Abstract
Although submerged vegetation is considered to be the most suitable refuge against predators and form of foraging habitat for small fishes, submerged plants are often scarce or lacking in turbid eutrophic lakes. To evaluate emergent (Zizania latifolia) and floating-leaved (Nelumbo nucifera) vegetation as refuge areas against predators and as foraging habitats for small fishes, we investigated the fauna, abundance, and size distribution of the fish community as well as the abundance of possible prey for small fishes in beds of each vegetation type in a eutrophic shallow lake: Lake Teganuma in Japan. The leaves and stems of N. nucifera occupied an area 4.2 times larger than that of Z. latifolia. The high coverage of the water surface with plants most likely induced the hypoxia found in the N. nucifera bed. The diversity of small fishes was greater in the Z. latifolia bed with piscivorous fish than in the N. nucifera bed without piscivorous fish. The diversity of fish species in the vegetation was enhanced when there was an increased diversity of possible food sources rather than an absence of predators. Some aquatic insects of the same species had a much lower δ13C signature at hypoxic locations than at less hypoxic locations in the N. nucifera bed. Such site differences within a bed were not observed in the organisms caught in the Z. latifolia bed. The insects in hypoxic zones with a δ13C signature lower than −30 ‰ were more depleted in 13C than the surface sediment or attached algae, suggesting that the larvae in the hypoxic zones incorporated the organic materials generated by methane-oxidizing bacteria. We can therefore conclude that floating-leaved vegetation, especially a N. nucifera bed, is not suitable as a replacement for submerged vegetation because of its potential to induce hypoxia, which can decrease the diversity of the fish fauna.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquatic macrophytes play an important role in structuring the ecosystems of freshwater shallow lakes. Littoral habitats dominated by aquatic macrophytes provide refuge against predators and serve as foraging habitats for small fishes (Jacobsen and Perrow 1998; Meerhoff et al. 2003). Aquatic macrophytes can be categorized into four functional groups: emergent, floating-leaved, submerged, and free-floating (Lacoul and Freedman 2006). Comparing these four functional groups, Meerhoff et al. (2003) found that the dominant omnivorous–planktivorous fishes, particularly the smallest size classes, preferred submerged plants rather than free-floating plants.
The organic materials produced by macrophytes and the periphyton that attaches to macrophytes are not the only food sources for the small fishes that live in macrophyte beds. The zooplankton that use the aquatic macrophytes as shelter are an important food source for omnivorous–planktivorous fishes. Submerged vegetation is considered the most suitable type of vegetation for zooplankton to use as a daytime refuge (van Donk and van de Bund 2002), although some studies indicate that stands of floating-leaved and emergent vegetation can also offer protection (Moss et al. 1998; Nurminen et al. 2001; Cazzanelli et al. 2008).
Dissolved oxygen concentrations of less than 4 mg L−1 are intolerable to pelagic fishes and crustaceans, while levels of less than 2 mg L−1 are intolerable to benthic fishes and levels of less than 0.8 mg L−1 are intolerable to macrozoobenthos (e.g., Fisheries Agency 2009). In a dense floating-leaved water-chestnut (Trapa natans L.) bed, the dissolved oxygen concentration in the bottom water often fell below 2 mg L−1 during the summer but never fell below 1.0 mg L−1 (Kornijów et al. 2010). Consequently, an abundant macrozoobenthos community was found in the water-chestnut bed, with a higher diversity in the sediments (30 taxa) than on the stems (28 taxa) or floating leaves (20 taxa). Whether the dense macrozoobenthos population reflected the absence of predatory fish due to hypoxia or contributed to the production of the fish that visited the floating-leaved vegetation was not determined by Kornijów et al. (2010).
Although submerged macrophytes are considered to be well suited for use as a refuge against predators, and create foraging habitats for small fishes, submerged macrophytes are often scarce or lacking in turbid eutrophic lakes (Scheffer et al. 1993). Other functional types, such as emergent and floating-leaved vegetation, may serve the same function as submerged vegetation and thus act as a refuge and foraging habitat for small fishes, as has been reported for zooplankton (Cazzanelli et al. 2008). The availability of these types of vegetation for fishes strongly depends on the degree of oxygenation at a site. In a study of five emergent macrophyte species, hypoxia increased with increasing macrophyte coverage area for all of the emergent macrophyte species (Bunch et al. 2010), suggesting that emergent macrophytes do not provide a suitable habitat for all fishes because of the oxygen conditions in the macrophyte beds.
The lotus, Nelumbo nucifera (Gaertn.), is a plant in the Nelumbonaceae family that is native to Japan. It is a popular plant for water gardens worldwide, and is cultivated not only in artificial settings but also in natural ponds and lakes. The lotus has both floating and emerging leaves, and the large floating leaves of N. nucifera shade the underlying zone. The consequent strong light attenuation leads to the depletion of submerged macrophytes. Submerged macrophytes and emerged helophytes were replaced by N. nucifera within 10 years after the introduction of N. nucifera to a small lake in Italy (Masstrantuono and Mancinelli 1999). Complete coverage by N. nucifera floating leaves leading to the exclusion of submerged macrophytes likely also induces more oxygen depletion in the bottom water by preventing water circulation and inhibiting primary production by other plants, including phytoplankton. This effect was reported for a bed of American lotus, Nelumbo lutea (Willdenow) Persoon, where the dissolved oxygen concentration during August dropped to 0.81 mg L−1 and the mean daily concentration was 1.6 mg L−1 (Turner et al. 2010).
The aim of this study was to assess and compare the temporal patterns of fish distribution in two different functional types of macrophytes, floating-leaved and emergent vegetation, and to determine whether each vegetation type can provide any refuge against piscivorous fish or serve as a foraging habitat for small fishes. To achieve this goal, we described the fauna, abundance, and size distribution of the fish community in each vegetation bed as well as the possible prey for small fishes. We simultaneously measured the oxygen dynamics in floating-leaved vegetation meadows during the early summer, when oxygen stress can impact juvenile fishes. Stable carbon and nitrogen isotope ratios of fishes, plants, particulate materials, zooplankton, insects, and sediments were measured to identify the fishes’ food in each vegetation type. Our hypotheses were that (1) the species diversity of small fishes would be greater in the habitats without piscivorous fish, and (2) the total density of small fishes should correlate with the degree of oxygenation.
Materials and methods
Study site
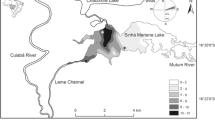
Lake Teganuma, with an area of 6.5 km2, a mean depth of 0.86 m, and a maximum depth of 3.8 m, is a freshwater lake that lies 30 km northeast of Tokyo (35°52′N and 140°01′E; Fig. 1). The main lake flow is from west to east, and the water discharge from the Tega River occurs at the easternmost edge. Submerged macrophytes had disappeared by 1972 (Asama 1989). At present, Lake Teganuma is inhabited by three species of floating-leaved macrophytes [Nelumbo nucifera, Trapa japonica Flerov, and Eichhornia crassipes (Marts) Solms] and three species of emergent macrophytes [Typha angustifolia L., Zizania latifolia (Griseb.) Turcz. ex Stapf., and Phragmites australis (Cav.) Trin. ex Steudel]. N. nucifera is the most dominant floating-leaved macrophyte, occupying 18.65 ha of the lake surface.
To compare the role of vegetation for small fishes between floating-leaved N. nucifera beds and beds of emergent plants, we chose the nearby Z. latifolia bed as the representative emergent vegetation. Among the three emergent species of vegetation [T. angustifoli, Z. latifolia, and P. australis], the Z. latifolia clumps had the highest shoot density and the largest amounts of trapped litter, causing stagnant water in the center of the clump (Asaeda et al. 2005). The N. nucifera bed was also expected to be highly stagnant. We used two sampling points within the stands of the N. nucifera bed (L1 and L2, Fig. 1) and one reference site (L3) at the edge of the N. nucifera bed. L2 faced the waterway. Likewise, the points O1 and O2 were defined in the stands of the Z. latifolia bed for sampling in July 2009. For the surveys in June and August 2010, we moved the sampling points to another Z. latifolia bed that was about 200 m closer to the N. nucifera bed than the previous points. M1 was set in the midpoint of the Z. latifolia bed, and M2 was set at the edge of the bed. The depths of the sampling points in the N. nucifera bed were 0.63 m (L1), 0.93 m (L2), and 1.75 m (L3), while the depths of those in the Z. latifolia bed were 0.78 m (O1), 0.76 m (O2), 0.41 m (M1), and 0.63 m (M2).
Field observations
The shoot density was counted using a 1 m × 1 m quadrat set randomly at three places in both the N. nucifera bed (L1) and the Z. latifolia bed (M1) on 25 June and 12 August 2010. We distinguished between the shoots of floating leaves, emerging leaves, and flowers for N. nucifera. The shoot and leaf diameters of N. nucifera were measured on 12 August 2010.
The temperature and pH were measured in situ with sensors (WM-22EP, TOA), and the dissolved oxygen concentration (DO) was determined using Winkler’s method at L1 (20 cm above the bottom) and L2 (50 cm above the bottom) in the N. nucifera bed on 21 July 2009. The temperature, dissolved oxygen concentration, pH, conductivity, chlorophyll a concentration, and turbidity were monitored with an in situ sensor (Datasonde 5X, Hydrolab) placed 30 cm above the bottom in the N. nucifera bed at site L1. The data were recorded every 30 min from 16 June to 24 July 2010. To compare the water quality inside and at the periphery of the N. nucifera bed (using the sites L2 and L3) with that of the open water (at sites P1, P2, and P3; Fig. 1), the temperature, dissolved oxygen, pH, and conductivity were measured in situ with a multiparameter water quality meter (DKK-TOA Corporation’s model WQC-24) using a boat on 11 August 2010. We measured the water 20 cm below the surface and 30 cm above the lake bottom. We did not measure the bottom water at L2 because the depth was very shallow, or at L3 because we could not vertically submerge the sensor without disturbing the water mass due to the dense stands of N. nucifera.
Sampling
Because the distributions of many fish species typically vary spatially due to feeding methods and the size structure of the population, we studied the fish community using single-point sampling with various items of fishing equipment (see Table 1) to sample as many different fishes as possible in July 2009. To collect large piscivorous fishes, a set net (1.6-m long pathway of 10 mm mesh and 1.2-m long catch net of 3 mm mesh) was placed at both the northeast mouth of the waterway in the N. nucifera bed and outside of O1 in the Z. latifolia bed overnight in July 2009. A cast net (3.1 m in radius, 12 mm mesh) was also operated three times at the same location as the set net operation.
The collection of small fishes within the vegetation was conducted three times: July 2009, June 2010, and August 2010. After analyzing the fish samples caught in July 2009, we expected that juvenile fishes would dominate in June and that most of the small fishes would be fully grown in August. We set three fish traps (0.25 m × 0.35 m of 1.5 mm mesh in 2009 and 0.24 m × 0.42 m of 2 mm mesh in 2010) at each sampling point for 2 h in the morning. Collection was also conducted by two people using a hand net (0.37 m × 0.32 m of 1 mm mesh in 2009 and 0.36 m × 0.30 m of 2 mm mesh in 2010) for 20 min at each sampling point. All fishes were kept cool in an ice-filled cooler and brought to the laboratory within 3 h after the collection. The crustaceans that were caught with the hand net and fish traps were recorded and used for stable isotope analysis. The number, weights, and total lengths of the fishes and other organisms were determined, and the organisms were then kept frozen until they were processed for stable isotope analysis.
The sediments used to sample the macrozoobenthos were collected with an Ekman–Birge sediment sampler (15 cm × 15 cm); sampling was performed twice at each of the sampling points (L1, L2, O1, O2) in July 2009. The sediments were sieved at the shore with a 1-mm meshed standard sieve. In the laboratory, the residue was sorted and identified under a binocular dissecting microscope, and the identified fractions were counted, weighed while wet, and frozen for later stable isotope analysis. Because the amount of macrozoobenthos quantitatively sampled in a single collection using the Ekman–Birge sediment sampler was not enough to allow the stable isotope ratios to be analyzed, we combined the samples collected at L1 and L2 and at O1 and O2. We collected the sediments with shovels and sieved with a 1-mm meshed standard sieve until we had collected enough macrozoobenthos in June and August 2010. The sediment was sampled using this method at L1, L2, and L3 in the N. nucifera bed as well as at M1 and M2 in the Z. latifolia bed.
In addition to collecting the macrozoobenthos in the sediments and particulate materials, we collected insects from the canopy of the vegetation that we identified as a possible food source for small fishes. We categorized them as terrestrial insects and captured them using a 0.5-mm mesh hand net with the same frame as that used for fishes in 2010. We established a 5 m × 5 m quadrat and kept sweeping in the canopy of the vegetation for 10 min. All of the insects obtained during the sweeping were kept cool and brought to the laboratory.
Surface water was collected with either a pump or a bucket and sequentially filtered with 1-mm, 200-μm, and 100-μm mesh sieves on the boat until the filtrate contained a substantial amount of particles, as determined by a visual inspection, in July 2009. We repeated the sampling until about 300 mL of filtrate were collected.
The algae attached to the shoots were sampled by cleaning with a brush in July 2009 at L1 and in June 2010 at L1 and M1. To estimate the contribution of the shoots to the isotopic composition of the plant, the stable isotopes of each shoot were also analyzed in June 2010.
Analytical procedures
For the stable isotope analysis, the fishes and shrimps were dissected and only the muscle was analyzed. For the other organisms, the whole body was analyzed. After being freeze-dried, all of the animal samples except for the rotifers were soaked in a mixture of chloroform and methanol (2:1 by volume) for 24 h to remove lipids. The samples were then dried in an oven set at 50 °C for 1 day. All of the samples were homogenized and powdered with an agate mortar and pestle.
To determine the stable isotope ratios, the samples were combusted at 1020 °C in an elemental analyzer (Fisons Instruments EA1108), and the combustion products (CO2 and N2) were introduced into an isotope-ratio mass spectrometer (Finnigan DELTAplus) using a He carrier. The ratios 13C/12C and 15N/14N were expressed relative to the Vienna Pee Dee Belemnite (V-PDB) standard for carbon and N2 in air for nitrogen. The ratios 13C/12C and 15N/14N were calculated using the following formula:
where R = 13C/12C or 15N/14N.
The machine drift during the analyses was checked using l-α-alanine (δ13C = 20.93 ‰, δ15N = 7.61 ‰) every six samples. The accuracy of the values was determined using interlaboratory-determined nitroarginine following the method of Minagawa et al. (1984) for δ13C (−22.27 ‰) and IAEA-N1 for δ15N (0.54 ‰). The samples were measured twice with an SD of ≤0.5 ‰ for both δ13C and δ15N.
Results and discussion
Hypoxia in floating-leaved vegetation beds
DO was higher than 10 mg L−1 in the open water at sites P1, P2, and P3 (Fig. 1) and at the edge of the N. nucifera bed (L3) on 11 August 2010 (Table 2). However, the DO at L2 in the N. nucifera bed facing the waterway was just over half the DO levels observed at those sites. Among the 1822 sample measurements taken from 16 June to 24 July 2010 at L1 (Fig. 2), 96 % had DO concentrations of <4 mg L−1, and 58 % had concentrations of <2 mg L−1.
Temporal changes in the temperature, dissolved oxygen concentration, pH, conductivity, and chlorophyll a concentration at 30 cm above the lake bottom in a Nelumbo nucifera bed at Lake Teganuma (L1 in Fig. 1) measured every 30 min from 16 June to 24 July 2010 (n = 1822). The precipitation data were observed at Abiko station of Meteorological Agency (35°51.8″N, 140°06.6″E), which is about 7 km from the study site
Based on the densities and the diameters of stems and leaves (Table 3), leaves of N. nucifera covered 1220 cm2 per 10,000 cm2 of the water area, and the stems occupied 29.3 cm2 per 10,000 cm2 in August 2010. Asaeda et al. (2005) reported that Zizania latifolia clumps had a higher shoot density and larger amounts of trapped litter than the other emergent macrophytes in Lake Teganuma, causing stagnant water in the center of the Z. latifolia clump. They also reported that the average major and minor axes of a Z. latifolia stem were 42 and 20 mm, respectively. Based on this stem size, Z. latifolia stems occupied 291 cm2 per 10,000 cm2 of water area. Thus, the leaves and stems of N. nucifera occupied a 4.2-fold larger area than those of Z. latifolia. The large coverage of the water surface by plants most likely induced the hypoxia found in the N. nucifera bed by decreasing the photosynthesis of epiphytes due to shading and by weakening the vertical mixing of water.
Diversity of fishes in the Nelumbo nucifera bed and the Zizania latifolia bed
During the surveys conducted in July 2009, June 2010, and August 2010, four species were caught with the fish traps and hand net inside the N. nucifera bed, while six species were caught with the same gear inside the Z. latifolia bed (Table 4). However, a marked difference was found between the fish fauna of the periphery of the N. nucifera bed and the fish fauna of the periphery of the Z. latifolia bed, where the fishes were caught with the set net and cast net: only two species were caught at the periphery of the N. nucifera bed, while 13 species were caught at the periphery of the Z. latifolia bed. Considering all of the species caught either inside each bed or at its periphery, all four species caught in the N. nucifera bed were reported to be omnivores by Kawanabe and Mizuno (1989), while the 14 species caught in the Z. latifolia bed included three piscivores: Anguilla japonica (Temminck and Schlegell), Hemibarbus barbus (Temminck and Schlegel), and Channa argus (Cantor); and two planktivore–detritivores: Carassius cuvieri (Temminck and Schlegel) and Mugil cephalus cephalus L.
The total number of fishes collected with the hand net and fish trap during July 2009 was 158 (comprising 4 species) in the N. nucifera bed and 458 (comprising 14 species) in the Z. latifolia bed. Because the public survey that was conducted in 2004 found 16 fish species across Lake Teganuma (Chiba Prefecture 2005), the results of this study show that the Z. latifolia bed, which contained 14 species either inside or at the periphery of the vegetation, serves as a habitat for most of the fish species in Lake Teganuma.
The N. nucifera bed should be a superior shelter for small fishes compared to the Z. latifolia bed because the emergent and floating leaves should hide the small fishes from birds. Moreover, no piscivorous fishes were caught either inside or at the periphery of the N. nucifera bed, while these predatory fishes were present both inside and at the periphery of the Z. latifolia bed. Nonetheless, more fish species were found in the Z. latifolia bed than in the N. nucifera bed. Our results suggest that the presence of fewer species in the N. nucifera bed is due not to the inferior function of the bed as shelter but to other factors that differentiate the beds.
Planktivore–detritivores were caught only at the periphery of the Z. latifolia bed, and all of the small fishes caught inside the beds were omnivores, except for the piscivorous A. japonica, which was caught inside the Z. latifolia bed. Thus, the interiors of the vegetation beds of both floating-leaved and emergent macrophytes did not serve as foraging habitats for planktivorous fishes and benthic-feeding fishes. The size distributions of the omnivorous fishes found inside both vegetation beds showed that both juvenile and grown fishes utilize the vegetation (Table 5).
Pseudorasbora parva was the most dominant fish in both vegetation types during all of the samplings performed in July 2009, June 2010, and August 2010 using the fish trap and hand net (Table 5). In the N. nucifera bed, P. parva was exclusively dominant. In August 2010, 203 individuals of P. parva and 18 individuals of other fish species were caught in the N. nucifera bed, while 52 individuals of P. parva and 33 individuals of other fish species were caught in the Z. latifolia bed. The number of fishes caught with either the fish trap or hand net was greater in the Z. latifolia bed than in the N. nucifera bed during the samplings of July 2009 and June 2010, but many more fish were caught in the N. nucifera bed during the sampling in August 2010.
These results suggest that both the N. nucifera bed and the Z. latifolia bed serve as habitats for omnivorous fishes at various growth stages, and that the conditions in the N. nucifera bed are more favorable for P. parva.
Food for the fishes in the Nelumbo nucifera bed and the Zizania latifolia bed
The aquatic insects utilizing the water area and sediments of the beds comprised Chironomidae larvae and a water strider. Although we could not quantitatively compare the abundances of the Chironomidae larvae, previous reports of surveys of the same beds showed that the abundance of Chironomidae larvae is ten times larger in the Z. latifolia bed than in the N. nucifera bed (Consultation Association for Conservation of Lake Teganuma 2010). Terrestrial insects utilized the abovewater vegetation, and the dead bodies of terrestrial insects were floating on the surface of the water. These insects included adult Chironomidae, spiders, flies and beetles.
The fishes always showed a greater range of trophic levels in the Z. latifolia bed than in the N. nucifera bed throughout all of the samplings in this study (Tables 6, 7, 8). The wider trophic range observed for the Z. latifolia bed was mainly due to the lowest trophic level represented, which was always lower than the lowest level represented in the N. nucifera bed. The lowest trophic levels of the fishes at the samplings in July 2009, June 2010, and August 2010 were 2.96, 3.10, and 3.28 in the N. nucifera bed, respectively, but 2.41, 2.83, and 2.33 in the Z. latifolia bed. Fishes that partially depend on primary producers (with a trophic level of nearly 1.0) utilized the resources in the Z. latifolia bed, while food resources with a trophic level of >2 (i.e., secondary producers) were only used by fishes in the N. nucifera bed. For example, three fish species had trophic levels of around 3.2 in the N. nucifera bed in July 2009 (Table 6). Possible food sources that can have trophic levels between 2.0 and 2.4 were restricted to the aquatic insect Chironomus plumosus, a larvae with a trophic level of 2.00, and the terrestrial insect Donacia lenzi, which has a trophic level of 2.15. The POM, sediment organic matter, and attached algae—with trophic levels of <1.8—were not food resources of the fishes in the N. nucifera bed.
Another reason for this difference is the absence of piscivorous fish in the N. nucifera bed. The largest trophic level of 3.62 for omnivorous fish was found for Rhinogobius sp. OR in August 2010 in the N. nucifera bed, while Channa argus, a piscivorous fish that was caught in the Z. latifolia bed in July 2009, had a trophic level of 3.70.
Thus, our hypothesis that the species diversity of small fishes would be greater in the habitat without piscivorous fish was rejected. The diversity of fish species in the vegetation beds was enhanced when there was an increased range of possible food sources rather than an absence of predators.
Adaptation by Pseudorasbora parva to the hypoxic conditions of the Nelumbo nucifera bed
Within the same fish species, the δ13C value in the Nelumbo nucifera bed was lower than it was in the Zizania latifolia bed (Tables 6, 7, 8). One possible reason for the lower δ13C of the fishes in the N. nucifera bed is that those fishes depend on a limited number of food sources, and one of these food sources—Chironomus plumosus larvae—possessed a significantly lower δ13C value in the N. nucifera bed than in the Z. latifolia bed. The average δ13C signature of the Pseudorasbora parva caught at L1 in the N. nucifera bed in June 2010 was −28.00 ‰, which was the lowest average value found in the fishes caught during this survey (Table 7). In contrast, the average δ13C of the P. parva caught at L2 in the N. nucifera bed in June 2010 was −25.41 ‰. Likewise, some aquatic insects of the same species showed much a lower δ13C value at L1 than at L2 in June 2010: δ13C of Chironomus plumosus was −30.21 ‰ at L1 and −25.61 ‰ at L2, and δ13C of Aquarius paludum was −30.12 ‰ at L1 and −27.92 ‰ at L2. Some of the terrestrial insects also had lower δ13C values at L1 than L2; for example, adults of Chironomus yoshimatsui had values of −31.83 ‰ at L1 and −26.06 ‰ at L2. Such site differences within a bed were not observed between the organisms caught at M1 and M2 in the Z. latifolia bed.
Organisms with δ13C of less than −30 ‰ were more depleted in 13C than the surface sediment observed in July 2009 (−26.89 ‰, Table 6) or the attached algae at L1 sampled in June 2010 (−28.33 ‰, Table 7). Because low δ13C was only observed in chironomids and their predator (A. paludum), and was only found at L1, where the dissolved oxygen concentration dropped below 2 mg L−1 (Table 2), it is likely that the chironomids at L1 incorporated the organic materials generated by methane-oxidizing bacteria, which exhibit rather low δ13C values (Jones and Grey 2011; Yasuno et al. 2012).
The probability of 13C-depleted chironomid larvae being consumed in situ by P. parva may be low because P. parva cannot dig and consume the larvae in the anoxic sediments where methane is generated. Because we found dead bodies of insects floating in the surface water in the N. nucifera bed, we assume that P. parva feed on these dead aquatic and terrestrial insects at the surface, where the fatal effect of anoxic water during feeding is minimized. The δ13C of the P. parva caught at L1 showed a wider range (−29.24 to −26.44 ‰) than the corresponding values of P. parva caught at the other sampling points in June 2010 (Fig. 3a). The range became even wider in August 2010 (−29.20 to −22.91 ‰, Fig. 3b). These wide ranges can be explained by the adaptability of P. parva to a wide range of foods, including dead insects on the surface of the water. The results of this study also contribute to the small body of evidence that methane may contribute appreciable carbon to the pelagic food webs in lakes (Jones and Grey 2011).
Total lengths (cm) and δ13C values of Pseudorasbora parva sampled in the Nelumbo nucifera bed (L1, L2) and the Zizania latifolia bed (M1, M2) at Lake Teganuma in June 2010 (a) and in August 2010 (b). The sampling stations are shown in Fig. 1
Pseudorasbora parva has invaded 32 countries from Central Asia to North Africa in less than 50 years (Gozlan et al. 2010). The species has been described as an omnivore but has also been considered to be planktivorous with a broad diet (Gozlan et al. 2010). Our data showed the plasticity of the food habits of P. parva as well as its tolerance of hypoxic conditions. These characteristics may have enabled P. parva to spread worldwide. Our hypothesis that the total density of small fishes should correlate with the degree of oxygenation and therefore be lower in the floating-leaved N. nucifera bed than in the emergent Z. latifolia bed was not supported. Our data showed that the largest fish abundance, mostly consisting of P. parva, was found at the most hypoxic site, L1, followed by L2 in the N. nucifera bed, and a much lower abundance was found in the Z. latifolia bed in August 2010 (Table 5).
Conclusion
Emergent and floating-leaved vegetation serve the same functions as submerged vegetation, acting as a refuge and foraging habitat for zooplankton (Cazzanelli et al. 2008). In this study, a Z. latifolia bed (emergent vegetation), was found to be utilized by fishes across a wide trophic range, so the species richness in the emergent vegetation bed was far greater than that in the nearby floating-leaved vegetation bed composed of N. nucifera. Strong hypoxia, with dissolved oxygen levels of <2 mg L−1, was observed in the N. nucifera bed during the summer. Only omnivorous fishes were found in the N. nucifera bed, and P. parva dominated at the most hypoxic site, where it presented a higher biomass than the same species did in the Z. latifolia bed. Thus, floating-leaved vegetation may only serve the same function as submerged vegetation for certain omnivorous fish with high hypoxic tolerance.
In shallow turbid lakes, the depth ranges for each vegetation type before the eutrophication seen in modern times were reported to be 0–1 m for emergent vegetation, 1–3 m for floating-leaved vegetation, and 2–4 m for submerged vegetation (Yoshimura 1937). This implies that planting floating-leaved vegetation may inhibit the revival of submerged vegetation due to the competition for available light at depths of 2–3 m (where they overlap). Floating-leaved vegetation—especially N. nucifera beds—is not a suitable alternative vegetation habitat for fishes that use submerged vegetation because of its potential to induce hypoxia and its superior competitive strength to other vegetation types (Masstrantuono and Mancinelli 1999). Because N. nucifera has been introduced to both artificial and natural water areas for gardening purposes worldwide, its control merits further study in order to minimize any resulting simplification of fish fauna.
References
Asaeda T, Fujino T, Manatunge J (2005) Morphological adaptations of emergent plants to water flow: a case study with Typha angustifolia, Zizania latifolia and Phragmites australis. Freshw Biol 50:1991–2001
Asama S (1989) Ecology of Lake Teganuma. Sai, Chiba (in Japanese)
Bunch AJ, Allen MS, Gwinn DC (2010) Spatial and temporal hypoxia dynamics in dense emergent macrophytes in a Florida Lake. Wetlands 30:429–435
Cazzanelli M, Warming TP, Christoffersen KS (2008) Emergent and floating-leaved macrophytes as refuge for zooplankton in a eutrophic temperate lake without submerged vegetation. Hydrobiologia 605:113–122
Chiba Prefecture (2005) Conservation project for fisheries target species in land water area. Business report of land water fisheries station for FY2004. Chiba Prefecture, Chiba, pp 7–8 (in Japanese)
Consultation Association for Conservation of Lake Teganuma (2010) Report on the Nelumbo nucifera bed at Lake Teganuma. NS Environment Co. Ltd., Tokyo (in Japanese)
Fisheries Agency (2009) Technical guideline for improvement of fisheries field in lakes. Fisheries Agency, Tokyo (in Japanese)
Gozlan RE, Andreou D, Asaeda T, Beyer K, Bouhadad R, Burnard D, Caiola N, Cakic P, Djikanovic V, Esmaeili HR, Falka I, Golicher D, Harka A, Jeney G, Kováč V, Musil J, Nocita A, Povz M, Poulet N, Virbickas T, Wolter C, Tarkan AS, Tricarico E, Trichkova T, Verreycken H, Witkowski A, Zhang C, Zweimueller I, Britton JR (2010) Pan-continental invasion of Pseudorasbora parva: towards a better understanding of freshwater fish invasions. Fish Fish 11:315–340
Jacobsen L, Perrow MR (1998) Predation risk from piscivorous fish influencing the diel use of macrophytes by planktivorous fish in experimental ponds. Ecol Freshw Fish 7:78–86
Jones RI, Grey J (2011) Biogenic methane in freshwater food webs. Freshw Biol 56:213–229
Kawanabe H, Mizuno N (1989) Freshwater fishes in Japan. Yama-kei, Tokyo (in Japanese)
Kornijów R, Strayer DL, Caraco NF (2010) Macroinvertebrate communities of hypoxic habitats created by an invasive plant (Trapa natans) in the freshwater tidal Hudson River. Fundam Appl Limnol 176:199–207
Lacoul P, Freedman B (2006) Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev 14:89–136
Masstrantuono L, Mancinelli T (1999) Long-term changes of zoobenthic fauna and submerged vegetation in the shallow Lake Monterosi (Italy). Limnologica 29:160–167
Meerhoff M, Mazzeo N, Moss B, Rodriguez-Gallego L (2003) The structuring role of free-floating versus submerged plants in a subtropical shallow lake. Aquat Ecol 37:377–391
Minagawa M, Winter DA, Kaplan IR (1984) Comparison of Kjeldahl and combustion methods for measurement of nitrogen isotope ratios in organic matter. Anal Chem 56:1859–1861
Moss B, Kornijów R, Measey GJ (1998) The effects of nymphaeid (Nuphar lutea) density and predation by perch (Perca fluviatilis) on the zooplankton communities in a shallow lake. Freshw Biol 39:689–697
Nurminen L, Horppila J, Tallberg P (2001) Seasonal development of the cladoceran assemblage in a turbid lake: the role of emergent macrophytes. Arch Hydrobiol 151:127–140
Scheffer M, Hosper SH, Meijer ML (1993) Alternative equilibria in shallow lakes. Trends Ecol Evol 8:275–279
Turner AM, Cholak EJ, Groner M (2010) Expanding American lotus and dissolved oxygen concentrations of a shallow lake. Am Midl Nat 164:1–8
van Donk E, van de Bund WJ (2002) Impact of submerged macrophytes including charophytes on phyto- and zooplankton communities: allelopathy versus other mechanisms. Aquat Bot 72:61–274
Vander Zanden MJ, Rasmussen JB (1999) Primary consumer δ13C and δ15N and the trophic position of aquatic consumers. Ecology 80:1395–1404
Yasuno N, Shikano S, Muraoka A, Shimada T, Ito T, Kikuchi E (2012) Seasonal increase of methane in sediment decreases δ13C of larval chironomids in a eutrophic shallow lake. Limnology 13:107–116
Yoshimura S (1937) Limnology. Sanseidoh, Tokyo (in Japanese)
Acknowledgments
We thank Yu Tabayahsi and Hidenori Naruoka for their technical assistance. Tomoko Hiratsuka, Akihisa Katsumura, Kazuki Abukawa, Takashi Komuro, Ryotaro Nakagawa, and Takashi Hori helped with the sampling and field observations. This study was supported by the River Fund of the Foundation of River and Watershed Environment Management (FOREM), Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Yoshinori Taniguchi.
Rights and permissions
About this article
Cite this article
Yamaki, A., Yamamuro, M. Floating-leaved and emergent vegetation as habitat for fishes in a eutrophic temperate lake without submerged vegetation. Limnology 14, 257–268 (2013). https://doi.org/10.1007/s10201-013-0403-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10201-013-0403-2