Abstract
The long non-coding RNAs (lncRNAs) are the crucial regulators of human chronic diseases. Therefore, approaches such as antisense oligonucleotides, RNAi technology, and small molecule inhibitors have been used for the therapeutic targeting of lncRNAs. During the last decade, phytochemicals and nutraceuticals have been explored for their potential against lncRNAs. The common lncRNAs known to be modulated by phytochemicals include ROR, PVT1, HOTAIR, MALAT1, H19, MEG3, PCAT29, PANDAR, NEAT1, and GAS5. The phytochemicals such as curcumin, resveratrol, sulforaphane, berberine, EGCG, and gambogic acid have been examined against lncRNAs. In some cases, formulation of phytochemicals has also been used. The disease models where phytochemicals have been demonstrated to modulate lncRNAs expression include cancer, rheumatoid arthritis, osteoarthritis, and nonalcoholic fatty liver disease. The regulation of lncRNAs by phytochemicals can affect multi-steps of tumor development. When administered in combination with the conventional drugs, phytochemicals can also produce synergistic effects on lncRNAs leading to the sensitization of cancer cells. Phytochemicals target lncRNAs either directly or indirectly by affecting a wide variety of upstream molecules. However, the potential of phytochemicals against lncRNAs has been demonstrated mostly by preclinical studies in cancer models. How the modulation of lncRNAs by phytochemicals produce therapeutic effects on cancer and other chronic diseases is discussed in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The long non-coding RNAs (lncRNAs) are highly conserved and potentially functional molecules with an ability to regulate gene expression in a cis- or trans-manner [1,2,3,4]. During the past decade, lncRNAs have emerged as the key player for normal and pathological conditions. The lncRNAs play a crucial role in cell-cycle regulation, innate immunity, and pluripotency [5]. The lncRNAs, transcribed by RNA pol II, are ≥ 200 nucleotides in length [6]. Normally located in the cytosol and the nucleus, the lncRNAs undergo post-transcriptional modifications such as polyadenylation, capping, and splicing [7,8,9,10]. The lncRNAs play a crucial role in diverse biological processes such as epigenetic regulation [11, 12], transcriptional regulation of gene expression [13, 14], organization of protein complexes, cell–cell communications, and the formation of nuclear sub-structures [15]. The lncRNAs also play a role during development [16, 17], somatic cell reprogramming, and stem cell pluripotency [17, 18]. Although the mechanism of lncRNAs function varies under different conditions, studies suggest that lncRNAs and miRNAs can display potential cross-talk especially during carcinogenesis [19,20,21,22].
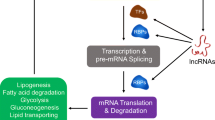
Often expressed in a development-, tissue-, or disease-specific manner, lncRNAs can be targeted therapeutically [23,24,25,26,27,28]. Indeed, strategies such as antisense oligonucleotides (ASOs), RNAi technology, and small molecule inhibitors have been used for lncRNAs’ targeting [29, 30]. The lncRNAs have also been used for the selective killing of cancer cells [31]. During recent years, phytochemicals derived from natural sources have demonstrated potential against lncRNAs. The phytochemicals are reported to be cost-effective with an ability to modulate multiple cell signaling pathways [32, 33]. Moreover, these agents have been consumed for ages and, thus, are known to be safe. The sources of phytochemicals include fruits, vegetables, spices, cereals, etc. The consumption of fruits and vegetables is associated with reduced risk of chronic diseases [34,35,36,37,38]. Phytochemicals can affect lncRNA expression either directly or indirectly through the involvement of miRNAs, protein kinases, enzymes, and transcription factors (Table 1). In the cancer model, phytochemicals can suppress the expression of oncogenic lncRNAs or can restore the functions of tumor suppressor lncRNAs. The modulation of lncRNAs by phytochemicals can produce therapeutic effects in some cancer types (Table 2). The disease models where phytochemicals have been demonstrated to modulate lncRNAs include cancer, rheumatoid arthritis, osteoarthritis, and nonalcoholic fatty liver disease (Fig. 1). In disease models, phytochemicals can both up-regulate and down-regulate lncRNAs (Fig. 2). The most common phytochemicals known to have potential to target lncRNAs include curcumin, resveratrol, sulforaphane, berberine, EGCG, gambogic acid, genistein, paclitaxel (taxol), quercetin, sanguinarine, silibinin, anacardic acid, and calycosin (Fig. 3). Moreover, the modulation of lncRNAs by phytochemicals can lead to the inhibition of survival, proliferation, migration, invasion, metastasis, and epithelial-to-mesenchymal transition (Fig. 4). The modulation of lncRNAs expression by phytochemicals can also lead to chemosensitization and radiosensitization of cancer cells (Fig. 4). How phytochemicals affect lncRNA expression in diverse diseases is discussed in the following section. The positives and negatives associated with the targeting of lncRNAs by phytochemicals are also discussed.
A list of lncRNAs modulated by phytochemicals. CASC2 cancer susceptibility 2, CDKN2B-AS1 CDKN2B antisense RNA 1, GAS5 growth arrest-specific 5, GUCY2GP guanylate cyclase 2G pseudogene, H2BFXP H2B histone family member X pseudogene, HOTAIR HOX transcript antisense RNA, LINC00623 long intergenic non-protein-coding RNA 623, LINC01116 long intergenic non-protein-coding RNA 1116, linc-PINT long intergenic non-protein-coding RNA-p53 induced transcript, MALAT1 metastasis-associated lung adenocarcinoma transcript-1, MEG3 maternally expressed gene 3, MIR155HG MIR155 host gene, NEAT1 nuclear-enriched abundant transcript 1, PANDAR promoter of CDKN1A antisense DNA damage-activated RNA, PCAT29 prostate cancer-associated transcript 29, PVT1 plasmacytoma variant translocation 1, ROR regulator of reprogramming, ST7OT1 ST7 overlapping transcript 1, TUG1 taurine up-regulated gene 1, ZFAS1 zinc finger antisense 1
Effects of phytochemicals on lncRNA expression
Phytochemicals can modulate multiple cell signaling molecules including kinases, adhesion molecules, cell-cycle regulators, receptors, miRNAs, etc. [32, 39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. During the last 5 years, phytochemicals have also been reported to modulate lncRNA expression. The common phytochemicals known to have potential to target lncRNAs include curcumin, resveratrol, sulforaphane, berberine, EGCG, gambogic acid, genistein, paclitaxel (taxol), quercetin, sanguinarine, silibinin, anacardic acid, and calycosin. In the following section, we have discussed the effects of phytochemicals on lncRNA expression in human disease models.
Curcumin
Curcumin (diferuloylmethane) is a yellow-color polyphenol derived from the yellow spice turmeric (Curcuma longa) [57]. The biological activities of this polyphenol have been reported against various human diseases including cancer, diabetes, cardiovascular disorders, obesity, and neurodegenerative diseases. This pleiotropic molecule can affect several signaling molecules such as adhesion molecules, enzymes, growth factors, inflammatory molecules, kinases, reductases, receptors, transcription factors, chemokines, DNA, RNA, and proteins involved in cell-cycle regulation, survival, and drug resistance [58]. Recent studies suggest that curcumin can also modulate lncRNAs in human disease models. The common lncRNAs modulated by curcumin include AF086415, AK056098, AK095147, AK294004, FLJ36000, GUCY2GP, H19, H2BFXP, HOTAIR, LINC00623, LOC100506835, MEG3, MUDENG, PANDAR, PVT1, RP1-179N16.3, and ZRANB2-AS2.
The oncogenic H19 is constitutively present in multiple myeloma [59] and in breast [60], gallbladder [61], esophageal [62], ovarian [63], and lung [62, 64] cancers. The expression of H19 also correlates with NF-κB activation [59, 65]. Curcumin suppressed the expression of oncogenic H19 in tumor cell lines such as Cal-27, Detroit-562, HCT-116, HeLa, Hep-2, and SW-620 without exerting any effect on normal cells [66]. Curcumin was also found to suppress H19 and c-Myc, and to enhance p53 expression in gastric cancer cells [67]. The polyphenol exhibited anti-proliferative activities and induced apoptosis in gastric cancer cells. Curcumin-induced p53 up-regulation and anti-proliferative effects were reversed by the ectopic expression of H19. When c-Myc was overexpressed, curcumin-induced down-regulation of H19 was reversed. It can be concluded that curcumin inhibits the proliferation of gastric cancer cells by negatively regulating the c-Myc/H19 pathway. The regulator of reprogramming (ROR) is an lncRNA that functions to regulate the activity and reprogramming of pluripotent stem cells. The activity of ROR is tightly regulated by stem cell related molecules such as SOX2, OCT4, and NANOG [68]. ROR is an oncogene with constitutive expression in multiple cancer types such as breast cancer [69], gallbladder cancer [70], nasopharyngeal carcinoma [71], pancreatic cancer [68], and prostate cancer [72]. Curcumin is reported to produce inhibitory effects on prostate cancer stem cells by suppressing ROR expression [73]. Curcumin can also up-regulate linc-PINT, which is frequently down-regulated in acute lymphoblastic leukemia (ALL) [74] and suppresses the migration capacity of most cancer cells [75]. Growth arrest specific 5 (GAS5) is a tumor suppressor lncRNA with potential to induce apoptosis and suppress the proliferation of tumor cells [76]. The expression of this lncRNA is significantly enhanced during the growth arrest of the tumor cells [77]. Curcumin can also modulate GAS5 expression in breast cancer cells [78].
The promoter of CDKN1A antisense DNA damage-activated RNA (PANDAR) is an lncRNA with 1506 nucleotides in length [79]. With a function to promote proliferation and migration, this lncRNA is up-regulated in several cancer types including bladder, gastric, and colorectal cancers [80,81,82,83,84]. Whether PANDAR contributes to the efficacy of curcumin against colorectal cancer was investigated [85]. An identical expression pattern of PANDAR was observed in CRC tissues and in normal tissues. The proliferation of CRC DLD-1 cells was not affected by the knockdown of PANDAR. Curcumin at lower doses induced senescence and up-regulated PANDAR without any effect on apoptosis in DLD-1 cells. Curcumin’s effect on apoptosis under the elevated level of PANDAR was investigated. The silencing of PANDAR enhanced apoptosis and attenuated senescence in curcumin-treated DLD-1 cells. Overall these results suggest that low-dose curcumin can induce PANDAR. Furthermore, PANDAR silencing can also switch cells from senescence to apoptosis partly by stimulating the expression of the p53-up-regulated modulator of apoptosis (PUMA). Further experiments will demonstrate the involvement of PUMA in PANDAR mediated apoptosis in CRC cells under curcumin treatment. HOX transcript antisense intergenic RNA (HOTAIR) is located at mammalian HOXC gene locus, and is associated with tumor progression and metastasis by binding and targeting polycomb repressive complex 2 [86]. Curcumin can suppress HOTAIR-induced migration of renal cell carcinoma (RCC) cells [87].
In certain cases, nanocurcumin has also been tested for its efficacy against lncRNA. For example, dendrosomal curcumin (DNC) with improved bioavailability [88, 89] can induce the tumor suppressor maternally expressed gene 3 (MEG3) in hepatocellular cancer (HCC) [90]. Under normal conditions, MEG3 is expressed at low level partly due to methylation of its promoter region. Although expressed at low level, MEG3 is known to stimulate p53, and can suppress proliferation, invasion, and migration of cancer cells [91]. The up-regulation in MEG3 expression by DNC was mediated through enhanced expression of miR-29a and miR-185 that down-regulated the expression of DNA methyltransferases (DNMTs) such as DNMT1, DNMT3A, and 3B. It was concluded that induction of DNA hypomethylation and MEG3 by DNC could be an effective choice for epigenetic therapy of HCC.
Curcumin is also known to sensitize cancer cells to chemotherapy and radiotherapy through modulation of lncRNA expression. Polycomb Repressive Complex 2 (PRC2) consisting of the Enhancer of Zeste Homolog-2 (EZH2) is reported to maintain the cancer stem cell population by regulating stemness-associated genes [92, 93]. EZH2 can interact with lncRNAs leading to resistance-associated phenomenon such as epithelial–mesenchymal transition and cancer stemness [94,95,96,97,98]. An interesting study was aimed to delineate the underlying mechanism of gemcitabine resistance in pancreatic ductal adenocarcinoma (PDAC) cell line [99]. The plasmacytoma variant translocation 1 (PVT1) is an oncogenic lncRNA that stabilizes the MYC protein [100]. Curcumin-sensitized chemoresistant PDAC cells were linked with the inhibition of EZH2 and lncRNA PVT1 [99]. Consistent with these observations, PVT1 is known to play a role in the sensitization of human pancreatic cancer cells to gemcitabine [98]. Curcumin also suppressed the spheroid formation by resistant cells and down-regulated several self-renewal driving genes, indicating the potential of this polyphenol against cancer stem cells (CSCs). Curcumin also attenuated gemcitabine-resistant tumor growth in vivo. Because CSCs contribute to chemoresistance [92, 101,102,103,104,105], the combination of curcumin and chemotherapy appears promising. However, further validation is required before these observations can be translated to the clinic. The extracellular vesicles (EVs) containing lncRNA and miRNAs are known to induce drug resistance in cancer cells [106,107,108]. Whether curcumin can overcome the cisplatin resistance in ovarian cancer was investigated [109]. The EVs from cisplatin-resistant ovarian cancer cells without or with curcumin treatment were analyzed. The EVs were found to induce drug resistance in ovarian cancer cells that were weakened by curcumin treatment. Furthermore, curcumin up-regulated MEG3 expression and induced demethylation in its promoter region. Curcumin also significantly reduced miR-214 in cells and in EVs that were associated with weakened chemoresistance. It was concluded that MEG3 could reduce drug resistance in ovarian cancer cells by suppressing EVs mediated transfer of miR-214. However, further studies using multiple cell lines and other preclinical models are required before these observations can be validated in the clinic.
Curcumin can radiosensitize nasopharyngeal CNE-2 carcinoma cells [110]. Furthermore, curcumin significantly up-regulated the expression of lncRNAs such as GUCY2GP, H2BFXP, and LINC00623, while the expression of ZRANB2-AS2, LOC100506835, and FLJ36000 IncRNA was down-regulated [110]. In another study, curcumin-induced radiosensitization of nasopharyngeal carcinoma cells was mediated partly through modulation of lncRNAs such as AF086415, AK056098, AK095147, AK294004, MUDENG, and RP1-179N16.3 [110].
In summary, curcumin’s ability to modulate lncRNA expression has provided a new molecular basis for its biological activities. However, the studies have been performed mostly in the cancer models. Curcumin’s potential to modulate lncRNAs in the other disease models remains to be explored. Future studies should also elucidate if curcumin can effectively regulate lncRNA expression in human subjects.
Resveratrol
Resveratrol is a polyphenolic phytoalexin derived from berries, grapes, peanuts, pistachio, plums, and white hellebore [111]. Although resveratrol exists in both cis- and trans-isomeric forms, the latter is of considerable interest [112]. The pleiotropic activities of this polyphenol originate from its ability to modulate several oncogenic signaling cascades [113,114,115].
The prostate cancer-associated transcript 29 (PCAT29) is a tumor suppressor lncRNA that is frequently down-regulated in prostate cancer tumors possibly through androgen signaling [116]. The lower levels of PCAT29 have also been observed in DU145 and LNCaP cells as compared to normal prostate cells [116]. This lncRNA is reported to inhibit proliferation and migration of prostate cancer cells [117, 118]. Whether resveratrol exhibits its anti-cancer activities against prostate cancer through modulation of PCAT29 was examined [116]. IL-6 was found to activate STAT3 and reduce the level of PCAT29 in both DU145 and LNCaP cells. The PCAT29 expression was enhanced by the inhibition of miR-21, which is downstream to STAT3. Resveratrol treatment stimulated the basal level of PCAT29 expression. Furthermore, the IL-6-induced suppression of PCAT29 was also reversed by resveratrol. Concomitantly, the viability of DU145 and LNCaP cells was also suppressed by resveratrol. Thus, the IL-6/STAT3/miR-21 pathway could regulate both the expression and function of PCAT29 and resveratrol induces expression and the functions of PCAT29 through the inhibition of this signaling pathway [116]. In another study, resveratrol modulated the expression of lncRNAs in lung cancer A549 cells [119]. Among various lncRNAs, AK001796 was overexpressed in lung cancer tissues and cell lines. However, resveratrol treatment reduced the expression of AK001796 in lung cancer cells. Furthermore, the knockdown of AK001796 was associated with a significant reduction in the viability of lung cancer cells and reduced tumor growth. The lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is reported crucial for the progression of several cancer types including those of liver, renal, cervical, colorectal, bladder, and osteosarcoma [120]. Originally discovered as a prognostic marker for lung cancer patients, MALAT1 is now reported to be evolutionary conserved [121]. However, mice deficient in MALAT1 lack any obvious phenotype under normal physiological conditions [122, 123]. In CRC cell lines, resveratrol inhibited the invasion and metastasis of CRC cells through MALAT1-mediated Wnt/β-catenin signaling and its downstream targets [124]. Some other lncRNAs known to be up-regulated in response to resveratrol include MEG3, ST7OT1, NEAT1, and MIR155HG in glioma cell lines [125].
Paclitaxel (Taxol)
Paclitaxel (brand name Taxol) is an anti-cancer agent that was first isolated from the bark of the Pacific yew tree in 1971 [126]. Approved in 1993 for its anti-cancer activities, taxol is an antimitotic agent that blocks tumor growth by stopping cell division. Taxol has been found effective against several cancer types such as breast, ovarian, pancreatic, non-small cell lung cancer, and AIDS-related Kaposi sarcoma [127, 128]. During recent years, this antimitotic agent was also demonstrated to modulate lncRNAs expression.
The tumor suppressor GAS5 is significantly lower in breast cancer tissues than in the adjacent non-tumor tissues [129]. The decreased expression of GAS5 correlates with TNM stage and lymph-node metastasis of breast cancer. GAS5 expression was also significantly low in paclitaxel-resistant breast cancer cells. Furthermore, GAS5 was positively correlated with p21 but in a negative manner with CDK6. The overexpression of GAS5 in paclitaxel-resistant breast cancer cells suppressed the migration and invasion, and enhanced susceptibility to paclitaxel. In the tumor-bearing nude mouse models, GAS5 overexpression enhanced the inhibitory effect of paclitaxel on tumor growth and lung metastasis by reversing the EMT. It was concluded that a decreased expression of GAS5 promotes lung metastasis of breast cancer by inducing EMT, thereby suggesting the therapeutic potential of this lncRNA against breast cancer [129]. In ERα-positive breast cancer cells, the high expression of H19 was correlated with paclitaxel (PTX) resistance [130]. H19 attenuated paclitaxel-induced apoptosis by inhibiting the transcription of BIK and NOXA (pro-apoptotic genes). Furthermore, H19 suppressed the promoter activity of BIK by recruiting EZH2 and by trimethylating the histone H3 at lysine 27. H19 was found to be one of the downstream target molecules of ERα. Overall, these observations suggest that the ERα–H19–BIK axis is crucial for the development of paclitaxel chemoresistance in ERα-positive breast cancer cells. One study was aimed to investigate the effects of lncRNA RP11-770J1.3 and transmembrane protein 25 (TMEM25) on paclitaxel-resistant human breast cancer (MCF-7/PR) cell line [131]. The parental MCF-7 cells (paclitaxel sensitive) were also used for the comparison. A higher expression of RP11-770J1.3 and TMEM25 was observed in MCF-7/PR cells. The MCF-7/PR cells were sensitized to paclitaxel after the gene silencing of RP11-770J1.3 and TMEM25. In agreement with these observations, the expression of MDR1/P-gp, MRP, and BCRP was also suppressed. Thus, RP11-770J1.3 and TMEM25 represent a novel target for enhancing the sensitivity of resistant breast cancer cells to paclitaxel. Similarly, MAPT-AS1 lncRNA can correlate with the growth, invasion, and paclitaxel resistance in ER-negative breast cancer cells [132]. The genetic polymorphisms of GAS5 can also predict the response of nasopharyngeal carcinoma patients to paclitaxel [133]. The inhibition of MA-linc1 enhances cell death in cancer cells induced by paclitaxel [134].
The RNA-sequencing in the A2780 ovarian cancer cell line and the A2780/PTX paclitaxel-resistant cell line was carried out [135]. Results indicated that five lncRNAs were up-regulated, while four lncRNAs were down-regulated in both multidrug-resistant ovarian and colon cancer cell lines. Furthermore, the lncRNA CTD-2589M5.4 was co-expressed with the multidrug-resistant genes (ABCB1, ABCB4, ABCC3, and ABCG2). Nuclear-enriched abundant transcript 1 (NEAT1) can act as both oncogene and tumor suppressor depending upon the cancer type [136, 137]. NEAT1 can also contribute to paclitaxel resistance of ovarian cancer cells partly by up-regulating ZEB1 expression and sponging miR-194 [138]. Some lncRNAs are dysregulated in paclitaxel-resistant lung adenocarcinoma cells as compared to parental A549 cells [139].
ZNFX1 antisense RNA 1 (ZFAS1) is known to act both as an oncogene and as a tumor suppressor in multiple cancer types [140,141,142,143]. ZFAS1 can modulate notch signaling and various other tumor-associated genes, and induce epithelial-to-mesenchymal transition in multiple cancer types [144,145,146,147]. The elevated expression of the lncRNA, ZFAS1, is observed in gastric cancer specimens as compared to the para-carcinoma tissues [135]. The knockdown of ZFAS1 can suppress the growth, proliferation, cell-cycle progression, migration, and invasion. Furthermore, the ZFAS1 gene silencing suppressed Wnt/β-catenin signaling and enhanced the sensitivity of SGC7901 gastric cancer cells to paclitaxel. Similarly, PVT1 is expressed at a higher level in human gastric cancer tissues than in adjacent non-cancerous tissues [148]. The expression level of PVT1 was also reported to be high in SGC7901 paclitaxel-resistant cells compared with that observed in SGC7901 cells [148].
The tumor suppressor TUSC7 can enhance the sensitivity of endometrial carcinoma to paclitaxel by targeting miR-23b [149]. Paclitaxel is also known to reduce the expression of CDKN2B-AS1, HOTAIR, and MALAT1 laryngeal squamous cell carcinoma [150]. PVT1 can affect the response of cervical cancer cells to paclitaxel by regulating EMT [151]. CCAT1 controls the sensitivity of nasopharyngeal carcinoma (NPC) cells to paclitaxel via miR-181a/CPEB2 axis [152]. Some other lncRNAs associated with paclitaxel resistance include H19 in breast cancer [153]; SNHG12 in NSCLC [152]; XR_938728, XR_947831, XR_938392, XR_948297, NR_036503, NR_073113, and NR_103801 in ovarian cancer [152]; LINC00672 in endometrial cancer [154]; n375709 in nasopharyngeal carcinoma [155]; HIF1A-AS2 and AK124454 in triple-negative breast cancer [156]; linc-ROR in breast cancer [157]; KCNQ1OT1 and ANRIL in lung adenocarcinoma [158]; and RP11-381N20.2 in cervical cancer [159].
Overall, these results suggest that lncRNAs contribute to paclitaxel resistance and, thus, could be targeted to enhance the sensitivity of cancer cells.
Epigallocatechin gallate
Epigallocatechin gallate (EGCG) is a type of catechin chiefly present in green tea. This catechin has been extensively studied for its potential health benefits by both preclinical and clinical studies [160,161,162,163,164,165,166,167]. The tea catechins have been closely linked with the maintenance of normal LDL-cholesterol level [168]. EGCG can modulate multiple cell signaling pathways in tumor cells [169, 170].
One study was aimed to elucidate the possible role of lncRNAs in the cholesterol modulatory effects of EGCG in hepatocytes [171]. When HepG2 cells were treated with EGCG, 15 genes related to cholesterol metabolism and 285 lncRNAs were dysregulated. Bioinformatic analyses revealed five matched lncRNA–mRNA pairs for five differentially expressed lncRNAs and four differentially expressed mRNA. The identification of lncRNA AT102202 and its potential mRNA target, 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) was of particular importance. The quantitative PCR analyses revealed a down-regulation in the mRNA level of HMGCR and an up-regulation in AT102202. Furthermore, silencing of AT102202 was associated with an increased expression of HMGCR. The authors of this study concluded that AT102202 is involved in the improvement of cholesterol metabolism by EGCG. However, further studies using the animal models are required before these claims can be translated to the clinic.
Platinum-based chemotherapy, such as cisplatin (cDDP), has been used for non-small cell lung cancer (NSCLC) patients [172]. Copper transporter 1 (CTR1) facilitates cDDP internalization in tumor cells [173,174,175]. The association of cDDP uptake with CTR1 levels has been confirmed by some studies [175, 176]. Interestingly, whereas CTR1 up-regulation can sensitize tumor cells to platinum drugs, its down-regulation contributes to resistance [175]. EGCG has been reported to induce CTR1 expression in ovarian cancer cells and mouse xenografts [177]. EGCG can also enhance the sensitivity of ovarian cancer cells to cDDP [177]. In another study, EGCG was found to up-regulate CTR1 expression and to increase platinum accumulation in NSCLC cells (H460, H1299, and A549), cDDP-resistant A549 cells and in a nude mouse xenograft model [156]. EGCG also enhanced the cell growth inhibitory effects of cisplatin both in vitro and in vivo. While miRNA hsa-mir-98-5p suppressed CTR1 expression, the lncRNA NEAT1 positively regulated CTR1 expression. The hsa-mir-98-5p harbors specific complementary binding sites for NEAT1. NEAT1 was found to compete with hsa-mir-98-5p and enhanced EGCG-induced CTR1 in NSCLC. Overall, these results suggest that NEAT1 plays a crucial role in sensitizing NSCLC cells to cisplatin. Thus, EGCG could be used as an effective adjuvant for lung cancer chemotherapy.
Genistein
Genistein is a dietary isoflavone known to modulate cell signaling pathways such as JAK/STAT, AKT, and Wnt pathway [178,179,180,181]. Genistein also acts as protein tyrosine kinase inhibitor and exhibits activities against multiple cancer types [182,183,184,185,186,187]. One study was aimed to investigate the mechanism by which the isoflavones such as calycosin and genistein exhibit activities against breast cancer [188]. Both genistein and calycosin inhibited proliferation and induced apoptosis in MCF-7 cells. However, calycosin was more effective as compared to genistein. Furthermore, both isoflavones decreased AKT phosphorylation and HOTAIR expression. Calycosin was concluded to be superior in inhibiting breast cancer growth in comparison to genistein. It was also concluded that the suppression of AKT phosphorylation and HOTAIR expression contribute to the anti-cancer activities of these isoflavones. However, more experiments are required to support these claims.
In renal cell carcinoma, genistein can suppress HOTAIR expression while up-regulating miR-141 expression [189]. MiR-141 has been inversely correlated with the tumorigenicity and invasiveness of several cancer types [190]. Conversely, the oncogenic role of HOTAIR has been demonstrated by some studies [191,192,193]. The observations that genistein down-regulates HOTAIR and up-regulates miR-141 further support the anti-cancer property of this soy isoflavone. In prostate cancer PC3 and DU145 cell lines, genistein down-regulated HOTAIR expression [194]. Furthermore, the gene silencing of HOTAIR was associated with a decrease in the proliferation, migration and invasion, while an induction in cell-cycle arrest and apoptosis was observed. The tumor suppressor miR-34a was also up-regulated by genistein in prostate cancer cells. Overall, up-regulation in miR-34a and suppression in HOTAIR may contribute to the anti-cancer activities of genistein against prostate cancer.
Silibinin
Silibinin is an active constituent of silymarin, which is derived from the seeds of milk thistle (Silybum marianum). Chemically, silibinin is a polyphenolic flavonolignan with potential against a variety of cancer types such as bladder [195, 196], brain [197], breast [198, 199], colon [200, 201], kidney [202, 203], lung [204, 205], pancreas [206], prostate [198, 207, 208], and skin [209, 210] cancers. The potential of silibinin against human bladder cancer cells was examined [211]. Silibinin significantly suppressed multi-steps of tumor development such as proliferation, migration, and invasion. Furthermore, this molecule also induced apoptosis in UM-UC-3 and T24 human bladder cancer cells. Silibinin also suppressed the actin cytoskeleton and PI3K/AKT signaling pathways, both of which cross-talk via RAS oncogene. Silibinin also reduced histone H3 lysine 4 (H3K4) trimethylation and H3 acetylation at the KRAS promoter suggesting the role of this agent in histone modifications. Furthermore, silibinin significantly attenuated the expression of oncogenic lncRNAs, HOTAIR, and ZFAS1 without any effect on MALAT1, MEG3, and GAS5. The use of wortmannin (PI3K inhibitor) suppressed HOTAIR expression in human bladder cancer cells [211]. Consistent with these observations, HOTAIR is linked with the recurrence of bladder cancer [212]. HOTAIR is also up-regulated by KRAS [213] and PI3K pathways [15]. Thus, silibinin may exert its effects through the modulation of oncogenic lncRNAs. It is also likely that multiple signaling pathways modulated by silibinin contribute to its activities against bladder cancer. Whether silibinin exhibits anti-cancer activities through modulation of HOTAIR in other cancer types remains to be explored.
Emodin
Emodin, an active anthraquinone isolated especially from Rhamnus frangula, is known to exhibit anti-cancer activities by some preclinical studies [214]. Furthermore, emodin can sensitize resistant cancer cells to chemotherapeutic agents. This anthraquinone has also demonstrated potential against osteoarthritis (OA), which is a chronic disease involving adipose tissues, articular cartilage, ligaments, subchondral bone, synovium, and tendons [215]. Characterized by pain, joint dysfunction and deformity, OA constitutes the leading cause of disability and compromises patients’ life quality [215,216,217]. The potential of emodin against OA was examined in vitro [218]. The murine chondrogenic ATDC5 cells were treated with lipopolysaccharide to mimic the OA model. The effects of emodin on viability, apoptosis, and release of cytokines (TNF-α, IL-6, and MCP-1) in LPS-treated ATDC5 cells were examined. The expression of taurine-up-regulated gene 1 (TUG1) lncRNA, and Notch and NF-κB signaling pathways were also examined in emodin-treated ATDC5 cells. The LPS stimulation induced a decrease in cell viability, an increase in apoptosis and pro-inflammatory cytokines expression, and alterations in the expression of apoptosis-related proteins. LPS-induced changes in these parameters were all mitigated by emodin in ATDC5 cells. While TUG1 was up-regulated, the NF-κB and Notch pathways were inhibited by emodin treatment. An up-regulation in TUG1 expression by emodin was found to inactivate Notch and NF-κB pathways. These observations provide a new mechanism for the therapeutic potential of emodin against OA. The previous studies have demonstrated that TUG1 functions as an oncogene in multiple cancer types [219,220,221]. For example, TUG1 modulates cancer cell proliferation and invasion by targeting miR-219, miR-145/ZEB1, and Wnt/β-catenin signaling pathways [222,223,224]. Whether emodin modulates the functions of TUG1 in cancer models remains to be elucidated.
Gambogic acid
Gambogic acid (GA) is a xanthonoid derived from the resin of Garcinia. This xanthonoid exhibits anti-inflammatory, antioxidant, antiviral, and parasiticidal activities [225]. GA also exhibit anti-cancer activities with minimal toxicity to normal cells [226,227,228]. Exposure of bladder cancer cells to GA induces apoptosis in bladder cancer cells by inhibiting EZH2 methyltransferase expression [229].
The lncRNA GAS5 negatively correlates with the clinical stage of bladder cancer [230]. Furthermore, GAS5 overexpression reduces viability and induces apoptosis in EJ and T24 bladder cancer cells. Mechanistically, GAS5 represses EZH2 transcription by direct interaction and recruitment of E2F4 to the EZH2 promoter. Moreover, GAS5-induced down-regulation in EZH2 was associated with overexpression of miR-101. Furthermore, GA induces GAS5 expression and produces pro-apoptotic effects in bladder cancer cells. Interestingly, GA-induced apoptosis in bladder cancer cells was suppressed by knockdown of GAS5. Overall these results suggest that GAS5 functions as a tumor suppressor by inhibiting EZH2 expression. In addition, induction of GAS5 by gambogic acid may contribute to its anti-cancer activities against bladder cancer.
Anacardic acid
Anacardic acid is a phenolic lipid chiefly present in cashew nuts. Chemically, anacardic acid is a mixture of saturated and unsaturated organic molecules [231]. This polyphenol has demonstrated potential against some cancer types including breast cancer [232,233,234]. The potential regulators involved in the activities of anacardic acid against ER-positive MCF-7 and triple-negative MDA-MB-231 cells was examined by next generation transcriptomic sequencing (RNA-Seq) and network analysis [233]. While 80 genes were dysregulated including lncRNA MIR22HG in MCF7 cells, 886 genes were identified in MDA-MB-231 cells in response to anacardic acid. The genes down-regulated by anacardic acid in both cell lines included SCD, INSIG1, and TGM2, while the up-regulated genes were PDK4, GPR176, and ZBT20. The molecular modeling indicated that anacardic acid could inhibit monounsaturated fatty acid biosynthesis in both cell lines and enhance endoplasmic reticulum stress in MDA-MB-231 cells. Furthermore, anacardic acid inhibited TNFα-induced NF-κB reporter activity in MCF-7 cells. Overall, this study uncovered new targets of anacardic acid that may contribute to its anti-proliferative and pro-apoptotic activities against breast cancer.
Berberine
Berberine is an alkaloid derived chiefly from herbs [235]. It has demonstrated potential against various conditions including cancer, diabetes, cardiovascular diseases, infectious diseases, and depression [236,237,238,239,240]. One study examined the therapeutic effects of berberine against nonalcoholic fatty liver disease (NAFLD), which is a common liver disorder [241]. Whether berberine can modulate the expression of mRNAs and lncRNAs in a high-fat diet (HFD)-induced steatotic animal model was examined. Berberine was found to reverse the expression pattern of a list of steatotic liver associated genes including 881 mRNAs and 538 lncRNAs. These observations suggest that berberine may produce a global effect on hepatic gene expression. Berberine was found to regulate a list of genes related to liver metabolism and NAFLD. More specifically, Nrf2 was strongly correlated with the lncRNA MRAK052686 and both of these were down-regulated in the steatotic liver. Furthermore, berberine completely reversed the reduced expression of MRAK052686 and Nrf2. The protein-coding gene Zbtb20, which regulates glucose homeostasis harbor MRAK052686 in its 3′UTR region. Berberine prevented oleic acid-induced steatosis in human Huh7 cells by reversing ZBTB20 expression. Overall, these observations provide new mechanistic insights into the therapeutic effects of berberine against NAFLD.
Quercetin
Quercetin is a dietary flavonoid with potential anti-cancer activities [242, 243]. This flavonoid can also prevent and protect the oxidative stress and β-cell damage induced by streptozotocin in the rat pancreas [244]. The flavonoid has demonstrated potential in the management of arthritis [245], and can inhibit the release of macrophage-derived cytokines and nitric oxide [246].
Rheumatoid arthritis (RA), a chronic disease of the joint, is characterized by the proliferation of cytokines and chemokines producing synoviocytes [247]. RA compromises the expectancy and quality of life and is also a cause of atherosclerosis [248]. The hallmarks of RA are the expansion of fibroblast-like synoviocytes (FLS) and leukocytic infiltration of the synovium [249, 250]. In one study, quercetin decreased the viability and induced apoptosis in RAFLS [251]. Consistent with these observations, an increase in MALAT1 expression was observed after quercetin treatment. The knockdown of MALAT1 enhanced the activation of PI3K/AKT pathway and reduced apoptosis. It is likely that the induction of MALAT1 contributes to quercetin-induced apoptosis in RAFLS. However, more studies are required to support this claim.
Sanguinarine
Sanguinarine is an alkaloid with anti-microbial, anti-fungal, anti-inflammatory, and anti-tumor activities [252]. This alkaloid has demonstrated significant anti-cancer activities against non-small cell lung cancer [253], pancreatic cancer [254], gastric cancer [255], and breast cancer [256]. Conversely, the alkaloid can also produce carcinogenic effects [257]. One study investigated the possible anti-tumor activities and the underlying mechanism of sanguinarine’s action against epithelial ovarian cancer [258]. Sanguinarine suppressed the viability, migration, and invasion, and induced apoptosis in SKOV3 cells. The alkaloid also induced the expression of cancer susceptibility candidate 2 (CASC2) lncRNA, the silencing of which reversed the effects of sanguinarine. While ovarian cancer tissues and cells expressed low levels of CASC2, an increased expression of eukaryotic translation initiation factor 4A3 (EIF4A3) was observed. EIF4A3 could bind to CASC2; the knockdown of EIF4A3 reversed the effects of sanguinarine plus CASC2 silencing. Sanguinarine also markedly reduced the activation of PI3K/AKT/mTOR or NF-κB activation cascades; both these effects were reversed by CASC2 silencing. Furthermore, the effects of sanguinarine plus CASC2 silencing on the modulation of NF-κB and PI3K/AKT/mTOR pathways were reversed by the EIF4A3 knockdown. Overall, these results suggest the anti-tumor activities of sanguinarine against epithelial ovarian cancer cells may be mediated through CASC2–EIF4A3 axis and/or PI3K/AKT/mTOR and NF-κB signaling pathways. Because CASC2 is well-known tumor suppressor with reduced expression in multiple cancer types [259,260,261,262,263,264,265,266], up-regulation of this lncRNA provides a potential avenue for anti-cancer drug development. That sanguinarine can up-regulate CASC2 further support its anti-cancer activities. Whether sanguinarine modulates CASC2 expression in cancer patients remains to be elucidated.
Sulforaphane
Sulforaphane (SFN) is an isothiocyanate group of organosulfur compounds obtained from the cruciferous vegetables [267]. In one study, normal human prostate epithelial cells and SFN-treated prostate cancer cells were subjected to whole-genome RNA-sequencing [268]. SFN modulated the expression of lncRNAs associated with cell-cycle regulation, signal transduction, and metabolism. Notably, the expression of LINC01116, which is an oncogene and overexpressed in several cancer types [268, 269], was significantly suppressed by SFN. The knockdown of LINC01116 significantly decreased the proliferation of prostate cancer cells and up-regulated the expression of genes involved in glycolysis (GAPDH), chromatin structure (H2AFY), and autophagy (MAP1LC3B2). The disruption of LINC01116 using CRISPR/CAS9 method suppressed the colony-forming ability of PC-3 cells by fourfold. The computational analyses indicated that LINC01116 could potentially interact with target genes through ssRNA:dsDNA triplexes. Overall, these results suggest that the modulation of lncRNAs by SFN may contribute to its activities against prostate cancer.
Bharangin
Bharangin is a diterpenoid quinonemethide derived from the roots of a medicinal plant, Pygmacopremna herbacea [270,271,272]. The parts of the plant are known to exhibit a range of biological activities [273,274,275]. The plant extract has also been shown to exhibit activities against breast cancer, leukemia, lymphoma, and multiple myeloma [276,277,278,279]. Recently, our group demonstrated that the diterpenoid can modulate the expression of long non-coding RNAs in breast cancer cells [60]. While the expression of tumor suppressor lncRNAs such as growth arrest specific-5 (GAS-5) and maternally expressed-3 (MEG-3) was induced, the expression of H19 (oncogenic lncRNA) was suppressed by the diterpenoid. We also observed that the diterpenoid suppresses the NF-κB activation induced by okadaic acid in breast cancer cells. It is likely that bharangin exhibits anti-cancer activities by modulating lncRNA expression and abrogating NF-κB activation. We are further exploring the in-depth mechanism for the activities of bharangin against breast cancer.
Conclusions and future prospects
Despite enormous expenses in the health sector, chronic diseases continue to affect millions of people worldwide. As most chronic diseases are caused by chronic inflammation, long-term treatment is required. The US-FDA has approved multiple drugs against chronic diseases such as steroids, statins, and metformin. However, the long-term use of these drugs is associated with numerous side effects. Moreover, these drugs are highly expensive and cannot be afforded by low-income and middle-income people. The phytochemicals derived from spices, fruits, vegetables, cereals, and medicinal plants have been consumed since ancient time. Thus, the safety of these agents is well proven. Moreover, these agents are readily available and produce minimum toxicity. Modern science has provided a molecular basis for the efficacy of these phytochemicals.
As discussed in this review, lncRNAs have emerged as a crucial player in the pathogenesis of chronic diseases with over 18,000 publications listed on PubMed database, most of which appeared during the last decade. The fact that lncRNAs exhibit cell/tissue/tumor-specific expression makes them potential target for the therapeutic development. However, lncRNAs are not very specific in the context of human diseases. For example, MALTA1 is dysregulated during multiple disease conditions such as cancer, cardiovascular diseases, and neurological disorders. During the last 5 years, phytochemicals have been shown to target lncRNAs. In most of the studies, the phytochemicals were found to up-regulate or down-regulate the expression of specific lncRNAs. Although most of the studies have been performed in cancer models, phytochemicals have also been demonstrated to modulate lncRNAs in the other disease models such as rheumatoid arthritis, osteoarthritis, and nonalcoholic fatty liver. In some cases, modifications have been performed to enhance phytochemicals bioavailability and efficacy against lncRNAs. Whether phytochemicals modulate lncRNAs in human subjects remains to be explored. The phytochemicals discussed in this review have been shown to hit several other disease-associated molecular targets. Because most chronic diseases are caused by dysregulation of multiple genes, phytochemicals possess promise against these diseases.
In summary, the discovery of lncRNAs has opened new avenue for the treatment of chronic human diseases. This has also provided a new molecular basis for the pleiotropic activities of phytochemicals. However, the in-depth mechanism by which phytochemicals modulate lncRNAs is lacking. Whether phytochemicals regulate copy number, subcellular localization, and protein-binding capacity of lncRNAs remains to be elucidated. Future studies in this direction would lead to a more deeper understanding of the beneficial effects of phytochemicals against chronic diseases. Future studies should also examine if phytochemicals target lncRNAs in normal cells. Eventually, this would lead to a more effective approach for the disease treatment.
Abbreviations
- 3′UTR:
-
Three prime untranslated region
- AIDS:
-
Acquired immunodeficiency syndrome
- AKT:
-
AKT8 virus oncogene cellular homolog
- ALL:
-
Acute lymphoblastic leukemia
- ANRIL:
-
Antisense non-coding RNA in the INK4 locus
- ASOs:
-
Antisense oligonucleotides
- BCRP:
-
Breast cancer resistance protein
- BIK:
-
Bcl-2-interacting killer
- CAS9:
-
CRISPR-associated protein 9
- CASC2:
-
Cancer susceptibility candidate 2
- CDK6:
-
Cyclin-dependent kinase 6
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- CTR1:
-
Copper transporter 1
- DNA:
-
Deoxyribo nucleic acid
- dsDNA:
-
Double-stranded deoxyribonucleic acid
- EGCG:
-
Epigallocatechin gallate
- EIF4A3:
-
Eukaryotic translation initiation factor 4A3
- EMT:
-
Epithelial-to-mesenchymal transition
- ERα:
-
Estrogen receptor α
- FLS:
-
Fibroblast-like synoviocytes
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GAS5:
-
Growth arrest-specific 5
- GUCY2GP:
-
Guanylate cyclase 2G homolog pseudogene
- H2AFY:
-
H2A histone family member Y
- H2BFXP:
-
H2B histone family member X pseudogene
- H3K4:
-
Histone H3 lysine 4
- HFD:
-
High-fat diet
- HMGCR:
-
3-Hydroxy-3-methylglutaryl-coenzyme A reductase
- HOTAIR:
-
HOX transcript antisense intergenic RNA
- IL-6:
-
Interleukin 6
- INSIG1:
-
Insulin-induced gene 1
- JAK:
-
Janus kinase
- LINC:
-
Long intergenic non-protein-coding RNA
- linc-PINT:
-
Long intergenic non-protein-coding RNA p53 induced transcript
- LncRNA:
-
Long non-coding RNA
- MAP1LC3B2:
-
Microtubule-associated proteins 1A/1B light chain 3B
- MCP-1:
-
Monocyte chemoattractant protein-1
- MDR1/P-gp:
-
Multidrug resistance protein 1/P-glycoprotein 1
- MEG3:
-
Human maternally expressed gene 3
- MIR155HG:
-
MicroRNA155 host gene
- miRNA:
-
MicroRNA
- mRNA:
-
Messenger RNA
- MRP:
-
Multidrug resistance-associated protein
- mTOR:
-
Mammalian target of rapamycin
- NAFLD:
-
Nonalcoholic fatty liver disease
- NEAT1:
-
Nuclear paraspeckle assembly transcript 1
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- Nrf2:
-
Nuclear factor erythroid 2-related factor 2
- NSCLC:
-
Non-small-cell lung carcinoma
- PANDAR:
-
Promoter of CDKN1A antisense DNA damage-activated RNA
- PDK4:
-
Pyruvate dehydrogenase kinase 4
- PI3K:
-
Phosphoinositide 3-kinase
- PUMA:
-
p53 up-regulated modulator of apoptosis
- PVT1:
-
Plasmacytoma variant translocation gene
- RA:
-
Rheumatoid arthritis
- RNA pol II:
-
RNA polymerase II
- RNA:
-
Ribo nucleic acid
- RNAi:
-
RNA interference
- ROR:
-
Regulator of reprogramming
- ST7OT1:
-
ST7 antisense RNA 1
- STAT:
-
Signal transducer and activator of transcription
- TGM2:
-
Transglutaminase 2
- TMEM25:
-
Transmembrane protein 25
- TNF-α:
-
Tumor necrosis factor alpha
- TNM:
-
Tumor nodes and metastasis
- TUG1:
-
Taurine-up-regulated gene 1
- TUSC7:
-
Tumor suppressor candidate 7
- Zbtb20:
-
Zinc finger and BTB domain-containing protein 20
- ZEB1:
-
Zinc-finger E-box-binding homeobox 1
- ZFAS1:
-
ZNFX1 antisense RNA 1
References
Fatica A, Bozzoni I (2014) Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet 15(1):7
Hung T, Chang HY (2010) Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol 7(5):582–585
Nagano T, Fraser P (2011) No-nonsense functions for long noncoding RNAs. Cell 145(2):178–181
Khalil AM, Guttman M, Huarte M, Garber M, Raj A, Morales DR, Thomas K, Presser A, Bernstein BE, Van Oudenaarden A (2009) Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci 106(28):11667–11672
Guttman M, Amit I, Garber M, French C, Lin MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP (2009) Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458(7235):223
Li Y, Wang X (2016) Role of long noncoding RNAs in malignant disease. Mol Med Rep 13(2):1463–1469
Jandura A, Krause HM (2017) The new RNA world: growing evidence for long noncoding RNA functionality. Trends Genet 33(10):665–676
Beaulieu YB, Kleinman CL, Landry-Voyer A-M, Majewski J, Bachand F (2012) Polyadenylation-dependent control of long noncoding RNA expression by the poly (A)-binding protein nuclear 1. PLoS Genet 8(11):e1003078
Guttman M, Rinn JL (2012) Modular regulatory principles of large non-coding RNAs. Nature 482(7385):339
Yin Q-F, Yang L, Zhang Y, Xiang J-F, Wu Y-W, Carmichael GG, Chen L-L (2012) Long noncoding RNAs with snoRNA ends. Mol Cell 48(2):219–230
Nagano T, Mitchell JA, Sanz LA, Pauler FM, Ferguson-Smith AC, Feil R, Fraser P (2008) The air noncoding RNA epigenetically silences transcription by targeting G9a to chromatin. Science 322(5908):1717–1720
Zhao J, Sun BK, Erwin JA, Song J-J, Lee JT (2008) Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science 322(5902):750–756
Martianov I, Ramadass A, Barros AS, Chow N, Akoulitchev A (2007) Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 445(7128):666
Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R (2008) Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature 454(7200):126
Chen L-L, Carmichael GG (2010) Decoding the function of nuclear long non-coding RNAs. Curr Opin Cell Biol 22(3):357–364
Loewer S, Cabili MN, Guttman M, Loh Y-H, Thomas K, Park IH, Garber M, Curran M, Onder T, Agarwal S (2010) Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet 42(12):1113
Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L (2011) lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477(7364):295
Ng SY, Johnson R, Stanton LW (2012) Human long non-coding RNAs promote pluripotency and neuronal differentiation by association with chromatin modifiers and transcription factors. EMBO J 31(3):522–533
Deng K, Wang H, Guo X, Xia J (2015) The cross talk between long, non-coding RNAs and microRNAs in gastric cancer. Acta Biochim Biophys Sin 48(2):111–116
Salmena L, Poliseno L, Tay Y, Kats L, Pandolfi PP (2011) A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 146(3):353–358
Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi Y, Guo J (2014) Long noncoding RNA associated-competing endogenous RNAs in gastric cancer. Sci Rep 4:6088
Zhuang M, Gao W, Xu J, Wang P, Shu Y (2014) The long non-coding RNA H19-derived miR-675 modulates human gastric cancer cell proliferation by targeting tumor suppressor RUNX1. Biochem Biophys Res Commun 448(3):315–322
Leucci E, Vendramin R, Spinazzi M, Laurette P, Fiers M, Wouters J, Radaelli E, Eyckerman S, Leonelli C, Vanderheyden K, Rogiers A, Hermans E, Baatsen P, Aerts S, Amant F, Van Aelst S, van den Oord J, de Strooper B, Davidson I, Lafontaine DL, Gevaert K, Vandesompele J, Mestdagh P, Marine JC (2016) Melanoma addiction to the long non-coding RNA SAMMSON. Nature 531(7595):518–522
Anastasiadou E, Jacob LS, Slack FJ (2018) Non-coding RNA networks in cancer. Nat Rev Cancer 18(1):5–18
Fu X, Ravindranath L, Tran N, Petrovics G, Srivastava S (2006) Regulation of apoptosis by a prostate-specific and prostate cancer-associated noncoding gene, PCGEM1. DNA Cell Biol 25(3):135–141
Kurian L, Aguirre A, Sancho-Martinez I, Benner C, Hishida T, Nguyen TB, Reddy P, Nivet E, Krause MN, Nelles DA (2015) Identification of novel long non-coding RNAs underlying vertebrate cardiovascular development. Circulation 131(14):1278–1290
Casero D, Sandoval S, Seet CS, Scholes J, Zhu Y, Ha VL, Luong A, Parekh C, Crooks GM (2015) Long non-coding RNA profiling of human lymphoid progenitor cells reveals transcriptional divergence of B cell and T cell lineages. Nat Immunol 16(12):1282
Sánchez Y, Huarte M (2013) Long non-coding RNAs: challenges for diagnosis and therapies. Nucleic Acid Ther 23(1):15–20
Leucci E (2018) Cancer development and therapy resistance: spotlights on the dark side of the genome. Pharmacol Ther 189:22–30
Chandra Gupta S, Nandan Tripathi Y (2017) Potential of long non-coding RNAs in cancer patients: from biomarkers to therapeutic targets. Int J Cancer 140(9):1955–1967
Mizrahi A, Czerniak A, Levy T, Amiur S, Gallula J, Matouk I, Abu-lail R, Sorin V, Birman T, de Groot N (2009) Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J Transl Med 7(1):69
Bishayee A, Sethi G (2016) Bioactive natural products in cancer prevention and therapy: progress and promise. Semin Cancer Biol 40–41:1–3
Sethi G, Tergaonkar V (2009) Potential pharmacological control of the NF-kappaB pathway. Trends Pharmacol Sci 30(6):313–321
Reddy L, Odhav B, Bhoola K (2003) Natural products for cancer prevention: a global perspective. Pharmacol Ther 99(1):1–13
Block G, Patterson B, Subar A (1992) Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer 18(1):1–29
Benetou V, Orfanos P, Lagiou P, Trichopoulos D, Boffetta P, Trichopoulou A (2008) Vegetables and fruits in relation to cancer risk: evidence from the Greek EPIC cohort study. Cancer Epidemiol Prev Biomark 17(2):387–392
Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC (2008) Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study. Int J Cancer 122(10):2330–2336
Steinmetz KA, Potter JD (1996) Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc 96(10):1027–1039
Gupta SC, Kim JH, Prasad S, Aggarwal BB (2010) Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 29(3):405–434
Deorukhkar A, Krishnan S, Sethi G, Aggarwal BB (2007) Back to basics: how natural products can provide the basis for new therapeutics. Expert Opin Investig Drugs 16(11):1753–1773
Yang SF, Weng CJ, Sethi G, Hu DN (2013) Natural bioactives and phytochemicals serve in cancer treatment and prevention. Evid Based Complement Altern Med eCAM 2013:698190
Tang CH, Sethi G, Kuo PL (2014) Novel medicines and strategies in cancer treatment and prevention. Biomed Res Int 2014:474078
Hsieh YS, Yang SF, Sethi G, Hu DN (2015) Natural bioactives in cancer treatment and prevention. Biomed Res Int 2015:182835
Yarla NS, Bishayee A, Sethi G, Reddanna P, Kalle AM, Dhananjaya BL, Dowluru KS, Chintala R, Duddukuri GR (2016) Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol 40–41:48–81
Hasanpourghadi M, Looi CY, Pandurangan AK, Sethi G, Wong WF, Mustafa MR (2017) Phytometabolites targeting the warburg effect in cancer cells: a mechanistic review. Curr Drug Targets 18(9):1086–1094
Shanmugam MK, Warrier S, Kumar AP, Sethi G, Arfuso F (2017) Potential role of natural compounds as anti-angiogenic agents in cancer. Curr Vasc Pharmacol 15(6):503–519
Shanmugam MK, Kannaiyan R, Sethi G (2011) Targeting cell signaling and apoptotic pathways by dietary agents: role in the prevention and treatment of cancer. Nutr Cancer 63(2):161–173
Aggarwal BB, Sethi G, Baladandayuthapani V, Krishnan S, Shishodia S (2007) Targeting cell signaling pathways for drug discovery: an old lock needs a new key. J Cell Biochem 102(3):580–592
Jung YY, Hwang ST, Sethi G, Fan L, Arfuso F, Ahn KS (2018) Potential anti-inflammatory and anti-cancer properties of farnesol. Molecules (Basel, Switzerland) 23(11):E2827
Merarchi M, Sethi G, Fan L, Mishra S, Arfuso F, Ahn KS (2018) Molecular targets modulated by fangchinoline in tumor cells and preclinical models. Molecules (Basel, Switzerland) 23(10):E2538
Sethi G, Shanmugam MK, Warrier S, Merarchi M, Arfuso F, Kumar AP, Bishayee A (2018) Pro-apoptotic and anti-cancer properties of diosgenin: a comprehensive and critical review. Nutrients 10(5):E645
Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS (2017) The role of resveratrol in cancer therapy. Int J Mol Sci 18(12):E2589
Tewari D, Nabavi SF, Nabavi SM, Sureda A, Farooqi AA, Atanasov AG, Vacca RA, Sethi G, Bishayee A (2018) Targeting activator protein 1 signaling pathway by bioactive natural agents: possible therapeutic strategy for cancer prevention and intervention. Pharmacol Res 128:366–375
Shanmugam MK, Lee JH, Chai EZ, Kanchi MM, Kar S, Arfuso F, Dharmarajan A, Kumar AP, Ramar PS, Looi CY, Mustafa MR, Tergaonkar V, Bishayee A, Ahn KS, Sethi G (2016) Cancer prevention and therapy through the modulation of transcription factors by bioactive natural compounds. Semin Cancer Biol 40–41:35–47
Shanmugam MK, Nguyen AH, Kumar AP, Tan BK, Sethi G (2012) Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: potential role in prevention and therapy of cancer. Cancer Lett 320(2):158–170
Shrimali D, Shanmugam MK, Kumar AP, Zhang J, Tan BK, Ahn KS, Sethi G (2013) Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett 341(2):139–149
Gupta SC, Patchva S, Koh W, Aggarwal BB (2012) Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39(3):283–299
Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB (2011) Multitargeting by curcumin as revealed by molecular interaction studies. Nat Prod Rep 28(12):1937–1955
Sun Y, Pan J, Zhang N, Wei W, Yu S, Ai L (2017) Knockdown of long non-coding RNA H19 inhibits multiple myeloma cell growth via NF-κB pathway. Sci Rep 7(1):18079
Awasthee N, Rai V, Verma SS, Sajin Francis K, Nair MS, Gupta SC (2018) Anti-cancer activities of Bharangin against breast cancer: evidence for the role of NF-kappaB and lncRNAs. Biochim Biophys Acta Gen Subj 1862 12:2738–2749
Zhang L, Yang F, J-H Yuan, S-X Yuan, W-p Zhou, Huo X-s XuD, H-s Bi, Wang F, S-h Sun (2012) Epigenetic activation of the MiR-200 family contributes to H19-mediated metastasis suppression in hepatocellular carcinoma. Carcinogenesis 34(3):577–586
Hibi K, Nakamura H, Hirai A, Fujikake Y, Kasai Y, Akiyama S, Ito K, Takagi H (1996) Loss of H19 imprinting in esophageal cancer. Cancer Res 56(3):480–482
Wang S-H, Ma F, Tang Z-h WuX-C, Cai Q, Zhang M-D, Weng M-Z, Zhou D, Wang J-D, Quan Z-W (2016) Long non-coding RNA H19 regulates FOXM1 expression by competitively binding endogenous miR-342-3p in gallbladder cancer. J Exp Clin Cancer Res 35(1):160
Kondo M, Suzuki H, Ueda R, Osada H, Takagi K, Takahashi T (1995) Frequent loss of imprinting of the H19 gene is often associated with its overexpression in human lung cancers. Oncogene 10(6):1193–1198
Pan J (2017) LncRNA H19 promotes atherosclerosis by regulating MAPK and NF-kB signaling pathway. Eur Rev Med Pharmacol Sci 21(2):322–328
Novak Kujundžić R, Grbeša I, Ivkić M, Katdare M, Gall-Trošelj K (2008) Curcumin downregulates H19 gene transcription in tumor cells. J Cell Biochem 104(5):1781–1792
Liu G, Xiang T, Wu QF, Wang WX (2016) Curcumin suppresses the proliferation of gastric cancer cells by downregulating H19. Oncol Lett 12(6):5156–5162
H-x Zhan, Wang Y, Li C, J-w Xu, Zhou B, J-k Zhu, H-f Han, Wang L, Wang Y-S, Hu S-Y (2016) LincRNA-ROR promotes invasion, metastasis and tumor growth in pancreatic cancer through activating ZEB1 pathway. Cancer Lett 374(2):261–271
Hou P, Zhao Y, Li Z, Yao R, Ma M, Gao Y, Zhao L, Zhang Y, Huang B, Lu J (2014) LincRNA-ROR induces epithelial-to-mesenchymal transition and contributes to breast cancer tumorigenesis and metastasis. Cell Death Dis 5(6):e1287
Wang S-H, Zhang M-D, Wu X-C, Weng M-Z, Zhou D, Quan Z-W (2016) Overexpression of LncRNA-ROR predicts a poor outcome in gallbladder cancer patients and promotes the tumor cells proliferation, migration, and invasion. Tumor Biol 37(9):12867–12875
Li L, Gu M, You B, Shi S, Shan Y, Bao L, You Y (2016) Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci 107(9):1215–1222
Chen S, Zhu J, Wang F, Guan Z, Ge Y, Yang X, Cai J (2017) LncRNAs and their role in cancer stem cells. Oncotarget 8(66):110685
Liu T, Chi H, Chen J, Chen C, Huang Y, Xi H, Xue J, Si Y (2017) Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene 631:29–38
Garitano-Trojaola A, San José-Enériz E, Ezponda T, Unfried JP, Carrasco-León A, Razquin N, Barriocanal M, Vilas-Zornoza A, Sangro B, Segura V (2018) Deregulation of linc-PINT in acute lymphoblastic leukemia is implicated in abnormal proliferation of leukemic cells. Oncotarget 9(16):12842
Marín-Béjar O, Mas AM, González J, Martinez D, Athie A, Morales X, Galduroz M, Raimondi I, Grossi E, Guo S (2017) The human lncRNA LINC-PINT inhibits tumor cell invasion through a highly conserved sequence element. Genome Biol 18(1):202
Pickard M, Williams G (2015) Molecular and cellular mechanisms of action of tumour suppressor GAS5 LncRNA. Genes 6(3):484–499
Yin D, He X, Zhang E, Kong R, De W, Zhang Z (2014) Long noncoding RNA GAS5 affects cell proliferation and predicts a poor prognosis in patients with colorectal cancer. Med Oncol 31(11):253
Esmatabadi MJD, Motamedrad M, Sadeghizadeh M (2018) Down-regulation of lncRNA, GAS5 decreases chemotherapeutic effect of dendrosomal curcumin (DNC) in breast cancer cells. Phytomedicine 42:56–65
Hung T, Wang Y, Lin MF, Koegel AK, Kotake Y, Grant GD, Horlings HM, Shah N, Umbricht C, Wang P (2011) Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 43(7):621
Zhan Y, Lin J, Liu Y, Chen M, Chen X, Zhuang C, Liu L, Xu W, Chen Z, He A (2016) Up-regulation of long non-coding RNA PANDAR is associated with poor prognosis and promotes tumorigenesis in bladder cancer. J Exp Clin Cancer Res 35(1):83
Ma P, Xu T, Huang M, Shu Y (2016) Increased expression of LncRNA PANDAR predicts a poor prognosis in gastric cancer. Biomed Pharmacother 78:172–176
Peng W, Fan H (2015) Long non-coding RNA PANDAR correlates with poor prognosis and promotes tumorigenesis in hepatocellular carcinoma. Biomed Pharmacother 72:113–118
Xu Y, Jiang X, Cui Y (2017) Upregulated long noncoding RNA PANDAR predicts an unfavorable prognosis and promotes tumorigenesis in cholangiocarcinoma. OncoTargets Ther 10:2873
Lu M, Liu Z, Li B, Wang G, Li D, Zhu Y (2017) The high expression of long non-coding RNA PANDAR indicates a poor prognosis for colorectal cancer and promotes metastasis by EMT pathway. J Cancer Res Clin Oncol 143(1):71–81
Chen T, Yang P, Wang H, He Z-Y (2017) Silence of long noncoding RNA PANDAR switches low-dose curcumin-induced senescence to apoptosis in colorectal cancer cells. OncoTargets Ther 10:483
Li X, Wu Z, Mei Q, Guo M, Fu X, Han W (2013) Long non-coding RNA HOTAIR, a driver of malignancy, predicts negative prognosis and exhibits oncogenic activity in oesophageal squamous cell carcinoma. Br J Cancer 109(8):2266
Pei C-S, Wu H-Y, Fan F-T, Wu Y, Shen C-S, Pan L-Q (2014) Influence of curcumin on HOTAIR-mediated migration of human renal cell carcinoma cells. Asian Pac J Cancer Prev APJCP 15(10):4239–4243
Babaei E, Sadeghizadeh M, Hassan ZM, Feizi MAH, Najafi F, Hashemi SM (2012) Dendrosomal curcumin significantly suppresses cancer cell proliferation in vitro and in vivo. Int Immunopharmacol 12(1):226–234
Mirgani MT, Isacchi B, Sadeghizadeh M, Marra F, Bilia AR, Mowla SJ, Najafi F, Babaei E (2014) Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int J Nanomed 9:403
Zamani M, Sadeghizadeh M, Behmanesh M, Najafi F (2015) Dendrosomal curcumin increases expression of the long non-coding RNA gene MEG3 via up-regulation of epi-miRs in hepatocellular cancer. Phytomedicine 22(10):961–967
Zhou Y, Zhang X, Klibanski A (2012) MEG3 non-coding RNA: a tumor suppressor. J Mol Endocrinol 48(3):R45–R53
Rajeshkumar N, Rasheed ZA, García-García E, López-Ríos F, Fujiwara K, Matsui WH, Hidalgo M (2010) A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol Cancer Ther 9(9):2582–2592
Huang C, Yu W, Wang Q, Cui H, Wang Y, Zhang L, Han F, Huang T (2015) Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med 106(3):143–149
Bardeesy N, DePinho RA (2002) Pancreatic cancer biology and genetics. Nat Rev Cancer 2(12):897
Zhou Q, Chen J, Feng J, Wang J (2016) Long noncoding RNA PVT1 modulates thyroid cancer cell proliferation by recruiting EZH2 and regulating thyroid-stimulating hormone receptor (TSHR). Tumor Biol 37(3):3105–3113
Wang D, Ding L, Wang L, Zhao Y, Sun Z, Karnes RJ, Zhang J, Huang H (2015) LncRNA MALAT1 enhances oncogenic activities of EZH2 in castration-resistant prostate cancer. Oncotarget 6(38):41045
Zhang K, Sun X, Zhou X, Han L, Chen L, Shi Z, Zhang A, Ye M, Wang Q, Liu C (2015) Long non-coding RNA HOTAIR promotes glioblastoma cell cycle progression in an EZH2 dependent manner. Oncotarget 6(1):537
You L, Chang D, Du H-Z, Zhao Y-P (2011) Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem Biophys Res Commun 407(1):1–6
Yoshida K, Toden S, Ravindranathan P, Han H, Goel A (2017) Curcumin sensitizes pancreatic cancer cells to gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1 expression. Carcinogenesis 38(10):1036–1046
Tseng Y-Y, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell TC (2014) PVT1 dependence in cancer with MYC copy-number increase. Nature 512(7512):82
Avan A, Crea F, Paolicchi E, Funel N, Galvani E, Marquez VE, Honeywell RJ, Danesi R, Peters GJ, Giovannetti E (2012) Molecular mechanisms involved in the synergistic interaction of the EZH2 inhibitor 3-deazaneplanocin A (DZNeP) with gemcitabine in pancreatic cancer cells. Mol Cancer Ther 11(8):1735–1746
Hong SP, Wen J, Bang S, Park S, Song SY (2009) CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer 125(10):2323–2331
Ottinger S, Klöppel A, Rausch V, Liu L, Kallifatidis G, Gross W, Gebhard MM, Brümmer F, Herr I (2012) Targeting of pancreatic and prostate cancer stem cell characteristics by Crambe crambe marine sponge extract. Int J Cancer 130(7):1671–1681
Sharma N, Nanta R, Sharma J, Gunewardena S, Singh KP, Shankar S, Srivastava RK (2015) PI3K/AKT/mTOR and sonic hedgehog pathways cooperate together to inhibit human pancreatic cancer stem cell characteristics and tumor growth. Oncotarget 6(31):32039
Xia P, Xu X-Y (2015) PI3K/Akt/mTOR signaling pathway in cancer stem cells: from basic research to clinical application. Am J Cancer Res 5(5):1602
Takahashi K, Yan IK, Kogure T, Haga H, Patel T (2014) Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio 4(1):458–467
Nawaz M, Fatima F, Nazarenko I, Ekström K, Murtaza I, Anees M, Sultan A, Neder L, Camussi G, Valadi H (2016) Extracellular vesicles in ovarian cancer: applications to tumor biology, immunotherapy and biomarker discovery. Expert Rev Proteomics 13(4):395–409
Yeung CLA, Tsuruga T, Yeung T-L, Kwan S-Y, Leung CS, Li Y, Lu ES, Kwan K, Wong K-K, Schmandt R (2016) Exosomal transfer of stroma-derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun 7:11150
Zhang J, Liu J, Xu X, Li L (2017) Curcumin suppresses cisplatin resistance development partly via modulating extracellular vesicle-mediated transfer of MEG3 and miR-214 in ovarian cancer. Cancer Chemother Pharmacol 79(3):479–487
Wang Q, Fan H, Liu Y, Yin Z, Cai H, Liu J, Wang Z, Shao M, Sun X, Diao J (2014) Curcumin enhances the radiosensitivity in nasopharyngeal carcinoma cells involving the reversal of differentially expressed long non-coding RNAs. Int J Oncol 44(3):858–864
Gupta SC, Kannappan R, Reuter S, Kim JH, Aggarwal BB (2011) Chemosensitization of tumors by resveratrol. Ann N Y Acad Sci 1215(1):150–160
Borriello A, Bencivenga D, Caldarelli I, Tramontano A, Borgia A, Zappia V, Della Ragione F (2014) Resveratrol: from basic studies to bedside. In: Advances in nutrition and cancer. Springer, New York, pp 167–184
Kulkarni SS, Cantó C (2015) The molecular targets of resveratrol. Biochim Biophys Acta (BBA) Mol Basis Dis 1852(6):1114–1123
Britton RG, Kovoor C, Brown K (2015) Direct molecular targets of resveratrol: identifying key interactions to unlock complex mechanisms. Ann N Y Acad Sci 1348(1):124–133
Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P (2006) Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127(6):1109–1122
Al Aameri RF, Sheth S, Alanisi EM, Borse V, Mukherjea D, Rybak LP, Ramkumar V (2017) Tonic suppression of PCAT29 by the IL-6 signaling pathway in prostate cancer: reversal by resveratrol. PLoS One 12(5):e0177198
Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, Laxman B, Asangani IA, Grasso CS, Kominsky HD (2011) Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 29(8):742
Malik R, Patel L, Prensner JR, Shi Y, Iyer MK, Subramaniyan S, Carley A, Niknafs YS, Sahu A, Han S (2014) The lncRNA PCAT29 inhibits oncogenic phenotypes in prostate cancer. Mol Cancer Res 12(8):1081–1087
Yang Y, Xu H, Huang W, Ding M, Xiao J, Yang D, Li H, Liu XY, Chu L (2015) Targeting lung cancer stem-like cells with TRAIL gene armed oncolytic adenovirus. J Cell Mol Med 19(5):915–923
Gutschner T, Hämmerle M, Diederichs S (2013) MALAT1—a paradigm for long noncoding RNA function in cancer. J Mol Med 91(7):791–801
Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E (2003) MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 22(39):8031
Zhang B, Arun G, Mao YS, Lazar Z, Hung G, Bhattacharjee G, Xiao X, Booth CJ, Wu J, Zhang C (2012) The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep 2(1):111–123
Nakagawa S, Ip JY, Shioi G, Tripathi V, Zong X, Hirose T, Prasanth KV (2012) Malat1 is not an essential component of nuclear speckles in mice. RNA (New York, NY) 18(8):1487–1499
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, Sun J, Cai J, Qin J, Ren J (2013) Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One 8(11):e78700
Liu Q, Sun S, Yu W, Jiang J, Zhuo F, Qiu G, Xu S, Jiang X (2015) Altered expression of long non-coding RNAs during genotoxic stress-induced cell death in human glioma cells. J Neurooncol 122(2):283–292
Fumoleau P, Seidman AD, Trudeau ME, Chevallier B, Huinink WTB (1997) Docetaxel: a new active agent in the therapy of metastatic breast cancer. Expert Opin Investig Drugs 6(12):1853–1865
de Weger VA, Beijnen JH, Schellens JH (2014) Cellular and clinical pharmacology of the taxanes docetaxel and paclitaxel–a review. Anticancer Drugs 25(5):488–494
Wani MC, Horwitz SB (2014) Nature as a Remarkable Chemist: a personal story of the discovery and development of Taxol®. Anticancer Drugs 25(5):482
Ding Y, Duan K, Chen S (2017) Low expression of lncRNA-GAS5 promotes epithelial–mesenchymal transition of breast cancer cells in vitro. J South Med Univ 37(11):1427–1435
Si X, Zang R, Zhang E, Liu Y, Shi X, Zhang E, Shao L, Li A, Yang N, Han X (2016) LncRNA H19 confers chemoresistance in ERα-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget 7(49):81452
Li Y, Wang Y, Wang H, Zhang L, Ding Y, Chen S, Yang Q, Chen C (2017) Effects of lncRNA RP11-770J1. 3 and TMEM25 expression on paclitaxel resistance in human breast cancer cells. J Zhejiang Univ Med Sci 46(4):364–370
Pan Y, Pan Y, Cheng Y, Yang F, Yao Z, Wang O (2018) Knockdown of LncRNA MAPT-AS1 inhibits proliferation and migration and sensitizes cancer cells to paclitaxel by regulating MAPT expression in ER-negative breast cancers. Cell Biosci 8(1):7
Guo Z, Wang Y, Zhao Y, Jin Y, An L, Wu B, Liu Z, Chen X, Zhou H, Wang H (2017) Genetic polymorphisms of long non-coding RNA GAS5 predict platinum-based concurrent chemoradiotherapy response in nasopharyngeal carcinoma patients. Oncotarget 8(37):62286
Bida O, Gidoni M, Ideses D, Efroni S, Ginsberg D (2015) A novel mitosis-associated lncRNA, MA-linc1, is required for cell cycle progression and sensitizes cancer cells to paclitaxel. Oncotarget 6(29):27880
Xu J, Wu J, Fu C, Teng F, Liu S, Dai C, Shen R, Jia X (2018) Multidrug resistant lncRNA profile in chemotherapeutic sensitive and resistant ovarian cancer cells. J Cell Physiol 233(6):5034–5043
Fang J, Qiao F, Tu J, Xu J, Ding F, Liu Y, Akuo BA, Hu J, Shao S (2017) High expression of long non-coding RNA NEAT1 indicates poor prognosis of human cancer. Oncotarget 8(28):45918
Zeng C, Xu Y, Xu L, Yu X, Cheng J, Yang L, Chen S, Li Y (2014) Inhibition of long non-coding RNA NEAT1 impairs myeloid differentiation in acute promyelocytic leukemia cells. BMC Cancer 14(1):693
An J, Lv W, Zhang Y (2017) LncRNA NEAT1 contributes to paclitaxel resistance of ovarian cancer cells by regulating ZEB1 expression via miR-194. OncoTargets Ther 10:5377
Tian X, Zhang H, Zhang B, Zhao J, Li T, Zhao Y (2017) Microarray expression profile of long non-coding RNAs in paclitaxel-resistant human lung adenocarcinoma cells. Oncol Rep 38(1):293–300
Liu F, Gao H, Li S, Ni X, Zhu Z (2017) Long non-coding RNA ZFAS1 correlates with clinical progression and prognosis in cancer patients. Oncotarget 8(37):61561
Hansji H, Leung EY, Baguley BC, Finlay GJ, Cameron-Smith D, Figueiredo VC, Askarian-Amiri ME (2016) ZFAS1: a long noncoding RNA associated with ribosomes in breast cancer cells. Biol Direct 11(1):62
Zhang Z, Weaver DL, Olsen D, Peng Z, Ashikaga T, Evans MF (2016) Long non-coding RNA chromogenic in situ hybridisation signal pattern correlation with breast tumour pathology. J Clin Pathol 69(1):76–81
Askarian-Amiri ME, Crawford J, French JD, Smart CE, Smith MA, Clark MB, Ru K, Mercer TR, Thompson ER, Lakhani SR, Vargas AC, Campbell IG, Brown MA, Dinger ME, Mattick JS (2011) SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA (New York, NY) 17(5):878–891
Gao K, Ji Z, She K, Yang Q, Shao L (2017) Long non-coding RNA ZFAS1 is an unfavourable prognostic factor and promotes glioma cell progression by activation of the Notch signaling pathway. Biomed Pharmacother 87:555–560
Nieto M, Huang R, Jackson R, Thiery J (2016) EMT. Cell 166(2016):21–45
Thorenoor N, Faltejskova-Vychytilova P, Hombach S, Mlcochova J, Kretz M, Svoboda M, Slaby O (2016) Long non-coding RNA ZFAS1 interacts with CDK1 and is involved in p53-dependent cell cycle control and apoptosis in colorectal cancer. Oncotarget 7(1):622
Wei Y-H, Fu Y, Luo H-J, Li R, Li H-Y, Zhang Z, Zhu Y-H, Gao Y, Liu X-L (2017) Higher expression of ZFAS1 is associated with poor prognosis in malignant melanoma and promotes cell proliferation and invasion. Int J Clin Exp Pathol 10(4):4640–4646
Ding J, Li D, Gong M, Wang J, Huang X, Wu T, Wang C (2014) Expression and clinical significance of the long non-coding RNA PVT1 in human gastric cancer. OncoTargets Ther 7:1625
Shang C, Lang B, Ao CN, Meng L (2017) Long non-coding RNA tumor suppressor candidate 7 advances chemotherapy sensitivity of endometrial carcinoma through targeted silencing of miR-23b. Tumor Biol 39(6):1010428317707883
Chen H, Xin Y, Zhou L, J-m Huang, Tao L, Cheng L, Tian J (2014) Cisplatin and paclitaxel target significant long noncoding RNAs in laryngeal squamous cell carcinoma. Med Oncol 31(11):246
Shen C-J, Cheng Y-M, Wang C-L (2017) LncRNA PVT1 epigenetically silences miR-195 and modulates EMT and chemoresistance in cervical cancer cells. J Drug Target 25(7):637–644
Wang Q, Zhang W, Hao S (2017) LncRNA CCAT1 modulates the sensitivity of paclitaxel in nasopharynx cancers cells via miR-181a/CPEB2 axis. Cell Cycle 16(8):795–801
Zhu Q-N, Wang G, Guo Y, Peng Y, Zhang R, Deng J-L, Li Z-X, Zhu Y-S (2017) LncRNA H19 is a major mediator of doxorubicin chemoresistance in breast cancer cells through a cullin4A-MDR1 pathway. Oncotarget 8(54):91990
Li W, Li H, Zhang L, Hu M, Li F, Deng J, An M, Wu S, Ma R, Lu J (2017) LINC00672 contributes p53-mediated gene suppression and promotes endometrial cancer chemosensitivity. J Biol Chem 292(14):5801–5813
Ren S, Li G, Liu C, Cai T, Su Z, Wei M, She L, Tian Y, Qiu Y, Zhang X (2016) Next generation deep sequencing identified a novel lncRNA n375709 associated with paclitaxel resistance in nasopharyngeal carcinoma. Oncol Rep 36(4):1861–1867
Jiang Y-Z, Liu Y-R, Xu X-E, Jin X, Hu X, Yu K-D, Shao Z-M (2016) Transcriptome analysis of triple-negative breast cancer reveals an integrated mRNA-lncRNA signature with predictive and prognostic value. Cancer Res 76(8):2105–2114
Chen Y-M, Liu Y, Wei H-Y, Lv K-Z, Fu P (2016) Linc-ROR induces epithelial–mesenchymal transition and contributes to drug resistance and invasion of breast cancer cells. Tumor Biol 37(8):10861–10870
Ren K, Xu R, Huang J, Zhao J, Shi W (2017) Knockdown of long non-coding RNA KCNQ1OT1 depressed chemoresistance to paclitaxel in lung adenocarcinoma. Cancer Chemother Pharmacol 80(2):243–250
Zou S, Du X, Lin H, Wang P, Li M (2018) Paclitaxel inhibits the progression of cervical cancer by inhibiting autophagy via lncRNARP11-381N20. 2. Eur Rev Med Pharmacol Sci 22(10):3010–3017
Wu D, Wang J, Pae M, Meydani SN (2012) Green tea EGCG, T cells, and T cell-mediated autoimmune diseases. Mol Aspects Med 33(1):107–118
Riegsecker S, Wiczynski D, Kaplan MJ, Ahmed S (2013) Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci 93(8):307–312
Suganuma M, Okabe S, Sueoka N, Sueoka E, Matsuyama S, Imai K, Nakachi K, Fujiki H (1999) Green tea and cancer chemoprevention. Mutat Res Fundam Mol Mech Mutagen 428(1):339–344
Kuroda Y, Hara Y (1999) Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res Rev Mutat Res 436(1):69–97
Stoner GD, Mukhtar H (1995) Polyphenols as cancer chemopreventive agents. J Cell Biochem 59(S22):169–180
Tran PL, Kim S-A, Choi HS, Yoon J-H, Ahn S-G (2010) Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer 10(1):276
Yang CS (1997) Inhibition of carcinogenesis by tea. Nature 389(6647):134
Dong Z, W-y Ma, Huang C, Yang CS (1997) Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols,(–)-epigallocatechin gallate, and theaflavins. Cancer Res 57(19):4414–4419
Momose Y, Maeda-Yamamoto M, Nabetani H (2016) Systematic review of green tea epigallocatechin gallate in reducing low-density lipoprotein cholesterol levels of humans. Int J Food Sci Nutr 67(6):606–613
Mukhtar H, Ahmad N (1999) Mechanism of cancer chemopreventive activity of green tea. Proc Soc Exp Biol Med 220(4):234–238
Rahmani AH, Allemailem KS, Aly SM, Khan MA (2015) Implications of green tea and its constituents in the prevention of cancer via the modulation of cell signalling pathway. Biomed Res Int 2015:925640
Liu G, Zheng X, Xu Y, Lu J, Chen J, Huang X (2015) Long non-coding RNAs expression profile in HepG2 cells reveals the potential role of long non-coding RNAs in the cholesterol metabolism. Chin Med J 128(1):91
Gridelli C, Sacco PC (2016) Novel cytotoxic drugs in advanced nonsmall cell lung cancer. Curr Opin Oncol 28(2):110–114
Larson CA, Blair BG, Safaei R, Howell SB (2008) The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol 75(2):324–330
Tsai C-Y, Larson CA, Safaei R, Howell SB (2014) Molecular modulation of the copper and cisplatin transport function of CTR1 and its interaction with IRS-4. Biochem Pharmacol 90(4):379–387
Kalayda GV, Wagner CH, Jaehde U (2012) Relevance of copper transporter 1 for cisplatin resistance in human ovarian carcinoma cells. J Inorg Biochem 116:1–10
Kim ES, Tang X, Peterson DR, Kilari D, Chow C-W, Fujimoto J, Kalhor N, Swisher SG, Stewart DJ, Wistuba II (2014) Copper transporter CTR1 expression and tissue platinum concentration in non-small cell lung cancer. Lung Cancer 85(1):88–93
Wang X, Jiang P, Wang P, Yang CS, Wang X, Feng Q (2015) EGCG enhances cisplatin sensitivity by regulating expression of the copper and cisplatin influx transporter CTR1 in ovary cancer. PLoS One 10(4):e0125402
Liss MA, Schlicht M, Kahler A, Fitzgerald R, Thomassi T, Degueme A, Hessner M, Datta MW (2010) Characterization of soy-based changes in Wnt-frizzled signaling in prostate cancer. Cancer Genomics Proteomics 7(5):245–252
Li Y, Sarkar FH (2002) Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res 8(7):2369–2377
Kim E-K, Kwon K-B, Song M-Y, Seo S-W, Park S-J, Ka S-O, Na L, Kim K-A, Ryu D-G, So H-S (2007) Genistein protects pancreatic β cells against cytokine-mediated toxicity. Mol Cell Endocrinol 278(1–2):18–28
Yan G-R, Yin X-F, Xiao C-L, Tan Z-L, Xu S-H, He Q-Y (2011) Identification of novel signaling components in genistein-regulated signaling pathways by quantitative phosphoproteomics. J Proteomics 75(2):695–707
Hu X-J, Xie M-Y, Kluxen FM, Diel P (2014) Genistein modulates the anti-tumor activity of cisplatin in MCF-7 breast and HT-29 colon cancer cells. Arch Toxicol 88(3):625–635
Pons DG, Nadal-Serrano M, Blanquer-Rossello MM, Sastre-Serra J, Oliver J, Roca P (2014) Genistein modulates proliferation and mitochondrial functionality in breast cancer cells depending on ERalpha/ERbeta ratio. J Cell Biochem 115(5):949–958
Hwang YW, Kim SY, Jee SH, Kim YN, Nam CM (2009) Soy food consumption and risk of prostate cancer: a meta-analysis of observational studies. Nutr Cancer 61(5):598–606
De Souza PL, Russell PJ, Kearsley JH, Howes LG (2010) Clinical pharmacology of isoflavones and its relevance for potential prevention of prostate cancer. Nutr Rev 68(9):542–555
Chen J, Liu L, Hou R, Shao Z, Wu Y, Chen X, Zhou L (2011) Calycosin promotes proliferation of estrogen receptor-positive cells via estrogen receptors and ERK1/2 activation in vitro and in vivo. Cancer Lett 308(2):144–151
Tian J, Duan Y, Bei C, Chen J (2013) Calycosin induces apoptosis by upregulation of RASD1 in human breast cancer cells MCF-7. Horm Metab Res 45(08):593–598
Chen J, Lin C, Yong W, Ye Y, Huang Z (2015) Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol Biochem 35(2):722–728
Chiyomaru T, Fukuhara S, Saini S, Majid S, Deng G, Shahryary V, Chang I, Tanaka Y, Enokida H, Nakagawa M (2014) Long noncoding RNA HOTAIR is targeted and regulated by microRNA-141 in renal carcinoma cells. J Biol Chem 289:12550–12565
Neves R, Scheel C, Weinhold S, Honisch E, Iwaniuk KM, Trompeter H-I, Niederacher D, Wernet P, Santourlidis S, Uhrberg M (2010) Role of DNA methylation in miR-200c/141 cluster silencing in invasive breast cancer cells. BMC Res Notes 3(1):219
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, Tsai M-C, Hung T, Argani P, Rinn JL (2010) Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 464(7291):1071
Kogo R, Shimamura T, Mimori K, Kawahara K, Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S (2011) Long non-coding RNA HOTAIR regulates Polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res 71(20):6320–6326
Kim K, Jutooru I, Chadalapaka G, Johnson G, Frank J, Burghardt R, Kim S, Safe S (2013) HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene 32(13):1616
Chiyomaru T, Yamamura S, Fukuhara S, Yoshino H, Kinoshita T, Majid S, Saini S, Chang I, Tanaka Y, Enokida H (2013) Genistein inhibits prostate cancer cell growth by targeting miR-34a and oncogenic HOTAIR. PLoS One 8(8):e70372
Zeng J, Sun Y, Wu K, Li L, Zhang G, Yang Z, Wang Z, Zhang D, Xue Y, Chen Y (2011) Chemopreventive and chemotherapeutic effects of intravesical silibinin against bladder cancer by acting on mitochondria. Mol Cancer Ther 10(1):104–116
Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang T, Zhang L, Chen Y, Gao Y, Wang B (2013) Silibinin inhibits β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis via dual-blocking epithelial–mesenchymal transition and stemness. Cell Signal 25(12):2625–2633
Bosch-Barrera J, Sais E, Cañete N, Marruecos J, Cuyàs E, Izquierdo A, Porta R, Haro M, Brunet J, Pedraza S (2016) Response of brain metastasis from lung cancer patients to an oral nutraceutical product containing silibinin. Oncotarget 7(22):32006
Lu W, Lin C, King TD, Chen H, Reynolds RC, Li Y (2012) Silibinin inhibits Wnt/β-catenin signaling by suppressing Wnt co-receptor LRP6 expression in human prostate and breast cancer cells. Cell Signal 24(12):2291–2296
Kim S, Jeon M, Lee J, Han J, Oh SJ, Jung T, Nam SJ, Kil WH, Lee JE (2014) Induction of fibronectin in response to epidermal growth factor is suppressed by silibinin through the inhibition of STAT3 in triple negative breast cancer cells. Oncol Rep 32(5):2230–2236
Bhatia V, Falzon M (2015) Restoration of the anti-proliferative and anti-migratory effects of 1, 25-dihydroxyvitamin D by silibinin in vitamin D-resistant colon cancer cells. Cancer Lett 362(2):199–207
Kumar S, Raina K, Agarwal C, Agarwal R (2014) Silibinin strongly inhibits the growth kinetics of colon cancer stem cell-enriched spheroids by modulating interleukin 4/6-mediated survival signals. Oncotarget 5(13):4972
Li L, Gao Y, Zhang L, Zeng J, He D, Sun Y (2008) Silibinin inhibits cell growth and induces apoptosis by caspase activation, down-regulating survivin and blocking EGFR–ERK activation in renal cell carcinoma. Cancer Lett 272(1):61–69
Liang L, Li L, Zeng J, Gao Y, Chen Y-L, Wang Z-Q, Wang X-Y, Chang LS, He D (2012) Inhibitory effect of silibinin on EGFR signal-induced renal cell carcinoma progression via suppression of the EGFR/MMP-9 signaling pathway. Oncol Rep 28(3):999–1005
Ramasamy K, Dwyer-Nield LD, Serkova NJ, Hasebroock KM, Tyagi A, Raina K, Singh RP, Malkinson AM, Agarwal R (2010) Silibinin prevents lung tumorigenesis in wild-type but not in iNOS−/− mice: potential of real-time micro-CT in lung cancer chemoprevention studies. Clin Cancer Res 17(4):753–761
Corominas-Faja B, Oliveras-Ferraros C, Cuyàs E, Segura-Carretero A, Joven J, Martin-Castillo B, Barrajón-Catalán E, Micol V, Bosch-Barrera J, Menendez JA (2013) Stem cell-like ALDHbright cellular states in EGFR-mutant non-small cell lung cancer: a novel mechanism of acquired resistance to erlotinib targetable with the natural polyphenol silibinin. Cell Cycle 12(21):3390–3404
Shukla SK, Dasgupta A, Mehla K, Gunda V, Vernucci E, Souchek J, Goode G, King R, Mishra A, Rai I (2015) Silibinin-mediated metabolic reprogramming attenuates pancreatic cancer-induced cachexia and tumor growth. Oncotarget 6(38):41146
Zi X, Zhang J, Agarwal R, Pollak M (2000) Silibinin up-regulates insulin-like growth factor-binding protein 3 expression and inhibits proliferation of androgen-independent prostate cancer cells. Cancer Res 60(20):5617–5620
Polachi N, Bai G, Li T, Chu Y, Wang X, Li S, Gu N, Wu J, Li W, Zhang Y (2016) Modulatory effects of silibinin in various cell signaling pathways against liver disorders and cancer—a comprehensive review. Eur J Med Chem 123:577–595
Bhatia N, Agarwal C, Agarwal R (2001) Differential responses of skin cancer-chemopreventive agents silibinin, quercetin, and epigallocatechin 3-gallate on mitogenic signaling and cell cycle regulators in human epidermoid carcinoma A431 cells. Nutr Cancer 39(2):292–299
Singh RP, Agarwal R (2005) Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer 41(13):1969–1979
Imai-Sumida M, Chiyomaru T, Majid S, Saini S, Nip H, Dahiya R, Tanaka Y, Yamamura S (2017) Silibinin suppresses bladder cancer through down-regulation of actin cytoskeleton and PI3K/Akt signaling pathways. Oncotarget 8(54):92032
Yan T-H, Lu S-W, Huang Y-Q, Que G-B, Chen J-H, Chen Y-P, Zhang H-B, Liang X-L, Jiang J-H (2014) Upregulation of the long noncoding RNA HOTAIR predicts recurrence in stage Ta/T1 bladder cancer. Tumor Biol 35(10):10249–10257
Xue Y, Ma G, Gu D, Zhu L, Hua Q, Du M, Chu H, Tong N, Chen J, Zhang Z (2015) Genome-wide analysis of long noncoding RNA signature in human colorectal cancer. Gene 556(2):227–234
Mueller S, Schmitt M, Dekant W, Stopper H, Schlatter J, Schreier P, Lutz W (1999) Occurrence of emodin, chrysophanol and physcion in vegetables, herbs and liquors. Genotoxicity and anti-genotoxicity of the anthraquinones and of the whole plants. Food Chem Toxicol 37(5):481–491
Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J (2016) Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol 12(10):580
Zhou Y, Ming J, Li Y, Du X, Deng M, He B, Zhou J, Wang G, Liu S (2018) Surfactant protein D attenuates nitric oxide-stimulated apoptosis in rat chondrocyte by suppressing p38 MAPK signaling. Biochem Biophys Res Commun 495(1):526–532
Rufino AT, Rosa SC, Judas F, Mobasheri A, Lopes MC, Mendes AF (2013) Expression and function of K (ATP) channels in normal and osteoarthritic human chondrocytes: possible role in glucose sensing. J Cell Biochem 114(8):1879–1889
Liang Z, Ren C (2018) Emodin attenuates apoptosis and inflammation induced by LPS through up-regulating lncRNA TUG1 in murine chondrogenic ATDC5 cells. Biomed Pharmacother 103:897–902
Li T, Liu Y, Xiao H, Xu G (2017) Long non-coding RNA TUG1 promotes cell proliferation and metastasis in human breast cancer. Breast Cancer 24(4):535–543
Zhang E, He X, Yin D, Han L, Qiu M, Xu T, Xia R, Xu L, Yin R, De W (2017) Increased expression of long noncoding RNA TUG1 predicts a poor prognosis of gastric cancer and regulates cell proliferation by epigenetically silencing of p57. Cell Death Dis 7(2):e2109
Sun J, Ding C, Yang Z, Liu T, Zhang X, Zhao C, Wang J (2016) The long non-coding RNA TUG1 indicates a poor prognosis for colorectal cancer and promotes metastasis by affecting epithelial–mesenchymal transition. J Transl Med 14(1):42
Yan G, Wang X, Yang M, Lu L, Zhou Q (2017) Long non-coding RNA TUG1 promotes progression of oral squamous cell carcinoma through upregulating FMNL2 by sponging miR-219. Am J Cancer Res 7(9):1899
Lei H, Gao Y, Xu X (2017) LncRNA TUG1 influences papillary thyroid cancer cell proliferation, migration and EMT formation through targeting miR-145. Acta Biochim Biophys Sin 49(7):588–597
Liu Q, Liu H, Cheng H, Li Y, Li X, Zhu C (2017) Downregulation of long noncoding RNA TUG1 inhibits proliferation and induces apoptosis through the TUG1/miR-142/ZEB2 axis in bladder cancer cells. OncoTargets Ther 10:2461
Zhao L, Guo Q-L, You Q-D, Wu Z-Q, Gu H-Y (2004) Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull 27(7):998–1003
Zhao K, Zhang S, Song X, Yao Y, Zhou Y, You Q, Guo Q, Lu N (2017) Gambogic acid suppresses cancer invasion and migration by inhibiting TGFβ1-induced epithelial-to-mesenchymal transition. Oncotarget 8(16):27120
Wang X, Lu N, Yang Q, Gong D, Lin C, Zhang S, Xi M, Gao Y, Wei L, Guo Q (2011) Studies on chemical modification and biology of a natural product, gambogic acid (III): determination of the essential pharmacophore for biological activity. Eur J Med Chem 46(4):1280–1290
Shahabipour F, Caraglia M, Majeed M, Derosa G, Maffioli P, Sahebkar A (2017) Naturally occurring anti-cancer agents targeting EZH2. Cancer Lett 400:325–335
Wang Y, Xiang W, Wang M, Huang T, Xiao X, Wang L, Tao D, Dong L, Zeng F, Jiang G (2014) Methyl jasmonate sensitizes human bladder cancer cells to gambogic acid-induced apoptosis through down-regulation of EZH 2 expression by miR-101. Br J Pharmacol 171(3):618–635
Wang M, Guo C, Wang L, Luo G, Huang C, Li Y, Liu D, Zeng F, Jiang G, Xiao X (2018) Long noncoding RNA GAS5 promotes bladder cancer cells apoptosis through inhibiting EZH2 transcription. Cell Death Dis 9(2):238
Paul V, Yeddanapalli LM (1954) Olefinic nature of anacardic acid from Indian cashew-nut shell liquid. Nature 174(4430):604
Brown JA, Bourke E, Eriksson LA, Kerin MJ (2016) Targeting cancer using KAT inhibitors to mimic lethal knockouts. Biochem Soc Trans 44(4):979–986
Schultz DJ, Krishna A, Vittitow SL, Alizadeh-Rad N, Muluhngwi P, Rouchka EC, Klinge CM (2018) Transcriptomic response of breast cancer cells to anacardic acid. Sci Rep 8(1):8063
Tan J, Jiang X, Yin G, He L, Liu J, Long Z, Jiang Z, Yao K (2017) Anacardic acid induces cell apoptosis of prostatic cancer through autophagy by ER stress/DAPK3/Akt signaling pathway. Oncol Rep 38(3):1373–1382
Chang W (2017) Non-coding RNAs and berberine: a new mechanism of its anti-diabetic activities. Eur J Pharmacol 795:8–12
Jeong Y, You D, Kang H-G, Yu J, Kim SW, Nam SJ, Lee JE, Kim S (2018) Berberine suppresses fibronectin expression through inhibition of c-Jun phosphorylation in breast cancer cells. J Breast Cancer 21(1):21–27
Liu D, Zhang Y, Liu Y, Hou L, Li S, Tian H, Zhao T (2018) Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp Clin Endocrinol Diabetes 126(8):513–520
Lin Y, Sheng M, Ding Y, Zhang N, Song Y, Du H, Lu N, Yu W (2018) Berberine protects renal tubular cells against hypoxia/reoxygenation injury via the Sirt1/p53 pathway. J Nat Med 72(3):715–723
Nonaka M, Murata Y, Takano R, Han Y, Kabir MHB, Kato K (2018) Screening of a library of traditional Chinese medicines to identify anti-malarial compounds and extracts. Malaria J 17(1):244
Zhu X, Sun Y, Zhang C, Liu H (2017) Effects of berberine on a rat model of chronic stress and depression via gastrointestinal tract pathology and gastrointestinal flora profile assays. Mol Med Rep 15(5):3161–3171
Yuan X, Wang J, Tang X, Li Y, Xia P, Gao X (2015) Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med 13(1):24
Hollman PC, Gaag MV, Mengelers MJ, Van Trijp JM, De Vries JH, Katan MB (1996) Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med 21(5):703–707
Choi J-A, Kim J-Y, Lee J-Y, Kang C-M, Kwon H-J, Yoo Y-D, Kim T-W, Lee Y-S, Lee S-J (2001) Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol 19(4):837–844
Coskun O, Kanter M, Korkmaz A, Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol Res 51(2):117–123
Natarajan V, Krithica N, Madhan B, Sehgal PK (2011) Formulation and evaluation of quercetin polycaprolactone microspheres for the treatment of rheumatoid arthritis. J Pharm Sci 100(1):195–205
Mamani-Matsuda M, Kauss T, Al-Kharrat A, Jm Rambert, Fawaz F, Thiolat D, Moynet D, Coves S, Malvy D, Mossalayi MD (2006) Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem Pharmacol 72(10):1304–1310
Nanki T, Nagasaka K, Hayashida K, Saita Y, Miyasaka N (2001) Chemokines regulate IL-6 and IL-8 production by fibroblast-like synoviocytes from patients with rheumatoid arthritis. J Immunol 167(9):5381–5385
Gabriel SE, Michaud K (2009) Epidemiological studies in incidence, prevalence, mortality, and comorbidity of the rheumatic diseases. Arthritis Res Ther 11(3):229
Kurowska M, Rudnicka W, Kontny E, Janicka I, Chorazy M, Kowalczewski J, Ziółkowska M, Ferrari-Lacraz S, Strom TB, Maśliński W (2002) Fibroblast-like synoviocytes from rheumatoid arthritis patients express functional IL-15 receptor complex: endogenous IL-15 in autocrine fashion enhances cell proliferation and expression of Bcl-xL and Bcl-2. J Immunol 169(4):1760–1767
Chen S, Yang Y, Feng H, Wang H, Zhao R, Liu H (2014) Baicalein inhibits interleukin-1β-induced proliferation of human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation 37(1):163–169
Pan F, Zhu L, Lv H, Pei C (2016) Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med 38(5):1507–1514
Slaninová I, Pěnčíková K, Urbanová J, Slanina J, Táborská E (2014) Antitumour activities of sanguinarine and related alkaloids. Phytochem Rev 13(1):51–68
Wei G, Xu Y, Peng T, Yan J, Wang Z, Sun Z (2017) Sanguinarine exhibits antitumor activity via up-regulation of Fas-associated factor 1 in non-small cell lung cancer. J Biochem Mol Toxicol 31(8):e21914
Ma Y, Yu W, Shrivastava A, Alemi F, Lankachandra K, Srivastava RK, Shankar S (2017) Sanguinarine inhibits pancreatic cancer stem cell characteristics by inducing oxidative stress and suppressing sonic hedgehog-Gli-Nanog pathway. Carcinogenesis 38(10):1047–1056
Zhang R, Wang G, Zhang PF, Zhang J, Huang YX, Lu YM, Da W, Sun Q, Zhu JS (2017) Sanguinarine inhibits growth and invasion of gastric cancer cells via regulation of the DUSP4/ERK pathway. J Cell Mol Med 21(6):1117–1127
Kalogris C, Garulli C, Pietrella L, Gambini V, Pucciarelli S, Lucci C, Tilio M, Zabaleta ME, Bartolacci C, Andreani C (2014) Sanguinarine suppresses basal-like breast cancer growth through dihydrofolate reductase inhibition. Biochem Pharmacol 90(3):226–234
Croaker A, King GJ, Pyne JH, Anoopkumar-Dukie S, Simanek V, Liu L (2017) Carcinogenic potential of sanguinarine, a phytochemical used in ‘therapeutic’ black salve and mouthwash. Mutat Res Rev Mutat Res 774:46–56
Zhang S, Leng T, Zhang Q, Zhao Q, Nie X, Yang L (2018) Sanguinarine inhibits epithelial ovarian cancer development via regulating long non-coding RNA CASC2–EIF4A3 axis and/or inhibiting NF-κB signaling or PI3K/AKT/mTOR pathway. Biomed Pharmacother 102:302–308
Li P, Xue W-J, Feng Y, Mao Q-S (2016) Long non-coding RNA CASC2 suppresses the proliferation of gastric cancer cells by regulating the MAPK signaling pathway. Am J Transl Res 8(8):3522
He X, Liu Z, Su J, Yang J, Yin D, Han L, De W, Guo R (2016) Low expression of long noncoding RNA CASC2 indicates a poor prognosis and regulates cell proliferation in non-small cell lung cancer. Tumor Biol 37(7):9503–9510
Wang R, Li Y, Zhu G, Tian B, Zeng W, Yang Y, Li Z (2017) long noncoding rNa casc2 predicts the prognosis of glioma patients and functions as a suppressor for gliomas by suppressing Wnt/β-catenin signaling pathway. Neuropsychiatr Dis Treat 13:1805
Pei Z, Du X, Song Y, Fan L, Li F, Gao Y, Wu R, Chen Y, Li W, Zhou H (2017) Down-regulation of lncRNA CASC2 promotes cell proliferation and metastasis of bladder cancer by activation of the Wnt/β-catenin signaling pathway. Oncotarget 8(11):18145
Huang G, Wu X, Li S, Xu X, Zhu H, Chen X (2016) The long noncoding RNA CASC2 functions as a competing endogenous RNA by sponging miR-18a in colorectal cancer. Sci Rep 6:26524
Zhou J, Huang H, Tong S, Huo R (2017) Overexpression of long non-coding RNA cancer susceptibility 2 inhibits cell invasion and angiogenesis in gastric cancer. Mol Med Rep 16(4):5235–5240
Baldinu P, Cossu A, Manca A, Satta MP, Sini MC, Rozzo C, Dessole S, Cherchi P, Gianfrancesco F, Pintus A (2004) Identification of a novel candidate gene, CASC2, in a region of common allelic loss at chromosome 10q26 in human endometrial cancer. Hum Mutat 23(4):318–326
Baldinu P, Cossu A, Manca A, Satta MP, Sini MC, Palomba G, Dessole S, Cherchi P, Mara L, Tanda F (2007) CASC2a gene is down-regulated in endometrial cancer. Anticancer Res 27(1A):235–243
Zhang Y, Talalay P, Cho C-G, Posner GH (1992) A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci 89(6):2399–2403
Beaver LM, Kuintzle R, Buchanan A, Wiley MW, Glasser ST, Wong CP, Johnson GS, Chang JH, Löhr CV, Williams DE (2017) Long noncoding RNAs and sulforaphane: a target for chemoprevention and suppression of prostate cancer. J Nutr Biochem 42:72–83
Fang Y, Huang Z, Li H, Tan W, Zhang Q, Wang L, Wu J (2018) LINC01116 promotes the progression of epithelial ovarian cancer via regulating cell apoptosis. Eur Rev Med Pharmacol Sci 22(16):5127–5133
Sankaram A, Rao G (1978) Bharangin, a novel diterpenoid quinonemethide from Pygmacopremna herbaceae. In: Proceedings of IUPAC 11th international symposium of chemistry of natural products, vol 1978, pp 97–100
Sankaram AVB, Marthanda Murthi M, Bhaskaraiah K, Narsimha Rao GL, Subramanyam M, Shoolery J (1988) Bharangin, a novel diterpenoid quinonemethide from Pigmacopremna herbaceae (Roxb.) Moldenke. Tetrahedron Lett 29(2):245–248
Sathish T, Brahmaiah P, Sathya K, Bhojaraju P, Naik NG, Kezia D, Prakasam RS (2009) A novel RP-HPLC method for the determination of bharangin in Ghantu bharangi crude extracts. Pak J Pharm Sci 22(1):68–73
Kirtikar KR, Basu BD (1918) Indian medicinal plants, vol 3. Lalit Mohan Basu, Allahabad, pp 883–884
Narayanan N, Thirugnanasambantham P, Viswanathan S, Reddy MK, Vijayasekaran V, Sukumar E (2000) Antipyretic, antinociceptive and anti-inflammatory activity of Premna herbacea roots. Fitoterapia 71(2):147–153
Nayar RC, Yoganarsimhan SN, Subramanyam K (1976) Pharmacognosy of a local market sample of bharangin: Pygmacopremna herbaceae. Indian J Pharm 38:39–44
Boonyaratanakornkit L, Chantaptavan V (1993) Identification and specification of khao-yen-neua and khao-yen-tai. Thai J Pharm Sci 1:79–90
Itharat A, Singchangchai P, Ratanasuwan P (1998) Wisdom of Southern Thai traditional doctors, vol 126. Prince of Songkla University, Songkla
Ravishankar K, Pai K, Isha D, Setty M, Manjula S, Ramalingayya G (2008) An appraisal of the antitumor activity of alcoholic extract of Premna herbacea Roxbin Ehrlich’s ascitic carcinoma model. Indian J Pharmacol 40(Suppl 2):67
Gupta SC, Kannappan R, Kim J, Rahman GM, Francis SK, Raveendran R, Nair MS, Das J, Aggarwal BB (2011) Bharangin, a diterpenoid quinonemethide, abolishes constitutive and inducible nuclear factor-κB (NF-κB) activation by modifying p65 on cysteine 38 residue and reducing inhibitor of nuclear factor-κB α kinase activation, leading to suppression of NF-κB-regulated gene expression and sensitization of tumor cells to chemotherapeutic agents. Mol Pharmacol 80(5):769–781
Acknowledgements
The authors would like to thank Richard Heather and Pokhrel Arya from UNMC High School Alliance Program at the University of Nebraska Medical Center, USA, for thoroughly reading the article. SCG is thankful to the Science and Engineering Research Board (ECR/2016/000034) and University Grants Commission [No.F. 30-112/2015 (BSR)] for the financial assistance. Dr. Challagundla’s laboratory is supported in whole or part from the NIH/NCI Grant (K22CA197074-01); the Nebraska State DHHS (LB506); IDeA Award from the NIH/NIGMS (P30 GM106397); UNMC Pediatric Cancer Research Center; Fred and Pamela Buffett Cancer Center’s Pilot Grant (P30 CA036727) in conjunction with the UNMC Pediatric Cancer Research Center; Leukemia Research Foundation Grant and the Department of Biochemistry and Molecular Biology start-up at UNMC. SM, SSV, and NA are supported from ICMR New Delhi (3/1/3/JRF-2016/LS/HRD-65-80388), DBT New Delhi (DBT/2017/BHU/786), and BHU Varanasi (R/Dev/IX-Sch-BHU Res Sch 2018-19), respectively.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mishra, S., Verma, S.S., Rai, V. et al. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell. Mol. Life Sci. 76, 1947–1966 (2019). https://doi.org/10.1007/s00018-019-03053-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-019-03053-0