Abstract
Several studies have shown that submerged macrophytes provide a refuge for zooplankton against fish predation, whereas the role of emergent and floating-leaved species, which are often dominant in eutrophic turbid lakes, is far less investigated. Zooplankton density in open water and amongst emergent and floating-leaved vegetation was monitored in a small, eutrophic lake (Frederiksborg Slotssø) in Denmark during July–October 2006. Emergent and floating-leaved macrophytes harboured significantly higher densities of pelagic as well as plant-associated zooplankton species, compared to the open water, even during periods where the predation pressure was presumably high (during the recruitment of 0+ fish fry). Zooplankton abundance in open water and among vegetation exhibited low values in July and peaked in August. Bosmina and Ceriodaphnia dominated the zooplankton community in the littoral vegetated areas (up to 4,400 ind l−1 among Phragmites australis and 11,000 ind l−1 between Polygonum amphibium stands), whereas the dominant species in the pelagic were Daphnia (up to 67 ind l−1) and Cyclops (41 ind l−1). The zooplankton density pattern observed was probably a consequence of concomitant modifications in the predation pressure, refuge availability and concentration of cyanobacteria in the lake. It is suggested that emergent and floating-leaved macrophytes may play an important role in enhancing water clarity due to increased grazing pressure by zooplankton migrating into the plant stands. As a consequence, especially in turbid lakes, the ecological role of these functional types of vegetation, and not merely that of submerged macrophyte species, should be taken into consideration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Eutrophication in shallow freshwater lakes is frequently responsible for dense algal biomasses during summer (Jeppesen et al., 2007a). This, in turn, might result in a decline or disappearance of submerged macrophytes, typically through shading, with cascading consequences on biotic community structure, food web interactions and water quality (Carpenter et al., 1985). However, it is empirically demonstrated that within a range of nutrient concentrations the presence of macrophytes is likely to limit if not prevent the occurrence of high phytoplankton biomasses, favouring a clear water state (Scheffer et al., 1993; Jeppesen et al., 1998). Among a number of stabilising mechanisms likely to be responsible for successful dominance by macrophytes and increased water clarity is an enhanced grazing pressure by zooplankton that migrates into the macrophyte beds (Søndergaard & Moss, 1998). Zooplankton generally occurs in greater numbers inside or around the edges of macrophyte beds than outside. According to several studies (i.e. Timms & Moss, 1984; Stansfield et al., 1997; Burks et al., 2002) macrophytes are likely to offer a daytime refuge for zooplankton against fish predation, with major consequences on food web interactions. Hence, the refuge effect and the consequently enhanced grazing by zooplankton may well play a key-role in restoration programmes and management of lakes.
So far, investigations on the role of macrophytes as refuge for zooplankton have mainly focused on submerged species, as they may provide optimal shelter due to structural complexity. However, especially in turbid lakes, where submerged vegetation is often scarce or lacking, other functional types, such as emergent and floating-leaved species, may play an important role in determining the ecological status of a lake (Nurminen et al., 2001, 2007). In spite of that, such species are sometimes removed in order to lower the release of nutrients in the lake. The variable information on the contribution of emergent macrophytes as refuge for zooplankton seems poor and not suitable for making any conclusive statements (Burks et al., 2006). The aim of this study was therefore to assess and compare the temporal pattern of zooplankton distribution in two different functional types of macrophytes, emergent and floating-leaved, and to determine whether they can provide any refuge for zooplankton against fish predation. To achieve this, the abundance and community structure of zooplankton larger than 200 μm were monitored in eutrophic Frederiksborg Slotssø (Denmark) during the summer maximum in plant density and fish predation pressure (July–October 2006). Since 2005, the lake has been undergoing a restoration program that involves precipitation of phosphorus by addition of aluminium chloride to the water surface and selective removal of planktivorous fish. Therefore, it is hypothesised that the reduced phosphorous concentration leads to improved water quality that in turn will promote the zooplankton community to develop larger and more efficient grazers of phytoplankton. In an optimal situation, this may lead to a severe reduction in cyanobacterial blooms.
Materials and methods
Study site
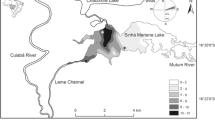
Frederiksborg Slotssø covers an area of 22.3 ha (maximum depth = 9 m, average depth = 3.5 m), with a total volume of 7.3 × 105 m3 (Rasmussen, 2001). The water basin is shallow; about 90% of its area has a water depth of 0–4 m (Fig. 1) (Andersen & Jacobsen, 1979). The lake is eutrophic, with TP and TN concentrations of 0.14 and 1.3 mg l−1, respectively (Rasmussen, 2001). It is monomictic, with stratification usually occurring from May to September. The lake is occasionally ice covered in winter and stratified in summer (Andersen & Jacobsen, 1979; Jespersen et al., 1988; Christoffersen et al., 1993). The total area with plant coverage is estimated to approximately 1% of the total surface area (own unpublished data).
Sampling and experimental design
The experiment started at the end of June 2006, with the routine sampling of phytoplankton and water quality parameters. At the beginning of July macrophyte occurrence was assessed and the density of plants was estimated as percent volume infested (PVI) in two different sites with similar physical conditions where Phragmites australis and floating-leaved Polygonum amphibium occurred. This was calculated by multiplying the percentage value of macrophyte cover (visual estimation) by the plant height divided by the water depth (Canfield et al., 1984). The PVI was estimated a second time in early October.
Zooplankton sampling was carried out weekly from July to October both in open water (from the deepest area of the lake) and in Phragmites and Polygonum stands (at a water depth ranging between 60 and 75 cm) (Fig. 1). At each macrophyte site, three replicate samples (the distance between sampling points was about 1.5 m) were taken using a tube sampler (length 55 cm, diameter 15 cm). In the open water each replicate sample was obtained by three pooled sub-samples taken from different depths (surface, 4 and 6.5 m) in order to get a good approximation of the whole water column.
Zooplankton abundance was estimated from selective filtration (mesh size 200 μm) of 3 l of water samples, and animals retained were fixed with acid Lugol’s solution. The animals were identified to species level and counted under an inverted microscope using 40× magnification. Generally three to four sub-samples were counted, but where less than 100 animals occurred, the entire sample was assessed.
Depth-integrated water samples for phytoplankton biomass estimation were taken at the deepest point of the lake from 2 to 4 depths (depending on the position of the boundary layer) in the epilimnion, using a 5-l water sampler. Sub-samples (100 ml) were preserved with 1–2 ml of Lugol’s solution. Samples were poured into counting chambers (5, 10 or 25 ml) and the most numerous taxa (usually 10–20) were counted using an inverted microscope. Linear dimensions were measured for at least 20 individuals of each counted taxon, and biovolume was calculated by fitting the individual taxa to geometric forms (Utermöhl, 1958).
Water transparency was measured weekly from the deepest area of the lake using a Secchi disc. Water temperature (±0.1°C), oxygen content (±0.2 mg l−1) and pH (±0.2 unit) were measured every second week at the surface and at 1–2-m intervals through the water column using a Multi-Sonde multiprobe (Hydrolab, USA). Water samples for chlorophyll a measurements were filtered through GF/C filters, and the filters were subsequently wrapped in aluminium foil, kept cold and frozen as soon as possible. Extraction procedures followed Jespersen & Christoffersen (1987) and the extracts were spectrophotometrically analysed.
Statistical analyses
For statistical analysis, all data concerning zooplankton abundance were logarithmically transformed to normalise the distribution and stabilise heterogeneous variances. Zooplankton densities in the different sites and enclosures were compared using analysis of variance for repeated measurements (rmANOVA). Tukey’s HSD was used as the post hoc test for multiple comparisons. Homogeneity of variance for rmANOVA was tested with Cochran’s C-test and Bartlett’s test. Where assumptions of homogeneity of variance were violated, zooplankton densities were compared by nonparametric Mann–Whitney U-tests. When not otherwise specified, the level of significance used was P < 0.05. The statistical analyses were performed with “Statistica” software (StatSoft, ver. 6.0).
Results
Macrophyte community
The macrophyte community was composed of emergent Phragmites australis and floating-leaved Polygonum amphibium, whereas submerged species were absent. The sampling amongst Phragmites was carried out in the northern part of the lake (Fig. 1), where the reeds formed a long belt. In the sampling area, the PVI was 40% at the beginning of the experiment and 50% by the end of the study. Minor aggregations of the species were also present in the southern part of the lake, but these were not sampled. Polygonum formed a dense bed just in front of the castle situated in the western part of the water basin. The PVI in the sampling area was 60 and 70%, at the beginning and end of the sampling periods, respectively.
Environmental parameters
The average temperature of the water column was 19.7°C at the beginning of July and declined progressively from August to the end of the study, where the temperature reached 17.1°C. Chlorophyll a was 44 μg l−1 at the beginning of July and quickly rose to 150 μg l−1 in late July–early August. In late August the level of chlorophyll a dropped to 80 μg l−1 and furthermore decreased to 60 μg l−1 in September. The Secchi depth exhibited the highest value at the beginning of July (1.55 m) but declined to 0.27 m (lowest value registered) by the end of the month. A second smaller peak was recorded in mid-August (1.04 m). In September, the water transparency was stable at 0.8–0.9 m (Fig. 2).
Cyanobacteria dominated the phytoplankton community in late June and July (from 83 to 99% of the total phytoplankton biovolume), declined in August (16%) and increased again in September (84%) (Table 1 and Fig. 3). The genus Microcystis spp. was dominant in late June (41%) and September (80%), whereas another cyanobacterial species, Anabaena planctonica, was dominant in late July (96%), when the phytoplankton density peaked with 88.1 mm3 l−1. Cryptomonas spp. was the dominant genus in August (69%), where it reached a biomass of 5.4 mm3 l−1.
Distribution of zooplankton in the open water and the littoral vegetated zones
The overall density of cladocerans was on average over 60 times higher in the presence of plants than in open water throughout the sampling period (Fig. 3). This difference was significant in July, part of August and September. In mid-August, Ceriodaphnia (Fig. 4A) and Bosmina longirostris (Fig. 4D) showed peaks of 4,400 ind l−1 in the Phragmites belt, and 11,000 ind l−1 between the Polygonum plants. However, in July, the dominant genus was Cyclops spp. (up to 81 ind l−1) in both plant species (Fig. 5). In the open water, the dominant zooplankton species were Daphnia cucullata (up to 67 ind l−1) and Cyclops spp. (41 ind l−1).
Total cladoceran densities (Fig. 4H) amongst the two different plant species did not differ significantly. Nonetheless, at the species level, some distinctions emerged. The patterns of habitat use by Scapholeberis mucronata, Sida crystallina, chydorids and Cyclops spp. (Fig. 4C, E, F, G) showed that Polygonum beds were favoured, since the abundance of these species was significantly lower in Phragmites, especially in late summer. On the other side, Ceriodaphnia was significantly more abundant amongst Phragmites in early and late summer. A similar pattern was shown by Bosmina, as it was significantly more abundant between Phragmites than Polygonum plants during September. In mid-August, however, Ceriodaphnia and Bosmina showed the highest peaks of abundance amongst Polygonum. In that period, the abundance of Bosmina in the Phragmites belt was significantly lower.
Discussion
Top-down control of zooplankton
Predation pressure on zooplankton is high in the shallow, eutrophic Lake Slotssø because of high densities of cyprinids (mainly roach, Rutilus rutilus L. but also bream, Abramis brama L.), while the predaceous fish species perch (Perca fluviatilis L.) and pike (Esox lucius, L.) are low in density although pike-perch (Lucioperca lucioperca, L.) is more abundant (Müller & Jensen 2004). Fish predation on zooplankton is strong in turbid eutrophic lakes, since the abundance of planktivorous fish generally increases with nutrient concentration (Jeppesen et al., 2006). Besides, planktivorous fish are able to exert a higher predation impact on zooplankton in shallow than in deep lakes, because shallow lakes contain a higher biomass of fish per unit volume (Jeppesen et al., 1998). Fish density seems to decide which zooplankton species perform diurnal migrations and their size range (Jeppesen et al., 2007b). In Frederiksborg Slotssø, the low abundance of large-bodied zooplankton (Daphnia spp.) and the strong preference for the macrophyte habitat of even small pelagic species like Ceriodaphnia and Bosmina are indications of a considerable predation pressure. This is in accordance with the finding by Lauridsen et al. (1996), who studied cladoceran composition and migration in 2-, 10- and 25-m macrophyte enclosures established in the littoral zone of the shallow, fish-rich Lake Stigsholm (Denmark). The authors observed a significant diel horizontal migration by Ceriodaphnia and Bosmina, both species seeking refuge in the plant beds during the daytime and moving to the pelagic during the night.
The much lower density of zooplankton found in Frederiksborg Slotssø in July, compared to later in the summer, is likely to correspond to recruitment of 0+ fish (Cryer et al., 1986; Burks et al., 2002). High shares of Cyclops spp. in the crustacean assemblage were an indication of relevant predation pressure as well. In July, the dominant genus was Cyclops spp. both in the littoral and in the open water in Frederiksborg Slotssø. Copepods are less threatened by fish predation than cladocerans, because the latter exhibit a very poor escape ability in response to attack by planktivorous fish (Winfield et al., 1983).
Invertebrate predators may also have influenced the zooplankton abundance. Leptodora kindtii and Polyphemus pediculus were found in both open water and littoral habitat. However, the modest abundances (only sporadically >20 ind l−1) of the two species registered throughout the study period suggest that the invertebrates were less important as a predatory risk than planktivorous fish in this lake. Night samplings are missing, so potential predation by Chaoborus cannot be excluded. A previous study in Frederiksborg Slotssø (Christoffersen, 1990) assessed that Chaoborus exert a poor top-down control on zooplankton in the lake, as predation was mainly limited to small-sized species (e.g. copepod nauplii and Chydorus spp.).
Habitat choice by zooplankton
During the study period, the zooplankton abundance peaked in August, whereas very low densities (<50 ind l−1) were observed during July, when the zooplankton community in the littoral zone was dominated by Cyclops spp. Through the summer, as emergent macrophyte stands developed fully and edible phytoplankton concentration increased, the cladoceran abundance rose accordingly. Among the vegetation, the density of especially Ceriodaphnia and Bosmina increased remarkably (up to 11,000 ind l−1), while it remained much lower in the open water. The abundance of the two species was, on average throughout the study period, respectively, 1,000-fold (Cerodaphnia) and more than 250-fold (Bosmina) higher in the littoral vegetated zones than in open water. Also Scapholeberis, Sida and the chydorids showed the highest densities in the macrophyte stands, with an overall preference for the Polygonum habitat rather than the Phragmites belt. The heterogeneity in terms of shelter provided by the two plant species is marked for Sida and chydorids, which on average occurred with over 7-fold higher densities in the Polygonum bed compared to the Phragmites belt. Pleuroxus truncatus, which frequently occurred in significant numbers (up to 100 ind l−1) amongst the Polygonum, was absent in the Phragmites belt. The selection of habitat may well be influenced by the higher structural complexity, and therefore, higher shelter offered by the Polygonum beds compared with the Phragmites belt. This in accordance with the speculations by Burks et al. (2006) who suggest that emergent (and free-floating) macrophytes may be involved in affecting the spatial distribution of the zooplankton community. Furthermore, the PVI of Polygonum (60–70%) was higher than the one of Phragmites (40–50%) during summer, and the abundance of zooplankton is generally positively related to increasing PVI (Stansfield et al., 1997; Jeppesen et al. 1998). Additionally, Polygonum compared to Phragmites may provide a more suitable habitat for Sida, as this plant-associated grazer has been reported hanging to the leaves of aquatic macrophytes during daytime (Vuille, 1991; Nurminen et al., 2007). However, because of the higher PVI and structural complexity, it was more difficult to avoid hitting macrophytes while sampling among Polygonum than Phragmites. Therefore, an overestimation of Sida abundance (and possibly of plant-associated chydorids as well) in the Polygonum bed cannot be excluded.
The reason why significantly higher numbers of zooplankton occurred in the plant beds, rather than in open water, seems to be related to a lower predation risk in the macrophyte cover. Evidence for this is provided by several studies (Timms & Moss, 1984; Stansfield et al., 1997; Jeppesen et al., 1998; Burks et al., 2002). Stansfield et al. (1997) suggest the formation of a predator-free space among macrophytes as the mechanism supporting the refuge effect. A major reason for macrophyte avoidance by fish is probably related to a decline in foraging efficiency with increasing habitat complexity (Winfield, 1986). The extent of such a decline is clearly species dependent. For instance, 0+ perch is able to feed more efficiently than juvenile roach in structured environments (Winfield, 1986). In addition to offering structural complexity, vegetation can sometimes provide refuge for zooplankton because physical–chemical conditions such as pH, oxygen and temperature may limit fish predation efficiency (Burks et al., 2002).
Nonetheless, macrophytes might also have a repellent effect on pelagic zooplankton. Daphnia, which will avoid macrophytes where no fish are present (Pennak, 1966; Lauridsen & Lodge, 1996), occurred with significantly higher densities in open water than in between plants throughout the study period. Lauridsen & Lodge (1996) found that both chemical and structural cues may contribute to Daphnia avoidance of macrophytes. In Frederiksborg Slotssø, the maximum depth of 9 m in combination with the low transparency (usually <1 m) likely allowed Daphnia to employ vertical rather than horizontal migration, as supported by Lauridsen & Lodge (1996), who confirm that Daphnia may use plant beds as a refuge only in those lakes where vertical migration is restricted.
Interactions between zooplankton and phytoplankton
A bloom of cyanobacteria, with dominance of large colonial and filamentous species, developed during the study period in Frederiksborg Slotssø. Cyanobacteria have low nutritious value and tend to inhibit zooplankton feeding by mechanical interference and/or through the direct toxicity of their toxins (Christoffersen, 1996). Consequently, the growth and reproduction of zooplankton, especially large-bodied species (e.g. Daphnia), are decreased (Rohrlack et al., 2003).
At the end of July, cyanobacteria represented 99% of the total volume of phytoplankton (88.1 mm3 l−1), and the filamentous species Anabaena planctonica alone represented 96% of total phytoplankton. The abundance of zooplankton in the corresponding period was very low (Fig. 3), probably because of negative effects by cyanobacteria and low availability of edible phytoplankton (only 1.0 mm3 l−1 of non-cyanobacteria species). On the other hand, at the end of August the contribution of cyanobacteria to the total phytoplankton biomass had decreased to 16%. This was mainly due to a decrease in the biomass of Anabaena planctonica. The concentration of edible phytoplankton, especially Cryptomonas spp., increased (6.5 mm3 l−1) during the same period. Synchronously, the shift in algal composition most likely contributed to the remarkable peak in zooplankton abundance. The decline of cyanobacteria was probably the result of unfavourable growth conditions, induced by increased mixing of the water column and decreased temperatures (Mischke, 2003). In September, an intermediate abundance of zooplankton seemed to reflect intermediate values of edible algae (3.1 mm3 l−1) and contribution of cyanobacteria (84%, dominant genus Microcystis) to the phytoplankton community. Thus, the observed pattern of zooplankton abundance was conceivably influenced by the availability of edible phytoplankton and by negative effects of blooming cyanobacteria.
Nonetheless, when cladocerans occur with relatively high abundance, they may by their grazing be able to reduce the phytoplankton biomass (Stansfield et al., 1997). In enclosure experiments in Frederiksborg Slotssø, Christoffersen et al. (1993) observed that high densities of cladocerans in enclosures without fish were able to control phytoplankton biomass and even prevent cyanobacteria from blooming. The dominance of cladocerans, especially daphnids, resulted in high grazing pressure with high qualitative (wide size range ingested) and quantitative (high specific filtering rates) effects. This, in turn, resulted in improved water quality (high transparency and low pH). The macrophyte-avoidance observed in the present study does not support the hypothesis that plant beds may offer a daytime refuge for Daphnia against fish predation. However, the remarkable densities of other cladocerans (e.g. Ceriodaphnia, Bosmina and Sida) harboured inside the plant beds suggest that the latter may still promote water clarity due to enhanced grazing pressure on phytoplankton. The negative effect of macrophytes on phytoplankton biomass by providing a diurnal refuge for zooplankton is likely to extend beyond the border of plant beds because of the diel migration. As proposed by Lauridsen et al. (1996) in large enclosure experiments, diel horizontal migration of Ceriodaphnia and Bosmina from dense macrophyte beds covering only 3% of the lake may be enough to double the grazing potential of zooplankton in open water. The capacity of the pelagic zooplankton to control phytoplankton in the open water is further reinforced by the fact that aquatic plants favour piscivorous fish such as pike at the expense of planktivorous fish and thereby indirectly support zooplankton and its grazing pressure on phytoplankton (Burks et al., 2002).
Conclusions
These results provide evidence that emergent and floating-leaved vegetation, and not only submerged species, may act as daytime refuge for migrating zooplankton. This is especially interesting in turbid lakes, where submerged macrophytes are often scarce or lacking due to low light penetration and are replaced by emergent and/or floating-leaved species. Emergent and floating-leaved plants in Frederiksborg Slotssø harboured not only typically plant-associated species (e.g. Sida, Pleuroxus), but also potential pelagic grazers (e.g. Ceriodaphnia, Bosmina) possibly exerting diel horizontal migration in and out of the littoral vegetated zone. Polygonum compared to Phragmites stands seemed to provide a more suitable refuge for several species (especially Sida and chydorids), likely due to higher PVI, structural complexity and morphology. The abundance of crustacean zooplankton was probably controlled in some periods by fish predation and in others by the occurrence of cyanobacterial blooms, which likely decreased feeding, growth and reproduction of grazers.
Emergent and floating-leaved macrophytes are usually regarded as less important than submerged species for restoration purposes, and they are sometimes harvested in order to remove nutrients or improve the recreational aspect of lakes. However, our data indicate that such functional types of vegetation might play an important ecological role as they may provide a predator-free space and thereby result in enhanced water transparency through algal control by migrating zooplankton.
References
Andersen, J. M. & O. S. Jacobsen, 1979. Production and decomposition of organic matter in eutrophic Frederiksborg Slotssø, Denmark. Archiv Für Hydrobiologie 85: 511–542.
Burks, R. L., D. M. Lodge, E. Jeppesen, et al., 2002. Diel horizontal migration of zooplankton: costs and benefits of inhabiting the littoral. Freshwater Biology 47: 343–365.
Burks, R. L., G. Mulderij, E. Gross, et al., 2006. Center stage: the crucial role of macrophytes in regulating trophic interactions in shallow lake wetlands. In Bobbink, R., J. T. A. Verhoeven & D. E. Whigham (eds), Wetlands: functioning, biodiversity conservation, and restoration. Springer-Verlag, Berlin, Germany: 37–59.
Canfield, D., E. J. V. Shireman, D. E. Colle, et al., 1984. Prediction of chlorophyll a concentrations in Florida lakes—importance of aquatic macrophytes. Canadian Journal of Fisheries and Aquatic Sciences 41: 497–501.
Carpenter, S. R., J. F. Kitchell & J. R. Hodgson, 1985. Cascading trophic interactions and lake productivity. Bioscience 35: 634–639.
Christoffersen K., 1990. Evaluation of Chaoborus predation on natural populations of herbivorous zooplankton in a eutrophic lake. Hydrobiologia 200: 459–466.
Christoffersen K., 1996. Ecological implications of cyanobacterial toxins in aquatic food webs. Phycologia 35: 42–50.
Christoffersen, K., B. Riemann, A. Klysner, et al., 1993. Potential role of fish predation and natural populations of zooplankton in structuring a plankton community in eutrophic lake water. Limnology and Oceanography 38: 561–573.
Cryer, M., G. Peirson & C. R. Townsend, 1986. Reciprocal interactions between roach, Rutilus rutilus, and zooplankton in a small lake—prey dynamics and fish growth and recruitment. Limnology and Oceanography 31: 1022–1038.
Jeppesen, E., T. L. Lauridsen, T. Kairesalo, M. R. Perrow, 1998. Impact of submerged macrophytes on fish–zooplankton interactions in lakes. In Jeppesen, E., Ma. Søndergaard, Mo. Søndergaard & K Christoffersen (eds), The structuring role of submerged macrophytes in lakes. Springer-Verlag, NY, USA: 91–114.
Jeppesen, E., Z. Pekcan-Hekim, T. L. Lauridsen, et al., 2006. Habitat distribution of fish in late summer: changes along a nutrient gradient in Danish lakes. Ecology of Freshwater Fish 15: 180–190.
Jeppesen, E., M. Søndergaard, M. Meerhoff, et al., 2007a. Shallow lake restoration by nutrient loading reduction—some recent findings and challenges ahead. Hydrobiologia 584: 239–252.
Jeppesen, E., M. Meerhoff, B. A. Jacobsen, et al., 2007b. Restoration of shallow lakes by nutrient control and biomanipulation—the successful strategy varies with lake size and climate. Hydrobiologia 581: 269–285.
Jespersen, A. M. & K. Christoffersen, 1987. Measurements of chlorophyll a from phytoplankton using ethanol as extraction solvent. Archiv Für Hydrobiologie 109: 445–454.
Jespersen, A. M., K. Christoffersen, B. Riemann, 1988. Annual carbon fluxes between phyto-, zoo-, and bacterio-plankton in eutrophic, Lake Frederiksborg Slotssø, Denmark. Verhandlunge, International Verein Limnologie 23: 440–444.
Lauridsen, T. L. & D. M. Lodge, 1996. Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator mediated use of macrophyte habitat. Limnology and Oceanography 41(4): 794–798.
Lauridsen, T. L., L. J. Pedersen, E. Jeppesen, et al., 1996. The importance of macrophyte bed size for Cladoceran composition and horizontal migration an a shallow lake. Journal of Plankton Research 18: 2283–2294.
Mischke, U., 2003. Cyanobacteria associations in shallow polytrophic lakes: influence of environmental factors. Acta Oecologica International Journal of Ecology 24(Suppl. 1): S11–S23.
Müller, J. P. & Jensen, H. J., 2004. The fish stocks in Frederiksborg Slotssø, September 2004. Internal report (in Danish), 30 pp.
Nurminen, L., J. Horppila & P. Tallberg, 2001. Seasonal development of the Cladoceran assemblage in a turbid lake: the role of emergent macrophytes. Archiv Für Hydrobiologie 151: 127–140.
Nurminen, L., J. Horppila & Z. Pekcan-Hekim, 2007. Effect of light and predator abundance on the habitat choice of plant-attached zooplankton. Freshwater Biology 52: 539–548.
Pennak, R. W., 1966. Structure of zooplankton populations in littoral macrophyte zone of some Colorado lakes. Transactions of the American Microscopical Society 85: 329–349.
Rasmussen, H. U., 2001. Frederiksborg Slotssø, 1999—Vandmiliøovervågning Nr. 85. Frederiksborg Amt, Teknik & Miljø Miljøafdelingen, Frederiksborg County, Denmark (in Danish), 48 pp.
Rohrlack, T., K. Christoffersen, P. E. Hansen, et al., 2003. Isolation, characterization, and quantitative analysis of microviridin J, a new Microcystis metabolite toxic to Daphnia. Journal of Chemical Ecology 29: 1757–1770.
Scheffer, M., S. H. Hosper, M. L. Meijer, et al., 1993. Alternative equilibria in shallow lakes. Trends in Ecology & Evolution 8: 275–279.
Søndergaard, M. & B. Moss, 1998. Impact of submerged macrophytes on phytoplankton in shallow freshwater lakes. In Jeppesen, E., Ma. Søndergaard, Mo. Søndergaard & K. Christoffersen (eds), The structuring role of submerged macrophytes in lakes. Springer Verlag, NY, USA: 115–132.
Stansfield, J. H., M. R. Perrow, L. D. Tench, et al., 1997. Submerged macrophytes as refuges for grazing Cladocera against fish predation: observations on seasonal changes in relation to macrophyte cover and predation pressure. Hydrobiologia 342: 229–240.
Statistica (ver. 6.0), 2003. Statsoft, Scandinavia, Uppsala, Sweden.
Timms, R. M. & B. Moss, 1984. Prevention of growth of potentially dense phytoplankton populations by zooplankton grazing in the presence of zooplanktivorous fish. In: A shallow wetland ecosystem. Limnology and Oceanography 29: 472–486.
Utermöhl, H., 1958. Zur Vervollkommung der quantitatven Phytoplankont-Metodik. Mitt. Unt. Ver. Limnol. 9: 1–38.
Vuille, T., 1991. Abundance, standing crop and production of microcrustacean population (Cladocera, Copepoda) in the littoral zone of lake Biel, Switzerland. Archiv Für Hydrobiologie 123: 165–185.
Winfield, I. J., 1986. The influence of simulated aquatic macrophytes on the zooplankton consumption rate of juvenile roach, Rutilus rutilus, rudd, Scardinius erythrophthalmus, and perch, Perca fluviatilis. Journal of Fish Biology 29(Suppl. A): 37–48.
Winfield, I. J., G. Peirson, M. Cryer, et al., 1983. The behavioural basis of prey selection by under yearling breams (Abramis brama (L.)) and roach (Rutilus rutilus (L.)). Freshwater Biology 13: 139–149.
Acknowledgements
We wish to thank Charlotte Møller and Nils Willumsen, both from the Freshwater Biological Laboratory, for technical assistance and Erik Jeppesen from the National Environmental Research Institute, and two anonymous referees for valuable comments to an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S. I. Dodson
Rights and permissions
About this article
Cite this article
Cazzanelli, M., Warming, T.P. & Christoffersen, K.S. Emergent and floating-leaved macrophytes as refuge for zooplankton in a eutrophic temperate lake without submerged vegetation. Hydrobiologia 605, 113–122 (2008). https://doi.org/10.1007/s10750-008-9324-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9324-1





 —) in Frederiksborg Slotssø (July–October 2006)
—) in Frederiksborg Slotssø (July–October 2006)

