Abstract
Beta-diversity measures have been used to understand patterns of community distribution in natural ecosystems. Recent studies included different facets of beta-diversity analyses, e.g. trait- and phylogeny-based. Here, we used ostracod communities to evaluate the influence of environmental and spatial factors structuring different facets of beta-diversity and their components (Beta-total, replacement and richness-difference) of ostracod communities associated with different macrophyte life forms. We test the hypotheses (1) that the influence of environmental factors is higher for ostracod beta-diversity facets of communities associated with submerged plants compared to emergent and floating plants and (2) that the influence of spatial factors is higher in communities associated with rooted, compared to non-rooted plants. Ostracods were sampled from five life forms of macrophytes, including emergent, rooted floating, rooted submerged, free submerged and free floating in 25 floodplain lakes. Our results showed that the environmental factors turned out to be important for all beta-diversity facets of ostracod communities, mainly for those associated with submerged macrophytes, thus corroborating the first hypothesis. We also found that spatial factors’ influence on ostracod beta-diversity was not related to whether the plant is rooted or not, thus refuting our second hypothesis. We also found differences in factors structuring each of the beta-diversity facets, showing the importance to include these three approaches (species-, traits- and phylogeny-based) in ecological surveys. Finally, we highlight the importance of considering different macrophyte life forms in biodiversity surveys for the preservation and management of the diversity of these plants and their associated communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the patterns of community distribution in natural ecosystems, as well as the factors (e.g., local and regional) structuring such patterns, are amongst the main goals of ecological studies (Holyoak et al. 2005). Beta-diversity, which is defined as “the variability in species composition amongst sampling units for a given area” (Anderson et al. 2006), is one of the main concepts that has been used to evaluate such patterns of community distribution (Anderson et al. 2011). Dissimilarities between communities have been attributed to two different process, nl. species replacement or turnover (species are replaced by other species from site to site; Baselga 2010); and richness differences (the gain or loss of species from site to site; Legendre 2014).
In general, studies including beta-diversity measures are based on the taxonomic identity of species (Leibold et al. 2004). However, the communities can be similar in their compositional diversity (based on taxonomic identity of species) and, at the same time, they can differ in their functional and/or phylogenetic diversity (Krasnov et al. 2019), or vice versa (Weinstein et al. 2014). Thus, to overcome this information gap, several authors have measured other aspects of biodiversity, including trait- and phylogenetic-based analyses (Perez Rocha et al. 2018; Alahuhta et al. 2019; Cai et al. 2019; Krasnov et al. 2019). The use of traits helps to understand how species can live, the interaction amongst them, and their contribution to ecosystem functioning (Cadotte et al. 2011). On the other hand, phylogenetic beta-diversity analyses, which measure how phylogenetic relationships amongst species change across space (Graham and Fine 2008), provide information about shared phylogenetic history between two communities (Graham et al. 2009). Besides, this approach helps to identify the ability of communities to generate new evolutionary solutions in the face of environmental changes (Forest et al. 2007). Integrating these different facets in beta-diversity analyses provides complementary and valuable information about community structuring (Alahuhta et al. 2019).
Riverine floodplains are excellent ecosystems to study patterns of community distributions, owing to their wide variety of environments, such as rivers, channels and closed/open lakes, high habitat heterogeneity and high biological diversity (Junk et al. 1989). Aquatic macrophytes are abundant in South American floodplains and they play a role as food resource (Neiff and Casco 2003), in nutrient cycling (Thomaz and Cunha 2010), as a habitat for many organisms, such as fishes (Walker et al. 2013) and associated invertebrates (Rocha and Por 1998; Thomaz and Cunha 2010; Fontanarossa et al. 2013).
Macrophytes have been grouped, according to their life form, in emergent, rooted floating, rooted submerged, free submerged and free floating (Pott and Pott 2000; Souza et al. 2017). Each life form is located in different regions of the water column, mainly related to water characteristics (e.g., chemical and physical variables), leading to different habitat suitability for associated communities (Yamaki and Yamamuro 2013). This variation in environmental characteristics (environmental factors) of the water, for example in temperature, pH, dissolved oxygen and electrical conductivity, are expected to influence the occurrence and structure of associated faunal communities (Nagorskaya and Keyser 2005; Liberto et al. 2012; Mesquita-Joanes et al. 2012). In addition, the influence of spatial factors (e.g., dispersal limitation) is different between aquatic macrophytes, according to their life form (Alahuta and Heino 2013). The efficiency of dispersal might be related to the capacity of the plants to break themselves up (e.g., fixed plants) and regrow from broken dispersed fragments (Bornette and Puijalon 2011) or to detach from the littoral areas of floodplain lakes (e.g., free floating plants), drift in the main channel of rivers and reach other environments (Bulla et al. 2011).
Several studies have evaluated the relationship between the taxonomic identity or structural complexity of the macrophytes (e.g., measured by fractal dimension) and the structure of associated communities (McAbendroth et al. 2005; Matsuda et al. 2015), and it is well-known that the more complex the plant, the higher the diversity of associated animal communities (Thomaz et al. 2008). However, few studies have tested the effects of the different life forms of macrophytes on associated communities (Meerhoff et al. 2003; Cazzanelli et al. 2008; Choi et al. 2014). Thus, the aim of the present study was to evaluate factors affecting the beta-diversity facets (species-, traits- and phylogeny-based) and their components (Beta-total, replacement and richness difference) of ostracod communities associated with macrophytes and to compare these factors amongst different macrophyte life forms. From that, we also assess if the choice of the type of habitat (here: different macrophyte life forms) is an important factor to be considered in studies evaluating beta-diversity of associated animal communities.
We used ostracod (Crustacea) communities, one of the most abundant groups in freshwater ecosystems (Thomaz et al. 2008; Bornette and Puijalon 2011; Liberto et al. 2012; Mazzini et al. 2014; Higuti and Martens 2016; Pereira et al. 2017), associated with five life forms of macrophytes, as a model group. We test two main hypotheses: (1) submerged plants (free submerged and rooted submerged) will have a more pronounced influence on the effects of environmental factors on the beta-diversity of associated ostracod communities than emergent and floating (free floating and rooted floating) plants. This is expected, because aquatic abiotic variables (e.g. water transparency, dissolved oxygen and water temperature) will have a larger impact on submerged plants, and thus also on associated (animal) communities; (2) the influence of the spatial factors (such as dispersal limitation) on ostracod beta-diversity facets will be higher for fixed (emergent, rooted floating and rooted submerged), compared to non-fixed plants in the sediment (free submerged and free floating). This is based on the expectation that fixed plants have lower dispersal capacity through the region, compared to the unrooted, floating macrophyte life forms. The distribution of ostracod communities associated with these different life forms in macrophytes will thus also be affected by this. Finally, we also test if there are mismatches in the factors (environmental and spatial) structuring each beta-diversity facet (and their components) in each macrophyte life form.
Material and methods
Study site
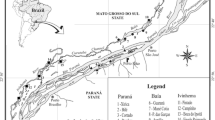
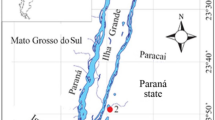
The Upper Paraná River section is 230 km long and can reach 20 km of width, including several secondary channels and floodplain lakes (Agostinho et al. 2004). It has an extensive floodplain, approximately 60 km long, located within the Brazilian territory (Paraná and Mato Grosso do Sul states). The study area is a river-floodplain system, which encompasses three different river systems: Ivinhema, Paraná and Baía, each one with its peculiar geology, hydrology and limnology (Souza Filho 2009) (Electronic Supplementary Material, Table S1). We selected 25 permanently connected lakes along the Upper Paraná River floodplain, with eight lakes in the Ivinhema system, eight lakes in the Baía system and nine lakes in the Paraná system (Fig. 1).
Sampling and laboratory analysis
Ostracod communities associated with macrophytes were sampled between March 20th and 22nd in 2018. We collected samples of five life forms of macrophytes: emergent (EM), rooted floating (RF), free floating (FF), rooted submerged (RS) and free submerged (FS). The macrophytes species belonging to each life form, are shown in the Electronic Supplementary Material (Table S2), and they were classified according to their life form following Pott and Pott (2000).
To standardize our sampling effort, we collected one sample of each macrophyte life form in each lake (when present). The sampling sites of each lake (one for each macrophyte life form) were chosen visually, based on the largest macrophyte bed of each life form. This selection was made because the larger the patches, the higher the chance of a good representation of the ostracod community in terms of diversity (e.g., richness, density and composition). However, not all life forms of macrophytes were always found in all lakes (see Electronic Supplementary Material, Table S2). A total of 75 samples were collected in 25 lakes, with 20 RF samples, 19 FF samples, 17 EM samples, 10 FS samples and 9 RS samples.
Ostracods associated with emergent macrophytes were sampled with a rectangular hand net (28 × 14 cm and mesh size of 160 µm), which was moved amongst the plants. Macrophytes with RF, FF, RS and FS life forms were removed manually from the water and transferred to a plastic bucket (Campos et al. 2017). Either the entire plants or only the roots were washed to remove the ostracods. The material retained in the bucket was washed through a hand net (mesh size of 160 µm). The sampling methods have been shown as adequate to represent associated fauna (Higuti et al. 2009, 2010; Castillo-Escrivà et al. 2016b; Campos et al. 2017). All material was preserved in 70º ethanol buffered with sodium tetraborate. In the laboratory, ostracods were sorted using a stereoscopic microscope and were identified down to species level (Martens and Behen 1994 and articles included therein; Rossetti and Martens 1998; Higuti and Martens 2012a, 2012b, 2014; Higuti et al. 2013; Ferreira et al. 2020).
Ostracod traits and taxonomic information
Ostracods were classified according to the functional traits: locomotion mode, body size, presence/absence of spines, general body morphology and reproductive mode (Electronic Supplementary Material, Tables S3). The locomotion mode was differentiated, considering the presence/absence or the length of natatory setae on the Antenna, in two categories: swimmer (long setae) and non-swimmer (reduced setae or setae absent, Meisch 2000). The body size classification was based on the length (L) and height (H) of the ostracod carapace. These measurements were obtained mostly using scanning electron microscopy (SEM) and in some cases with a stereoscope microscope. The species were categorized as small (L ≤ 0.54 mm and/or H ≤ 0.32 mm), medium (0.55 ≤ L ≤ 1.32 mm and/or 0.33 ≤ H ≤ 0.72 mm) and large (L > 1.32 mm and/or H > 0.72 mm) (Matsuda et al. 2015). Spines of any size were classified as present/absent, according to visual observations of the ostracod carapace in a stereoscope microscope and SEM. Amongst the species treated here, all spines (when present) were relatively large. Reproductive mode was differentiated in parthenogenic, sexual and mixed reproduction. Body morphology was distinguished in flat (laterally compressed) and rounded in dorsal or ventral view, according to Martens and Behen (1994) and articles included therein; Rossetti and Martens (1998); Meisch (2000); Higuti and Martens 2012a, b, 2014; Higuti et al. (2013) and Ferreira et al. (2020). All these traits were chosen owing to their importance in life history characteristics (e.g., body size, Matsuda el al. 2015), dispersal limitation (e.g., locomotion mode and body morphology, Matsuda et al. 2015), defence against predators (e.g. presence of spines) and population establishment (e.g., reproductive mode, Horne et al. 1998) of ostracod species. Besides, these are the traits for which we have complete information for all taxa found in our survey.
We used taxonomic distance based on the path lengths in the Linnean taxonomic trees as a proxy for phylogeny (Clarke and Warwick 1998; Winter et al. 2013). Six taxonomic levels were included in this taxonomic tree: species, genus, tribe, subfamily, family and superfamily, and we followed Meisch et al. (2019) for higher taxonomy (Electronic Supplementary Material, Table S4).
Environmental and spatial factors
Chemical and physical variables, such as pH and electrical conductivity (µS cm−1) (YSI 63), dissolved oxygen (mg L−1) and water temperature (ºC) (YSI 550A oximeter), and water transparency (black and white Secchi disk) were measured in situ. The perimeter (in km) of the lakes and length of the meandering channel that connect each lake to the main river (in km) were obtained through the Google Earth image program, all from images taken during the dry season. All these variables (see Electronic Supplementary Material, Table S1), were considered “environmental factors”.
We generated the “spatial factors” calculating matrices of Euclidean distances (“overland”, derived from geographical coordinates) of the sites. These matrices were submitted to the PCNM method (Principal Coordinates of Neighbour Matrices) for the construction of the eigenvectors (explanatory spatial variables, Borcard and Legendre 2002). The PCNM analysis was truncated by minimum distance that kept all sampling sites connected (minimum spanning tree procedure, Landeiro et al. 2011). In the present study, the first PCNMs generated represent larger scales of amplitude, whereas the latter ones represent smaller scales of amplitude. Campos et al. (2019) compared three methods to generate spatial effects on ostracod metacommunities, “overland”, “watercourse” (considering distances amongst the environments, following watercourse) and Asymmetric Eigenvectors Maps (AEM, considering the sampling sites connected in the watercourse, following the flow direction of the river), and showed that the results for “overland” were similar to the other methods.
Data analysis
We performed rarefaction curves to evaluate if the sampling effort for each macrophyte life form (20 RF samples, 19 FF samples, 17 EM samples, 10 FS samples and 9 RS samples) was sufficient to represent the associate ostracod communities in terms of richness over all 25 lakes. For that, we used Hill numbers through the function iNEXT (iNEXT R-package, Hsieh et al. 2016). The iNEXT R-package uses Chao 2 (for incidence data or “reference sample”) to estimate the number of undetected species. We used q = 0 to estimate species richness and the maximum extrapolated size was up to 100% of the reference sample size. According to Heck et al. (1975), the occurrence of 50–75% of the estimated richness might be satisfactory (considering that most of the common species were recorded). We assessed possible differences in the ostracod composition amongst the life forms of macrophytes to highlight the relevance of analyzing each life form separately. For this, we first visualized the (dis)similarity in ostracod composition amongst the macrophytes life forms using the Principal Coordinate Analysis (PCoA, Legendre and Legendre 1998), based on a presence/absence matrix and Jaccard index. For testing such (dis)similarity, we performed a Multivariate Permutational Variance Analysis (PERMANOVA, Anderson 2006), using a total of 999 permutations. After that, we used a pairwise PERMANOVA to evaluate ostracod composition (dis)similarity amongst the macrophytes life forms. Finally, we performed Multivariate Dispersion Analysis (PERMDISP, Anderson 2006) to evaluate variability of the dispersion of ostracod communities amongst the macrophytes life forms. PERMDISP was based on a presence/absence matrix and Jaccard index, and the significance amongst the groups (macrophytes life forms) was tested using a total of 999 permutations. When PERMDISP results show significant differences in the variability of dispersal between groups, it can be related to differences in the scatter of data, indicating that the comparisons between groups (e.g., in PERMANOVA results) must be interpreted with caution. PERMANOVA and PERMDISP were performed using the vegan R-package (Oksanen et al. 2017).
We used distance-based redundancy analysis (db-RDA, Legendre and Anderson 1999), as well as variation partitioning procedures (Legendre et al. 2005) to evaluate the influence of environmental and spatial factors on ostracod beta-diversity facets, amongst different life forms of macrophytes. Before the analyses, we checked the multicollinearity amongst the environmental variables (environmental factors) using the variance inflation factor (VIF). Variables with VIF > 10 should be removed (Oksanen et al. 2017).
We used the function beta (BAT R-package, Cardoso et al. 2015) according to Perez Rocha et al. (2018) to generated three dissimilarity matrices (with Jaccard family measures): total beta-diversity (B-tot) and its components, replacement (B-repl) and richness difference (Rich-Diff), for each beta-diversity facet. These dissimilarity matrices were based on:
-
1)
Species: sites x species matrices of presence-absence of ostracods;
-
2)
Traits: we used the Gower distance (Gower 1971) to calculate between‐species distances, based on the traits data, using the function gowdis (FD R-package, Laliberté et al. 2014). Subsequently, this species‐by‐species matrix was subjected to a hierarchical clustering (UPGMA agglomeration method) procedure using the function hclust (STATS R-package);
-
3)
Phylogeny: we used ostracod taxonomic information as a proxy for phylogeny. We used the function taxa2dist (vegan R-package) to calculate taxonomic distance between ostracod species. Subsequently, this species‐by‐species matrix was also subjected to a hierarchical clustering (UPGMA agglomeration method) procedure using the function hclust (STATS R-package). The BAT R-package requires an hclust object to calculate beta-diversity metrics, thus clustering (phylogenetic tree, such as traits tree) was necessary (Perez Rocha et al. 2018).
After that, average beta-diversity (B-tot) and their components (B-repl and Rich-Diff), based on species, traits and phylogeny, were calculated using the function beta.multi (BAT R-package). This function returns three values (also named average values), which represent the total multi-site dissimilarity across the sites (B-tot) and its B-repl and Rich-Diff components. Finally, each of the dissimilarity matrices of each ostracod beta-diversity facet was used in the db-RDA and variation partitioning. The environmental and spatial factors, which should be included in the analyses, were selected using the function ordiR2step (p < 0.05, 999 permutations, vegan R-package). The db-RDA analyses were performed using the function capscale (vegan R-package). Subsequently, ostracod community variation in each of the beta-diversity measures (B-tot, B-repl and Rich-Diff) was partitioned in purely environmental (E|S) and spatial factors (S|E), and in a component explained by the intersection of these factors (E ∩ S). The results were adjusted R2 values and the significance of the components was tested using the Anova function (p < 0.05, vegan R-package). We used the sqrt.dist correction in all db-RDA analyses, for negative eigenvalues (Legendre 2014). All the analyses described above were performed separately for ostracod communities associated with each life form of macrophytes, using R 3.4 software (R Core Team 2017).
Results
Abiotic factors
The mean of the chemical and physical variables was more different between the Ivinhema and Paraná systems (Electronic Supplementary Material, Table S1). The Ivinhema system had the highest mean value of water temperature, lake perimeter and length of the meandering channel, while the Paraná system had the lowest values for the same variables. On the other hand, the Paraná system had the highest mean values of electrical conductivity, pH and water transparency, while the Ivinhema system had the lowest values for these variables. Mean values of all the variables in the Baía system were intermediate amongst the three systems. Lake perimeter, length of the meandering channel, dissolved oxygen and electrical conductivity were the variables that had the highest coefficient of variation amongst the lakes of all systems (see Electronic Supplementary Material, Table S1).
According to the spatial variables, 8 PCNMs were generated for EM, 9 PCNMs for RF, 6 PCNMs for FF and FS, and 4 PCNMs for RS. In EM, PCNMs 1–4 indicate broad-scaled and 5–8 fine-scaled patterns; in RF, PCNMs 1–5 indicate broad-scaled and 6–9 fine-scaled patterns; in FF and FS, PCNMs 1–3 indicate broad-scaled and 4–6 fine-scaled patterns; and in RS, PCNMs 1–2 indicate broad-scaled and 3–4 fine-scaled patterns.
Ostracod communities
We recorded 38 species of ostracods associated with 13 macrophyte species. Higher ostracod richness was found in RF (35 species), followed by FF (33), EM (30), RS (27) and FS (24) (Table 1). The observed richness represents between 82.17% (FS) and 98.45% (EM) of the estimated richness and the rarefaction curves show asymptotic levelling‐off (Fig S1). These results suggest that the sampling effort was adequate to represent the ostracod communities in each macrophyte life form.
The PCoA and PERMANOVA results show significant differences in ostracod composition amongst the macrophyte life forms (F = 2.02; p = 0.001, Fig. S2). The pairwise PERMANOVA results show differences in the ostracod composition between free floating plants and all other macrophyte life forms (Table S5). The PERMDISP results show no significant differences in the variability of the dispersion of ostracod communities amongst the macrophytes life forms (F = 0.64, P = 0.65). The average distance from the centroid is similar amongst the macrophytes life forms: RS = 0.54, RF and FS = 0.52, EM = 0.51 and FF = 0.49. PERMDISP results thus show that differences in PERMANOVA results are not influenced by differences in the scatter of ostracod data amongst the macrophytes life forms (e.g., difference in the number of samples amongst macrophytes life forms), thus indicating that the data of these groups are comparable.
Average values of total beta-diversity and its components
In this section, we compare both differences amongst macrophytes life forms and amongst beta-diversity facets. The average values (derived from the dissimilarity matrices) of total species-based ostracod beta-diversity are higher than the traits- and phylogeny-based ones (Table 2). The total species-based beta-diversity (B-tot, average values ranging between 0.689 and 0.754) is mostly explained by B-repl in all macrophyte life forms (ranging between 55 and 67% of B-tot). Traits-based total beta-diversity (B-tot, average values ranging between 0.478 and 0.577) is more explained by B-repl in FF, EM and RS (60%, 57% and 54% of B-tot, respectively), and by Rich-Diff in FS and RF (55% and 52% of B-tot, respectively). Finally, phylogeny-based total beta-diversity (B-tot, average values ranging between 0.507 and 0.593) is more explained by B-repl in EM, RS, FF and RF (58%, 57%, 57% and 51% of B-tot, respectively), and by Rich-Diff in FS (61% of B-tot, Table 2).
Comparing factors affecting ostracod beta-diversity facets amongst macrophyte life forms
The influence of the environmental and spatial factors structuring ostracod beta-diversity facets are variable amongst different life forms of macrophytes (Fig. 2). The total percentage of explained variation (R2) of the factors (sum of pure environmental, pure spatial and shared fraction) had the highest values in FS (up to 86%), followed by RF (up to 49%), FF (up to 44%), RS (up to 43%) and EM (up to 27%).
Results of variation partitioning analyses showing the relative contributions (% of explanation) of the environmental, spatial, shared fraction between environmental and spatial factors and the unexplained fraction for each beta diversity measure (B-tot beta total, B-repl replacement and Rich-Diff richness difference), amongst the facets of ostracod beta diversity, in different macrophyte life forms
Variation partitioning shows that the environmental factors are important for ostracod beta-diversity facets in all macrophyte life forms, while higher percentages of explanation are found in submerged life forms (FS up to 68% and RS up to 32%), than in floating and emergent life forms (FF up to 19%, RF up to 13% and EM up to 19%). Spatial factors are mostly non-significant in structuring ostracod beta-diversity. However, significant percentages of explanation of these factors are found for B-repl in RF and FS (up to 30%), and for Rich-Diff in EM and FS (up to 21%). We do not observe higher percentages of explanation of spatial factors in rooted life forms (EM, RF and RS), as compared to the free-living ones (FF and FS, Fig. 2). Shared fractions between environmental and spatial factors are high (up to 21%, Fig. 2) in some life forms, mainly in FF and RS.
Factors affecting each facet of ostracod beta-diversity
In general, the influence of the environmental factors is high for B-repl (up to 68%) and low for B-tot (< 24%) and Rich-Diff (< 12%) for all beta-diversity facets. However, this influence is variable amongst the beta-diversity facets. Frequently, the percentages of explanation of environmental factors are higher for B-tot (ranging between 5 and 23%) and B-repl (ranging between 12 and 68%) for traits-based beta-diversity, than for those of species- (B-tot ranging between 0 and 15%, B-repl ranging between 8 and 47%) and phylogeny-based beta-diversity (B-tot ranging between 4 and 11%, B-repl ranging between 6 and 42%). As environmental factors were always less explanatory for Rich-Diff, we do not see differences in their influence amongst the beta-diversity facets. The percentages of explanation of spatial factors are always low (< 31%) for all beta-diversity facets. Higher values for such effects of spatial factors are observed for B-repl (up to 30%) as compared to Rich-Diff (up to 21%) and B-tot (up to 7%). The influence of spatial factors is higher for phylogeny-based beta-diversity (up to 30%) than for species- (up to 21%) and traits-based beta-diversity (up to 24%, Fig. 2).
Selected environmental and spatial factors
In this section, we compare selected environmental and spatial variables in the db-RDA models, both amongst macrophytes life forms and amongst ostracod beta-diversity facets. Considering the environmental factors, the selected variables are quite similar amongst the beta-diversity facets but quite different amongst the macrophytes life forms (Table 2). Furthermore, the environmental variables are frequently significant for B-tot and for B-repl components, but are rarely significant for the Rich-Diff component, for all beta-diversity facets in all macrophytes life forms. The most frequently selected environmental variables are electrical conductivity (e.g. for B-repl of all beta-diversity facets in almost all macrophytes life forms), pH (e.g., for B-repl of species- and traits-based beta-diversity in FS and FF; and for B-tot of trait- and phylogeny-based beta-diversity in RF, FF and FS), dissolved oxygen (e.g., for B-tot and B-repl of all beta-diversity facets in FF), and water temperature (e.g., for B-tot of species- and phylogeny-based beta-diversity in RF, Table 2). Considering the spatial factors, the generated PCNMs are rarely selected for species-based beta-diversity in all macrophytes life forms (Table 2). For traits-based beta diversity, the selected PCNMs are variable amongst macrophytes life, representing fine-scaled (e.g. PCNM6 for B-repl in EM) broad-scaled (e.g., PCNM1 for Rich-Diff in FS), and both fine- and broad-scaled spatial patterns (e.g. PCNM1, PCNM3, PCNM5 and PCNM6 for B-repl in RF). Similarly, for phylogeny-based beta-diversity, the selected PCNMs represent fine-scaled (e.g., PCNM5 for B-repl in FS), broad-scaled and both fine- (e.g., PCNM1 for Rich-Diff and PCNM3 for B-tot in EM) and broad-scaled spatial patterns (e.g. PCNM1, PCNM2, PCNM3, PCNM5 and PCNM6 for B-repl in RF, Table 2).
Discussion
Environmental and spatial effects amongst different macrophyte life forms
Environmental factors significantly structure ostracod beta-diversity facets and their percentages of explanation were higher for submerged macrophytes life forms, compared to the floating and emergent ones, supporting the first hypothesis of the present study. This pattern of percentage of explanation of the environmental factors may reflect the presence and repartition of the life forms in the water column, which are important factors in determining habitat structure (Choi et al. 2014). Such occupancy creates a higher variation in chemical and physical characteristics of the water around the roots (or even around the entire plant). For example, submerged macrophytes can live in a great variety of depths, compared to floating macrophytes, because they are adapted to regions where transparency is higher (Meerhoff et al. 2003), apart from the fact that they themselves also have the capacity to increase the water clarity by reducing nutrients (Scheffer et al. 1993). On the other hand, floating macrophytes (FF or RF) penetrate less in the vertical dimension as compared to the EM ones, and might furthermore reduce the light penetration in the water column owing to the floating leaves that form patches on the water surface (Cattaneo et al. 1998; Cremona et al. 2008). Floating macrophytes can still supply oxygen to the water around the roots, as they can transport oxygen from the atmosphere to these structures (Rehman et al. 2017). Consequently, ostracod species (or sets of traits and phylogenetic lineages of ostracods) may have been sorted, mainly in submerged life forms, owing to their physiological tolerances to certain environmental conditions. For example, some ostracod species are very sensitive to low concentrations of dissolved oxygen, as shown by Ruiz et al. (2013), and we observe lower concentrations of this variable around submerged macrophytes (see also below in “Selected environmental and spatial factors”).
In addition, we find that environmental factors had a higher influence on ostracod beta-diversity facets in FS macrophytes than in other life forms. The FS life form was represented by Utricularia foliosa, which is known as a “carnivorous plant”. This plant genus is adapted to life in aquatic regions that are poor in nutrients, and they remove these directly from the water or from their prey (Adamec 2008). Guisande et al. (2000) showed that there is a relationship between the decrease of zooplankton abundance and the increase in the number of bladders (for prey) per leaf in U. foliosa, as an adaptation to improve its carnivory and input of nutrient. These authors also showed that higher electrical conductivity had a negative influence on the bladder production of U. foliosa. Although we did not measure bladder productivity of U. foliosa in the present study, we infer that electrical conductivity may have indirectly influenced ostracod communities by increasing (or decreasing) the quantity of these plant structures. For example, some species of ostracods may have been more easily trapped by plants with higher numbers of bladders, which have led to such higher environmental influence on beta-diversity facets in our survey.
Spatial factors have some importance in structuring ostracod beta-diversity facets and we did not find higher values of spatial effects on ostracods associated with fixed macrophyte life forms, indicating that ostracods might present some dispersal limitation, regardless of the plant dispersal capacity, thus refuting our second hypothesis. Despite the fact that ostracods in general are good passive dispersers, especially the species of the family Cyprididae (Meisch 2000; Brochet et al. 2010; Pereira et al. 2017), several studies have also found spatial effects on ostracod communities, related to dispersal limitation (Castillo-Escrivà et al. 2016a, 2017; Campos et al. 2019).
We highlight that the environmental and spatial factors discussed here explain at most 25% of the variability in ostracod beta-diversity in some macrophyte life forms (e.g., EM) in the Upper Paraná River floodplain. A high shared fraction of environmental and spatial factors in some cases (e.g., 21% in species-based beta-diversity in RS), suggests that part of the environmental gradient was spatially structured, which may, for example, have led to a decrease in the effect of environmental or spatial factors.
Differences amongst the beta-diversity facets and their components
Our results show that there were mismatches in the factors structuring each of the ostracod beta-diversity facets in each of the macrophyte life forms. This is so, because communities consist not only of different taxonomic assemblages but also of species with different traits (Alahuhta et al. 2019). Thus, using different data (either species-, traits- or phylogeny-based) on beta-diversity metrics can show species responding differently to the environmental gradient (e.g., variation in habitat formed by the different macrophyte life forms), thus generating a mismatch in the factors affecting each beta-diversity facet (Devictor et al. 2010). Similarly, Cai et al. (2019) found that the effect of the factors structuring the beta-diversity of freshwater molluscs, such as geography, energy and environment, were different amongst the three facets. However, such pattern of mismatches in the factors affecting the beta-diversity facets depends on the biological group under study. For example, Perez Rocha et al. (2018) found that local environment and space were factors that affected all the facets of macroinvertebrates beta-diversity in streams of western Finland in a similar way.
Considering the total beta-diversity, our results indicate that high species-based beta-diversity does not necessarily indicate high traits- and phylogeny-based beta-diversity. This might be related to the redundancy in the set of traits of ostracod species amongst the lakes, or even to the lack of information about other (unknown) ostracod traits (such as feeding preferences, behavioral responses to light or predators, parasites and burrowing abilities), which might have led to a lower traits-based diversity. Braghin et al. (2018), analysing zooplankton communities in the lakes of the Paraná River, also found lower functional beta-diversity, because species that were replaced amongst the environments probably had functional redundancy. Likewise, the set of ostracod species amongst the environments may have closely related taxonomic levels (same genus, same subfamily, …), which led to a lower phylogeny-based beta-diversity. According to Graham and Fine (2008), if lineages have conserved phylogenetic niches, species might be expected to be sorted by habitat, whereas if their phylogenetic niche is variable, closely related species are predicted to exist in different habitats. Our results indicated that (phylogenetically) closely related ostracod species might present conserved phylogenetic niches.
The fact that the replacement component was the main driver of the ostracod beta-diversity facets, in almost all macrophyte life forms, confirms that the variation in the environmental conditions replaces species (or traits and lineages of ostracod) from one lake to another. This might be associated with the difference in ecological conditions throughout the systems of the Paraná River floodplain (such as electrical conductivity which was the most variable amongst the systems). For example, the Paraná system can present lower environmental heterogeneity than the Ivinhema and Baía systems (Higuti et al. 2009), because of the effects resulting from dam regulation (Braghin et al. 2018), which evens-out or even eliminates small water level changes that are still present in the other two rivers.
Selected environmental and spatial factors
Most studies evaluating the influence of environmental factors on ostracod community structure are species-based, and this information is less applicable using the other facets of ostracod beta-diversity. Only one previous study (Marmonier et al. 1994) showed that environmental variables (such as variation of habitat characteristics) had an effect on traits-based diversity of ostracods, and the distribution of the set of traits of this community was found to be related to the habitat type. The selected variables in the present study, such as dissolved oxygen, electrical conductivity, pH and water temperature, are known to be important for the structure of ostracod communities in species-based studies. For example, such studies have found a correlation between ostracod community attributes and dissolved oxygen (Nagorskaya and Keyser 2005; Higuti et al. 2017) and electrical conductivity (Liberto et al. 2012). pH was relatively acidic in some lakes of the Upper Paraná River (Table S1), and this parameter was probably selected because low values of pH might affect freshwater ostracod valve calcification. Most ostracod species prefer alkaline or only slightly acidic waters (Ruiz et al. 2013; Mesquita-Joanes et al. 2012 and references therein). Finally, water temperature can affect the (length of the) life history and resulting body size of the adult organisms, i.e. the development rate of species might accelerate with increasing temperature (Aguilar-Alberola and Mesquita-Joanes 2014; Castillo-Escrivà et al. 2016a).
The spatial factors selected here indicate that dispersal limitation might have influenced some ostracod beta-diversity facets (and their components), from narrow (e.g., within systems of the Paraná River floodplain) to broad (e.g., amongst systems) scales of variation, differently in the different macrophyte life forms.
Conclusion
Environmental factors are significantly structuring ostracod beta-diversity facets, mainly for submerged macrophytes. This is probably related to the variation in water chemical and physical characteristics around the roots (or entire body) of these life forms, which replaces species (or ostracod traits and lineages), according to their ecological niche, thus affecting beta-diversity patterns. Furthermore, environmental and spatial factors have different influence on each of the beta-diversity facets, thus highlighting the importance to include these three approaches (species-, traits- and phylogeny-based) in ecological surveys. Therefore, biological communities associated with different macrophyte life forms should be considered in local as well as regional biodiversity surveys, owing to the variation in the factors affecting these communities associated with each macrophyte life form. In addition, we stress the importance of preservation and management of the different macrophyte life forms in river-floodplain ecosystems, as they provide higher diversity of available habitat for associated biological communities. Besides, we predict that if more dams are constructed (e.g., in Paraná River and its adjacent tributaries) and the diversity of macrophyte life forms is negatively affected, direct effects might change the structure of associated communities such as invertebrates and small fish.
Availability of data and material
The authors declare that they can make the data available.
References
Adamec L (2008) Mineral nutrient relations in the aquatic carnivorous plant Utricularia australis and its investment in carnivory. Fund Appl Limnol 171(3):175–183. https://doi.org/10.1127/1863-9135/2008/0171-0175
Agostinho AA, Gomes LC, Veríssimo S, Okada EK (2004) Flood regime, dam regulation and fish in the upper paraná river: effects on assemblage attributes, reproduction and recruitment. Rev Fish Biol Fisher 14:11–19. https://doi.org/10.1007/s11160-004-3551-y
Aguilar-Alberola JA, Mesquita-Joanes F (2014) Breaking the temperature-size rule: Thermal effects on growth, development and fecundity of a crustacean from temporary waters. J Therm Biol 42:15–24. https://doi.org/10.1016/j.jtherbio.2014.02.016
Alahuhta J, Heino J (2013) Spatial extent, regional specificity and metacommunity structuring in lake macrophytes. J Biogeogr 40(8):1572–1582. https://doi.org/10.1111/jbi.12089
Alahuhta J, Erős T, Kärnä OM, Soininen J, Wang J, Heino J (2019) Understanding environmental change through the lens of trait-based, functional, and phylogenetic biodiversity in freshwater ecosystems. Environ Rev 27(2):263–273. https://doi.org/10.1139/er-2018-0071
Anderson MJ (2006) Distance-Based tests for homogeneity of multivariate dispersions. Biometrics 62(1):245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9(6):683–693. https://doi.org/10.1111/j.1461-0248.2006.00926.x
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of β diversity: a roadmap for the practicing ecologist. Ecol Lett 14(1):19–28. https://doi.org/10.1111/j.1461-0248.2010.01552.x
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Global Ecol Biogeogr 19(1):134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Model 153(1):51–68. https://doi.org/10.1016/S0304-3800(01)00501-4
Bornette G, Puijalon S (2011) Response of aquatic plants to abiotic factors: a review. Aquat Sci 73:1–14. https://doi.org/10.1007/s00027-010-0162-7
Braghin LSM, Almeida BA, Amaral DC, Canella TF, Gimenez BCG, Bonecker CC (2018) Effects of dams decrease zooplankton functional β-diversity in river-associated lakes. Freshwater Biol 63(7):721–730. https://doi.org/10.1111/fwb.13117
Brochet AL, Gauthier-Clerc M, Guillemain M, Fritz H, Waterkeyn A, Baltanás Á, Green AJ (2010) Field evidence of dispersal of branchiopods, ostracods and bryozoans by teal (Anas crecca) in the Camargue (southern France). Hydrobiologia 637:255–261. https://doi.org/10.1007/s10750-009-9975-6
Bulla CK, Gomes LC, Miranda LE, Agostinho AA (2011) The ichthyofauna of drifting macrophyte mats in the Ivinhema River, upper Paraná River basin. Brazil Neotrop Ichthyol 9(2):403–409. https://doi.org/10.1590/S1679-62252011005000021
Cadotte MW, Carscadden K, Mirotchnick N (2011) Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol 48(5):1079–1087. https://doi.org/10.1111/j.1365-2664.2011.02048.x
Cai Y, Zhang Y, Hu Z, Deng J, Qin B, Yin H, Wang X, Gong Z, Heino J (2019) Metacommunity ecology meets bioassessment: assessing spatio-temporal variation in multiple facets of macroinvertebrate diversity in human-influenced large lakes. Ecol Indic 103:713–721. https://doi.org/10.1016/j.ecolind.2019.03.016
Campos R, Conceição EO, Pinto MBO, Bertoncin APS, Higuti J, Martens H (2017) Evaluation of quantitative sampling methods in pleuston: an example from ostracod communities. Limnologica 63:36–41. https://doi.org/10.1016/j.limno.2017.01.002
Campos R, Conceição EO, Martens K, Higuti J (2019) Extreme drought periods can change spatial effects on periphytic ostracod metacommunities in river-floodplain ecosystems. Hydrobiologia 828:369–381. https://doi.org/10.1007/s10750-018-3825-3
Cardoso P, Rigal F, Carvalho JC (2015) BAT - Biodiversity assessment tools, an R package for the measurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol Evol 6(2):232–236. https://doi.org/10.1111/2041-210X.12310
Castillo-Escrivà A, Rueda J, Zamora L, Hernández R, Moral M, Mesquita-Joanes F (2016a) The role of watercourse versus overland dispersal and niche effects on ostracod distribution in Mediterranean streams (eastern Iberian Peninsula). Acta Oecol 73:1–9. https://doi.org/10.1016/j.actao.2016.02.001
Castillo-Escrivà A, Valls L, Rochera C, Camacho A, Mesquita-Joanes F (2016b) Spatial and environmental analysis of an ostracod metacommunity from endorheic lakes. Aqua Sci 78(4):707–716. https://doi.org/10.1007/s00027-015-0462-z
Castillo-Escrivà A, Valls L, Rochera C, Camacho A, Mesquita-Joanes F (2017) Disentangling environmental, spatial, and historical effects on ostracod communities in shallow lakes. Hydrobiologia 787:1–12. https://doi.org/10.1007/s10750-016-2945-x
Cattaneo A, Galanti G, Gentinetta S, Romo S (1998) Epiphytic algae and macroinvertebrates on submerged and floating-leaved macrophytes in an Italian lake. Freshwater Biol 39(4):725–740. https://doi.org/10.1046/j.1365-2427.1998.00325.x
Cazzanelli M, Warming TP, Christoffersen KS (2008) Emergent and floating-leaved macrophytes as refuge for zooplankton in a eutrophic temperate lake without submerged vegetation. Hydrobiologia 605:113–122. https://doi.org/10.1007/s10750-008-9324-1
Choi JY, Jeong KS, La GH, Kim SK, Joo GJ (2014) Sustainment of epiphytic microinvertebrate assemblage in relation with different aquatic plant microhabitats in freshwater wetlands (South Korea). J Limnol 73(1):197–202. https://doi.org/10.4081/jlimnol.2014.736
Clarke KR, Warwick RM (1998) A taxonomic distinctness index and its statistical properties. J Appl Ecol 35(4):523–531. https://doi.org/10.1046/j.1365-2664.1998.3540523.x
Cremona F, Dolors P, Marc L (2008) Biomass and composition of macroinvertebrate communities associated with different types of macrophyte architectures and habitats in a large fluvial lake. Fund Appl Limnol 171(2):119–130. https://doi.org/10.1127/1863-9135/2008/0171-0119
Devictor V, Mouillot D, Meynard C, Jiguet F, Thuiller W, Mouquet N (2010) Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: The need for integrative conservation strategies in a changing world. Ecol Lett 13(8):1030–1040. https://doi.org/10.1111/j.1461-0248.2010.01493.x
Ferreira VG, Higuti J, Martens K (2020) Taxonomic revision of Strandesia ss (Crustacea, Ostracoda) from four Brazilian floodplains, with the description of three new species. Zootaxa 4760(1):1–74. https://doi.org/10.11646/zootaxa.4760.1.1
Fontanarrosa MS, Chaparro GN, O’Farrell I (2013) Temporal and spatial patterns of macroinvertebrates associated with small and medium-sized free-floating plants. Wetlands 33(1):47–63. https://doi.org/10.1007/s13157-012-0351-3
Forest F, Grenyer R, Rouget M, Davies J, Cowling D, Faith DP, Balmford A, Manning JC, Proches S, Bank MV, Reeves G, Hedderson TAJ, Savolainen V (2007) Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 445:757–760. https://doi.org/10.1038/nature05587
Gower JC (1971) A general coefficient of similarity and some of its properties. Biometrics 27(4):857–871. https://doi.org/10.2307/2528823
Graham CH, Fine PVA (2008) Phylogenetic beta diversity: linking ecological and evolutionary processes across space in time. Ecol Lett 11(12):1265–1277. https://doi.org/10.1111/j.1461-0248.2008.01256.x
Graham CH, Parra JL, Rahbek C, McGuire JA (2009) Phylogenetic structure in tropical hummingbird communities. P Natl Acad Sci Usa 106:19673–19678. https://doi.org/10.1073/pnas.0901649106
Guisande C, Andrade C, Granado-Lorencio C, Duque SR, Avellaneda N (2000) Effects of zooplankton and conductivity on tropical Utricularia foliosa investment in carnivory. Aquat Ecol 34:137–142. https://doi.org/10.1023/A:1009966231287
Heck KL, van Belle G, Simberloff D (1975) Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology 56:1459–1461. https://doi.org/10.2307/1934716
Higuti J, Martens K (2012a) Description of a new genus and species of Candonopsini (Crustacea, Ostracoda, Candoninae) from the alluvial valley of the Upper Paraná River (Brazil, South America). Eur J Taxon 33:1–31. https://doi.org/10.5852/ejt.2012.33
Higuti J, Martens K (2012b) On a new cypridopsine genus (Crustacea, Ostracoda, Cyprididae) from the upper paraná river floodplain (Brazil). Zootaxa 3391(1):23–38. https://doi.org/10.11646/zootaxa.3391.1.2
Higuti J, Martens K (2014) Five new species of Candoninae (Crustacea, Ostracoda) from the alluvial valley of the Upper Paraná River (Brazil, South America). Eur J Taxon 106:1–36. https://doi.org/10.5852/ejt.2014.106
Higuti J, Martens K (2016) Invasive South American floating plants are a successful substrate for native central African Pleuston. Bio Invasions 18:1191–1201. https://doi.org/10.1007/s10530-016-1061-1
Higuti J, Lansac-Tôha FA, Velho LFM, Martens K (2009) Biodiversity of non-marine ostracods (Crustacea, Ostracoda) in the alluvial valley of the upper Paraná River, Brazil. Braz J Biol 69(2):661–668. https://doi.org/10.1590/S1519-69842009000300020
Higuti J, Declerck SAJ, Lansac-Tôha FA, Velho LFM, Martens K (2010) Variation in ostracod (Crustacea, Ostracoda) communities in the alluvial valley of the upper Paraná River (Brazil) in relation to substrate. Hydrobiologia 644:261–278. https://doi.org/10.1007/s10750-010-0122-1
Higuti J, Schön I, Audenaert L, Martens K (2013) On the Strandesia obtusata/elliptica lineage (Ostracoda, Cyprididae) in the alluvial valley of the upper Paraná River (Brazil), with the description of three new species. Crustaceana 86(2):182–211. https://doi.org/10.1163/15685403-00003160
Higuti J, Conceição EO, Campos R, Ferreira VG, Rosa J, Pinto MBO, Martens K (2017) Periphytic community structure of Ostracoda (Crustacea) in the river-floodplain system of the Upper Paraná River. Acta Limnol Bras 29:e120. https://doi.org/10.1590/s2179-975x12217
Holyoak M, Leibold MA, Mouquet N, Holt RD, Hoopes M (2005) A framework for large scale community ecology. In: Holyoak M, Leibold MA, Holt RD (eds) Metacommunities: spatial dynamics and ecological communities, 1st edn. The University of Chicago Press, Chicago, pp 1–31
Horne DJ, Danielopol DL, Martens K (1998) Reproductive behaviour. In: Martens K (ed) Sex and parthenogenesis: evolutionary ecology of reproductive modes in non-marine ostracods. Backhuys Publish, Netherlands, pp 157–196
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (H ill numbers). Methods Ecol Evo 7(12):1451–1456. https://doi.org/10.1111/2041-210X.12613
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. Can Spec Publ Fish Aquat Sci 106(1):110–127
Krasnov BR, Shenbrot GI, Korallo-Vinarskaya NP, Vinarski MV, Warburton EM, Khokhlova IS (2019) The effects of environment, hosts and space on compositional, phylogenetic and functional beta-diversity in two taxa of arthropod ectoparasites. Parasitol Res 118:2107–2120. https://doi.org/10.1007/s00436-019-06371-1
Laliberté E, Legendre P, Shipley B, Laliberté ME (2014) Measuring functional diversity from multiple traits, and other tools for functional ecology. R Pack Vers 1:10–12
Landeiro VL, Magnussum WE, Melo AS, Espírito-Santo HMV, Bini LM (2011) Spatial eigenfunction analyses in stream networks: do watercourse and overland distances produce different results? Freshwater Biol 56(6):1184–1192. https://doi.org/10.1111/j.1365-2427.2010.02563.x
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Global Ecol Biogeogr 23(11):1324–1334. https://doi.org/10.1111/geb.12207
Legendre P, Anderson MJ (1999) Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecol Monogr 69(1):1–24. https://doi.org/10.1890/0012-9615(1999)069[0001:DBRATM]2.0.CO;2
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science, Amsterdam
Legendre P, Borcard D, Peres-Neto PR (2005) Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecol Monogr 75(4):435–450. https://doi.org/10.1890/05-0549
Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: a framework for multi-scale community ecology. Ecol Lett 7(7):601–613. https://doi.org/10.1111/j.1461-0248.2004.00608.x
Liberto R, Mesquita-Joanes F, César I (2012) Dynamics of pleustonic ostracod populations in small ponds on the Island of Martín García (Río de la Plata, Argentina). Hydrobiologia 688:47–61. https://doi.org/10.1007/s10750-011-0600-0
Marmonier P, Bodergat AM, Dolédec S (1994) Theoretical habitat templets, species traits, and species richness: ostracods (Crustacea) in the upper rhône river and its floodplain. Freshwater Biol 31:341–355. https://doi.org/10.1111/j.1365-2427.1994.tb01745.x
Martens K, Behen F (1994) A Checklist of the recent non-marine ostracods (Crustacea, Ostracoda) from the Inland Waters of South America and Adjacent Islands. Trav sci Mus nat hist nat Luxembourg 22:1–81
Matsuda JT, Lansac-Tôha FA, Martens K, Velho LFM, Mormul RP, Higuti J (2015) Association of body size and behavior of freshwater ostracods (Crustacea, Ostracoda) with aquatic macrophytes. Aquat Ecol 49:321–331. https://doi.org/10.1007/s10452-015-9527-2
Mazzini I, Ceschin S, Abati S, Gliozzi E, Piccari F, Rossi A (2014) Ostracod communities associated to aquatic macrophytes in an urban park in Rome. Italy Int Rev Hydrobiol 99(6):425–434. https://doi.org/10.1002/iroh.201301728
McAbendroth L, Ramsay PM, Foggo A, Rundle SD, Bilton DT (2005) Does macrophyte fractal complexity drive invertebrate diversity, biomass and body size distributions? Oikos 111(2):279–290. https://doi.org/10.1111/j.0030-1299.2005.13804.x
Meerhoff M, Mazzeo N, Moss B, Gallego R (2003) The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquat Ecol 37:377–391. https://doi.org/10.1023/B:AECO.0000007041.57843.0b
Meisch C (2000) Freshwater Ostracoda of Western and Central Europe. In: Schwoerbel J, Zwick P (eds) Süsswasserfauna von Mitteleuropa. Spektrum Akademischer Verlag, Berlin, Germany, pp 1–522
Meisch C, Smith RJ, Martens K (2019) A subjective global checklist of the extant non-marine Ostracoda (Crustacea). Eur J Taxon 492:1–135. https://doi.org/10.5852/ejt.2019.492
Mesquita-Joanes F, Smith AJ, Viehberg FA (2012) The ecology of Ostracoda across levels of biological organisation from individual to ecosystem: a review of recent developments and future potential. In: Horne DJ, Holmes JA, Rodríguez-Lázaro J, Viehberg FA (eds) Ostracoda as proxies for quaternary climate change. Elsevier, Amsterdam, pp 15–35
Nagorskaya L, Keyser D (2005) Habitat diversity and ostracod distribution patterns in Belarus. Hydrobiologia 538:167–178. https://doi.org/10.1007/s10750-004-4959-z
Neiff ASP, Casco SL (2003) Biological agents that accelerate winter decay of Eichhornia crassipes Mart. Solms. in northeastern Argentina. In: Thomas SM, Bini LM (eds) Ecologia e Manejo de macrófitas aquáticas. Editora da Universidade Estadual de Maringá, Maringá, pp 127–144
Oksanen J, Blanchet FG, Kindt R (2017) Vegan: Community ecology package. Version 2.3–3. https://cran.r-project.org/web/packages/vegan/ (Accessed 26 Mar 2019)
Pereira LC, Lansac-Tôha FA, Martens K, Higuti J (2017) Biodiversity of ostracod communities (Crustacea, ostracoda) in a tropical floodplain. Inland Waters 7(3):323–332. https://doi.org/10.1080/20442041.2017.1329913
Perez Rocha M, Bini LM, Domisch S, Tolonen KT, Jyrkänkallio-Mikkola J, Soininen J, Hjort J, Heino J (2018) Local environment and space drive multiple facets of stream macroinvertebrate beta diversity. J Biogeogr 45(12):2744–2754. https://doi.org/10.1111/jbi.13457
Pott VJ, Pott A (2000) Plantas aquáticas do Pantanal. EMBRAPA Comunicação para transferência de Tecnologia, Corumbá, MS
R Core Team (2017) R: A language and environment for statistical com- puting. Vienna, Austria: R Foundation for Statistical Computing. Retrieved from https://www.R-project.org/ (Accessed 26 June 2019)
Rehman F, Pervez A, Mahmood Q, Nawab B (2017) Wastewater remediation by optimum dissolve oxygen enhanced by macrophytes in constructed wetlands. Ecol Eng 102:112–126. https://doi.org/10.1016/j.ecoleng.2017.01.030
Rocha CEF, Por FD (1998) Preliminary comparative data on the fauna of the pleuston in the Southern Pantanal, Brazil, with emphasis on the microcrustaceans. Verh Internat Verein Limnol 26:2137–2140. https://doi.org/10.1080/03680770.1995.11901121
Rossetti G, Martens K (1998) Taxonomic revision of the Recent and Holocene representatives of the family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bull Inst R Sc Nat Belg Biol 68:55–110
Ruiz F, Abad M, Bodergat AM, Carbonel P, Rodríguez-Lázaro J, González-Regalado ML, Toscano A, García EX, Prenda J (2013) Freshwater ostracods as environmental tracers. Int J Environ Sci Technol 10(5):1115–1128. https://doi.org/10.1007/s13762-013-0249-5
Scheffer M, Hosper SH, Meijer ML, Jeppesen MB (1993) Alternative equilibria in shallow lakes. Trends Ecol Evol 8:275–279. https://doi.org/10.1016/0169-5347(93)90254-M
Souza DC, Cunha ER, Murillo RA, Silveira MJ, Pulzatto MM, Dainez-Filho MS, Lolis LA, Thomaz SM (2017) Species inventory of aquatic macrophytes in the last undammed stretch of the Upper Paraná River, Brazil. Acta Limnol Bras 29:e115. https://doi.org/10.1590/s2179-975x6017
Souza Filho E (2009) Evaluation of the Upper Paraná River discharge controlled by reservoirs. Braz J Biol 69(2):707–716. https://doi.org/10.1590/S1519-69842009000300024
Thomaz SM, Cunha ER (2010) The role of macrophytes in habitat structuring in aquatic ecosystems: methods of measurement, causes and consequences on animal assemblages’ composition and biodiversity. Acta Limnol Bras 22(2):218–236. https://doi.org/10.4322/actalb.02202011
Thomaz SM, Dibble ED, Evangelista LR, Higuti J, Bini LM (2008) Influence of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lagoons. Freshwater Biol 53(2):358–367. https://doi.org/10.1111/j.1365-2427.2007.01898.x
Walker PD, Wijnhoven S, Velde GV (2013) Macrophyte presence and growth form influence macroinvertebrate community structure. Aquat Bot 104:80–87. https://doi.org/10.1016/j.aquabot.2012.09.003
Weinstein BG, Tinoco B, Parra JL, Brown LM, McGuire JA, Stiles FG, Graham CH (2014) Taxonomic, phylogenetic, and trait beta diversity in South American Hummingbirds. Am Nat 184(2):211–224. https://doi.org/10.1086/676991
Winter M, Devictor V, Shweiger O (2013) Phylogenetic diversity and nature conservation: where are we? Trends Ecol Evol 28(4):199–204. https://doi.org/10.1016/j.tree.2012.10.015
Yamaki A, Yamamuro M (2013) Floating-leaved and emergent vegetation as habitat for fishes in a eutrophic temperate lake without submerged vegetation. Limnology 14:257–268. https://doi.org/10.1007/s10201-013-0403-2
Acknowledgements
We thank the Centre of Research in Limnology, Ichthyology and Aquaculture (Nupélia) and the Graduate Program in Ecology of Inland Water Ecosystems (PEA) of the State University of Maringá (UEM) for logistic support. We also thank Dr Fernando M. Lansac-Toha and Dr Danielle K. Petsch for statistical support and Jaime Luiz Lopes Pereira (Nupélia) for the production of the map. R.C., V.G.F. and E.O.C. would like to thank CAPES, and J.R. would like to thank to CNPq for granting their scholarships. The State University of Maringá (UEM, Maringá) and the Royal Belgian Institute of natural Sciences (RBINS, Brussels) have a bilateral Memorandum of Understanding regarding collaborative Scientific Research. Two anonymous referees offered important comments that improved the paper significantly.
Funding
This work was funded by Ministry of Science and Technology (MCT)/National Council for Scientific and Technological Development (CNPq) through the Long-Term Ecological Research (LTER, site 6) and Academic Excellency Program (PROEX)/Coordination for the Improvement of Higher Education Personnel (CAPES).
Author information
Authors and Affiliations
Contributions
RC, JH and KM designed the research. All authors contributed to the ostracod sampling. RC, VGF, JR and EOC contributed to the ostracod sorting and identification. RC wrote the first draft of the manuscript and all authors contributed to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Consent for publication
All authors significantly contributed to further manuscript revisions and gave final approval for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Campos, R., Rosa, J., Ferreira, V.G. et al. Macrophyte life forms influence the effects of environmental and spatial factors on the beta-diversity of associated ostracod communities (Crustacea). Aquat Sci 83, 27 (2021). https://doi.org/10.1007/s00027-021-00777-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-021-00777-9