Abstract
The gradual rise in the human population and an ever-increasing demand for high-value products and alternative fuels has pushed industries into discovering new bioactive compounds and process technologies. Microalgae, an emerging bioresource with distinctive metabolite composition and an efficient growth rate, thus offer a unique platform to meet these demands of sustainable and alternative sources of food and energy. Till date several species of microalgae have been evaluated for their application as cosmeceuticals, pharmaceuticals, nutraceuticals, and biofuels owing to the major bioactive profiles including proteins, polysaccharides, polyunsaturated fatty acids (PUFAs), functional pigments, and vitamins. In addition, the prospect of genetically modified microalgae holds a greater promise to the future for high-value products and third-generation biofuels.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
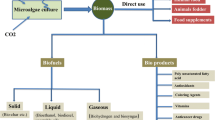
The cosmetic industry is predicted to exceed more than US$ 390 billion by 2025, with a compound annual growth rate (CAGR) of 4.3% from 2016 to 2022. Changing lifestyles, the rising economy, and the ineluctable health benefits from natural ingredients are the major factors impacting the thriving trend in the global cosmetics market. Growing awareness about the ill effects of toxic constituents and the developing need for a green lifestyle has further shifted the oversight towards natural products derived from cosmetics. Cosmetics and cosmeceuticals are increasingly using active ingredients from natural sources which present great biodiversity of desired and safe components. Microalgae have come a long way as a unique potential with several primary bioactive compounds of cosmeceutical, pharmaceutical, and nutraceutical importance such as proteins, polysaccharides, pigments, lipids, and vitamins (Agrawal and Verma 2022; Andrade 2018; Morais et al. 2015). Owing to a range of bioactive compounds displaying antioxidant, immunomodulatory, and anticancer activities, microalgae are currently explored for utilizing these properties in cosmetic application including anti-aging, sunscreens, wound healing, and anti-acne treatments (Mehariya et al. 2021; Yarkent et al. 2020; Pangestuti and Kim 2011). Numerous microalgae have prospected for cosmeceutical properties, for example, Spirulina platensis extracts showed wound healing activities in HS2 keratinocyte cells (Gunes et al. 2017), and Spirulina extract loaded PCL nanofiber improved skin regeneration by increasing fibroblasts viability and modulating (reactive oxygen species) ROS levels. Furthermore, antiproliferative and anticancer properties of Spirulina and Chlorella are also widely reported (Cha et al. 2008; Czerwonka et al. 2018; El-fayoumy et al. 2021; Fayyad et al. 2019; Kyadari et al. 2013). Notably, application of microalgae in thalassotherapy as a marine cosmetic for the therapeutic and revitalizing schemes are novel frontiers in microalgal biotechnology (Mourelle et al. 2017) (Fig. 3.1).
Another distinct application of microalgal bioresource includes the demanding area of biofuels. In the coming years, depletion of fossil fuels as well as concerns of global warming further necessitates an urgent requirement for alternative energy sources (Kotasthane 2017). In comparison to crop-based biofuels, Microalgal derived biofuels have several advantages as follows: (1) It reduces pressure on fertile agricultural land which can be instead used for agricultural practices to nourish the growing population. (2) Unlike lignocellulose-rich crop biomass which requires several pretreatment processes, microalgae with low levels of lignocellulose provide a cost-effective raw material for biofuel extraction. (3) Adaptability of microalgae in extreme conditions and wastewater provides feasible bioenergy source as well as scope for greener technological advancements. (4) Finally the prospect of genetically modified microalgae and strain optimization studies can further enhance the bio-products development in area of biodiesel, bioethanol, biogas, and bio-oil (Bhardwaj et al. 2020; Molinuevo-Salces et al. 2019; Adegboye et al. 2021; Goswami et al. 2021b; Hannon et al. 2010). In this chapter we briefly discuss the wide range of bioactive compounds with potential role in cosmeceuticals as well as capture the overview of different microalgal species involved in cosmeceuticals and biofuels generation. Additionally, we also discuss numerous bio-products that have potential role in biofuel development.
3.2 Microalgae as Cosmeceuticals
In view of the changing “greener” lifestyles and the growing awareness for natural ingredients, microalgae emerge as the popular choice for the cosmeceutical industry. The potential of microalgae as a rich source of pigments, proteins, vitamins, minerals, polysaccharides, and fatty acids provides a direct benefit for human health, especially as a cosmetic product. Microalgal bioresource offers a unique platform for novel bioactive molecules in the cosmeceutical industry, a combination of cosmetics and pharmaceuticals, i.e., possessing health benefits. Microalgae represent a diverse group of unicellular prokaryotic (Prochlorophyta and Cyanophyta) and eukaryotic (Chlorophyta, Bacillariophyta, Phaeophyta, Rhodophyta, and Chrysophyta) microorganisms characterized by a wide range of bioactive metabolites with antioxidant, anti-inflammatory, and immune-modulatory properties. Extracts from microalgae such as Arthrospira, Chlorella, Nannochloropsis, Spirulina, and Dunaliella have already been established as an ingredient in skincare compositions (Stolz and Obermayer 2005). Exsymol (Monaco) used protein-rich extract from Spirulina platensis with anti-aging properties. Spirulina firming masks produced by Optimum Derma Aciditate (Lithuania) improve skin moisture balance and immunity (Chakdar et al. 2012). Dermochlorella D, an amino acid concentrate from Chlorella vulgaris produced by Codif (France), promotes activation of collagen synthesis and reduces stretch marks and vascular imperfections (Ryu et al. 2015). The cosmeceutical value of microalgae is conferred due to the feasibility of growing them in extreme conditions and also due to the wide range of chemicals with favorable biological activity (Mobin and Chowdhury 2019). Although a plethora of microalgae have been identified and utilized in industrial operations for cosmeceuticals, yet these represent only a percentage of their substantial diversity. To date more than 25,000 novel bioactive compounds have been discovered from different oceanic regions of the world (Blunt et al. 2016). These bioactive metabolites are categorized into different major groups, viz., polysaccharides, fatty acids, pigments, proteins, peptides, etc. (Table 3.1).
3.2.1 Polysaccharides
Polysaccharides are the most abundant biochemical molecule made up of thousands of monosaccharide units. These polymeric compounds can be categorized as homo-polysaccharides or hetero-polysaccharides based on the single or different monosaccharide unit that makes the compound. The glycosidic bonds that frequently occur in these compounds are β-1,4 or β-1,3 and α-1,4, α-1,2, or α-1,6 linkages. The α-glucans (starch and floridean starch) have been reported in green, glaucophyte, charophyte, cryptomonad, dinophyte, red microalgae, and cyanobacteria (Mobin and Chowdhury 2019). Similarly, the β-glucans (chrysolaminarin and paramylon) have been found in euglenophytes, haptophytes, Chlorella genus, Skeletonema diatom, and also Porphyridium and Nostoc flagelliforme (Mourelle et al. 2017). The cosmetic action provided by β-1,3-glucans includes blood cholesterol levels reduction, antioxidant activities, as well as immune stimulator (Koller et al. 2014). Xia et al. (2014) discovered a novel β-glucans, chrysolaminarin, named CL2 obtained from Odontella aurita that showed strong antioxidant activities. Until recently, at an industrial level, Algatech has licensed their rights to the production of β-1,3-glucans from fermented microalgae Euglena gracilis. High concentration and purity of the biochemical in Euglena gracilis compared to common sources in the market such as oats, mushrooms, and yeast make them potential and effective bioresource with powerful health benefits (for an example see https://www.algatech.com/algatech-product/bioglena-beta-glucan). In a cosmetic formulation, use of hydroxy acid (HA) is promoted to provide skin moisturization; however, since it can be only extracted from plants and animals, the cost is relatively high. Studies have shown the application of polysaccharides from marine algal species like Saccharina japonica and Chondrus crispus can provide a much more cost-effective alternative to hydroxy acid (Wang et al. 2015). As reported by several authors, water-soluble polysaccharides from Spirulina platensis are another potential raw material for the cosmeceutical industry with strong antioxidant activities (Chaiklahan et al. 2013; Kurd and Samavati 2015).
Microalgal exopolysaccharides are complex glycopolymers that protect microorganisms against extreme environmental stress conditions like dehydration as it allows them to retain cellular water. It is thus widely accepted that this property of the microalgal exopolysaccharide can be utilized for moisturization and hydration of the skin (Stoyneva-Gärtner et al. 2020). Although only a few studies have been conducted on these lines, extracellular sulfated polysaccharide from red microalgae Porphyridium sp. has drawn prominent interest based on their remarkable nutritional, therapeutic, and cosmetic activity. Estee and Companies (2003) reported the in vitro and in vivo anti-inflammatory activity of polysaccharide material from Porphyridium sp. The bioactive sulfated polysaccharide prevented the polymorphonuclear leukocyte (PMN) migration involved in skin inflammation. In another study, the antioxidant properties of sulfated polysaccharide extract from Porphyridium sp. have been reported. At a concentration of 2 mg ml−1 and 10 mg ml−1, Porphyridium polysaccharide gave antioxidant activity of 45% and 90%, respectively (Tannin-Spitz et al. 2005). Furthermore, the sulfated polysaccharide from Porphyridium sp. is also believed to be an excellent alternative to hyaluronic acid as a bio-lubricant with antioxidant activities (Raposo et al. 2013a). Based on the significant cosmetic properties, sulfated polysaccharides thus make a suitable candidate as a cosmetic bioresource (Arad and van Moppes 2013). Notably in a study conducted on polysaccharide, extract of Caulerpa microphysa (Chlorophyta) suggested excellent anti-inflammatory and wound healing with good moisture-holding capacity. Caulerpa microphysa extract (CME) showed complete inhibition of β-hexosaminidase, a critical inflammatory mediator in allergic reactions thus suggesting the anti-allergic potential of CME (Lee et al. 2021). Table 3.2 presents a list of microalgal polysaccharides and their specific features and cosmetic action.
3.2.2 Fatty Acids
Traditionally, microalgae are considered a rich source of lipids, especially the long unsaturated fatty acids such as γ-linolenic acid, arachidonic acid, stearidonic acid, and ω-3 fatty acids: eicosapentaenoic acid (EPA) (ω-3 C 20:5) and docosahexaenoic acid (DHA) (ω-3 C 22:6), etc. (Mobin and Chowdhury 2019). The total lipid production in microalgae may reach up to 30–70% of their dry weight depending on the species type (Balasubramaniam et al. 2021). Microalgal species like Chlorella emersonii, Dunaliella tertiolecta, Nannochloropsis sp., Porphyridium cruentum, Botryococcus braunii, and Neochloris oleoabundans show high lipid content of more than 60% of their dry weight. Freshwater microalgal species like Chlorella minutissima, Chlorella protothecoides, Chlorella vulgaris, Scenedesmus dimorphus, and Scenedesmus obliquus and marine microalgal species like Dunaliella salina, Nannochloris sp., Crypthecodinium cohnii, Isochrysis galbana, Nitzschia sp., Phaeodactylum tricornutum, Schizochytrium sp., and Skeletonema costatum display moderate lipid content in the range of 40–60% of their dry weight. Microalgae that display lower lipid content, i.e., below 40% of their dry weight, include Arthrospira maxima, Spirulina platensis, Dunaliella primolecta, Chlorella sorokiniana, Ankistrodesmus sp., Chaetoceros muelleri, Cylindrotheca sp., C. cohnii, Ellipsoidion sp., Euglena gracilis, Haematococcus pluvialis, Monodus subterraneus UTEX 151, Monallanthus salina, Oocystis pusilla, Pavlova salina, Thalassiosira pseudonana, and Tetraselmis suecica (Mobin and Chowdhury 2019; Maity et al. 2014). Evidently, two genera of microalgae, viz., Dunaliella and Chlorella, show differential total lipid content at a species level: Dunaliella tertiolecta (more than 60%), Dunaliella salina (40–60%), and Dunaliella primolecta (less than 40%) and also Chlorella emersonii (more than 60%), Chlorella vulgaris (40–60%), and Chlorella sorokiniana (less than 40%). A greater emphasis must be thus given to the microalgal strains with high lipid content to empower cost-effectiveness for economic production.
The unique fatty acid profiles from microalgae can be broadly categorized into polar and neutral lipids. The polar lipids are mainly constituted of membrane lipids such as phospholipids and glycolipids in a microalgal cell. On the contrary neutral lipids are localized in the cytoplasm as triacylglycerols (TAGs) (Yao et al. 2012). Long-chain polyunsaturated fatty acid (PUFA) usually belongs to polar lipids, whereas a neutral lipid consists of monounsaturated fatty acids (MUFAs) and saturated fatty acids (SFAs) in the triacylglycerols (TAGs). Based on the percentages of characteristic fatty acids such as C 16:0/C16:1 and eicosapentaenoic acid (EPA) (ω-3 C 20:5) for neutral (TAGs) and polar fatty acids, respectively, fatty acid profiles of different microalgae can be compared. Nannochloropsis oceanica fatty acid percentage values in terms of r2 of C16:0 and EPA were 0.94 and 0.97, respectively. Similarly, Chlorella pyrenoidosa r2 values for C18:1/ C18:3 with TAG content were 0.91 and 0.99, respectively. This method of correlation thus allows researchers to precisely quantify TAGs in microalgae (Shen et al. 2016). Several species of microalgae have been explored for their rich lipids content and their subsequent application in the cosmetic industry. Gamma-linolenic acid from Spirulina platensis functions as the potential bioactive compound with anti-aging, anti-wrinkle, collagen synthesis, anti-inflammatory, and antioxidant activities (Hoseini et al. 2013). Porphyridium cruentum is an excellent source of polyunsaturated fatty acids (PUFAs) that provides photoprotection against the high intensity and UV light as well as prevents skin dehydration. ω-3 PUFAs, namely, eicosapentaenoic acid (EPA, 20:5 ω3) and docosahexaenoic acid (DHA, 22:6 ω3), from Isochrysis galbana show anti-inflammatory effect. Furthermore, several extracts of lipids, PUFAs, and pigments from Isochrysis galbana have been claimed to have skin and hair care properties (Abdoul-latif et al. 2021; Bonfanti et al. 2018). Microalgal squalenes have been reported to have moisturizing, antioxidant, and anti-aging properties. A large number of cosmetic products, viz., lipstick, lotions, eye pencil, eye shadows, eye makeup remover, and perfumes, utilize the squalene in their formulations. Botryococcus braunii, Schizochytrium mangrovei, and Thraustochytrium sp. are a few microalgae species that produce these thriving molecules. Microalgal galactolipids, i.e., monogalactosyl diacylglycerol (MGDG) and digalactosyldiacylglycerol (DGDG), present another unique bioactive resource with cosmeceutical applications based on its anti-inflammatory activities. Bruno et al. compared dose-dependent anti-inflammatory activity of MGDG and DGDG and concluded that eicosapentaenoic acid (EPA) presence in MGDG further enhanced the activity (Bruno et al. 2005). Microalgal species such as Chlorella minutissima, Chaetoceros, Cyclotella, Ellipsidion, Isochrysis, Monochrysis, Monoraphidium, Nannochloris, Nannochloropsis, Nitzschia, Phaeodactylum, Porphyridium, Skeletonema, and Thalassiosira are the potential sources of the galactolipids (Asraful et al. 2020).
3.2.3 Pigments
Pigments are another widely explored bioactive resource from the microalgal biomass. Fat-soluble pigments such as chlorophyll and carotenoids including β-carotene, astaxanthin, and fucoxanthin as well as water-soluble phycobiliproteins such as allophycocyanin, phycocyanin, phycoerythrin, and phycoerythrocyanin are the most ubiquitous in the microalgal cell factory. Agardhiella, Arthrospira, Chlorella, Dunaliella, Haematococcus, Muriellopsis, Nannochloropsis, Nostoc, Phaeodactylum, Porphyridium, Polysiphonia, Scenedesmus, and Spirulina are major microalgal genera that produce these wide ranges of bioactive pigments. They are commonly present in cyanobacteria (blue-green algae), Rhodophyta (red algae), and unicellular eukaryotic algae (cryptomonads). Phycobiliprotein’s properties as an antioxidant and free radical scavenging activities have drawn major attention in cosmetics as well as in food, pharmaceuticals, and biomedical applications. Furthermore, microalgal pigments also exhibit anti-inflammatory, antiangiogenic, antiviral, antiobesity, antidiabetic, anticancer, anti-osteoporotic, and neuro- and hepatic-protective activities (Pailliè-Jiménez et al. 2020). At present Dainippon Ink and Chemicals Inc. (Japan) utilizes Arthrospira spp. with a brand name of Linablue as a food colorant as well as cosmetic applications (Saini et al. 2021; Morocho-Jácome et al. 2020). Algenist (CA, USA) introduced a novel vitamin C from Spirulina which enhances skin tone, smoothens texture, and prevents photoaging (for an example see BLUE ALGAE VITAMIN C™ Skinclarity Brightening Serum (algenist.com)). Because of their pH and heat stability characteristics, phycobiliproteins are used as a natural colorant in eyeliners, lipsticks, and other cosmetic formulations (Balboa et al. 2015).
There is an increasing demand for carotenoids derived from microalgae considering their pharmaceutical and cosmetic role. Chlorella, Dunaliella, Haematococcus, and Muriellopsis are the routine sources for carotenoids. Astaxanthin extracted from Haematococcus pluvialis is reported to have antioxidant biological activity as well known to reduce skin pigmentation. A clinical study on two human subjects, 30 women aged 20–55 years old and 36 men aged 20–60 years old, concluded astaxanthin’s role in improving elasticity, skin texture, moisture content of corneocyte layer, and corneocyte condition as well as reducing skin wrinkle and age spot size in both subjects (Goswami et al. 2021c; Tominaga et al. 2012). In another study, astaxanthin was found to significantly suppress transepidermal water loss and wrinkle formation thus preventing photoaging caused due to UV-A radiation (Komatsu et al. 2017). AstaPure is a commercial product of astaxanthin extracted from Haematococcus pluvialis utilized by Algatech (Israel) as topical creams and emulsions because of its cosmeceutical activity on skin health, eye health, and immunity ((Morocho-Jácome et al. 2020), also see AstaPure Natural Astaxanthin l astaxanthin manufacturer l bulk astaxanthin (algatech.com). Fucoxanthin mainly found in Cercis siliquastrum, H. fusiformis, L. japonica, Undaria pinnatifida, and Sargassum fulvellum also offers antioxidant properties. Fucoxanthin extracted from Phaeodactylum tricornutum showed protection against oxidative damage (Kawee-ai et al. 2013). A study reported the anti-pigmentary activity of fucoxanthin by inhibiting tyrosinase, a key enzyme in melanogenesis, and also preventing the UV-B radiation-induced skin pigmentation (Shimoda et al. 2010).
The most common carotene produced by the halotolerant microalga Dunaliella salina is β-carotene, i.e., up to 10% of its dry weight biomass. Apart from pro-vitamin A activity, β-carotene is also accepted as a cosmeceutical because of its properties like antioxidants, anticancer, anti-inflammatory, and immune modulators (Raposo et al. 2013b). Guruvayoorappan and Kuttan (2007) demonstrated the antiangiogenic activity of β-carotene on B16F-10 cell lines. The study showed that β-carotene prevented neovascularization, i.e., the formation of tumor-directed capillaries by tailoring the expression of proinflammatory cytokines such as matrix metalloproteinase 2 (MMP-2) and MMP-9 and also preventing nuclear translocation of the transcription factor. In another study, β-carotene exhibited antioxidant and anti-inflammatory activity on H. pylori-infected human gastric epithelial AGS cell lines. H. pylori-infected cell lines showed increased expression of matrix metalloproteinases (MMPs), key molecules in metastasis and cancer invasion. However, on treatment with β-carotene, the expression of MMPs was significantly controlled primarily by reducing the ROS levels in the cell line (Bae et al. 2021).
3.2.4 Proteins and Peptides
Mycosporine-like amino acids (MAAs) are water-soluble, low molecular weight (0.188 to 1.05 kDa) bioactive resources found in cyanobacteria, microalgae, macroalgae (chlorophyta and Rhodophyta), and several other marine organisms (Wada et al. 2015). Oligosaccharide-linked mycosporine-like amino acids were first reported in Nostoc commune which also showed significant photoprotection against UV-B radiation. These molecules have a characteristic UV-A and UV-B absorption, a critical feature for cosmeceutical application as sunscreens (Bohm et al. 1995). Schmid et al. (2006) characterized MAAs-based sunscreens (Helioguard 365) extracted from Porphyra umbilicalis in in vitro and in vivo conditions and demonstrated the anti-aging and antioxidant properties of the biochemical. Helioguard 365 containing porphyra-334 and shinorine showed protection from irradiation-induced DNA damage and prevented lipid peroxidation ultimately providing smoothness and firmness of the skin. In another study porphyra-334 extracted from P. yezoensis showed significant antioxidant properties with ROS production inhibition as well as induced type I collagen and elastin suggesting a role as an anti-wrinkle or anti-aging agent (Ryu et al. 2014). A commercial product named HELIONORI® with mycosporine-like amino acids (MAAs) as an important ingredient is derived from Porphyra umbilicalis. The cosmetic product protects against UV-A-induced skin damage and DNA damage and also prevents premature photoaging ((Figueroa 2021), e.g., see HELIONORI® - GELYMA)). Until recently, MAAs has been reported in Chlorella, Pseudochlorella, Stichococcus, Apatococcus, Bracteacoccus, Coccomyxa, Elliptochloris, Pabia, Prasiolopsis, and Pseudococcomyxa (Stoyneva-Gärtner et al. 2020).
Biopeptides are low molecular weight (3 kDa) short peptides derived from plants, animals, and microalgal sources. Chlorella and Spirulina are a few microalgal genera that are currently explored for biopeptides with cosmeceutical properties. Chlorella-derived peptides showed inhibitory activity towards UV-B-induced matrix metalloproteinase-1 (MMP-1) involved in the photoaging process and also restored collagen and TbRII (transforming growth factor, TGF-β, receptor) preventing further damage to the skin (Chen et al. 2011). In another study, Chlorella-derived peptide (CDP) prevented UV-C-induced cytotoxicity and DNA damage through inhibition of caspase-3 activity and Fas-associated death domain (FADD) expression (Shih and Cherng 2012). Sadeghi et al. (2018) reported spirulina-based peptides with antimicrobial and anticancer properties. In this study spirulina-based peptides (<3 kDa peptide fraction) showed prominent dose-dependent inhibition of SW480 cells (human colon adenocarcinoma cell), and the same fraction also demonstrated significant antibacterial activity against Escherichia coli and Staphylococcus aureus. Furthermore a phycobiliprotein-derived bioactive peptide, SpirPep1, attained significant (angiotensin-converting enzyme) ACE inhibitory activity thus demonstrating anti-tumor and antihypertensive activity (Anekthanakul et al. 2019).
3.3 Microalgae for Biofuel Production
Due to environmental concerns including increasing greenhouse emissions and the world energy crisis, a need for an alternative source of energy with CO2 mitigation potential has arisen. Microalgae provide a unique feedstock for biofuels production that can outcompete the traditional fossil fuels and crop-based biofuels providing a sustainable approach to the energy crisis as well as increasing the efficiency of biofuel production. In developing countries like India with 120 million tonnes of petroleum consumption per year, at least 21% of the agricultural land for biofuels production is necessary (Mondal et al. 2017). However, with microalgal bioresource, less than 2–3% of the agricultural land would be required considering the high oil yield productivity in terms of area utilized. Compared to the oil yield of crops such as caster, maize, oil palm, and physic nut which range from 172 to 5366 oil liters/hectare/year, microalgae with high oil yield can produce up to 136,900 oil liters/hectare/year (Medipally et al. 2015). Significantly microalgal land use efficiency for biofuel production was 338 times greater than corn biodiesel (Lum et al. 2013). Furthermore the CO2 capture efficiency of microalgae is estimated to be 10–50 times higher than that of terrestrial plants (Li et al. 2008). Studies report CO2 capture from many microalgal species such as Chlorella kessleri, Chlorella emersonii, Chlorococcum littorale, Galdieria partita, and Synechococcus PCC7942. The photoautotrophic potential of microalgae thus provides an added advantage to the utilization of greenhouse gas like CO2 in the upstream application for biofuels like bioethanol, biodiesel, biogas, biohydrogen, bio-oil, and syngas.
3.3.1 Biodiesel
Major biodiesel standards specification like US specification (ASTM D6751), European biodiesel specification (EN 14214), and Indian biodiesel specification define biodiesel as a mixture of monoalkyl esters of long-chain fatty acids (Mondal et al. 2017). Transesterification is a primary process for the conversion of triglycerides (oils) generated from microalgae to biodiesel using different catalysts such as alcohols, acids, and enzymes. Tang et al. (2011) reported Dunaliella tertiolecta as a potential feedstock for biodiesel production extracted using methanol and chloroform. Fatty acid methyl ester (FAME) profile showed the significant percentage of methyl linolenate (C18:3), methyl palmitate (C16:0), methyl oleate (C18:1), and methyl linoleate (C18:2) with the highest cell density at 2–6% of CO2 concentration. A study conducted on biodiesel production from Spirulina platensis using acid and methanol reported a viscosity value of 4.8 in accordance with the EN 14214 standard thus affirming its quality as a biodiesel substitute to fossil-derived fuels (El-Shimi et al. 2013). Investigation on the choice of the solvent for lipids extraction in Nannochloropsis sp. showed chloroform/methanol (1:2 v/v) with the highest percentage yield of 60.37% (Rahmanpour and Shariati 2015). Extraction process is one of the major factors affecting biodiesel yield apart from the choice of solvents. A study on microalgae Schizochytrium limacinum validated that direct transesterification of microalgal biomass using methanol and sulfuric acid along with chloroform, hexane, and petroleum ether produced higher biodiesel yield (10–20%) compared to indirect extraction-transesterification method using chloroform and methanol as extracting agent and additional sulfuric acid as transesterification agent (Johnson and Wen 2009). In another study using Scenedesmus sp. for in situ or direct transesterification process, a higher biodiesel production was recorded for alkaline catalyst (55.07 ± 2.18%) than acidic catalyst (48.41 ± 0.21%) (Kim et al. 2014). Nutrient limitation is another factor that can impact the efficiency of biodiesel production in microalgae by lipid accumulation, i.e., converting excess carbon into storage lipids. In one study nitrogen limitation increased the biodiesel yield in Chlorella sp. and Desmodesmus quadricaudatus. For Chlorella the unsaturated fatty acids increased from 37.92% to 41.6% in nitrogen-free medium, and similarly for Desmodesmus quadricaudatus, saturated fatty acids increased from 51.62% to 66.92% in nitrogen-free medium (Shafik et al. 2015).
3.3.2 Bioethanol
On account of low ligno- and hemicellulose content in microalgae compared to lignocellulosic biomass observed in conventional crops, microalgae with high levels of carbohydrates provide an appropriate choice for bioethanol production. Bioethanol production from biomass requires processes such as pretreatment, saccharification or enzymatic hydrolysis, fermentation, and distillation. Mild pretreatment is required for microalgal biomass owing to the absence of lignocellulose in algal biomass. During the process of pretreatment and saccharification, the complex polysaccharide is broken down into fermentable monomeric sugars. Several methods of pretreatment and saccharification are employed, i.e. physical or mechanical (ultrasonication, high-pressure homogenization, autoclave, bead beating), chemical (acidic hydrolysis, alkaline hydrolysis, and supercritical CO2), or enzymatic treatment (Agrawal et al. 2020; Kumar et al. 2020a; Phwan et al. 2018; Behera et al. 2015). Pretreatment studies using alkaline hydrolysis produced a maximum reducing sugar concentration of 88 mg/g dried biomass and 81 mg/g dried biomass in Chlorella sp. and Tetraselmis suecica, respectively (Behera et al. 2015). In another study, microalgae Chlorococcum infusionum was used for alkaline pretreatment which produced the highest glucose yield of 350 mg/g dried biomass with the highest bioethanol yield of 0.26 g/g dried biomass (Harun et al. 2011). Khan et al. (2017) reported that calcium oxide (CaO) treatment before acid and enzymatic hydrolysis significantly doubled the yield of monomeric sugars in Microcystis aeruginosa. Furthermore, a high temperature of 120 °C (autoclave disruption treatment) combined with acidic treatment showed the highest disruption and sugar extraction efficiency for microalgae Scenedesmus obliquus (Miranda et al. 2012). Jeon et al. (2013) investigated the ultrasonic pretreatment prior to microbial fermentation for bioethanol feedstock production in Scenedesmus obliquus YSW15. The results demonstrated that ultrasonic treatment at 15 min duration increased the dissolved carbohydrates concentration at 0.12 g/g dried biomass further increasing the bioethanol yield through microbial fermentation. Scanning electron microscope (SEM) images confirmed that rupture of microalgal cell wall allowed fermenting microbes to ingress the microalgal cellular system thus further enhancing the treatment efficiency. After pretreatment and saccharification, microbial fermentation process then converts the fermentable monosaccharides into bioethanol and other bio-products. Several fermenting microorganisms have been utilized for the process, viz., yeast and fungi such as Saccharomyces cerevisiae, Schizosaccharomyces pombe, Kluyveromyces fragilis, Kluyveromyces marxianus, etc. as well as bacteria such as Escherichia coli, Klebsiella oxytoca, and Zymomonas mobilis. A study on bioethanol production from red microalgae Porphyridium cruemtum depicted that simultaneous saccharification and fermentation (SSF) was a better method than separate hydrolysis and fermentation (SHF). A bioethanol yield of 65.4 and 70.3% using Saccharomyces cerevisiae KCTC 7906 was achieved for seawater Porphyridium cruemtum and freshwater Porphyridium cruemtum, respectively (Miyamoto et al. 1979). Using Escherichia coli as a microbial fermenting organism, three marine algae Chlorella vulgaris, Chlamydomonas reinhardtii, and Undaria pinnatifida were explored for bioethanol production. The highest bioethanol yield of 0.4 g/g biomass was observed for acidic and enzymatic pretreated Chlorella vulgaris biomass (Lee et al. 2011). Many other fermenting microorganisms such as Saccharomyces bayanus, Saccharomyces cerevisiae S288C, E. coli KO11, and Zymomonas mobilis have been utilized for fermentation treatment for microalgae such as Chlorococcum sp., Chlamydomonas reinhardtii UTEX 90, Chlorella variabilis, and Chlorella vulgaris FSP-E, respectively, at different pretreatment procedures and fermentation conditions (Phwan et al. 2018).
3.3.3 Biogas
Owing to the presence of polysaccharides such as agar, alginate, carrageenan, laminarin, and mannitol and low cellulose content, microalgae present a superior bioresource for biogas extraction. Biogas production occurs through several anaerobic fermentation steps involving primarily conversion of insoluble high molecular weight organic molecules into soluble organic fraction. In the subsequent steps, volatile fatty acids and alcohols are released by acidogenesis; these volatile organic compounds are then converted into acetic acid and hydrogen and finally conversion of acetic acid and hydrogen into methane and carbon dioxide gas. The entire process of anaerobic fermentation requires microorganisms for biodegradation such as acidogenic bacteria, acetogenic bacteria, and methanogens (Behera et al. 2015). It is essential that the hydrolysis of the microalgal cells must be efficient enough to increase the biodegradation of microalgal biomass. A study on anaerobic biodegradation of Scenedesmus obliquus and Phaeodactylum tricornutum under similar experimental conditions showed that different microalgae have different biodegradation efficiencies owing to their unique physiology and thermal tolerance (Zamalloa et al. 2012). Several works have been reported for the methane and biogas yield from microalgae such as Scenedesmus obliquus, Chlorella vulgaris, Nannochloropsis salina, Spirulina maxima, Phaeodactylum tricornutum, Isochrysis galbana, etc. (Jankowska et al. 2017; Goswami et al. 2021d). A study on methane gas production from Spirulina maxima reported that mechanical and thermochemical treatments had a positive effect on acidogenic bacteria; however, it had no subsequent effect on the methanogens (Samson and Leduy 1983). Similarly, thermal pretreatment of Scenedesmus sp. showed a significant anaerobic biodegradability of 48% when the temperature was increased from 70 to 90̊ C thus establishing the effect of higher temperature on the biodegradation efficiency (González-Fernández et al. 2012). Another condition that increases the yield of biogas like methane from microalgae was a reduction of sodium concentration in the biodegradation mixture. Santos et al. (2014) described a 71.5% increase in methane yield by removing inhibitory sodium in the anaerobic digestion mixture in microalgae Isochrysis galbana. Another important variable that affects the yield of methane biogas includes the inoculum/extract (I/S) ratio. A consistent I/S ratio of 1 produced effective specific methane productivity of 0.304–0.557 L CH4/g volatile solids in five microalgae, viz., Chlorella vulgaris UTEX 395, Phaeodactylum tricornutum CCMP 632, Nannochloropsis sp., Nannochloropsis salina, and Nanofrustulum sp. (Zhao et al. 2014).
3.3.4 Biohydrogen
As an alternative energy and fuel source, hydrogen is advantageous as its combustion only releases water thus mitigating CO2 levels and reducing greenhouse emissions (Nagarajan et al. 2021). Hydrogenases are the main enzymes involved in biohydrogen production in microalgae. These enzymes accept electrons through many sources like photosynthesis; an approach of direct photolysis is applied where the electrons released during photolysis of water are utilized by the enzymes for biohydrogen production. Also, other approaches include indirect photolysis; in this mechanism electrons released during the fermentative metabolism of stored carbon catalyze the biohydrogen production (Limongi et al. 2021; Goswami et al. 2021a; Nagarajan et al. 2021). A study on thermophilic algae Mastigocladus laminosus described the inhibition of hydrogen production yield due to hydrogen consumption by oxygen and carbon monoxide. Conclusively at the higher temperatures, the decrease in oxygen concentration enhanced the biohydrogen yield by 50% (Miyamoto et al. 1979). Kose and Oncel (2014) further enhanced the biohydrogen production in green microalgae Chlamydomonas reinhardtii. Using genetically engineered Chlamydomonas reinhardtii with mutations in D1 protein, a higher biohydrogen yield rate was generated 1.3 ± 0.5 mL/L.h compared to the wild strain with twofold lower yield rate of 0.57 ± 0.2 mL/L.h. The mutated D1 protein blocks PSII repair system preventing the generation of oxygen and subsequently increasing the activity of oxygen sensitive hydrogenases. Apart from Chlamydomonas reinhardtii, biohydrogen production is also reported in Chlorella fusca, Chlamydomonas moewusii, Chlorococcum littorale, Lobochlamys culleus, Scenedesmus obliquus, and Tetraselmis subcordiformis (Limongi et al. 2021).
3.3.5 Bio-Oil and Syngas
Bio-oil or biocrude oil is liquid fuel derived from organic biomass at anaerobic and high-temperature conditions (Behera et al. 2015). Pyrolysis is the main method for bio-oil production as it provides high-temperature conditions (400–600 °C) in the absence of oxygen. Microalgae Chlorella protothecoides has been investigated for biocrude oil production with highest yield of 52% produced at a temperature of 500 °C for a relatively short time of 5 min (Peng et al. 2000). Hydrothermal liquefaction is generally at a lower temperature (250–350 °C) under high-pressure conditions. In a study, a maximum biocrude yield of 65 wt% ash-free dry weight (AFDW) was produced at 350 °C in 5 min for halophytic microalga Tetraselmis sp. (Eboibi et al. 2014). Furthermore, in a study on high-protein high-ash microalgae, Cyanobacteria sp. and Bacillariophyta sp., a significant bio-oil yield of 21.1% and 18.21% per dry biomass weight was observed at 325 °C for 45–60 min (Huang et al. 2016). At a temperature of 350 °C, Nannochloropsis sp. generated the highest bio-yield of 43% weight at a holding time of 60 minutes (Brown et al. 2010). A study investigating optimum thermochemical liquefaction operations in Spirulina platensis reported a remarkable biocrude quality at par with the petroleum crude. At 350–380 °C, the biocrude product has similar energy properties with density of 34.7–39.9 MJ/kg compared to that of petroleum (42.9 MJ/kg) (Jena et al. 2011). The thermochemical conversion of microalgal biomass can be directed to bio-oil as well as syngas production through hydrothermal liquefaction and hydrothermal gasification, respectively (Barreiro et al. 2013). Gasification is operated at high temperatures of 800–1000 °C which converts biomass into combustible gas mixtures like carbon dioxide, carbon monoxide, methane, hydrogen, and nitrogen. In a study, hydrothermal gasification of Spirulina platensis produced syngas mixtures of methane, carbon dioxide, carbon monoxide, and hydrogen. Using ruthenium catalyst in supercritical water, it was then possible to separate the methane from syngas producing a methane-rich biogas that can be further explored for biofuel properties (Stucki et al. 2009). Duman et al. (2014) reported catalytic steam gasification of Nannochloropsis oculata and seaweeds including Fucus serratus and Laminaria digitata which produced maximum hydrogen gas yields of 413 cc/g, 937 cc/g, and 1036 cc/g algal residue. The syngas produced hydrogen as the major component with carbon monoxide and methane in trace amounts. The method thus offers promising means to maximize hydrogen production from micro- and macroalgal biomass.
3.4 Future Perspectives
To attain the promising utilization of microalgal bioresource, persistent efforts are being carried out in the genetic modification of microalgae. Genetic engineering of microalgae aims to provide novel strain with exclusive features like high lipids yield, greater biomass accumulation, higher expression of bioactive compounds, superior CO2 capture, and possible role in wastewater treatment. Several steps are required in the genetic manipulation of microalgae. The choice for genetic transformation techniques such as electroporation, particle bombardment, etc. is an important barrier to strain improvement. Chlamydomonas reinhardtii has been utilized in the past for genetic transformation through electroporation. Apart from transformation, efficient expression of our choice gene through codon optimization and promoter selection is also remarkably important (Chaturvedi et al. 2020; Barrera and Mayfield 2013). Barrera and Mayfield (2013) investigated overexpression of malic enzyme in Phaeodactylum tricornutum generating genetically improved transgenic cell with 2.5-fold higher lipid yield as well as higher biomass, thus suggesting novel prospects in biodiesel production. In another study enhanced lipid content of 46.4–52.9% was achieved in Chlorella ellipsoidea by overexpressing GmDof4 transcription factor from soybean (Glycine max) (Zhang et al. 2014). Couso et al. (2011) reported the stable increase in carotenoids production by overexpression of exogenous phytoene synthase gene in the Chlamydomonas reinhardtii. In recent times genetic improvement of the microalgal bioresource is currently pursued by the development of gene-editing tools like clustered regularly interspaced short palindromic repeats (CRISPR/Cas9) (Kumar et al. 2020a, b). CRISPR/Cas9-based technology was utilized for the expression of omega-3 fatty acid desaturase (fad3) gene in Chlorella sorokiniana and Chlorella vulgaris FSP-E. Higher lipid content of 46% (w/w) in C. vulgaris FSP-E was achieved using the novel genetic tool (Couso et al. 2011). Success of genome editing has also been reported in Nannochloropsis oceanica, Phaeodactylum tricornutum, and Chlamydomonas reinhardtii (Vazquez-Villegas et al. 2018). Although genetic tools provide a propitious opportunity for better products development from microalgae, it must be also stringently regulated and monitored to prevent compromise on human health and safety issues. In silico simulation studies show that upon escape to the natural habitat, these genetically superior microalgae with excellent growth kinetics can cause the formation of harmful algal blooms, creating a nutritional challenge to zooplanktons and ultimately giving rise to ecological imbalance (Flynn et al. 2013). Thus as more industries are exploring genetically modified microalgae for commercial applications, a need to monitor appropriate regulation and risk assessment has arisen. A new greener technology with minimal environmental pollution as well as highly regulated road map for the microalgal industry is thus the need of the hour.
3.5 Conclusions
The enormous bioactive compounds in microalgae provide a plethora of opportunities for several applications including biomedical, cosmetics, nutraceuticals, and biofuels. Prominent among the bioactive components include polysaccharides, fatty acids, pigments, proteins, and peptides. Till date, several interesting cosmetic formulations have been developed utilizing the unique metabolites present in microalgae. A wide range of health benefits with antioxidants, immune-modulatory, and anticancer activities provides a much needed drive to the cosmeceutical industry. The prospect of next-generation fuels such as biodiesel, bioethanol, biogas, etc. has allowed industries to explore the microalgal diversity. Although numerous microalgal species have been utilized for several applications, many are yet to be characterized. Industries have utilized several approaches to amplify the yield such as nutrient limitation, photochemical parameters, and genetic modification. Favorably numerous microalgae have shown promising yield thus providing scope for future developments.
Abbreviations
- PUFAs:
-
polyunsaturated fatty acids
- CAGR:
-
compound annual growth
- US:
-
United States of America
- MAAs:
-
mycosporine-like amino acids
- HA:
-
hydroxy acids
- PMNS:
-
polymorphonuclear leukocyte
- mg:
-
milligram
- ml:
-
milliliter
- kDa:
-
kilodaltons
- EPA:
-
eicosapentaenoic acid
- DHA:
-
docosahexaenoic acid
- TAGs:
-
triacylglycerols
- MUFAs:
-
monounsaturated fatty acids
- SFAs:
-
saturated fatty acids
- MGDG:
-
monogalactosyl diacylglycerol
- DGDG:
-
digalactosyldiacylglycerol
- MW:
-
molecular weight
- UV:
-
ultraviolet radiation
- FADD:
-
Fas-associated death domain
- MMPs:
-
matrix metalloproteinases
- CDP:
-
Chlorella-derived peptide
- ACE:
-
angiotensin-converting enzyme
- CaO:
-
calcium oxide
- CO2:
-
carbon dioxide
- CME:
-
Caulerpa microphysa extract
- FAME:
-
fatty acid methyl ester
- V/V:
-
volume per volume
- Mg/gm:
-
milligram per gram
- cc/g:
-
cubic centimeter per gram
- AFDW:
-
ash-free dry weight
- MJ/kg:
-
megajoules per kilogram
- SEM:
-
scanning electron microscope
- PSII:
-
photosystem II
- CRISPR:
-
clustered regularly interspaced short palindromic repeats
- SSF:
-
saccharification and fermentation
- SHF:
-
separate hydrolysis and fermentation
- CH4:
-
methane
References
Abdoul-latif FM, Oumaskour K, Boujaber N (2021) Formulations of a cosmetic product for hair care based on extract of the microalga Isochrysis galbana: in vivo and in vitro activities. J Anal Sci Appl Biotechnol 3(1):15–19. https://doi.org/10.48402/IMIST.PRSM/jasab-v3i1.25015
Abe K, Hattori H, Hirano M (2007) Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata Var. Multistriata. Food Chem 100(2):656–661. https://doi.org/10.1016/j.foodchem.2005.10.026
Adegboye MF, Ojuederie OB, Talia PM, Babalola OO (2021) Bioprospecting of microbial strains for biofuel production: metabolic engineering, applications, and challenges. Biotechnol Biofuels 14(1):1–21. https://doi.org/10.1186/s13068-020-01853-2
Agrawal K, Bhatt A, Bhardwaj N, Kumar B, Verma P (2020) Algal biomass: potential renewable feedstock for biofuels production–part I. In: In biofuel production technologies: critical analysis for sustainability. Springer, Cham, pp 203–237
Agrawal K, Verma P (2022) An overview of various algal biomolecules and its applications. In: An integration of phycoremediation processes in wastewater treatment. Elsevier, Amsterdam, pp 249–270
Agustina S, Aidha NN, Oktarina E (2021) The extraction of antioxidants from Chlorella vulgaris for cosmetics. IOP Conf Ser Mater Sci Eng 1011:1. https://doi.org/10.1088/1757-899X/1011/1/012057
Andrade LM (2018) Chlorella and Spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process Technol 6(1):45–58. https://doi.org/10.15406/mojfpt.2018.06.00144
Anekthanakul K, Senachak J, Hongsthong A, Charoonratana T, Ruengjitchatchawalya M (2019) Natural ACE inhibitory peptides discovery from spirulina (Arthrospira platensis) strain C1. Peptides 118:170107. https://doi.org/10.1016/j.peptides.2019.170107
Arad S, Levy-Ontman O (2010) Red microalgal cell-wall polysaccharides: biotechnological aspects. Curr Opin Biotechnol 21(3):358–364. https://doi.org/10.1016/j.copbio.2010.02.008
Arad SM, van Moppes D (2013) Novel sulfated polysaccharides of red microalgae: basics and applications. In: Handbook of microalgal culture: applied phycology and biotechnology. Wiley, Chicester, pp 406–416. https://doi.org/10.1002/9781118567166.ch21
Asraful AM, Jing Liang X, Wang Z (2020) Microalgae biotechnology for food, health and high value products. Springer, Singapore. https://doi.org/10.1007/978-981-15-0169-2
Bae S, Lim JW, Kim H (2021) β-carotene inhibits expression of matrix Metalloproteinase-10 and invasion in Helicobacter pylori-infected gastric epithelial cells. Molecules 26:6. https://doi.org/10.3390/molecules26061567
Balasubramaniam V, Gunasegavan RDN, Mustar S, Lee JC, Noh MFM (2021) Isolation of industrial important bioactive compounds from microalgae. Molecules 26(4):1–45. https://doi.org/10.3390/molecules26040943
Balboa EM, Enma Conde M, Soto L, Pérez-armada L, Domínguez H (2015) Cosmetics from marine sources. Appl Marine Biotechnol 44:1015–1042
Barreiro L, Diego WP, Ronsse F, Brilman W (2013) Hydrothermal liquefaction (HTL) of microalgae for biofuel production: state of the art review and future prospects. Biomass Bioenergy 53:113–127. https://doi.org/10.1016/j.biombioe.2012.12.029
Barrera DJ, Mayfield SP (2013) High-value recombinant protein production in microalgae. In: Handbook of microalgal culture: applied phycology and biotechnology. Wiley, Chicester, pp 532–544. https://doi.org/10.1002/9781118567166.ch27
Behera S, Singh R, Arora R, Sharma NK, Shukla M, Kumar S (2015) Scope of algae as third generation biofuels. Front Bioeng Biotechnol 2:1–13. https://doi.org/10.3389/fbioe.2014.00090
Bhardwaj N, Komal A, Pradeep V (2020) Algal biofuels: An economic and effective alternative of fossil fuels. In: Microbial strategies for techno-economic biofuel production. Springer, Cham, pp 207–227
Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR (2016) Marine natural products. Nat Prod Rep 33:382. https://doi.org/10.1039/c5np00156k
Bohm GA, Pfleiderer W, Boger P, Scherer S (1995) Structure of a novel oligosaccharide-Mycosporine-amino acid ultraviolet a/B sunscreen pigment from the terrestrial cyanobacterium Nostoc commune. J Biol Chem 270(15):8536–8539. https://doi.org/10.1074/jbc.270.15.8536
Bonfanti C, Cardoso C, Afonso C, Matos J, Garcia T, Tanni S, Bandarra NM (2018) Potential of microalga Isochrysis galbana: bioactivity and bioaccessibility. Algal Res 29:242–248. https://doi.org/10.1016/j.algal.2017.11.035
Brown TM, Duan P, Savage PE (2010) Hydrothermal liquefaction and gasification of Nannochloropsis Sp. Energy Fuel 24(6):3639–3646. https://doi.org/10.1021/ef100203u
Bruno A, Rossi C, Marcolongo G, Di Lena A, Venzo A, Berrie CP, Corda D (2005) Selective in vivo anti-inflammatory action of the Galactolipid Monogalactosyldiacylglycerol. Eur J Pharmacol 524(1–3):159–168. https://doi.org/10.1016/j.ejphar.2005.09.023
Burg A, Oshrat LO (2015) Salt effect on the antioxidant activity of red microalgal sulfated polysaccharides in soy-bean formula. Mar Drugs 13(10):6425–6439. https://doi.org/10.3390/md13106425
Caicedo Y, Suarez C, Gelves G (2020) Evaluation of preliminary plant design for Chlorella vulgaris microalgae production focused on cosmetics purposes. J Phys Conf Ser 1655:1. https://doi.org/10.1088/1742-6596/1655/1/012086
Campiche R, Sandau P, Kurth E, Massironi M, Imfeld D, Schuetz R (2018) Protective effects of an extract of the freshwater microalga Scenedesmus rubescens on UV-irradiated skin cells. Int J Cosmet Sci 40(2):187–192. https://doi.org/10.1111/ics.12450
Cengiz S, Sevilay. (2019) Scenedesmus Obliquus: a potential natural source for cosmetic industry. Int J Sec Metabol 6(2):129–136. https://doi.org/10.21448/ijsm.545771
Cha KH, Koo SY, Lee D-U (2008) Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J Agric Food Chem 56(22):10521–10526. https://doi.org/10.1021/jf802111x
Chaiklahan R, Chirasuwan N, Triratana P, Loha V, Tia S, Bunnag B (2013) Polysaccharide extraction from Spirulina Sp. and its antioxidant capacity. Int J Biol Macromol 58:73–78. https://doi.org/10.1016/j.ijbiomac.2013.03.046
Chakdar H, Jadhav SD, Dhar DW, Pabbi S (2012) Potential applications of blue green algae. J Sci Ind Res 71:13–20
Chaturvedi V, Goswami RK, Verma P (2020) Genetic engineering for enhancement of biofuel production in microalgae. In: Biorefineries: a step towards renewable and clean energy. clean energy production technologies. Springer, Cham, pp 539–559
Chatzikonstantinou M, Kalliampakou A, Gatzogia M, Flemetakis E, Katharios P, Labrou NE (2017) Comparative analyses and evaluation of the cosmeceutical potential of selected Chlorella strains. J Appl Phycol 29(1):179–188. https://doi.org/10.1007/s10811-016-0909-1
Chen C-L, Liou S-F, Chen S-J, Shih M-F (2011) Protective effects of Chlorella-derived peptide on UVB-induced production of MMP-1 and degradation of procollagen genes in human skin fibroblasts. Regul Toxicol Pharmacol 60(1):112–119. https://doi.org/10.1016/j.yrtph.2011.03.001
Couso I, Vila M, Rodriguez H, Vargas MA, León R (2011) Overexpression of an exogenous phytoene synthase gene in the unicellular alga Chlamydomonas reinhardtii leads to an increase in the content of carotenoids. Biotechnol Prog 27(1):54–60. https://doi.org/10.1002/btpr.527
Czerwonka A, Kaławaj K, Sławińska-Brych A, Lemieszek MK, Bartnik M, Wojtanowski KK, Zdzisińska B, Rzeski W (2018) Anticancer effect of the water extract of a commercial spirulina (Arthrospira platensis) product on the human lung cancer A549 cell line. Biomed Pharmacother 106:292–302. https://doi.org/10.1016/j.biopha.2018.06.116
de Andrade JC, de Andrade LM (2017) An overview on the application of genus Chlorella in biotechnological processes. J Adv Res Biotechnol 2(1):1–9. https://doi.org/10.15226/2475-4714/2/1/00117
Duman G, Uddin MA, Yanik J (2014) Hydrogen production from algal biomass via steam gasification. Bioresour Technol 166(April):24–30. https://doi.org/10.1016/j.biortech.2014.04.096
Eboibi BE, Lewis DM, Ashman PJ, Chinnasamy S (2014) Effect of operating conditions on yield and quality of biocrude during hydrothermal liquefaction of halophytic microalga Tetraselmis Sp. Bioresour Technol 170:20–29. https://doi.org/10.1016/j.biortech.2014.07.083
El-fayoumy EA, Shanab SMM, Gaballa HS, Tantawy MA, Shalaby EA (2021) Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement Med Therap 21(1):51. https://doi.org/10.1186/s12906-020-03194-x
El-Shimi HI, Attia NK, El-Sheltawy ST, El-Diwani GI (2013) Biodiesel production from Spirulina platensis microalgae by in-situ transesterification process. J Sustain Bioenergy Syst 03(03):224–233. https://doi.org/10.4236/jsbs.2013.33031
Estee T, Companies L (2003) From red microalgae have Anti-inflammatory properties In vitro and In vivo, vol 104, pp 13–22
Fayyad RJ, Mohammed Ali AN, Dwaish AS, Al-Abboodi AKA (2019) Anticancer activity of Spirulina platensis Methanolic extracts against L20b and Mcf7 human cancer cell lines. Plant Archiv 19(April):1419–1426
Figueroa FL (2021) Mycosporine-like amino acids from marine resource. Mar Drugs 19(18):18. https://doi.org/10.3390/md19010018
Fimbres-Olivarria D, Carvajal-Millan E, Lopez-Elias JA, Martinez-Robinson KG, Miranda-Baeza A, Martinez-Cordova LR, Enriquez-Ocaña F, Valdez-Holguin JE (2018) Chemical characterization and antioxidant activity of sulfated polysaccharides from Navicula Sp. Food Hydrocoll 75:229–236. https://doi.org/10.1016/j.foodhyd.2017.08.002
Flynn KJ, Mitra A, Greenwell HC, Sui J (2013) Monster potential meets potential monster: pros and cons of deploying genetically modified microalgae for biofuels production. Interface Focus 3(1):20120037. https://doi.org/10.1098/rsfs.2012.0037
González-Fernández C, Sialve B, Bernet N, Steyer JP (2012) Thermal pretreatment to improve methane production of Scenedesmus biomass. Biomass Bioenergy 40:105–111. https://doi.org/10.1016/j.biombioe.2012.02.008
Goswami, Kumar R, Agrawal K (2021d) Microalgae-based biofuel-integrated biorefinery approach as sustainable feedstock for resolving energy crisis. In: Verma P (ed) bioenergy research: commercial opportunities & challenges. Springer, Cham, pp 267–293
Goswami RK, Agrawal K, Mehariya S, Verma P (2021b) Current perspective on wastewater treatment using Photobioreactor for Tetraselmis Sp.: An emerging and foreseeable sustainable approach. Environ Sci Pollut Res 5:1–33. https://doi.org/10.1007/s11356-021-16860-5
Goswami RK, Agrawal K, Verma P (2021c) An overview of microalgal carotenoids: advances in the production and its impact on sustainable development. In: Bioenergy research: evaluating strategies for commercialization and sustainability. Wiley Library, London, pp 105–128
Goswami RK, Mehariya S, Karthikeyan OP, Verma P (2021a) Advanced microalgae-based renewable biohydrogen production systems: a review. Bioresour Technol 320:124301. https://doi.org/10.1016/j.biortech.2020.124301
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21(5):210–216. https://doi.org/10.1016/S0167-7799(03)00078-7
Gunes S, Tamburaci S, Dalay MC, Gurhan ID (2017) In vitro evaluation of Spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities. Pharm Biol 55(1):1824–1832. https://doi.org/10.1080/13880209.2017.1331249
Guruvayoorappan C, Kuttan G (2007) β-Carotene inhibits tumor-specific angiogenesis by altering the cytokine profile and inhibits the nuclear translocation of transcription factors in B16F-10 melanoma cells. Integr Cancer Ther 6(3):258–270. https://doi.org/10.1177/1534735407305978
Guzmán S, Gato A, Lamela M, Freire-Garabal M, Calleja JM (2003) Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res 17(6):665–670. https://doi.org/10.1002/ptr.1227
Halaj M, Paulovičová E, Paulovičová L, Jantová S, Cepák V, Lukavský J, Capek P (2019) Extracellular biopolymers produced by Dictyosphaerium family—chemical and Immunomodulative properties. Int J Biol Macromol 121:1254–1263. https://doi.org/10.1016/j.ijbiomac.2018.10.116
Hannon M, Gimpel J, Tran M, Rasala B, Mayfield S (2010) Biofuels from algae: challenges and potential importance; challenges of algal. Biofuels 1(5):763–784
Harun R, Jason WSY, Cherrington T, Danquah MK (2011) Exploring alkaline pre-treatment of microalgal biomass for bioethanol production. Appl Energy 88(10):3464–3467. https://doi.org/10.1016/j.apenergy.2010.10.048
Hashtroudi MS, Shariatmadari Z, Riahi H, Ghassempour A (2013) Analysis of Anabaena vaginicola and Nostoc calcicola from northern Iran, as rich sources of major carotenoids. Food Chem 136(3–4):1148–1153. https://doi.org/10.1016/j.foodchem.2012.09.055
Hoseini SM, Khosravi-Darani K, Mozafari MR (2013) Nutritional and medical applications of Spirulina microalgae. Mini-Rev Med Chem 13(8):1231–1237. https://doi.org/10.2174/1389557511313080009
Huang Y, Chen Y, Xie J, Liu H, Yin X, Chuangzhi W (2016) Bio-oil production from hydrothermal liquefaction of high-protein high-ash microalgae including wild cyanobacteria Sp. and cultivated Bacillariophyta Sp. Fuel 183:9–19. https://doi.org/10.1016/j.fuel.2016.06.013
Jankowska E, Sahu AK, Oleskowicz-Popiel P (2017) Biogas from microalgae: review on Microalgae’s cultivation, harvesting and pretreatment for anaerobic digestion. Renew Sustain Energy Rev 75:692–709. https://doi.org/10.1016/j.rser.2016.11.045
Jena U, Das KC, Kastner JR (2011) Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresour Technol 102(10):6221–6229. https://doi.org/10.1016/j.biortech.2011.02.057
Jeon BH, Choi JA, Kim HC, Hwang JH, Abou-Shanab RAI, Dempsey BA, Regan JM, Kim JR (2013) Ultrasonic disintegration of microalgal biomass and consequent improvement of bioaccessibility/bioavailability in microbial fermentation. Biotechnol Biofuels 6(1):1–9. https://doi.org/10.1186/1754-6834-6-37
Johnson MB, Wen Z (2009) Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuel 23(10):5179–5183. https://doi.org/10.1021/ef900704h
Kane SN, Mishra A, Dutta AK (2016) Preface: international conference on recent trends in physics (ICRTP 2016). J Phys Conf Ser 755:1. https://doi.org/10.1088/1742-6596/755/1/011001
Kawee-ai A, Kuntiya A, Kim SM (2013) Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat Prod Commun 8(10):1381–1386. https://doi.org/10.1177/1934578x1300801010
Khan MI, Lee MG, Shin JH, Kim JD (2017) Pretreatment optimization of the biomass of Microcystis aeruginosa for efficient bioethanol production. AMB Express 7:1. https://doi.org/10.1186/s13568-016-0320-y
Kim GV, Choi W, Kang D, Lee S, Lee H (2014) Enhancement of biodiesel production from marine alga, Scenedesmus Sp. through in situ transesterification process associated with acidic catalyst. Biomed Res Int 2014:5. https://doi.org/10.1155/2014/391542
Kim HM, Jung JH, Kim JY, Heo J, Cho DH, Kim HS, An S, An IS, Bae S (2019) The protective effect of violaxanthin from Nannochloropsis oceanica against ultraviolet B-induced damage in Normal human dermal fibroblasts. Photochem Photobiol 95(2):595–604. https://doi.org/10.1111/php.13030
Kim SK (2014) Marine Cosmeceuticals. J Cosmet Dermatol 13(1):56–67. https://doi.org/10.1111/jocd.12057
Koller M, Muhr A, Braunegg G (2014) Microalgae as versatile cellular factories for valued products. Algal Res 6(PA):52–63. https://doi.org/10.1016/j.algal.2014.09.002
Komatsu T, Sasaki S, Manabe Y, Hirata T, Sugawara T (2017) Preventive effect of dietary Astaxanthin on UVA-induced skin Photoaging in hairless mice. PLoS One 12(2):1–16. https://doi.org/10.1371/journal.pone.0171178
Kose A, Oncel SS (2014) Biohydrogen production from engineered microalgae Chlamydomonas reinhardtii. Adv Energy Res 2(1):1–9. https://doi.org/10.12989/eri.2014.2.1.001
Kotasthane T (2017) Potential of microalgae for sustainable biofuel production. J Marine Sci Res Develop 07(01):10.4172/2155-9910.1000223
Kumar B, Bhardwaj N, Agrawal K, Verma P (2020a) Bioethanol production: generation-based comparative status measurements. In: Biofuel production technologies: critical analysis for sustainability. Springer, New York, pp 155–201
Kumar G, Shekh A, Jakhu S, Sharma Y, Kapoor R, Sharma TR (2020b) Bioengineering of microalgae: recent advances, perspectives, and regulatory challenges for industrial application. Front Bioeng Biotechnol 8:914. https://doi.org/10.3389/fbioe.2020.00914
Kurd F, Samavati V (2015) Water soluble polysaccharides from Spirulina platensis: extraction and in vitro anti-cancer activity. Int J Biol Macromol 74:498–506. https://doi.org/10.1016/j.ijbiomac.2015.01.005
Kwang HC, Koo Song YI, Lee DU (2008) Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J Agric Food Chem 56(22):10521–10526. https://doi.org/10.1021/jf802111x
Kyadari M, Fatma T, Azad R, Velpandian T (2013) Evaluation of antiangiogenic and antiproliferative potential of the organic extract of green algae Chlorella pyrenoidosa. Indian J Pharm 45(6):569–574. https://doi.org/10.4103/0253-7613.121366
Lee S, Younghoon O, Kim D, Kwon D, Lee C, Lee J (2011) Converting carbohydrates extracted from marine algae into ethanol using various ethanolic Escherichia coli strains. Appl Biochem Biotechnol 164(6):878–888. https://doi.org/10.1007/s12010-011-9181-7
Lee MC, Yeh HY, Shih WL (2021) Extraction procedure, characteristics, and feasibility of Caulerpa microphysa (Chlorophyta) polysaccharide extract as a cosmetic ingredient. Mar Drugs 19:9. https://doi.org/10.3390/md19090524
Letsiou S, Kalliampakou K, Gardikis K, Mantecon L, Infante C, Chatzikonstantinou M, Labrou NE, Flemetakis E (2017) Skin protective effects of Nannochloropsis gaditana extract on H2O2-stressed human dermal fibroblasts. Front Mar Sci 4(JUL):1–15. https://doi.org/10.3389/fmars.2017.00221
Li P, Liu Z, Ren X (2001) Chemical characterisation of the released polysaccharide from the cyanobacterium Aphanothece halophytica GR02. J Appl Phycol 13(1):71–77. https://doi.org/10.1023/A:1008109501066
Li Q, Wei D, Liu D (2008) Perspectives of microbial oils for biodiesel production. Appl Microbiol Biotechnol 80(5):749–756. https://doi.org/10.1007/s00253-008-1625-9
Limongi AR, Viviano E, De Luca M, Radice RP, Bianco G, Martelli G (2021) Biohydrogen from microalgae: production and applications. Appl Sci 11(4):1–14. https://doi.org/10.3390/app11041616
Lum KK, Kim J, Lei XG (2013) Dual potential of microalgae as a sustainable biofuel feedstock and animal feed. J Animal Sci Biotechnol 4(1):1–7. https://doi.org/10.1186/2049-1891-4-53
Ma NL, Lam SS, Zaidah R (2015) The application of algae for cosmeceuticals in the omics age. Genom Proteom Metabol Nutraceut Funct Foods 2018:476–488. https://doi.org/10.1002/9781118930458.ch37
Maity JP, Bundschuh J, Chen CY, Bhattacharya P (2014) Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: present and future perspectives—a mini review. Energy 78:104–113. https://doi.org/10.1016/j.energy.2014.04.003
Marino T, Iovine A, Casella P, Martino M, Chianese S, Larocca V, Musmarra D, Molino A (2020) From Haematococcus pluvialis microalgae a powerful antioxidant for cosmetic applications. Chem Eng Trans 79:271–276. https://doi.org/10.3303/CET2079046
Matsui MS, Muizzuddin N, Arad S, Marenus K (2003) Sulfated polysaccharides from red microalgae have anti-inflammatory properties in vitro and in vivo. Appl Biochem Biotechnol 104(1):13–22. https://doi.org/10.1385/abab:104:1:13
Medipally SR, Yusoff F, Banerjee S, Shariff M (2015) Feedstock for biofuel production. Wiley, Beverly, p 13
Mehariya S, Goswami RK, Karthikeyan O, Karthikeyan P, Verma P (2021) Microalgae for high-value products: a way towards green nutraceutical and pharmaceutical compounds. Chemosphere 280:130553. https://doi.org/10.1016/j.biortech.2020.124301
Miranda JR, Passarinho PC, Gouveia L (2012) Pre-treatment optimization of Scenedesmus obliquus microalga for bioethanol production. Bioresour Technol 104:342–348. https://doi.org/10.1016/j.biortech.2011.10.059
Miyamoto K, Hallenbeck PC, Benemann JR (1979) Hydrogen production by the thermophilic alga Mastigocladus laminosus: effects of nitrogen, temperature, and inhibition of photosynthesis. Appl Environ Microbiol 38(3):440–446. https://doi.org/10.1128/aem.38.3.440-446.1979
Mobin A, Chowdhury H (2019) Commercially important bioproducts from microalgae and their international bioproducts commercially important microalgae and their current applications – from a review current applications – a review assessing the. Energy Procedia 160(2018):752–760. https://doi.org/10.1016/j.egypro.2019.02.183
Molinuevo-Salces B, Riaño B, Hernández D, Cruz García-González M (2019) Microalgae and wastewater treatment: advantages and disadvantages. In: Microalgae biotechnology for development of biofuel and wastewater treatment. Springer, Singapore, pp 505–533. https://doi.org/10.1007/978-981-13-2264-8_20
Mondal M, Goswami S, Ghosh A, Gunapati Oinam ON, Tiwari PD, Gayen K, Mandal MK, Halder GN (2017) Production of biodiesel from microalgae through biological carbon capture: a review. 3 Biotech 7(2):1–21. https://doi.org/10.1007/s13205-017-0727-4
Morais MG, Da De B, Vaz S, De Morais EG, Costa JAV (2015) Biologically active metabolites synthesized by microalgae. Biomed Res Int 2015:1–15. https://doi.org/10.1155/2015/835761
Morocho-Jácome AL, Ruscinc N, Martinez RM, Monteiro JC, de Carvalho T, de Almeida S, Rosado C, Costa JG, Velasco MVR, Baby AR (2020) (Bio)technological aspects of microalgae pigments for cosmetics. Appl Microbiol Biotechnol 104:9513. https://doi.org/10.1007/s00253-020-10936-x
Mourelle ML, Gómez CP, Legido JL (2017) The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 4:46. https://doi.org/10.3390/cosmetics4040046
Nagarajan D, Cheng DD, Chen CY, Lee DJ, Chang JS (2021) Biohydrogen production from microalgae—major bottlenecks and future research perspectives. Biotechnol J 16:5. https://doi.org/10.1002/biot.202000124
Pailliè-Jiménez ME, Stincone P, Brandelli A (2020) Natural pigments of microbial origin. Front Sustain Food Syst 4:1–8. https://doi.org/10.3389/fsufs.2020.590439
Pangestuti R, Kim S-K (2011) Biological activities and health benefit effects of natural pigments derived from marine algae. J Funct Foods 3(4):255–266. https://doi.org/10.1016/j.jff.2011.07.001
Park SB, Yun JH, Ryu AJ, Yun J, Kim JW, Lee S, Choi S et al (2021) Development of a novel Nannochloropsis strain with enhanced Violaxanthin yield for large-scale production. Microb Cell Fact 20(1):1–11. https://doi.org/10.1186/s12934-021-01535-0
Peng W, Qingyu W, Pingguan T (2000) Effects of temperature and holding time on production of renewable fuels from pyrolysis of Chlorella protothecoides. J Appl Phycol 12:147–152. https://doi.org/10.1023/A:1008115025002
Phwan CK, Ong HC, Chen WH, Ling TC, Ng EP, Show PL (2018) Overview: comparison of pretreatment technologies and fermentation processes of bioethanol from microalgae. Energ Conver Manage 173:81–94. https://doi.org/10.1016/j.enconman.2018.07.054
Ragusa I, Nardone GN, Zanatta S, Bertin W, Amadio E (2021) Spirulina for skin care: a bright blue future. Cosmetics 8(1):1–19. https://doi.org/10.3390/cosmetics8010007
Rahmanpour O Shariati A (2015) Comparison of different solvents used in nanochloropsis algae oil extraction comparison of different solvents used in nanochloropsis algae oil extraction Gas Engineering Department, Petroleum University of Technology, Ahwaz, Iran, June
Raposo J, De MF, De Morais RMSC, De Morais AMMB (2013a) Bioactivity and applications of Sulphated polysaccharides from marine microalgae. Mar Drugs 11:233. https://doi.org/10.3390/md11010233
Raposo J, De MF, De Morais RMSC, De Morais AMMB (2013b) Health applications of bioactive compounds from marine microalgae. Life Sci 93(15):479–486. https://doi.org/10.1016/j.lfs.2013.08.002
Ruiz-Domínguez MC, Espinosa C, Paredes A, Palma J, Jaime C, Vílchez C, Cerezal P (2019) Determining the potential of Haematococcus pluvialis oleoresin as a rich source of antioxidants. Molecules 24(22):1–17. https://doi.org/10.3390/molecules24224073
Ryu BM, Himaya SWA, Kim S-K (2015) Applications of microalgae-derived active ingredients as cosmeceuticals. In: Handbook of marine microalgae. Elsevier, Amsterdam, pp 309–316
Ryu J, Park S-j, Kim I-h, Youn HE, Choi E, Nam T-j (2014) Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int J Mol Med 34:796–803. https://doi.org/10.3892/ijmm.2014.1815
Sadeghi S, Jalili H, Ranaei Siadat SO, Sedighi M (2018) Anticancer and antibacterial properties in peptide fractions from hydrolyzed Spirulina protein. J Agric Sci Technol 20(4):673–683
Saini KC, Yadav DS, Mehariya S, Rathore P, Kumar B, Marino T, Leone GP, Verma P, Musmarra D, Molino A (2021) Overview of extraction of astaxanthin from Haematococcus pluvialis using CO2 supercritical fluid extraction technology Vis-A-Vis quality demands. In: Global perspectives on astaxanthin. Academic Press, Elsevier, Amsterdam, pp 341–354
Samson R, Leduy A (1983) Influence of mechanical and thermochemical pretreatments on anaerobic digestion of Spirulina maxima algal biomass. Biotechnol Lett 5(10):671–676. https://doi.org/10.1007/BF01386360
Santos NO, Oliveira SM, Alves LC, Cammarota MC (2014) Methane production from marine microalgae Isochrysis galbana. Bioresour Technol 157:60–67. https://doi.org/10.1016/j.biortech.2014.01.091
Schmid BD, Schürch C, Zülli F (2006) Mycosporine-like amino acids from red algae protect against premature skin-aging abstract 1–4
Senni K, Pereira J, Gueniche F, Delbarre-Ladrat C, Sinquin C, Ratiskol J, Godeau G, Fischer AM, Helley D, Colliec-Jouault S (2011) Marine polysaccharides: a source of bioactive molecules for cell therapy and tissue engineering. Mar Drugs 9(9):1664–1681. https://doi.org/10.3390/md9091664
Shafik H, Saad M, El-Serehy H (2015) Impact of nitrogen regime on fatty acid profiles of Desmodesmus quadricaudatus and Chlorella Sp. and ability to produce biofuel. Acta Bot Hungar 57(1–2):205–218. https://doi.org/10.1556/ABot.57.2015.1-2.16
Shen PL, Wang HT, Pan YF, Meng YY, Wu PC, Xue S (2016) Identification of characteristic fatty acids to quantify triacylglycerols in microalgae. Front Plant Sci 7:FEB2016. https://doi.org/10.3389/fpls.2016.00162
Shih MF, Cherng JY (2012) Protective effects of chlorella-derived peptide against UVC-induced cytotoxicity through inhibition of Caspase-3 activity and reduction of the expression of phosphorylated FADD and cleaved PARP-1 in skin fibroblasts. Molecules 17(8):9116–9128. https://doi.org/10.3390/molecules17089116
Shimoda H, Tanaka J, Shan SJ, Maoka T (2010) Anti-pigmentary activity of Fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J Pharm Pharmacol 62(9):1137–1145. https://doi.org/10.1111/j.2042-7158.2010.01139.x
Silva MR, Oliveira B, Moura YAS, Converti A, Porto ALF, de Araújo Viana Marques D, Bezerra RP (2021) Assessment of the potential of Dunaliella microalgae for different biotechnological applications: a systematic review. Algal Res 58(July):102396. https://doi.org/10.1016/j.algal.2021.102396
Staats N, De Winder B, Mur LR, Stal LJ (1999) Isolation and characterization of extracellular polysaccharides from the epipelic diatoms Cylindrotheca closterium and Navicula salinarum. Eur J Phycol 34(2):161–169. https://doi.org/10.1080/09670269910001736212
Stolz P, Obermayer B (2005) Manufacturing microalgae for skin care. Cosmet Toilet 120(3):99–106
Stoyneva-Gärtner M, Uzunov B, Gärtner G (2020) Enigmatic microalgae from Aeroterrestrial and extreme habitats in cosmetics: the potential of the untapped natural sources. Cosmetics 7:1–22. https://doi.org/10.3390/cosmetics7020027
Stucki S, Vogel F, Ludwig C, Haiduc AG, Brandenberger M (2009) Catalytic gasification of algae in supercritical water for biofuel production and carbon capture. Energ Environ Sci 2(5):535–541. https://doi.org/10.1039/b819874h
Suárez ER, Syvitski R, Kralovec JA, Noseda MD, Barrow CJ, Stephen Ewart H, Lumsden MD, Grindley BT (2006) Immunostimulatory polysaccharides from Chlorella pyrenoidosa. A new Galactofuranan. Measurement of molecular weight and molecular weight dispersion by DOSY NMR. Biomacromolecules 7(8):2368–2376. https://doi.org/10.1021/bm060365x
Sun L, Chu J, Sun Z, Chen L (2016) Physicochemical properties, immunomodulation and antitumor activities of polysaccharide from Pavlova viridis. Life Sci 144:156–161. https://doi.org/10.1016/j.lfs.2015.11.013
Sun Y, Wang H, Guo G, Yinfang P, Yan B (2014a) The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr Polym 113:22–31. https://doi.org/10.1016/j.carbpol.2014.06.058
Sun L, Wang L, Li J, Liu H (2014b) Characterization and antioxidant activities of degraded polysaccharides from two marine Chrysophyta. Food Chem 160:1–7. https://doi.org/10.1016/j.foodchem.2014.03.067
Sushytskyi L, Lukáč P, Synytsya A, Bleha R, Rajsiglová L, Capek P, Pohl R, Vannucci L, Čopíková J, Kaštánek P (2020) Immunoactive polysaccharides produced by heterotrophic mutant of green microalga Parachlorella Kessleri HY1 (Chlorellaceae). Carbohydr Polym 246:5. https://doi.org/10.1016/j.carbpol.2020.116588
Tang H, Nadia Abunasser MED, Garcia MC, Simon Ng KY, Salley SO (2011) Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Appl Energy 88(10):3324–3330. https://doi.org/10.1016/j.apenergy.2010.09.013
Tannin-Spitz T, Bergman M, Van-Moppes D, Grossman S, Arad S (2005) Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J Appl Phycol 17(3):215–222. https://doi.org/10.1007/s10811-005-0679-7
Tominaga K, Hongo N, Karato M, Yamashita E (2012) Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim Pol 59(1):43–47. https://doi.org/10.18388/abp.2012_2168
Tran D, Doan N, Louime C, Giordano M, Portilla S (2014) Growth, antioxidant capacity and Total carotene of Dunaliella salina DCCBC15 in a low cost enriched natural seawater medium. World J Microbiol Biotechnol 30(1):317–322. https://doi.org/10.1007/s11274-013-1413-2
Vazquez-Villegas P, Torres-Acosta MA, Garcia-Echauri SA, Aguilar-Yanez JM, Rito-Palomares M, Ruiz-Ruiz F (2018) Genetic manipulation of microalgae for the production of bioproducts. Front Biosci 10(2):254–275. https://doi.org/10.2741/e821
Vega J, Bonomi-Barufi J, Gómez-Pinchetti JL, Figueroa FL (2020) Cyanobacteria and red macroalgae as potential sources of antioxidants and UV radiation-absorbing compounds for cosmeceutical applications. Mar Drugs 18:12. https://doi.org/10.3390/md18120659
Vieira MV, Derner RB, Lemos-Senna E (2021) Preparation and characterization of Haematococcus pluvialis carotenoid-loaded PLGA Nanocapsules in a gel system with antioxidant properties for topical application. J Drug Deliv Sci Technol 61:102099. https://doi.org/10.1016/j.jddst.2020.102099
Wada N, Sakamoto T, Matsugo S (2015) Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 4:603. https://doi.org/10.3390/antiox4030603
Wang HMD, Chen CC, Huynh P, Chang JS (2015) Exploring the potential of using algae in cosmetics. In: Bioresource technology. Elsevier, Amsterdam. https://doi.org/10.1016/j.biortech.2014.12.001
Xia S, Gao B, Li A, Xiong J, Ao Z, Zhang C (2014) Preliminary characterization, antioxidant properties and production of chrysolaminarin from marine diatom Odontella aurita. Mar Drugs 12(9):4883–4897. https://doi.org/10.3390/md12094883
Yao S, Brandt A, Egsgaard H, Gjermansen C (2012) Neutral lipid accumulation at elevated temperature in conditional mutants of two microalgae species. Plant Physiol Biochem 61:71–79. https://doi.org/10.1016/j.plaphy.2012.09.007
Yarkent Ç, Gürlek C, Oncel SS (2020) Potential of microalgal compounds in trending natural cosmetics: a review. Sustain Chem Pharm 17:5. https://doi.org/10.1016/j.scp.2020.100304
Zakaria NNA, Zamzurie NA, Harith ZT (2021) Evaluation of sunscreen cream incorporated with Astaxanthin from Haematococcus Pluvialis in different storage conditions. In: IOP conference series: Earth and environmental science, vol 756, p 1. https://doi.org/10.1088/1755-1315/756/1/012078
Zamalloa C, Boon N, Verstraete W (2012) Anaerobic digestibility of Scenedesmus obliquus and Phaeodactylum tricornutum under mesophilic and thermophilic conditions. Appl Energy 92:733–738. https://doi.org/10.1016/j.apenergy.2011.08.017
Zhang J, Hao Q, Bai L, Jin X, Yin W, Song L, Ling X et al (2014) Overexpression of the soybean transcription factor GmDof4 significantly enhances the lipid content of chlorella Ellipsoidea. Biotechnol Biofuels 7(1):128. https://doi.org/10.1186/s13068-014-0128-4
Zhao B, Ma J, Zhao Q, Laurens L, Jarvis E, Chen S, Frear C (2014) Efficient anaerobic digestion of whole microalgae and lipid-extracted microalgae residues for methane energy production. Bioresour Technol 161:423–430. https://doi.org/10.1016/j.biortech.2014.03.079
Competing Interests
All the authors declare that they have no competing interests.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Rai, A.K., Gurung, S.A. (2022). Microalgal Promise to the Next Generation: A Dual Potential Perspective as Cosmeceuticals and Biofuels. In: Verma, P. (eds) Micro-algae: Next-generation Feedstock for Biorefineries. Clean Energy Production Technologies. Springer, Singapore. https://doi.org/10.1007/978-981-19-0793-7_3
Download citation
DOI: https://doi.org/10.1007/978-981-19-0793-7_3
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-0792-0
Online ISBN: 978-981-19-0793-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)