small-effect QTL), prediction accuracy of 0.5 was achieved using RR-BLUP model, when one of the parents was common among the hybrids to be evaluated (Liu et al. 2016). Prediction accuracy using RR-BLUP reached 0.89–1.00, when both the parental lines were the same in several trials. In hybrid wheat also, RR-BLUP was shown to be the best of all the available prediction models and gave a prediction accuracy of 0.63, which is fairly high (Zhao et al. 2013). In another study, the prediction accuracy for hybrid wheat yield was highest (0.62–0.65) when both the male and female parents were included in the training set across different models and cross-validation schemes. It was also shown that the addition of pedigree data and g x e interaction to the prediction model significantly enhanced the prediction accuracy (Basnet et al. 2018).
The accuracy of genomic prediction for both heading time and plant height was low, when estimated using RR-BLUB and BayesCπ (Zhao et al. 2014). However, using weighted best linear unbiased prediction (W-BLUP), the prediction accuracy was improved. Using G-BLUP and involving parents and hybrids in the test set, a genomic prediction accuracy of 0.89 for grain yield was reported by Zhao et al. (2015). In another study, using the hybrids alone (not with parents) derived from a full diallel experiment (26 parental lines), the predictive ability for grain yield estimated using G-BLUM was high (0.52) for 80–90% hybrids using the remaining 10–20% hybrids as a training set (Baenziger et al. 2019).
The predictability for the trait with low heritability can be improved if indicator correlated traits that have higher heritability are included in the genomic prediction model. Multiple trait genomic prediction (MTGP) versus single-trait genomic prediction (STGP) models has been tested for prediction of Fusarium head blight resistance (FHBr) in wheat hybrids (Schulthess et al. 2018). In this study, plant height and heading date were used as indicator traits. High phenotypic and genotypic correlations along with improvement in predictabilities made plant height a better indicator than heading date for FHB severity. The availability of good indicator traits could reduce the impact of genotype x environmental interaction of STGP for hybrids less related to the estimations set. Thus, models and approaches have been developed where genomic prediction of the hybrid performance for even low heritability complex traits like grain yield could be made more accurately. In future, hopefully, further progress will be made to allow prediction of wheat hybrids with superior performance, which will reduce the hybrid production cost and will give higher genetic gain.
Hybrid seed production is a limitation: two new approaches for cost-effective hybrid seed production (blend hybrid and POWERPOLLEN)
A major limitation in the success of hybrid wheat is the economics of seed production, which has not been efficient and cost-effective (Whitford et al. 2013). This is mainly due to the self-pollinated nature of the crop, where outcrossing is restricted; seeding rate is another limitation. A hybrid variety program is generally successful in crops, where either the seed is not the commercial/marketable product, or else where only small quantity of seed is needed for raising the hybrid crop, as in case of vegetables and fruit crops. Economics suggests that relative to the level of heterosis, more important and decisive factors for the success of a hybrid crop like wheat are seeding rate and low cost of hybrid seed production (Pickett and Galvey 1997). In wheat, many approaches were tried to address this issue; of these approaches, ‘blend hybrids’ and ‘POWERPOLLEN’ seem to be two promising approaches, which will be described in brief.
Blend hybrids
Wilson (1997) proposed a ‘blend-hybrid’ strategy for low-cost production of hybrid seed in wheat. In this strategy, the commercial hybrid seed is a physical mixture of the seed obtained from a field having a small proportion of male parent (5–20%) and large proportion of male-sterile females (80–95%), since such a small proportion of male parent is sufficient to provide enough pollen for the large population of male-sterile female parent. In such a blend-planted hybrid seed production field, the pollen needs to travel a relatively small distance.
Male/female blend ratio
Wilson (1997) also observed that seed production with male–female blend ratio of 20:80 was significantly higher than the seed production with 50:50 strip plot schemes. However, no improvement in seed production efficiency was observed with further enhancement of male parent from 20 to 35 or 50%. Likewise, Koekemoer et al. (2011) reported an average increase of 46–76% in seed production under blend-production system compared to strip male/female ratio with 20:80–50:50 ratio. These results show that optimum blend of male and female parents is crucial to maximize the hybrid seed production with minimum cost. Another important aspect in this system is that the flowering nick between male and female parents should be perfectly optimized. Ideally, the female parent should flower 2–3 days earlier than the male. Also, the male parent should be an excellent pollen producer, and the female should be a highly efficient pollen receptor.
Blend hybrid and regulatory system
Although the ‘blend-hybrid’ concept was proposed during the late 1990s, apparently no commercial blend hybrids have so far been released. However, varietal blends are used in Nebraska, USA, so that in the year 2016, 2.4% area under wheat was covered by varietal blend (Baenziger et al. 2017). The failure to implement this technology has generally been attributed to strict regulatory provisions imposed by seed certification authorities in different countries. In most countries, seed blend having proportion of > 20% for the male parent is considered unacceptable by the regulatory system. This concern can be addressed by reducing proportion of male seed in the blend to 10% or lesser. In order to produce pure seed from the blend-production system, we can use male and females with different height profiles, the male being taller than the female, where the tall males are eliminated after flowering (Fig. 3). This approach will ensure that the F1 seed contains minimum proportion of seed from the male parent.
Blend-hybrid program in wheat may also require careful selection of Rht genes for dwarfing in the hybrid seed production (Würschum et al. 2017a, b). It was shown that although both Rht1 and Rht24 loci reduce plant height but Rht1 reduces anther extrusion and the cross-pollination ability, while Rht24 has no such adverse effect. This suggests that different Rht genes may be used in the female or male pool of a hybrid breeding program for efficient seed set. Introduction of a preferred gene like Rht24 may be facilitated using marker-assisted selection (MAS). Such a manipulation of dwarfing genes will further facilitate blend-hybrid approach.
Blend-hybrid approach also eliminates the cost incurred in the transport of large quantities of hybrid seed to long distances. In the blend-hybrid approach, the breeder or the seed company can provide or market the parental blend of seed to the local seed producers, including small seed companies and farmer groups. These companies can then produce hybrid seed at their own location by following the simple approach of blend-hybrid seed production and then use/sell blend-hybrid seed produced at their own location, thus reducing the cost of transport of large quantities of hybrid seed. A policy change having permission to use blend hybrid will certainly encourage hybrid seed researchers and institutions (both public and private) to deploy more resources and investment in hybrid wheat.
POWERPOLLEN technology
PowerPollenTM technology was developed by an AgTech Startup with the same name in Ankeny, Iowa, USA. The technology was proposed recently in maize, but can also be used for hybrid seed production in wheat (http://www.powerpollen.com/). In this approach, pollen is preserved for 8–10 months at an appropriate temperature; the preserved pollen is used in combination with advanced pollen handling automation such that preserved pollen is applied directly to the inflorescence of the female parent (silks in case of maize; spikes in case of wheat) to increase the yield and purity of hybrid seed. With this technology, environmental risks are reduced and producers can have a greater control over seed production (http://www.powerpollen.com/). In normal hybrid seed production, a breeder has to wait for the right stage of plants for pollen to shed. But in this technology, a breeder can use stored pollen and apply to female plants as soon as the female parent is ready to receive the pollen. Therefore, this approach enables much higher control over the entire pollination process, reduces labour cost needed for detasseling (although this will not apply in case of wheat) and also prevents pollen contamination (https://www.agupdate.com/agriview/news/crop/powerpollen-launched-to-boost-yields/article_65492f5b-c8dd-590c-89c8-4746c35bc1b9.html).
Based on the results of three seasons of testing conducted, PowerPollen was shown to provide an average increase in seed yields by more than 20% and reduction in contamination by ~ 50% (http://www.powerpollen.com/). According to another estimate in maize, PowerPollen technology was shown to reduce the cost of seed production by about $3250 per hectare as a result of increased yield and purity, and reduction of detasseling costs (https://powerpollen.com/). The ‘PowerPollen’ start-up is hopeful of utilizing the approach of PowerPollen™ in wheat and rice also.
Perspectives and conclusions
Hybrid wheat giving higher grain yield is certainly looked upon as a possible solution for any future problem of wheat shortage, which cannot be completely ruled out. However, only limited progress has been made in this direction, although as with rice, success with hybrid rye has been reported (Geiger and Miedaner 2009; Miedaner and Laidig 2019). However, to achieve greater success in the area of hybrid wheat, some of the following areas need attention. (1) Enhancement of seed production efficiency to make wheat seed business attractive and sustainable. The ‘blend-hybrid’ concept has the potential to make hybrid seed production more economical, which is currently 2–3 times more expensive than the seed of the pure lines (Akel et al. 2018b). Also, exploration and beneficial utilization of phenology and reproductive biology (anther extrusion, amount of pollen production, prolonged pollen viability, increased female receptivity, etc.) may allow higher seed set in female-sterile parent in the production block leading to reduced cost of hybrid seed. Visual anther extrusion involved in pollen dispersal outside the florets shows high correlation (r = 0.76; p < 0.001) with seed set on male-sterile female parent in wheat suggesting its importance in cheap hybrid seed production (Boeven et al. 2018). Baenziger et al. (2019) evaluated > 3000 wheat lines for anther extrusion and many good pollinators were identified. High genetic variability and quantitative inheritance (controlled mainly by small-effect QTLs) of visual anther extrusion suggest the possibility of its improvement through selection both in common wheat and in the durum wheat (Langer et al. 2014; Boeven et al. 2016b; Muqaddasi et al. 2017; Akel et al. 2018b). (2) The extent of heterosis in wheat (i.e. 15–25% over mid-parent, and 10–15% over commercial parent) is comparable to heterosis achieved in maize during early years (1920s–1940s) of hybrid maize breeding (Duvick 1997). This may be improved in future for developing high-yielding wheat hybrids through understanding of the diverse functional haplotypes and their association with heterosis and by development of heterotic groups. (This is being addressed by JC Rief, IPK, Gatersleben, Germany; M. Geyer, personal communication.) (3) Genomics-based prediction of single-cross performance can increase the genetic gains per unit time. Also, the recent simulation studies and quantitative genetic theory suggest that the reciprocal recurrent genomic selection may increase long-term selection gain of hybrid breeding by implementing a two-step selection strategy involving population improvement and hybrid development (Rembe et al. 2018). (4) Speed breeding may also help in rapid development of parental lines via backcrossing combined with molecular marker-based selection for key traits such as fertility restoration and enhanced cross-pollination. (5) Replacement of CHA by CMS/GMS or a transgenic system for reduction of seed production cost to enable screening of many testcrosses in many different environments (M. Geyer, personal communication). (6) Technologies like split-gene and genome editing may also provide attractive GMO/non-GMO solutions to the problems like oligogenic (rather than monogenic) fertility restoration. In fact, investments and efforts towards the development of novel/transgenic technologies for successful production of commercial hybrid wheat seed at a cheaper rate should help in making hybrid wheat varieties a commercial success. Therefore, in the next 10–20 years, newer and affordable hybrid varieties may become reality to augment wheat production and to give better returns to the farmers. A proactive public–private partnership and long-term investment and commitment will certainly help to make these expectations a reality.
Author contribution statement
PKG conceived, outlined, wrote the first draft of the review and edited it in several rounds of reading. HSB edited/revised the whole manuscript and wrote part of the review. GS and VG helped in finalizing the Figures, Tables and the References. BP suggested the inclusion of heterotic groups/patterns and seed production sections. AKJ and BRB read/edited the manuscript and wrote new approaches for cost effective seed production and parts of the future prospects.
References
Abdelkhalik AF, Shishido R, Nomura K, Ikehashi H (2005) QTL-based analysis of heterosis for grain shape traits and seedling characteristics in an indica–japonica hybrid in rice (Oryza sativa L.). Breed Sci 55:41–48
Adugna A, Nanda GS, Singh K, Bains NS (2004) A comparison of cytoplasmic and chemically-induced male sterility systems for hybrid seed production in wheat (Triticum aestivum). Euphytica 135:297–304
Aimin Z, Xiuling N, Dongchen L (2001) Advances of hybrid wheat breeding in China. Cereal Res Commun 9:343–350
Akel W, Thorwarth P, Mirdita V, Weissman EA, Liu G, Würschum T, Longin CFH (2018a) Can spelt wheat be used as heterotic group for hybrid wheat breeding? Theor Appl Genet 131(4):973–984
Akel W, Rapp M, Thorwarth Würschum T, Longin CFH (2018b) Hybrid durum wheat: heterosis of grain yield and quality traits and genetic architecture of anther extrusion. Theor Appl Genet. https://doi.org/10.1007/s00122-018-3248-6
Allen E, Gilbertson LA, Houmerd NM, Huarg S, Ivashuta SI, Robert JK (2007) Methods for producing hybrid seed. Patent WO/2007/047016 Monsanto Company, MO (US)
An G, Mitra A, Choi HK, Costa MA, An K, Thornburg RW, Ryan CA (1989) Functional analysis of the 3′ control region of the potato wound-inducible proteinase inhibitor II gene. Plant Cell 1:115–122
Baenziger PS, Rose D, Santra D, Xu L (2017) Improving small grains varieties for Nebraska. State breeding and quality evaluation report to the Nebraska Wheat Development, Utilization and Marketing Board, Nebraska, USA
Baenziger PS, Belamkar V, Easterly A, Garst N, Stoll H, Ibrahim AMH, Rudd JC, Adhikari A, Opena GB, Basent B, Longin F, Reif J, Sarazin Jean-Benoit, Poland J (2019) Developing the tools for hybrid wheat: American perspective. EWG breeding methods-workshop on hybrid wheat held on February 19, 2019 at University of Hoeinheim, Stuttgart, Germany
Bahl PN, Maan SS (1973) Chromosomal location of male fertility restoring genes in six lines of common wheat. Crop Sci 13:317–320
Basnet BR, Crossa J, Dreisigacker S, Perez-Rodriguez P, Manes Y, Singh RP, Camarillo-Castillo F, Murua M (2018) Hybrid wheat prediction using genomic, pedigree and environmental variables interaction models. Plant Gen. https://doi.org/10.3835/plantgenome2018.07.0051
Bernardo R (1994) Prediction of maize single-cross performance using RFLPs and information from related hybrids. Crop Sci 34:20–25
Bertin P, Gregoire D, Massart S, de Froidmont D (2001) Genetic diversity among European cultivated spelt revealed by microsatellites. Theor Appl Genet 102:148–156
Bertin P, Gregoire D, Massart S, de Froidmont D (2004) High level of genetic diversity among spelt germplasm revealed by microsatellite markers. Genome 47:1043–1052
Boeven PH, Longin CF, Wurschum T (2016a) A unified framework for hybrid breeding and establishment of heterotic groups in wheat. Theor Appl Genet 129:1231–1245
Boeven PHG, Longin CFH, Leiser WL, Kollers S, Ebmeyer E, Würschum T (2016b) Genetic architecture of male floral traits required for hybrid wheat breeding. Theor Appl Genet 129:2343–2357
Boeven PHG, Wurschum T, Rudloff J, Ebmeyer E, Longin CFH (2018) Hybrid seed set in wheat is a complex trait but can be improved indirectly by selection for male floral traits. Euphytica 214:110
Castillo A, Atienza SG, Martin AC (2014) Fertility of CMS wheat is restored by two Rf loci located on a recombined acrocentric chromosome. J Exp Bot 65:6667–6677
Changping Z (2013) Research and application of hybrid wheat in China. Eng Sci 11:19–21
Chu SH, Jiang W, Lee J, Chin JH, Koh HJ (2012) QTL analysis of heterosis for grain yield and yield related traits in indica-japonica crosses of rice (Oryza sativa L). Genes Genom 34:367–377
Cisar G, Cooper D (2002) Hybrid wheat. In: Curtis BC, Rajaram S, Macpherson HG (eds) Bread wheat: improvement and production. Food and Agriculture Organization of the United Nations, Rome, pp 157–174
Conner TW, Fabbri BJ, Huang J (2002) Methods for translational repression of gene expression in plants. Patent US0062499. Monsanto Company, MO (US)
Crow JF (1948) Alternative hypotheses of hybrid vigor. Genetics 33:477–487
Dotson SB, Lanahan MB, Smith AG, Kishore GM (1996) A phosphonate monoester hydrolase from Burkholderia caryophilli PG2982 is useful as a conditional lethal gene in plants. Plant J 10:383–392
Du HL, Maan SS, Hammond JJ (1991) Genetic analysis of male-fertility restoration in wheat. III: effects of neuploidy. Crop Sci 31:319–322
Duvick DN (1997) Heterosis: feeding people and protecting natural resources. In: Book of Abstracts. The Genetics and Exploitation of Heterosis in Crops. An International Symposium, Mexico City. 17–22 Aug 1997. CIMMYT, Mexico, DF, pp 6–9
East EM (1936) Heterosis. Genetics 21:375–397
Fischer S, Mohring J, Schön CC, Piepho H-P, Klein D, Schipprack W, Utz HF, Melchinger AE, Reif JC (2008) Trends in genetic variance components during 30 years of hybrid maize breeding at the University of Hohenheim. Plant Breed 127:446–451
Garcia AAF, Wang S, Melchinger AE, Zeng ZB (2008) Quantitative trait loci mapping and the genetic basis of heterosis in maize and rice. Genetics 180:1707–1724
Geiger HH, Miedaner T (2009) Rye breeding. In: Carena MJ (ed) Cereals. Springer, Berlin, pp 157–181
Geyer M, Bund A, Albrecht T, Hartl L, Mohler V (2016) Distribution of the fertility-restoring gene Rf3 in common and spelt wheat determined by an informative SNP marker. Mol Breed 36:167
Geyer M, Albrecht T, Hartl L, Mohlar V (2018) Exploring the genetics of fertility restoration controlled by Rf1 in common wheat (Triticum aestivum L.) using high-density linkage maps. Mol Genet Genom 293:451–462
Gowda M, Kling C, Würschum T, Reif JC (2010) Hybrid breeding in durum wheat: heterosis and combining ability. Crop Sci 50:2224–2230
Gowda M, Longin CFH, Lein V, Reif JC (2012) Relevance of specific versus general combining ability in winter wheat. Crop Sci 52:2494–2500
Gowda M, Zhao Y, Würschum T et al (2013) Relatedness severely impacts accuracy of marker assisted selection for disease resistance in hybrid wheat. Heredity 112:552–561
Guan RX, Liu DC, Zhang AM (2001) Genetic analysis and identification of RAPD markers of fertility restorer gene Rf6 for the T. timopheevii cytoplasmic male sterility of wheat. J Agric Biotechnol 9:159–162
Hawkes T, Pline-Srnic W, Dale R, Friend E, Hollinshead T, Howe P, Thompson P, Viner R, Greenland A (2011) D-glufosinate as a male sterility agent for hybrid seed production. Plant Biotech J 9:301–314
He ZH, Rajaram S, Huang GZ (1995) Wheat and wheat breeding in China: an overview. In: He ZH, Rajaram S, Xin ZY, Huang GZ (eds) 2001 A history of wheat breeding in China. CIMMYT, Mexico, DF, pp 1–14
Hermsen JGT (1965) Towards a more efficient utilization of genic male sterility in breeding hybrid barley and wheat. Euphytica 14:221–224
Hershey HP, Stoner TD (1991) Isolation and characterization of cDNA clones for RNA species induced by substituted benzenesulfonamides in corn. Plant Mol Biol 17:679–690
Hohn CE, Lukaszewski AJ (2016) Engineering the 1BS chromosome arm in wheat to remove the Rf multi locus restoring male fertility in cytoplasms of Aegilops kotschyi, Ae. uniaristata and Ae. mutica. Theor Appl Genet 129:1769–1774
Hull FH (1945) Recurrent selection for specific combining ability in corn. J Am Soc Agron 37:134–145
Jiang Y, Schmidt RH, Zhao Y, Reif JC (2017) A quantitative genetic framework highlights the role of epistatic effects for grain-yield heterosis in bread wheat. Nat Genet 49:1741–1746
Jinks JL, Jones RM (1958) Estimation of the components of heterosis. Genetics 43:223–234
Keil M, Sanches-Serrano J, Schell J, Willmitzer L (1986) Primary structure of a proteinase inhibitor II gene from potato. Nucleic Acids Res 14:5641–5650
Kempe K, Gils M (2011) Pollination control technologies for hybrid breeding. Mol Breed 27:417–437
Kempe K, Rubtsova M, Gils M (2014) Split-gene system for hybrid wheat seed production. Proc Natl Acad Sci 111:9097–9102
Kihara H, Tsunewaki K (1966) Basic studies on hybrid wheat breeding, carried out at the National Institute of Genetics. Seiken Ziho 18:55–63
Kihara H, Tsunewaki K (1967) Genetic principles applied to the breeding of crop plants. In: Brink RA (ed) Heritage from Mendel. University Wisconsin Press, Madison, pp 403–418
Koekemoer FP, Van Eeden E, Bonjean AP (2011) An overview of hybrid wheat production in South Africa and review of current worldwide wheat hybrid developments. In: Bonjean AP, Angus WJ, Van Ginkel M (eds) The world wheat book: a history of plant breeding. Lavoisier, Paris, pp 907–950
Koemel JE, Guenzi AC, Carver BF et al (2004) Hybrid and pure line hard winter wheat yield and stability. Crop Sci 44:107–113
Kojima T, Tsujimoto H, Ogihara Y (1997) High-resolution RFLP mapping of the fertility restoration (Rf3) gene against Triticum timopheevi cytoplasm located on chromosome 1BS of common wheat. Genes Genet Syst 72:353–359
Kriete G, Niehaus K, Perlick AM, Puhler A, Broer I (1996) Male sterility in transgenic tobacco plants induced by tapetum-specific deacetylation of the externally applied non-toxic compound N-acetyl-L-phosphinothricin. Plant J 9:809–818
Langer SM, Longin CFH, Würschum T (2014) Phenotypic evaluation of floral and flowering traits with relevance for hybrid breeding in wheat (Triticum aestivum L.). Plant Breed 133:433–441
Li XL, Liu LK, Hou N, Liu GQ, Liu CG (2005) SSR and SCAR markers linked to the fertility-restoring gene for a D2-type cytoplasmic male-sterile line in wheat. Plant Breed 124:413–415
Liable CA (1974) Chemical methods for pollen control. In: Proceedings of the 29th annual corn and sorghum research conference publication, vol 29, pp 174–182
Liu B, Deng J (1986) A dominant gene for male sterility in wheat. Plant Breed 97:204–209
Liu CG, Wu YW, Zhang CL, Ren SX, Zhang Y (1997) A preliminary study on the effects of Aegilops crassa cytoplasm on the characters of common wheat. J Genet Genom 24:241–247
Liu CG, Hou N, Liu GQ, Wu YW, Zhang CL, Zhang Y (2002) Studies on fertility genetic characters in D2-type CMS lines of common wheat. J Genet Genom 29:638–645
Liu CG, Hou N, Liu LK, Liu JC, Kang XS, Zhang AM (2006) A YA type cytoplasmic male sterile source in common wheat. Plant Breed 125:437–440
Liu G, Zhao Y, Gowda M, Longin CFH, Reif JC, Mette MF (2016) Predicting hybrid performance for quality traits through genomic-assisted approaches in Central European wheat. PLoS ONE 11:e0158635
Livers RW (1964) Fertility restoration and its inheritance in cytoplasmic male sterile wheat. Science 144:420
Longin CFH (2016) Future of wheat breeding is driven by hybrid wheat and efficient strategies for pre-breeding on quantitative traits. In: Zhang D (ed) Plant biotechnology and its applications, e-ISSN:2320-0189 and p-ISSN:2347-2380
Longin CF, Mühleisen J, Maurer HP, Zhang H, Gowda M, Reif JC (2012) Hybrid breeding in autogamous cereals. Theor Appl Genet 125:1087–1096
Longin CFH, Gowda M, Mühleisen J, Ebmeyer E, Kazman E, Schachschneider R, Schacht J, Kirchhoff M, Zhao Y, Reif JC (2013) Hybrid wheat: quantitative genetic parameters and consequences for the design of breeding programs. Theor Appl Genet 126:2791–2801
Longin CFH, Mi X, Wurschum T (2015) Genomic prediction in wheat: optimum allocation of test resources and comparison of breeding strategies for line and hybrid breeding. Thoer Appl Genet 128:12097–12306
Lucken KA (1987) Hybrid wheat. In: Heyne EG (ed) Wheat and wheat improvement. American Society of Agronomy, Madison, pp 444–452
Lukaszewski AJ (2017) Chromosomes 1BS and 1RS for control of male fertility in wheats and triticales with cytoplasms of Aegilops kotschyi, Ae. mutica and Ae. uniaristata. Theor Appl Genet 130:2521–2526
Ma ZQ, Sorrells ME (1995) Genetic analysis of fertility restoration in wheat using restriction fragment length polymorphisms. Crop Sci 35:1137–1143
Maan SS, McCracken EU (1968) Meiotic instability of common wheat strains derived from Triticum timopheevii zhuk crosses. Euphytica 17:445–450
Maan SS, Luchen KA, Bravo JM (1984) Genetic analyses of male-fertility restoration in wheat. I. Chromosomal location of Rf genes. Crop Sci 24:17–20
Mahajan V, Nagarjan S (1998) Opportunities in hybrid wheat-A review. PINSA B64:51–58
Mantle PG, Swan DJ (1995) Effect of male sterility on ergot disease spread in wheat. Plant Pathol 44:392–395
Mariani C, De Beuckeleer M, Truettner J, Leemans J, Goldberg RB (1990) Induction of male sterility in plants by a chimaeric ribonuclease gene. Nature 374:737–741
Mariani CV, Gossele V, de Beuckeleer M, de Block M, Goldberg RB, de Greef W, Leemans J (1992) A chimaeric ribonuclease-inhibitor gene restores fertility to male sterile plants. Nature 357:384–387
Martin AC, Atienza SG, Ramırez MC, Barro F, Martín A (2008) Male fertility restoration of wheat in Hordeum chilense cytoplasm is associated with 6HchS chromosome addition. Aust J Agri Res 59:206–213
Martin AC, Atienza SG, Ramírez MC, Barro F, Martín A (2010) Molecular and cytological characterization of an extra acrocentric chromosome that restores male fertility of wheat in the msH1 CMS system. Theor Appl Genet 121:1093–1101
Matuschke I, Qaim M (2006) Adoption and impact of hybrid wheat in India. Presented at ‘International Association of Agricultural Economists Conference’ held during August 12–18, 2006 at Gold Cost, Australia
McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers J, Morris C, Appels R, Xia XC (2013) Catalogue of Gene Symbols for Wheat. In: The 12th International Wheat Genet Symposium September 8–13, 2013 Yokohama, Japan, pp 1–31
McRae DH (1985) Advances in chemical hybridisation. Plant Breed Rev 3:169–191
Melchinger AE, Gumber RK (1998) Overview of heterosis and heterotic groups in agronomic crops. In: CSSA special publication: concepts and breeding of heterosis in crop plants, pp 29–44
Melchinger AE, Utz HF, Piepho HP, Zeng ZB, Schön CC (2007) The role of epistasis in the manifestation of heterosis: a systems-oriented approach. Genetics 177:1815–1825
Mette MF, Gils M, Longin CFH, Reif JC (2015) Hybrid breeding in wheat. In: Ogihara Y, Takumi S, Handa H (eds) Advances in wheat Genetics: from genome to field. Springer, Tokyo, pp 225–232
Miedaner T, Laidig F (2019). Hybrid breeding in rye (Secale cereale L.). In: Al-Khayri et al JM (eds) Advances in plant breeding strategies. Vol 5 Cereals and legumes. Springer, Switzerland, pp 1–31
Mühleisen J, Piepho H-P, Maurer HP, Longin CFH, Reif JC (2014) Yield stability of hybrids versus lines in wheat, barley, and triticale. Theor Appl Genet 127:309–316
Mukai Y, Tsunewaki K (1979) Basic studies on hybrid wheat breeding.VIII. A new male sterility-fertility restoration system in common wheat utilizing the cytoplasms of Aegilops kotschyi and Ae. variabilis. Theor Appl Genet 54:153–160
Müller T, Schierscher-Viret B, Fossati D, Branant C, Schori A, Keller B (2018) Unlocking the diversity of genebanks: whole-genome marker analysis of Swiss bread wheat and spelt. Theor Appl Genet 131:407–416
Muqaddasi QH, Brassac J, Börner A, Pillen K, Roder MS (2017) Genetic architecture of anther extrusion in spring and winter wheat. Front Plant Sci 8:754
Murai K (1992). Basic studies on hybrid wheat breeding utilizing Aegilops crassa cytoplasm. Ph.D. dissertation. Kyoto University, Japan
Murai K (2002) Comparison of two fertility restoration systems against photoperiod-sensitive cytoplasmic male sterility in wheat. Plant Breed 121:363–365
Murai K, Tsunewaki K (1993) Photoperiod-sensitive cytoplasmic male sterility in wheat with Aegilops crassa cytoplasm. Euphytica 67:41–48
Murai K, Takumo S, Koga H, Ogihara Y (2002) Pistillody, homeotic transformation of stamens into pistil-like structures, caused by nuclear–cytoplasm interaction in wheat. Plant J 29:169–181
Murai K, Ohta H, Kurushima M, Ishikawa N (2016) Photoperiod-sensitive cytoplasmic male sterile elite lines for hybrid wheat breeding, showing high cross-pollination fertility under long-day conditions. Euphytica 212:313–322
Ni F, Qi J, Hao Q et al (2017) Wheat Ms2 encodes for an orphan protein that confers male sterility in grass species. Nat Commun 8:15121
Nielsen NH, Backes G, Stougaard J, Andersen SU, Jahoor A (2014) Genetic diversity and population structure analysis of European hexaploid bread wheat (Triticum aestivum L.) varieties. PLoS ONE 9:e94000
Niu N, Zhang GS, Liu HW, Wang JW, Li HX (2003) Inheritance on restoration performance of non-1BL/1RS male sterile line of Nian type in wheat. Acta Botanica Boreali-Occidentalia Sinica 23:608–614
O’Keefe DP, Tepperman JM, Dean C, Leto KJ, Erbes DL, Odell JT (1994) Plant expression of a bacterial cytochrome P450 that catalyzes activation of a sulfonylurea pro-herbicide. Plant Physiol 105:473–482
Parodi PC, de los Angeles Gaju M (2009) Male sterility induced by the chemical hybridizing agent clofencet on wheat, Triticum aestivum and T. turgidum var. durum. Cien Inv Agr 36:267–276
Pickett AA (1993) Hybrid wheat: results and problems. Adv Plant Breed Suppl J Plant Breed 15:1–259
Pickett AA, Galvey NV (1997) A further evaluation of hybrid wheat. Plant Var Seeds 10:15–32
Powers L (1944) An expansion of Jones’s theory for the explanation of heterosis. Am Nat 78:275–280
Qian CM, Xu A, Uiang GH (1986) Effects of low temperatures and genotypes on pollen development in wheat. Crop Sci 26:43–46
Qu Z, Li L, Luo J, Wang P, Yu S et al (2012) Qtl mapping of combining ability and heterosis of agronomic traits in rice backcross recombinant inbred lines and hybrid crosses. PLoS ONE 7:e28463
Reif JC, Hallauer AR, Melchinger AE (2005) Heterosis and heterotic patterns in maize. Maydica 50:215–223
Reif JC, Gumpert F, Fischer S, Melchinger AE (2007) Impact of genetic divergence on additive and dominance variance in hybrid populations. Genetics 176:1931–1934
Rembe M, Zhao Y, Jian Y, Reif JC (2018) Reciprocal recurrent genomic selection: an attractive tool to leverage hybrid wheat breeding. Theor Appl Genet. https://doi.org/10.1007/s00122-018-3244-x
Richey FD (1942) Mock-dominance and hybrid vigor. Science 96:280–281
Robertson LD, Curtis BC (1967) Monosomic analysis of fertility restoration in common wheat (T. aestivum L.). Crop Sci 9:493–495
Ru Z-G, Zhang L-P, Hu T-Z, Liu H-Y, Yang Q-K, Weng M-L, Wang B, Zhao C-P (2015) Genetic analysis and chromosome mapping of a thermo-sensitive genic male sterile gene in wheat. Euphytica 201:321–327
Schmidt W, Johnson VA, Mann SS (1962) Hybrid wheat. Neb Agric Exp Stn Q 9:9
Schulthess AW, Zhao Y, Longin CFH, Reif JC (2018) Advantage and limitations of multiple-trait genomic prediction for Fusarium head blight severity in hybrid wheat (Triticum aestivum L.). Theor Appl Genet 131:685–701. https://doi.org/10.1007/s00122-017-3029-7
Siedler H, Messmer MM, Schachermayr GM, Winzeler H, Winzeler M, Keller B (1994) Genetic diversity in European wheat and spelt breeding material based on RFLP data. Theor Appl Genet 88:994–1003
Singh SK, Chatrath R, Misra B (2010) Perspective of hybrid wheat research: a review. Indian J Agric Sci 80:1013–1027
Singh SP, Srivastava R, Kumar J (2015) Male sterility systems in wheat and opportunities for hybrid wheat development. Acta Physiol Plant 37:1713
Sinha P, Tomar SM, Vinod Singh VK, Balyan HS (2013) Genetic analysis and molecular mapping of a new fertility restorer gene Rf8 for Triticum timopheevi cytoplasm in wheat (Triticum aestivum L.) using SSR markers. Genetica 141:431–441
Sun B, Zhang A, Bonjean AP (2001) Chinese wheat pool (in) the world wheat book. In: Bonjean AP, Angus WJ (eds) A history of wheat breeding. Lavoisier Publishing Inc, Paris, pp 667–701
Tahir CM, Tsunewaki K (1969) Monosomic analysis of Triticum spelta var duhameliamum, a fertility-restorer for T. timopheevi cytoplasm. Jpn J Genet 44:1–9
Tsunewaki K (2015) Fine mapping of the first multi-fertility-restoring gene, Rf multi, of wheat for three Aegilops plasmons, using 1BS-1RS recombinant lines. Theor Appl Genet 128:723–732
Tucker EJ, Baumann U, Kouidri A et al (2017) Molecular identification of the wheat male fertility gene Ms1 and its prospects for hybrid breeding. Nat Commun 8:869
Virmani SS, Edwards JB (1983) Current status and future prospects for breeding hybrid rice and wheat. Adv Agron 36:145–214
Whitford R, Fleury D, Reif JC, Garcia M, Okada T, Korzun V, Langridge P (2013) Hybrid breeding in wheat: technologies to improve hybrid wheat seed production. J Exp Bot 64:5411–5428
Wilson JA (1984) Hybrid wheat breeding and commercial seed development. Plant Breeding Rev 2:303–319
Wilson P (1997) Hybrid wheat development in Australia and a proposal for hybrid wheat blends for developing countries. In: Book of abstracts. The genetics and exploitation of heterosis in crops. An international symposium, Mexico City. 17-22 Aug. 1997. CIMMYT, Mexico, DF, pp 216–217
Wilson JA, Ross WM (1962) Male sterility interaction of the Triticum aestivum nucleus and Triticum timopheevi cytoplasm. Wheat Inf Serv 14:29–30
Würschum T, Boeven PHG, Langer SM, Longin CFH, Leiser WL (2015) Multiply to conquer: copy number variations at Ppd-B1 and Vrn-A1 facilitate global adaptation in wheat. BMC Genet 16:96
Würschum T, Leiser WL, Longin CFH (2017a) Molecular genetic characterization and association mapping in spelt wheat. Plant Breed 136:214–223
Würschum T, Leiser WL, Weissmann S, Maurer HP (2017b) Genetic architecture of male fertility restoration of Triticum timopheevii cytoplasm and fine-mapping of the major restorer locus Rf3 on chromosome 1B. Theor Appl Genet 130:1253–1266
Xia XC, Reif JC, Hoisington D, Melchinger AE, Frisch M, Warburton ML (2004) Genetic diversity among CIMMYT maize inbred lines investigated with SSR markers: I. Lowland tropical maize. Crop Sci 44:2230–2237
Xiao J, Li J, Yuan L, Tanksley SD (1995) Dominance is the major genetic basis of heterosis in rice as revealed by QTL analysis using molecular markers. Genetics 140:745–754
Xie F, He Z, Esguerra MQ, Qiu F, Ramanathan V (2014) Determination of heterotic groups for tropical Indica hybrid rice germplasm. Theor Appl Genet 127:407–417
Xin XY, Wang WX, Yang JS, Luo XJ (2011) genetic analysis of heterotic loci detected in a cross between indica and japonica rice (Oryza sativa L.). Breed Sci 61:380–388
Xing QH, Ru ZG, Zhou CJ, Liang FS, Yang DE, Jin DM, Wang B (2003) Genic analysis and molecular mapping of thermosensitive genic male sterile gene (wtms1) in wheat. Theor Appl Genet 107:1500–1504
Xiyue S, Peng F, Lingjian M, Yajun X, Shudong L, Beiru H (2002) A comparison of wheat CMS lines of Ae. kotschyi cytoplasm of no 1B/1R type and 1B/1R type. Acta Univ Agric Boreali-occidentalis 30:1–4
Yang L, Liu B, Zhai H, Wang S, Liu H et al (2009) Dwarf male-sterile wheat: a revolutionary breeding approach to wheat. In: Shu QY (ed) Induced plant mutations in the genomics era. Food and Agriculture Organization of the United Nations, Rome, pp 370–372
Yen FS, Evans LE, Larter EH (1969) Monosomic analysis of fertility restoration in three restorer lines of wheat. Can J Genet Cytol 11:531–546
Yuan Q, Zhiying D, Peng T, Tian J (2012) QTL-based analysis of heterosis for number of grains per spike in wheat using DH and immortalized F2 populations. Euphytica 188:387–395
Zehr B (2001) Emerging biovision: Hybrid wheat. Paper presented at MSSRF-FAO expert meeting. Chennai, June 25–28
Zhang GS (1992) Breeding of wheat male sterile line having Aegilops uniaristata cytoplasm. Chin SciBull 7:641–645
Zhao Y, Zeng J, Fernando R, Reif JC (2013) Genomic prediction of hybrid wheat performance. Crop Sci 53:802–810
Zhao Y, Mette MF, Gowda M, Longin CFH, Reif JC (2014) Bridging the gap between marker-assisted and genomic selection of heading time and plant height in hybrid wheat. Heredity 112:638–645
Zhao Y, Liu G, Maurer HP et al (2015) Genome-based establishment of a high-yielding heterotic pattern for hybrid wheat breeding. Proc Natl Acad Sci 112:15624–15629
Zhou W, Frederic LK, Leslie LD, Wang S (2005) SSR markers associated with fertility restoration genes against Triticum timopheevii cytoplasm in Triticum aestivum. Euphytica 141:33–40
Zhou KJ, Wang SH, Feng YQ, Liu ZX, Wang GX (2006) The 4E-ms system of producing hybrid wheat. Crop Sci 46:250–255
Zhuo-Kun L, Kuan-Gang X, Zhan-Ling Z, Jin-Liang L, Shu-Xiao H, Bin T, Qian-Quian Y, Ji-Chun T (2010) Analysis of plant height heterosis based on QTL mapping in wheat. Acta Agron Sin 36:771–778
Acknowledgements
This review had a long incubation period, during which the authors received necessary facilities from Head, Department of Genetics and Plant Breeding, CCS University (Meerut, India); HSB continued to hold the position of INSA Senior Scientist, starting in 2015; PKG was initially awarded INSA Senior Scientist position during 2015–2017 and later continued to hold the position of Hony Senior Scientist, starting in June 2017; VG was awarded the position of INSPIRE Faculty by DST [DST/INSPIRE/04/2017/000413] and SG worked as SRF in an ICAR-NASF Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Albrecht E. Melchinger.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
XYZ 4E-ms system for production of hybrid wheat. ms1 recessive male-sterile gene located on chromosome 4BS of wheat; fertility can be restored to the homozygous male-sterile mutant (Z line) through the action of Ms1 dominant fertility restorer gene on alien chromosome 4E of Agropyron elongatum ssp. ruthenicum Beldie (Reproduced with permission from Whitford et al. 2013) (TIFF 111 kb)
Supplementary Figure 2a
Pollination control systems based on conditional male sterility. (a) The induction of male sterility through conversion of a non-phytotoxic substance, i.e. protoxin (N-acetyl-L-phosphinothricin) into a phytotoxin (L-phosphinothricin) in the anthers catalysed by the action of the transgene argE from Escherichia coli (encodes N-acetyl-L-ornithine deacetylase) having exclusive expression in male reproductive tissue (T, tapetum-specific promoter) (JPEG 50 kb)
Supplementary Figure 2b
Pollination control systems based on conditional male sterility. (b) Conditional male sterility through herbicide treatment. A transgene-impairing herbicide tolerance (HerbR) is constitutively expressed (C, constitutive promoter). Its inactivation in male tissue results in the selective destruction of cells necessary for pollen development after herbicide treatment. Transgene inactivation is achieved by male-specific expression (MS, male-specific promoter) of a protein that binds to a 50 UTL operator region (OP) of the HerbR-mRNA. Alternatively, a micro-RNA (miRNA) that binds specifically to an exogenous recognition site (RS) of the HerbR-mRNA may lead to suppression of the protein in the male reproductive tissue (modified after Kempe and Gils 2011) (JPEG 64 kb)
Supplementary Figure 3
Dual-component barnase-barstar system (SeedLink hybridization system) for production of hybrid wheat. Tapetal cell-specific expression (Ta) of barnase (bar) induces male sterility. Herbicide resistance gene (HR) allows selection of male-sterile individuals by herbicide spray. Barstar inhibitor (barstar) inactivates barnase resulting in restored fertility (Reproduced with permission from Whitford et al. 2013) (TIFF 92 kb)
Supplementary Figure 4a
(a) Vector constructs used in split-barnase technology (based on T-DNA; only T-DNA part of the vector is shown here); A is the barnase expression vector called provector; B (pICH13130) is the phiC31-integrase expression vector; (C) in F1 plants obtained by crossing two parents (one with the provector and the other with phiC31); (D) assembly of active barnase cytotoxin via the intein-mediated trans-splicing of two translated precursor protein molecules, Bar-N and Bar-C (with HPTII, hygromycin phosphotransferase; IntN and IntC). For details, see text. LB and RB, T-DNA left and right borders; ocs, octopine synthase terminator; NLS, SV40 T antigen nuclear localization signal (amino acids PKKKRKV); nos, nopaline synthase terminator; ocs, octopine synthase terminator; phiC31, phage phiC31 recombinase coding sequence; PSK, intron PSK7-i3 from Petunia hybrida; Ptap, tapetum-specific promoter from rice; Pubi, maize ubiquitin 1 promoter; S, (GGGGS)3 flexible peptide linker; UBQ, intron UBQ10-i1 from Arabidopsis thaliana (TIFF 69 kb)
Supplementary Figure 4b
(b) Experimental design for the production of hybrid seed using split-barnase approach. Genotypes that have an expected male-sterile phenotype are shown in green. (A) shows development of male-sterile line; (B) represents the strategy for maintaining the male-sterile line, and (C) shows hybrid seed production (reproduced from Kempe et al. 2014) (TIFF 95 kb)
Supplementary Figure 5
Pioneer-DuPont Seed Production Technology (SPT) for production of hybrid wheat. ms45, male sterility inducing gene; Ms45, a dominant fertility restorer gene; SC, seed colour marker; SC allows the visual separation of transgenic maintainer seed from non-transgenic male-sterile seed; PGI is the pollen germination inhibitor. F1 hybrid is non-transgenic (Reproduced with permission from Whitford et al. 2013) (TIFF 104 kb)
Rights and permissions
About this article
Cite this article
Gupta, P.K., Balyan, H.S., Gahlaut, V. et al. Hybrid wheat: past, present and future. Theor Appl Genet 132, 2463–2483 (2019). https://doi.org/10.1007/s00122-019-03397-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-019-03397-y