Abstract
Large river floodplains are convenient model systems to test for variation in animal and plant community structure, as they have a variety of habitats and substrates and are generally dynamic systems through the occurrence of flood pulses with varying intensity. South American floodplain systems furthermore have unique types of substrates, in the form of root systems of floating macrophytes. Here, we investigate the variation in ostracod (small, bivalved crustaceans) communities in relation to substrates and related environmental variables. Sampling was effected in 2004 in the alluvial valley of the upper Paraná River, Brazil, in the wet and dry seasons. Five different substrates, including littoral sediment and four macrophyte species root and leaf systems, in four hydrological systems and a variety of habitat types, were sampled. Fifty-four species of Ostracoda were found. Variation partitioning analysis (RDA) showed that ostracod communities significantly differed between different substrates, mainly between the littoral and plants with small root systems (Eichhornia azurea) on the one hand, and plants with large and complex root systems on the other hand (Eichhornia crassipes and Pistia stratiotes). RDA analyses indicated that the pleuston (biotic communities associated with root systems of floating plants) of E. crassipes comprised more non-swimming species than the pleuston of the smaller roots of P. stratiotes, but species-level Kruskal–Wallis analyses could not detect significant differences between both macrophyte species. Also habitat type and hydrological systems contributed to variation amongst ostracod communities, but less so than the factor substrate. Abiotic factors also contributed to variation, but the ranges of all measured water chemistry variables were narrow. This uniformity in abiotic factors, which might be owing to the occurrence of large flooding events, unites all water bodies, even those that are generally separated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Floodplains associated with large rivers, such as the upper Paraná River, are convenient model systems to investigate change and variability in animal communities. The upper Paraná River Floodplain consists of four fluvial systems, which can have a variety of habitat types: rivers, connecting channels, open lakes, and closed lakes. Each of these habitat types has various substrates: benthic (littoral as well as profundal benthic, but the latter are often hypoxic and/or anoxic for at least part of the year—Higuti, 2004) or different species of floating macrophytes, a feature typical of South American floodplains (Thomaz et al., 2004a). The root systems of such plants can host rich communities of invertebrates and vertebrates. Such communities are referred to as pleuston (Por, 1995; Esteves, 1998; Por & Rocha, 1998; Higuti et al., 2007). Several of these floating plants, native to South America, have meanwhile become invasive species on other continents. Eichhornia crassipes, for example, is now recognized as a hazardous alien element in many African and Asian water bodies, to the extent that its presence might endanger entire ecosystems (Barreto et al., 2000; Jin et al., 2003). Yet, nowhere in non-Neotropical environments have rich pleuston communities developed in the root systems as they do in South America. It appears that local invertebrate faunas mostly fail to adapt and exploit the opportunities presented by these floating invasive species (Barreto et al., 2000).
A second typical feature of floodplains is the regular occurrence of flood pulses, periods where water levels rise suddenly, sometimes several metres overnight, for a period of time, to disappear equally suddenly. Several studies have demonstrated the potential importance of such flood pulses in structuring aquatic communities in floodplains, such as benthos, phytoplankton, zooplankton, fish and/or macrophytes (Twombly & Lewis 1987, 1989; Junk et al., 1989; Neiff, 1990; Oliveira & Calheiros, 2000; Agostinho et al., 2004; Higuti, 2004; Lansac-Tôha et al., 2004, Train & Rodrigues, 2004, Thomaz et al., 2004a, b, c, 2007; Boschilia et al., 2008). The existence of pleuston is thought to be a putative adaptation to neutralize possible detrimental effects of flood pulses on meiobenthic groups, such as acute anoxia (Higuti et al., 2007). Thomaz et al. (2007) argue that exceptionally high floods actually increase similarity among aquatic habitats in river floodplain systems, as high water levels unite, and homogenize, the water bodies, which are isolated during periods of low-water levels. However, most flood events are of lower magnitude, and only affect open lakes, channels and rivers. Flooding used to be seasonal, and directly linked to natural climatic wet and dry season alterations. Due to the constructions of several dams upriver of the upper Paraná floodplain, flooding patterns are now largely artificial and dependent on when water is released from the dams. If flood pulses are important for community structure, then they should show larger effects on open than on closed lakes, considering that only pulses of high magnitude would produce effect in closed lakes. Moreover, the effects would be more evident in benthic communities than in pleuston.
We organized sampling campaigns in wet and dry seasons, so that seasonality was included as a factor potentially affecting variability of ostracod communities.
Martens & Behen (1994) summarized the literature on South American recent, non-marine ostracods and listed 260 species in 53 genera; of these 96 species in 32 genera were reported from Brazil (Martens et al., 1998). Recent research on the Brazilian ostracod fauna has increased this number to 108 species in 35 genera (Würdig & Pinto, 2001; Pinto et al., 2003, 2004, 2005a, b, 2008; Higuti et al., 2009a). Nevertheless, many undescribed species await description, as is exemplified by the present study, where about a dozen of the species found are new to science. The global diversity of extant non-marine ostracods is presently estimated in approximately 2000 species (Martens et al., 2008) and the known contribution to this diversity in the Neotropical (275 species) is at present under estimation. Ostracods are abundantly present in the root systems of the floating plants in the river floodplain system of the Upper Paraná, as shown in recent articles by Higuti et al. (2007, 2009b), where they have investigated the effects of flood pulses on species richness.
Here, we investigate variation in the ostracod communities of the upper Paraná alluvial valley, in relation to five different substrates in a variety of hydrological systems and habitat types. Specific hypotheses tested and questions addressed, comprise: (1) to which extent do ostracod communities vary with different substrates (benthic littoral substrate and macrophyte root systems of four different species of floating plants); (2) are these differences related to biological properties of different ostracod groups (e.g. swimming versus non-swimming) or of the macrophytes (e.g. size and complexity of root system); (3) do ostracod communities vary among river systems, habitat types and seasons, or are they related to abiotic factors; (4) is there an effect of seasonality of flood pulses on ostracod communities, for example: is flood-related seasonality more obvious in open than in closed lakes?
Materials and methods
Study area
The upper Paraná River consists of a large braided channel, with an extensive floodplain and high-sediment accumulation in its bed, creating sand bars and islands of diverse sizes (from some hundreds of metres to several kilometres in length) and a floodplain with a width between 3 and 6 km in the study area (Agostinho et al., 1994). The floodplain reaches a maximum width of 20 km and, apart from the main channel of the Paraná river, comprises several secondary channels, lakes and tributaries (including Ivinheima and Baía Rivers) (Agostinho & Zalewski, 1996). These three river systems (Paraná, Ivinheima and Baía) are influenced by flooding events. Such flooding events used to be natural and directly related to rainfall during rainy season, both at the actual floodplain and further upstream. Meanwhile, several dams have been built upstream of the Paraná River floodplain, and flood events are now caused by controlled water release by these dams.
Further away from the main channel, and not connected to it, is the Taquaruçu system, which comprises exclusively closed lakes (Souza Filho & Stevaux, 2004) (Fig. 1). The origin of the lakes of the Taquaruçu system is still unknown, although some authors suggested that colluvial processes of an old drainage network formed these lakes in the lower to middle Pleistocene (Justus, 1985; Pires-Neto et al., 1994; Stevaux et al., 2004).
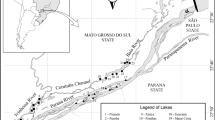
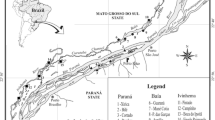
Satellite image (LANDSAT 7—ETM 1999) of 48 sampled environments from the alluvial valley of upper Paraná River. Locality codes are the same shown in Table 1. Inset shows position in Brazil
Field sampling and laboratory analysis
Ostracods were collected during March, July and November of 2004 throughout the alluvial valley of the upper Paraná River. In total, we collected 132 samples at 48 sites in four fluvial systems (Paraná, Ivinheima, Baía and Taquaruçu). The sites mostly represent four habitat types (closed lake, open lake, channel and river) and five substrate types (littoral = shallow benthic, less than 1 m deep, sometimes with submerged vegetation; root systems of Eichhornia crassipes, E. azurea, Pistia stratiotes, or Salvinia spp.). Only monospecific stands of these plants were sampled. We also obtained some additional samples from one habitat type (streams) as well as some further substrate types (Hydrocotyle ranunculoides, Oxycaryum cubense and mixed floating macrophytes) (Table 1). Although we recorded the species present in these additional samples (cf. Table 2), we did to not take them into account in the data analysis (Table S1).
We sampled littoral ostracods by hauling a rectangular net (28 cm × 14 cm, mesh size ~160 μm) close to the sediment–water interface for approximately 1 min (same for all littoral samples). Floating vegetation was collected by hand, and comparable amounts of roots were thoroughly washed in a bucket. The residues were washed in the same handnet. In the laboratory, we subsequently washed samples over two sieves with different mesh size (2 and 0.25 mm, respectively) and we preserved the material by retaining in the 0.25 mm sieve with alcohol (70% final concentration). Subsamples were taken with a Folsom fractioner, and ¼ of samples was counted. Species richness was always estimated from the total sample, i.e. all specimens in the sample were identified. Valves and appendages were examined using scanning electron microscopy and optical microscope, respectively. Ostracods were identified down to species level, using available primary ostracod literature (see Martens & Behen, 1994 and articles comprised therein; Rossetti & Martens 1996, 1998; Pinto et al., 2003, 2004, 2005a; Savatenalinton & Martens, 2009).
For each sampling station, descriptive aspects of the environments (types of substrate and habitat, degree of connectivity) and key limnological factors, such as pH (pHmeter-Digimed), electrical conductivity (conductivimeter-Digimed), dissolved oxygen concentration and water temperature (oxymeter-YSI) were measured. Two major elements, calcium and magnesium, were quantified for the November 2004 sampling, using the spectrometry technique of atomic absorbance. Sampling, collection and preservation were done to determine Ca and Mg concentrations, followed procedures as described in the literatures (Rainwater & Thatcher, 1960; Wagner, 1976; Souza & Derisio, 1977).
Water level data were obtained from Brazilian National Water Agency (Agência Nacional de Águas—ANA) and Itaipu Binacional.
Description of substrates
Five types of substrate were sampled, including shallow littoral (<1 m deep, on sand or mud) and root systems from four floating macrophyte species. Benthic samples in the middle of the lakes and channels (2–3 m deep) were also collected (with Ekman grab) during the first sampling period, but these sediments were mostly anoxic and contained no or very few living ostracods. So, this sampling was abandoned during the second and third sampling period.
The sampled floating macrophytes differ strongly in the morphology and size of their root systems. E. crassipes has the most complex and the largest root systems, up to a metre long and voluminous, capturing substantial amounts of sediment. Pistia has smaller roots (10–15 cm long). Salvinia has submerged systems of modified leaves, which also harbour pleuston communities (~5 cm long). E. azurea has small, narrow patches of short roots (5–10 cm) along the stems; the latter are moreover attached to the sediment (S. M. Thomaz, unpubl. data). The other three macrophyte species investigated here are free floating.
Data analysis
The main purpose of our analysis was to investigate the association between ostracod community composition and substrate type, with special attention for differences among floating macrophytes with different root systems. Given that our data were collected in different seasons, hydrological systems and habitat types, we organized our analysis in such a way as to maximally take into account spatial and temporal interdependence of our data and to control for collinearity among explanatory variables. We also tried to assess the relative importance of each of the investigated categories of explanatory variables in explaining community variation. For this, we applied variation partitioning on the ostracod abundance matrix (expressed as catch per unit of effort—CPUE) using redundancy analysis (RDA). RDA analysis can be considered as a multivariate extension of multiple regression, with multiple dependent variables (Legendre & Legendre, 1998). We first tested the significance of a global RDA-model incorporating the entire set of explanatory variables, and assessed the fraction of total community variation explained by this model. If, and only if, the global model deemed significant, we then proceeded by estimating the total fraction of variation uniquely explained by each variable category separately (i.e., substrate type, hydrological system, habitat type and season), specifying the other variable categories as covariables (partial RDA). In this way, we took a conservative stance by ruling out potentially spurious associations due to collinearity among explanatory variables. RDA analyses were performed on log (x + 1)-transformed data using the program Canoco v4.5 (Lepš and Šmilauer, 2003). Significant effects of categorical variables in the variation partitioning analysis were further explored through multiple RDA-comparisons for each pairwise combination of category levels. Significance tests were performed through random Monte Carlo permutations in CANOCO v4.5, where permutations were restricted to blocks for seasons and hydrological system in order to take into account spatial and temporal dependency in the data. Species responses to variables were also further explored and verified graphically with boxplots and with univariate techniques (Kruskal–Wallis ANOVA—with STATISTICA 7.1).
Rarefaction of specimens versus species was calculated using the program EcoSim.
Results
Of the 54 species found in the present survey (Table 2), at least 12 are new species and also 3 genera are new. To allow identification, an atlas of valve morphology of the different species was compiled using scanning electron microscopy. A summary of valve shapes of most of the species is given in Figure S1 (in online supplementary material) to the present article for future reference to species and genera presently left in open nomenclature. In the present survey, 33 species belong to the Cyprididae, 10 to Candonidae, 8 to Darwinulidae and 3 to Limnocytheridae. Rarefaction analysis on richness shows that all substrates tend to approximate saturation levels with the present sampling effort (Fig. 2).
Variation partitioning
The global RDA model, incorporating all explanatory variables, was highly significant and explained 42.5% of ostracod community variation (F = 4.638, P = 0.002; Table 3). Each of the explanatory variable categories significantly explained a unique part of community variation (Table 3). Collinearity among explanatory variables (i.e. variation explained by two or more variable categories in common) contributed with 12% to the explained variation (Table 3). Substrate type contributed most to total explained community variation (11%), followed by abiotic variables (6%), habitat type (5%), hydrological system (5%) and season (3%). The same analysis was performed on the datamatrix without the data from Taquaruçu (as this system has only closed lakes and only a subset of the substrates), and the results were similar to those obtained for the full set analysis (Table 3).
Substrate type
Ostracod communities differed significantly among substrate types according to the variation partitioning analysis (Table 3) and pairwise comparisons between substrate types (Fig. 3, Table 4). The first axis of the RDA analysis was twice as important as the second axis and represented 10% of the community variation; this axis mainly differentiated the ‘littoral’ from P. stratiotes or E. crassipes (Fig. 3). A large number of species tended to be positively associated with P. stratiotes or E. crassipes, whereas less species tended to be specifically associated with the littoral and E. azurea in the analyses (Fig. 3). This was tested at family level (Cyprididae, Candonidae, Limnocytheridae and Darwinulidae), and it is clear that littoral and E. azurea are nearly always significantly different from E. crassipes and P. stratiotes (Fig. 4). At the species level, 18 out of 50 species tested showed consistent differences between substrate types (Table 5). Again, mostly differences between littoral and macrophytes (e.g. Diaphanocypris meridana) and between macrophytes with dense root systems (E. crassipes, P. stratiotes) and the others were evident.
Biplot of a redundancy analysis, showing the association between ostracod community structure and substrate. Triangles represent centroids and indicate the average location of samples taken in the same substrate type. For an explanation of species codes, see Table 2
The root systems of P. stratiotes and of E. crassipes are both well developed; yet the latter root systems are still several times larger than those of the former. It is, therefore, interesting to see that most species in P. stratiotes tend to be good swimmers (almost all are Cyprididae, and thus mostly have long natatory setae on the Antenullae and the Antennae), whereas many members of the ostracod community associated with E. crassipes are non-swimming, such as darwinulids and candonids. This difference between species with these divergent biological aspects in these two pleuston communities was tested at the family level (Cyprididae, Candonidae, Darwinulidae) with Kruskal–Wallis (KW) analyses, but no significant difference was found between the communities in E. crassipes and P. stratiotes (see Fig. 4). Also at species-level analyses, differences between E. crassipes and P. stratiotes were significant in one (swimming) species only, Cypridopsis vidua sp.2.
River systems, habitat type, seasonality and abiotic factors
Ostracod communities differed significantly among all pairwise comparisons of river systems, but Baía and Paraná were most differentiated from each other (Fig. 5, Table 4), with Baía by far being the system with the most species associated with it.
Biplot of a redundancy analysis, showing the association between ostracod community structure and system. Triangles represent centroids and indicate the average location of samples taken in the same system. For an explanation of species codes, see Table 2
RDA analyses (Fig. 6), using habitat type as explanatory variable, indicated that the most important axis of variation reflects the difference between closed lakes (with relatively few species positively associated with this habitat type) and open environments, such as channels and open lakes. However, habitat type was to an important degree collinear with substrate types, and the factor ‘habitat type’ explains only 5.4% of the variation if substrate type is corrected for by specifying it as covariable in the analysis (F = 2.511, P = 0.001). These results indicate that habitat type explains part of the variation in the ostracod community data, although part of this explained variation may potentially reflect differences in substrate type among habitat type. Nevertheless, according to the variation partitioning results, habitat type also seems to contribute significantly to the faunal variation, independent of substrate type (Table 3). Rivers do not seems to have many species specifically associated with them, whereas channels and open lakes have the most specifically associated taxa.
Biplot of a redundancy analysis, showing the association between ostracod community structure and habitat type. Triangles represent centroids and indicate the average location of samples taken in the same habitat type. For an explanation of species codes, see Table 2
RDA analyses, with the set of abiotic variables as dependent variables and substrate type, season and fluvial system as explanatory variables, revealed no significant association between substrate type and environmental variables, but important associations with both season and system (25%) were found (Fig. 7). The effect of habitat type on these variables was also significant, but less so. pH and temperature were higher in autumn (March) than in spring (November). Paraná had a significantly higher pH than Baía, whereas Taquaruçu had higher levels of dissolved oxygen. Rivers had higher oxygen and pH than closed lakes; streams and open lakes were intermediary.
Biplot of a redundancy analysis, showing the association between ostracod community structure and abiotic variables. Triangles represent centroids and indicate the average location of samples taken in the same habitat type. For an explanation of species codes, see Table 2
When only corrected for season, abiotic variables explained 11.7% of ostracod community variation (P = 0.001, F = 2.908). However, after correction for season, substrate, hydrological system and habitat type, the remaining amount of community variation explained by abiotic variables equalled only 6.4% (F = 2.088, P = 0.001). This is so, because environmental variables showed a considerable degree of collinearity with the other factors, especially season and system. Nevertheless, conductivity, and to a lesser extend dissolved oxygen and pH, still showed some unique association with ostracod community variation.
Flood pulses in the upper Paraná River system are no longer natural and associated with wet-dry seasons, but are a result of water release of upstream dams. The irregular pattern of flooding (both in timing and in intensity) is illustrated for the flooding in Paraná and Ivinheima, during the sampling period (2004), as well as for the same systems over the period 2000–2007 (Fig. 8). We tested if seasonality was more pronounced in open than in closed systems, but there was no significant interaction between both variables.
Plots of water level fluctuations in the Upper Paraná River floodplain. A Paraná River, January 2000–January 2007. B Paraná River, January–December 2004. C Ivinheima River, January 2000–January 2007. D Ivinheima River, January–December 2004. Note the March, July and November samplings in 2004. Source: Brazilian National Water Agency (Agência Nacional de Águas—ANA) and Itaipu Binacional
Discussion
Diversity
We found 54 ostracod species in 132 samples from 48 sites; the latter included open and closed lakes, channels, rivers and one stream. Rarefaction analyses showed that species richness approached plateau values for all substrates, so sampling effort was sufficient, certainly for substrates such as the root systems of E. crassipes and for the litoral (Fig. 2). Comparable surveys have yielded comparable diversities: 47 ostracod species from 33 European lakes (Viehberg, 2006), 29 species from 36 Canadian lakes (Bunbury & Gajewski, 2005), 30 species from 100 lakes in Tibet (Mischke et al., 2007) and 47 species from 106 samples in Western Mongolia (including lakes, springs, streams and pools) (Van der Meeren et al., 2010). Yet, this is less than one could have expected and this for several reasons.
First, because the present survey is in the sub-tropics and one could expect higher species richness than in more temporate, or even subarctic, settings. Second, we have sampled a larger variety of habitats than most of the studies cited above. Finally, several ‘species’ in our present survey are in open nomenclature. In this, we have followed a rather non-conservative view in that most (stable) morphotypes have been called ‘species’. Recently, it was shown that in ostracod groups with mixed reproduction, mostly in Cyprididae, classical species should be regarded as species complexes, with sometimes dozens of putative cryptic species. For example, in Eucypris virens, a common European species, Bode et al. (2009) identified almost 40 potential cryptic species. Most of these could not be identified using their morphology. It is thus not certain to which extent some of the species cited here in open nomenclature can be viewed as ‘classical’ species, or should be seen as cryptic species in species complexes. Most of the Northern Hemisphere studies cited above use conservative, classical species.
Other potential reasons for this relatively low number of species are discussed below, and include relatively narrow gradients in abiotic variables and possible homogenizing effects of very large flood events (see also Thomaz et al., 2007).
From the saturation curves, observed ostracods richness appeared to be highest in the littoral. Amongst the floating macrophytes, richness appeared to follow root size and complexity, with E. crassipes having the highest and E. azurea the lowest richness, while P. stratiotes and Salvinia spp. being intermediate (see below).
General variation partitioning
Our global model explained about 42% of the total community variation, indicating that our analyses give important indications along which gradients communities are structured. The categoric variables used in our model (substrate, system, habitat type, seasonality and abiotic variables), rather than being direct drivers of community structure themselves, seem to represent important latent causal variables. Although our analyses do not necessarily allow to link community variation to the specific ecological mechanisms that shape ostracod communities, and should, therefore, mainly be considered as exploratory, they reveal an important fraction of the structure in the ostracod communities and give important indications on which the design of future studies can be based. For example, it would seem that abiotic variables are less important for ostracods than in several other studies (see below), whereas effects of different root systems on ostracod communities are obvious, but remain ill understood.
The results of our analysis also proved robust. The Taquaruçu system, for example, appears highly aberrant (it is well-separated from the three main riverine systems, only contains closed lakes and thus also contains a limited subset of investigated substrates), but its exclusion from the general variation partitioning analysis hardly changed the results.
Effects of substrates on ostracod communities
The littoral of (mostly closed) lakes has a specific ostracod community, and at least some species, like Physocypria schubarti, are significantly attracted to it. Root systems of the floating plants also offer substrate to ostracod communities. Our analyses show that the ostracod communities from littoral substrates differ from the pleuston of almost all of the floating macrophytes. This was confirmed for most species for which there were significant differences in abundance (as CPUE) between substrates (Table 5).
Different substrates housed different ostracod communities in the aquatic habitats of the alluvial valley of the upper Paraná River. Our RDA analysis indicated significant differences among each possible pair of substrate type. Furthermore, the first axis of the RDA analysis represented a main gradient from littoral (no roots), over plant systems with very small (E. azurea) to small (Salvinia spp.) rootsystems, to plants with larger (P. stratiotes) and very large (E. crassipes) root systems.
The effect of aquatic macrophyte habitat complexity on invertebrate abundance and richness in tropical lakes was demonstrated by Thomaz et al. (2008), and their results indicate that habitat complexity as exemplified by different architectures of aquatic plants, significantly affects both number of taxa and density. Ostracods were ill represented in that study, but the present results seem to indicate that size and complexity of rootsystems can have major effects on ostracod communities in the pleuston. Similar studies on other animal and plant groups show the importance of the substrate type and/or structural complexity on the distribution of richness and abundance of invertebrates (Iversen et al., 1985; Cyr & Downing, 1988; Botts & Cowell, 1993; Taniguchi et al., 2003; Taniguchi & Tokeshi, 2004; Declerck et al., 2007; Thomaz et al., 2008).
Eichhornia crassipes has by far the largest root system, which also captures floating sediment particles. This is less so for P. stratiotes, the macrophyte with the second largest root system in the present study. The E. crassipes root system thus resembles a real sediment substrate, and a rich ostracod community is attracted to this substrate type.
When comparing the communities associated with both P. stratiotes and E. crassipes (Fig. 3), it appears that almost all species significantly associated with P. stratiotes are swimming Cyprididae, whereas the majority of species associated with E. crassipes are non-swimming, crawling Candonidae, Limnocytheridae and Darwinulidae. This would intuitively make sense, as the large root systems of the latter macrophyte species could accommodate non-swimming species, whereas the smaller root systems of the other macrophytes, including P. stratiotes, can only be colonized by swimming species. However, subsequent KW tests at family and at species levels do not corroborate this pattern. Further, experimental research will be required to test if this pattern holds true.
A study carried out in Gentil lagoon (Rio Grande do Sul, Brazil), also demonstrated an elevated abundance of darwinulids associated with aquatic macrophytes (Albertoni & Würdig, 1996). According to Würdig & Freitas (1988), darwinulid species adapt to sandy and sandy–muddy sediment as substrates rich in vegetal fragments and decomposing organic matter, even with low oxygen content. The large root systems of E. crassipes could be placed into this category, as they are hard substrate, contain muddy sediment and large amounts of organic matter, some autochtonous (dead roots), some allochtonous.
Eichhornia crassipes has successfully invaded many water bodies in South East Asia, North America and Africa, to the extent that it has become a serious threat to ecosystem health in such places (Ogutu-Ohwayo et al., 1997; Coetzee et al., 2003; Jin et al., 2003; Center et al., 2005). Nevertheless, isolated attempts to recover meiobenthos from the root systems of such alien invaders showed that almost no local faunas have thus far invaded this potential habitat (K.M., unpubl. data). Toxic substances produced by the plants have been cited as reasons for the ‘empty pleuston’ in alien Eichhornia (Jin et al., 2003), which means that South American meiobenthos first had to develop tolerance against such toxins to invade Eichhornia (and other) root system. This requires substantial physiological adaptations, which must have occurred independently in various animal groups, certainly in at least five different ostracod families.
There is an apparent absence of truly benthic ostracod communities in the sampled lakes, channels and rivers. In the lakes, this is most likely related to the anoxic nature of most of the deeper (>4 m) benthic habitats, especially during high lake levels after mega-flood pulses (see below). In rivers, the sediment is highly unstable (although apparently well oxygenated), because of the changing currents with flood pulse over the year (Thomaz et al., 2004a, b, c). Freshwater ostracods are ill-adapted to such unstable sediments, hence the absence of benthic ostracod communities. Ostracods in the true river habitats can be found amongst macrophytes and in the littoral.
Effects of other variables
Most of the explanatory variable categories considered in our study show some degree of co-linearity. The system Taquaruçu has only one habitat type, closed lakes, which have less substrates (more littoral and less E. crassipes). Also some abiotic variables determine the environment of river systems (e.g. higher pH in Paraná, higher DO in Taquaruçu), etc. Yet, our variation partitioning analysis shows that each of these variable categories has some independent, unique contribution to the total ostracod community variation.
Surprisingly, closed lakes have the lowest diversity (Higuti et al., 2009b) and have few characteristic species. Generally, there are more lacustrine (lentic) than lotic ostracod species (Meisch, 2000), but in the upper Paraná alluvial valley, rivers, channels and open lakes have a higher diversity and especially channels and open lakes hold more species than closed lakes (Higuti et al., 2009b) (Table S1). Open lakes and channels could be seen as the ‘best of both worlds’, an ecotone between lentic and lotic habitats, with better nutrient supply and higher probabilities for colonization than isolated, lentic habitats, and with less flow-induced stress than in lotic habitats.
The lack of species characteristic to rivers is surprising (Fig. 8), given the fact that this is the habitat type with the highest diversity and richness (Higuti et al., 2009b). Maybe riverine faunas are a random collection of species washed out of other habitat types, and no truly rheophylic species exist in the Paraná alluvial valley ostracod communities. Rivers can be seen as the main avenue of ostracod dispersal rather than dispersal of drought resistant stages through wind, or biotic vectors (McKenzie, 1971; Sandberg & Plusquellec, 1974; De Deckker, 1977; Horne & Martens, 1998; Lopez et al., 2002). The effects of river-mediated dispersal might explain at least part of the variation not accounted for by the factors analysed here (~60%, see above).
The hydrological systems Baía and Paraná are most different from each other. One of the most characteristic aspects of the Baía River is its low water velocity (0.1–0.5 m s−1 at its mouth, compared to the Paraná River channel with 0.9–1 m s−1, Thomaz et al., 2004a). Therefore, although the system of Baía is fundamentally lotic, it approaches semi-lentic stage, with vast stands of macrophytes, even in the main river channel. This could be the reason why more species seem associated to this system than to any of the other three fluvial systems (Fig. 5), despite its relatively low pH.
Physical and chemical properties of water are generally thought to be major driving factors in ostracod distribution, as ostracods need to calcify their valves after each moult (nine times in total in podocopids) and this imposes important physiological stress if water chemistry is not adequate (e.g. low HCO3 levels, low pH, etc.). In the present study, we measured only few variables and these had limited ranges of measurements, yet water chemistry was still the second most important factor associated with ostracod community structure. Other studies have also found that water chemistry strongly correlate with ostracod diversity and species distributions (Mezquita et al., 2001). Viehberg (2006) identified temperature as a main driver of species assemblages in Northern German lakes. Mischke et al. (2007) investigated a broad range of Central Asian lakes, with a wide range of salinities, and could single out electrical conductivity as a local factor determining ostracod communities. Mourguiart & Carbonel (1994) identified lake depth, and Kiss (2007) identified macrophyte habitat structure as determinants of ostracods species distributions.
Since only a limited number of variables were measured in the present study, other, unmeasured factors could have even more pronounced effects in this category (for example nutrients by Van der Meeren et al., 2010). Higuti et al. (2009c), however, showed that hydrodynamic fluctuations of systems and habitats are more important to darwinulid distribution than abiotic variables of the water bodies.
Seasonality of flood pulses
Large river floodplains consist of a variety of habitats, including lenthic and lotic ones and open and closed ones. The open systems, more in particular the open lakes, should receive a higher impact of flood pulses, as they are connected to main channels or even directly to rivers themselves. Such flood pulses were normally associated with alternating wet and dry periods during the year. Therefore, our sampling was designed to incorporate this seasonality with major sampling being effected in March (dry season) and November (rainy season). It was thus postulated that open lakes would reflect flood-related seasonality more than closed lakes. Although we detected some systematic differences between the ostracods communities of March and November, temporal variation was overall rather low and the degree of temporal variation did not vary amongst open and closed lakes. Three possible underlying reasons for this lack of interaction between habitat connectivity and seasonality could be put forward.
First, flooding has not been following natural cycles for the last decade or so, and depended on water release from upstream dams in the upper Paraná River. Our two major sampling campaigns were carried out during relatively low lake stands and major floods only occurred after the November sampling campaign. Thus, no difference in lake stands/flood intensity occurred during our two major sampling periods.
Second, apart from regular and moderate flood pulses of 1–2 m water level changes, also major flood pulses (>4 m) occur, roughly once a year (Fig. 8). Such flood pulses not only connect open lakes, but occasionally also flood closed lakes (except in Taquaruçu, where no connection to channels or rivers occurs anymore). Such mega-floods may increase habitat similarity (Thomaz et al., 2007) and nullify the effects of seasonality. However, as such events occur roughly annually, it is surprising that differences amongst communities persist at all.
Finally, most of the ostracod communities occurred in pleuston of floating macrophyte root systems. Such floating plants simply follow water level increases and decreases. It has been postulated (Higuti et al., 2007) that the origin of pleuston communities might (at least in part) be an adaptation to neutralize the detrimental effects of flooding on benthic communities. Mega-floods may render benthos anoxic virtually overnight, but pleuston simply follows water level changes and thus escapes the detrimental side-effects. The uncoupling of ‘habitat type’ and ‘seasonality’ effects on ostracod communities might thus be a consequence of this particular habitat type investigated here. The present results would then offer support to the ‘pleuston refugia’ hypothesis of Higuti et al. (2007).
Conclusions
Based on the present knowledge, the design of future studies should focus on (experimental) testing of the relevance of specific drivers of community structure. The present study clearly showed that substrate type has an effect on ostracod communities in the pleuston of floating macrophytes. It now remains to be determined which aspects of these root systems cause the difference: size, complexity or chemical substances. In addition, it is also unclear which other variables cause the ~60% as yet unexplained variation in ostracod community structure, observed in the present study. These could be abiotic factors, such as unmeasured variables of water chemistry, but could also be linked to biotic factors such as predation, competition and parasites.
References
Agostinho, A. A. & M. Zalewski, 1996. A planície alagável do alto rio Paraná: importância e preservação (Upper Paraná River Floodplain: Importance and Preservation). EDUEM, Maringá.
Agostinho, A. A., H. F. Julio Jr. & M. Petrere Jr., 1994. Itaipu reservoir (Brazil): impacts of the impoundment on the fish fauna and fisheries. In Cowx, I. G. (ed.), Rehabilitation of Freshwater Fisheries. Bodman, Fishing News Book, Oxford: 171–184.
Agostinho, A. A., L. M. Bini, L. C. Gomes, H. F. Júlio Jr., C. S. Pavanelli & C. S. Agostinho, 2004. Fish assemblages. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its Floodplain: Physical Aspects. Ecology and Conservation. Backhuys Publishers, Leiden: 223–246.
Albertoni, E. F. & N. L. Würdig, 1996. Comunidade de ostracodes associada à macrófitas aquáticas na lagoa do Gentil, Tramandaí, RS. Acta Limnologica Brasiliensis 8: 103–114.
Barreto, R., R. Charudattan, A. Pomella & R. Hanada, 2000. Biological control of neotropical aquatic weeds with fungi. Crop Protection 19: 697–703.
Bode, S. N. S., S. Adolfsson, D. K. Lamatsch, E. R. D. Bautz, M. J. F. Martins, O. Schmit, J. Vandekerkhove, F. Mezquita, T. Namiotko, G. Rossetti, I. Schön, R. K. Butlin & K. Martens, 2009. Exceptional cryptic diversity and multiple origins of parthenogenesis in a freshwater ostracod. Molecular Phylogenetics and Evolution 54: 542–552.
Boschilia, S. M., E. F. Oliveira & S. M. Thomaz, 2008. Do aquatic macrophytes co-occur randomly? An analysis of null models in a tropical floodplain. Oecologia 156: 203–214.
Botts, P. S. & B. C. Cowell, 1993. Temporal patterns of abundance of epiphytic invertebrates on Typha shoots in a subtropical lake. Journal of the North American Benthological Society 12: 27–39.
Bunbury, J. & K. Gajewski, 2005. Quantitative analysis of freshwater ostracode assemblages in southwestern Yukon Territory, Canada. Hydrobiologia 545: 117–128.
Center, T. D., T. K. Van, F. A. Dray Jr., S. J. Franks, M. T. Rebelo, P. D. Pratt & M. B. Rayamajhi, 2005. Herbivory alters competitive interactions between two invasive aquatic plants. Biological Control 33: 173–185.
Coetzee, J., M. Byrne & M. Hill, 2003. Failure of Eccritotarsus catarinensis, a biological control agent of waterhyacinth, to persist on pickerelweed, a non-target host in South Africa, after forced establishment. Biological Control 28: 229–236.
Cyr, H. & J. A. Downing, 1988. The abundance of phytophilous invertebrates on different species of submerged macrophytes. Freshwater Biology 20: 365–374.
De Deckker, P., 1977. The distribution of the “giant” ostracods (Family: Cyprididae, Baird, 1845) endemic to Australia. In Löffler, H. & D. L. Danielopol (eds), Aspects of Ecology and Zoogeography of Recent and Fossil Ostracoda. Junk, The Hague: 285–294.
Declerck, S., M. Vanderstukken, A. Pals, K. Muylaert & L. de Meester, 2007. Plankton biodiversity along a gradient of productivity and its mediation by macrophytes. Ecology 88: 2199–2210.
Esteves, F. A., 1998. Fundamentos de Limnologia. Interciência, Rio de Janeiro.
Higuti, J., 2004. Composition, density and habitats of benthic chironomid larvae. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its Floodplain: Physical Aspects. Ecology and Conservation. Backhuys Publishers, Leiden: 209–221.
Higuti, J., L. F. M. Velho, F. A. Lansac-Tôha & K. Martens, 2007. Pleuston communities are buffered from regional flood pulses: the example of ostracods in the Paraná River floodplain, Brazil. Freshwater Biology 52: 1930–1943.
Higuti, J., C. Meisch & K. Martens, 2009a. On Paranacypris samambaiensis n.gen. n.sp. (Crustacea, Ostracoda), the first South American psychrodromid, from the alluvial valley of the Upper Paraná River, Brazil. Journal of Natural History 43: 769–783.
Higuti, J., F. A. Lansac-Tôha, L. F. M. Velho & K. Martens, 2009b. Biodiversity of non-marine ostracods (Crustacea, Ostracoda) in the alluvial valley of the upper Paraná River, Brazil. Brazilian Journal of Biology 69(2 suppl): 661–668.
Higuti, J., F. A. Lansac-Tôha, L. F. M. Velho, R. L. Pinto, L. C. G. Vieira & K. Martens, 2009c. Composition and distribution of Darwinulidae (Crustacea, Ostracoda) in the alluvial valley of the upper Paraná River, Brazil. Brazilian Journal of Biology 69(2): 253–262.
Horne, D. J. & K. Martens, 1998. An assessment of the importance of resting eggs for the evolutionary success of Mesozoic non-marine cypridoidean Ostracoda (Crustacea). Advances in Limnology 52: 549–561.
Iversen, T. M., J. Thorup, T. Hansen, J. Lodal & J. Olsen, 1985. Quantitative estimates and community structure of invertebrates in a macrophyte rich stream. Archiv für Hydrobiologie 102: 291–301.
Jin, Z. H., Y. Y. Zhuang, S. G. Dai & T. L. Li, 2003. Isolation and identification of extracts of Eichhornia crassipes and their allelopathic effects on algae. Bulletin of Environmental Contamination Toxicology 71: 1048–1052.
Junk, W. J., P. B. Bayley & R. E. Sparks, 1989. The flood pulse concept in river-floodplain systems. In Dodge, D. P. (ed.), Proceedings of the International Large River Symposium (LARS). Canadian Special Publication of Fisheries and Aquatic Sciences, Otawa: 110–127.
Justus, J. O., 1985. Subsídios para a interpretação morfogenética através da utilização de imagens de radar. Master Thesis, Universidade Federal da Bahia: 204 pp.
Kiss, A., 2007. Factors affecting spatial and temporal distribution of Ostracoda assemblages in different macrophyte habitats of a shallow lake (Lake Fehér, Hungary). Hydrobiologia 585: 89–98.
Lansac-Tôha, F. A., C. C. Bonecker & L. F. M. Velho, 2004. Composition, species richness and abundance of the zooplankton community. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its Floodplain: Physical Aspects. Ecology and Conservation. Backhuys Publishers, Leiden: 145–190.
Legendre, P. & P. Legendre, 1998. Numerical Ecology. Developments in Environmental Modelling, Vol. 20. Elsevier, The Netherlands.
Lepš, J. & P. Šmilauer, 2003. Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press, Cambridge.
Lopez, L. C. S., D. A. Gonçalves, A. Mantovani & R. I. Rios, 2002. Bromeliad ostracods pass through amphibian (Scinaxax perpusillus) and mammalian guts alive. Hydrobiologia 485: 209–211.
Martens, K. & F. Behen, 1994. A checklist of the non-marine ostracods (Crustacea, Ostracoda) from the inland waters of South America and adjacent islands. Travaux Scientifiques du Musée d’ Histoire Naturelle de Luxembourg 22: 1–81.
Martens, K., N. L. Würdig & F. Behen, 1998. Maxillopoda. Non-marine Ostracoda. In Young, P. S. (ed.), Catalogue of Crustacea of Brazil. Museu Nacional, Rio de Janeiro: 45–65.
Martens, K., I. Schön, C. Meisch & D. J. Horne, 2008. Global biodiversity of non-marine Ostracoda (Crustacea). Hydrobiologia 595: 185–193.
McKenzie, K. G., 1971. Paleozoogeography of freshwater Ostracoda. Bulletin du Centre de Recherchers de Pau 5: 207–237.
Meisch, C., 2000. Freshwater Ostracoda of Western and Central Europe. Spektrum Akademischer Verlag GmbH, Heidelberg, Berlin.
Mezquita, F., H. I. Griffiths, M. I. Dominguez & M. A. Lozano-Quilis, 2001. Ostracoda (Crustacea) as ecological indicators: a case study from Iberian Mediterranean brooks. Archiv für Hydrobiologie 150: 545–560.
Mischke, S., U. Herzschuh, G. Massmann & C. Zhang, 2007. An ostracod-conductivity transfer function for Tibetan lakes. Journal of Paleolimnology 38: 509–524.
Mourguiart, P. & P. Carbonel, 1994. A quantitative method of palaeolake-level reconstruction using ostracod assemblages: an example from the Bolivian Altiplano. Hydrobiologia 288: 183–193.
Neiff, J. J., 1990. Ideas para la interpretacion ecológica del Paraná. Interciencia 15: 424–441.
Ogutu-Ohwayo, R., R. E. Hecky, A. S. Cohen & L. Kaufman, 1997. Human impacts on the African Great Lakes. Environmental Biology of Fishes 50: 117–131.
Oliveira, M. D. & D. F. Calheiros, 2000. Flood pulse influence on phytoplankton communities of the south Pantanal floodplain, Brazil. Hydrobiologia 427: 101–112.
Pinto, R. L., C. E. F. Rocha & K. Martens, 2003. On two new species of the genus Vestalenula Rossetti & Martens, 1998 (Crustacea, Ostracoda, Darwinulidae) from semi-terrestrial habitats in São Paulo State (Brazil). Zoological Journal of the Linnean Society 139: 305–313.
Pinto, R. L., C. E. F. Rocha & K. Martens, 2004. On the genus Penthesilenula Rossetti & Martens, 1998 (Crustacea, Ostracoda, Darwinulidae) from (semi-) terrestrial habitats in São Paulo State (Brazil), with the description of a new species. Journal of Natural History 38: 2567–2589.
Pinto, R. L., C. E. F. Rocha & K. Martens, 2005a. On the evolution of the genus Microdarwinula Danielopol, 1968 (Ostracoda, Darwinulidae) with the description of a new species from semi-terrestrial habitats in São Paulo State (Brazil). Crustaceana 78: 975–986.
Pinto, R. L., C. E. F. Rocha & K. Martens, 2005b. On new terrestrial ostracods (Crustacea, Ostracoda) from Brazil, primarily from São Paulo State. Zoological Journal of the Linnean Society 145: 145–173.
Pinto, R. L., C. E. F. Rocha & K. Martens, 2008. On the first terrestrial ostracod of the Superfamily Cytheroidea (Crustacea, Ostracoda): description of Intrepidocythere ibipora n. gen. n. sp. from the forest leaf litter in São Paulo State, Brazil. Zootaxa 1828: 29–42.
Pires-Neto, A. G., A. Bartorelli & M. S. Vargas, 1994. A planície do rio Paraná. Boletim Paranaense de Geociências 42: 217–229.
Por, F. D., 1995. The Pantanal of Mato Grosso, World’s Largest Wetland. Kluwer Academic Publishers, Dordrecht.
Por, F. D. & C. E. F. Rocha, 1998. The Pleustal, a third limnic biochore and its neotropical centre. Internationale Vereinigung füer Theoretische und Angewandte Limnologie Verhandlungen 26: 1876–1881.
Rainwater, F. H. & L. L. Thatcher, 1960. Methods for Collection and Analysis a Water Samples. United States Government Printing Office, Washington.
Rossetti, G. & K. Martens, 1996. Redescription and morphological variability of Darwinula stevensoni (Brady & Robertson, 1870) (Crustacea, Ostracoda). Bulletin van het Koninklijk Belgisch Instituut voor Natuurwetenschappen, Biologie 66: 73–92.
Rossetti, G. & K. Martens, 1998. Taxonomic revision of the Recent and Holocene representatives of the Family Darwinulidae (Crustacea, Ostracoda), with a description of three new genera. Bulletin de L′Institut Royal des Sciences Naturelles de Belgique, Biologie 68: 55–110.
Sandberg, P. A. & P. L. Plusquellec, 1974. Notes on the anatomy and passive dispersal of Cyprideis (Cytheracea, Ostracoda). Geocience and Man 6: 1–26.
Savatenalinton, S. & K. Martens, 2009. Generic revision of Cypricercinae McKenzie, 1971 (Crustacea, Ostracoda), with the description of three new genera and one new species and a phylogenetic analysis of the subfamily. Hydrobiologia 632: 1–48.
Souza, H. B. & J. C. Derisio, 1977. Guia técnico de coleta de amostras de água. Companhia de Tecnologia de Saneamento Ambiental – CETESB, São Paulo.
Souza Filho, E. E. & J. C. Stevaux, 2004. Geology and geomorphology of the Baía-Curutuba-Ivinheima River complex. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its Floodplain: Physical Aspects. Ecology and Conservation. Backhuys Publishers, Leiden: 1–29.
Stevaux, J. C., E. E. Souza-Filho, S. Medeanic & G. Yamskikh, 2004. The quaternary history of the Paraná River and its floodplain. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and Its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 31–53.
Taniguchi, H. & M. Tokeshi, 2004. Effects of habitat complexity on benthic assemblages in a variable environment. Freshwater Biology 49: 1164–1178.
Taniguchi, H., S. Takano & M. Tokeshi, 2003. Influences of habitat complexity on the diversity and abundance of epiphytic invertebrates on plants. Freshwater Biology 48: 718–728.
Thomaz, S. M., L. M. Bini, T. A. Pagioro, K. J. Murphy, A. M. Santos & D. C. Souza, 2004a. Aquatic macrophytes: diversity, biomass and decomposition. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its Floodplain: Physical Aspects. Ecology and Conservation. Backhuys Publishers, Leiden: 331–352.
Thomaz, S. M., T. A. Pagioro, L. M. Bini, M. C. Roberto & R. R. A. Rocha, 2004b. Limnological characterization of the aquatic environments and the influence of hydrometric levels. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its Floodplain: Physical Aspects. Ecology and Conservation. Backhuys Publishers, Leiden: 75–102.
Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), 2004c. The Upper Paraná River and its Floodplain: Physical Aspects, Ecology and Conservation. Backhuys Publishers, Leiden: 393 pp.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13.
Thomaz, S. M., E. D. Dibble, L. R. Evangelista, J. Higuti & L. M. Bini, 2008. Influence of aquatic macrophyte habitat complexity on invertebrates abundance and richness in tropical lagoons. Freshwater Biology 53: 358–367.
Train, S. & L. C. Rodrigues, 2004. Phytoplanktonic assemblages. In Thomaz, S. M., A. A. Agostinho & N. S. Hahn (eds), The Upper Paraná River and its Floodplain: Physical Aspects. Ecology and Conservation. Backhuys Publishers, Leiden: 103–124.
Twombly, S. & W. M. Lewis Jr., 1987. Zooplankton abundance and species composition in Laguna la Orsinera, a Venezuelan floodplain lake. Archiv für Hydrobiologie 1: 87–107.
Twombly, S. & W. M. Lewis Jr., 1989. Factors regulating cladoceran dynamics in a Venezuelan floodplain lake. Journal of Plankton Research 11: 317–333.
Van der Meeren, T., J. E. Almendinger, E. Ito & K. Martens, 2010. The ecology of ostracodes (Ostracoda; Crustacea) in western Mongolia. Hydrobiologia 641:253–273.
Viehberg, F. A., 2006. Freshwater ostracod assemblages and their relationship to environmental variables in waters from northeast Germany. Hydrobiologia 571: 213–224.
Wagner, R., 1976. Sampling and sample preparation water. Fresenius Zeitschrift fuer Analytische Chemie 228: 315–321.
Würdig, N. L. & S. M. F. Freitas, 1988. Distribuição espacial e temporal da comunidade de ostrácodes na lagoa emboaba, Rio Grande do Sul, Brasil. Acta Limnologica Brasiliensis 2: 677–700.
Würdig, N. L. & I. D. Pinto, 2001. Pseudocandona pumilis sp. nov. (Ostracoda). Ecological data and distribution in the Tramandai lagunar system, RS, Southern Brasil. Nauplius 7: 39–51.
Acknowledgments
Julien Cillis (Brussels, Belgium) offered technical assistance with the scanning electron micrographs. Mrs Sukonthip Savatenalinton (University of Ghent, Belgium) assisted the first author in many laboratorial activities; Dr. Ricardo Lourenço Pinto (University of São Paulo) assisted in the field sampling. Several researchers of the Laboratory of Limnology of Nupélia helped with the measurements of abiotic variables; Dirceu Galli (University of Maringá) assisted with the Ca and Mg analyses. Jaime Luiz Lopes Pereira (Nupélia, University of Maringá) assisted in the production of the map and the plates. This project was supported by CNPq (proc. 472434/03-9 and through the Long-Term Ecological Research (LTER) Program) and Nupélia. S.A.J.D. is a postdoctoral fellow with the Research Foundation—Flanders (FWO-Vlaanderen). The Royal Belgian Institute of Natural Sciences financed several trips of KM to Maringá.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: B. Oertli
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Higuti, J., Declerck, S.A.J., Lansac-Tôha, F.A. et al. Variation in ostracod (Crustacea, Ostracoda) communities in the alluvial valley of the upper Paraná River (Brazil) in relation to substrate. Hydrobiologia 644, 261–278 (2010). https://doi.org/10.1007/s10750-010-0122-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0122-1