Abstract
The present research was aimed at the synthesis and screening of 35 novel 2-(substituted phenyl imino)-5-benzylidene-4-thiazolidinones having different substitutions at imino phenyl and arylidene groups. The title compounds were synthesized by Knoevenagel condensation at the 5th position of the 4-thiazolidinone ring, in the presence of sodium acetate. The structures were assigned on the basis of spectral data. The compounds were screened for in vivo anti-inflammatory, antinociceptive and in vitro free-radical scavenging activities. The compounds exhibited significant activities when compared with standard drugs. The distinctive property of the derivatives was that none of them had an acidic group, like conventional NSAIDs, but exhibited significant in vivo activity in acute inflammation models. Further, the active compounds of each series were docked against cyclooxygeanase (COX)-2 enzyme using Glide module of Maestro 11.1 program. It was evident from the docking results that 3-chlorophenylimino and 2-chloro moiety on 5-benzylidene nucleus of the 4-thiazolidinone derivative (30) could easily fit into the COX-2-binding pocket, considered as critical interaction for COX-2 inhibition. Interestingly, some of the compounds exhibited the potential of becoming dual action or even triple action drug candidates, which could target degenerative disorders associated with excessive free-radical generation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An imbalance between cellular production of reactive oxygen species (ROS) and body’s antioxidant defence mechanism always leads to oxidative stress. ROS such as superoxide radical, peroxynitryl, hydroxyl radical, and hydrogen peroxide are constantly produced as a result of the metabolic reactions in living systems. Pathogenesis of a number of diseases has been reported to involve oxygen-derived free-radicals or ROS (Halliwell and Gutteridge 2015). In addition to playing a role in direct tissue damage, their generation may also amplify body’s general inflammatory response and promote further cell injury (Clarkson and Tremblay 1988). The role of free radicals in inflammatory process is well known (Flohe et al. 1985). A number of non-steroidal anti-inflammatory drugs have been reported to act either as inhibitors of free-radical production or as radical scavengers (Saldanha et al. 1990). Thus, compounds with antioxidant properties may offer protection in rheumatoid arthritis and inflammation and lead to potentially effective drugs. Treatment of various diseases could benefit from the use of drugs that combine antioxidant and antiinflammatory activity. This has already been proved for a number of commercially available non-steroidal anti-inflammatory drugs, which simultaneously possess radical scavenging properties (Orhan and Şahin 2001; Seth et al. 2014).
Substituted 4-thiazolidinones, in addition to being anti-inflammatory agents (Taranalli et al. 2008; Bhati and Kumar 2008) and free-radical scavengers (Shih and Ke 2004), also possess a series of other activities like aldose reductase inhibitory (Maccari et al. 2011), antidegenerative (Panico et al. 2011), antimicrobial (Vicini et al. 2006; Omar et al. 2010), and antihypertensive activity (Bhandari et al. 2009). Substitutions at various positions of this nucleus have been made and, in particular 2-imino-5-arylidene 4-thiazolidinones, have been reported to possess antidegenerative and antinflammatory activity (Ottana et al. 2005; Ottanà et al. 2007; Singh et al. 2015). 2,5-Disubstituted 4-thiazolidinones act as lipooxygenase and cyclooxygeanase (COX) inhibitors (Geronikaki et al. 2008; Chakraborti et al. 2010), with some authors reporting selective COX-2 inhibition (Ottana et al. 2005). Some of the synthesized compounds were evaluated for antimicrobial activity by our research group and proved to be potent antimicrobial agents (Chawla et al. 2012a, b). On the basis of these reports, it was decided to synthesize some 2,5-disubstituted 4-thiazolidinones and evaluate them for anti-inflammatory, antinociceptive, and free-radical scavenging activities.
Experimental section
General
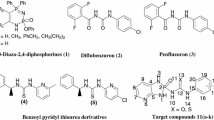
The synthetic route to obtain 2,5-disubstituted 4-thiazolidinone derivatives (Scheme 1) was through the chloroacetylation of substituted anilines (1a-e), using chloroacetyl chloride to form 2-chloro-N-(substituted phenyl) acetamide (2a-e), which upon heterocyclization with ammonium thiocyanate afforded 2-(substituted phenyl imino) thiazolidine-4-one (3a-e). Synthesis of 2-(substituted phenyl imino)-5-substituted arylidene-thiazolidine-4-ones (1–35) involved Knoevenagel reaction, and these compounds were obtained by condensation of (3a-e) with various aryl aldehydes in glacial acetic acid in the presence of sodium acetate (Vicini et al. 2008). The compounds were characterized using UV, IR, 1HNMR, 13C nuclear magnetic resonance (NMR), and high-resolution mass spectra (HRMS) analysis.
Starting material, reagents, and solvents were procured from Aldrich, Himedia, and SD Fine Chemicals and used as such. Melting points were determined by open capillary method and are uncorrected. The λmax values were recorded on a Shimadzu 1700 UV-Visible spectrophotometer. The infrared (IR) spectra were recorded on a Shimadzu 8400 S FTIR spectrophotometer by KBr pellet technique. 1HNMR and 13C NMR spectra were recorded on a Bruker Advance DRX-300 spectrophotometer, using tetramethyl silane as the internal standard and DMSO-d6 as the solvent. Chemical shift (δ) values are reported in ppm. Splitting patterns were designated as follows: s (singlet), d (doublet); m (multiplet). HRMS studies were performed on a JEOL-Accu TOF JMS-T100LC spectrometer. Progress of the reactions was monitored on precoated TLC silica gel-G plates and the spots were detected in an iodine chamber.
Synthesis of 2-chloro-N-(substituted phenyl) acetamide (2a-e)
Ice-cold chloroacetyl chloride (52 mL, 0.46 mol) was added drop-wise to substituted aniline (0.2 mol) (1a-e) with stirring, under anhydrous conditions. The reaction mixture was stirred at room temperature for 4 h. The semisolid residue was neutralized with sodium bicarbonate solution. The contents were filtered off and washed thoroughly with cold water. The crude mass was air dried and recrystallized from ethanol.
Synthesis of 2-(substituted phenyl imino) thiazolidin-4-one (3a-e)
A solution of 2-chloro-N-(substituted phenyl) acetamide (0.10 mol) and ammonium thiocyanate (15.22 g, 0.20 mol) was refluxed in ethanol for 5 h and allowed to stand overnight. The precipitate was filtered, washed with water and recrystallized from 1,4-dioxane (Omar et al. 2010).
2-(3-chloro-4-fluorophenylimino) thiazolidin-4-one (3a)
Brown powder (14.67 g, 60%); mp 212–215 °C (from dioxane); Rf value 0.72 (Ethyl acetate: Chloroform 9:1); IR (KBr) (v cm−1): 1701 (-C═O):, 3400 (-NH- stretch), 1641 (-NH- bend), 1618 (C═N), 1248(C–N), 2922 (C–H stretch (arom.), 860 (C–H bend (arom. para substituted), 650 and 800 (C–H bend (arom. meta substituted), 1438 (CH2), 1060 (Ar–Cl), 1191(Ar-F); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.02 (s, 1H, NH), 4.05 (s, 2H, CH2), 8.12 (d, 1H, J = 25.6 Hz, H-5), 7.45 (s, 1H, H-2), 7.10 (d, 1H, J = 5.5 Hz,H-6); 13C NMR (DMSO-d6, CDCl3): 167.2 (CONH, amide), 36.3 (CH2), 159.2 (C═N, imino), 142.2 (C–N), 113.6–116.9 (3CH, benzene), 120.1 (C–Cl, benzene), 150.2 (C–F, benzene); Mass: M+1 peak at 245.5.
2-(2-nitrophenylimino) thiazolidin-4-one (3b)
Bright orange crystals (17.07 g, 72%); mp 161–164 °C (dioxane); Rf value: 0.87 (Ethyl acetate: Chloroform 9:1); IR(KBr, cm−1) ν:1731(C═O), 3463 (NH st), 1593 (NH bend), 1257 (C–N), 1639 (C═N), 1342 (Ar–NO2 Sym), 1514 (Ar–NO2 Asym), 3001 (C–H arom.), 765 (C–H ortho arom.); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.54 (s, 1H, NH), 4.02 (s, 2H, CH2), 7.73–7.56 (m, 2H, H-4 and 5), 7.20 (d, 1H, J = 7.5 Hz, H-6); 13C NMR (DMSO-d6, CDCl3): 167.5 (CONH, amide), 36.5 (CH2), 150.4 (C═N, imino), 142.8 2 (C–N), 146.8 (C–NO2 benzene), 126.4–132.1(4CH, benzene; Mass: M++1 peak at 238.
2-(4-nitrophenylimino) thiazolidin-4-one (3c)
Yellowish orange crystals (17.74 g, 79%); mp 230–233 °C (dioxane); Rf value: 0.72 (Ethyl acetate: Chloroform 9:1); IR(KBr, cm−1) ν: 1677 (C═O), 3413 (NH str.), 1510 (NH bend), 1245 (C–N), 1643 (C═N), 1342 (Ar–NO2 Sym), 1413 (Ar–NO2 Asym), 3260 (C–H arom.), 746 (C–H para arom.); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.65 (s, 1H, NH), 4.09 (s, 2H, CH2), 7.54–7.48 (m, 2H, H-2 and 6), 7.95–7.86 (m, 2H, H-3 and 5); 13C NMR (DMSO-d6, CDCl3): 166.9 (CONH, amide), 36.2 (CH2), 155.8 (C═N, imino), 150.5 (C–N), 146.9 (C–NO2 benzene),121.5–125.4 (4CH, benzene); Mass: M+1 peak at 238.
2-(4-chlorophenylimino) thiazolidin-4-one (3d)
Reaction time 5 h; Rust colored powder (19.94 g, 88%); mp 200–203 °C (dioxane); Rf value: 0.81 (Ethyl acetate: Chloroform 9:1); IR(KBr, cm−1) ν: 1733 (C═O), 3413(NH str.), 1598(NH bend), 1257 (C–N); 1637 (C═N) 2921(C–H arom.), 862 (C–H para arom.); 1150 (Ar–Cl); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.08 (s, 1H, NH), 4.01 (s, 2H, CH2), 7.50–7.34 (m, 2H, H-2 and 6), 7.66–7.58 (m, 2H, H-3 and 5); 13C NMR (DMSO-d6, CDCl3 167.4 (CONH, amide), 36.4 (CH2), 150.5 (C═N, imino), 145.8 (C–N), 136.9 (C–Cl benzene), 124.2 (2H CH, benzene) 132.4 (2H CH, benzene); Mass: M+1 peak at 227.
2-(3-chlorophenylimino) thiazolidin-4-one (3e)
Dark brown powder (13.59 g, 60%); mp 201–208 °C (from dioxane); Rf value: 0.74 (Ethyl acetate:Chloroform 9:1); IR (KBr) (ν cm−1): -C═O: 1701; -NH- stretch 3409; -NH- bend 1573; -C–N 1247; C═N 1612; C–H stretch (arom.) 3062; CH2: 1446 Ar–Cl 1095; C–H bend meta (arom.) 775, 867; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.06, (s, 1H, NH), 4.01 (s, 2H, CH2), 7.61 (s, 1H, H-2), 7.65 (d, 1H, H-4), 7.08-6.99 (m, 1H, H-5), 7.23 (d, 1H, H-6); 13C NMR (DMSO-d6, CDCl3): 167.8 (CONH, amide), 36.3 (CH2), 150.4 (C═N, imino), 149.8 (C–N),136.2 (C–Cl benzene), 123.4–132.4 (4H CH, benzene); Mass: M+1 peak at 227.
Synthesis of 2-(substituted phenyl imino-5-substituted arylidene 4-thiazolidinones) (1–35)
The 2-(substituted phenyl imino) thiazolidin-4-one (3a-e) (0.004 mol) was stirred in 35 mL of acetic acid, in the presence of sodium acetate (0.008 mol). Various aryl aldehydes (0.006 mol) were then added and refluxed for different time periods using standard Knoevenagel reaction procedure. The progress of the reaction was monitored by TLC. The reaction mixture was cooled to room temperature and the precipitated solid was filtered, washed thoroughly with water and recrystallized from 1,4-dioxane (Omar et al. 2010).
2-(3-chloro-4-fluorophenylimino)-5-(benzylidene)-4-thiazolidinone (1)
Light brown crystals (843 mg, 62%); mp 208–210 °C (from dioxane); Rf value: 0.69 (Toluene: Ethanol, 8:2); λmax 278 nm; IR(KBr, cm−1) ν: -C═O 1674; -NH- stretch 3419; -NH bend 1550; -C–N 1244; C═N 1630; stretch (arom.) 3001; C═C 1633; Ar–Cl 1050, Ar-F 1164; C–H bend (arom. ortho) 758; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.3 (s, 1H, NH), 8.09 (d, 1H, J = 29.4 Hz, H-5), 7.85 (s, 1H, CH), 7.65 (d,2H, J = 12.3 Hz, H-3’and 5’), 7.29 (d, 1H, J = 5.1 Hz, H-4’), 7.15 (s, 1H, H-2), 7.07 (d, 2H, J = 3.1 Hz, H- 2’and 6’); 13C NMR (DMSO-d6, CDCl3): 168.4 (CONH, amide), 115.9 (C═C, ethylene), 145.2 (C═C),163.5 (C═N, imino), 126.6–129.5 (5CH, benzene), 133.6 (1C, benzene), 147.2 (C–N), 120.3–125.8 (3CH, benzene), 126.5 (C–Cl), 159.8 (C–F); HRMS calcd for C16H10ClFN2OS: 332.0186, found 333.0165; Anal.Calcd.: C, 58.87; H, 3.49; N, 8.08; Found: C, 58.77; H, 3.42; N, 8.01.
2-(3-chloro-4-fluorophenylimino)-5-(2-chlorobenzylidene)-4-thiazolidinone (2)
Off-white crystals (1.0 g, 67%); mp 195–200 °C (from dioxane); Rf value: 0.69 (Toluene: Ethanol, 8:2); λmax 274 nm; IR(KBr, cm−1) ν: C═O: 1703; -NH- stretch: 3253; -NH bend: 1587; C═N: 1616; -C–N: 1242; C–H stretch (arom.): 3053; C–H bend (arom. meta): 648, 759; C–H bend (arom. para): 819; C–H bend (arom. ortho): 750; C═C: 1643; Ar–Cl: 1100; Ar-F: 1242; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.358 (s, 1H, NH); 8.31 (d, 1H, J = 14.1, H-5; 7.8 (s, 1H, CH); 7.269 (t, 1H, J = 2.1, H-4’); 7.156 (d, 1H, J = 4.8, H-6’); 7.04 (m, 2H, J = 5.4, H-3’ and 5’); 6.962 (s, 1H, H-2); 13C NMR (DMSO-d6, CDCl3): 168.5 (CONH, amide), 115.7 (C═C, ethylene), 142.9 (C═C), 163.6 (C═N, imino), 132.8 (1C,benzene), 135.2 (C–Cl), 126.1–129.8 (4CH, benzene),146.3 (C–N), 118.6–122.8 (3H benzene), 124.8 (C–Cl), 158.9 (C–F); HRMS calcd for: C16H9Cl2FN2OS: 365.9797, found 366.9628; Anal.calcd.: C, 53.56; H, 2.91; N, 7.35; found: C, 53.45; H, 2.89; N, 7.38.
2-(3-chloro-4-fluorophenylimino)-5-(4-chlorobenzylidene)-4-thiazolidinone (3)
Light brown crystals (957 mg, 64%); mp 215–220 °C (from dioxane); Rf value: 0.71 (Toluene: Ethanol, 8:2); λmax 347 nm; IR (KBr, cm−1) ν: -C═O: 1690; -NH- stretch: 3137; -NH bend: 1587; -C–N: 1257; C–H stretch (arom.): 3053; C–H bend (arom. para): 879, 817; (arom. meta): 669, 786; C═C: 1654; Ar-F: 1257; Ar–Cl: 1100; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.1 (s, 1H, NH); 8.09 (d, 1H, J = 24, H-5); 7.74 (s, 1H, CH); 7.66 (d, 2H, J = 8.7, H-3’ and 5’); 7.47 (d, 2H, J = 9.3, H-2’ and 6’); 7.29 (d, 1H, J = 5.0, H-5); 7.15 (d, 1H, J = 3.9, H-6); 6.962 (s, 1H, H-2); 13C NMR (DMSO-d6, CDCl3): 168.4 (CONH, amide), 115.8 (C═C, ethylene), 142.5 (C═C), 163.2 (C═N, imino), 133.1 (1C,benzene), 133.6 (C–Cl), 128.2–129.5 (4H benzene), 145.3 (C–N), 118.3–122.8 (3H benzene), 124.3 (C–Cl), 158.4 (C–F); HRMS calcd for C16H9Cl2FN2OS: 365.9797, found 366.9652; Anal. calcd.: C, 53.56; H, 2.91; N, 7.35; found: C, 53.51; H, 2.87; N, 7.31
2-(3-chloro-4-fluorophenylimino)-5-(4-hydroxybenzylidene)-4-thiazolidinone (4)
Yellowish white crystals (981 mg, 69%); mp 210–213 °C (from dioxane); Rf value: 0.73 (Toluene: Ethanol, 8:2); λmax 267 nm; IR (KBr, cm−1) ν: -C═O: 1703; -NH- stretch: 3406; - NH- bend: 1585; -C–N: 1259; C–H stretch (arom.): 3055; C–H bend (arom. para): 819, 883; (arom. meta): 653, 744; Ar-F: 1191; C═C: 1643; Ar–Cl: 1056; Ar-OH: 3500; C–O: 1220; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.8 (s, 1H, NH); 8.0 (s, 1H, CH); 7.15 (d, 2H, J = 4.5, H-3’ and 5’); 6.95 (d, 2H, J = 3.6, H-2’ and 6’); 7.70 (m, 3H, H-2, 5 and 6); 13C NMR (DMSO-d6, CDCl3): 168.6 (CONH, amide), 115.6 (C═C, ethylene), 163.4 (C═N, imino), 142.5 (C═C), 116.8 (2H benzene), 131.2 (2H benzene), 128.8 (1C benzene), 158.7 (C–OH), 145.3 (C–N), 118.8–122.7 (3H benzene), 124.3 (C–Cl), 157.4 (C–F); HRMS calcd for C16H10ClFN2O2S: 348.0136, found 349.0015; Anal. calcd.: C, 56.28; H, 3.33; N, 7.72; found: C, 56.21; H, 3.29; N, 7.75.
2-(3-chloro-4-fluorophenylimino)-5-(2-nitrobenzylidene)-4-thiazolidinone (5)
Reaction time 5 h; Rust colored crystals (976 mg, 63%); mp 189–191 °C (from dioxane); Rf value: 0.66 (Toluene: Ethanol 8:2); λmax 267 nm; IR (KBr, cm−1) ν: -C═O: 1703; -NH- stretch: 3436; - NH- bend: 1585; C═N: 1614; -C–N: 1259; C–H stretch (aromatic): 3053; C–H bend (arom. ortho): 750; C–H bend (arom. meta): 651, 786; C–H bend (arom. para): 819; C═C: 1643, Ar–NO2: 1346(sym), 1504 (asym); Ar–Cl: 1056; Ar-F: 1191; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.9 (s, 1H, NH); 8.268 (s, 1H, CH); 8.234 (d, 1H, J = 4.2, H-5); 7.249 (d, 1H, J = 6.6, H-3’); 6.98 (m, 2H, J = 4.8, H-4’ and 5’); 7.149 (s, 1H, H-2); 8.18 (d, 1H, J = 5.1, H-6’); 8.05 (d, 1H, J = 3.9, H-6); 13C NMR (DMSO-d6, CDCl3): 168.4 (CONH, amide), 115.9 (C═C, ethylene), 163.4 (C═N, imino), 142.6 (C═C), 127.8 (2C benzene), 123.4 (1C benzene), 127.9 (1C benzene), 135.23 (1C benzene), 148.2 (C–NO2), 146.2 (C–N), 118.8–122.7 (3C benzene), 124.3 (C–Cl), 157.4 (C–F); HRMS calcd for C16H9ClFN3O3S: 377.7774, Found 377.9906; Anal. calcd.: C, 52.11; H, 2.83; N, 10.72; found: C, 52.13; H, 2.80; N, 10.74.
2-(3-chloro-4-fluorophenylimino)-5-(3-nitrobenzylidene)-4-thiazolidinone (6)
Light brown crystals (1.0 g, 63%); mp 240–245 °C (from dioxane); Rf value: 0.72 (Toluene: Ethanol, 8:2); λmax 339 nm; IR (KBr, cm−1) ν: -C═O: 1681; -NH- stretch: 3421; -NH- bend: 1637; C═N: 1600; -C–N: 1263; C═C: 1637; C–H stretch (arom.): 3045; C–H bend (arom. meta): 669, 750; C–H bend (arom. para): 819; Ar–NO2: 1350 (sym), 1498 (asym); Ar–Cl: 1056; Ar-F: 1184; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.4 (s, 1H, NH); 8.39 (d, 1H, J = 11.4, H-5); 8.24(d, 1H, J = 3.3, H-5’); 8.06 (d, 1H, J = 8.1, H-4’); 7.822 (d, 1H, J = 7.8, H-6); 7.822 (m, 2H, J = 14.7, H-2’ and 6’); 7.575 (s, 1H, CH); 13C NMR (DMSO-d6, CDCl3): 168.1 (CONH, amide), 116.2 (C═C, ethylene), 163.2 (C═N, imino), 142.1 (C═C), 122.7–123.1 (2C benzene), 130.2 (1C benzene), 136.5 (1C benzene), 135.8 (1C benzene), 147.4 (C–NO2), 146.1 (C–N), 118.5–122.4 (3C benzene), 124.3 (C–Cl), 157.1 (C–F); HRMS calcd for C16H9ClFN3O3S: 377.7774, found 378.0016; Anal. calcd.: C, 52.11; H, 2.83; N, 10.72; found: C, 52.14; H, 2.81; N, 10.70.
2-(3-chloro-4-fluorophenylimino)-5-(4-hydroxy-3-methoxybenzylidene)-4-thiazolidinone (7)
Mustard colored crystals (898 mg, 58%); mp 198–201 °C (from dioxane); Rf value: 0.77 (Toluene: Ethanol, 8:2); λmax 267 nm; IR (KBr, cm−1) ν: -C═O 1728, -NH- stretch 3251, -NH bend 1614, C═N 1585, -C–N 1259, C═C 1650; C–H stretch (arom.) 3040; Ar-OH 3452 (Broad band); C–H bend (arom. ortho) 765; C–H bend (arom. meta) 821 and 871, C–H bend (arom. para) 805; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.91 (s, 1H, NH), 7.78 (s, 1H, H- 2), 7.40-6.91 (m, 2H, H- 5 and 6), 7.781 (s, 1H, CH), 7.67 (s, H-2’), 7.58 (m, 2H, H = 5’ and 6’), 10.20 (s, OH); 13C NMR (DMSO-d6, CDCl3): 168.3 (CONH, amide), 116.4 (C═C, ethylene), 142.2 (C═C), 163.2 (C═N, imino), 111.2–116.9 (2C benzene), 130.1 (1C benzene), 123.5 (1C benzene), 147.5 (C–OH), 149.9 (1C benzene), 56.6 (OCH3), 146.3 (C–N), 119.8- 122.6 (3C benzene), 124.5 (C–Cl), 158.1 (C–F); HRMS calcd for C17H12ClFN2O3S: 378.0241, found 380.9928; Anal. calcd.: C, 55.03; H, 3.59; N, 7.13; found: C, 55.06; H, 3.62; N, 7.10.
2-(2-nitrophenylimino)-5-benzylidene-4-thiazolidinone (8)
Light brown powder (945 mg, 69%); mp 238–240 °C (dioxane); Rf value: 0.69 (Toluene: Ethanol, 8:2); λmax: 285 nm; IR(KBr, cm−1) ν: 1726 (C═O), 3423 (NH str.), 1593(NH bend), 1251(C–N), 1643 (C═N), 3009 (C–H str. arom.), 1633(C═C), 1344 (Ar–NO2 Sym), 1519 (Ar–NO2 Asym); 761, 326 (C–H arom. ortho); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.74 (s, 1H, NH), 8.04 (d, 1H, J = 8.1 Hz, H-3), 7.51(s, 1H, CH), 7.73–7.64 (m, 2H, H-4 and 5), 7.30 (d, 1H, J = 7.5 Hz, H-6), 7.30–7.18 (m, 2H, H-2’ and 6’), 7.44–7.31 (m, 2H, H-3’ and 5’), 7.39 (m, 1H, J = 7.2 Hz, H-4’); 13C NMR (DMSO-d6, CDCl3): 168.8 (CONH, amide), 116.6 (C═C, ethylene), 163.2 (C═N, imino), 142.6 (C═C), 136.5 (1C benzene), 127.7–129.2 (5C benzene), 142.3 (C–N), 145.9 (C–NO2), 125.5 (1C benzene), 128.6–131.3 (3C benzene); HRMS calcd for C16H11N3O3S: 325.3418, found 326.0326; Anal. calcd.: C, 60.17; H, 3.86; N, 12.38; found: C, 60.14; H, 3.85; N, 12.39.
2-(2-nitrophenylimino)-5-(2-chlorobenzylidene)-4-thiazolidinone (9)
Rust powder (986 mg, 65%); mp 242–247 °C (dioxane); Rf value: 0.69 (Toluene: Ethanol, 8:2); λmax: 331 nm; IR (KBr, cm−1) ν: 1720 (C═O); 3423 (NH str.), 1562 (NH bend), 1658(C═N), 1253(C–N), 1643(C═C), 1342 (Ar–NO2 Sym), 1517 (Ar–NO2 Asym); 1041 (Ar–Cl), 761, 779 (C–H arom. ortho); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.9 (s, 1H, NH), 8.04 (d, 1H, J = 8.1 Hz, H-3), 7.85 (s, 1H, CH), 7.72–7.60 (m, 2H, H-4 and 5), 7.97 (d, 1H, J = 7.8 Hz, H-6), 7.28 (d, 1H, J = 7.8 Hz, H-3’), 7.61–7.46 (m, 2H, H-4’ and 5’), 7.17 (d, 1H, J = 7.5 Hz, H-6’); 13C NMR (DMSO-d6, CDCl3): 168.5 (CONH, amide), 116.4 (C═C, ethylene), 163.3 (C═N, imino), 142.5 (C═C), 133.5 (1C benzene), 126.3–129.7 (4C benzene), 134.2 (C–Cl), 142.4 (C–N), 145.9 (C–NO2), 125.6–131.5 (4C benzene); HRMS calcd for C16H10ClN3O3S: 359.7869, found 360.2831; Anal. calcd.: C, 54.62; H, 3.24; N, 11.24; found: C, 54.64; H, 3.22; N, 11.22.
2-(2-nitrophenylimino)–5-(4-chlorobenzylidene)-4-thiazolidinone (10)
Buff colored powder (1.04 g, 69%); mp 205–210 °C (dioxane); Rf value: 0.85 (Toluene: Ethanol, 8:2); λmax: 285 nm; IR (KBr, cm−1) ν: 1722 (C═O); 3450 (NH str.); 1255 (C–N); 2997 (C–H arom.), 1643 (C═C), 1344 (Ar–NO2 Sym), 1519 (Ar–NO2 Asym); 1057 (Ar–Cl), 757(C–H ortho), 800 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.79 (s, 1H, NH), 8.04 (d, 1H, J = 8.1 Hz, H-3), 7.54 (s, 1H, CH), 7.97–7.67 (m, 2H, J = 7.5 Hz, H-4 and 5), 7.97 (d, 1H, J = 8.1 Hz, H-6), 7.40–7.38 (m, 2H, H-2’and 6’), 7.35–7.16 (m, 2H, H-3’ and 4’); 13C NMR (DMSO-d6, CDCl3): 168.6 (CONH, amide), 116.1 (C═C, ethylene), 163.4 (C═N, imino), 142.3 (C═C), 128.4–129.4 (4C benzene), 133.6 (1C benzene), 133.9 (C–Cl), 142.6 (V-N), 126.5–131.4 (4C benzene), 145.5 (C–NO2); HRMS calcd for C16H10ClN3O3S: 359.7869, found 360.0325; Anal. calcd.: C, 54.62; H, 3.24; N, 11.24; found: C, 54.64; H, 3.26; N, 11.27.
2-(2-nitrophenylimino)-5-(4-hydroxybenzylidene)-4-thiazolidinone (11)
Reaction time: 8.5 h; Brown powder (1.04 g, 72%); mp 210–215 °C (dioxane); Rf value: 0.67 (Toluene: Ethanol, 8:2); λmax: 280 nm; IR (KBr, cm−1) ν: 1703(C═O), 3338(NH str.), 1276(C–N), 1600(C═N), 3026(C–H arom.), 1656(C═C), 1342 (Ar–NO2 Sym), 1514 (Ar–NO2 Asym), 3326 (Ar-OH Broad), 765(C–H arom. ortho), 805 (C–H para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.34 (s, 1H, NH), 8.01 (d, 1H, H-3), 7.59 (s, 1H, CH), 7.81–7.67 (m, 3H, H-4, 5, 6), 7.37–7.19 (m, 2H, H-2’ and 6’), 7.17-6.85 (m, 2H, H-3’ and 5’), 10.18 (s, OH); 13C NMR (DMSO-d6, CDCl3): 168.3 (CONH, amide), 116.5 (C═C, ethylene), 163.1 (C═N, imino), 142.4 (C═C), 127.4 (1C benzene), 130.4 (2C benzene), 116.1 (2C benzene), 158.2 (C–OH), 142.3 (C–N), 145.1 (C–NO2), 125.3–128.2 (2C benzene), 130.2–131.2 (2C benzene); HRMS calcd for C16H11N3O4S: 341.3412, found 342.0561; Anal. calcd.: C, 57.46; H, 3.69; N, 11.82; found: C, 57.43; H, 3.67; N, 11.84.
2-(2-nitrophenylimino)-5-(2-nitrobenzylidene)-4-thiazolidinone (12)
Light brown powder (936 mg, 60%); mp 230–235 °C (dioxane); Rf value: 0.81(Toluene: Ethanol 8:2); λmax: 284 nm; IR (KBr, cm−1) ν: 1726 (C═O), 3415(NH str.), 1257(C–N), 1600(C═N), 3001 (C–H arom.), 1643(C═C), 1342 (Ar–NO2 Sym), 1514 (Ar–NO2 Asym), 763, 801 (C–H arom. ortho); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.88 (s, 1H, NH), 8.16 (d, 1H, J 8.1 Hz H-3), 8.15–7.95 (m, 2H, H-4 and 5), 8.01 (d, 1H, J = 8.1 Hz, H-6), 7.92 (s, 1H, CH), 7.25 (d, 1H, J = 7.8 Hz, H-3’), 7.71–7.61 (m, 2H, H-4’ and 5’), 7.79 (d, 1H, J = 7.5 Hz, H-6’); 13C NMR (DMSO-d6, CDCl3): 168.6 (CONH, amide), 116.2 (C═C, ethylene), 163.4 (C═N, imino), 142.3 (C═C), 127.5 (2C benzene), 123.4 (1C benzene), 128.9 (1C benzene), 134.9 (1C benzene), 147.9 (C–NO2), 142.8 (C–N), 147.3 (C–NO2), 126.3–131.2 (4C benzene); HRMS calcd for C16H10N4O5S: 370.3394, found 371.0268; Anal. calcd.: C, 53.12; H, 3.15; N, 14.58; found: C, 53.14; H, 3.11; N, 14.56.
2-(2-nitrophenylimino)-5-(3-nitrobenzylidene)-4-thiazolidinone (13)
Reaction time: 9 h; Light orange powder (982 mg, 63%); mp 230–235 °C (dioxane); Rf value: 0.71 (Toluene: Ethanol, 8:2); λmax: 284 nm; IR (KBr, cm−1) ν: 1726(C═O), 3452 (NH str.), 1253 (C–N), 1600(C═N), 3060 (C–H arom.), 1665(C═C), 1350 (Ar–NO2 Sym), 1519 (Ar–NO2 Asym), 745(C–H ortho), 795, 821 (C–H arom. meta); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.91 (s, 1H, NH), 8.24 (d, 1H, J = 7.8 Hz, H-3), 7.78–7.73 (m, 2H, H-4 and 5), 8.05 (d, 1H, J = 7.5 Hz, H-6), 7.88 (s, 1H, CH); 8.39 (s, 1H, H-2’), 7.93 (d, 1H, J = 7.5 Hz, H-4’), 7.42 (t, 1H, J = 7.8 Hz, H-5’), 7.32 (d, 1H, J = 7.5 Hz, H-6’); 13C NMR (DMSO-d6, CDCl3): 168.2 (CONH, amide), 116.6 (C═C, ethylene), 163.2 (C═N, imino), 142.6 (C═C), 136.5 (1C benzene), 134.2 (1C benzene), 122.9–123.9 (2C benzene), 134.9 (1C benzene), 147.4 (C–NO2), 142.3 (C–N), 145.5 (C–NO2), 125.3 (1C benzene), 128.2–131.2 (3C benzene); HRMS calcd for C16H10N4O5S: 370.3394, found 371.2654; Anal. calcd.: C, 53.12; H, 3.15; N, 14.58; found: C, 53.15; H, 3.13; N, 14.56.
2-(2-nitrophenylimino)-5-(4-hydroxy-3-methoxybenzylidene)-4-thiazolidinone (14)
Reaction time: 10 h; Mustard powder (985 mg, 63%); mp 245–250 °C (dioxane); Rf value: 0.73 (Toluene: Ethanol, 8:2); λmax: 283 nm; IR (KBr, cm−1) ν: 1726 (C═O), 3452 (NH str.), 1253(C–N), 1600(C═N), 3060 (C–H arom.), 1665 (C═C), 1342 (Ar–NO2 Sym), 1514 (Ar–NO2 Asym), 3452 (Ar-OH Broad band); 765 (C–H arom. ortho); 821, 871 (C–H arom. meta), 805 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.12 (s, 1H, NH), 7.83 (d, 1H, J = 3.9 Hz, H-3), 8.30–8.01(m, 3H, H-4, 5, and 6), 7.25(s, 1H, CH), 6.93 (s, 1H, H-2), 7.43–7.39 (m, 2H, H-5’ and 6’), 9.85 (s, OH), 4.009 (s, 3H, OCH3); 13C NMR (DMSO-d6, CDCl3): 168.4 (CONH, amide), 116.4 (C═C, ethylene), 163.5 (C═N, imino), 142.4 (C═C), 128.5 (1C benzene), 111.7 (1C benzene), 116.9 (1C benzene), 122.2 (1C benzene), 147.6 (C–OH), 149.4 (1C benzene), 56.4 (CH3), 142.4 (C–N), 145.3 (C–NO2), 125.7 (1C benzene), 128.6 (1C benzene), 131.3 (1C benzene), 130.5 (1C benzene); HRMS calcd for C17H13N3O5S: 371.3672, found 372.1055; Anal. calcd.: C, 56.10; H, 3.92; N, 10.90; found: C, 56.13; H, 3.90; N, 10.93.
2-(4-nitrophenylimino)-5-benzylidene-4-thiazolidinone (15)
Light brown (986 mg, 72%); mp 280–283 °C (dioxane); Rf value: 0.72 (Toluene: Ethanol, 8:2); λmax: 285 nm; IR(KBr, cm−1) ν: 1665 (C═O), 3269(NH str.), 1565(NH bend), 1244 (C–N), 1631(C═N), 3016 (C–H arom.), 1631 (C═C), 1342 (Ar–NO2 Sym), 1519 (Ar–NO2 Asym), 748 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz): (δ, ppm): 12.74 (s, 1H, NH), 7.40 (d, 1H, J = 8.1 Hz, H-2), 7.95 (d, 1H, J = 6.9 Hz, H-3), 7.45–7.40 (m, 2H, H-5 and 6), 7.51 (s, 1H, CH), 7.18–7.15 (m, 2H, H-2’ and 6’), 7.76–7.71 (m, 3H, H-4’, 3’ and 5’); 13C NMR (DMSO-d6, CDCl3): 168.6 (CONH, amide), 116.4 (C═C, ethylene),163.3 (C═N, imino), 142.4 (C═C), 135.5 (1C benzene), 128.9 (2C benzene), 127.4–128.9 (3C benzene), 154.3 (C–N), 146.2 (C–NO2), 123.6 (2C benzene), 125.3 (2C benzene); HRMS calcd for C16H11N3O3S: 325.3418, found 326.0695; Anal. calcd.: C, 60.83; H, 4.82; N, 11.82; found: C, 60.85; H, 4.80; N, 11.85.
2-(4-nitrophenylimino)-5-(2-chlorobenzylidene)-4-thiazolidinone (16)
Reaction time: 7 h; Rust powder (986 mg, 65%); mp 250–255 °C (dioxane); Rf value: 0.69 (Toluene: Ethanol, 8:2); λmax: 255 nm; IR (KBr, cm−1) ν: 1665(C═O), 3270(NH str.), 1244(C–N), 1631(C═N), 3014 (C–H arom.), 1631 (C═C), 1342 (Ar–NO2 Sym), 1517 (Ar–NO2 Asym), 1114 (Ar–Cl), 748 (C–H arom. ortho), 794 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.90, (s, 1H, NH), 7.46, (s, 1H, H-2), 8.04 (d, 1H, J = 8.1 Hz, H-3), 7.72–7.96 (m, 2H, H-5 and 6), 7.86(s, 1H, CH), 7.71–7.29 (d, 2H, H-3’ and 6’), 7.43 (t, 2H, J = 9.6 Hz, H-4’ and 5’); 13C NMR (DMSO-d6, CDCl3): 168.4 (CONH, amide), 116.3 (C═C, ethylene), 163.4 (C═N, imino), 142.1 (C═C), 133.5 (1C benzene), 134.9 (C–Cl), 129.1–129.7 (2C benzene), 126.6 (1C benzene), 127.2 (1C benzene), 154.9 (C–N), 123.5 (2C benzene), 125.6 (2C benzene), 146.8 (C–NO2); HRMS calcd for C16H10ClN3O3S: 359.7869, found 360.5326; Anal. calcd.: C, 54.62; H, 3.24; N, 11.24; found: C, 54.64; H, 3.20; N, 11.22.
2-(4-nitrophenylimino)-5-(4-chlorobenzylidene)-4-thiazolidinone (17)
Reaction time: 8 h; Light yellow (1.05 g, 69%); mp 245–250 °C (dioxane); Rf value: 0.85 (Toluene: Ethanol, 8:2); λmax: 254 nm; IR (KBr, cm−1) ν: 1665(C═O), 3274(NH str.), 1244 (C–N), 1565 (C═N), 3018(C–H arom.), 1643 (C═C), 1344 (Ar–NO2 Sym), 1517 (Ar–NO2 Asym), 1114 (Ar–Cl); 817, 854 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.79 (s, 1H, NH), 7.71 (s, 1H, H-2), 8.04 (d, 1H, J = 8.1 Hz, H-3), 7.40 (t, 1H, J = 7.5 Hz, H-5), 7.30 (d, 1H, J = 7.8 Hz, H-6), 7.54 (s, 1H, CH), 7.31–7.16 (m, 2H, H-2’ and 6’), 7.96–7.71 (d, 2H, H-3’ and 5’); 13C NMR (DMSO-d6, CDCl3): 168.3 (CONH, amide), 116.1 (C═C, ethylene), 163.2 (C═N, imino), 142.5 (C═C), 133.6 (1C benzene), 129.3 (2C benzene), 128.4 (2C benzene), 133.9 (C–Cl), 155.5 (C–N), 123.3 (2C benzene), 125.3 (2C benzene), 146.5 (C–NO2); HRMS calcd for C16H10ClN3O3S: 359.7869, found 360.0356; Anal. calcd.: C, 54.62; H, 3.24; N, 11.24; found: C, 54.64; H, 3.22; N, 11.21.
2-(4-nitrophenylimino)–5-(4-hydroxybenzylidene)-4-thiazolidinone (18)
Reaction time: 8 h; Light yellow powder (1.03 g, 72%); mp > 320 °C (dioxane); Rf value: 0.70 (Toluene: Ethanol, 8:2); λmax: 254 nm; IR (KBr, cm−1) ν: 1665(C═O), 3272 (NH str.), 1244(C–N), 1565 (C═N), 3014 (C–H arom.), 1631(C═C), 1340 (Ar–NO2 Sym), 1517 (Ar–NO2 Asym), 3413 (Ar-OH Broad band), 794, 856 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.34 (s, 1H, NH), 7.38 (s, 1H, H-2), 8.03–7.77 (m, 3H, H-3,5,6), 7.60(s, 1H, CH), 7.38–7.17 (m, 2H, H-2’ and 6’), 7.71–7.59 (m, 2H, H-3’ and 5’), 10.18 (s, OH); 13C NMR (DMSO-d6, CDCl3): 168.3 (CONH, amide), 116.1 (C═C, ethylene), 163.2 (C═N, imino), 142.4 (C═C), 127.5 (1C benzene), 130.9 (2C benzene), 116.1, 116.1 (2C benzene), 157.2 (C–OH), 155.3 (C–N), 123.5 (2C benzene), 125.4 (2C benzene), 146.6 (C–NO2); HRMS calcd for C16H11N3O4S: 341.3412, found 342.0532; Anal. calcd.: C, 57.46; H, 3.69; N, 11.82; found: C, 57.44; H, 3.67; N, 11.80.
2-(4-nitrophenylimino)-5-(2-nitrobenzylidene)-4-thiazolidinone (19)
Reaction time: 9 h; Light brown (936 mg, 60%); mp 250–252 °C (dioxane); Rf value: 0.76 (Toluene: Ethanol, 8:2); λmax: 255 nm; IR (KBr, cm−1) ν: 1677 (C═O); 3278(NH- str.), 1244 (C–N), 1599 (C═N), 2983 (C–H arom.), 1643 (C═C), 1385(Ar–NO2 Sym), 1510 (Ar–NO2 Asym), 745 (C–H arom. ortho), 800 (C–H para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.88 (s,1H, NH), 7.96 (s, 1H, H-2), 8.16 (d, 1H, J = 8.1 Hz, H-3), 7.80 (t, 1H, J = 7.2 Hz, H-5), 7.26 (d, 1H, J = 7.8 Hz, H-6), 7.92 (s, 1H, CH), 8.01 (d, 1H, J = 8.01 Hz, H-3’), 7.80 (t, 1H, J = 7.2 Hz, H-4’), 7.69 (t, 1H, J = 7.8 Hz, H-5’), 7.26 (d, 1H, J = 7.8 Hz, H-6’); 13C NMR (DMSO-d6, CDCl3): 168.1 (CONH, amide), 116.4 (C═C, ethylene), 163.1 (C═N, imino), 142.6 (C═C), 127.5 (2C benzene), 123.7 (1C benzene), 128.1 (1C benzene), 134.6 (1C benzene), 147.9 (C–NO2), 155.4 (C–N), 123.3 (2C benzene),, 125.4 (2C benzene), 146.6 (C–NO2); HRMS calcd for C16H10N4O5S: 370.3394, found 371.0649; Anal. calcd.: C, 53.12; H, 3.15; N, 14.58; found: C, 53.14; H, 3.13; N, 14.60.
2-(4-nitrophenylimino)-5-(3-nitrobenzylidene)-4-thiazolidinone (20)
Reaction time: 9 h; Light brown (982 mg, 63%); mp > 310 °C (dioxane); Rf value: 0.80 (Toluene: Ethanol, 8:2); λmax: 255 nm; IR (KBr, cm−1) ν: 1677 (C═O); 3278 (NH str.), 1242 (C–N), 1596 (C═N), 2945 (C–H arom.), 1643(C═C), 1344 (Ar–NO2 Sym), 1514 (Ar–NO2 Asym), 748 (C–H arom. para), 808, 845 (C–H arom. meta); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.91 (s, 1H, NH), 8.39 (s, 1H, H-2), 8.24 (d, 1H, J = 7.8 Hz, H-3), 7.75 (t, 1H, J = 6.6 Hz, H-5), 7.32 (d, 1H, J = 7.5 Hz, H-6), 7.88 (s, 1H, CH), 8.39 (s, 1H, H-2’), 8.05 (d, 1H, J = 7.5 Hz, H-4’), 7.42 (t, 1H, J = 7.8 Hz, H-5’), 7.93 (d, 1H, J = 7.5 Hz, H-6’); 13C NMR (DMSO-d6, CDCl3): 168.7 (CONH, amide), 116.1 (C═C, ethylene), 163.6 (C═N, imino), 142.5 (C═C), 136.5 (1C benzene), 122.9 (1C benzene), 123.4 (1C benzene),, 129.6 (1C benzene), 134.2 (1C benzene), 147.7 (C–NO2), 155.4 (C–N), 123.3 (2C benzene),, 125.1 (2C benzene),, 146.6 (C–NO2); HRMS calcd for C16H10N4O5S: 370.3394, found 371.3654; Anal. calcd.: C, 53.12; H, 3.15; N, 14.58; found: C, 53.10; H, 3.12; N, 14.57.
2-(4-nitrophenylimino)-5-(4-hydroxy-3-methoxybenzylidene)-4-thiazolidinone (21)
Mustard powder (985 mg, 63%); mp 270–275 °C (dioxane); Rf value: 0.78 (Toluene: Ethanol, 8:2); λmax: 254 nm; IR (KBr, cm−1) ν: 1665 (C═O), 3271 (NH str.), 1244 (C–N), 1565 (C═N), 3016 (C–H arom.), 1631 (C═C), 1340 (Ar–NO2 Sym), 1517 (Ar–NO2 Asym), 3423 (Ar-OH Broad band), 745 (C–H arom. ortho), 856, 871 (C–H arom. meta), 795 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 12.00 (s, 1H, NH), 7.48 (s, 1H, H-2), 7.40–6.91(m, 3H, H-3, 5 and 6), 7.82 (s, 1H,H, CH), 7.67 (s, 1H, H-2’), 7.58 (m, 2H, H = 5’ and 6’), 9.85 (s, OH), 4.066 (s, 3H, OCH3); 13C NMR (DMSO-d6, CDCl3): 168.1 (CONH, amide), 116.2 (C═C, ethylene), 163.2 (C═N, imino), 142.4 (C═C), 128.5 (1C benzene), 111.6 (1C benzene), 116.2 (1C benzene), 123.2 (1C benzene), 149.7 (1C benzene), 147.1 (C–OH), 56.3 (OCH3), 155.3 (C–N), 123.3(2C benzene), 125.1 (2C benzene), 146.2 (C–NO2); HRMS calcd for C17H13N3O5S: 371.3672, found 372.3265; Anal. calcd.: C, 56.10; H, 3.92; N, 10.90; found: C, 56.12; H, 3.94; N, 10.93.
2-(4-chlorophenylimino)-5-benzylidene-4-thiazolidinone (22)
Buff powder (958 mg, 69%); mp 340–342 °C (dioxane), Rf value: 0.72 (Toluene: Ethanol 8:2), λmax: 346 nm, IR(KBr, cm−1) ν: 1674 (C═O), 3413 (NH str.), 1564 (NH bend), 1245 (C–N), 1564 (C═N), 2963 (C–H arom.), 1639 (C═C), 1087 (Ar–Cl), 761 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.31 (s, 1H, NH), 7.06–7.51 (m, 4H, H-2,3,5,6), 6.57 (s, 1H, CH), 7.57–7.65 (m, 2H, H-2’,6’), 7.65–8.01 (m, 3H, H = 3’,4’,5’); 13C NMR (DMSO-d6, CDCl3): 168.6 (CONH, amide), 116.3 (C═C, ethylene), 165.2 (C═N, imino), 142.5 (C═C), 136.8 (1C benzene), 128.8 (2C benzene), 128.7 (2C benzene), 127.1 (1C benzene), 147.8 (C–N), 122.6 (2C benzene), 130.3 (1C benzene), 132.3 (C–Cl); HRMS calcd for C16H11ClN2OS: 314.7893, found 315.0595; Anal. calcd.: C, 62.69; H, 4.97; N, 8.12; found: C, 62.67; H, 4.98; N, 8.14.
2-(4-chlorophenylimino)-5-(2-chlorobenzylidene)-4-thiazolidinone (23)
Off-white powder (1.10 g, 72%); mp 310–312 °C (dioxane), Rf value: 0.69 (Toluene: Ethanol 8:2), λmax: 276 nm, IR(KBr, cm−1) ν: 1724 (C═O), 3413 (NH str.), 1490 (NH bend), 1245 (C–N), 1606 (C═N), 2908 (C–H arom.), 1649 (C═C), 1091 (Ar–Cl), 756 (C–H arom. ortho), 825 (C–H arom. para); 1HNMR(DMSO-d6, 300 MHz) (δ, ppm): 11.784 (s, 1H, NH), 7.628–7.593 (m, 2H, H-2 & 6), 6.98–7.07 (m, 2H, H-3,5), 7.92 (s, 1H, CH), 7.57 (d, 1H, J = 9.3 H-3’), 7.44–7.60 (m, 2H, H = 4’,5’), 7.546 (d, 1H, J = 9.3, H-6’); 13C NMR (DMSO-d6, CDCl3): 168.2 (CONH, amide), 116.1 (C═C, ethylene), 163.5 (C═N), 142.2 (C═C), 133.4 (1C benzene), 134.4 (1C benzene), 129.8 (1C benzene), 129.1 (1C benzene), 126.7 (1C benzene), 127.9 1C benzene), 147.4 (C–N), 122.4 (2C benzene), 130.7 (2C benzene), 132.8 (C–Cl); HRMS calcd for C16H10Cl2N2OS: 349.2344, found 349.0219; Anal. calcd.: C, 56.21; H, 3.33; N, 7.71; found: C, 56.23; H, 3.31; N, 7.73.
2-(4-chlorophenylimino)-5-(4-chlorobenzylidene)-4-thiazolidinone (24)
Reaction time: 5 h; Off-white crystals (1.05 g, 68%); mp 320–325 °C (dioxane), Rf value: 0.90 (Toluene: Ethanol 8:2), λmax: 263 nm, IR(KBr, cm−1) ν: 1722 (C═O), 3413 (NH str.), 1505 (NH bend), 1247 (C–N), 1605 (C═N), 3051 (C–H arom.), 1649 (C═C), 1091 (Ar–Cl), 732 (C–H arom. ortho), 821 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 10.908 (s, 1H, NH), 7.05–7.46 (m, 4H, H-2,3,5,6), 7.63 (s, 1H, CH), 7.54–7.78 (m, 4H, H-2’,3’,5’,6’); 13C NMR (DMSO-d6, CDCl3): 168.7 (CONH, amide), 116.4 (C═C, ethylene), 163.1 (C═N), 142.3 (C═C), 133.4 (1C benzene), 129.8 (2C benzene), 128.1 (2C benzene), 133.9 (C–Cl), 147.2 (C–N), 122.3 (2C benzene), 130.4 (2C benzene), 133.3 (C–Cl); HRMS calcd for C16H10Cl2N2OS: 349.2344, found 349.0219; Anal. calcd.: C, 56.21; H, 3.33; N, 7.71; found: C, 56.23; H, 3.31; N, 7.74.
2-(4-chlorophenylimino)-5-(4-hydroxybenzylidene)-4-thiazolidinone (25)
Brown powder (1.09 g, 75%); mp 235–240 °C (dioxane), Rf value: 0.71 (Toluene: Ethanol 8:2), λmax = 269 nm, IR(KBr, cm−1) ν: -C═O 1701; -NH- stretch 3421; -NH- bend 1505; -C–N 1255; C═N 1575; C–H stretch (aromatic) 2979; C═C 1639; Ar–Cl 1080; Ar-OH 3421 (Broad band); C–H bend (arom. para) 740 and 821; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.268 (s, 1H, NH); 6.85–7.07 (m, 4H, H-2,3,5,6); 7.897 (s, 1H, CH); 7.23–7.43 (m, 2H, H-2’,6’); 7.730 (d, 2H, J 6.3, H = 3’ and 5’); 10.288 (s, OH); 13C NMR (DMSO-d6, CDCl3): 168.4 (CONH, amide), 116.9 (C═C, ethylene), 163.5 (C═N), 142.1 (C═C), 127.9 (1C benzene), 130.9 (2C benzene), 117.1 (2C benzene), 157.9 (C–OH), 147.8 (C–N), 122.6 (2C benzene), 130.2 (2C benzene), 132.4 (C–Cl); HRMS calcd for C16H11ClN2O2S: 330.7887, found 331.0555; Anal. calcd.: C, 59.21; H, 3.80; N, 8.12; found: C, 59.19; H, 3.81; N, 8.11.
2-(4-chlorophenylimino)-5-(2-nitrobenzylidene)-4-thiazolidinone (26)
Rust crystals, (1.05 g, 66%); mp 218–220 °C (dioxane), Rf value: 0.79 (Toluene: Ethanol 8:2), λmax: 278 nm, IR (KBr, cm−1) ν: 1678(C═O), 3255 (NH str.), 1500 (NH bend), 1238 (C–N), 1550 (C═N), 2983 (C–H arom.), 1618 (C═C), 1083(Ar–Cl), 1338 (Ar–NO2 Sym), 1517 (Ar–NO2 Asym), 756 (C–H arom. ortho), 821 (C–H arom. para);1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.272 (s, 1H, NH), 7.31–7.52 (m, 4H, H-2,3,5,6), 7.92 (s, 1H, CH), 7.79 (d, 1H, J = 7.5 Hz, H-3’), 7.76–8.05(m, 2H, H = 4’ and 5’), 7.511 (d, 1H, J = 8.4 Hz, H-6’); 13C NMR (DMSO-d6, CDCl3): 168.2 (CONH, amide), 116.4 (C═C, ethylene), 163.3 (C═N), 142.5 (C═C), 127.4 (2C benzene), 134.1 (1C benzene), 128.6 (1C benzene), 123.8 (1C benzene), 147.9 (C–NO2), 147.6 (C–N), 122.8 (2C benzene), 130.6 (2C benzene), 132.9 (C–Cl); HRMS calcd for C16H11ClN2O2S: 359.7869, found 360.0568; Anal. calcd.: C, 54.62; H, 3.24; N, 11.24; found: C, 54.60; H, 3.22; N, 11.21.
2-(4-chlorophenylimino)-5-(3-nitrobenzylidene)-4-thiazolidinone (27)
Buff powder (984 mg, 62%); mp 280–285 °C (dioxane), Rf value: 0.84 (Toluene: Ethanol 8:2), λmax: 343 nm, IR (KBr, cm−1) ν: 1681 (C═O), 3263 (NH str.), 1500 (NH bend), 1238 (C–N), 1512 (C═N), 2975 (C–H arom.), 1637 (C═C), 1091 (Ar–Cl), 1349 (Ar–NO2 Sym), 1512 (Ar–NO2 Asym), 821 (C–H arom. meta), 745 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 10.99 (s, 1H, NH), 7.07–7.51 (m, 2H, H-2,3,5,6), 8.36 (s, 1H, CH), 8.434 (s, 1H, H-2’), 8.07 (d, 1H, J = 7.5 Hz, H-4’), 7.57–7.84 (m, H-5’,6’); 13C NMR (DMSO-d6, CDCl3): 168.2 (CONH, amide), 116.4 (C═C, ethylene), 163.1 (C═N), 142.6 (C═C), 136.9 (1C benzene), 122.9 (1C benzene), 129.1 (1C benzene), 134.9 (1C benzene), 147.9 (C–NO2), 123.4 (1C benzene), 147.8 (C–N), 122.3 (2C benzene), 130.2 (2C benzene), 133.4 (C–Cl); HRMS calcd for C16H11ClN2O2S: 359.7869, found 360.0568; Anal. calcd.: C, 54.62; H, 3.24; N, 11.24; found: C, 54.61; H, 3.21; N, 11.22.
2-(4-chlorophenylimino)-5-(4-hydroxy-3-methoxybenzylidene)-4-thiazolidinone (28)
Bright yellow crystals (810 mg, 51%); mp 220–228 °C (dioxane), Rf value: 0.79 (Toluene: Ethanol 8:2), λmax: 344 nm, IR (KBr, cm−1) ν: 1691 (C═O), 3421 (NH str.), 1263 (C–N), 1505 (C═N), 2974 (C–H arom.), 1647 (C═C), 1091 (Ar–Cl), 3473 (Ar-OH Broad band), 823 (C–H arom. meta), 756 (C–H arom. para); 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 10.9 (s, 1H, NH), 6.80–7.45 (4H, H-2,3,5,6), 7.580 (s, 1H, CH), 7.635 (s, 1H, H-2’), 7.63–7.91 (m, 2H, H-5’,6’), 4.01 (s, 3H, OCH3), 9.770 (s, OH); 13C NMR (DMSO-d6, CDCl3): 168.9 (CONH, amide), 116.3 (C═C, ethylene), 163.4 (C═N), 142.5 (C═C), 128.9 (1C benzene), 112.9 (1C benzene), 122.8 (1C benzene), 116.1 (1C benzene), 147.4 (C–OH), 149.9 (1C benzene), 56.9 (OCH3), 147.4 (C–N), 122.9 (2C benzene), 130.6 (2C benzene), 132.4 (C–Cl); HRMS calcd for C17H13ClN2O3S: 360.8147, found 361.0767; Anal. calcd.: C, 57.68; H, 4.03; N, 7.47; found: C, 57.66; H, 4.01; N, 7.45.
2-(3-chlorophenylimino)-5-benzylidene-4-thiazolidinone (29)
Reaction time 8 h; Brown crystals (1.0 g, 72%); mp 260–262 °C (from dioxane); Rf value: 0.72 (Toluene: Ethanol, 8:2); λmax 262 nm; IR (KBr, cm−1) ν: -C═O 1679; -NH- stretch 3267; -NH bend 1504; -C–N 1244; C═N 1595; C–H stretch (arom.) 3022; C═C 1629; Ar–Cl 1076; C–H bend (arom. meta) 851 and 875; 1HNMR (DMSO-d6, 300 MHz): (δ, ppm): 11.31, (S, 1H, NH), 7.509 (s, 1H, H-2), 7.084-6.985 (m, 3H, H-4,5,6), 7.084 (s, 1H, CH), 7.748–7.509 (m, 5H arom.); 13C NMR (DMSO-d6, CDCl3): 168.1 (CONH, amide), 116.3 (C═C, ethylene),, 163.5 (C═N), 135.9 (1C benzene), 142.4 (C═C), 128.9 (2C benzene), 129.2 (2C benzene), 127.1 (1C benzene), 149.4 (C–N), 120.9 (1C benzene), 122.1(1C benzene), 134.8 (1C benzene), 127.6 (1C benzene), 131.6 (C–Cl); HRMS calcd for C16H11ClN2OS: 314.0281, found 315.0583; Anal. calcd.: C, 62.10; H, 3.98; N, 8.52; found: C, 62.11; H, 3.96; N, 8.55.
2-(3-chlorophenylimino)-5-(2-chlorobenzylidene)-4-thiazolidinone (30)
Brown crystals (923 mg, 60%); mp 240–242 °C (from dioxane); Rf value: 0.72 (Toluene: Ethanol, 8:2); λmax 263 nm; IR (KBr, cm−1) ν: -C═O 1722; -NH- stretch 3423; -NH bend 1509; -C–N 1247; C═N 1599; C–H stretch (arom.) 2966; C═C 1654; Ar–Cl 1039; C–H bend (arom. meta) 862; C–H bend (arom. para) 765.; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.7 (s, 1H, NH), 7.593 (s, 1H, H-2), 7.204 (d, 1H, J 28.5, H-4), 7.071-6.985 (m, 2H, H-5 and 6), 7.628 (s, 1H, CH), 7.577 (d, 1H, J 3.9, H-3’), 7.923–7.723 (m, 3H, H-4’, 5’, 6’); 13C NMR (DMSO-d6, CDCl3): 165.1 (CONH, amide), 116.4 (C═C, ethylene), 163.2 (C═N), 142.5 (C═C), 133.9 (1C benzene), 127.1 (1C benzene), 126.4 (1C benzene), 129.4 (1C benzene), 129.1 (1C benzene), 134.9 (C–Cl), 150.8 (C–N), 122.4 (1C benzene), 120.9 (1C benzene), 131.6 (1C benzene), 127.6 (1C benzene), 134.8 (C–Cl); HRMS calcd for C16H10Cl2N2OS: 347.9891, found 349.0123; Anal. calcd.: C, 56.21; H, 3.33; N, 7.71; found: C, 56.23; H, 3.30; N, 7.73.
2-(3-chlorophenylimino)-5-(4-chlorobenzylidene)-4-thiazolidinone (31)
Reaction time 9 h; Brown crystals (1.07 g, 70%); mp 240–245 °C (from dioxane); Rf value: 0.74 (Toluene: Ethanol, 8:2); λmax 348 nm; IR (KBr, cm−1) ν: -C═O 1672; -NH- stretch 3265; -NH bend 1463; -C–N 1251; C═N 1631; C–H stretch (arom.) 3053; C═C 1631; Ar–Cl 1091; C–H bend (arom. meta) 821; C–H bend (arom. para) 763; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 10.01 (s, 1H, NH), 7.542 (s, 1H, H-2), 7.063 (d, 1H, J 7.5,H-4), 7.45 (d, 2H, J 7.5, H-5 and 6), 7.633 (s, 1H, CH), 7.633–7.441 (m, 4H, H-2’, 3’, 5’, and 6’); 13C NMR (DMSO-d6, CDCl3): 168.1 (CONH, amide), 116.6 (C═C, ethylene), 163.4 (C═N), 142.1 (C═C), 133.9 (1C benzene), 129.9 (2C benzene), 128.4 (2C benzene), 133.1 (C–Cl), 150.9 (C–N), 120.9 (1C benzene), 122.4 (1C benzene), 127.6 (1C benzene), 131.6 (1C benzene), 134.8 (C–Cl); HRMS calcd for C16H10Cl2N2OS: 347.9891, found 349.0103; Anal. calcd.: C, 56.21; H, 3.33; N, 7.71; found: C, 56.22; H, 3.35; N, 7.74.
2-(3-chlorophenylimino)-5-(4-hydroxybenzylidene)-4-thiazolidinone (32)
Reaction time 7 h; Mustard yellow crystals (1.05 g, 72%); mp 310–315 °C (from dioxane); Rf value: 0.796 (Toluene: Ethanol, 8:2); λmax 365 nm; IR (KBr, cm−1) ν: -C═O 1677; -NH- stretch 3413; -NH bend 1500; -C–N 1244; C═N 1564; C–H stretch (aromatic) 3026; C═C 1650; Ar–Cl 1091; Ar-OH 3469 (Broad band); C–H bend (arom. meta) 825, 850; C–H bend (arom. para) 764; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 10.288, (s, 1H, NH), 7.460 (s, 1H, H-2), 7.56 (d, 1H, J 7.5, H-4), 7.258–7.041 (m, 2H, H-5 and 6), 7.433 (s, 1H, CH), 7.741–7.604 (m, 4H, arom.), 10.181 (s, 1H, OH); 13C NMR (DMSO-d6, CDCl3): 168.5 (CONH, amide), 116.4 (C═C, ethylene), 163.6 (C═N), 142.6 (C═C), 127.9 (1C benzene), 130.9 (2C benzene), 115.4 (2C benzene), 157.9 (C–OH), 150.2 (C–N), 122.1 (1C benzene), 127.4 (1C benzene), 131.2 (1C benzene), 120.3 (1C benzene), 134.9 (C–Cl); HRMS calcd for C16H11ClN2O2S: 330.0230, found 331.05; Anal. calcd.: C, 59.21; H, 3.80; N, 8.12; found: C, 59.20; H, 3.83; N, 8.10.
2-(3-chlorophenylimino)-5-(2-nitrobenzylidene)-4-thiazolidinone (33)
Dark Brown crystals (1.0 g, 63%); mp 200–205 °C (from dioxane); Rf value: 0.72 (Toluene: Ethanol, 8:2); λmax 274 nm; IR (KBr, cm−1) ν: -C═O 1685; -NH- stretch 3265; -NH bend 1523; -C–N 1236; C═N 1598; C–H stretch (arom.) 3062; C═C 1639; Ar–Cl 1091; Ar–NO2 1342(sym) 1523 (asym); C–H bend (arom. meta) 772 and 857; C–H bend (arom. ortho) 754; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.05 (s, 1H, NH), 7.716 (s, 1H, H-2), 7.701 (d, 1H, J 8.7, H-4), 7.687–7.638 (m, 2H, H-5 and 6), 7.497 (s, 1H, CH), 7.762 (d, 1H, J 13.5, H-3’), 7.884–7.785 (m, 3H, arom.); 13C NMR (DMSO-d6, CDCl3): 168.3 (CONH, amide), 116.2 (C═C, ethylene), 163.2 (C═N), 142.4 (C═C), 127.6 (2C benzene), 135.4 (1C benzene), 123.9 (1C benzene), 129.4 (1C benzene), 148.9 (C–NO2), 151.1 (C–N), 123.9 (1C benzene), 128.2 (1C benzene), 121.3 (1C benzene), 132.3 (1C benzene), 135.4 (C–Cl); HRMS calcd for C16H10ClN3O3S: 359.0131, found 360.0572; Anal. calcd.: C, 54.62; H, 3.24; N, 11.24; found: C, 54.65; H, 3.22; N, 11.21.
2-(3-chlorophenylimino)-5-(3-nitrobenzylidene)-4-thiazolidinone (34)
Brown crystals (1.03 g, 65%); mp 275–280 °C (from dioxane); Rf value: 0.72 (Toluene: Ethanol, 8:2); λmax 342 nm; IR (KBr, cm−1) ν: -C═O 1677; -NH- stretch 3261; -NH bend 1527; -C–N 1242; C═N 1598; C–H stretch (arom.) 2964; C═C 1629; Ar–Cl 1114; Ar–NO2 1352 (sym) 1510 (asym); C–H bend (arom. meta) 765 and 859; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 11.01 (s, 1H, NH), 7.726 (s, 1H, H-2), 7.611–7.510 (m, 3H, H-4,5 and 6), 7.611 (s, 1H, CH), 7.812 (s, 1H, H-2’), 7.828 (d, 1H, 7.925–7.844 (m, 2H, H-5’ and 6’); 13C NMR (DMSO-d6, CDCl3): 168.5 (CONH, amide), 116.9 (C═C, ethylene), 163.6 (C═N),142.2 (C═C), 137.6 1C benzene), 123.9 (1C benzene), 124.4 (1C benzene), 130.4 (1C benzene), 135.9 (1C benzene), 149.1 ((C–N), 149.9 (C–N), 123.2 (1C benzene), 120.9 (1C benzene), 127.2 (1C benzene), 131.9 (1C benzene), 135.1 (C–Cl); HRMS calcd for C16H10ClN3O3S: 359.0131, found 360.0572; Anal. calcd.: C, 54.62; H, 3.24; N, 11.24; found: C, 54.60; H, 3.21; N, 11.22.
2-(3-chlorophenylimino)-5-(4-hydroxy-3-methoxybenzylidene)-4-thiazolidinone (35)
Dark brown crystals (955 mg, 60%); mp 225–230 °C (from dioxane); Rf value: 0.73 (Toluene: Ethanol, 8:2); λmax 375 nm; IR (KBr, cm−1) ν: -C═O 1701; -NH- stretch 3265; -NH bend 1497; -C–N 1255; C═N 1583; C–H stretch (arom.) 2905; C═C 1641; Ar–Cl 1033; Ar-OH 3425 (Broad band); C–H bend (arom. meta) 795, 857; C–H bend (arom. para) 744; 1HNMR (DMSO-d6, 300 MHz) (δ, ppm): 10.9 (s, 1H, NH), 7.253 (s, 1H, H-2), 7.580–7.431 (m, 3H, H-4, 5, and 6), 7.045 (s, 1H, CH), 7.301 (s, 1H, H-2’), 3.839 (s, 3H, OCH3), 9.81 (s, 1H, OH), 7.081-6.966 (d, 2H, H-5’ and 6’); 13C NMR (DMSO-d6, CDCl3): 167.5 (CONH, amide), 114.9 (C═C, ethylene), 162.6(C═N), 142.9 (C═C), 129.6 (1C benzene), 113.9 (1C benzene), 117.4 (1C benzene), 147.4(C–OH), 147.9 (1C benzene), 57.6 (OCH3), 150.1 (C–N), 123.9 (1C benzene), 123.9 (1C benzene), 129.2 (1C benzene), 132.5 (1C benzene), 136.1 (C–Cl); HRMS calcd for C17H13ClN2O3S: 360.0335, found 361.0759; Anal. calcd.: C, 57.68; H, 4.03; N, 7.47; found: C, 57.66; H, 4.05; N, 7.45.
Pharmacological evaluation
The experiments were performed using Wistar rats of either sex weighing 100–150 g, and albino mice of either sex weighing 25–30 g. Standard food pellets and water were given ad libitum. The animals were acclimatized for 14 days under laboratory conditions. The animals were maintained under natural day and night cycle before performing the tests. Indomethacin and Ibuprofen were used as reference drugs for anti-inflammatory and antinociceptive activities, respectively. Test compounds dissolved in polyethylene glycol PEG 400 were administered orally through suitable gavages. Ethical clearance was obtained from Institutional Animal Ethics Committee (IAEC) before performing the experiments, and the same were conducted as per the Indian National Science Academy Guidelines (Reg. No. BBDNITM/IAEC/Clear/04/2010).
Anti-inflammatory activity
Test compounds were evaluated for anti-inflammatory activity by carrageenan-induced rat paw edema method (Davies et al. 1946). The rats received a sub plantar injection of 0.1 mL saline, containing 1% carrageenan, in the right hind paw. A suspension of test compounds (50 mg/kg) in PEG 400 was administered orally, 30 min before carrageenan injection. Control animals received the same volume of vehicle. The volume of the paw was measured by a plethysmometer immediately after the injection. Subsequent readings of the volume of the same paw were recorded at 1, 2, 4 h intervals and compared with the initial readings. The percent anti-inflammatory activity was calculated according to the following formula:
where Vt and Vc are volumes of edema in drug treated and control groups, respectively.
Statistical analysis was carried out using Graph Pad Prism 3.0 (Graph Pad Software, San Diego, CA). Significance of statistical differences of control and test groups was verified using analysis of variance (ANOVA) followed by Dunnett’s test. Values are expressed as mean ± S.E.M; n = 6. The compounds were considered to show significant effect (Table 1).
Antinociceptive activity
Antinociceptive activity of the test compounds, at a dose of 50 mg/kg, was studied by tail flick method in mice (Davies et al. 1946; Crofford et al. 1994). Albino mice of either sex, weighing 25–30 grams, were used for the study. Ibuprofen 40 mg/kg was used as the standard. After oral dose of the standard and test compounds, reaction time was noted at 0, 1, 2, and 4 h time intervals. Basal reaction time to radiant heat was taken by placing the tip (last 1–2 cm) of the tail on the radiant heat source. Withdrawal of the tail from the source was taken as the end point. A cutoff period of 10–12 s was observed to prevent damage to the tail. All the values are expressed as means ± S.E.M. The results were analyzed by ANOVA (Dunett’s test). Values are expressed as mean ± S.E.M; n = 6. The compounds exhibited significant effect (Table 2).
where, Tc is the reaction time in the control group, Tt is the reaction time in treated mice and Ct is the cutoff time. Cutoff time was set at 15 seconds to prevent tissue damage.
Acute toxicity studies
The animals were tested for any kind of toxicity of the test compounds and were observed for 14 days prior to performing the pharmacological studies (Dixion 1985). Title compounds were found to be non-toxic and were well tolerated by the experimental animals. The compounds showed a high safety margin. The approximate lethal dose ALD50 of the compounds was found to be >500 mg/kg.
Antioxidant activity
The test compounds were evaluated for in vitro free radical scavenging capacity at concentrations of 2, 4, 6, 8, and 10 µg/mL in methanol through Diphenyl-1-picrylhydrazyl (DPPH) assay method (Mensor et al. 2001; Oyaizu 1986; Soare et al. 1997). The reduction capability of DPPH radical is determined by a decrease in its absorbance at 517 nm induced by antioxidants. The compounds showed promising antioxidant activity in comparison to ascorbic acid as the standard (Table 3).
Molecular docking studies
Methodology
The molecular docking studies were performed on the human COX-2 (PDB ID: 5KIR), which contains rofecoxib (Vioxx) as co-crystallized ligand. The resolution of the protein was found to be 2.69 Å (Orlando and Malkowski 2016). The protein preparation was done using the protein preparation wizard of Maestro (version 11.1) program available in the Schrodinger software. Hydrogen atoms were added and bond orders were assigned to the protein–ligand complex at a consistent physiological pH (7.0). The optimization of the interactions between the side chains and ligand with side chains was done. The energy minimization of the X-ray structure was done at the root mean square deviation value of 0.3 Å. This energy minimization also ensured the placement of hydrogen atoms in an optimized geometry (Friesner et al. 2006). The ligand preparation was done using the Ligprep module where the SDF files of the imported ligands were generated for their isomers, tautomers, with a maximum of 10 conformations for each ligand using latest OPLS3 force field (Glide 2018; Ligprep 2018; Harder et al. 2015). The minimized structure of the enzyme was subjected to the grid generation module where the active site of the enzyme was taken into consideration and a grid was prepared for the docking of the ligands. The docking studies were performed using Ligand Docking module which takes up grid as a grid file and ligprep file to generate the scores for the generated conformations by ligand binding to the active site in comparison with the standard, rofecoxib. The scores generated were the glide scores that comprise of the interactions between protein and ligand. These interactions include the van der Waals (vdW) (contact term), a Coulomb (Coul) (electrostatic), the H Bond (hydrogen bond), the Lipo term (lipophilic), the Metal (metal), the RotB (penalty for freezing rotatable bonds), the BuryP (penalizes buried polar groups) and Site (polar group in the active site) interactions. The conformations with good scores were generated.
Compound portrayed interaction in rofecoxib binding site of human COX-2
The recognition pattern for the most potent investigational compounds 23, 24, 26, 30, and 31 identified as COX-2 inhibitors, molecular docking based experiments were conducted using Glide module of Mastero 11.1 (Licensed Version 2017) (Maestro 2018). The docking study on the COX-2 was performed to investigate the binding pattern of the potent identified COX-2 inhibitors. The flexible docking experiment was performed using glide module of the MAESTRO 11.1 (Maestro 2018). It was observed that the active compound 30 best fitted to the rofecoxib COX-2 active site. The active site residues and binding pattern of the active molecules was found to be similar to that of the standard, rofecoxib. The active molecules were found to be having B (thiazole ring) binding as the planar (Fig. 1a) while in the inactive molecules the ring B was tilted (Fig. 1b). The binding pattern thus clarifies the differences between the active and inactive molecules.
The interactions with the binding site residues and compound 30 in the active site have been found to be similar to that of rofecoxib (Fig. 2).
The binding pattern not only gave an idea about the arrangement of the molecule in the active site but also picturized important interactions between the molecules and actives site residues. It was observed that similar types of interactions were observed for the active molecule 30 and rofecoxib. The planar ring A, containing a chloro group, was observed to be aligned parallel to the ARG120 residue and π-cation interactions were observed. Hydrogen bonding was observed between the NH group of ligand and O group of TYR355 amino acid residue and also perpendicular π–π stacking interactions were observed between ring A and TYR355. PHE518 ring energetically favors the π-anion interactions with the ring B. Similar type of π–π interactions were observed in the COX-2 active site residues TRP387 and TYR385 with ring C of compound 30 (Fig. 3).
The binding of the active molecule 30 and favorable interactions have been shown in the Fig. 3a, b. The comparative study has been made between the active and inactive pattern. The docking score of the active compounds are enumerated in Table 4.
Prediction of physiochemical parameters
The compounds synthesized were predicted for the pharmaceutical properties with the help of QikProp module in MAESTRO 11.4 (1) (Schrödinger 2018). The pharmaceutical properties include Lipinski rule of 5 and other parameters such as bioavailability and pharmacokinetics, etc. The module suggested that all the compounds followed the Lipinski rule of 5. Moreover, the partition coefficient (QPlogPo/w) predicted was in the normal range, i.e. −2.0 to 6.5. For all the synthesized compounds, the QPlogPo/w was found to be 1.5–4.5. QPPCaco represents the gut cell absorption of the compounds and ranges from 25 to 500 for poor gut absorbed compounds while > 500 for the compounds with good gut absorption. Some of the compounds were predicted to have poor gut absorption, such as compound 10, 11, 14, 17, 18, and 26 (Table 5). The human oral absorption depends on the number of rotatable bonds, number of metabolites, logP, cell permeability, and solubility. The number 1 depicts low oral absorption, 2 represents medium oral absorption, and 3 represents normal oral absorption. The percentage of normal oral absorption ranges from 70 to 100%. It was observed that all the compounds had normal oral absorption.
Results and discussion
Novel 2-(substituted phenyl imino)-5-benzylidene-4-thiazolidinones, having different substitutions at imino phenyl and arylidene groups, have been synthesized and evaluated in this study. The bathochromic shift attributable to C═N chromophoric group was obtained at 285–375 nm in the UV spectra of synthesized compounds, arising owing to the nitro and the chloro groups. The observed peaks in IR spectra (cm−1) of lactam -NH at 3200, 1550, C═O at 1670 and C═C at 1633 agreed with the structures. In 1HNMR spectra (δ, ppm), -NH proton observed at 11.8–12.7 showed substitution at 2nd position instead of 3rd position, which could be attributed to a lactam proton (Vicini et al. 2008; Bruno et al. 2002; Ishida et al. 1990; Momose et al. 1991). The presence of methine hydrogen (=CH) at 7.70–7.75 further confirmed the proposed structure. The HRMS characterization of the synthesized compounds further confirmed the success of synthesis. The compounds were formed through amino-imino tautomerism involving heteroaryl thiazolidinones and their benzylidene derivatives (Scheme 2) and E/Z isomerism of final compounds was studied on the basis of 1HNMR and IR spectral data, and confirmed on the basis of literature.
Compounds (1–35) were evaluated for anti-inflammatory, antinociceptive, and antioxidant activities. Anti-inflammatory activity was evaluated in vivo by carrageenan-induced rat paw edema model (Winter et al. 1962) at a dose of 50 mg/kg (per oral), whereas antinociceptive activity was carried out in vivo using tail flick method (Davies et al. 1946; Dandiya and Menon 1963) at a dose of 50 mg/kg (per oral) while antioxidant screening was done in vitro at concentrations of 2, 4, 6, 8, and 10 µg/mL using DPPH assay method (George et al. 2008). The results are shown in Tables 2–4. The compounds have shown varying degree of anti-inflammatory activity ranging from 16.91% to 61%; some compounds (5, 7, 23, 24, 26, 28 with 60.29%, 61.02%, 60.09%, 60.29%, 60.29%, 60.29%, respectively) being comparable to the standard, Indomethacin (10 mg/kg) i.e., 69.11%. Moreover, most of the compounds exhibited significant antinociceptive activity, comparable to the standard drug, Ibuprofen (20 mg/kg). Promising free-radical scavenging activity was recorded for the synthesized compounds when compared with the standard. Acute toxicity studies were carried out as per OECD guidelines and ALD50 of the compounds was > 500 mg/kg (Dixion 1985). The selected test dose (50 mg/kg) was decided on the basis of ALD50 value, i.e., 1/10th of the determined LD50 (500 mg/kg).
Compounds (22–28) formed by the reaction of p-chloro aniline were found to possess more potential in the series. Among the synthesized compounds, it was observed that substitution at the arylidene ring influenced the activity. Structure activity relationship studies revealed that arylidene ring containing electron withdrawing groups like -Cl and -NO2 demonstrated better anti-inflammatory activity than electron donating groups like -OH and -OCH3. Positions of these groups also affected the activity, with -Cl at para and -NO2 at meta positions showing maximum activity. The findings matched well with the previous reports on the same nucleus (Ottana et al. 2005; Crofford et al. 1994). Electron withdrawing groups like -Cl and -NO2 demonstrated better activity than electron donating groups like -OH and -OCH3. Chloro group at the para position led to more active compounds (3, 10, 17, 24, 31) while nitro group at the meta position also behaved in the same manner (6, 13, 20, 27, 34). Compound 7 proved to be the most potent with the highest anti-inflammatory activity (61.02%). Antinociceptive activity was not much affected by the presence of electron withdrawing or electron-releasing groups. Compounds 6, 13, 17, 20, 22, 23, 24, 26, 27, 29, 30, 31, and 34 showed significant antinociceptive activity. The activity did not vary much with substituents on the arylidene ring. However, the compounds (22–28) starting from 4-chloro aniline proved to be more potent antinociceptive agents than the other synthesized derivatives. Compounds with p-chloro (24) and m-nitro (27) groups at arylidene ring proved to be the most potent with the highest antinociceptive activity (64%). Compounds (1–7) with Cl and F groups on benzene ring attached to imino group at position-2 showed maximum antiinflammatory–antinociceptive activity. It has been reported that 5-arylidene 4-thiazolidinones act as anti-inflammatory agents by inhibiting COX-2 enzyme (Ottana et al. 2005). Thus, the mechanism of action of the synthesized compounds may also be through COX-2 inhibition.
The synthesized compounds exhibited the potential to scavenge free radicals in varying capacities in the DPPH assay. Activity was influenced by substitutions on arylidene moeity present at position-5 of the 4-thiazolidinone nucleus. Compounds with hydroxy substitution, either alone or in addition to a methoxy group, emerged as better antioxidants in every series (4, 7, 11, 14, 18, 21, 25, 28, 32, and 35). This superiority may be due to the presence of electron cloud on the aryl rings which can easily donate electron to DPPH. The free-radical scavenging activity of the synthesized compounds, which lack hydroxy or methoxy group is attributable to the presence of free NH group at position-3 and unsaturated double bonds, which can donate hydrogen and electrons, respectively. Similar findings have been reported by Hossain and Bhattacharya (Hossain and Bhattacharya 2007). Donation of hydrogen leaves the compounds in their radical form and their structure gets stabilized owing to electronic conjugation, thus favouring the reaction to occur. Tominaga et al. (2005) have reported that the presence of free hydroxyl group in the aryl moiety is one of the factors in determining the DPPH-scavenging activity of the compound, where protection of the free-hydroxyl group drastically reduces the DPPH-scavenging activity. IC50 of the synthesized derivatives was also calculated and compounds 4, 14, and 21 were found to be the most potent antioxidant agents, with IC50 (2.39, 2.33, 2.33 µg/mL) comparable to ascorbic acid (1.08 µg/mL). The docking study on COX-2 was performed to investigate the binding pattern of the potent identified COX-2 inhibitors. It was observed that the active compound 30 best fits to the rofecoxib COX-2 active site. The active site residues and binding pattern of the active molecules was found to be similar to the standard, rofecoxib. The active molecules were found to be having B (thiazole ring) binding in the planar (Fig. 1a), whereas in the inactive molecules the ring B was tilted (Fig. 1b). The binding pattern thus clarifies the differences between the active and inactive molecules. The interactions with the binding site residues and compound 30 in the active site have been found to be similar to that of rofecoxib (Fig. 2).
Conclusions
A total of 35 2,5-disubstituted 4-thiazolidinones (1–35) have been synthesized and evaluated for in vivo anti-inflammatory, antinociceptive activities and in vitro free-radical scavenging properties using standard protocols. The compounds showed promising anti-inflammatory, antinociceptive, and free-radical scavenging activities. Docking studies revealed that the interactions with the binding site residues and compound 30 in the active site have been found to be similar to that of rofecoxib. The planar ring A containing chloro group was observed to be aligned parallel to the ARG120 residue and π-cation interactions were observed. Hydrogen bonding has been observed between the NH group of ligand and O group of TYR355 amino-acid residue and also perpendicular π–π stacking interactions were observed between ring A and TYR355. PHE518 ring energetically favors the π-anion interactions with the ring B. Similar type of π–π interactions were observed in the COX-2 active site residues TRP387 and TYR385 with ring C of compound 30. As neurodegenerative disorders including inflammation may be associated with free radicals like reactive oxygen species, compounds containing both or either of these activities may prove to be very useful. Drugs possessing both activities can help in better patient compliance. These compounds exhibit potential to counter three diseases at the same time none of them had an acidic group, thereby being non-ulcerogenic. The outstanding properties of these compounds may open new horizons for further studies at molecular level to ascertain their mode of action.
References
Bhandari SV, Bothara KG, Patil AA, Chitre TS, Sarkate AP, Gore ST, Dangre SC, Khachane CV (2009) Design, synthesis and pharmacological screening of novel antihypertensive agents using hybrid approach. Bioorg Med Chem 17:390–400
Bhati SK, Kumar A (2008) Synthesis of new substituted azetidinoyl and thiazolidinoyl-1, 3, 4-thiadiazino (6, 5-b) indoles as promising anti-inflammatory agents. Eur J Med Chem 43:2323–2330
Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolo F, Ottana R, Vigorita M (2002) Synthesis and aldose reductase inhibitory activity of 5-arylidene-2, 4-thiazolidinediones. Bioorg Med Chem 10:1077–1084
Chakraborti AK, Garg SK, Kumar R, Motiwala HF, Jadhavar PS (2010) Progress in COX-2 inhibitors: a journey so far. Curr Med Chem 17:1563–1593
Chawla P, Singh R, Saraf SK (2012a) Effect of chloro and fluoro groups on the antimicrobial activity of 2, 5-disubstituted 4-thiazolidinones: a comparative study. Med Chem Res 21:3263–3271
Chawla P, Singh R, Saraf SK (2012b) Syntheses and evaluation of 2, 5-disubstituted 4-thiazolidinone analogues as antimicrobial agents. Med Chem Res 21:2064–2071
Clarkson PM, Tremblay I (1988) Exercise-induced muscle damage, repair, and adaptation in humans. J Appl Physiol 65:1–6
Crofford LJ, Wilder RL, Ristimäki A, Sano H, Remmers EF, Epps HR, Hla T (1994) Cyclooxygenase-1 and-2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester and corticosteroids. J Clin Investig 93:1095–1101
Dandiya P, Menon M (1963) Studies on central nervous system depressants.(III). Influence of some tranquillizing agents on morphine analgesia. Arch Int Pharmacodyn Ther 141:223
Davies O, Raventos J, Walpole A (1946) A method for the evaluation of analgesic activity using rats. Br J Pharmacol 1:255–264
Dixion W (1985) The up and down method or small samples. J Am Stat Assoc 60:967–978
Flohe L, Beckmann R, Giertz H, Loschen G (1985) Oxygen-centered free radicals as mediators of inflammation. In: Sies H (ed). Oxidative stress. Academic Press, London, p 403–436
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein− ligand complexes. J Med Chem 49:6177–6196
George S, Parameswaran MK, Chakraborty R, Ravi TK (2008) Synthesis and evaluation of the biological activities of some 3-{[5-(6-methyl-4-aryl-2-oxo-1, 2, 3, 4-tetrahydropyrimidin-5-yl)-1, 3, 4-oxadiazol-2-yl]-imino}-1, 3-dihydro-2H-indol-2-one derivatives. Acta Pharm 58:119–129
Geronikaki AA, Lagunin AA, Hadjipavlou-Litina DI, Eleftheriou PT, Filimonov DA, Poroikov VV, Alam I, Saxena AK (2008) Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition. J Med Chem 51:1601–1609
Glide (2018) Schrödinger Release 2018-1. Schrödinger, LLC, New York, NY
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine, 5th edn. Oxford University Press, USA
Harder E, Damm W, Maple J, Wu C, Reboul M, Xiang JY, Wang L, Lupyan D, Dahlgren MK, Knight JL (2015) OPLS3: a force field providing broad coverage of drug-like small molecules and proteins. J Chem Theory Comput 12:281–296
Hossain SU, Bhattacharya S (2007) Synthesis of O-prenylated and O-geranylated derivatives of 5-benzylidene2, 4-thiazolidinediones and evaluation of their free radical scavenging activity as well as effect on some phase II antioxidant/detoxifying enzymes. Bioorg Med Chem Lett 17:1149–1154
Ishida T, In Y, Inoue M, Tanaka C, Hamanaka N (1990) Conformation of (Z)-3-carboxymethyl-[(2E)-2-methyl-3-phenylpropenylidene] rhodanine (epalrestat), a potent aldose reductase inhibitor: X-ray crystallographic, energy calculational, and nuclear magnetic resonance studies. J Chem Soc Perkin Trans 2:1085–1091
Ligprep (2018) Schrödinger Release 2018-1. Schrödinger, LLC, New York, NY
Maccari R, Del Corso A, Giglio M, Moschini R, Mura U, Ottanà R (2011) In vitro evaluation of 5-arylidene-2-thioxo-4-thiazolidinones active as aldose reductase inhibitors. Bioorg Med Chem Lett 21:200–203
Maestro (2018) Schrödinger Release 2018-1. Schrödinger, LLC, New York, NY
Mensor LL, Menezes FS, Leitão GG, Reis AS, Santos TCD, Coube CS, Leitão SG (2001) Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res 15:127–130
Momose Y, Meguro K, Ikeda H, Hatanaka C, Oi S, Sohda T (1991) Studies on antidiabetic agents. X. Synthesis and biological activities of pioglitazone and related compounds. Chem Pharm Bull 39:1440–1445
Omar K, Geronikaki A, Zoumpoulakis P, Camoutsis C, Soković M, Ćirić A, Glamočlija J (2010) Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg Med Chem 18:426–432
Orhan H, Şahin G (2001) In vitro effects of NSAIDS and paracetamolon oxidative stress-related parameters of human erythrocytes. Exp Toxicol Pathol 53:133–140
Orlando BJ, Malkowski MG (2016) Crystal structure of rofecoxib bound to human cyclooxygenase-2. Sect Acta Crystallogr F Struct Biol Comm 72:772–776
Ottana R, Maccari R, Barreca ML, Bruno G, Rotondo A, Rossi A, Chiricosta G, Di Paola R, Sautebin L, Cuzzocrea S (2005) 5-Arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorg Med Chem 13:4243–4252
Ottanà R, Maccari R, Ciurleo R, Vigorita MG, Panico AM, Cardile V, Garufi F, Ronsisvalle S (2007) Synthesis and in vitro evaluation of 5-arylidene-3-hydroxyalkyl-2-phenylimino-4-thiazolidinones with antidegenerative activity on human chondrocyte cultures. Bioorg Med Chem 15:7618–7625
Oyaizu M (1986) Studies on products of browning reaction, Jpn. J Nutr Diet 44:307–315
Panico AM, Vicini P, Geronikaki A, Incerti M, Cardile V, Crascì L, Messina R, Ronsisvalle S (2011) Heteroarylimino-4-thiazolidinones as inhibitors of cartilage degradation. Bioorg Chem 39:48–52
Saldanha L, Elias G, Rao M (1990) Oxygen radical scavenging activity of phenylbutenones and their correlation with anti-inflammatory activity. Arzneimittelforschung 40:89–91
Schrödinger (2018) Release 2018-4. QikProp, Schrödinger, LLC, New York, NY
Seth K, Garg SK, Kumar R, Purohit P, Meena VS, Goyal R, Banerjee UC, Chakraborti AK (2014) 2-(2-Arylphenyl) benzoxazole as a novel anti-inflammatory scaffold: synthesis and biological evaluation. ACS Med Chem Lett 5:512–516
Shih MH, Ke FY (2004) Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg Med Chem 12:4633–4643
Singh N, Tripathi AC, Tewari A, Kumar SarafSK (2015) Ulcerogenicity devoid novel non-steroidal anti-inflammatory agents (NSAIDS): syntheses, computational studies, and activity of 5-aryliden-2-imino-4-thiazolidinones. Med Chem Res 24:1927–1941
Soare JR, Dinis TC, Cunha AP, Almeida L (1997) Antioxidant activities of some extracts of Thymus zygis. Free Radic Res 26:469–478
Taranalli A, Bhat A, Srinivas S, Saravanan E (2008) anti-inflammatory, analgesic and antipyretic activity of certain thiazolidinones. Indian J Pharm Sci 70:159–164
Tominaga H, Kobayashi Y, Goto T, Kasemura K, Nomura M (2005) DPPH radical-scavenging effect of several phenylpropanoid compounds and their glycoside derivatives. Yakugaku Zasshi 125:371–375
Vicini P, Geronikaki A, Anastasia K, Incerti M, Zani F (2006) Synthesis and antimicrobial activity of novel 2-thiazolylimino-5-arylidene-4-thiazolidinones. Bioorg Med Chem 14:3859–3864
Vicini P, Geronikaki A, Incerti M, Zani F, Dearden J, Hewitt M (2008) 2-Heteroarylimino-5-benzylidene-4-thiazolidinones analogues of 2-thiazolylimino-5-benzylidene-4-thiazolidinones with antimicrobial activity: Synthesis and structure–activity relationship. Bioorg Med Chem 16:3714–3724
Winter CA, Risley EA, Nuss GW (1962) Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111:544–547
Acknowledgements
The authors are thankful to Central Drug Research Institute (CDRI) India for spectral characterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chawla, P., Kalra, S., Kumar, R. et al. Novel 2-(substituted phenyl Imino)-5-benzylidene-4-thiazolidinones as possible non-ulcerogenic tri-action drug candidates: synthesis, characterization, biological evaluation And docking studies. Med Chem Res 28, 340–359 (2019). https://doi.org/10.1007/s00044-018-02288-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-018-02288-z