Abstract
A series of new 5-aryliden-2-imino-4-thiazolidinones (5a–e and 6a–e) were synthesized via a three-step reaction and characterized by physicochemical and spectral data. The uniqueness of the derivatives lies in the fact that none of them had an acidic group, like conventional NSAIDS, but exhibited significant in vivo activity in acute inflammation models. In particular, 5-(3-chlorobenzyliden)-2-(pyridin-2-yl-imino)-4-thiazolidinone(5a) and 5-(3-chlorobenzyliden)-2-(5-methylisoxazol-3-yl-imino)-4-thiazolidinone (6a) showed remarkable paw oedema inhibition (67.76 and 74.47 % oedema inhibition, respectively, after 3 h) comparable to that of Ibuprofen (74.56 % oedema inhibition, after 3 h) at half of the dose of the standard drug. Also, compounds 5a (72.86 %) and 6a (80.20 %) were found to possess significant inhibition of albumin denaturation when screened for in vitro anti-inflammatory activity. In addition, these compounds were docked into the known active site of COX-2 protein using Glide XP and QPLD algorithms, and the binding-free energy was calculated using Prime MM/GBSA simulation methods. The combined use of molecular docking and MM/GBSA methods gave a good correlation between the predicted binding-free energy and experimentally determined biological activities. It was also evident from the docking results that 2-methylisoxazolylimino or 2-(pyridin-2-yl-imino substitution and 3-chloro moiety on 5-benzylidin nucleus of these 4-thiazolidinone derivatives can easily occupy the COX-2 binding pocket, considered as the critical interaction for COX-2 inhibition. Moreover, pharmacokinetic properties of all the synthesized compounds were predicted, with good results. Further, the synthesized derivatives showed neither acute toxicity nor symptoms of gastric ulceration, at extended doses, owing to the absence of an acidic group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammation is a multi-factorial process. It reflects the response of an organism to various stimuli and is related to many disorders such as arthritis, asthma, and psoriasis, which require prolonged or repeated treatment. Although several mediators support the inflammatory processes, the main target of nonsteroidal anti-inflammatory drugs (NSAIDs) is cyclooxygenase (COX) enzyme. Traditional NSAIDs act via the inhibition of the COX-1 isoenzyme or the combined inhibition of COX-1 and COX-2 isoenzymes (Geronikaki et al., 2008; Leval et al., 2002; Vane and Botting 1987). Because COX-2 isoenzyme was found to be over expressed during inflammation, drug investigation was focused on selective COX-2 inhibition, hoping to prevent inflammation by sidestepping the undesired side effect of COX-1 or non-selective inhibitors. Recently, highly selective COX-2 inhibitors belonging to the classes of diarylheterocycles and methanesulfonanilides have been developed and marketed (de Leval et al., 2000; Graul et al., 1997). Such kind of compounds have shown interesting stereoselective anti-inflammatory/analgesic activities, together with better gastrointestinal safety profile than known NSAIDs, suggesting they might preferentially interact with inducible COX-2 isoform. Suitable selectivity assays as well as molecular modeling studies had also supported this hypothesis (Ottana et al., 2002). Thiazolidinone derivatives possessing anti-inflammatory and analgesic activities have been reported in the literature (Goel et al., 1999; Kumar et al., 2003, 2007; Ottana et al., 2005; Previtera et al., 1987; Vazzana et al., 2004; Vigorita et al., 2003). In addition to the above mentioned activities, thiazolidinone derivatives possessing antitumour, antimicrobial, anticancer, anti-HIV, and various other activities have also been reported (Agrawal et al., 2013; Chawla et al., 2011a, b; Chen et al., 2009; Gouveia et al., 2009; Havrylyuk et al., 2010; Omar et al., 2010; Rawal et al., 2007; Sadashiva et al., 2009; Tripathi et al., 2014; Verma and Saraf 2008; Zhang et al., 2009; Zhou et al., 2008). Moreover, pyridine and isoxazole are other important pharmacodynamic heterocyclic nuclei which, when incorporated in different heterocyclic templates, have been reported to possess potent anti-inflammatory activity (Balsamo et al., 2003; Haviv et al., 1983; Hosni and Abdulla 2008; Sondhi et al., 2008; Thirumurugan et al., 2010). Thus, it was decided to synthesize a new series of structurally novel 4-thiazolidinone derivatives by incorporating pyridine and isoxazole moieties at 2-position of the thiazolidinone nucleus. The structural variations were selected by introducing different arylidene substituents at the 5-position of the thiazolidinone nucleus. The effect of chloro, methoxy, hydroxy, and fluoro substituents of the arylidene moiety on the anti-inflammatory profile was considered. The groups were selected on the basis of previous SAR studies on thiazolidinone nucleus (Geronikaki et al., 2008; Ottana et al., 2005), and also with a view at the most frequent substituents in coxibs structure. The proposed 5-arylidene-2-imino-thiazolidin-4-one derivatives can be considered as diarylheterocyclic coxib like compounds, with promising anti-inflammtory potential.
Results and discussion
Chemistry
2-Chloro-N-(etheroaryl) acetamides (1 and 2) were synthesized using a reported procedure (Geronikaki and Theofilidis 1992), and these 2-chloro-N-(etheroaryl) acetamides were then reacted with heteroaromatic amines, in the presence of ammonium thiocyanate and refluxing ethanol, to efficiently produce 2-(heteroarylimino) thiazolidin-4-ones (3 and 4). The 5-aryliden-2-imino-4-thiazolidinones (5a–e and 6a–e) were obtained by refluxing 2-(heteroarylimino) thiazolidin-4-ones with appropriate aldehydes in buffered glacial acetic acid (Scheme 1).
All the synthesized compounds 5a–e and 6a–e were characterized by melting point, elemental analysis, and spectral data (IR, MS, 1H NMR, and 13C NMR). The suggested mechanisms of the heterocyclization step and the theoretically tautomeric forms of key intermediate 2-iminothiazoldin-4-ones (3 and 4) have been shown in Scheme 2. The features of a γ-lactam heterocycle for the compounds of series 3 and 4 were supported by the IR spectral data. Characteristic peaks of γ-lactam heterocyclic compounds were determined to be 3,174–3,072 cm−1 (multiple band) for the –NH group, and a strong band of the –C=O group in the 1,725–1,687 cm−1 region (Ottana et al., 2005; Pavia et al., 2007). IR spectra of all the compounds were in accordance with the reported values for the characteristic groups.
All the compounds (5a–e and 6a–e) can exist as potential E and Z geometrical isomers; the Z conformation of the 5 exocyclic C=C double bond was assigned on the basis of 1H NMR spectroscopy and on the basis of tha literature data for analogous 4-thiazolidinones and 2,4- thiazolidinediones (Bruno et al., 2002; Ottana et al., 2005). The 1H NMR spectra of the compounds 5a–e and 6a–e showed only one kind of methine proton that, deshielded by the adjacent C=O group, was detected at 6.172–8.525 ppm, at higher chemical shift values than the expected ones for E isomers that have a methane proton with a lesser deshielding effect. The –NH proton observed at 12.322–12.729 ppm showed the substitution on 2-position rather than on 3-position, in agreement with a lactam proton since an imine proton appears at a much higher field (about 9.70) (Scheme 3).
Anti-inflammatory activity
The novel 4-thiazolidinones (5a–e and 6a–e) were explored for their in vivo anti-inflammatory activity by carrageenan-induced rat paw oedema model and in vitro screening by inhibition of albumin denaturation. For in vivo screening, injection of carrageenan into the rat paw produced a marked increase of volume from the first hour, reaching its maximal effect after 3–4 h. Pretreatment of rats with synthesized compounds, administered (50 mg/kg body weight, p.o) 1 h before the carrageenan injection, significantly attenuated inflammatory response.
The percent inhibition of inflammation was calculated for each compound at different intervals of time and compared with that of the standard. All the synthesized compounds showed maximum percent of oedema inhibition at third hour after carrageenan administration (Table 1). The 3-chloroarylidene derivative (6a) proved to be the most effective compound of the series, achieving an inhibition level (74.47 % oedema inhibition, after 3 h) similar to that of the standard drug, ibuprofen (74.56 % oedema inhibition, after 3 h) at half the dose of the standard. Moreover, the comparison between the two series of 5-arylidene-2-imino-thiazolidin-4-ones, i.e., 5-arylidene-2-(pyridin-2-yl-imino)-4-thiazolidinones (5a–e) and 5-arylidene-2-(5-methylisoxazole-3-yl-imino)-4-thiazolidinones (6a–e), indicated that the compounds substituted with 5-methyl-3-amino isoxazole moiety at the 2-position were more active as compared to those having substitution of 2-aminopyridine at the 2-position. The compounds substituted at para position showed less activity than the meta-substituted compounds at the same interval of the time. Compounds having 4-OCH3 substituents (5b and 6b) also showed very good oedema inhibition, (60.24 and 67.20 %, respectively), at the third hour. Addition of a hydroxyl group at the para position resulted in less active compounds (47.40 %, 5d and 50.80 %, 6d oedema inhibition, respectively) compared to the other compounds of the series. The replacement of the 4-hydroxy substituent with an electron withdrawing group, such as chlorine and fluorine, increased the activity of the compounds (p-Cl (5c); 56.32 %, p-F (5e); 56.68 %, p-Cl (6c); 61.32 %, p-F (6e); 55.40 %, oedema inhibition, respectively). The maximum percent inhibition found after 3 h was plotted for each compound and is shown in Fig. 1.
All the synthesized compounds (5a–e and 6a–e) were also screened in vitro, for their anti-inflammatory potential, using inhibition of albumin denaturation, and the results are summarized in Table 1. The compounds 5a and 6a emerged as the most potent compounds of the series, showing almost similar trend as observed for in vivo activity.
None of the synthesized compounds showed evidence of acute toxicity up to a dose level 500 mg/kg b.w., suggesting their good safety margin. Also, these derivatives were found to possess no ulcerogenicity, in comparison to that exhibited by the standard drug Ibuprofen, when tested.
5-Aryliden-2-imino-4-thiazolidinone derivatives were evaluated for the influence of log P value on the anti-inflammatory effect. 3-Chloroarylidene derivative, 5a was the most lipophilic (log P; 2.85) as well as one of the most active (74.47 % oedema inhibition) compounds of the series. Introduction of the hydroxyl group at para position reduced both lipophilicity and the potency (5d: log P; 1.95, 47.40 %, and 6d: log P; 1.38, 50.80 % oedema inhibition). Hence, it was found that the activity of a compound was in direct relation with its lipophilicity (Table 2).
All the synthesized compounds were hydrolyzed more in SIF than the SGF, thereby indicating their stability in the gastro-intestinal tract. In SGF, the maximum hydrolyzed compound of the series was 5c (40.67 %) while the least hydrolyzed compound was 6b (3.69 %). In SIF, compound 5c (96.42 %) was the maximum hydrolyzed while 6e (13.79 %) was the least hydrolyzed.
The inflammation induced by carrageenan is believed to be biphasic. The early phase (up to 1 h) involves the release of histamine, serotonin, and bradykinin, and the late phase (over 1 h) is due to the release of prostaglandin like substances. Therefore, the decrease of inflammation in the second phase indicates the inhibition of cyclooxygenase enzyme (Salvemini et al., 1996; Sauzem et al., 2008). Carrageenan-induced paw oedema was significantly prevented in a time-dependent manner by previous treatment with compounds 5a–e and 6a–e (Table 1). None of the tested compounds exhibited inhibitory effect up to 1 h after the carrageenan injection. However, all the compounds inhibited increase in paw volume from the third to the fifth hour after carrageenan administration. This inhibition of paw oedema, only in the late phase of inflammation, suggests that the 5-arylidine-2-imino-thiazolidin-4-ones (5a–e and 6a–e) may act on the prostaglandin biosynthesis by inhibition of COX-2 enzyme, which is involved in the late phase of carrageenan-induced inflammation.
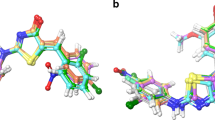
In order to ascertain COX-2 enzyme inhibition and nature of ligand–receptor interaction, docking experiments were carried out into the active site of COX-2 enzyme using Glide-Extra Precision (XP) mode from Schrödinger Inc. Results of the study showed good comparative scores (Table 2) and a remarkable correlation (−0.844114526) with in vitro data (Table 3). To validate the docking protocol, re-docking experiment was performed in which the ligand conformation of Celicoxib was extracted from the crystal structure of the corresponding COX-2/ligand complex and later docked back into the binding pocket. Glide-XP was able to perfectly reproduce the experimental position (Fig. 2) of the ligand (with a Glide score of −11.2541 and RMSD of 0.4382 from native co-crystallized structure), confirming the ability of the method to accurately predict the binding conformation. Also, to study the accurate role of electrostatic charges in determining the ligand bound conformation, QPLD has been performed which combines the docking power of Glide with the accuracy of Q Site. QPLD scores were found to be in good correlation with the results of in vitro studies and evidenced a noteworthy correlation coefficient of −0.882300416 with QPLD scores (Table 3). Moreover, MM/GBSA approach was employed to study the energetics of biomolecular systems and especially to determine the binding-free energies in bimolecular complex (i.e., protein-drug complex). A reasonable correlation of MM/GBSA scores with in vivo (−0.682696804) as well as in vitro (−0.730207507) was observed (Table 3). Finally, results of the docking studies showed that compounds 5a and 6a were the most promising compounds in the series, with good scoring functions (Table 2).
The most stable ligand–receptor complex configurations and the interactions of the most active compounds 5a, and 6a with amino acid residues have been depicted in Figs. 2, 3, 4, 5, 6, 7 and 8. Compounds 5a and 6a clearly preferred a single binding position in the typical binding pocket of COX-2, which were the best predictions since these had the lowest energy among the others. In such an orientation, the bound conformation of compound 6a was engaged in hydrogen bonding with Tyr341 and Arg499; polar interaction with Gln178, Ser339, Ser516, and His75; charged (positive) interaction with Arg106 and Arg499 and was involved in hydrophobic contacts with the residues of the binding pocket Val509, Tyr341, Ala513, Leu517, Val335, Tyr334, Trp373, Phe367, Tyr371, Leu370, Met508, Leu338, Ile503, Ala502, and Phe504 (Figs. 4, 6). However, that of compound 5a involved hydrogen bonding with Tyr341 and Arg106; polar interaction with Ser339, Ser516, and His75, charged (positive) interaction with Arg106 and Arg499, hydrophobic interaction with Val509, Tyr341, Ala513, Leu345, Met99, Val102, Leu517, Val335, Tyr334, Phe367, Tyr371, Trp373, Met508, Leu370, Leu338, and Phe504 (Figs. 3, 5).
Previous studies had shown that the COX-2 binding site was 25 % larger than that of COX-1, due to the substitution of a single amino acid (valine for isoleucine at position 523), thus creating a secondary internal pocket not accessible in COX-1(Gierse et al., 1996). It was suggested that the selectivity of Celecoxib for COX-2 over COX-1 might result from additional contacts with residues along this deeper binding region. In fact, the structural analysis of Celecoxib/COX-2 complex indicated that the selective inhibitor molecule interacted with amino acid residues located at the bottom of the COX-2 gorge. It is noteworthy that compounds 5a and 6a were able to reach such a gorge, rationalizing the COX- 2 selectivity.
Also, in silico pharmacokinetic properties of the synthesized compounds 5a–e and 6a–e were predicted using Qik Prop program from Schrodinger Inc., which showed promising results (Table 4). Therefore, all the synthesized derivatives can be good drug candidates as they fulfill the ADME prerequisites.
Conclusion
The synthetic strategy adopted here is a versatile approach for the preparation of thiazolidinone derivatives. The results obtained expound that the different synthesized derivatives can be utilized as promising anti-inflammatory agents when a differently substituted arylidene moiety is inserted at position-5 (5a–e and 6a–e). However, compounds substituted with isoxazole moiety at position-2 (6a–e) were found to be more potent anti-inflammatory agents than the compounds substituted with aminopyridine moiety (5a–e). In particular, on the substitution with meta-chloroarylidene (6a), a good in vivo (74.47 % oedema inhibition, 3 h), in vitro (80.20 % inhibition of denaturation) anti-inflammatory profile was achieved reaching Ibuprofen level but half at the dose. The compounds substituted at para position showed less potency than the meta-substituted compounds at the same intervals of time. Also, a straight relationship was observed between log P and anti-inflammatory activity, i.e., more lipophilic derivatives were found to possess better activity. The synthesized thiazolidin-4-one derivatives neither possess any acidic functional group such as carboxyl nor a functional group susceptible to hydrolysis. Moreover, the results of the stability studies in SGF indicated that the compounds were stable in SGF, thus indicating their stability in the gastro-intestinal tract. ADME characteristics of the synthesized compounds were also approximated by in silico methods and found to be within the range. All the compounds were found to inhibit paw oedema only in the late phase (3 h) of inflammation, suggesting that they were involved in the inhibition of the prostaglandin biosynthesis, similar to coxib-like compounds, which was also proven by different docking algorithms (such as XP-Glide, MM/GBSA, and QPLD) with good docking scores. Their interaction to COX-2 enzyme binding site showed excellent correlation with the anti-inflammatory activity. None of the compound in the synthesized series was found to possess ulcerogenicity or acute toxicity at extended dose levels (upto 500 mg/kg b.w.) suggesting their safety in terms of undesired side effects, such as ulceration, associated with most of the NSAIDs.
Experimental
Chemistry
Synthetic starting material, reagents and solvents were of reagent grade and procured from S.D. Fine Chemicals, Mumbai and were used without further purification. Melting range was determined using melting point apparatus by open capillary method and is uncorrected.
The progress of the reactions was monitored by performing thin layer chromatography (TLC) on silica gel G plates, using iodine vapors and UV light as the visualizing agents. UV spectra were recorded on Shimadzu UV-1700 spectrophotometer. IR spectra were recorded, using KBr pellet technique, on a FTIR-8400S spectrophotometer (Shimadzu) and the reported values are expressed in cm−1. Mass spectra were recorded on a JEOL-AccuTOF JMS-T100LC spectrometer having a DART (Direct Analysis in Real Time) source. 1H-NMR spectra were recorded on a Bruker DRX-300 (at 300 MHz) spectrometer and 13C-NMR data were recorded on Advance-400 MHz, Bruker (Switzerland) spectrometer. Chemical shifts are reported as δ (ppm) relative to TMS as the internal standard. Elemental analysis was performed on an Elemental Vario EL III analyzer, for C, H and N. The found values were within the range of the theoretical ones. The spectral and elemental analysis was carried out at the Sophisticated Analytical Instrument Facility (SAIF), Central Drug Research Institute (CDRI), Lucknow.
General procedure for synthesis of 2-chloro-N-(etheroaryl) acetamide (1 and 2)
To a solution of appropriate amine (0.02 mol) in 6.5 mL dimethylformamide (DMF), a cooled solution of chloroacetylchloride (2.5 mL, 0.03 mol) in 6.5 mL DMF was added drop wise. The reaction mixture was refluxed on a water bath at 50 °C for 4 h. The completion of the reaction was monitored by TLC. The residue was washed with 10 % aqueous sodium bicarbonate solution followed by cold water. The crude product was dried and crystallized from ethanol (Geronikaki and Theofilidis 1992).
2-Chloro-N-(pyridin-2-yl) acetamide (1)
Yield: 15.28 %. mp: 180–182 °C, TLC: methanol/diethylether (0.5:9.5). IR (KBr) ν max/cm−1: 3064.75 (N–H), 1770.53 (C=O), 1649.02 (C=N), 773.40 (C–Cl). C7H7ClN2O (170.02); Observed molecular ion peak [M+1]+: 171.03.
2-Chloro-N-(5-methylisoxazol-3-yl) acetamide (2)
Yield: 25.97 %. mp: 192–194 °C, TLC: methanol/diethylether (0.5:9.5). IR (KBr) νmax/cm−1: 3222.83 (N–H), 1712.67 (C=O), 1639.38 (C=N), 1205.43 (C–O), 756.04 (C–Cl). C6H7ClN2O2 (174.02); Observed molecular ion peak [M+1]+: 175.0.
General procedure for synthesis of 2-(heteroarylimino) thiazolidin-4-ones (3 and 4)
A solution of 2-chloro-N-(etheroaryl) acetamide (5 mmol) and ammonium thiocyante (0.759 g, 10 mmol) in 20 mL of absolute ethanol was refluxed for 1 h and allowed to stand overnight. The completion of the reaction was monitored by TLC. The precipitate was filtered, washed with water, and recrystallized from dioxane (Vicini et al., 2008).
2-(Pyridin-2-yl-imino) thiazolidin-4-ones (3)
Yield: 19.25 %. mp: 194–198 °C, TLC: methanol/diethylether (0.5:9.5). IR (KBr) ν max/cm−1: 3058.89 (N–H stretch), 1687.60 (C=O lactam), 1623.95 (C=N stretch), 1556.45 (N–H bend). C8H7N3OS (193.03); Observed molecular ion peak [M+1]+: 194.08.
2-(5-Methylisoxazole-3-yl-imino) thiazolidin-4-ones (4)
Yield: 55.26 %. mp: 204–206 °C, TLC: methanol/diethylether (0.5:9.5). IR (KBr) ν max/cm−1: 3415.70 (N–H stretch), 1724.24 (C=N stretch), 1635.52 (N–H bend), 1600.81 (C=O lactam), 1197.71 (C–O stretch). C7H7N3O2S (195.03); Observed molecular ion peak [M+1]+: 196.04.
General procedure for synthesis of 5-arylidene-2-(heteroarylimino)-4-thiazolidinones (5a–e and 6a–e)
A well-stirred solution of 2-(heteroarylimino) thiazolidin-4-one (4 mmol) in 35 mL of acetic acid was buffered with sodium acetate (8 mmol) and added to the appropriate arylaldehyde (6 mmol). The solution was refluxed over different periods till the completion of the reaction. The reaction mixture was then cooled to room temperature and the solid precipitate was filtered, washed with water and then recrystallized from dioxane. The completion of the reaction was monitored by TLC using the solvent system hexane:ethylacetate (2:1) (Vicini et al., 2008).
5-(3-Chlorobenzyliden)-2-(pyridin-2-yl-imino)-4-thiazolidinone (5a)
Reaction time: 18 h. Yield: 50.75 %, mp: 192–194 °C. IR (KBr) ν max/cm−1: 3060.82 (N–H stretch), 1710.74 (C=O lactam), 1625.88 (C=N stretch), 1556.45 (N–H bend). 1H NMR (CDCl3–DMSO; δ ppm): 12.531 (s, 1H), 8.508–8.521 (d, 1H), δ 7.848–7.895 (t, 1H), 7.735 (s, 1H), 7.438–7.641 (m, 4H), 7.193–7.233 (t, 2H). 13C NMR (CDCl3–DMSO; δ ppm): 175.21 (CONH, amide), 117.04 (C=C, ethylene), 165.07 (C=N, imino), 127.5–130.04 (4CH, benzene), 133.05 (2C, benzene), 159.26 (C=N, pyridine), 150.30 (CH, pyridine), 116.25 (2CH, pyridine), 135.96 (CH, pyridine). MS [M+1]+: 316. Anal. Calcd. for C15H10ClN3OS (315.02): C, 57.05; H, 3.19; N, 13.31. Found C, 57.45; H, 3.16; N, 13.46.
5-(4-Methoxybenzyliden)-2-(pyridin-2-yl-imino)-4-thiazolidinone (5b)
Reaction time: 17 h. Yield: 23.33 %; mp: 237–240 °C. IR (KBr) ν max/cm−1: 3467.77 (N–H stretch), 1706.88 (C=O lactam), 1627.81 (C=N stretch), 1560.30 (N–H bend). 1H NMR (CDCl3–DMSO; δ ppm): 12.363 (s, 1H), 8.509–8.521 (d, 1H), 7.834–7.882 (t, 1H), 70619–70649 (d, 3H), 7.179–7.217 (m, 2H), 7.117–7.147 (d, 2H). 13C NMR (CDCl3–DMSO; δ ppm): 174.71 (CONH, amide), 115.46 (C=C, ethylene), 164.23 (C=N, imino), 114.05 (2CH, benzene), 130.41 (2CH, benzene), 126.01 (C, benzene), 160.63 (2CH, benzene), 56.12 (CH3, methyl) 160.33 (C=N, pyridine), 151.02 (CH, pyridine), 118.3 (2CH, pyridine), 136.11 (CH, pyridine). MS [M+1]+: 312.1. Anal. Calcd. for C16H13N3O2S (311.07): C, 61.72; H, 4.21; N, 13.50. Found C, 61.97; H, 4.16; N, 13.46.
5-(4-Chlorobenzyliden)-2-(pyridine-2-yl-imino)-4-thiazolidinone (5c)
Reaction time: 10 h. Yield: 68.99 %; mp: 216–218 °C. IR (KBr) ν max/cm−1: 3475.49 (N–H stretch), 1710.74 (C=O lactam), 1685.67 (C=N stretch), 1625.88 (N–H bend). 1H NMR (CDCl3–DMSO; δ ppm): 8.503–8.516 (d, 1H), 7.845–7.894 (t, 1H), 7.613–7.703 (m, 5H), 7.196–7.233 (m, 2H). 13C NMR (CDCl3–DMSO; δ ppm): 175.69 (CONH, amide), 118.04 (C=C, ethylene), 166.13 (C=N, imino), 129.35–136.11 (6CH, benzene), 161.37 (C=N, pyridine), 151.02 (CH, pyridine), 117.04 (2CH, pyridine), 134.22 (CH, pyridine). MS [M+1]+: 316. Anal. Calcd. for C15H10ClN3OS (315.02): C, 57.05; H, 3.19; N, 13.31. Found C, 56.21; H, 3.36; N, 13.04.
5-(4-Hydroxybenzyliden)-2-(pyridine-2-yl-imino)-4-thiazolidinone (5d) (Wang et al., 2008)
Reaction time: 22 h. Yield: 33.69 %; mp: 220–223 °C. IR (KBr) ν max/cm−1: 3404.13 (N–H stretch), 1600.81 (C=O lactam), 1581.52(C=N stretch), 1552.59 (N–H bend). 1H NMR (CDCl3-DMSO; δ ppm): 12.322 (s, 1H), 10.239 (s, 1H), 8.512-8.525 (d, 1H), 7.827–7.874 (t, 1H), 7.510–7.562 (t, 3H), 7.169–7.208 (m, 2H), 6.922–6.951 (d, 2H). 13C NMR (CDCl3-DMSO; δ ppm): 175.88 (CONH, amide), 118.32 (C=C, ethylene), 165.07 (C=N, imino), 115.77 (2CH, benzene), 131.17 (2CH, benzene), 159.84 (C, benzene), 156.89 (C=N, pyridine), 148.98 (CH, pyridine), 113.75 (2CH, pyridine), 137.18 (CH, pyridine). MS [M+1]+: 298.08. Anal. Calcd. for C15H11N3O2S (297.06): C, 57.05; H, 3.19; N, 13.31. Found C, 57.93; H, 3.33; N, 13.39.
5-(4-Fluorobenzyliden)-2-(pyridine-2-yl-imino)-4-thiazolodinone (5e)
Reaction time: 15 h. Yield: 29.69 %; mp: 198-200 °C. IR (KBr) ν max/cm−1: 3064.68 (N–H stretch), 1710.74 (C=O lactam), 1683.74 (C=N stretch), 1558.38 (N–H bend), 1234.36 (C–F stretch). 1H NMR (CDCl3–DMSO; δ ppm): 12.473 (s, 1H), 8.507–8.520 (d, 1H), 7.844–7.893 (t, 1H), 7.665–7.760 (m, 3H), 7.388–7.446 (t, 2H), 7.205–7.230 (d, 2H). 13C NMR (CDCl3–DMSO; δ ppm): 171.21 (CONH, amide), 121.04 (C=C, ethylene), 162.07 (C=N, imino), 130.04 (C, benzene), 115.77 (2CH, benzene), 131.17 (2CH, benzene), 159.84 (C, benzene), 159.32 (C=N, pyridine), 150.48 (CH, pyridine), 116.25 (2CH, pyridine), 135.96 (CH, pyridine). MS [M+1]+: 300.08. Anal. Calcd. for C15H10 FN3OS (299.05): C, 60.19; H, 3.37; N, 14.04.
5-(3-Chlorobenzyliden)-2-(5-methylisoxazol-3-yl-imino)-4-thiazolidinone (6a)
Reaction time: 17 h. Yield: 93.70 %; mp: 198–200 °C. IR (KBr) ν max/cm−1: 3060.82 (N–H stretch), 1710.74 (C=O lactam), 1625.88 (C=N stretch), 1556.45 (N–H bend), 1184.21 (C–O strech). 1H NMR (CDCl3–DMSO; δ ppm): 12.729 (s, 1H), 7.698 (s, 2H), 7.521–7.518 (m, 3H), 6.233 (s, 3H), 2.405 (d, 3H). 13C NMR (CDCl3–DMSO; δ ppm): 175.21 (CONH, amide), 117.91 (C=C, ethylene), 166.54 (C=N, imino), 127.29–130.64 (4CH, benzene), 133.77 (2C, benzene), 173.46 (C, isoxazole), 168.63 (C, isoxazole), 101.27 (CH, isoxazole), 13.86 (CH3, methyl). MS [M+1]+: 320.02. Anal. Calcd. for C14H10N3O2S (319.06): C, 52.59; H, 3.15; N, 13.14. Found C, 52.93; H, 3.07; N, 13.26.
5-(4-Methoxybenzyliden)-2-(5-methylisoxazol-3-yl-imino)-4-thiazolidinone (6b)
Reaction time: 16 h. Yield: 46.00 %; mp: 230–232 °C. IR (KBr) ν max/cm−1: 3120.61 (N–H stretch), 1714.60 (C=O lactam), 1639.38 (C=N stretch), 1595.02 (N–H bend), 1174.57 (C–O strech). 1H NMR (CDCl3–DMSO; δ ppm): 7.625 (s, 1H), 7.555–7.583 (d, 2H), 7.098–7.127 (d, 2H), 6.172 (s, 1H), 2.500 (s, 3H). 13C NMR (CDCl3–DMSO; δ ppm): 175.21 (CONH, amide), 117.91 (C=C, ethylene), 166.54 (C=N, imino), 114.05 (2CH, benzene), 131.07 (2CH, benzene), 125.23 (C, benzene), 161.25 (2CH, benzene), 57.01 (CH3, methyl), 173.72 (C, isoxazole), 167.44 (C, isoxazole), 102.09 (CH, isoxazole), 14.52 (CH3, methyl). MS [M+1]+: 316.10. Anal. Calcd. for C15H13N3O3S (315.06): C, 57.13; H, 4.16; N, 13.33. Found C, 56.01; H, 4.07; N, 13.41.
5-(4-Chlorobenzyliden)-2-(5-methylisoxazol-3-yl-imino)-4-thiazolidinone (6c)
Reaction time: 8 h. Yield: 43.13 %; mp: 224–26 °C. IR (KBr) ν max/cm−1: 2925.81 (N–H stretch), 1724.24 (C=O lactam), 1637.45 (C=N stretch), 1600.81 (N–H bend), 1093.56 (C–O strech). 1H NMR (CDCl3-DMSO; δ ppm): 7.695 (s, 1H), 7.625 (s, 4H), 6.223 (s, 1H), 2.402 (s, 3H). 13C NMR (CDCl3–DMSO; δ ppm): 178.02 (CONH, amide), 119.23 (C=C, ethylene), 165.66 (C=N, imino), 129.35–130.39 (4CH, benzene), 133.48 (2C, benzene), 171.28 (C, isoxazole), 168.11 (C, isoxazole), 101.55 (CH, isoxazole), 16.10 (CH3, methyl). MS [M+1]+: 321.01. Anal. Calcd. for C14H10ClN3O3S (320.07): C, 52.59; H, 3.15; N, 13.14. Found C, 53.04; H, 3.12; N, 13.07.
5-(4-Hydroxybenzyliden)-2-(5-methylisoxazol-3-yl-imino)-4-thiazolidinone (6d)
Reaction time: 18 h. Yield: 55.41 %; mp: 196–198 °C. IR (KBr) ν max/cm−1: 3253.69, (N–H stretch), 1679.88, (C=O lactam), 1631.67, (C=N Stretch), 1577.66 (N–H bend), 1174.57, (C–O stretch). 1H NMR (CDCl3–DMSO; δ ppm): 12.501 (s, 1H), 10.287 (s, 1H), 7.612 (s, 1H), 7.458–7.486 (d, 2H), 6.918–6.946 (d, 2H), 6.221 (s, 1H), 2.403 (s, 3H). 13C NMR (CDCl3–DMSO; δ ppm): 175.67 (CONH, amide), 117.14 (C=C, ethylene), 165.69 (C=N, imino), 131.17 (2CH, benzene), 115.76 (2CH, benzene), 124.69 (C, benzene), 159.84 (C, benzene), 177.04 (C, isoxazole), 167.51 (C, isoxazole), 100.16 (CH, isoxazole), 14.33 (CH3, methyl). MS [M+1]+: 302.16. Anal. Calcd. for C14H10ClN3O3S (301.05): C, 55.89; H, 3.68; N, 13.95. Found C, 55.66; H, 3.52; N, 13.87.
5-(4-Fluorobenzyliden)-2-(5-methylisoxazol-3-yl-imino)-4-thiazolidinone (6e)
Reaction time: 11 h. Yield: 38.38 %; mp: 174–176 °C. IR (KBr) ν max/cm−1: 3124.47, (N–H stretch), 1618.46, (C=O lactam), 1600.81 (N–H bend), 1641.31, (C=N Stretch), 1193.85, (C–O stretch), 1240.14 (C–F, stretch). 1H NMR (CDCl3-DMSO; δ ppm): 12.671 (s, 1H), 7.664–7.719 (m, 3H), 7.373–7.19 (t, 2H), 6.227 (s, 1H), 2.405 (s, 3H). 13C NMR (CDCl3–DMSO; δ ppm): 173.21 (CONH, amide), 119.04 (C=C, ethylene), 168.07 (C=N, imino), 131.22 (C, benzene), 116.08 (2CH, benzene), 131.77 (2CH, benzene), 160.12 (C, benzene), 172.58 (C, isoxazole), 165.44 (C, isoxazole), 101.92 (CH, isoxazole), 18.26 (CH3, methyl). MS [M+1]+: 304.10. Anal. Calcd. for C14H10FN3O2S (303.05): C, 55.44; H, 3.32; N, 13.85.
Pharmacology
In vivo anti-inflammatory screening
Male Wistar albino rats, weighing 175–300 g, were housed in polypropylene cages with steel net, under standard living conditions of temperature 25 ± 2 °C and relative humidity of 55 ± 5, with regular 12 h light and 12 h dark cycles and allowed free access to standard laboratory food and water. All animals were treated humanely in accordance with the guidelines laid down by the Institutional Animal Ethics Committee (IAEC) (Raghvan 2000). The experimental protocols were approved by the IAEC (BBDGEI/IAEC/13/2011).
Group of five animals were used for control and treated rats. The test compounds (at a dose of 50 mg/kg) and the standard drug, ibuprofen (at the dose 100 mg/kg), were used for the study by suspending in 1.0 % sodium carboxymethyl cellulose (NaCMC) and administered orally (p.o.). The paw of the same animal that is not induced for the oedema was used as control and the control group received 1 ml of 1.0 % NaCMC, orally. After 1 h of treatment, 0.1 ml of 1 % carrageenan solution in normal saline was injected subcutaneously into the plantar side of left hind paw to induce inflammation in tested animals. The paw volume was measured, by plethysmometer, immediately after injection and at the time intervals of 1, 3, 5, and 7 h, respectively. The mean paw volume of each group at different time intervals was statically evaluated and compared with that of the control group at the same time intervals. The percent inhibition of each compound was calculated by the following equation; (Ottana et al., 2005)
where V t = volume of paw of tested group, and V c = volume of paw of control group.
In vitro anti-inflammatory screening
The synthesized compounds were screened for their anti-inflammatory activity using inhibition of albumin denaturation technique, which was studied according to Mizushima and Kobayashi (Mizushima and Kobayashi 1968) with a slight modification. The standard drug and test compounds were dissolved in a minimum quantity of DMF and diluted with phosphate buffer (pH 7.4). Final concentration of DMF in all solutions was less than 2.5 %. Test solution (1 mL), containing different concentrations of drug, was mixed with 1 mL of 1 % albumin solution in phosphate buffer and incubated at 27 ± 1 °C in a water bath for 15 min. Denaturation was induced by keeping the reaction mixture at 60 ± 1 °C in the water bath for 10 min. After cooling, the turbidity was measured at 660 nm using a UV–Visible Spectrophotometer UV-1700 (Shimadzu). Percent inhibition of denaturation was calculated from control, where no drug was added. Each experiment was done in triplicate and the average was taken. Ibuprofen was used as the standard drug.
The percent inhibition of albumin denaturation was calculated using following formula;
where V t = Mean absorbance of test sample; V c = Mean absorbance of control
Ulcerogenicity
Ulcerogenicity newly synthesized compounds was evaluated by the method of (Verma et al., 1981). For this, Wistar albino rats were fasted for 24 h prior to drug administration, and then all the animals were sacrificed 8 h after drug treatment. Their stomachs and small intestines were microscopically examined to assess the incidence of hyperemia, shedding of epithelium, petechial and frank hemorrhages and erosion or discrete ulceration, with or without perforation. The presence of any one of these criteria was considered to be an evidence of ulcerogenic activity.
Acute toxicity study
Acute toxicity of synthesized compounds was investigated, in BALB/c albino mice, according to the method of Smith (Smith 1960). The test compounds were administered orally in separate groups of animals and after 24 h of drug administration, mortality in each group was observed.
Partition coefficient
Partition coefficient of the synthesized compounds was determined by “Shake Flask Method” (Aulton 2002; Harrold and Yee 2005). Absorbance of each compound was plotted against respective concentrations to draw a standard curve. Regression analysis was performed and equations for a straight line were obtained for each compound. Concentration of the compounds in organic phase (octanol) and aqueous phase was calculated by straight line equation.
In vitro hydrolysis
To determine the stability of compounds in the GI tract, all the compounds were subjected to simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) in vitro. Both SGF and SIF were prepared as per the USP method (The United State Pharmacopoeia. USP-NF-XXV 2002). For this, 10 mg of the synthesized compound was dispersed/dissolved in 100 ml of SGF/SIF, and then the resulting mixture was shaken in an orbital shaker at a temperature of 37 ± 2 °C. Sampling was done at time intervals of 15 min for 1.5 h (SGF)/and 2 h (SIF) and the volume was replaced after each withdrawal. The withdrawn samples were suitably diluted, filtered and analyzed spectrophotometrically at the designed absorption maxima of each compound. Percent hydrolysis by subtracting the per cent remaining from 100 % was calculated.
Docking studies
The crystal structure of COX-2 (PDB ID: 3LN1), retrieved from the Protein Data Bank (PDB), (Wang et al., 2010) was used for molecular docking. The crystal structure of the COX-2 protein was subsequently optimized and minimized with the “protein preparation wizard” workflow. The ligands were built using Maestro 9.3 build panel and prepared by LigPrep 2.5 version v25111(Schrödinger, LLC, USA) application that uses optimized potential liquid simulations (OPLS) 2005 force field, and it gave the corresponding energy minima 3D conformers of the ligands. The default settings were used for all other parameters. All ligand atoms, but no protein atoms, were allowed to move during the calculations. In order to gain some structural insights into the binding mode of the ligand and establish a correlation with biological activity, all the synthesized compounds were computationally docked into the X-ray crystal structure of macromolecule target, COX-2 enzyme active site using Glide-XP and QPLD algorithms, present in Maestro user interface of Schrödinger Inc (Maestro, version 9.3, Schrödinger, LLC, New York, NY, 2012).
Post docking calculations
The post docking calculations for the docked complexes were performed by Prime MM/GBSA module of Schrodinger suite, which predicts the free energy of binding for a given receptor and a set of given ligands. As the docking algorithms provide good quality binding sites, an energy function with a well-defined description of binding contributions was utilized to re-score the docking results. The total free energy of binding is then expressed by equation given below:
where ΔE MM is the change of the gas phase MM energy upon binding, and includes ΔE internal (bond, angle, and dihedral energies), ΔE Elect, (electrostatic), and ΔE VDW (van der Waals) energies. ΔG solv is the change of the solvation free energy upon binding, and includes the electrostatic solvation free energy ΔG solvGB (polar contribution calculated using generalized Born model), and the nonelectrostatic salvation component ΔG solvSA (nonpolar contribution estimated by solvent accessible surface area). Finally, TΔS is the change of the conformational entropy upon binding (Lyne et al., 2006; Munoz et al., 2012).
In silico prediction of pharmacokinetic properties
Nearly 40 % of drug candidates fail in clinical trials due to poor ADME (absorption, distribution, metabolism, and excretion) properties. These late-stage failures contribute significantly to the rapidly escalating cost of new drug development. The ability to detect problematic candidates early can dramatically reduce the amount of wasted time and resources, and streamline the overall development process. QikProp, version 3.5, Schrödinger, LLC, New York, NY, 2012, program was used for in silico prediction of pharmacokinetic properties of the synthesized compounds.
Statistical analysis
All the values of the experimental results are expressed as mean ± SD and analyzed by one-way ANOVA followed by Dunnett’s test for the possible significant (P < 0.01) identification between various groups. Statistical analysis was carried out using Graph pad prism 3.0 (Graph pad software, San Diego, CA).
References
Agrawal OP, Sonar PK, Saraf SK (2013) 4-Thiazolidinone and 1-thia-3,4,9-triaza fluorene conjugates: synthesis, characterization and antimicrobial screening. Med Chem Res 22:1972–1978
Aulton ME (2002) Pharmaceutics: the science of dosage form design, 2nd edn. Churchill Livingstone, Edinburg
Balsamo A, Coletta I, Guglielmotti A, Landolfi C, Mancini F, Martinelli A, Milanese C, Minutolo F, Nencetti S, Orlandini E, Pinza M, Rapposelli S, Rossello A (2003) Synthesis of heteroaromatic analogues of (2-aryl-1-cyclopentenyl-1-alkylidene)-(arylmethyloxy)amine COX-2 inhibitors: effects on the inhibitory activity of the replacement of the cyclopentene central core with pyrazole, thiophene or isoxazole ring. Eur J Med Chem 38:157–168
Bruno G, Costantino L, Curinga C, Maccari R, Monforte F, Nicolo F, Ottana R, Vigorita MG (2002) Synthesis and aldose reductase inhibitory activity of 5-arylidene-2,4-thiazolidinediones. Bioorg Med Chem 10:1077–1084
Chawla P, Singh R, Saraf SK (2011a) Effect of chloro and fluoro groups on the antimicrobial activity of 2,5-disubstituted 4-thiazolidinones: a comparative study. Med Chem Res 21:3263–3271
Chawla P, Singh R, Saraf SK (2011b) Syntheses and evaluation of 2,5-disubstituted 4-thiazolidinone analogues as antimicrobial agents. Med Chem Res 21:2064–2071
Chen H, Bai J, Jiao L, Guo Z, Yin Q, Li X (2009) Design, microwave-assisted synthesis and HIV-RT inhibitory activity of 2-(2,6-dihalophenyl)-3-(4,6-dimethyl-5-(un)substituted-pyrimidin-2-yl)thiazolidin-4-ones. Bioorg Med Chem 17:3980–3986
de Leval X, Delarge J, Somers F, de Tullio P, Henrotin Y, Pirotte B, Dogne JM (2000) Recent advances in inducible cyclooxygenase (COX-2) inhibition. Curr Med Chem 7:1041–1062
Geronikaki A, Theofilidis G (1992) Synthesis of 2-(aminoacetylamino)thiazole derivatives and comparison of their local anaesthetic activity by the method of action potential. Eur J Med Chem 27:709–716
Geronikaki AA, Lagunin AA, Hadjipavlou-Litina DI, Eleftheriou PT, Filimonov DA, Poroikov VV, Alam I, Saxena AK (2008) Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition. J Med Chem 51:1601–1609
Gierse JK, McDonald JJ, Hauser SD, Rangwala SH, Koboldt CM, Seibert K (1996) A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem 271:15810–15814
Goel B, Ram T, Tyagi R, Bansal E, Kumar A, Mukherjee D, Sinha JN (1999) 2-Substituted-3-(4-bromo-2-carboxyphenyl)-5-methyl-4-thiazolidinones as potential anti-inflammatory agents. Eur J Med Chem 34:265–269
Gouveia FL, de Oliveira RM, de Oliveira TB, da Silva IM, do Nascimento SC, de Sena KX, de Albuquerque JF (2009) Synthesis, antimicrobial and cytotoxic activities of some 5-arylidene-4-thioxo-thiazolidine-2-ones. Eur J Med Chem 44:2038–2043
Graul A, Martel AM, Castaner J (1997) Celecoxib: anti-inflammatory, cycloxygenase-2 inhibitor. Drugs Future 22:711–714
Harrold MW, Yee NS (2005) Principles of Pharmacodynamics and Medicinal Chemistry. In: Hansch C, Sammes PG, Taylor JB, Ramsden CA (eds) Comprehensive medicinal chemistry, 6th edn. Elsevier Publication, New Delhi (India), pp 270–271
Haviv F, DeNet RW, Michaels RJ, Ratajczyk JD, Carter GW, Young PR (1983) 2-[(Phenylthio)methyl]pyridine derivatives: new antiinflammatory agents. J Med Chem 26:218–222
Havrylyuk D, Mosula L, Zimenkovsky B, Vasylenko O, Gzella A, Lesyk R (2010) Synthesis and anticancer activity evaluation of 4-thiazolidinones containing benzothiazole moiety. Eur J Med Chem 45:5012–5021
Hosni HM, Abdulla MM (2008) Anti-inflammatory and analgesic activities of some newly synthesized pyridinedicarbonitrile and benzopyranopyridine derivatives. Acta Pharm 58:175–186
Kumar A, Bansal D, Bajaj K, Sharma S, Archana, Srivastava VK (2003) Synthesis of some newer derivatives of 2-amino benzoic acid as potent anti-inflammatory and analgesic agents. Bioorg Med Chem 11:5281–5291
Kumar A, Rajput CS, Bhati SK (2007) Synthesis of 3-[4’-(p-chlorophenyl)-thiazol-2’-yl]-2-[(substituted azetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorg Med Chem 15:3089–3096
Leval X, Julemont F, Delarge J, Pirotte B, Dogne JM (2002) New trends in dual 5-LOX/COX inhibition. Curr Med Chem 9:941–962
Lyne PD, Lamb ML, Saeh JC (2006) Accurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM–GBSA scoring. J Med Chem 49:4805–4808
Mizushima Y, Kobayashi M (1968) Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol 20:169–173
Munoz C, Adasme F, Alzate-Morales JH, Vergara-Jaque A, Kniess T, Caballero J (2012) Study of differences in the VEGFR2 inhibitory activities between semaxanib and SU5205 using 3D-QSAR, docking, and molecular dynamics simulations. J Mol Graph Model 32:39–48
Omar K, Geronikaki A, Zoumpoulakis P, Camoutsis C, Sokovic M, Ciric A, Glamoclija J (2010) Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg Med Chem 18:426–432
Ottana R, Mazzon E, Dugo L, Monforte F, Maccari R, Sautebin L, De Luca G, Vigorita MG, Alcaro S, Ortuso F, Caputi AP, Cuzzocrea S (2002) Modeling and biological evaluation of 3,3’-(1,2-ethanediyl)bis[2-(4-methoxyphenyl)-thiazolidin-4-one], a new synthetic cyclooxygenase-2 inhibitor. Eur J Pharmacol 448:71–80
Ottana R, Maccari R, Barreca ML, Bruno G, Rotondo A, Rossi A, Chiricosta G, Di Paola R, Sautebin L, Cuzzocrea S, Vigorita MG (2005) 5-Arylidene-2-imino-4-thiazolidinones: design and synthesis of novel anti-inflammatory agents. Bioorg Med Chem 13:4243–4252
Pavia DL, Lampman GM, Kriz GS (2007) Spectroscopy, 1st edn. Cenage Learning India Private Limited, Australia
Previtera T, Basile M, Vigorita MG, Fenech G, Occhiuto F, Circosta C, de Pasquale RC (1987) 3,3′-Di [1,3-thiazolidine-4-one] system. II. Anti-inflammatory and anti-histaminic properties in new substituted derivatives. Eur J Med Chem 22:67–74
Raghvan PV (2000) Expert consultant, CPCSEA, OECD, Guideline No. 420
Rawal RK, Tripathi R, Katti SB, Pannecouque C, De Clercq E (2007) Design, synthesis, and evaluation of 2-aryl-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Bioorg Med Chem 15:1725–1731
Sadashiva CT, Chandra JN, Kavitha CV, Thimmegowda A, Subhash MN, Rangappa KS (2009) Synthesis and pharmacological evaluation of novel N-alkyl/aryl substituted thiazolidinone arecoline analogues as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Eur J Med Chem 44:4848–4854
Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT (1996) Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol 303:217–220
Sauzem PD, Machado P, Rubin MA, da S Sant’Anna G, Faber HB, de Souza AH, Mello CF, Beck P, Burrow RA, Bonacorso HG, Zanatta N, Martins MA (2008) Design and microwave-assisted synthesis of 5-trifluoromethyl-4,5-dihydro-1H-pyrazoles: novel agents with analgesic and anti-inflammatory properties. Eur J Med Chem 43:1237–1247
Smith QE (1960) Pharmacological screening tests progressive. In: Medicinal Chemistry, vol I. Butterworths, London
Sondhi SM, Jain S, Dinodia M, Kumar A (2008) Synthesis of some thiophene, imidazole and pyridine derivatives exhibiting good anti-inflammatory and analgesic activities. Med Chem 4:146–154
Thirumurugan P, Mahalaxmi S, Perumal P (2010) Synthesis and anti-inflammatory activity of 3-indolyl pyridine derivatives through one-pot multi component reaction. J Chem Sci 122:819–832
Tripathi AC, Gupta SJ, Fatima GN, Sonar PK, Verma A, Saraf SK (2014) 4-Thiazolidinones: The advances continue. Eur J Med Chem 72:52–77
The United State Pharmacopoeia. USP-NF-XXV (2002). United State Pharmacopoeial Convention Inc. Rockville, M.D
Vane J, Botting R (1987) Inflammation and the mechanism of action of anti-inflammatory drugs. Faseb J 1:89–96
Vazzana I, Terranova E, Mattioli F, Sparatore F (2004) Aromatic Schiff bases and 2,3-disubstituted-1,3-thiazolidin-4-one derivatives as antiinflammatory agents. ARKIVOC 5:364–374
Verma A, Saraf SK (2008) 4-thiazolidinone: a biologically active scaffold. Eur J Med Chem 43:897–905
Verma M, Sinha JN, Gujrati VR, Bhalla TN, Bhargava KP, Shanker K (1981) A new potent anti-inflammatory quinazolone. Pharmacol Res Commun 13:967–979
Vicini P, Geronikaki A, Incerti M, Zani F, Dearden J, Hewitt M (2008) 2-Heteroarylimino-5-benzylidene-4-thiazolidinones analogues of 2-thiazolylimino-5-benzylidene-4-thiazolidinones with antimicrobial activity: synthesis and structure-activity relationship. Bioorg Med Chem 16:3714–3724
Vigorita MG, Ottana R, Monforte F, Maccari R, Monforte MT, Trovato A, Taviano MF, Miceli N, De Luca G, Alcaro S, Ortuso F (2003) Chiral 3,3’-(1,2-ethanediyl)-bis[2-(3,4-dimethoxyphenyl)-4-thiazolidinones] with anti-inflammatory activity. Part 11: evaluation of COX-2 selectivity and modelling. Bioorg Med Chem 11:999–1006
Wang M, Liu Q, Yueyun ZLL, Zhou L, Sun M, Su H, Hua Y, Faming ZS (2008) Preparation of 5-membered heterocycles as regulators of glucagon-like peptide1 receptors (GLP1R). CN 101274918 A
Wang JL, Limburg D, Graneto MJ, Springer J, Hamper JR, Liao S, Pawlitz JL, Kurumbail RG, Maziasz T, Talley JJ, Kiefer JR, Carter J (2010) The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: the second clinical candidate having a shorter and favorable human half-life. Bioorg Med Chem Lett 20:7159–7163
Zhang X, Li X, Li D, Qu G, Wang J, Loiseau PM, Fan X (2009) Ionic liquid mediated and promoted eco-friendly preparation of thiazolidinone and pyrimidine nucleoside-thiazolidinone hybrids and their antiparasitic activities. Bioorg Med Chem Lett 19:6280–6283
Zhou H, Wu S, Zhai S, Liu A, Sun Y, Li R, Zhang Y, Ekins S, Swaan PW, Fang B, Zhang B, Yan B (2008) Design, synthesis, cytoselective toxicity, structure-activity relationships, and pharmacophore of thiazolidinone derivatives targeting drug-resistant lung cancer cells. J Med Chem 51:1242–1251
Acknowledgments
This study was funded by the All India Council for Technical Education (AICTE), New Delhi, India, under the Research Promotion Scheme (RPS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N., Tripathi, A.C., Tewari, A. et al. Ulcerogenicity devoid novel non-steroidal anti-inflammatory agents (NSAIDS): syntheses, computational studies, and activity of 5-aryliden-2-imino-4-thiazolidinones. Med Chem Res 24, 1927–1941 (2015). https://doi.org/10.1007/s00044-014-1270-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1270-z