Abstract
Microbial fuel cells (MFCs) is the bioelectrochemical typed approach in which bacterial species generate electricity and remove the metal ions from synthetic wastewater. To improve the performance of MFCs, a local oil palm trunk sap (OPTS) was used in the present study as an organic substrate to improve the bacterial activities. The present electrochemical and biological characterizations proved that OPTS can deliver good efficiency in MFCs operations. The maximum obtained power density was 0.37 mW/m2 and a current density of 55.26 mA/m2. Similarly, the obtained removal rate for Pb2+, Cd2+, Cr3+, Ni2+, Co2+ and Hg2+ was 75%, 70.10%, 75%, 80%, 78.10% and 60%, respectively. During biological characterization, conductive pili-type bacterial species such as Klebsiella pneumoniae, Bacillus species, Lysinibacillus, and Enterobacter were found for metal removal and energy generation. Additionally, the parameter optimization showed that room temperature and pH 7 are ideal conditions for the industrial-scale application of the MFCs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The two most universal issues today are environmental threats, such as water pollution and energy crises. Several physical, chemical, and biological techniques are applied to overcome the issues but still, the challenges remain. All conventional methods such as adsorption, photocatalysts, ozonation, electrochemical degradation, chemical precipitation, coagulation, monitoring natural recovery, electrolytic reduction, ion-exchange, and thermal treatment are used to treat wastewater (Owusu and Asumadu-Sarkodie 2016; Umar et al. 2020a; Yaqoob and Ibrahim 2019). Despite all developments, the performance was not at a satisfactory level. The above-mentioned methods showed some drawbacks such as the production of sludge, energy demand, high operating cost, and excessive involvement of chemicals during operation, etc. These drawbacks limit their applicability in the modern world (Umar et al. 2021a). With the continued efforts of experts, the microbial fuel cells (MFCs) was introduced to remediate the wastewater treatment by concurrently generating the energy (Bajracharya et al. 2016; Logan and Regan 2006). This approach seems to be promising because it does not require any special support such as external energy supply (Yaqoob et al. 2020c). The energy generation process is carried out using the bacterial species as a biocatalyst in MFCs to oxidize the organic substrate. The organic substrate is converted into the electric form via the bacterial metabolism process. The anode and cathode electrodes are separated by a proton permeable membrane (PEM) in MFCs (Logan and Rabaey 2012; Yaqoob et al. 2020a). This is also known as double chamber MFCs. In an anode chamber, the bacterial species oxidized the organic substrate to produced electrons and protons. The protons can transfer to the cathode directly via PEM while electrons travel through an outer circuit to take part in the reduction process at the cathode (Palanisamy et al. 2019; Yaqoob et al. 2020d). This approach offering several merits over other common approaches in terms of pollutant removal and energy generation such as no energy is required for operation, low generation of sludge, simple working in normal environments, and eco-friendly (Li et al. 2017). The present study was focused on the removal of toxic metals with energy generation using the synthetic wastewater. According to one report, Malaysians are using 99% surface water for their utility while 1% is coming from groundwater (Ab Razak et al. 2015).
Currently, Malaysia is suffering from toxic metal contamination in water resources (Low et al. 2016). A large amount of allocation is required to address this issue via conventional methods. Therefore, the MFCs can be an appropriate approach at an industrial scale for such struggling countries to overcomes these issues without big expenditure. In the literature review, it was found that reported studies on the removal of one metal at a time via MFCs with high removal efficiency (Zhang et al. 2020). With the experts' recommendations and revealed issues in MFCs, the organic substrate is one of the key factors in the success of MFCs operations. Several studies reported that instability of substarte to bacterial specie is another identified challenge the removal of metals via MFCs (Ahmad et al. 2020; Kumar et al. 2018; Yaqoob et al. 2021b). The removal of several toxic metals (such as Pb2+, Cd2+, Cr3+, Ni2+, Co2+, Hg2+) simultaneously is explored in this study. The present study focused on the utilization of oil palm trunk sap (OPTS). According to Yamada et al. (2010) the OPTS contains 85% simple sugar with other carbohydrates. Several articles already explained and proved that the OPTS is a high sugar content substrate (Kunasundari et al. 2017; Murata et al. 2013; Salim et al. 2013). Therefore, the utilization of OPTS as the organic substrate can be a perfect diet for a bacterial community to grow and respirate. The present study highlighted the utilization of OPTS as the organic substrate to remove the mixture of Pb2+, Cd2+, Cr3+, Ni2+, Co2+, Hg2+ ions from synthetic wastewater. Lastly, the working mechanism of MFCs (energy generation-transportation and metal removal) are well explained with future recommendations.
Experimental detail
Chemicals and materials
The OPTS (received from Prof. Dr. Rokiah Hashim’s research group in the School of Industrial Technology at Universiti Sains Malaysia), local pond water (collected from the Fajar Harapan lake, Universiti Sains Malaysia), commercial graphite rods (FUDA 2B Lead, NY, USA), cadmium nitrate tetrahydrate (R&M Chemical), lead nitrate (R&M Chemical), chromium nitrate (R&M Chemical), nickel nitrate hexahydrate (R&M Chemical), Nafion-117 membrane, cobalt nitrate hexahydrate (R&M Chemical), silver nitrate (R&M Chemical), copper nitrate (R&M Chemical), and glucose (AR, grade) are used throughout the study.
Preparation of inoculation source
The local pond water was collected from the Fajar Harapan lake, Universiti Sains Malaysia. The local pond water was filtered to remove the solid impurities and supplemented the different metal ions (50 mg/L each). This metal supplemented local pond water is known as synthetic wastewater in the present study. This synthetic wastewater was used as an inoculation source in MFCs in an anode chamber. The physicochemical properties of collected local pomd water and synthetic wastewater are measured and shown in Table 1. The conductivity, temperature, and pH were analyzed using an electrical meter (ECM) (Alpha-800 conductivity meter, Vernon Hills, IL, USA), thermometer (GH, ZEAL LTD; London, UK), and pH meter (EUTECH instrument-700; New York, NY, USA).
MFCs start and setup
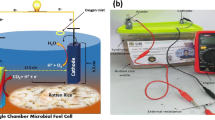
A 10 cm high double chamber MFCs was employed in this study. The width of the anode chamber was 9.5 cm while the width of the cathode chamber was 9.0 cm. The prepared inoculation source (350 mL) was supplemented in the anode chamber with an anode electrode while the cathode chamber was added by tap water (350 mL). The Nafion-17 membrane was used to separate the anode and cathode chambers. Before using the Nafion-117 membrane, it was washed according to the method explained by Liu and Logan (2004). Copper wire was used to make the outer circuit for electron transference. The graphite rod (GR series, FUDA 2B Lead, NY, USA) was used as anode and cathode electrodes throughout the operation. The distance between both electrodes was 7 cm. The size of the anode and cathode electrodes was 8 cm × 0.7 cm (heigh × radius) with 38.28 cm2 surface area of each electrode. The external resistance was 1000 Ω. According to Igboamalu et al. (2019), 1000 Ω external resistance was preferable for MFCs under room temperature with pH 6–7. The external oxygen was supplied at the cathode chamber to maintain the aerobic environment for a high reduction rate. The OPTS was provided to the anode chamber 5 mL/day. It works as fuel toward the bacterial community in the operation. The model and used reactor of the MFCs are shown in Fig. 1.
Electrochemical measurements
The voltage potential of the operation was measured by a digital multimeter (UNI-T UT33A, China). To interpret the voltage into current, Ohm’s law was followed. The equations which are used to measure the internal resistance (r), power density (PD), and current density (CD) are as follow (Yaqoob et al. 2021a):
where, I = current, r = internal resistance, A = cross-sectional area, V = voltage output, E = electromotive force (Emf). An open-circuit voltage (OCV) is equivalent to the Emf. The term "open circuit voltage" refers to the absence of external resistance between the anode and the cathode. While in closed-circuit there are specific external resistance present between the both electrodes. Further, the polarization behavior of the operation was observed after the pseudo-steady state of the reaction using the changeable resistance switch box from 10 kΩ to 100 Ω.
Further, the cyclic voltammetry (CV, Model BAS Epsilon Version 1.4; West Lafayette, IN, USA). and Electrochemical Impedance Spectroscopy (EIS; Gamry Reference 600; Warminster, PA, USA) was used to analyze the redox reaction potential and electron transfer mobility. In the CV analysis, the scan rate was 30 mV/s with a window range of potential from + 0.8 to − 0.8 V. During measurements, the glassy carbon and Pt wire were used as an operating electrode and counter electrode, respectively. The Ag/AgCl was used as a reference electrode. The CV measurements were carried out every 15 days. The total period of the reaction was 90 days. Additionally, the CV curves offer the opportunity to calculate the specific capacitance (Cp) value. The unit of Cp is F/g and can be calculated using Eq. 5. The Cp indicates the stability rate of the biofilm during the redox reaction:
where, A = CV area, m = loaded quantity, k = scan rate, \((V2-V1)\) = voltage range. The effect of the resistance of anode in the direction of voltage was studied by EIS. On the completion of the operation, the EIS test was performed within the 100 kHz–100 MHz frequency range.
Biological tests
The removal rate of supplemented metals was studied using the atomic adsorption spectrometer (AAS; PerkinElmer AAnalyst 400; Waltham, MA, USA). On every 10 days, 1.5 mL of inoculum source was collected for AAS analysis. The removal percentage was calculated through the following equation:
where, Minitial = the initial concentration of metal ions, Mfinal = the final concentration of metal ions.
To study the biofilm formation and its stability, scanning electron microscopy (SEM-Zeiss, DSM-960, Germany) was used. The SEM analysis of the treated anode with biofilm was carried out at the end of the operation. Moreover, the electron dispersive X-ray (EDX) was used to observe the biofilm composition on the completion of the operation. This was done to evaluate whether there is any metal on the surface of the anode electrode or otherwise. The EDX can ensure the produced biofilm around the anode surface is free from metal deposits. It is a clue that can ensure all metal ions are transformed into an insoluble state.
Finally, in a biological test, the bacterial identification process was performed to identify the bacterial species which are present on the surface of the anode. Around 1 mm biofilm was taken from an anode surface and dilute for mixed culture growth. The nutrient agar plates were used to grow the mixed culture and later pure culture bacterial species. The bacterial 16S rRNA genes were obtained through the polymerase chain reaction (PCR) method. The reverse primer (1492R) and forward primer (27F) was used to amplify the DNA of the PCR product. A cloning kit (TOPOTA, Invitrogen; Carlsbad, CA, USA) was used to clone the PCR amplified product. 16S rRNA microbial strains were deposited in GenBank after DNA sequencing analysis.
Multiple parameters optimizations
The multiple parameters optimizations studied were carried out. The pH and temperature were considered in the present study. First, pH the range from 4 to 10 was optimized in terms of energy generation and metal removal. The NaOH and H2SO4 was used to adjust the pH. A series of experiments was carried out using the OPTS as organic substrate, at room temperature, 1000 Ω external resistance, and synthetic wastewater in a double chamber MFCs. Each pH range voltage removal efficiency was calculated after 10 days. Similarly, temperature range from 20 to 35 °C was optimized with the OPTS as organic substrate, 1000 Ω external resistance, pH 6.25, and synthetic wastewater in a double chamber MFCs. In each temperature range, the voltage removal efficiency was calculated after 10 days.
Results and discussion
Electrochemical tests
Figure 2a showed the voltage generation trend under the closed-circuit condition for 90-day operation. The operation was successfully operated for three-cycle for more precise measurements. The fresh inoculation source was used in the beginning with a continuous supply of OPTS throughout the operation. In the beginning, the voltage was 1 mV on the first day with external resistance while the open-circuit voltage was 400 mV. As the OPTS was supplied, the voltage showed a gradually increasing trend. On the 24th day, the maximum voltage of 202 mV was achieved on the completion of the first cycle. On the completion of the first cycle, the voltage gradually started decreasing trend even the supplying of OPTS was continued. The reason for the reduction in voltage trend is it might be due to some bacterial species which are completing their life cycles. Similar, as the operation continued for the second and third cycles the same trend is observed in voltage generation. The maximum observed voltage on the second cycle was 166 mV (on the 44th day) and in the third cycle, it was the highest on the 67th day (134 mV). Upon the completion of the third cycle, the voltage generation trend started to reduce and reached even below the first-day value. It is an indication that the present operation showed maximum voltage was on the first cycle and reach its bacterial life completion time in the third cycle. Further, the voltage generation trend has also corresponded to the removal of toxic metals. The maximum voltage achieving point was a turning point of toxic metals where the toxic soluble state of the metal is converted into insoluble states. Yaqoob et al. (2020b) presented that Pb2+ was converted into Pb or PbO and they described the conversion process of metal with the support of voltage generation trend. They also followed a similar trend in describing the voltage generation trend. The reasons for the maximum voltage of 202 mV were also given such as (a) the continuous supply of OPTS which acts as a fuel for bacterial species to generate the electrons and protons, (b) the external supply of the oxygen at the cathode chamber which significantly increased the reduction reaction and increased the bacterial adaptability.
Figure 2b showed the polarization behavior which is describing the relationship between CD, PD voltage, and resistance. A different range of external resistance (100 Ω–10 kΩ) was used to plot the polarization slope. It is very common to measure the polarization behavior at high voltage. The polarization curve revealed that the voltage dropped from 10 to 100 kΩ while the CD and PD increased. Several studies were reported to follow a similar trend and reported the PD efficiency (Kumar et al. 2015; Yaqoob et al. 2020b, 2021b). In the present study, the cell design point was observed at 1000 Ω with a PD value of 0.37 mW/m2. The maximum achieved current density was 55.26 mA/m2. However, at 10 kΩ showed the PD value was 0.08 mW/m2 while at 100 Ω the PD value was 0.13 mW/m2. The low external resistance such as 100 Ω showed a high voltage generation but the stabilization of the voltage was not fast which leads to poor energy generation performance. Similar, at high external resistance, the destabilization was high but due to high external charge resistance, the voltage generation turned poor. It leads to low performance of energy generation. Therefore, a stable external resistance with internal resistance should be used. A stable electron transference with stable charge resistance can deliver high-power efficiency. The poor availability of oxygen at the cathode can also be a reason for low energy generation, therefore external oxygen supply is an appreciated step.
To study the redox potential and electronic resistance of the operation, CV and EIS studies were carried out. The CV was measured at a different time interval to evaluate the rate of oxidation and reduction during operation at different stages. Figure 3a showed the maximum reverse and forward scan rate. Different values of current were observed in forward as well as reverse scan. The observed highest forward scan was on the 90th day (7.5 × 10–6 mA) while in reverse scan the maximum current was − 1 × 10–5 mA on the 90th day. The forward and reverse scans correspond to the oxidation and reduction processes of the cell. The observation showed that both oxidation and reduction reactions were increased gradually. The maximum oxidation rate means that the generation of electrons and protons is the highest. The study also corresponds that the OPTS can easily be oxidized by bacterial species.
Further, to study the anode biofilm formation and stability rate, the Cp value was calculated. The increasing trend of the Cp value indicated that the biofilm was formed gradually. From the observation, as shown in Table 2 the maximum Cp value was observed on the 90th day. On the completion of each cycle, the voltage started to reduce. The reduction was occurred due to the instability of the biofilm. Days 45th and 75th showed that the biofilm was unstable due to the bacterial instabilities. A similar trend was explained by Abbas et al. (2018) to study the oxidative/reductive electron transfer activities via biofilm.
Figure 3b showed the EIS-Nyquist plots by employing the equivalent circuit model. This study showed the electron transference rate of the operation. The straight-line slope of the EIS indicated the poor transfer of electrons due to the presence of high charge resistance. While the semi-circle or bent slop of EIS indicated the stable and high electronic movement during the operation (Hung et al. 2019). In the present study, the semi-circle/bent-shaped EIS curve was found. It is indicated that the electronic mobility was high, and the charge resistance was poor. The charge resistance toward the electrolytes was low because the internal resistance was lower (809.9 Ω) than the external resistance (1000 Ω). The low internal resistance and low charge resistance can lead to the high transfer rate of electrons during the operation. Further, to increase the electron transfer rate, the electrode material should be made from a highly conductive material. Therefore, it is a need to pay special attention to the development of the anode electrode.
Removal of toxic metals studies
Table 3 indicates the removal trend of the toxic metals via MFCs for a 90-day operation. The removal trend was found to gradually increase. The OPTS was used as fuel for the bacterial community to remove the toxic metal ions into insoluble states. The present results were encouraging as compared to the previous studies. For example, Yaqoob et al. (2021b) reported around 60% removal of Pb2+ and 65.51% removal of Cd via MFCs using sweet potato waste as an organic substrate. In the present study, OPTS increased the removal efficiency to 75% for Pb2+, and 70.10% for Cd2+. Similarly, the removal efficiency for Cr3+, Ni2+, Co2+, and Hg2+ was 75%, 80%, 78.10%, and 60%, respectively, is achieved in the present study. The Hg removal efficiency is considered a unique attempt in the field of MFCs due to its highly toxic nature. In the present work, the OPTS supply empowered the bacterial community to remove more than half Hg successfully. Furthermore, the removal efficiency was observed to gradually increased in the beginning due to the fresh inoculation source. Later, the removal rate efficiency was quite fast until the 75th day. After the 75th day, the observation showed the removal rate was slow which might be an indication of the maximum removal has reached. During the operation, the number of electrons going toward the anode will increase the pH which therefore significantly lowered the exoelectrogens power. Another reason is, after the maximum removal efficiency is reached, the bacterial growth pattern showed a reduction in their life period. The bacterial community has a particular life pattern that is to convert into the death phase. Whenever the bacterial community started the death phase, the metal removal ability was reduced, and as a result, the removal efficiency was low. Several other eco-friendly parameters such as pH of the solution, external resistance, biofilm formation are also engaged in MFCs operation which requires careful study in the future.
Biological characterizations
In the biological analysis, the SEM–EDX is used to study the biofilm formation and its growth on the surface of the anode. The biofilm is known as a city of bacterial species, where it is a collection of bacteria at one specific place. The biofilm is one of the responsible factors to oxidize the organic substrate and later transfer the produced electrons via an anode electrode. The biofilm composition mostly contains 2–5% bacteria cells, 97% water content, and 3–6% extracellular polymeric substance (EPS’) (Singh and Songera 2012). Further, the EPS is the most important material in the oxidation process to generate the electron and protons. EPS consists of 1–60% (protein), 40–95% (polysaccharide), 10% (nucleic acids) and 40% (lipids). EPS is commonly known as a biofilm aging regulator (Di Martino 2018; Kumar et al. 2011). Once the supply of organic substrate is depleted, EPS will decrease resulting in low electron generation. Figure 4 showed the SEM images of both treated and untreated anode and cathode electrodes. The SEM image of the treated anode with biofilm (Fig. 4b) showed very dense and healthy growth of the bacterial community on the surface of the anode. This image shows that the bacterial community does not face toxic effects and the biofilm grows uninterrupted. Similarly, some bacterial communities are also found on the surface of the cathode (Fig. 4d). The reason for stable biofilm growth on the surface of the anode was the supply of OPTS to the bacterial communities. The SEM images of biofilms (Fig. 4b, d) showed both images having almost a similar morphology. They are showing tube/rod-shaped appendage. means there is a limited type of bacteria present on the samples. Several studies showed that the SEM images of biofilms with tube/rod-shaped appendages indicated the presence of conductive pili-typed bacterial species such as Acinetobacter, Bacillus, Proteus, Lysinibacillus, Leucobacter, Klebsiella sp. etc. (Yaqoob et al. 2020a, 2021c). The conductive pili-typed bacteria are those who use the pili to transfer the electrons. The pili is the body part of the bacteria which is conductive. The limited bacterial species are found in very dense conditions on the surface of biofilm because of the high sugar content from the organic substrate supplied. EDX study of the biofilm was carried out to detect the presence of the metals on the surface of the anode. From Fig. 5, few metals such as Cr3+, Cd2+, Pb2+, were found on the surface of the anodic biofilm. However, these metals are present in oxide forms. The solid form of metal is less toxic as compared to the metal ions. Overall, the study showed that during the MFCs operation the metal ions did not show any toxicity toward the bacterial community. Previously, Yaqoob et al. 20 have conducted studies on the MFCs operational biocompatibility factors.
In the biological study, the bacterial isolation and identification process was carried out to discover the bacterial species. The anode biofilm was used to identify the bacterial species. Table 4 showing the list of the identified bacterial species. There are few types of bacterial species which belong to the conductive pili-typed species. It means that the rod-shaped appendages as seen in SEM images are proving that there are conductive pili-typed species. Klebsiella pneumoniae, Lysinibacillus, and Bacillus are the most dominant species found on the surface of the biofilm. The identified bacterial are already well-proved exoelectrogens and metal-reducing species in previous literature in different studies. For example, Zhang et al. (2008) stated that K. pneumoniae sp. is well-known species in MFCs as exoelectrogens and metal-reducing species. Nandy et al. (2013) reported that the Lysinibacillus sp. served as a biocatalyst in bioelectrochemical systems and able to produce 85 mW/m2 energy power. Similarly, Mathivanan et al. (2016) also found the Lysinibacillus sp. and they called the Lysinibacillus sp. as the most effective species for copper and cadmium ions degradation from wastewater. Further, Nimje et al. (2009) observed the 0.000105 mW/m2 power density using the Bacillus strains as biocatalyst. However, several studies showed that the identified bacterial species (Table 4) are well exoelectrogens and metal-reducing species (Ayangbenro and Babalola 2020; Yaqoob et al. 2020b, 2021b).
The working mechanism of MFCs in the present study
The basic working principle of MFCs is dependent on bacterial species which served as a biocatalyst to oxidize the organic substrate to produce the electrons and protons (Abbas and Rafatullah 2021; Umar et al. 2020b, 2021b). Previously, many bacterial species, e.g., Acidobacteria, Proteobacteria, Firmicutes, fungi, and many others are considered as exoelectrogens (Abbas et al. 2017; Chuo et al. 2020; Yaqoob et al. 2020a). In the present work, OPTS which is a polysaccharide is used. Before oxidization, it was converted into simple sugar through the bioelectrogenesis process which was carried out by the bacterial community. The oxidation and reduction processes of the present study can be represented as:
The process of conversion of the OPTS into glucose and further glucose breakdown into pyruvate leads to the production of acetyl coenzyme A (acetyl CoA). Next, the acetyl CoA followed the Kreb’s cycle which produced three reduced nicotinamide adenine dinucleotide (NADH) and one reduced flavin adenosine dinucleotide (FADH2) molecules on completion of one Kreb’s cycle. The produced NADH and FADH2 followed the electron transport chain (ETC). Lastly, the 34-adenine triphosphate (ATP) molecules were produced (Abbas et al. 2017; Yaqoob et al. 2020b). The exoelectrogenic species have generally followed this route or process. The most common exoelectrogens are Geobacter sulfurreducens, Geobacter lovleyi, Shewanella putrefaciens, Rhodopseudomonas palustris, Geothrix fermentans, E. coli, and Shewanella oneidensis (Abbas et al. 2017). All exoelectrogens have to follow some mechanism to transfer the electrons to the anode surface as shown in Fig. 6.
-
1.
Direct electrons transfer The direct transportation of electrons is depending on two sub-categories. First, the electron transfer through conductive pili of bacterial species. This mechanism can be used only by the conductive pili-typed bacteria. The K. pneumoniae, Leucobacter, Bacillus species, Proteus species, and Acinetobacter species are well-known conductive pili-typed bacterial species. The conductive pili is a body part of the bacteria and it is conductive like a metal. Second direct electron transportation is using the redox-active proteins to transfer the electrons. The most dominant redox-active proteins are OmcB, OmcZ, OmcT, OmcE, and OmcS to transport the electrons (Igboamalu et al. 2019).
-
2.
Indirect electrons transfer The indirect electrons transfer mechanism proceeded via reduced and oxidized shuttles molecules. Some bacterial species generated their self-reduced and oxidized shuttles molecules to utilize to transfer the electrons. Several bacterial species families such as Geobacteraceae and Desulfuromonadaceae obeyed this mechanism. The self-electron shuttle molecules are MtrF, OmcA, MtrC, MtrE, and components.
However, the present study followed the direct electron transfer mechanism using the conductive pili to transfer the electrons. According to the biological test, specifically from the SEM images, it showed the rod-shaped morphology while bacterial identification showed conductive pili-type species. This is a sign that the transportation of electrons was done via conductive pili. On the other side, the protons are transferred directly to the cathode via PEM for the reduction reaction. Simultaneously, the metal ions are reduced into insoluble form during the redox reactions. The soluble metal ions were transferred into the insoluble state such as sludge as shown in Fig. 7. Generally, it can be written as:
-
Reduction of Pb2+ into Pb(s)
-
Reduction of Cd2+ into Cd(s)
-
Reduction of Cr3+ into Cr(s)
-
Reduction of Ni2+ into Ni(s)
-
Reduction of Co2+ into Co(s)
-
Reduction of Hg2+ into Hg(s)
Multiple parameters optimization
Figure 8a, b demonstrated the effect of pH on the voltage generation trend as well as the removal efficiency of metal ions. According to the present observation, the voltage was gradually increased from pH 4 to 7 but after pH 7 it started a reduction in the voltage generation. It means that the highly alkaline or acidic both are not favorable conditions for a stable operation. The maximum achieved voltage was 70 mV at pH 7 with the continued operation of MFCs. While pH 4 showed 30 mV but pH 10 showed less than 20 mV. The reason for the low voltage generation in the alkaline and acidic conditions is the disturbance in the bacterial growth which directly affects the biofilm formation. A weak and unstable biofilm will not lead to higher performance. Earlier, Huang et al. (2012) studied the optimization parameter and discussed the pH optimization in detail because it is one of the important factor of the operation. The report said pH 5.2 generated the acidic sludge which drastically disrupts the voltage output, while the highest voltage output was recorded at neutral pH. Similarly, the effect of pH was noticed in terms of metal removal. AS shown in Fig. 8b, the maximum removal efficiency of each metal was found the highest at pH 7. The acidic condition still offered higher removal efficiency of metals than the alkaline condition. The results for pH 6 were almost similar to pH 7, but the reduction in removal efficiency rate started to decrease from pH 8 and above. The lowest removal efficiency rate was recorded at pH 10. The reason is in alkaline conditions there are more chances of the formation of metal oxides directly from metal salts. Another reason is, the bacterial community required special requirements to survive in alkaline conditions as compared to the acidic medium (Asim et al. 2021). Overall, the neutral pH is found to be the most favorable condition.
Similar, Fig. 8c showed the effect of temperature variation. The temperature is also an important environmental factor that needs careful attention in a large-scale application. On an industrial scale, pH and temperature are the two most common factors which the operators need to control. For a lab-scale operation, the MFCs usually operated at room temperature.To optimize the temperature, the present study selected a very close temperature range to the room temperature. The obtained results showed that the temperature 25–30 °C was the most favorable temperature range. At 30 °C the maximum voltage generation was achieved, however at 25 °C was the favorable temperature for bacterial growth and biofilm formation. At 30 °C, the MFCs delivered 58 mV while at 25 °C showed its slightly lower value (55 mV). The temperature effect on metal removal is presented in Fig. 8d. The results showed that a temperature range of 25–30 °C is the ideal condition for the removal rate. A slight difference was found in the metal removal rate between 25 and 30 °C. Previously, Hsu et al. (2013) demonstrated that a temperature of 30 °C was the required environment for bacterial biofilm formation and working stability. It can be suggested that the room temperature and neutral pH is the most preferable condition for the MFCs operations at any scale.
Conclusion and future recommendations
The present study highlighted the performance of OPTS as an organic substrate in MFCs. The OPTS showed encouraging results in terms of metal removal efficiency. Due to the continued supply of the OPTS, the bacterial community developed a stable and healthy biofilm for a successful MFCs operation. According to the literature, there is a need for a stable organic substarte for the bacterial activities. From the electrochemical, biological, and physicochemical analysis, the OPTS found to be a good source of energy for the bacterial community in the MFCs operation. The bacterial community as observed during the bacterial identification process are well-known exoelectrogens with conductive pili. Although the energy generation was not high, the removal of metal was very promising. Energy generation was not a major issue as compared to electron transportation and energy storage. This is because offering the high sugar content-based substrate can improve the generation of electrons. The transportation of the produced electrons and energy storage remain critical challenges currently in the MFCs field. As the detailed mechanism of the MFCs was described in the present work, it is found that the electron transportation required highly conductive electrodes, particularly an anode to deliver the electrons. The highly conductive electrode material can capture the electrons more easily as compared to the less conductive material. Capturing the electrons from the bacterial cell and later transfer rapidly to the cathode is the main challenge. At present, several efforts are ongoing to fabricate the anode electrode using various materials. From the literature survey, graphene-derived metal oxide composite has the promising potential to offer a breakthrough in the field of MFCs. The graphene-derived metal oxide composites are highly conductive which can capture the electrons from conductive pili-typed species and later send them to cathode more effectively.
References
Ab Razak NH, Praveena SM, Aris AZ, Hashim Z (2015) Drinking water studies: a review on heavy metal, application of biomarker and health risk assessment (a special focus in Malaysia). J Epidemiol Glob Health 5(4):297–310
Abbas SZ, Rafatullah M (2021) Recent advances in soil microbial fuel cells for soil contaminants remediation. Chemosphere 272:129691
Abbas SZ, Rafatullah M, Ismail N, Syakir MI (2017) A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: mechanisms and future prospective. Int J Energy Res 41(9):1242–1264
Abbas SZ, Rafatullah M, Ismail N, Shakoori FR (2018) Electrochemistry and microbiology of microbial fuel cells treating marine sediments polluted with heavy metals. RSC Adv 8(34):18800–18813
Ahmad H, Parveen T, Ahmad A, Oves M, Ismail IM, Qari HA, Umar K, Mohamad Ibrahim MN (2020) Recent advances in metal decorated nanomaterials and their various biological applications: a review. Front Chem 8:341
Asim AY, Mohamad N, Khalid U, Tabassum P, Akil A, Lokhat D, Siti H (2021) A glimpse into the microbial fuel cells for wastewater treatment with energy generation. Desalin Water Treat 214:379–389
Ayangbenro AS, Babalola OO (2020) Genomic analysis of Bacillus cereus NWUAB01 and its heavy metal removal from polluted soil. Sci Rep 10(1):1–12
Bajracharya S, Sharma M, Mohanakrishna G, Benneton XD, Strik DP, Sarma PM, Pant D (2016) An overview on emerging bioelectrochemical systems (BESs): technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew Energy 98:153–170
Chuo SC, Mohamed SF, Mohd Setapar SH, Ahmad A, Jawaid M, Wani WA, Yaqoob AA, Mohamad Ibrahim MN (2020) Insights into the current trends in the utilization of bacteria for microbially induced calcium carbonate precipitation. Materials 13(21):4993
Di Martino P (2018) Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol 4(2):274
Hsu L, Chadwick B, Kagan J, Thacher R, Wotawa-Bergen A, Richter K (2013) Scale up considerations for sediment microbial fuel cells. RSC Adv 3(36):15947–15954
Huang L, Chai X, Quan X, Logan BE, Chen G (2012) Reductive dechlorination and mineralization of pentachlorophenol in biocathode microbial fuel cells. Biores Technol 111:167–174
Hung Y-H, Liu T-Y, Chen H-Y (2019) Renewable coffee waste-derived porous carbons as anode materials for high-performance sustainable microbial fuel cells. ACS Sustain Chem Eng 7(20):16991–16999
Igboamalu TE, Bezuidenhout N, Matsena MT, Chirwa E (2019) Microbial fuel cell power output and growth: effect of pH on anaerobic microbe consortium. Chem. Eng. Trans 76:1–6
Kumar MA, Anandapandian KTK, Parthiban K (2011) Production and characterization of exopolysaccharides (EPS) from biofilm forming marine bacterium. Braz Arch Biol Technol 54(2):259–265
Kumar R, Singh L, Wahid ZA, Din MFM (2015) Exoelectrogens in microbial fuel cells toward bioelectricity generation: a review. Int J Energy Res 39(8):1048–1067
Kumar R, Singh L, Zularisam A, Hai FI (2018) Microbial fuel cell is emerging as a versatile technology: a review on its possible applications, challenges and strategies to improve the performances. Int J Energy Res 42(2):369–394
Kunasundari B, Arai T, Sudesh K, Hashim R, Sulaiman O, Stalin NJ, Kosugi A (2017) Detoxification of sap from felled oil palm trunks for the efficient production of lactic acid. Appl Biochem Biotechnol 183(1):412–425
Li S, Cheng C, Thomas A (2017) Carbon-based microbial-fuel-cell electrodes: from conductive supports to active catalysts. Adv Mater 29(8):1602547
Liu H, Logan BE (2004) Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ Sci Technol 38(14):4040–4046
Logan BE, Regan JM (2006) Microbial fuel cells—challenges and applications. ACS Publications
Logan BE, Rabaey K (2012) Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 337(6095):686–690
Low KH, Koki IB, Juahir H, Azid A, Behkami S, Ikram R, Mohammed HA, Zain SM (2016) Evaluation of water quality variation in lakes, rivers, and ex-mining ponds in Malaysia. Desalin Water Treat 57(58):28215–28239
Mathivanan K, Rajaram R, Balasubramanian V (2016) Biosorption of Cd(II) and Cu(II) ions using Lysinibacillus fusiformis KMNTT-10: equilibrium and kinetic studies. Desalin Water Treat 57(47):22429–22440
Murata Y, Tanaka R, Fujimoto K, Kosugi A, Arai T, Togawa E, Takano T, Ibrahim WA, Elham P, Sulaiman O (2013) Development of sap compressing systems from oil palm trunk. Biomass Bioenergy 51:8–16
Nandy A, Kumar V, Kundu PP (2013) Utilization of proteinaceous materials for power generation in a mediatorless microbial fuel cell by a new electrogenic bacteria Lysinibacillus sphaericus VA5. Enzyme Microb Technol 53(5):339–344
Nimje VR, Chen C-Y, Chen C-C, Jean J-S, Reddy AS, Fan C-W, Pan K-Y, Liu H-T, Chen J-L (2009) Stable and high energy generation by a strain of Bacillus subtilis in a microbial fuel cell. J Power Sources 190(2):258–263
Owusu PA, Asumadu-Sarkodie S (2016) A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng 3(1):1167990
Palanisamy G, Jung H-Y, Sadhasivam T, Kurkuri MD, Kim SC, Roh S-H (2019) A comprehensive review on microbial fuel cell technologies: processes, utilization, and advanced developments in electrodes and membranes. J Clean Prod 221:598–621
Salim N, Hashim R, Sulaiman O, Nordin NA, Ibrahim M, Akil HM, Sato M, Sugimoto T, Hiziroglu S (2013) Effect of steaming on some properties of compressed oil palm trunk lumber. BioResources 8(2):2310–2324
Singh S, Songera DS (2012) A review on microbial fuel cell using organic waste as feed. Cibtech J Biotechnol 2(1):17–27
Umar K, Yaqoob A, Ibrahim M, Parveen T, Safian M (2020a) Environmental applications of smart polymer composites. Smart Polym Nanocompos Biomed Environ Appl 15:295–320
Umar MF, Abbas SZ, Mohamad Ibrahim MN, Ismail N, Rafatullah M (2020b) Insights into advancements and electrons transfer mechanisms of electrogens in benthic microbial fuel cells. Membranes 10(9):205
Umar K, Adnan R, Ibrahim MNM, Rashid M (2021a) Graphene oxide–ZnO nanocomposite: an efficient visible light photocatalyst for degradation of rhodamine B. Appl Nanosci 11(4):1291–1302
Umar MF, Rafatullah M, Abbas SZ, Mohamad Ibrahim MN, Ismail N (2021b) Advancement in benthic microbial fuel cells toward sustainable bioremediation and renewable energy production. Int J Environ Res Public Health 18(7):3811
Yamada H, Tanaka R, Sulaiman O, Hashim R, Hamid Z, Yahya M, Kosugi A, Arai T, Murata Y, Nirasawa S (2010) Old oil palm trunk: a promising source of sugars for bioethanol production. Biomass Bioenergy 34(11):1608–1613
Yaqoob AA, Ibrahim MNM (2019) A review article of nanoparticles; synthetic approaches and wastewater treatment methods. Int Res J Eng Technol 6:1–7
Yaqoob AA, Ibrahim MNM, Rodríguez-Couto S (2020a) Development and modification of materials to build cost-effective anodes for microbial fuel cells (MFCs): an overview. Biochem Eng J 164:107779
Yaqoob AA, Ibrahim MNM, Yaakop AS, Umar K, Ahmad A (2020b) Modified graphene oxide anode: a bioinspired waste material for bioremediation of Pb2+ with energy generation through microbial fuel cells. Chem Eng J 417:128052
Yaqoob AA, Khatoon A, Mohd Setapar SH, Umar K, Parveen T, Mohamad Ibrahim MN, Ahmad A, Rafatullah M (2020c) Outlook on the role of microbial fuel cells in remediation of environmental pollutants with electricity generation. Catalysts 10(8):819
Yaqoob AA, Mohamad Ibrahim MN, Rafatullah M, Chua YS, Ahmad A, Umar K (2020d) Recent advances in anodes for microbial fuel cells: an overview. Materials 13(9):2078
Yaqoob AA, Ibrahim MNM, Guerrero-Barajas C (2021a) Modern trend of anodes in microbial fuel cells (MFCs): an overview. Environ Technol Innov 23:101579
Yaqoob AA, Mohamad Ibrahim MN, Umar K, Bhawani SA, Khan A, Asiri AM, Khan MR, Azam M, AlAmmari AM (2021b) Cellulose derived graphene/polyaniline nanocomposite anode for energy generation and bioremediation of toxic metals via benthic microbial fuel cells. Polymers 13(1):135
Yaqoob AA, Serrà A, Ibrahim MNM, Yaakop AS (2021c) Self-assembled oil palm biomass-derived modified graphene oxide anode: an efficient medium for energy transportation and bioremediating Cd(II) via microbial fuel cells. Arab J Chem 14(5):103121
Zhang L, Zhou S, Zhuang L, Li W, Zhang J, Lu N, Deng L (2008) Microbial fuel cell based on Klebsiella pneumoniae biofilm. Electrochem Commun 10(10):1641–1643
Zhang J, Cao X, Wang H, Long X, Li X (2020) Simultaneous enhancement of heavy metal removal and electricity generation in soil microbial fuel cell. Ecotoxicol Environ Saf 192:110314
Acknowledgements
This article was financially supported by Universiti Sains Malaysia (Malaysia) under research grant no. 304/PKIMIA/6501153/E128.
Author information
Authors and Affiliations
Contributions
MNMI, ASY: conceptualization. AAY: methodology, writing-original draft preparation, visualization, electromicrobiology investigation. AA: english editing of manuscript. MNMI: supervision, funding acquisition. This article has been read and approved by all listed authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yaqoob, A.A., Ibrahim, M.N.M., Yaakop, A.S. et al. Application of microbial fuel cells energized by oil palm trunk sap (OPTS) to remove the toxic metal from synthetic wastewater with generation of electricity. Appl Nanosci 11, 1949–1961 (2021). https://doi.org/10.1007/s13204-021-01885-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13204-021-01885-6