Abstract
The Microbial Fuel Cell (MFC) is a contemporary technology that employs electrogenic microorganisms as a catalyst to convert chemical energy contained in the bonds of organic matter found in waste materials directly into electricity, without polluting the environment. An MFC is a bioelectrochemical system with unique characteristics that may be utilised for a number of purposes, including power generation, waste treatment and biosensors. Besides powering a wide range of electrical equipment, its advancements in chemical, electrochemical and microbiological characteristics have extended its applications in chemical generation, acid and alkali production, bioremediation, water desalination and other fields. Except for powering tiny sensor devices, MFCs encounter significant challenges in real-world use as power producers. In recent years, there has been a lot of research done to broaden the use of MFCs as biosensors. Unlike electrical applications, MFC biosensors have a good chance of becoming practical tools in a variety of analytical applications. MFCs-based biosensors are gaining popularity in various fields due to their ease of application and long-term viability in quality monitoring of the environment. This chapter examines the most recent advancements in MFC-based biosensors in terms of their concepts, principles, design, operating mechanisms, power sources, power generation process, along with their scope and benefits. We also highlight biosensing applications in a variety of disciplines, with a focus on the detection of biochemical oxygen demand (BOD), toxicity, microbial activity, biocorrosion-causing microbial biofilms, volatile fatty acids, etc. A brief discussion of the problems and opportunities of MFC-based biosensors is also included.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The rise of the industrial revolution, urbanisation and a scarcity of crude oil have prompted scientists to search for other energy sources. The development of ecologically friendly, sustainable and renewable energy supplies is required as a result of the current energy crisis and global warming. Solar, wind, biomass and nuclear energy are all examples of non-conventional, carbon-neutral energy sources that are being researched and used to a large extent. Microbial fuel cells (MFCs) have arisen as the next viable and environmentally benign energy source [116]. Exoelectrogenic bacteria that function as substrate oxidation half-reaction catalysers in MFCs directly transform the chemical energy available in an organic bioconvertible substrate into electric energy [27]. It immediately transforms chemical energy contained in the bonds of organic materials in wastes into electricity without polluting the environment [113, 116, 133]. MFCs may decrease environmental pollutants such as waste, reduce atmospheric carbon dioxide, and offer a renewable source of electricity all at the same time [41].
Electrode-reducing organisms are those that transport electrons from the cathode to the anode. They can transmit electrons directly through their outer membrane proteins, a mediator molecule in the solution or nanowires/pili overlaying the bacterium's outer surface. Electrode-oxidising organisms use electrons from the cathode to convert CO2 to acetate, for example [41].
In MFCs, microorganisms release electrons via substrate oxidation in the anode chamber, which are then transported to the cathode compartment via a conductive substance. The electrons interact with O2 at the cathode, and the protons are diffused across a proton exchange membrane. MFCs require a constant flow of electrons in the anode and a constant flow of electrons in the cathode. The difference between the anode voltage and the substrate redox potential determines how much metabolic energy bacteria can acquire [19]. The generated biofilm in the anodic chamber serves as the bioreceptor in an MFC-based biosensor, while the anode serves as the transducer. The electron flow rate is affected by the anodic biofilm's reaction to the disturbance, which is translated into a quantifiable signal [43].
Due to the vast range of applications, such as disease detection, health care, drug delivery, food quality and water quality monitoring and environmental monitoring, biosensor design and development has taken centre stage for researchers in recent decades [112]. The MFC biosensors, which are based on the activity of microorganisms in MFCs, have received much interest as a consequence of the fast development of microbiology and have become an alternate tool for rapid, sensitive and selective recognition of numerous analytes [44, 174]. It has evolved into one of the most significant ecological monitoring techniques [47]. Because of its capacity to self-regenerate and self-replicate, the whole-cell biosensor has a lot of potential for making cost-effective and long-term environmental monitoring. It has the exclusive capacity to provide bioavailability information, which is impossible to get using any other analytical approach [47, 143, 174]. Fluorescent proteins, fluorescence molecules and enzyme activity are commonly used as indicators in conventional whole-cell biosensors. These marker molecules should be assessed utilising electrically driven apparatus such as microscopes, spectrometers and other instruments to get a quantitative signal [47, 174]. The biosensor's response will then be represented by the equipment's measured electrical output. Traditional biosensors require an external power supply and expensive equipment, which severely limits their use in distant and long-standing environmental monitoring when a sufficient power supply and necessary analytical instruments are frequently unavailable.
MFC technology is a unique way of utilising bacteria to generate bioelectricity from organic waste and renewable biomass [97], which may be used directly in biosensing. MFC-based biosensors have piqued interest currently owing to benefits such as high stability, sensitivity and distant place applicability with no electrical supply, regardless of their role in wastewater treatment or energy production [10]. Because of its simplicity and long-term viability, MFC-based biosensors have received much interest in recent decades, with applications ranging from water quality monitoring (e.g. poisonous substance) to air quality monitoring (e.g. CO2) [43]. These MFC biosensors can detect characteristics and events in their environment and transform that information into signals. One of the most potential usages of MFC-allied technologies is the MFC-based biosensor, which has been investigated to quantity a range of parameters such as biochemical oxygen demand (BOD), dissolved oxygen (DO), chemical oxygen demand (COD), toxicants, microbial activity and volatile fatty acids (VFAs), among others [43].
In this chapter, we summarise the fundamentals, design, power sources, mechanism of electron transfer and operation of MFCs-based biosensors along with their application in various fields focusing on BOD, COD, VFAs, DO, toxicants, microbial activity, etc. Lastly, the further challenges and prospects of MFC-based biosensors in real time and onsite monitoring are also discussed.
2 Fundamentals, Configuration and Operation of Biosensors
2.1 Basic Principle of MFCs as Biosensors
A biosensor comprises three functional elements. A biorecognition element, a transducer and an electrical device with an amplifier, CPU and display are among the components [61]. The first part of the biosensor is the biological component, which is responsible for sensing the analyte and producing a signal. The biological component's response is then transmuted into a measurable signal by the second unit, the transducer, that is, the utmost important element in any sensing device. The detector, which magnifies and analyses the signals prior to presenting them on an electronic display device, is the biosensor's third component [122]. The different phases of a biosensor's signal processing, from sensing to transduction to display, as well as various types of bioreceptors and transducers, are depicted graphically in Fig. 1.

Reprinted from Ref. [112] with permission under Creative Commons Attribution (CC BY) license
A schematic illustration of a typical biosensor, which includes a bioreceptor, transducer, electrical system (amplifier followed by processor) and display (PC or printer), as well as numerous types of bioreceptors and transducers.
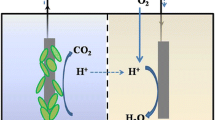
A biosensor is a system that joins a receptor and a transducer to transform a biochemical response into an electrical signal. MFCs are devices that employ metabolic activities of microorganisms to directly transform chemical energy in organic materials into electricity. An MFC consists of two electrodes, an anode and a cathode, that are connected by an electrolyte. An ion/proton exchange membrane (PEM) separates the electrodes, which are linked via an exterior circuit that contains an external load (Fig. 2). Anodophiles (electroactive bacteria) live as a biofilm on the device's anode, which functions as a bioreceptor. The anodophiles generate electrons, protons and CO2 by oxidising the biodegradable organic molecules in the feed solution. Electrons are extracellularly transported to the anode and travel via the exterior circuit in the direction of the cathode in the absence of oxygen, generating electricity. Water is formed when protons move via the PEM towards the cathode and combine with electrons and an electron acceptor (typically oxygen). The current produced by an MFC is directly proportional to the metabolic action of the electroactive biofilm on the anode shell. Any disruptions in their metabolic routes result in a change in the amount of power produced. This current variation may be linked to the precise disturbance applied if operational factors like temperature, pH and conductivity of the feeding fluid are maintained steady. The usage of MFCs as electrochemical microbial biosensors is based on this principle [40].

Reprinted from Ref. [40] under the Creative Commons Attribution License
A two-compartment MFC's operating principles. The anode's electroactive biofilm degrades an organic substrate, producing electrons, protons and CO2. The electrons are reduced by the cathode after passing through an external circuit.
MFCs are electrochemical systems that employ the redox metabolic processes of microbes to produce electricity. If the microorganisms in the anodic compartment are biologically active and there is a useable carbon supply, they produce a voltage discrepancy between the anode and the cathode, ensuring an electron flow driving force. This is the property that makes MFCs suitable for biosensor functions [32, 70, 82]. The anode compartment microorganisms function as biocatalysts, while the electrodes and PEM serve as transducers. Two essential ideas underpin the practical uses of MFC-based biosensors. The objective in the first circumstance is to find contaminated microbes. As a result, a sample's sterility may be continuously monitored and reported. A positive signal (electron production) is generated when a contaminating microbe appears, but no signal is observed in a sterile sample. In the second example, biosensing may be used to monitor the presence or appearance of a target chemical when a specific bacteria strain is employed as an essential element of a system that is sensitive to that chemical [40] by measuring the strength of the electrical signal generated during microorganism metabolism.
2.2 Design and Configuration
Electrodes, their connections, cells and a salt bridge are the fundamental components of MFCs. An ion exchange membrane replaces the salt bridge in a PEM in MFC. The system's efficiency and mobility have been improved by improving its handling, cost and power generation. A two-chamber MFC typically comprises an anode and a cathode compartment divided by a PEM, as illustrated in Fig. 3, but exposure of the cathode element directly to the open air removes the requirement for the cathodic portion in a single chamber. A two-chamber MFC, on the other hand, works in the water-cathode mode, while a single-chamber MFC works in the air-cathode mode. The main benefit of the two-chamber MFC over the one chamber is that the cathode's functioning may be increased by regulating purging pure O2, improving the flow rate, changing pH and supplying electron mediators to the cathode, resulting in total MFC performance improvement. Almost all current configurations are based on three primary configurations, which will be offered greater attention since they are critical to the MFC-based biosensor's history [55]. Customising the reactor configurations can enhance the performance of MFCs. The success of microbial fuel cell (MFC)-based biosensors is determined on the assemble and configuration of the fuel cell.

Reprinted from Ref. [46] with permission. Copyright (2020) Elsevier
Design architecture and basic components of the double-chamber MFCs-based biosensor.
MFCs are systems that transform biochemical energy directly into electricity employing microorganisms as catalysts. The single-chamber MFCs [94] which have developed from the original double-chamber layout in an effort to abolish a membrane are primarily made up of anodic chambers [26]. The single-chamber devices may also yield the best results. A basic MFC model can be single or double chambered depending on how the anode and cathode chambers are assembled. Aside from these two fundamental concepts, numerous design and structural changes have been done to improve the MFC prototype [119]. They are categorised into two forms based on their configuration: single-chamber MFCs and double-chamber MFCs.
Double-Chambered MFCs: In principle, a PEM separates the anodic and cathodic chambers of a double-chambered MFC, allowing proton movement from the anode towards the cathode while inhibiting O2 passage into the anode. As a result, this arrangement is widely utilised to treat waste and create electricity at the same time, which is useful in biosensing. Both the anode and the cathode are distinct compartments that are linked by a PEM which serves primarily as a proton transmission channel to complete the circuit between the two compartments [13, 176] (Fig. 3). This completes the reaction and inhibits the diffusion of O2 or any other oxidants from the cathode chamber. Double-chambered MFCs provide energy power output in a batch manner with a chemically specified medium such as an organic substrate solution [55] and can be used in biosensing.
Single-Chambered MFCs: The idea behind the construction of a single-chambered MFC, in which the anodic compartment is connected to a porous air exposed cathode, and they are separated from one another a PEM or by a gas diffusion layer, allowing passive O2 transport to the cathode. To complete the circuit, electrons are delivered to the porous cathode across an electrically conducting wire. Because the use of O2 as a last electron acceptor eliminates the requirement to aerate the cathode, single-chamber MFCs with an air-cathode assemblage have been developed. Researchers are interested in this sort of MFC arrangement (Fig. 4) because of numerous advantages, including lower internal resistance, simplicity of operation, increased O2 reduction amount on the cathode, improved proton circulation and decreased electrode spacing. This arrangement is more flexible since it requires less frequent regular change of oxidative medium and aeration [55].

Reprinted from Ref. [84] with permission. Copyright (2018) Elsevier
Design the architecture and basic components of an MFCs-based single-chambered biosensor.
Electroactive microorganisms are introduced into the anodic chamber, where they oxidise organic molecules to make electrons and protons. The anode captures electrons, which are subsequently sent through an exterior circuit to the cathode. To maintain charge balance, protons and other positive ion like K+, Na+ transfer to the cathode via the PEM [73]. Finally, oxygen serves as an electron acceptor, allowing electrons and protons to merge to produce water [15, 183].
Although lot of variations are available, there are mainly three groups of microbial biosensors categorised based on signal transducers: electrochemical, optical and MFCs [166]. The alter in electric potential, current and conductivity induced by microbial-analyte interaction is exploited by electrochemical transducers. Biosensors can be classified as potentiometric, amperometric or conductometric. The potentiometric transducer was developed by Mulchandani [111]. Ion selective electrodes are used in these transducers to convert the biochemical signal into an electrical signal. These are less sensitive, make greater relative errors and have a poor linear connection between the exported signal and the detected analyte concentration [166]. On the other hand, Amperometric microbial biosensors function at a set voltage in relation to a reference electrode, and the equivalent current is produced as a result of the oxidation or reduction of electroactive compounds on the electrode's surface [166]. This arrangement has been depicted by Yong et al. [173], Anu Prathap et al. [12] and Wang et al. [158], conductometric biosensors, as the name implies, measure changes in medium conductivity induced by the target analyte. Despite the fact that conductance quantification is highly sensitive, solution conductance detection is deemed nonspecific [166].
Sensor devices that employ optical principles such as bioluminescence, colorimetry and fluorescence to convert a biochemical interface into an appropriate output signal are known as optical biosensors [166]. The expression of bioluminescence and fluorescence in the target organism is possible thanks to genetic engineering. In biosensing, scientists explore the use of luciferase [29, 114, 142] and green fluorescent protein (GFP) applications [75, 159].
2.3 Operation/Working Mechanism
In 1910, Potter reported, ‘the disintegration of organic compounds by microorganisms is accompanied by the liberation of electrical energy’ [126]. Microbes perform metabolic processes (catabolism and anabolism) either in the accessibility of O2 (aerobic) or in the inaccessibility of O2 (anaerobic). Microbes use the accessible substrate (fermentation) by producing reducing equivalents [electrons (e−)/protons (H+] in the form of redox carriers, regardless of their metabolism. During respiration, these redox carriers assist in the generation of energy.
The chemical energy of decomposable organic compounds can be directly transformed into electrical energy in MFCs thanks to the metabolism of exoelectrogenic bacteria. The power produced can be quantified and/or used to build MFC-based biosensors for detecting decomposable organic compounds and/or hazardous compounds in water or wastewater [150]. The MFC works by oxidising organic materials using microorganisms as a biocatalyst [106]. In principle, bioelectrochemical systems may be used in self-contained effluent treatment amenities to convert wastewater, including organic materials, into electricity. Actually, bioelectrochemical systems can reduce the energy consumption of activated sludge treatment processes [50, 51] while simultaneously detecting them using MFC-based biosensors. The scientific idea is very appealing, and many research studies have been carried out in this area.
Unlike traditional sensors, bacteria in an MFC-based biosensor can sense the analyte and then respond to its output electric current, where the detection and electrical signal conversion steps are combined and can be accomplished in single phase without the use of a signal transducer or an outside energy source. The MFC-based biosensor's most intriguing feature is that it does not require a transducer to transform the output to an electric signal since the assessed signal is available as an electrical current. These distinct properties help to create disposable and portable biosensors that precisely suit the needs of long-term and distant sensing [148].
Microorganisms oxidise the substrate in the anode compartment of a conventional MFC, generating electrons that are then carried by the anode electrode across the exterior cable. The protons flow across the PEM while the electrons flow to the cathode. In the cathode, protons and electrons interact with O2 to produce water [113]. Considering acetate as an organic substrate, the subsequent reaction occurs in MFC.
Anode half-cell reaction (in the presence of microbes):
After that, the generated electrons reduce electron acceptors such as O2 at the cathode.
Cathode half-cell reaction:
There are several essential considerations for using an MFC as a biosensor that may differ from those for using it to generate energy. When the goal is to produce electricity, the attention is on improving fuel efficacy and current production, but when the goal is to employ the MFC as a biosensor, the aim will be on more sensitive recognition of the target chemicals [95]. The variation in electric signal per unit of change in analyte content concentration is defined as sensitivity, which is generally controlled by the anode's surface area (Eq. 3) [45].
Here, the difference in the current output is ΔI (μA), while the unit difference in the analyte content is Δc (mmol/l) and the surface area of the electrode is A (cm2). As a result, better sensitivities are linked to differences in current per unit change in target concentration. The biosensor should also generate a consistent and continuous current output, referred to as the baseline [147]. As a result, it is critical to keep a monitor on the anode's overpotential and the feed pH in the MFC. Anode voltages between −0.4 and −0.35 V versus the Ag/AgCl reference electrode give the best steady output current density, according to prior research. In the long-term procedure, the MFC biosensor stability must be confirmed. The outputs of MFC biosensors should be reproducible regardless of operating factors including temperature, pH and conductivity of liquid samples [2]. Furthermore, in order to attain 95% of the steady-state current, the response time must be very fast. Following the fermentation/toxic response, the needed recovery period from the employed disturbance must be minimal, and the starting baseline current need to be entirely restored.
To comprehend the outputs of an MFC biosensor, the use of artificial neural networks (ANNs) was recommended. ANNs are a type of mathematical prototype utilised to assess complex nonlinear connections between input and output records. In a batch mode MFC, ANN was able to properly identify butyrate, acetate, glucose and corn starch [54]. This model provides an effective method for determining target analytes from MFC signal responses. The advantages of MFCs over other sorts of biosensors are due to their mechanical and electrical simplicity of operation and construction. Because the pollutant, which runs in the feeding stream, is immediately recognised by a defined current variation through the system, no extra transducers are necessary to transform the biochemical/organic reaction into a signal. MFC biosensors have been in continuous operation onsite, providing real-time monitoring. Furthermore, the MFCs’ electrical power output makes them excellent for use as sustaining devices. They may be suitable for use in locations where there are no available energy sources [103].
3 Microbial Fuel Cells as Biosensors
The biosensors we have observed so far necessitate the use of a transducer that can convert bacterial analyte contact into a measurable signal. This necessitates expensive electrical equipment and external power supply, limiting the use of these biosensors in distant regions where external power is scarce. As a result, MFC-based biosensors have piqued the scientific community's interest as a viable alternative to conventional biosensors. MFCs were first conceived as a method of obtaining energy from the metabolic processes of anaerobic bacteria that oxidise organic molecules. However, because the MFCs’ power output is so low, significant research has been done in recent years to identify alternate applications for the MFCs. Biosensing in environmental monitoring is one such potential application that has been thoroughly investigated. MFC-based biosensors might be utilised as self-powered portable biosensors with a lot of applications in long-standing and remote environmental monitoring [148].
The chemical energy of biodegradable compounds may be directly transformed into electricity in MFCs thanks to the metabolism of exoelectrogenic microbes. The produced electricity may be employed directly to build MFC-based biosensors for the identification and monitoring of biodegradable organic components available in the target samples [150]. The alternative perspective is that any toxicant in the feedstock solution would impact microbial metabolic activity, and thus substrate intake rate, which is directly linked to an MFC's current output. As a result, any variation in the availability and content of toxicants in flowing water can be easily identified by observing perturbations in the electric current produced by MFCs [141, 146], saving time and money over traditional methods [63]. MFCs that produce an electrical signal in reaction to any input analyte can be utilised as a biosensing application in this case.
The biologically energetic anaerobic bacterial species in the anodic compartment functions as a biocatalyst in MFCs, acting as the biosensor's biological detection element. In the presence of a metabolizable organic nutrient source, these anaerobic microbes produce a detectable voltage difference between the electrodes, resulting in an electron flow [108, 109]. There is a difference in current production based on the interaction with the analyte. MFCs that will be utilised as biosensors are based on this fluctuation in output current. The current generated in MFCs can function as a transducer element [154], which is measurable and depends on the electron transmission kinetics of the microbes, in addition to the analyte concentration [105]. This property of MFC-based biosensors, where the assessment and signal detection stages are combined, reduces the need for a transducer for signal conversion [148], thus enhancing the benefits of using MFC as a biosensorics. Aside from the fact that an MFC biosensor does not require a separate sensing element and transducer, these systems have the extra benefit of allowing for online monitoring in both the laboratory and field settings because they can be used in flow-through and assay formats [136]. Furthermore, their improved stability and sensitivity, as well as their capacity to detect a wide range of chemicals, make them excellent biosensors [169].
4 Selection of Microorganisms for MFCs-Based Biosensors
Most microbes are incapable to deliver enough electrons outside of cells to generate efficient current because their exterior layers consist of nonconducting lipid membranes, lipopolysaccharides and peptidoglycans that impede electron transmission to the anode [101]. A critical stage in the development of a microorganism-based biosensor is the assortment of an appropriate microbe for detecting contaminants and their effects in the environment, as well as its inclusion into a suitable transducer. The most often utilised microorganisms for biosensors are bacteria and yeast [166]. To provide cost-effective detection, the selected microorganism ought to be vigorous and capable of precise pollutant detection at low concentrations. Whole-cell biosensors [12, 29, 89] and MFCs [45, 89, 140] biosensors have recently received a lot of interest.
The recent discovery of a MFC that is capable of generating electricity from organic materials trapped in sediments has shown that generating reasonable amounts of electricity for biosensing in distant locations is possible. Microorganisms that totally oxidise organic substances to CO2 with direct electron transmit to electrodes have been discovered. This means that biosensing might benefit from self-sustaining MFCs that can efficiently transform a huge variety of waste organic substances or recyclable biomass to electricity [98].
Some bacterial species such as Shewanella spp. [155] and Clostridium butyricumcan [121] have been demonstrated to be capable of self-mediating extracellular electron transfer utilising their own metabolites. In the meantime, direct electron transfer involving electroactive redox enzymes (cytochromes) has been found in several bacterial species, including Shewanella oneidensis [96], Shewanella putrefaciens [7], Rhodoferax ferrireducens [33], Geobacter sulfurreducens [21] and the oxygenic phototrophic cyanobacterium Synechocystis sp. PCC 6803 [58]. Exoelectrogens are bacteria that have developed electrically conducting molecular pili to enable direct electron transmission. S. oneidensis and G. sulfurreducens are two examples. S. oneidensis may also perform mediated electron transfer utilising a self-produced mediator, in addition to direct electron transfer. Exoelectrogens in MFCs are considered to actively utilise electrodes to preserve electrochemical energy needed for their development, ensuring elevated levels of fuel oxidation and electron transfer for electrical energy production [101].
In MFCs, a wide variety of microorganisms have been used as electron donors and acceptors. They include Phormidium sp., Chlorella vulgaris, Saccharomyces cerevisiae, Leptothrix discophora, Scenedesmus armatus, Rhodispirullum rubrum, Thiobacillus ferrooxidance, Desulfovibrio desulfuricans, Pseudomonas fluroscens, Geobacter metallireducens and some anaerobic bacteria [10, 71]. Genetic engineering has also grown in importance. We can modify microorganisms to enhance analyte detection systems or express them in different ones [111]. DNA segments coding for detecting mechanisms may be isolated and introduced into prototype organisms such as S. cerevisiae and Escherichia coli, which have optimal growth conditions. To get the highest expected signal detection, the organism and detecting configuration should be correctly integrated. Some of the species listed above have been genetically modified to generate considerably more current and long-term biomass production than their wild-type strains.
5 Power Sources of MFCs-Based Biosensors
Electrochemical batteries (e.g. lithium batteries) power the majority of commercial sensor and biosensor devices, which have a reduced and comparatively short life cycle and must be recharged or replaced on a regular basis [6]. To find out effective and self-renewable power supplies to generate adequate power for distant devices when battery substitution is neither possible nor accessible is a hot topic these days [48]. In this perspective, self-renewable MFCs seem to be a viable long-term energy source for remote monitoring biosensors and sensors. In distant sensors, supplanting traditional batteries with MFC power sources reduces operational costs and reduces environmental concerns significantly [63].
The MFC is a device in which microbes use organic substances as a nutrition supply, produce electrons/energy from the assimilation of organic compounds by microorganisms’ metabolic activity and discharge those electrons to an electrode, which generates electricity [17, 62, 132]. An MFC is an electrochemical bioreactor that uses the unique characteristics of the colony of bacteria located within the MFC chamber to produce electrical energy. An MFC is made up of a pair of conductive electrodes with a bacterial habitat between them. The electrogenic bacteria employed determine the cell's specific voltage potential and maximum output power [28, 94]. The bacteria that inhabit the MFC release electrons as a result of their metabolism of organic substances available in the environment, and the amount of power provided is dependent on the colony's health and organic nutrient sources. It is essential to consider the MFC's influencing elements in order to get desired results. Microorganisms and their metabolism; substrates and their concentration; electrode element and electrode shape, membrane type;; mechanism of electron transfer in an anodic compartment; functioning parameters such as pH, temperature and salt concentration; cathodic compartment’s electron acceptor and geometric layout of the MFC are the most vital parameters among the numerous factors affecting MFC performance in biosensing [5]. For the power supply of low-power embedded systems, MFC is a viable option to other fuel cells [22, 99] or accumulator technologies [125].
The hydrolysis of complex substrate such as proteins, carbohydrates and lipids in the anaerobic digestion chamber begins with the conversion of these molecules to hydrolysates which consists of amino acids, sugar and long-chain fatty acids. Hydrolytic microorganisms are the ones liable for this process. Hydrolysate conversion to simple organic acids, CO2 and H2 is the next step in anaerobic digestion, which is carried out by acidogens or fermentative bacteria. In the third phase of anaerobic digestion, acetogenic bacteria convert simple organic acids to acetate, carbon dioxide and hydrogen. The last phase in the anaerobic digestion method is the conversion of acetate to H2O, CO2 and electrons, which is carried out by electrogens, but acetoclastic methanogens can also convert acetate to methane and carbon dioxide. Furthermore, a kind of methanogen known as hydrogenotrophic methanogens may use electrogens to convert protons and carbon dioxide into methane [14, 18]. Figure 5. depicts the successive power-generating procedures. The electrons produced by this process are used in both the power production and biosensing processes.

Reprinted from Ref. [14] with permission. Copyright (2021) Elsevier
The sequential biodegradation processes of a complex substrate by different microorganisms are for power generation in MFC-based biosensors.
For the bacteria to operate and release the charges/electrons that the cathode needs to complete the electricity generation, the anode must be placed in an oxygen-poor layer. The cathode, on the other hand, is positioned on top of the media or aeration chamber because it must exchange oxygen with the environment while combining positive charges and receiving electrons from an external circuit. This process produces water as a by-product. As far as bacteria absorb nutrients from the medium, this process works [23].
6 Mechanism of Electron Transfer in MFCs
Electrons must be transferred from interior microbial cell membrane to the outer surface for MFC to operate, as electrodes cannot penetrate cell membranes because they are solid things. This can be accomplished by (a) utilising electrons that leave the cell membrane via membrane-attached redox enzymes or (b) transferring reduced substances physically. The process of electron transport to the electrode, however, leads it towards redox-active units capable of creating electric connections between the bacterial cell and the electrode [113]. Microorganisms can transport electrons to the electrodes via two major processes (Fig. 6).
Different mechanisms of electron transfer include short-range electron transfer via cytochrome b of the membrane (direct), electron shuttle mediated through intermediate molecules (indirect) and long-range electron transfers via the pili (direct) of a bacterium. Reprinted from Ref. [10] under Creative Commons Attribution License (CC BY)
6.1 Direct Electron Transfer
In the first kind of MFC, the bacterium directly transmits electrons from its membrane to the electrode, bypassing any intermediary fermentation product [88]. This is known as a direct transfer. Because these microorganisms are the catalysts in electron transfer, these MFCs need the use of a highly active microbial consortia, which can be mixed cultures. The transfer is facilitated by cytochrome proteins adsorbed on the bacterial cell wall. This kind of bacterium includes Rhodoferax ferrireducens [92] and Geobacter sulfurreducens [21].
The direct electron transfer method is based on the capability of several microbes, sometimes recognised as exoelectrogens, to carry electrons generated by organic matter oxidation directly to the anode. Bacterial membrane-bound-redox-active proteins (for example, c-type cytochromes and multi-heme proteins) and pili are both involved in this process [118]. The direct transfer of electrons produced during consumption from electroactive bacteria to the anode is the most significant process (Fig. 1). It was initially hypothesised that microorganisms might transport electrons to an electrode surface when cultures of Shewanella putrefaciens generated electrical energy while metabolising lactate [117]. Shewanella putrefaciens MR-1, a metal-reducing bacterium, was shown to pose cytochromes in its external surface. These transport proteins (cytochromes) were able to produce anodic current in the absence of terminal electron acceptors in anaerobic settings. In Shewanella, it was also revealed that external membrane cytochromes play a role in electron shuttle reduction [157].
A variety of exoelectrogens have been described to use direct electron transfer pathways to transport electrons to the anode, although Geobacter sulfurreducens has been the most thoroughly investigated in this respect, owing to its genome being sequenced. Around 110 genes in the G. sulfurreducens genome are thought to encode for type-c cytochromes, which are supposed to perform a key function in extracellular electron transport mechanism [4]. A research found that a mediatorless MFC injected with Desulfovibrio desulfuricans and supplied with electrochemically cured graphite felt electrodes had the maximum current density of 233 mA/m2. D. desulfuricans cytochrome-c was implicated in the effective transport of electrons to the electrode surface [68].
In single-layer biofilms, most of the cells are in tight proximity to electrodes and are therefore engaged in current generation directly. Because just a few cells may directly reach electrode surfaces in multiple layer biofilms, long-array electron transport techniques such as nanowire/pili are utilised. The thick pili network has metal-like conduction, which is responsible for conductive biofilms’ high current production. Various bacteria, including G. sulfurreducens, generate conductive pili. The pili such as type-IV are nanowires engaged in the transfer of electrons between chambers in the biofilm and to the electrode's surface. Charge transmission from cell to cell through pili was recently demonstrated by electrostatic force microscopy to be comparable to that of carbon nanotubes [4].
In the case of heterogeneous cultures, exoelectrogens employ direct interspecies electron transfer for intermediate electron transmission. Direct interspecies electron transmission is facilitated by pilus and pilus-linked c-type cytochrome OmcS. Earlier transcriptomic and genetic investigations showed that G. sulfurreducens and G. metallireducens developed conductive aggregates when metabolising ethanol, and that the aggregates exchanged electrons during syntrophic interaction [34]. Tetrathiobacter, Clostridium, Aeromonas and Desulfovibrio anolyte communities were investigated for bioelectricity production. Proteobacteria and Firmicutes have a syntrophic relationship, according to community analysis. Fermentation and ferredoxin-facilitated electron transport to the electrode were both aided by Clostridium. Sulphate-reducing–sulphur-oxidising bacteria such as Aeromonas, Desulfovibrio and Tetrathiobacter transport electrons directly to the electrode [81].
6.2 Indirect Electron Transfer
Soluble molecules are used to mediate electron transport in indirect electron transfer. Artificial exogenous redox mediators and soluble electron shuttles produced by microbes are the two most common types of mediated electron transfer [25].
6.2.1 Using Artificial Exogenous Redox Mediators
A fermentative microbe generates alcohols, carbon dioxide, hydrogen or ammonia as by-products in an indirect MFC or mediator-based MFC. Electrons produced during substrate catabolism are utilised in anaerobic circumstances to reduce transitional products like protons or acid to make hydrogen or alcohol, correspondingly. As a result, the fermentative bacteria are unable to give electrons to the anode directly. An external mediator that can shuttle within the cell membrane and the anode is necessary to use this bacterium on the anode. Some typical exogenous electron mediators shuttling within the anode and the cell membrane of fermentative bacteria are thionine, benzylviologen, 2-hydroxy-1,4-naphthoquinone and 2,6-dichlorophenolindophenol [87] (Fig. 6).
Because of the numerous drawbacks involved with this strategy, it has been widely abandoned. Cohen suggested the use of inorganic or organic compounds such as benzoquinone or potassium ferricyanide to assist electron transport from cells to electrodes to solve the low current production problem in 1930 [42]. Benetto and his co-workers revived this technique in the 1980s, and it gained a lot of popularity. Because of their potential as mediators, a wide variety of molecules based on phenoxazine, quinones, phenazines and phenothiazine were chosen [113, 120].
Artificial mediators have a number of drawbacks, including low current densities (10–100 µA/cm2) and the requirement for frequent supplements, which is both ecologically and technologically impractical. As a result of these drawbacks, this technique has mostly been abandoned. Artificial mediators are no longer required for mediated electron transfer using natural electron shuttles and direct electron transfer processes, according to most experts [25].
Soluble electron shuttles secreted by microorganisms
Bacteria without pili can generate electricity by secreting secondary metabolites such as pyocyanins, flavins and quinones, which function as endogenous soluble electron shuttles. These electron shuttles communicate with cytochromes to transport electrons to the electrode. G. sulfurreducens secretes riboflavin, which forms a connection with OM c-Cysts and therefore performs an essential part in the extracellular electron transfer mechanism [100]. Pseoudomonas aeruginosa strain KRPI was previously reported to generate phenazine-1-carboxamide and pyocyanin to transport electrons through the cell membrane. A glycolipid surfactant named Sophorolipid was recently introduced to the system, which improved the penetrability of the cell membrane and boosted pyocyanin synthesis. As a result, compared to the control, power output rose fourfold [138].
Microorganisms such as Lactobacillus, Enterococcus and Pseudomonas aeruginosa have been found to produce electron shuttles (i.e. phenazines by Pseudomonas aeruginosa) that aid in electron transport to the anode. Shewanella oneidensis has been found to use an electron shuttle-like mechanism to reduce extracellular Fe3+ [130]. The production of natural mediators such as riboflavin and pyocyanin is an energy-requiring process that bacteria do when they are stressed (Fig. 6).
MFC has also employed thicker cell walls including microorganisms such as gram-positive bacteria like Bacillus sp. and yeast like Pichia stipitis. To discover key mediators in extracellular electron transport, researchers studied exoelectrogenic Bacillus sp. WS-XY1 and P. stipitis. Both bacteria employ flavins to mediate extracellular electron transport, according to the findings [34, 113].
The processes by which electrically active bacteria get electrons from the cathode are far less well understood than the processes by which similar bacteria get electrons from the anode. Nonetheless, it has been proven that bacteria get electrons from the cathode via a different process than electron transport to the anode. The S. oneidensis MR-1 employed riboflavin as an endogenous electron shuttle to transmit electrons to Cr(VI) inside the cathode (in the existence of lactate in aerobic states) [163]. Two methods have been documented for accepting electrons from the cathode: direct and indirect electron transfer. Electroactive microbes are in direct bodily interaction with the surface of the cathode in direct electron transfer, and electrons are accepted utilising OM c-Cysts. Indirect electron transfer, on the other hand, happens through soluble electron shuttle mediators, in which an oxidised mediator molecule is reduced to the cathode surface. Furthermore, electrons are transported to the bacteria via reduced mediator molecules [80]. A gene involved in electron transport from the cathode has been identified. A research found that the GSU2374 gene was expressed in cathodic biofilm. This gene is thought to code for a monohaem-c-type cytochrome (PccH). Moreover, mutation analysis has shown that biofilms that lack this gene are unable to absorb electrons from the cathode. Despite this, biofilms continue to contribute electrons when employed on the anode, indicating the gene's critical function in electron transfer mechanism from the cathode to the microorganisms [131].
7 Merits and Scopes of MFCs Biosensors
Enzymes are the most often utilised biological sensing component in biosensor manufacturing. Due to the time-consuming, labour-intensive and expensive process of enzyme purification, purified enzymes are not a good alternative for biosensor development. In a traditional biosensor, several enzymes are required to produce the detectable product of the cofactor/coenzyme, whereas microorganisms (MFCs) provide an excellent alternative. Because the cell has a high number of enzymes and co-factors, it can digest and detect huge amounts of substances, but this might impair selectivity. MFCs may be readily controlled and altered to consume/degrade novel substrates under culture conditions. Furthermore, advances in recombinant DNA technology have opened up more options for modifying microorganisms to increase enzyme performance, making microbes an effective biosensing element [85].
MFC has the potential to be used for sustainable effluent treatment, as well as concurrent power generation from renewable biomass and biosensing. MFC can cope with a wide range of waste streams, including industrial, agricultural and municipal wastewaters [30, 78]. The current generation now includes stackable MFCs. Erable et al. [52] conducted research throughout the world to enhance the energy density of MFCs and get them more cost-effective to deploy on a broad scale. MFC may be utilised for a range of applications besides to wastewater treatment, such as BOD biosensors and bacterial account. As a BOD and toxicity detection biosensor, MFC enhanced with electrochemically active microbes have been employed. Toxic chemicals including arsenic, cadmium, lead, chromium (VI), mercury, surfactant, cyanide and organophosphorus compounds induce changes in the electric current signals of MFCs, making toxicity in the water simpler to detect. Current generation was shown to be relational to the concentration of hazardous and biodegradable waste at low concentrations. To examine the condition of wastewater, hazardous chemicals in the aqueous system might be combined with BOD measurements [110]. Sediment-based MFCs have recently showed potential in the management of artificial wetlands. The current generated by the sediment MFC may be stored in capacitors and then utilised to power remote sensors via a power management system. The underwater monitoring devices were designed to be powered by a solid-phase MFC. Low-power biomedical devices implanted in people have also been found to benefit from MFCs for supplying long-term, steady power [16]. The Saccharomyces cerevisiae-biocatalysed micro-MFCs may generate energy from glucose in the bloodstream. This MFC can be used to track food spoiling. As a result, the current generation has been revealed to be rising at a faster rate with an escalating extent of contamination. This type of technology might potentially be useful for quickly detecting and counting bacteria in contaminated food [30].
Finally, the most significant benefit of an MFC is that it is able to produce burning and pollution-free electricity directly from biomass organic substance, which may be used in the sensing process. Microorganisms use enzymatic processes to transform the energy held in chemical bonds in organic compounds into bioelectrical energy in an MFC. As a result, MFC energy production is linked to bacteria's regular life activities [101].
8 Analytical Applications of MFC-Based Biosensors
Various variables, such as organic chemical modes, pH, temperature, toxicants, inhibitors and concentration, have an impact on MFC voltage and power output. This implies that, in addition to acting as a backup power supply for remote sensors, MFCs may also be employed as biosensors to find a variety of factors [169] described below. The capacity of MFCs to produce electrical current, as well as their ability to facilitate on the spot and real-time checking of different analytes, might allow them to function as efficient biosensors. We recapped the most recent advancements in numerous biosensor applications utilising MFCs including analysing BOD, detection of toxicants and DO, monitoring microbial activity, detection of microbial biofilms and VFA, which are very significant factors for usable water.
8.1 BOD Detection
The quantity of DO required by aerobic bio-organisms to break down organic substances accessible in a given water sample at a particular temperature during a certain period is referred to as BOD. Because it serves as an indicator for evaluating the effect of discharged waste on the ecosystem, BOD is an important water quality metric [79]. The greater the BOD number, the more organic matter or food available for oxygen-consuming bacteria. Unfavourable circumstances arise when the amount of DO intake by bacteria surpasses the amount of DO supply by aquatic organisms or diffused from the air. Reduction of DO puts aquatic creatures under stress, putting the habitat unfit for existence. Furthermore, severe depletion might result in hypoxia or anoxia. BOD is also widely utilised in wastewater treatment, where the breakdown of organic waste by microbes is a typical treatment method. In general, for direct environmental wastewater discharge, the maximum permitted concentration is approximately 10 mg/L BOD, whereas for sewer system discharge, the maximum allowable concentration is around 300 mg/L BOD [129].
The amount of biodegradable material in water, or BOD, is a common element in managing and evaluating the operation of a wastewater treatment facility. The conventional method of determining BOD takes 5–7 days and should only be done by professionals. Because this approach is labour-intensive and time-consuming, an alternate method for monitoring BOD onsite that is quick and easy is required. For the first time [69], suggested the usage of MFCs as a BOD sensor. The bacterium, Clostridium butyricum, was immobilised on the electrode surface in the anodic compartment, and a linear correlation was detected within current output from the MFC and BOD concentration, indicating that MFC-based BOD biosensors are feasible.
Following then, some kinds of MFC-based BOD sensors were described, as well as numerous microorganisms were employed [149, 172]. MFCs with electron mediators were also investigated as BOD biosensors [152], with the mediators assisting electron transfer from the microbial cells towards the electrode, however these biosensors were unstable over time due to the toxicity of the mediators to microbes. Chang et al. [31] demonstrated that a mediatorless MFC may be utilised to constantly assess the BOD of effluent for real-time examining using a mediatorless MFC. Furthermore, an MFC-based biosensor was described to have functioned in a stable manner for over 5 years [72], which was far longer than formerly stated BOD biosensors (7–140 days) [91]. This proved the benefits of MFC-based biosensors in the long run. Unlike traditional sensors, MFC-based biosensors directly employ the quantified voltage or current as output signals, making them easier to process and display. They may also be developed and used in distant places due to their capacity to generate electricity on their own [43].
Microbial decomposition of organic substances and their transformation into an electrical current were used to develop MFC-based BOD sensors [107]. One of these BOD biosensors was made as a low-cost, single-compartment MFC utilising anodic organic substrate and activated slurry, and its viability as an actual BOD monitoring device was confirmed [168]. When combined with synthetic wastewater, this system showed a constant voltage after 132 min, causing in a BOD rate of 200 mg/L. The response signal was observed to be enhanced and related to the trend of the increase in BOD content from 5 to 200 mg/L. After the BOD concentration exceeded 120 mg/L, the response signal remained constant. To address several of the constraints of the MFC-based BOD biosensor, a novel arrangement with enhanced features was designed, which used an exterior voltage to surmount internal resistance and permit microorganisms to amplify electricity generation [107]. This configuration was membrane-free to avoid pH fluctuations that would limit the sensor's applicability to low alkaline effluents. During a 20 h reaction period, BOD levels ranging from 32 to 1280 mg/L showed a linear relationship with charge. The sensing ability was lowered to a level of BOD of up to 320 mg/L when the response period was cut to 5 h. The need of exterior voltage equipment for MFC biosensors limits their use to remote monitoring in far-flung locales. Auto-generated power floating biosensors for actual water condition checking were developed as a result, removing the requirement for external power and allowing maintenance to be incorporated into other settings [123].
BOD sensors, on the other hand, are employed in the early detection of feed water condition. This technique is valuable for detecting the beginning of biofouling in reverse osmosis (RO) membranes used in saltwater purification [128].
Biofouling is one of the most severe troubles in desalination techniques, since it causes serious problems such as flux loss, short membrane lifetime and increased energy usage. Before starting the RO process, recent biofouling detection methods measure the silt total direct cell quantification [64], density index [9] and the biofilm growth rate [77].
As a result, detecting assimilable organic carbon (AOC) in the feed flow of RO plants is a useful method for estimating possible biofouling. AOC monitoring marine MFC biosensors was built, injected with a marine sediment bacterial strain, and examined for 36 days. In the range of 0–150 μmol/L (0–3600 μg/L) of AOC, the results indicated a linear connection between electrochemical signals and acetate concentration. Nonetheless, at high acetate concentrations ranging from 150 to 450 μmol/L (3600–10,800 μg/L) of AOC, this biosensor revealed a deviating linear relationship [49].
8.2 Toxicity Detection
The industrial revolution advances civilization, but it also introduces a plethora of new-to-nature compounds into the environment including water [124]. Many of them are hazardous to both people and other living things. The detection of toxicity in water is a key criterion in identifying the measures that must be taken to provide safe, high-quality water for human, animal and agricultural use. Off-site chemical analysis utilising physicochemical techniques such as high-performance liquid chromatography, gas chromatography-mass spectrometry and mass spectrometry is a traditional strategy [38, 61]. These techniques are often slow and ineffective for real-time detection. MFC-based biosensors are an excellent option since they are directly based on the toxicants’ biotoxicity impacts. The composition of wastewater can be quite complicated, and a wide range of poisonous substance may be available. Compounds that can change the pH of wastewater, such as acid mine drainage, where the pH can be down to 2.4, inorganic and organic compounds with extremely severe toxic properties, phenolic compounds, heavy metals and so on, are all potential toxicants [141]. Toxic contaminants can block the action of electrogens, causing the current produced by MFCs to be interrupted [74]. The current diminishes the more poisonous the chemical is to the bacteria. As a result, multiple toxicity sensors may be developed based on the connection between hazardous chemicals and current decrease amplitude [169]. Toxicity sensors are primarily utilised to evaluate if the concentration of hazardous chemicals in an effluent surpasses the regulatory highest concentration limit. As a result, the emphasis of MFC biosensor for toxicity testing is based on the detection limit of the contaminants rather than the linear range as in BOD. The detection threshold of MFC-based toxicity biosensors is yet far away from the World Health Organization's water quality standard (tens to hundreds of times higher) [67]. Heavy metals sensors, antibiotics detection sensors, organic toxicants sensors and acidic toxicity sensors are the four main types of MFC-based toxicity biosensors depending on the target pollutants.
Heavy metals detection
Pollution containing heavy metals has the potential to harm human health as well as the environment. Heavy metals, such as mercury and arsenic, for example, produce significant toxicity in the neurons and endocrine system, as well as heart problems, skin damage and cancer [61]. Heavy metallic elements have a lengthy half-life (ten to hundreds of years) and are difficult for microbes to eliminate or decrease. They will also accumulate in the human body as they go up the food chain, and once certain concentrations are reached, they may cause health issues, despite the fact that some of them are necessary for human health [151].
Heavy metal ions can limit microorganisms’ respiration activities [57], which is the basis for affecting the current generation of MFCs. Six heavy metals such as Hg2+, Cu2+, Zn2+, Cd2+, Cr3+ and Pb2+ions (2 mg/L) were evaluated in a double compartment MFC arrangement, and their resistant rates on current output were 12.56%, 13.99%, 8.81%, 9.29%, 5.59% and 1.95%, respectively [175]. Zhiheng [167] proposed a flat membrane-based MFC biosensor and verified it with two different ions (Ni2+ and Cr6+) to increase the sensitivity and stability of MFC-based biosensors. After adding 10 mg/L Cr6+ for 40 min, the voltage dropped to 40 mV from 180 mV, and after adding 20 mg/L Cr6+, the voltage dropped to 50 mV in 6 min. Injecting Ni2+ (20 mg/L) into the anolyte, on the other hand, only caused in a modest voltage decrease from 180 to 150 mV after 180 min. With a higher concentration (50 mg/L Ni2+), the voltage decrease occurred faster (45 min), but the change was in the same range.
Heavy metallic ions can compete for electrons with the anode in the anodic compartment, resulting in a small number of electrons being transported to the cathode in some MFCs designed for specific target compounds. Cr6+ is a terminal electron acceptor that may be reduced by Cr6+-reducing anaerobes in anaerobic circumstances [37]. When an MFC is made utilising Cr6+-reducing anaerobes, the cell voltage is anticipated to drop as the Cr6+ concentration rises. The Cr6+-reducing bacterium Ochrobactrum anthropi YC152 was injected into an MFC for the determination of Cr6+ as proof of concept (Guey-Horng [156]). The outcomes showed that the proposed biosensor can detect Cr6+ in the scale of 0.0125–5 mg/L quantitatively. Wu et al. established a comparable method utilising Exiguobacterium aestuarii YC211, a Cr6+-reducing bacteria with a linear range of 2.5–60 mg/L [160].
In contrast to the negative effects, there was also a positive connection between ions and MFC outputs. Iron-oxidising bacteria were a good example. Iron-oxidising bacterial consortia can employ iron (II) as the only electron source in the Anolyte. To build an MFC-based biosensor [153], injected this particular bacterial community onto the anode. Within the concentration range of 3–20 mM, a linear relationship between current output and Fe2+ was found.
Rather than detecting heavy metallic ions through their biological effects on electrogens, the biosensor may be constructed by using them as MFC cathode electron acceptors. In MFC sensors, the abiotic cathode-sensing element has recently been explored to detect heavy metallic ions (e.g. Cu2+, Cr6+). Shuai Zhao et al. [182] applied a sediment MFC (SMFC) to watch Cr6+ in industrial effluent and found that Cr6+ was decreased at the cathode. The linear response scale was 0.2–0.7 mg/L, which was significant for Cr6+ detection limit. The Cu2+, which acts as an electron acceptor and is finally accumulated on the surface of cathode as Cu (0), was also measured using the SMFC [161]. The voltage increases and the Cu2+ intensity (5–160 mg/L) was found to have a linear relationship (R2 = 0.87).
Heavy metallic ions in tap water are now monitored using MFC-based biosensors. In order to detect toxic shocks in tap water, an MFC biosensor based on O2-reducing bacterial cathodes was developed [127]. The detection limits for three heavy metallic ions (Cr6+, Hg2+ and Pb2+) were found to be in the range of 1–10 mg/L.
Antibiotics detection
Antibiotics are utilised in animal production, as a preventative measure in animal feed, and as therapeutic medicines. Only a tiny percentage of antibiotics consumed by fauna are metabolised, allowing a large part to accumulate in tissues or be excreted and released into the environment. Antibiotics in the environment may lead to antibiotic resistance, with the risk of transmission to humans via the food chain [61]. Antibiotics have saved millions of lives, but their inappropriate management and release into the environment has disrupted the normal evolution process, posing several safety concerns for microbial ecosystems and, as a result, humans [59]. Tracing and controlling antibiotic discharge and distribution has become a critical issue for future generations. MFC is one of the real-time techniques for detecting antibiotics in the field, among all the antibiotic sensors.
To detect tobramycin, Wenguo [162] built a single-chamber MFC with hydrophilic carbon fabric as the anode. There were no discernible effects at concentrations of 0.10, 0.24 and 0.47 g/L. However, as the concentration reached 0.93 g/L or above, the current production dropped significantly. After the addition of tobramycin, less than half of the initial current production could be preserved. Interestingly, depending on the tobramycin concentration, the current might be restored after hundreds of hours. Owing to the ‘self-healing’ nature of the electroactive biofilms in MFCs, this occurrence indicates the stability of MFC-based sensors for tobramycin (and perhaps other antibiotics) detection over long-term operation.
Schneider et al. [135] used tiny MFCs in a panel system to develop a rapid method to β-lactam antibiotics analysis. Two model bacteria, E. coli strain ATCC 25,922 and S. aureus strain ATCC 29,213, were employed to test hypothesis proof, and 10 separate β-lactam antibiotics (cefoxitin, ampicillin, cefazolin, cefoperazone, cefepime, cefuroxime, imipenem, ticarcillin and penicillin) were tested at concentrations varying from 1 to 75 μg/mL. 2–4 h after introducing the cell mix solution into the MFCs, the antibiologic effects of these drugs could be evaluated in terms of changes in cell voltage output, whereas the standard Kirby–Bauer disc diffusion technique for antibiotic testing needs 24–48 h.
A single-chamber MFC was utilised to test another frequently used antibiotic, levofloxacin [177]. The MFC biosensor can identify levofloxacin at up to 1000 μg/L using sodium acetate as the energy source in the anode. In the range of 0.1–100 μg/L, a linear association (R2 = 0.924) was found between current yield and levofloxacin antibiotic concentration. Furthermore, this MFC has been operational for over 14 months and continues to generate a consistent electrical production, illustrating the benefits of MFC-based biosensors for antibiotic detection in enduring usage.
Organic Toxicants detection
Organic toxicants in water, such as polycyclic aromatic hydrocarbons, organic phosphate compounds, organic nitrogen compounds and polychlorinated biphenyls (PCBs), can induce eutrophication and have negative consequences for public safety [83, 137, 170]. Kim et al. [74] used a double-chamber MFC to investigate the toxicity of diazinon and PCBs, finding inhibition of 61% and 38%, respectively, for diazinon and PCBs (1 mg/L). Weiyang Yang et al. [171] established a special micro-sized MFC for formaldehyde detection in water. A solid-state thin film Ag/AgCl reference electrode and a microscale air bubble trap were used in this micro-sized system to maintain an optimum anodic potential and counteract microscale air bubbles from accessing the MFC biosensor. The current dropped proportionately to the concentration of formaldehyde in the medium, which ranged from 0.001 to 0.10%, whereas the anode voltage was held constant at 0.20 V versus the reference electrode. A single-element paper MFC has recently been developed and tested for chemical detection in the water phase [39]. During the procedure, the recyclable carbon-based electrodes were imprinted on a single sheet of paper, with the anode merging into the liquid state and the cathode remaining in the gas phase. Because of the capillary force generated by the paper material, the paper basement served as both a divider between electrodes and a bridge for mass transfer. The addition of 0.1% (v/v) formaldehyde to the existing output resulted in an abrupt decrease in the current production. Furthermore, two MFCs may be printed on a single sheet of paper and linked in parallel by folding them back to back. The current output of the stacked MFCs was entirely dropped in 115 min, contrasted to 175 min for the single paper MFC, indicating that they were more sensitive to formaldehyde shock.
In contrast to the inhibitory impacts, Zhengjun [36] developed a double-chamber MFC utilising p-nitrophenol (PNP) as the only substrate that exhibits stimulatory effects. The anodic chamber was kept in an aerobic state by inoculating the reactor with an aerobic bacterium, Pseudomonas monteilii LZU-3. The cell voltage improved at higher PNP concentrations when the optimum operating parameters were used (pH of 7.8, external resistance of 1000 W and temperature of 30 °C). The PNP concentrations in the range of 16–44 mg/L were found to have the highest linear voltage relationship (R2 = 0.98). Even when PNP was combined with other aromatic molecules (5 mg/L of toluene, nitrobenzene and 2-nitrophenol), a linear relationship between PNP concentrations (9–36 mg/L) and cell voltage could be seen.
Acidic Toxicity detection
Acidic toxicity is of particular importance to be checked online, since numerous forms of hazardous chemicals in wastewater, such as mine drainage, induce a rapid shift in pH [148]. A low pH value inhibits microbial growth and activity and inhibits the growth of other aquatic plants and animals, reducing the water body's capacity to self-purify and deteriorating the water quality. A single-compartment air-cathode MFC was constructed and functioned in a continuous batch method by Yu et al. [141]. HCl was used to change the pH of the influent (i.e. the electrolyte in the working compartment). When the pH was kept within 3–4, the output voltage dropped quickly and then retrieved after the addition of HCl was stopped. Altering the pH of the influent to a value of 2, on the other hand, resulted in a voltage output crisis, which was most likely triggered by the total destruction of electrochemically active biofilm in high acidic circumstances. To detect acidic toxicity, [66] built a cathode shared MFC sensor arrangement. Because the cathode performance fluctuation was minimised, the detection credibility of this sensor assembly operating in non-stop mode might be guaranteed. After the MFC array attained a steady state, acidified anolyte was used to provide an acidic toxicity shock. The voltage dropped from 200 to 0 mV very quickly when the pH was lowered from 6 to 4. The threshold value of pH may vary depending on the biofilm composition, nonetheless, this phenomenon allows for a possible method of obtaining the pH in water, based on the disruption of MFC cell voltage.
Acid rain impact was also reported to be observed, likewise, to checking acidic toxicity in water. Rhizosphere microorganisms in plant MFCs (PMFCs) may produce electrical current by decomposing the organic defaecates of the rhizodeposits, thus any variations in the bioavailable substrate concentration could impact the electrical current [134]. Tian [86] used a mixture of concentrated HNO3 and H2SO4 solution to imitate acid rain and built PMFCs to assess acid rain damage. Artificial acid rain might harm rice plant leaves, lowering photosynthetic activity, which is linked to rhizospheric electrochemical activity. After pretended acid rain was sprayed on the leaves of plant, immediate and reproducible current decrease was recorded within 2 min, which was in good agreement with variations in rhizospheric organic concentration.
8.3 DO Detection
In natural waterways, oxygen is required for a variety of chemical, biological and metabolic processes. DO is without a doubt one of the most important and broadly used indicators of water quality [180]. The levels of DO in aquatic settings provide an essential quality indicator for biochemical and biological activities. To detect DO in water, various physical, chemical and electrochemical techniques have been developed [35]. Though electrochemical techniques are widely utilised due to their ease of application and high sensitivity, the biological applications of these technologies have been studied in a few research. The development of a submergible MFC biosensor for online and in situ quantification of DO in aquatic environments was one of the first efforts to employ a bioelectrochemical sensor for DO [180]. The sensor was powered using domestic effluent as a substrate. The functioning of the sensor was evaluated using tap water as a control at various DO concentrations. When employing an external resistance of 1000, the sensor produced a current intensity in the range of 5·60–462·20 mA/m2; as a result, it linearly rose with the increase of DO intensity up to 8.80 mg/L, with a reaction time of below 4 min for each measurement [49].
The quantity of dissolved oxygen (DO) in water is an important indication and a frequent criterion in water management [102]. Its fluctuations, for example, have been found to correlate the amount of organic contaminants flowing into a freshwater lake [11]. It also offers crucial information on biological and metabolic processes in the water ecosystem, and the DO level acts as a natural selecting pressure for various microbe lifestyles. Because Clark-type oxygen sensors are substantially influenced by ambient factors such as pressure, reliable DO measurement in the field is difficult [102]. MFCs are an alternate method for measuring DO. MFC-based biosensors are more robust against external conditions than Clark-type oxygen electrodes and can enable actual checking in the field. The cathode behaviour is the core concept of MFC-based DO measurement. The cathode efficiency is a performance limitation for MFCs [181] but oxygen, as the ultimate electron acceptor, has a substantial impact on the cathodic reduction rate and therefore the current output. Oh et al. [115] discovered a Monod-like kinetic connection between DO levels and current density, with a 1.74 mg/L half saturated DO value.
The ability to measure DO online can be useful for understanding the aquatic ecology. Periodic oxygen stratification has been discovered in several shallow freshwater nutrient enriched lakes in recent years, which ultimately leads to the creation of a lake's ‘dead zone’ [139, 179]. Monitoring DO levels in a lake can act as a primary warning system for the possibility of a ‘dead zone’. Song et al. developed a sediment-based multiple cathode MFC system for this purpose, which incorporates several cathodes positioned at various depths of water for in situ, non-stop and online monitoring of DO intensities and lake deepness [144, 145]. In the range of 0.0–9.0 mg/L, there was a direct connection (R2 = 0.9576) between voltage and DO [144, 145].
8.4 Microbial Activity Detection
Because the presence of Escherichia coli indicates faecal contamination, a precise quantification of E. coli can be regarded critical for detecting faecal contamination and safeguarding community health. An MFC was employed as an E. coli sensor, with specified E. coli enzymes, for instance, β-d-glucuronidase (GUS) and β-d-galactosidase (GAL) serving as biological monitoring components [76]. The GUS was measured using 4-nitrophenyl-β-d glucuronide and 8-hydroxyquinoline glucuronide as substrates, whereas GAL was measured using 4-aminophenyl β-d-galactopyranoside as a substrate. The detection process of these compounds is based on GUS or GAL hydrolysis, followed by electrochemical activation and an oxidation phase in the MFC's anode compartment. As the E. coli concentration approached the threshold level, the power produced by the MFC reactor increased dramatically [49].
Microorganism screening and phenotyping using traditional microbiological methods are quantitative but labour-intensive and time-consuming. MFC was recommended in this context as a quick and simple way to get first-hand information about the microbe and its overall lifestyle [3]. The process is based on microbial metabolic activity (and hence electron output to anode) that is uniquely influenced by the surrounding environmental conditions. Miller and Oremland [104] reported a study that used arsenate's ability to function as an anode for electrons to predict the existence of arsenate-respiring microbes in soda lakes. Abrevaya et al. [1] presented another hypothetical application: using MFC to identify live (micro) organisms on distant planets, provided they might likewise export electrons throughout their life process. An MFC has been demonstrated to be a suitable technique for bioprocess monitoring in more practical terms. Zhidan Liu et al. [93] used a flow-cell MFC to track anaerobic digestor functioning and discovered that current production variations were connected to fluctuations in working indicators such as pH, gas flow rate and COD. Furthermore, MFC current density was linear for acetate intensity up to 20 mM, according to Zhidan Liu et al. [93], with very little interference from other volatile fatty acids available in the anaerobic digestor. These findings suggested that an MFC-based biosensor might be used to monitor the anaerobic digestor's metabolic turnover rates of organic molecules.
8.5 Monitoring of the Corrosive Biofilms
In several industries, such as the gas and oil industry and water utilities, microbiologically induced corrosion (MIC) is a key concern [165]. According to [8], MIC is responsible for 20% of all corrosion damage. Microbial biofilms, particularly anaerobic bacteria, are the major source of MIC owing to their metabolic activity or metabolites. Most anaerobic MIC attacks fall into one of two categories: respiration or fermentation. Microorganisms that undertake anaerobic respiration are included in Type-I MIC. Sulphate is the terminal electron acceptor in respiration of Sulphate-Reducing Bactria (SRB). As electron donors, organic compounds such as volatile fatty acids are frequently employed. Type-II MIC is characterised by the secretion of caustic metabolic products such as organic acids. Because biocorrosion is usually accompanied by a low pH, it is easy to detect. Because oxygen is cut off to prevent extreme corrosion of carbon steel, gas and oil pipelines are always maintained anaerobic. The cathodic biofilm is fed by electrons from a solid-state anode. If a corrosive biofilm is formed to the cathode, such as SRB biofilm, the electrogenic biofilm will transport the biofilm to the cytol of sessile cells, reducing sulphate levels [164].
The ability to recognise corrosive biofilms is critical when deciding whether to employ biocides or mechanical pigs to combat them. Available biofilm sensors measure electrical resistance variations across a biofilm by applying an external electrical field. This, however, disrupts the biofilm metabolism. In addition, because these sensors cannot tell the difference between a mineral layer and a biofilm, a passive sensor that does not require an exterior voltage is preferred to avoid misleading findings [169].
Electrogenicity was presented by Gu [60] as a sign of the existence of a corrosive biofilm and their potential to attack metal. The cathodic biofilm is fed by electrons from a solid-state anode. If a corrosive biofilm attaches to the cathode, like an SRB biofilm, the electrogenic biofilm will transport the biofilm to the cytol of sessile cells, reducing sulphate [164]. After calibration, the open-circuit voltage can be utilised to determine if nitrate reduction, sulphate reduction or other chemical reactions are happening at the cathode. The capacity of the cathodic biofilm to transfer extracellular electrons, which is a deadlock for an electrogenic biofilm to biocorrode, is measured by closed-circuit current flow [178].
8.6 Volatile Fatty Acids (VFA) Detection
VFAs must be monitored closely in anaerobic digesters since their aggregation can cause pH decrease and reactor failure. VFAs are often tested offline using high-pressure liquid chromatography or gas chromatography, for example, pH titration [53] and headspace gas chromatography [20] are two online methods that can identify specific VFAs but need costly equipment.
VFA sensors have also been studied using microbial electrochemical systems. Acetate is a frequent substrate in MFC research, and biological anodes have already been demonstrated to convert other VFAs into electrical current [56, 90]. MFCs were enhanced with butyrate propionate and acetate, by Kaur et al. [70]. They used cyclic voltammetry to examine the response and discovered correlation between peak current and VFA concentration. Surprisingly, MFCs enhanced in acetate and propionate only responded electrochemically when fed with their respective substrates, but MFCs enriched in butyrate responded to all substrates. This implies that acetate or propionate-specific microbial electrochemical biosensors could be possible to develop. This is especially important since the ratio of propionate to acetate concentration is a critical process parameter whose rapid shift can serve as an early warning sign of anaerobic digester problems. It should be emphasised that in an anaerobic digester, maintaining electroactive microbial populations specialised to acetate or propionate is likely to be challenging. Using membranes that prevent pre-enriched biofilms from invasion by other species while allowing substrate transfer might be a strategy to preserve the biosensor's substrate specificity.
9 Challenges and Perspectives
The MFC-based biosensor has gained increasing attention as an analytical technique due to the use of whole cells and self-powering capability. Significant advances have been achieved in the field, particularly in the detection of toxicity and BOD. However, several issues must be resolved before it can be considered a mature sensing technology that is recognised by scientific communities and stakeholders.
First, additional research into the stability of MFC-based biosensors is urgently needed, as it is frequently ignored. MFC employs a self-renewable catalyst in the form of microorganisms. During long-term operation, bacteria can grow quickly in response to ecological alterations. As a result, the biosensors’ sensitivity, selectivity and repeatability may be compromised.
The limit of detection of MFC-based biosensors, particularly for toxicity measurement, is generally much lower than the World Health Organization's water quality standard. A promising method for improving MFC-based biosensors could be to screen bacteria with a high extracellular electron transfer rate. Creating genetically modified microbes might potentially be an option for improving biosensor performance. The discovery of electrogenic genes linked to electron transport and metabolism broadens the possibilities of MFC-based biosensors, allowing them to be used for different types of applications.
Furthermore, because water quality has a significant impact on the electric signal yield of MFC-based biosensors, intervention of signal may emerge, particularly in intricate aquatic settings. A change in BOD, for example, can mute the signal for toxic substances. Jiang et al. [65] investigated the impact of background organic material content on the functioning of MFC-based toxicity biosensors in a systematic way. They evaluated the signal output of two MFC biosensors with low and high organic material contents to a pre-made response chart to produce qualitative distinctions in order to prevent signal intervention in the collective shock of toxicity and BOD. To prevent the mutual shock of toxicity and BOD, Jiang et al. [66] used biocathode for toxicity examining. Majority of MFC-based toxicity biosensors can only measure overall toxicity, and just a few research looked at the toxicity of a specific agent. Pseudomonas monteilii LZU-3, used to detect PNP, is an example of a pure cultivated or genetically modified bacterium that may be utilised in MFC-based biosensors for specialised monitoring [36]. A multi-chamber MFC biosensor with distinct electrogenic microorganisms inoculated in each chamber was also constructed to detect distinct target toxic materials in the same flow [24].
Many recent research has focused on MFC system assimilation, such as the combination of multiple anode or cathode or multiple cell MFCs, combined with other chemical or physical processes, and extending MFCs with other biological methods to increase the operating performance of MFC. When compared to a single anode/cathode, these configurations can achieve better power density [46]. However, improving the selectivity of MFC-based biosensors remains a major issue. As a result, additional research using molecular biology or other contemporary approaches is needed to improve specificity and sensitivity.
10 Conclusions
Microbial fuel cells (MFCs) have appeared as the next viable and environmentally benign energy source and, over the last two decades, MFC-based biosensors have advanced at a breakneck pace as an analytical tool. MFCs-based biosensors are gaining popularity in various fields due to their ease of application and long-term viability in quality monitoring of the environment. These biosensors are often low cost, self-powered and capable of real-time remote monitoring. It also offers distinct advantages in many applications, including ease of fabrication, ease of operation, economical and in situ monitoring. MFC-based biosensors may become recognised as standard techniques. These biosensors are built on the activity of microbes in MFCs. They can transmit electrons directly through their outer membrane proteins or pili overlaying the bacterium's outer surface. MFC technology is a unique way of utilising bacteria to generate bioelectricity from organic waste and renewable biomass using the redox metabolic processes of microorganisms. They can detect characteristics and events in their environment and transform that information into signals. MFC-based biosensors have a lot of possibility for monitoring BOD, hazardous chemicals, DO, corrosive biofilm presence and corrosivity, microbial activity analysis, VFA and anaerobic digester performance or as an energy source for other sensors.
The capacity of MFCs to produce electrical current, as well as their ability to facilitate on the spot and real-time testing of different analytes, might allow them to function as efficient sensors. But till there are limitations and lots of scope available for further development to make them viable and perfect for analytical applications. A better understanding of biofilm characteristics and organism genetic manipulation might lead to novel ways to enhance the performance of MFC-based biosensors. Some of them may see actual deployment in the near future. Majority of biosensors function well in the lab but need to be tweaked for use in the field.
References
Abrevaya XC, Mauas PJ, Cortón E (2010) Microbial fuel cells applied to the metabolically based detection of extraterrestrial life. Astrobiology 10(10):965–971
Abrevaya XC, Sacco NJ, Bonetto MC, Hilding-Ohlsson A, Cortón E (2015a) Analytical applications of microbial fuel cells. Part I: biochemical oxygen demand. Biosens Bioelectron 63:580–590
Abrevaya XC, Sacco NJ, Bonetto MC, Hilding-Ohlsson A, Cortón E (2015) Analytical applications of microbial fuel cells. Part II: toxicity, microbial activity and quantification, single analyte detection and other uses. Biosens Bioelectron 63:591–601
Adhikari RY, Malvankar NS, Tuominen MT, Lovley DR (2016) Conductivity of individual Geobacter pili. RSC Adv 6(10):8354–8357
Aghababaie M, Farhadian M, Jeihanipour A, Biria D (2015) Effective factors on the performance of microbial fuel cells in wastewater treatment—A review. Environ Technol Rev 4(1):71–89
Agubra V, Fergus J (2013) Lithium ion battery anode aging mechanisms. Materials 6(4):1310–1325
AKim H, Park H, Hyun M (2002) A media-tor less microbial fuel cell using a metal reducing bacte-rium, Shewanella putrefaciense. Enzyme Microb Technol 30(2):145–152
AlAbbas FM, Williamson C, Bhola SM, Spear JR, Olson DL, Mishra B et al (2013) Influence of sulfate reducing bacterial biofilm on corrosion behavior of low-alloy, high-strength steel (API-5L X80). Int Biodeter Biodegrad 78:34–42
Alhadidi A, Kemperman A, Blankert B, Schippers J, Wessling M, Van der Meer W (2011) Silt density index and modified fouling index relation, and effect of pressure, temperature and membrane resistance. Desalination 273(1):48–56
Angelaalincy MJ, Navanietha Krishnaraj R, Shakambari G, Ashokkumar B, Kathiresan S, Varalakshmi P (2018) Biofilm engineering approaches for improving the performance of microbial fuel cells and bioelectrochemical systems. Front Energy Res 6:63
Ansa-Asare O, Marr I, Cresser M (2000) Evaluation of modelled and measured patterns of dissolved oxygen in a freshwater lake as an indicator of the presence of biodegradable organic pollution. Water Res 34(4):1079–1088
Anu Prathap M, Chaurasia AK, Sawant SN, Apte S (2012) Polyaniline-based highly sensitive microbial biosensor for selective detection of lindane. Anal Chem 84(15):6672–6678
Asensio Y, Montes I, Fernandez-Marchante C, Lobato J, Cañizares P, Rodrigo M (2017) Selection of cheap electrodes for two-compartment microbial fuel cells. J Electroanal Chem 785:235–240
Askari A, Vahabzadeh F, Mardanpour MM (2021) Quantitative determination of linear alkylbenzene sulfonate (LAS) concentration and simultaneous power generation in a microbial fuel cell-based biosensor. J Clean Prod 294:126349
Asim AY, Mohamad N, Khalid U, Tabassum P, Akil A, Lokhat D, Siti H (2021) A glimpse into the microbial fuel cells for wastewater treatment with energy generation. Desalin Water Treat 214:379–389
Babauta J, Renslow R, Lewandowski Z, Beyenal H (2012) Electrochemically active biofilms: facts and fiction A review. Biofouling 28(8):789–812
Barua E, Hossain MS, Shaha M, Islam E, Zohora FT, Protity AT et al (2018) Generation of electricity using microbial fuel cell (MFC) from sludge. Bangladesh J Microbiol 35(1):23–26
Batstone DJ, Keller J, Angelidaki I, Kalyuzhnyi SV, Pavlostathis SG, Rozzi A et al (2002) The IWA anaerobic digestion model no 1 (ADM1). Water Sci Technol 45(10):65–73
Beylier MR, Balaguer M, Colprim J, Pellicer-Nàcher C, Ni B, Smets B et al (2011) 6.27-Biological nitrogen removal from domestic wastewater. Compr Biotechnol 6:329–340
Boe K, Batstone DJ, Angelidaki I (2007) An innovative online VFA monitoring system for the anerobic process, based on headspace gas chromatography. Biotechnol Bioeng 96(4):712–721
Bond DR, Lovley DR (2003) Electricity production by geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol 69(3):1548–1555
Boyle D, Magno M, O'Flynn B, Brunelli D, Popovici E, Benini L (2011) Towards persistent structural health monitoring through sustainable wireless sensor networks. In: 2011 seventh international conference on intelligent sensors, sensor networks and information processing. IEEE, pp 323–328
Brunelli D, Tosato P, Rossi M (2016) Microbial fuel cell as a biosensor and a power source for flora health monitoring. In: 2016 IEEE sensors. IEEE, pp 1–3
Buck J, Silver M (2015) Microbially-based sensors for environmental monitoring. Google Patents
Calabrese Barton S, Gallaway J, Atanassov P (2004) Enzymatic biofuel cells for implantable and microscale devices. Chem Rev 104(10):4867–4886
Call D, Logan BE (2008) Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ Sci Technol 42(9):3401–3406
Capodaglio A, Molognoni D, Dallago E, Liberale A, Cella R, Longoni P et al (2013) Microbial fuel cells for direct electrical energy recovery from urban wastewaters. Sci World J
Chabert N, Ali OA, Achouak W (2015) All ecosystems potentially host electrogenic bacteria. Bioelectrochemistry 106:88–96
Chan AC, Ager D, Thompson IP (2013) Resolving the mechanism of bacterial inhibition by plant secondary metabolites employing a combination of whole-cell biosensors. J Microbiol Methods 93(3):209–217
Chandrasekhar K, Amulya K, Mohan SV (2015) Solid phase bio-electrofermentation of food waste to harvest value-added products associated with waste remediation. Waste Manag 45:57–65
Chang IS, Jang JK, Gil GC, Kim M, Kim HJ, Cho BW et al (2004) Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosens Bioelectron 19(6):607–613
Chang IS, Moon H, Jang JK, Kim BH (2005) Improvement of a microbial fuel cell performance as a BOD sensor using respiratory inhibitors. Biosens Bioelectron 20(9):1856–1859
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21(10):1229–1232
Chen S, Rotaru A-E, Liu F, Philips J, Woodard TL, Nevin KP et al (2014) Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Biores Technol 173:82–86
Chen Y-P, Liu S-Y, Yu H-Q (2007) A simple and rapid method for measuring dissolved oxygen in waters with gold microelectrode. Anal Chim Acta 598(2):249–253
Chen Z, Niu Y, Zhao S, Khan A, Ling Z, Chen Y et al (2016) A novel biosensor for p-nitrophenol based on an aerobic anode microbial fuel cell. Biosens Bioelectron 85:860–868
Cheung K, Gu J-D (2007) Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeter Biodegrad 59(1):8–15
Choi SH, Gu MB (2003) Toxicity biomonitoring of degradation byproducts using freeze-dried recombinant bioluminescent bacteria. Anal Chim Acta 481(2):229–238
Chouler J, Cruz-Izquierdo Á, Rengaraj S, Scott JL, Di Lorenzo M (2018) A screen-printed paper microbial fuel cell biosensor for detection of toxic compounds in water. Biosens Bioelectron 102:49–56
Chouler J, Di Lorenzo M (2015) Water quality monitoring in developing countries; can microbial fuel cells be the answer? Biosensors 5(3):450–470
Clark DP, Pazdernik NJ (2016) Chapter 12—Environmental biotechnology. In: Clark DP, Pazdernik NJ (eds) Biotechnology, 2nd edn. Academic Cell, Boston, pp 393–418. https://doi.org/10.1016/B978-0-12-385015-7.00012-0
Cohen B (1931) The bacterial culture as an electrical half-cell. J Bacteriol 21(1):18–19
Cui Y, Lai B, Tang X (2019) Microbial fuel cell-based biosensors. Biosensors 9(3):92
D’souza S (2001) Microbial biosensors. Biosens Bioelectron 16(6):337–353
Di Lorenzo M, Thomson AR, Schneider K, Cameron PJ, Ieropoulos I (2014) A small-scale air-cathode microbial fuel cell for on-line monitoring of water quality. Biosens Bioelectron 62:182–188
Do MH, Ngo HH, Guo W, Chang SW, Nguyen DD, Liu Y et al (2020) Microbial fuel cell-based biosensor for online monitoring wastewater quality: a critical review. Sci Total Environ 712:135612
Dong X, Wang P, Fang W, Su C-Y, Chen Y-H, Li L-J et al (2011) Growth of large-sized graphene thin-films by liquid precursor-based chemical vapor deposition under atmospheric pressure. Carbon 49(11):3672–3678
Donovan C, Dewan A, Peng H, Heo D, Beyenal H (2011) Power management system for a 2.5 W remote sensor powered by a sediment microbial fuel cell. J Power Sour 196(3):1171–1177
ElMekawy A, Hegab H, Pant D, Saint C (2018) Bio-analytical applications of microbial fuel cell—based biosensors for onsite water quality monitoring. J Appl Microbiol 124(1):302–313
ElMekawy A, Hegab HM, Dominguez-Benetton X, Pant D (2013) Internal resistance of microfluidic microbial fuel cell: challenges and potential opportunities. Biores Technol 142:672–682
ElMekawy A, Hegab HM, Losic D, Saint CP, Pant D (2017) Applications of graphene in microbial fuel cells: the gap between promise and reality. Renew Sustain Energy Rev 72:1389–1403
Erable B, Duţeanu NM, Ghangrekar MM, Dumas C, Scott K (2010) Application of electro-active biofilms. Biofouling 26(1):57–71
Feitkenhauer H, von Sachs J, Meyer U (2002) On-line titration of volatile fatty acids for the process control of anaerobic digestion plants. Water Res 36(1):212–218
Feng Y, Barr W, Harper W Jr (2013) Neural network processing of microbial fuel cell signals for the identification of chemicals present in water. J Environ Manag 120:84–92
Flimban SG, Ismail IM, Kim T, Oh S-E (2019) Overview of recent advancements in the microbial fuel cell from fundamentals to applications: design, major elements, and scalability. Energies 12(17):3390
Freguia S, Teh EH, Boon N, Leung KM, Keller J, Rabaey K (2010) Microbial fuel cells operating on mixed fatty acids. Biores Technol 101(4):1233–1238
Giller KE, Witter E, Mcgrath SP (1998) Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: a review. Soil Biol Biochem 30(10–11):1389–1414
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A et al (2006) Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc Natl Acad Sci 103(30):11358–11363
Gothwal R, Shashidhar T (2015) Antibiotic pollution in the environment: a review. Clean-Soil Air Water 43(4):479–489
Gu T (2012) Methods and devices for the detection of biofilms. World Intellectual Property Organization: Patent WO2012/149487
Hashem A, Hossain MM, Al Mamun M, Simarani K, Johan MR (2021) Nanomaterials based electrochemical nucleic acid biosensors for environmental monitoring: a review. Appl Surf Sci Adv 4:100064
Islam E, Hossain MS, Sarker PK, Towhid ST, Salimullah M, Hashem A (2020) Isolation and characterization of electrogenic bacteria from tannery wastewater. Bangladesh J Microbiol 37(1):23–27
Ivars-Barceló F, Zuliani A, Fallah M, Mashkour M, Rahimnejad M, Luque R (2018) Novel applications of microbial fuel cells in sensors and biosensors. Appl Sci 8(7):1184
Jeong S, Kim LH, Kim S-J, Nguyen TV, Vigneswaran S, Kim IS (2012) Biofouling potential reductions using a membrane hybrid system as a pre-treatment to seawater reverse osmosis. Appl Biochem Biotechnol 167(6):1716–1727
Jiang Y, Liang P, Liu P, Bian Y, Miao B, Sun X et al (2016) Enhancing signal output and avoiding BOD/toxicity combined shock interference by operating a microbial fuel cell sensor with an optimized background concentration of organic matter. Int J Mol Sci 17(9):1392
Jiang Y, Liang P, Liu P, Yan X, Bian Y, Huang X (2017) A cathode-shared microbial fuel cell sensor array for water alert system. Int J Hydrog Energy 42(7):4342–4348
Jiang Y, Yang X, Liang P, Liu P, Huang X (2018) Microbial fuel cell sensors for water quality early warning systems: fundamentals, signal resolution, optimization and future challenges. Renew Sustain Energy Rev 81:292–305
Kang CS, Eaktasang N, Kwon D-Y, Kim HS (2014) Enhanced current production by Desulfovibrio desulfuricans biofilm in a mediator-less microbial fuel cell. Biores Technol 165:27–30
Karube I, Matsunaga T, Mitsuda S, Suzuki S (1977) Microbial electrode BOD sensors. Biotechnol Bioeng 19(10):1535–1547
Kaur A, Kim JR, Michie I, Dinsdale RM, Guwy AJ, Premier GC et al (2013) Microbial fuel cell type biosensor for specific volatile fatty acids using acclimated bacterial communities. Biosens Bioelectron 47:50–55
Khatoon A, Mohd Setapar SH, Umar K, Parveen T, Mohamad Ibrahim MN, Rafatullah M (2020) Outlook on the role of microbial fuel cells in remediation of environmental pollutants with electricity generation. Catalysts 10(8):819
Kim BH, Chang IS, Gil GC, Park HS, Kim HJ (2003) Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotech Lett 25(7):541–545
Kim JR, Cheng S, Oh S-E, Logan BE (2007) Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ Sci Technol 41(3):1004–1009
Kim M, Hyun MS, Gadd GM, Kim HJ (2007) A novel biomonitoring system using microbial fuel cells. J Environ Monit 9(12):1323–1328
Kim M, Lim JW, Kim HJ, Lee SK, Lee SJ, Kim T (2015) Chemostat-like microfluidic platform for highly sensitive detection of heavy metal ions using microbial biosensors. Biosens Bioelectron 65:257–264
Kim T, Han J-I (2013) Fast detection and quantification of Escherichia coli using the base principle of the microbial fuel cell. J Environ Manag 130:267–275
Kooij DVD (1992) Assimilable organic carbon as an indicator of bacterial regrowth. J‐Am Water Works Assoc 84(2):57–65
Kumar AK, Reddy MV, Chandrasekhar K, Srikanth S, Mohan SV (2012) Endocrine disruptive estrogens role in electron transfer: bio-electrochemical remediation with microbial mediated electrogenesis. Biores Technol 104:547–556
Kumar R, Kumar A (2005) Water analysis| biochemical oxygen demand
Kumar R, Singh L, Zularisam A (2016) Exoelectrogens: recent advances in molecular drivers involved in extracellular electron transfer and strategies used to improve it for microbial fuel cell applications. Renew Sustain Energy Rev 56:1322–1336
Kumar SS, Malyan SK, Basu S, Bishnoi NR (2017) Syntrophic association and performance of Clostridium, Desulfovibrio, Aeromonas and Tetrathiobacter as anodic biocatalysts for bioelectricity generation in dual chamber microbial fuel cell. Environ Sci Pollut Res 24(19):16019–16030
Kumlanghan A, Liu J, Thavarungkul P, Kanatharana P, Mattiasson B (2007) Microbial fuel cell-based biosensor for fast analysis of biodegradable organic matter. Biosens Bioelectron 22(12):2939–2944
Le C, Zha Y, Li Y, Sun D, Lu H, Yin B (2010) Eutrophication of lake waters in China: cost, causes, and control. Environ Manag 45(4):662–668
Lee SH, Lee K-S, Sorcar S, Razzaq A, Grimes CA, In S-I (2018) Wastewater treatment and electricity generation from a sunlight-powered single chamber microbial fuel cell. J Photochem Photobiol A 358:432–440
Lei Y, Chen W, Mulchandani A (2006) Microbial biosensors. Anal Chim Acta 568(1–2):200–210
Li T, Wang X, Zhou Q, Liao C, Zhou L, Wan L et al (2018) Swift acid rain sensing by synergistic rhizospheric bioelectrochemical responses. ACS Sens 3(7):1424–1430
Li Y, Marshall A, Gostomski PA (2014) Gaseous pollutant treatment and electricity generation in microbial fuel cells (MFCs) utilising redox mediators. Rev Environ Sci Bio/Technol 13(1):35–51
Lincy MA, Kumar BA, Vasantha V, Varalakshmi P (2015) Microbial fuel cells: a promising alternative energy source. Oppor Challenges, 61
Liu B, Lei Y, Li B (2014) A batch-mode cube microbial fuel cell based “shock” biosensor for wastewater quality monitoring. Biosens Bioelectron 62:308–314
Liu H, Cheng S, Logan BE (2005) Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ Sci Technol 39(2):658–662
Liu J, Mattiasson B (2002) Microbial BOD sensors for wastewater analysis. Water Res 36(15):3786–3802
Liu Z, Du Z, Lian J, Zhu X, Li S, Li H (2007) Improving energy accumulation of microbial fuel cells by metabolism regulation using Rhodoferax ferrireducens as biocatalyst. Lett Appl Microbiol 44(4):393–398
Liu Z, Liu J, Zhang S, Xing X-H, Su Z (2011) Microbial fuel cell based biosensor for in situ monitoring of anaerobic digestion process. Biores Technol 102(22):10221–10229
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S et al (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40(17):5181–5192
Logan BE, Rabaey K (2012) Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337(6095):686–690
Logan BE, Regan JM (2006) Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14(12):512–518
Lovley DR (2006) Bug juice: harvesting electricity with microorganisms. Nat Rev Microbiol 4(7):497–508
Lovley DR (2006) Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol 17(3):327–332
Magno M, Boyle D, Brunelli D, Popovici E, Benini L (2013) Ensuring survivability of resource-intensive sensor networks through ultra-low power overlays. IEEE Trans Industr Inf 10(2):946–956
Malvankar NS, Lovley DR (2012) Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. Chemsuschem 5(6):1039–1046
Mao L, Verwoerd WS (2013) Selection of organisms for systems biology study of microbial electricity generation: a review. Int J Energy Environ Eng 4(1):1–18
Markfort CD, Hondzo M (2009) Dissolved oxygen measurements in aquatic environments: the effects of changing temperature and pressure on three sensor technologies. J Environ Qual 38(4):1766–1774
Melhuish C, Ieropoulos I, Greenman J, Horsfield I (2006) Energetically autonomous robots: food for thought. Auton Robot 21(3):187–198
Miller LG, Oremland RS (2008) Electricity generation by anaerobic bacteria and anoxic sediments from hypersaline soda lakes. Extremophiles 12(6):837–848
Modestra JA, Mohan SV (2014) Bio-electrocatalyzed electron efflux in Gram positive and gram negative bacteria: an insight into disparity in electron transfer kinetics. RSC Adv 4(64):34045–34055
Modin O, Aulenta F (2017) Three promising applications of microbial electrochemistry for the water sector. Environ Sci: Water Res Technol 3(3):391–402
Modin O, Wilén B-M (2012) A novel bioelectrochemical BOD sensor operating with voltage input. Water Res 46(18):6113–6120
Mohan SV, Velvizhi G, Krishna KV, Babu ML (2014) Microbial catalyzed electrochemical systems: a bio-factory with multi-facet applications. Biores Technol 165:355–364
Mohan SV, Velvizhi G, Modestra JA, Srikanth S (2014) Microbial fuel cell: critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew Sustain Energy Rev 40:779–797
Mook WT, Aroua MKT, Chakrabarti MH, Noor IM, Irfan MF, Low CTJ (2013) A review on the effect of bio-electrodes on denitrification and organic matter removal processes in bio-electrochemical systems. J Ind Eng Chem 19(1):1–13
Mulchandani A (2011) Microbial biosensors for organophosphate pesticides. Appl Biochem Biotechnol 165(2):687–699
Naresh V, Lee N (2021) A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 21(4):1109
Nawaz A, Hafeez A, Abbas SZ, Haq IU, Mukhtar H, Rafatullah M (2020) A state of the art review on electron transfer mechanisms, characteristics, applications and recent advancements in microbial fuel cells technology. Green Chem Lett Rev 13(4):101–117
Niazi JH, Kim BC, Ahn J-M, Gu MB (2008) A novel bioluminescent bacterial biosensor using the highly specific oxidative stress-inducible pgi gene. Biosens Bioelectron 24(4):670–675
Oh S, Min B, Logan BE (2004) Cathode performance as a factor in electricity generation in microbial fuel cells. Environ Sci Technol 38(18):4900–4904
Pandit S, Chandrasekhar K, Kakarla R, Kadier A, Jeevitha V (2017) Basic principles of microbial fuel cell: technical challenges and economic feasibility. In: Microbial applications, vol 1. Springer, pp 165–188
Pandit S, Khilari S, Roy S, Ghangrekar M, Pradhan D, Das D (2015) Reduction of start-up time through bioaugmentation process in microbial fuel cells using an isolate from dark fermentative spent media fed anode. Water Sci Technol 72(1):106–115
Pandit S, Khilari S, Roy S, Pradhan D, Das D (2014) Improvement of power generation using Shewanella putrefaciens mediated bioanode in a single chambered microbial fuel cell: effect of different anodic operating conditions. Biores Technol 166:451–457
Pant D, Van Bogaert G, Diels L, Vanbroekhoven K (2010) A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Biores Technol 101(6):1533–1543
Park D, Zeikus J (2000) Electricity generation in microbial fuel cells using neutral red as an electronophore. J Appl Environ Microb 66:1292–1297
Park HS, Kim BH, Kim HS, Kim HJ, Kim GT, Kim M et al (2001) A novel electrochemically active and Fe (III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7(6):297–306
Parkhey P, Mohan SV (2019) Biosensing applications of microbial fuel cell: Approach toward miniaturization. In: Microbial electrochemical technology. Elsevier, pp 977–997
Pasternak G, Greenman J, Ieropoulos I (2017) Self-powered, autonomous biological oxygen demand biosensor for online water quality monitoring. Sens Actuat B Chem 244:815–822
Petrie B, Barden R, Kasprzyk-Hordern B (2015) A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res 72:3–27
Porcarelli D, Brunelli D, Benini L (2012) Characterization of lithium-ion capacitors for low-power energy neutral wireless sensor networks. 2012 ninth international conference on networked sensing (INSS). IEEE, pp 1–4
Potter M (1910) On the difference of potential due to the vital activity of microorganisms. In: Proceedings of the University of Durham philosophical society, pp 245–249
Prévoteau A, Clauwaert P, Kerckhof F-M, Rabaey K (2019) Oxygen-reducing microbial cathodes monitoring toxic shocks in tap water. Biosens Bioelectron 132:115–121
Quek SB, Cheng L, Cord-Ruwisch R (2015) Microbial fuel cell biosensor for rapid assessment of assimilable organic carbon under marine conditions. Water Res 77:64–71
Realtech (2021) Biochemical oxygen demand (BOD). https://realtechwater.com/parameters/biochemical-oxygen-demand/. Accessed 17 June 2021
Rimboud M, Pocaznoi D, Erable B, Bergel A (2014) Electroanalysis of microbial anodes for bioelectrochemical systems: basics, progress and perspectives. Phys Chem Chem Phys 16(31):16349–16366
Rosenbaum M, Aulenta F, Villano M, Angenent LT (2011) Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Biores Technol 102(1):324–333
Saha TC, Protity AT, Zohora FT, Shaha M, Ahmed I, Barua E et al (2019) Microbial fuel cell (MFC) application for generation of electricity from dumping rubbish and identification of potential electrogenic bacteria. Adv Ind Biotechnol 2(10)
Sai ASV (2009) Microbial fuel cells: principles and applications. Altenergymag. https://www.altenergymag.com/article/2009/12/microbial-fuel-cellsprinciples-and-applications/587
Schamphelaire LD, Bossche LVD, Dang HS, Höfte M, Boon N, Rabaey K et al (2008) Microbial fuel cells generating electricity from rhizodeposits of rice plants. Environ Sci Technol 42(8):3053–3058
Schneider G, Czeller M, Rostás V, Kovács T (2015) Microbial fuel cell-based diagnostic platform to reveal antibacterial effect of beta-lactam antibiotics. Enzyme Microb Technol 73:59–64
Schneider G, Kovács T, Rákhely G, Czeller M (2016) Biosensoric potential of microbial fuel cells. Appl Microbiol Biotechnol 100(16):7001–7009
Schwarzenbach RP, Egli T, Hofstetter TB, Von Gunten U, Wehrli B (2010) Global water pollution and human health. Annu Rev Environ Resour 35:109–136
Shen H-B, Yong X-Y, Chen Y-L, Liao Z-H, Si R-W, Zhou J et al (2014) Enhanced bioelectricity generation by improving pyocyanin production and membrane permeability through sophorolipid addition in Pseudomonas aeruginosa-inoculated microbial fuel cells. Biores Technol 167:490–494
Shen Q, Zhou Q, Shang J, Shao S, Zhang L, Fan C (2014) Beyond hypoxia: occurrence and characteristics of black blooms due to the decomposition of the submerged plant Potamogeton crispus in a shallow lake. J Environ Sci 26(2):281–288
Shen Y, Wang M, Chang IS, Ng HY (2013) Effect of shear rate on the response of microbial fuel cell toxicity sensor to Cu(II). Biores Technol 136:707–710
Shen YJ, Lefebvre O, Tan Z, Ng HY (2012) Microbial fuel-cell-based toxicity sensor for fast monitoring of acidic toxicity. Water Sci Technol 65(7):1223–1228
Shin HJ (2010) Development of highly-sensitive microbial biosensors by mutation of the nahR regulatory gene. J Biotechnol 150(2):246–250
Si R-W, Zhai D-D, Liao Z-H, Gao L, Yong Y-C (2015) A whole-cell electrochemical biosensing system based on bacterial inward electron flow for fumarate quantification. Biosens Bioelectron 68:34–40
Song N, Jiang H-L (2018) Effects of initial sediment properties on start-up times for sediment microbial fuel cells. Int J Hydrog Energy 43(21):10082–10093
Song N, Yan Z, Xu H, Yao Z, Wang C, Chen M et al (2019) Development of a sediment microbial fuel cell-based biosensor for simultaneous online monitoring of dissolved oxygen concentrations along various depths in lake water. Sci Total Environ 673:272–280
Stein NE, Hamelers HM, van Straten G, Keesman KJ (2012) On-line detection of toxic components using a microbial fuel cell-based biosensor. J Process Control 22(9):1755–1761
Stein NE, Hamelers HV, Buisman CN (2010) Stabilizing the baseline current of a microbial fuel cell-based biosensor through overpotential control under non-toxic conditions. Bioelectrochemistry 78(1):87–91
Sun J-Z, Peter Kingori G, Si R-W, Zhai D-D, Liao Z-H, Sun D-Z et al (2015) Microbial fuel cell-based biosensors for environmental monitoring: a review. Water Sci Technol 71(6):801–809
Tan T, Li F, Neoh K, Lee Y (1992) Microbial membrane-modified dissolved oxygen probe for rapid biochemical oxygen demand measurement. Sens Actuat B Chem 8(2):167–172
Tardy GM, Lóránt B, Gyalai-Korpos M, Bakos V, Simpson D, Goryanin I (2021) Microbial fuel cell biosensor for the determination of biochemical oxygen demand of wastewater samples containing readily and slowly biodegradable organics. Biotech Lett 43(2):445–454
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Mol Clin Environ Toxicol, 133–164
Thurston C, Bennetto H, Delaney G, Mason J, Roller S, Stirling J (1985) Glucose metabolism in a microbial fuel cell. Stoichiometry of product formation in a thionine-mediated Proteus vulgaris fuel cell and its relation to coulombic yields. Microbiology, 131(6):1393–1401
Tran PHN, Luong TTT, Nguyen TTT, Nguyen HQ, Van Duong H, Kim BH (2015) Possibility of using a lithotrophic iron-oxidizing microbial fuel cell as a biosensor for detecting iron and manganese in water samples. Environ Sci Process Impacts 17(10):1806–1815
Vogrinc D, Vodovnik M, Marinšek-Logar R (2015) Microbial biosensors for environmental monitoring. Acta Agricult Slovenica 106(2):67–75
Von Canstein H, Ogawa J, Shimizu S, Lloyd JR (2008) Secretion of flavins by Shewanella species and their role in extracellular electron transfer. Appl Environ Microbiol 74(3):615–623
Wang G-H, Cheng C-Y, Liu M-H, Chen T-Y, Hsieh M-C, Chung Y-C (2016) Utility of Ochrobactrum anthropi YC152 in a microbial fuel cell as an early warning device for hexavalent chromium determination. Sensors 16(8):1272
Wang H, Ren ZJ (2013) A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol Adv 31(8):1796–1807
Wang X, Liu M, Wang X, Wu Z, Yang L, Xia S et al (2013) P-benzoquinone-mediated amperometric biosensor developed with Psychrobacter sp. for toxicity testing of heavy metals. Biosens Bioelectron 41:557–562
Wei T, Zhang C, Xu X, Hanna M, Zhang X, Wang Y et al (2013) Construction and evaluation of two biosensors based on yeast transcriptional response to genotoxic chemicals. Biosens Bioelectron 44:138–145
Wu L-C, Tsai T-H, Liu M-H, Kuo J-L, Chang Y-C, Chung Y-C (2017) A green microbial fuel cell-based biosensor for in situ chromium (VI) measurement in electroplating wastewater. Sensors 17(11):2461
Wu S, Deng H, Han C, Liu L, Zhong W (2018) A novel sediment microbial fuel cell based sensor for on-line and in situ monitoring copper shock in water. Electroanalysis 30(11):2668–2675
Wu W, Lesnik KL, Xu S, Wang L, Liu H (2014) Impact of tobramycin on the performance of microbial fuel cell. Microb Cell Fact 13(1):1–7
Xafenias N, Zhang Y, Banks CJ (2013) Enhanced performance of hexavalent chromium reducing cathodes in the presence of Shewanella oneidensis MR-1 and lactate. Environ Sci Technol 47(9):4512–4520
Xu D, Gu T (2014) Carbon source starvation triggered more aggressive corrosion against carbon steel by the Desulfovibrio vulgaris biofilm. Int Biodeter Biodegrad 91:74–81
Xu D, Li Y, Song F, Gu T (2013) Laboratory investigation of microbiologically influenced corrosion of C1018 carbon steel by nitrate reducing bacterium Bacillus licheniformis. Corros Sci 77:385–390
Xu X, Ying Y (2011) Microbial biosensors for environmental monitoring and food analysis. Food Rev Intl 27(3):300–329
Xu Z, Liu B, Dong Q, Lei Y, Li Y, Ren J et al (2015) Flat microliter membrane-based microbial fuel cell as “on-line sticker sensor” for self-supported in situ monitoring of wastewater shocks. Biores Technol 197:244–251
Yang G-X, Sun Y-M, Kong X-Y, Zhen F, Li Y, Li L-H et al (2013) Factors affecting the performance of a single-chamber microbial fuel cell-type biological oxygen demand sensor. Water Sci Technol 68(9):1914–1919
Yang H, Zhou M, Liu M, Yang W, Gu T (2015) Microbial fuel cells for biosensor applications. Biotech Lett 37(12):2357–2364
Yang M (2011) A current global view of environmental and occupational cancers. J Environ Sci Health C 29(3):223–249
Yang W, Wei X, Fraiwan A, Coogan CG, Lee H, Choi S (2016) Fast and sensitive water quality assessment: a μL-scale microbial fuel cell-based biosensor integrated with an air-bubble trap and electrochemical sensing functionality. Sens Actuat B Chem 226:191–195
Yang Z, Suzuki H, Sasaki S, Karube I (1996) Disposable sensor for biochemical oxygen demand. Appl Microbiol Biotechnol 46(1):10–14
Yong D, Liu C, Yu D, Dong S (2011) A sensitive, rapid and inexpensive way to assay pesticide toxicity based on electrochemical biosensor. Talanta 84(1):7–12
Yong Y-C, Zhong J-J (2009) A genetically engineered whole-cell pigment-based bacterial biosensing system for quantification of N-butyryl homoserine lactone quorum sensing signal. Biosens Bioelectron 25(1):41–47
Yu D, Bai L, Zhai J, Wang Y, Dong S (2017) Toxicity detection in water containing heavy metal ions with a self-powered microbial fuel cell-based biosensor. Talanta 168:210–216
Yaqoob AA, Ibrahim MNM, Yaakop AS, Ahmad A (2021) Application of microbial fuel cells energized by oil palm trunk sap (OPTS) to remove the toxic metal from synthetic wastewater with generation of electricity. Appl Nanosci 11(6):1949–1961
Zeng L, Li X, Shi Y, Qi Y, Huang D, Tadé M et al (2017) FePO4 based single chamber air-cathode microbial fuel cell for online monitoring levofloxacin. Biosens Bioelectron 91:367–373
Zhang C, Liang P, Jiang Y, Huang X (2015) Enhanced power generation of microbial fuel cell using manganese dioxide-coated anode in flow-through mode. J Power Sour 273:580–583
Zhang M, Yu Y, Yang Z, Shi X, Kong F (2012) The distribution of phytoplankton along trophic gradients and its mediation by available light in the pelagic zone of large eutrophic lakes. Can J Fish Aquat Sci 69(12):1935–1946
Zhang Y, Angelidaki I (2012) A simple and rapid method for monitoring dissolved oxygen in water with a submersible microbial fuel cell (SBMFC). Biosens Bioelectron 38(1):189–194
Zhao F, Harnisch F, Schröder U, Scholz F, Bogdanoff P, Herrmann I (2006) Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ Sci Technol 40(17):5193–5199
Zhao S, Liu P, Niu Y, Chen Z, Khan A, Zhang P et al (2018) A novel early warning system based on a sediment microbial fuel cell for in situ and real time hexavalent chromium detection in industrial wastewater. Sensors 18(2):642
Zhou T, Han H, Liu P, Xiong J, Tian F, Li X (2017) Microbial fuels cell-based biosensor for toxicity detection: a review. Sensors 17(10):2230
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hashem, A., Simarani, K., Marlinda, A.R., Hossain, M.A.M., Al Mamun, M., Johan, M.R. (2022). Application of Microbial Fuel Cells as Biosensors. In: Ahmad, A., Mohamad Ibrahim, M.N., Yaqoob, A.A., Mohd Setapar, S.H. (eds) Microbial Fuel Cells for Environmental Remediation. Sustainable Materials and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-19-2681-5_17
Download citation
DOI: https://doi.org/10.1007/978-981-19-2681-5_17
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-2680-8
Online ISBN: 978-981-19-2681-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)