Abstract
The utilization of renewable biological resources from living organisms to generate food, energy, and materials is a significant driver of bioeconomies. There is a growing awareness among consumers in the utilization of natural products obtained from microorganisms and plant-based materials to deliver novel functional product technologies that can contribute to industrial bioeconomy. Photosynthetic microorganisms, especially microalgae, are beneficial in yielding highly valuable metabolites, including lipids, proteins, pigments, and carbohydrates. Among these bioactive compounds, natural organic pigments such as chlorophyll, carotenoids, phycobiliproteins, astaxanthin, and xanthophyll are gaining importance in several cosmetic, food, and textile industries. This chapter gives an overview of microalgal pigments production and profiles high-value products that are obtained from microalgal pigments. Discussions on the utilization of microalgal pigments as viable ingredients in various product formulations, recent industrial applications of natural pigments, and the future of microalgal pigment-based economy is also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
The utilization of renewable biological resources from living organisms to generate food, energy, and materials is a significant driver of bioeconomies. There is a growing attention among consumers in the utilization of natural products obtained from microorganisms and plant-based materials to deliver novel functional product technologies that can contribute to industrial bioeconomy. The time required for plant growth and the tediousness in the extraction of valuable products are the drawbacks of plant-based novel functional products to boost the bio-based economies (Marchetti et al. 2014). Further, the factors of deforestation (Hoffmann et al. 2018), bioprospecting (Krishnaswamy 2018), and biopiracy (Efferth et al. 2016) also hinders the custom of using plants for the large-scale manufacture of commercial goods. These challenges in plant-based commercial products lead to the introduction of microorganisms, as a replacement, to extract valuable products (Patridge et al. 2016). Among microbes, algae are unique organisms that are similar to plants in several aspects, especially in photosynthesis. This exclusive property of algae, along with their rapid growth in favorable conditions with less nutrient requirement gained the attention of researchers to use them for natural product extraction and valuable compounds, as a better alternative to plants (Trantas et al. 2015).
Algae are known to humanity for more than a thousand years and are involved in human diet even before 14000 years, which was evident from archaeological excavation sites in Chile. Numerous pre-historic literatures from China, India, Ireland, and historic literatures by eminent scientists, including Newton and Turner, mentioned about algae, and its association with human food and medicine. It is noteworthy that the global production of seaweeds gained a profit of about USD 6.7 billion, out of which 95% are produced by commercial cultivation of aquatic lives for food called mariculture from China and Indonesia (Wells et al. 2017). Two main types of algae, namely, macro and micro algae are extensively present throughout the world, that are classified based on their size (Verawaty et al. 2017), whereas aquatic and terrestrial algae are the types that are classified based on their habitat (Bharathiraja et al. 2015; Ismail et al. 2017). In both the habitats, micro and macro algae possess the ability to grow extensively and, in several cases, the algal species helps to reduce the complex chemical compounds, especially wastes, and simplify them to reduce their toxic effect (Yu et al. 2019). Thus, the presence of algae either serves as a bioindicator of toxic content in the ecosystem (Parmar et al. 2016) or facilitates the in situ bioremediation processes (Vidyashankar and Ravishankar 2016).
In recent times, microalgae are widely used in several applications, compared to macroalgae, due to their rapid growth and smaller size. In addition, the advancements in the microalgal biotechnology increased their chances to be utilized in several sectors for natural product fabrication to replace toxic chemical products, food products, medicine, and nutraceuticals (Posten and Chen 2016). Photosynthetic microalgae are beneficial in yielding highly valuable metabolites such as lipids, proteins, carbohydrates, lipids, and pigments (Priyadarshani and Rath 2012). Among these bioactive compounds, natural organic pigments such as chlorophyll, carotenoids, phycobiliproteins, astaxanthin, and xanthophyll from microalgae are gaining importance in numerous cosmetic, food, and textile industries (Dufossé et al. 2005). Thus, this chapter gives a summary of microalgal pigment production and profiles high-value products that are obtained from microalgal pigments. Additionally, utilization of microalgal pigments as viable ingredients in various product formulations, recent industrial applications of natural pigments, and the future of microalgal pigment-based economy are also discussed.
14.2 Bioeconomy Based on Microalgae
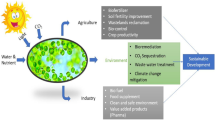
Microalgae is one of the marine organisms that are proven to have a wide economic value. Ease in culture methods, the lesser nutrient requirement for their growth and presence of highly valuable biomolecules makes microalgae as a profound agent to improve bio-based economy. These microalgae are cultured commercially for food, medicine, nutraceuticals, cosmetics, energy, and other industries as displayed in Fig. 14.1, that depend on microalgae for their growth and economic development.
14.2.1 Availability
The major photosynthesizers on earth are the microalgae that produce important various plant pigments. These natural colorants can replace the presently used synthetic colorants used in food, cosmetic, pharmaceutical, and nutraceutical industries. Cascading event of symbiosis between heterotrophic hosts gave rise to the diversified photosynthetic microalgae, which directs to the plethora of photosynthetic pigments capable of utilizing various trophic levels in submarine habitats. Nearly a quarter of the biomass in world’s total vegetation is constituted by phytoplankton. Further, some microalgae, also contributes to the food web by acting as effective primary producers. In an independent study conducted in the south east Asian region disclosed the abundance and spatial distribution, the major taxon that are identified are Chrysophyta, Cyanophyta, and Pyrrophyta (Kadim and Arsad 2016). The growth rate and distribution of microalgae are dependent on the environmental factor and prevailing conditions, the factors that drive growth potential varies with every species (Adenan et al. 2013). The parameters that affect the distribution are temperature, salinity, and water current (Cokrowati et al. 2014). Studies involving species distribution, abundance, biological diversity index, and dominance index indicate that the growth of microalgae is better in places with pristine environment and less polluted areas.
14.2.2 Opportunities and Threats
Microalgae are considered as a contemporary attraction across the world due to its varied areas of implications. Microalgae has shown a remarkable potential as value added products in biology, but the most challenging portions in the production of microalgae are enhancing growth rate, dewatering the culture for the production of biomass, pretreating the biomass, standardizing the fermentation process (Khan et al. 2018). In certain conditions, local weather can be a major limiting factor that affects the production rates; local species show better adaptability than the exotic better yielding variants. Few species like Scenedesmus performs consistently in large-scale production and it is considered as the validating species to study the biotic and a biotic factor favoring and diminishing the growth dynamics (Lee et al. 2014). Agar plating methods and micromanipulation show substantial improvement in the subsequent processing of samples. Pure cultures are often established after several attempts; hence, the preparatory cultures are retained till pure cultures are obtained. The three primary algal pigments, β-carotene, astaxanthin, and phycocyanin are produced on a large-scale to be utilized as organic food colorants. Besides the fact that the industry has established, applied researches in these pigments are still developing at a rapid rate (Perumal et al. 2015). Their impact on human health is to be explored to the maximum to understand the usage spectrum and applications (Eriksen 2016).
14.2.3 Food Industry
More than a century, microalgae are utilized as a food source among humans due to their exclusive nutrient content. The microalgae was proposed as an alternate source to meet the global demand of proteins in the 1950s, which leads to the first large quantity Chlorella export in 1960s and used them as protein-rich food among humans. The developments in the downstream process leads to the establishment of microalgal production facility units in countries including the US, India, Israel, Australia, and several countries in Asia (Enzing et al. 2014). Lately, microalgae serves as a sustainable source of food that can yield nutrients such as fatty acids, polysaccharides, sterols and polyhydroxyalkonates along with common nutrients required for growth in humans (Vigani et al. 2015). Microalgae has potential to yield a large quantity of lipids and proteins along with a portion of carbohydrates. Both these microalgae produced biomolecules, either in their raw or decontaminated form serves as a food source and has a wide market throughout the world. Commercially grown microalgae are used to extract lipid and proteins as food, as an alternate to their plant counterparts, to maintain sustainability and reduce deforestation (Vanthoor-Koopmans et al. 2013). In recent times, Spirulina and Chlorella are extensively used as a source of nutritional food, not only for man, but also for fishes in aquarium, pets including dogs, cats, certain birds, horses, cows, and in poultry. In addition, microalgal species, including Pavlova, Chaetoceros, Skeletonema, Tetraselmis and Nannochloropsis are used as feed in aquaculture of fishes. Moreover, microalgae are gaining acceptance among public as a raw nutritional diet, compared to other food sources. Thus, they are marketed as individual food product as Chlorella noodles or their extracts are mixed with other food products to improve their nutritional value (García et al. 2017). The low maintenance, low energy usage for growth, and high nutritional value compounds are the significant factors to use microalgae in most of the food industries to improve their economy and profit.
14.2.4 Medicine and Nutraceuticals
Literatures of eighteenth-century revealed that the presence of microalgae in the drinking water causes undesirable tastes and odor. Later, the reason was found to be the release of metabolites such as trans-2-cis-6-nonadienal from algal blooms in drinking water. Mostly, microalgae are considered as harmful microbes that can lead to diseases until the twentieth century. The use of microalgae such as Arthrospira and Nostoc for medicinal purposes has been started in the twentieth century. In China, Nostoc commune and Nostoc flagelliforme are reported to be the only edible species in their traditional medicine literatures. The herbal values of Nostoc have been still utilized by several traditional and folklore medicinal practices for disease treatments in countries such as Mongolia, Fiji, Japan, Philippines, Mexico, and Siberia (Borowitzka 2018). After twentieth century, microalgae such as Chlorella, Arthrospira, and Aphanizomenon has gained importance in the food and pharmaceutical sectors. Chlorella growth factor is a significant product from Chlorella that is proved to possess anticancer and antiaging properties (Panahi et al. 2016). In addition, C. pyrenoidosa with lysine and threonine are reported to be beneficial in curing famine edema (da Silva Ferreira and Sant’Anna 2017). Likewise, Chlorella extracts are reported to reduce cholesterol, hypertension (Fallah et al. 2018), anemia in pregnant women (Bito et al. 2016), improves ulcerative colitis (Freitas 2017) and enhance the immune system (Chidley and Davison 2018). The extracts from Chlorella, especially from C. vulgaris, possess ability to prevent harmful, disease causing bacterial growth and are used as antibiotics (Acurio et al. 2018).
The elevated protein, vitamin B12, provitamin A, linolenic acid, iron, and other minerals present in Arthrospira increases their potential to be beneficial as excellent microalgal nutraceutical agent (Stunda-Zujeva and Ruģele). Studies reported that the extracts obtained from Arthrospira species possess ability to reduce hypercholesterolemia (Sengupta et al. 2018), allergic rhinitis, including nasal congestion, sneezing and itching (Appel et al. 2018), along with anticancer (Srivastava et al. 2015), antioxidant, antidiabetic (Parveen 2016), and anti-inflammatory properties (Jensen et al. 2015). The high dietary value of these microalgae reduces nutritional deficiency, thereby elevates immunity by producing a large number of T-cells, cytokines, secretes IgA antibody, and by increasing the activity of natural killer cells (Seyidoglu et al. 2017). Moreover, Arthrospira possess enhanced antibacterial (Markou et al. 2016), antifungal (Keimes and Gunther 2016) and antiviral activities against disease causing microbes (Reichert et al. 2017). It has been reported that the calcium spirulan extracted from Arthrospira platensis facilitates the Herpes simplex virus (HSV-1) and human immunodeficiency virus (HIV-2) inhibition (Jena and Subudhi 2019). These calcium spirulan polysaccharides obtained from microalgae, also possess anticancer activity and are useful in the oral cancer theranostics (de la Jara et al. 2018). Further, it is noteworthy that the filamentous cyanobacterium Aphanizomenon flos-aquae are also used in the nutraceutical products due to their worthy nutrition and in pharmaceutical products due to their medicinal properties (Borowitzka 2018). The extracts of Aphanizomenon are reported to possess anticancer, antimicrobial (Srivastava 2015), antidiabetic, antioxidant (Hyun et al. 2016), and helps in the treatment of neurodegenerative (Nuzzo et al. 2018), cardiac and vascular ailments (Eid et al. 2018). These medicinal and nutritional values of these microalgae are produced as products by several companies which serves as a profitable bioeconomy, throughout the world.
14.2.5 Cosmetics
Apart from food and medical applications, microalgae are used to produce natural cosmetic products as it contains minerals, fatty acids, pigments, vitamins, protein,s and minerals for the structural and functional maintenance of skin. The bioactive components that are extracted from microalgae are reported to possess ability to protect skin against harsh environment as well as microbial attack and regenerate them. Microalgal bioactive compounds, including exopolysaccharides, chrysolaminarin, glucan, carotenes, asthaxanthin, lycopene, phycocyanin, ectoine, and biopterin glucose are used in the cosmetic product formulation. These bio-compounds from microalgae possess enhanced properties to be used as moisturizers, sunscreen lotions, free radical collector, antioxidants, lipsticks, deodorants, eye shadows, UV protectors, and for collagen repair which are beneficial to produce personal care products. These microalgal bioactive compounds are also used in thalassotherapy which utilizes seawater and marine elements including microalgae for skin care. Products such as Blue RetinolTM from D. salina for skin cell development and propagation, SILIDINE® from Porphyridium cruentum for improving skin aspect and decreasing redness and effects of rosacea and protein-rich Arthrospira extract as products for repairing skin damages. The advancements in the downstream processing of commercial microalgae have led to the emergence of skin and care, personalized cosmetic products such as GoldenChlorellaTM and AlgaPurTM algae oils. In future, the bioeconomy based on microalgae for cosmetic applications are expected to increase about three times as these products are comparatively less toxic and highly effective in skin care, compared to chemical-based cosmetic products (Mourelle et al. 2017).
14.2.6 Energy
The ability of microalgae to produce highly valuable and essential lipids has been mentioned in the previous sections. The ability of microalgae to accumulate lipid similar to vegetable oil, in their cell, makes them highly beneficial in biofuel production as an alternate fuel. It is noteworthy that the microalgae possesses potential to synthesize and store, 100 times higher quantity of lipids than any other organisms, especially plants (Mubarak et al. 2015). These lipids obtained from microalgae are processed and are widely used as biodiesel to encounter the growing fuel demand throughout the world. However, the production of microalgal biodiesel, specifically dimethyl ether, is still in its infancy and lab-scale stage. After the in situ trans-esterification process with methanol for biodiesel production from microalgal oil, the residual microalgal oil are also used for the biogas production (Salam et al. 2016). Microalgae are used to produce ethanol, methanol, hydrogen, and biodiesel as efficient biofuels. It has been reported that the biofuel production is high in green algae, compared to red, blue-green and yellow-green algae. Microalgal species, namely, Chlorella and Chlorococcum are proved to be extensively utilized for the distinctly classifiable biofuel production (Faried et al. 2017). Due to the depletion of conventional, non-renewable fossil fuels and demand for novel green fuels, the bioeconomy based on the microalgal biofuel are expected to increase gradually in the near future.
14.2.7 Other Industrial Applications
The pigments present in microalgae are the high valuable components that can be used for enormous applications. In food sectors, these pigments are beneficial as organic coloring agent (Dufossé 2018), paints as natural colors (Danaee et al. 2018), solar energy collectors to trap more sun energy (Dufossé 2016) and as fluorescent dyes in bioimaging of diseases (Della Rosa et al. 2018). In addition, the extracts of microalgae possess biologically valuable compounds that are beneficial as high-value co-products (Chew et al. 2017), stable biochemical isotope (Allen et al. 2017), biofertilizer (Garcia-Gonzalez and Sommerfeld 2016), bio-mitigation of carbon dioxide emission (Zhou et al. 2017), co-processing of wastewater (Puyol et al. 2017), and in eliminating organic pollutants (Matamoros et al. 2015). Even though a wide variety of microalgal highly valuable compounds are available and are under extensive research, each component possesses at least a drawback which hinders their transformation process from lab to large-scale production (Rizwan et al. 2018). Microalgal pigments are currently under extensive research as they are easily extracted from microalgae and requires less processing stages to fabricate the final product. The bioeconomy associated with microalgae are mostly depending on these pigments and each pigment are significantly helpful in various applications which are discussed in consecutive sections.
14.3 Economically Important Microalgal Pigments
14.3.1 General Characteristics
Microalgae is one of the photosynthesizing organisms that produce various pigments, namely, chlorophyll, carotenoids, astaxanthins, phycobiliproteins, and other lipids. These phycopigments are in the colors of green, yellow, brown, red, and are preferred over synthetic colors, due to their non-carcinogenicity and non-toxicity (Sekar and Chandramohan 2008). These pigments are the important source of natural colors compared to synthetic colorants.
Microalgal pigments such as chlorophyll, carotenoids, phycobiliproteins, astaxanthins, and other pigments are extensively used in different industries. For example, carotenoids obtained from Dunaliella, phycobiliproteins from Spirulina, red algae, cyanobacteria, and astaxanthin from Haematococcus are commonly used in food, pharmaceutical, nutraceutical, aquaculture, and cosmetic industry. Additionally, these pigments are utilized in medical research as receptors that are effective as antibodies (Santiago-Santos et al. 2004; Begum et al. 2015). Apart from performing the primary role in pigmentation-based metabolism and photosynthesis in algae, these pigments also display numerous advantageous anti-inflammatory, antioxidative, anti-carcinogenic, anti-obesity, anti-angiogenic and neuroprotective activity (Guedes et al. 2011). The major microalgal pigments, their source, biological effects and applications are mentioned in Table 14.1.
14.3.2 Chlorophylls
Chlorophyll molecules are green fat-soluble pigment, universally present in all algal groups and are classified into three major types, namely, a, b and c of chlorophyll. The basic structural unit of these molecules is a ring of porphyrin that are coordinated to a central magnesium atom. Each porphyrin ring consists of a tetrapyrrole ring having four carbon and one nitrogen in each pyrrole ring (Humphrey 2004). The structural differences in chlorophyll a (ring II has methyl group) and chl b (formyl group) is responsible for different absorption maxima (Scheer et al. 2004). Chlorophyll c is a reddish-brown pigment and similar to chlorophyll b. The pigment obtained from Chlorophyll a is blue and green in color, having an extreme optical absorption at 660–665 nm, whereas pigment from chlorophyll b is yellow and green in color with absorption maxima at 642–652 nm (Humphrey 1980; Begum et al. 2015). Chlorophyll a photosynthetic pigment is highly present in cyanobacteria and red algae. Chlorophyll b is present in chlorophyta, euglenophyta and marine microalgae, whereas chlorophyll c is present in dinoflagellates. The green alga Chlorella sp. is named as Emerald food as it has high (7%) chlorophyll content and is the main source of chlorophyll production (Bewicke and Potter 2009a). Chlorophyll b is the major pigment in alga Spirogyra, Chlorella, and Euglena gracilis, whereas Chlorophyll c is present in Fucus vesiculosus, Dunaliella salina, Amphidinium carterae, and Cryptomonas sp.
The chlorophyll extraction method traditionally involves solvents of organic origin, namely, acetone, ethanol, diethylformamide, and methanol that dissolve lipoproteins and lipids into extract phase. Pretreatment methods before extraction, including homogenization and sonication disrupts the cells, so that chlorophyll extraction process is efficient. Homogenization followed by the use of dimethyl formamide as solvent led to effectual chlorophyll a extraction from algae Stichococcus sp. and Chlorella sp. (Schumann et al. 2005). Phycopigment extraction via fluid at supercritical condition (SFE) is better than the solvent-based approaches, as no carcinogenic solvent is involved, and the extract has better purity with less processing steps. Being an inert, inflammable, cheap, and readily available, CO2 are used as SFE in multiple studies, where chlorophyll is extracted from microalgae Synechococcus sp. (Macias-Sanchez et al. 2009). Following extraction, polyethylene powder in chromatography column is used in an economically patented method for separation of chlorophyll a and b from other pigments. The final purification process involves sucrose filled column that yields highly pure and crystalline chlorophyll molecules.
14.3.2.1 Application of Chlorophylls
Chlorophyll-rich foods provide good nutritional value to humans as they contain heme, and thus increases the production of red blood cells (RBCs) (Solymosi and Mysliwa-Kurdziel 2017). Chlorophyll molecules are broadly utilized in cosmetic, pharmaceutical, and food sectors. Chlorophyll a, being a stable molecule has been used as a natural colorant, alternative to artificial colors. Spirulina platensis contains large amounts of chlorophyll, which can be utilized as an organic green color in food products and cosmetics (Godoy Danesi et al. 2011). In addition, Chlorophyllin which is a chlorophyll derivative is used as a fabric dye to add colors in wool and cotton.
Chlorophyll derivatives have also exhibited various health promoting activities, apart from being primarily used as natural colors. (Ferruzzi and Blakeslee 2007) have documented the medicinal use of these compounds owing to their anti-inflammatory and wound healing entities. Another experiment (Balder et al. 2006) revealed the characteristic of chlorophylls in decreasing the colorectal cancer risk. Chlorophyll molecules also exhibit antimutagenic property, due to production of carcinogen detoxifying enzyme and thus reducing the risk of cancer.
14.3.3 Carotenoids
In nature, carotenoids are widely spread as yellow, orange, or red colored pigments with aliphatic or alicyclic structures (Prasanna et al. 2007). There are more than 1000 carotenoids that are identified till date, and it comprises of about 0.1% of the total algal dry weight. Carotenoids are accessory light harvesting pigments as they transfer the energy to chlorophyll. Carotenoids are antioxidative molecules that deactivate reactive singlet oxygen that are released, when the cells are experiencing stress due to environmental conditions (Ciccone et al. 2013). Carotenoids can be classified based on their structural cyclization, hydrogenation, and chemical groups that are responsible for the bioactivity (Dutta et al. 2005).
Further, these colored pigments are categorized as major and minor carotenoid groups. Carotenoids that constitute the physical characteristics of xanthophylls are called major carotenoids, whereas the minor carotenoids are formed in large quantities by the microalgae due to firm external environmental stimulus (Eonseon et al. 2003). Most carotenoids contain C40 hydrocarbon backbone, having basic eight isoprenoid units with double bonds that are conjugated in the series. These carotenoids could be cyclic or linear molecules with or without oxygen. Further, carotene and xanthophylls are considered as two major carotenoids groups. Hydrocarbon carotenoids are called carotenes as they do not contain any oxygen compound or its subordinates in their structures, whereas oxygenated derivatives of carotene are xanthophylls with oxygen being in the group of hydroxyl form (e.g., Zeaxanthin), oxi-groups (e.g., echinenone) or in combinations (e.g., astaxanthin) (Del Campo et al. 2000). Other substituent groups such as epoxy is present in violaxanthin and diadinoxanthin; acetyl group is present in dinoxanthin and fucoxanthin in the structures. Additionally, xanthophylls being hydrophobic molecules are either membrane-linked or have exclusive protein interactions via non-covalent bonds and are commonly present in the membrane of thylakoids. Minor carotenoids are present in vesicles formed by lipids and possess extraplastidic property (Grossman et al. 1995).
For most carotenoids, three major absorption maxima have been recorded. The absorption maximum is affected by cyclization and oxygenation. The common cyanobacterial carotenoids are β-carotene (only cyclic compounds), zeaxanthin, ketocarotenoid, echinenone, and carotenoid glycoside myxoxanthophyll. Primary carotenoids of green algae include β-carotene, lutein, and violaxanthnin that are present within the chloroplasts along with chlorophyll. Till date, more than 600 xanthophylls have been reported with different oxygen containing groups and their combinations (Lemoine and Schoefs 2010). Certain green algae, including Chlorella sp. accumulates biomass of carotenoids and are considered as good sources of carotenoids (Bhosale and Bernstein 2005).
Dunaliella salina is the preferred microalgae for β-carotene production due to its highest carotenoid content (10% dry weight) (Prieto et al. 2011a). Under critical conditions such as nutrient limitation, high salt and light, these microalgae can produce 14% of β-carotene in its dry weight. Growth rate and yield of β-carotene by Dunaliella is increased, when seaweeds are cultivated at higher concentrations (Raja et al. 2004). Dunaliella cultures are used to extract β-carotene using biphasic aqueous or organic systems (Hejazi et al. 2003, 2004). Cyanobacterial species Synechocystis sp. PCC6803 and Synechococcus sp. PCC702 are good candidates that accumulates β-carotene after genetic modification (Macias-Sanchez et al. 2009). Moreover, the different strains of Synechococcus are reported to contain β-carotene, equinone and other phycopigments at varying extents (Kaur et al. 2009). Extraction of carotenoids is performed using organic solvents including acetone, methanol, or dimethyl sulfoxide (DMSO). Commercially, Dunaliella is cultivated throughout the world (Australia, Israel, USA, India, and China) for β-carotene production with Australia being the major producer (Kleinegris et al. 2011)
The initial step of carotenoid extraction and purification involves separation of algal biomass from liquid media by centrifugation, flocculation, and filtration. Later, the biomasses were processed by specific drying methods. Carotenoids are extracted from various microalgae, namely, D. salina, C. vulgaris, Spirulina pacifica and Nannochloropsis gaditana by supercritical fluid extraction approach (Macias-Sanchez et al. 2009; Careri et al. 2001). Likewise, the supercritical CO2 crystallization method with organic solvent and edible are utilized for the extraction of β-carotene from Dunaliella biomass. The marine algae Synechococcus sp. is evaluated in terms of effective operating pressure and temperature on efficiency of β-carotene extraction using supercritical CO2 (Montero et al. 2005). Multiple studies have shown that extraction processes that are operated at the temperature between 40–80 °C and pressure between 20–40 MPa, selectively separates carotenoid molecule from algal biomass (Khanra et al. 2018). Moreover, supercritical dimethyl ether is utilized for efficient fucoxanthin extraction from microalgae U. pinnatifida (Kanda et al. 2014).
14.3.3.1 Applications of Carotenoids
Commercially available carotenoids include β-carotene, zeaxanthin, lycopene, canthaxanthin, lutein, and astaxanthin (Sathasivam and Ki 2018), that are used as provitamins, antioxidants, immune boosters, antiageing, and anticancer agents. Out of all carotenoids, β-carotene is the most commonly occurring, first commercialized carotenoid with diverse biological functions. Carotenoids are not vitamins, but these molecules have provitamin A activity and performs diverse biological functions in humans (Pisal and Lele 2005; Vilchez et al. 2011). There are approximately 60 carotenoids that can act as precursors of vitamin (1 ug retinol = 2 ug β-carotene). In humans, β-carotene is converted to vitamin A through the activity of skin tissues. Further, vitamin A highly necessary and efficient in the human body, as it prevents cataracts, night blindness, skin diseases and boosts the immune system (Agarwal and Rao 2000). Multivitamin and healthy food formulations also contain β-carotene as provitamin A (Spolaore et al. 2006a)
β-carotene from Dunaliella has been approved as a natural colorant by the United States Food and Drug Administration (FDA) to be beneficial in food and cosmetic industries as well as drugs to cure diseases. These carotenes possess ability to elevate the appearances of food products. Furthermore, it is beneficial as a food supplement, as feed in poultry and aquaculture (Christaki et al. 2013) in powder form. Additionally, it is also used as a colorant and a provitamin supplement in pet foods (Cantrell et al. 2003). Further, β-carotene gained potential significance in the cosmetic industry as sunscreen lotions, nail paints, lipsticks, and anti-aging creams. An interesting property of carotenoids is their antioxidant activity to protect the cells from harmful free radicals by quenching and scavenging processes. The antioxidant property is due to the physical and chemical interactions of these pigments with cell membranes. Thus, these pigments act as immune modulators, prevents the onset of cancers, protects against life menacing diseases, including different forms of cancer (Rock 1997; Albanes et al. 1997; Tornwall et al. 2004). Certain carotenoids including astaxanthins also reduce the risk and progression of cancers. In particular, Dunaliella extracted β-carotene have 40% of 9-cis and 50% of all-trans stereoisomers that helps them in lowering the risks of certain cancers and degenerative diseases (Ausich 1992; Sandmann 2001).
The antioxidative properties of carotenoids allow them to protect brain cells from getting deteriorated and hence, prevents the process of aging. The specific role played by β-carotene in stimulating the immune system also prevent several other diseases such as cancers, premature aging, and arthritis. Additionally, β-carotene declines the memory loss in Alzheimer’s disease by acting as a better antioxidant to facilitate brain cells to survive (Mattson 2004). Further, a detailed health aid of β-carotene is investigated in in vivo models. Cognitive impairment was found to be prevented, when transgenic mice fed with β-carotene and lutein of Chlorella sp. (Nakashima et al. 2009). Furthermore, extracts of C. ellipsoidea and C. vulgaris containing β-carotene prevented development of colon cancer (Plaza et al. 2009). D. salina extracted β-carotene has been commercialized by an Australian company named Western Biotechnology, since 1986. Similarly, huge quantities of β-carotene are produced in India from cyanobacteria. Microalgal carotenoids are good competitors of synthetic carotenoid forms as they provide superior natural isomers, compared to the synthetic form. Along with carotene, other carotenoids help in decreasing the premenopausal cancer prevalence in the breast; cryptoxanthin lowers the chances of cervical cancer. Lycopene can reduce the menace of prostate and digestive tract cancers. Additionally, combination of β-carotene, lycopene, vitamin E, and C help the patients under cancer therapy in developing immunity. Lycopene with β-carotene helps in preventing coronary heart disease by inhibiting the harmful cholesterol and low-density lipoprotein (LDL) formation. Lycopene also reduces the risk of type II diabetes with β-carotene and cryptoxanthin. Oxygenated carotenoids (Xanthophyll) obtained from Dunaliella sp., have better anticancer properties and high bioactivity (Roodenburg et al. 2000). Lutein and Zeaxanthin inhibits the harmful UV radiations from sun and prevent loss of vision. Also, these carotenoids protect skin from photooxidation and hence can be used as nutraceutical or cosmetic ingredients that prevent oxidative stress-related diseases.
14.3.4 Phycobiliproteins
Phycobiliproteins (PBS), belongs to the family of water soluble, brilliantly colored, primary protein pigments in microalgal species (Glazer 1994). These pigments are arranged as macromolecular phycobilisomes complexes and are devoted to the outer thylakoid membrane surface for photosynthesis (Hemlata and Fatma 2009). These pigments constitute up to 50% of the soluble protein and comprise about 20% of the cell dry weight (Grossman et al. 1993). Bluish green allophycocyanin, bluish phycocyanin, purple colored phycoerythrin, and orange colored phycoerythrocyanin are the major microalgal phycobiliproteins (Sekar and Chandramohan 2008). The optical absorbance of allophycocyanin is 650–655 nm, phycocyanin is 615–640 nm, phycoerythrin is 565 ~ 575 nm and phycoerythrocyanin is 577 nm. Thus, phycobiliproteins absorb light in the visible spectrum and acts as accessory light collecting pigments during photosynthesis (Batista et al. 2006). Phycobiliproteins are made of α and β polypeptides, having a molecular weight of 15 kDa and 22 kDa, respectively, that associates non-covalently to form heterodimers (Glazer 1994).
Two polypeptide chains with methionine was present in c-phycocyanin protein that is isolated from Oscillatoria agardhii microalgae (Peters et al. 1992). These two polypeptide chains are linked by disulphide bonds and each chain contains a single chromophore group. The composition of amino acids and N-terminal sequences of both polypeptides are also found to be similar. In complete phycobilisome, Allophycocyanin is present in the core of phycobiliproteins that are joined to the disc-shaped hexameric phycocyanins to the proximal side and distal side phycoerythrins present in the core. The prosthetic groups in covalent arrangement with phycobilins led to exclusive phycobiliprotein colors (Sekar and Chandramohan 2008). About 10–15% of total phycobiliprotein consists of uncolored or linker polypeptides which are not only the structural components that stabilizes PBS, but also helps in the efficient energy flow to the reaction center of photosynthesis.
Most abundant phycobiliproteins produced by red algae Porphyridium are phycoerythrins, while cyanobacteria Arthrospira Spirulina have phycocyanins as the major pigment (Glazer 1994; Bermejo Roman et al. 2002). The core pigment in Nostoc strains is C-phycoerythrin and it makes 10% of the microalgal dry weight. A strain of Ananbena having 8.3% dry weight of phycoerythrin was isolated from coastal lagoons of Spain (Rodriguez et al. 1991). Heterocyst containing strains of cyanobacteria were found to be high producers of phycobiliproteins among 41 cyanobacterial species, including unicellular, colonial, filamentous (heterocystous and non-heterocystous) and heterotrichous strains (Kaushik 2000). Phycoerythrin and phycocyanin also have a direct effect on total phycobiliprotein production. Analysis of 20 Tolypothrix strains of their phycobilin content revealed significant differences at both inter- and intraspecies level (Prasanna et al. 2003).
Various potential algal species for phycobiliprotein production are screened and it was found that Spirulina fusiformis produced 6 to 46% of c-phycocyanin and about 26% in Spirulina platensis (Zhu et al. 2007; Madhyastha et al. 2006). Moreover, the high heterotrophic cell density, continuous and fed batch unicellular red algae culture of Galdieria sulphuraria also produced large quantities of phycocyanin (Graverholt and Eriksen 2007). For extraction of phycobiliproteins, cell disruption methods such as osmotic shock, enzyme treatment, or high-pressure homogenization is used. The crude extract is then directly used for purification of pigments. Moreover, a combination of methods is also used depending on the desired purity and source organism. The purified pigment is dried by lyophilization to prevent its denaturation. Recently, high-pressure homogenization is utilized for extraction of phycocyanin from S. platensis (Seo et al. 2013). Likewise, chromatography coupled with expanded bed adsorption technique has been used to extract phycocyanin in recent times (Ramoz et al. 2010). Various methods, including lysozyme treatment and nitrogen cavitation are utilized to obtain Synechococcus sp. extracted phycobiliproteins (Viskari and Colyer 2003; Gupta and Sainis 2010). Phycoerythrin isolation is performed by methods including sonication and freeze thaw method, using different cyanobacterial species (Pumas et al. 2011; Mishra et al. 2011).
14.3.4.1 Applications of Phycobiliproteins
Phycobiliproteins possess enhanced antioxidative, hepatoprotective, and neuroprotective entities (Spolaore et al. 2006a). These pigments are commercially used as organic dyes in various countries. They form an important component of fluorescent labeling for flow cytometry and histochemistry, due to their exclusive spectral entities (Kronick and Grossman 1983). Phycoerythrin due to its absorbance spectra is utilized as a secondary color in antibodies that are labeled in fluorescent colors (Sekar and Chandramohan 2008). Further, DNA and protein probes can be detected by using phycoerythrin labeled streptavidin (Diwu et al. 2012). Low-molecular weight cryptomonad derived phycobiliproteins (termed cryptoFluortrade mark dyes) finds applications in both extracellular and intracellular flow-based labeling systems. Also, these phycobiliproteins are beneficial as electrophoresis markers, focusers in chromatograph and iso-electric experiments, since they exhibit optical absorption in the visible light wavelength.
PBS is used as natural food colorants over synthetic dyes which exhibits carcinogenicity. Other benefits comprise their strong colors and elevated water solubility. The yellow fluorescent color of phycoerthrin is used for making transparent sugar-based lollipops and in other food decorations (Dufosse et al. 2005). S. platensis and Phorphyridium aerugineum extracted phycoerythrin is utilized as confectionary colors, gelatin desserts, and in cosmetic products. Another phycobiliprotein, S. platensis extracted C-phycocyanin is widely preferred as a colorant, compared to synthetic ones (Sekar and Chandramohan 2008) and marketed in Japan. It is beneficial as a chewing gum coloring agent, popsicles, and soft drinks to name a few. PBS is not permitted by FDA, however, desert lake technologies applied to grant generally regarded as safe (GRAS) status to CyaninPlus (C-PC) (FDA GRAS 2012). Additionally, it is used in cosmetic industries as lipsticks (Santiago-Santos et al. 2004). Phycocyanin is a pharmaceutically active phycopigment due to its antioxidant, neuroprotective, and hepatoprotective properties (Sekar and Chandramohan 2008). For instance, Aphanizomenon flos-aquae extracted phycocyanin has been found to be a robust antioxidative agent and is proven to reduce oxidative damage (Benedetti et al. 2004). It is capable of scavenging free radicals and also inhibits in vitro peroxidation of microsomal lipids (Romay et al. 2003). Further, phycocyanin significantly reduced hippocampal cell death in rats (Thaakur and Sravanthi 2010; Penton-Rol et al. 2011), decreased hepatic inflammation (González et al. 2003; Sekar and Chandramohan 2008) and decreased phagocytosis in Kupffer cell (Remirez et al. 2002).
Phycocyanin reduces edema, release of histamines, myeloperoxidase activity, prostaglandins and leukotriene in the inflamed tissues. Thus, phycocyanin is potentially beneficial in pathological conditions involving oxidative stress and inflammation. Phycocyanin also exhibits anticancer properties by reducing the tumor necrosis factor (TNF-α) in serum of the endotoxin treated mice and it also exhibited a neuroprotective effect in the cell cultures of rat cerebella granules (Sekar and Chandramohan 2008). In addition, phycocyanin derived from S. platensis exhibited dose and time-dependent growth inhibition of human leukemic K562 cells (Subhashini et al. 2004). Phycocyanin also led to prostate cancer cell apoptosis by enhancing the effect of anticancer medication topotecan (Gantar et al. 2012). Further, Phycocyanin reduced the spread of human hepatoma HepG2 cell line in another study by (Basha et al. 2008). Furthermore, R-phycoerythrin subunits were used for treating mouse tumor S180 cells and human SMC 7721 carcinoma cells (Huang et al. 2002). Allophycocyanin (APC) at concentrations not toxic to the host cells, inhibited cytopathic effects by inducing enterovirus-71 as reported by (Shih et al. 2003). Along with phycocyanin, allophycocyanin also has antioxidative and anti-inflammatory properties and hence, APC is also a potential therapeutic agent.
14.3.5 Astaxanthin
Astaxanthin with C40H52O4 as molecular formula is a xanthophyll carotenoid and a red fat-soluble pigment. Haematococcus pluvialis is one of the best green algal sources of organic astaxanthin as it represents 90% of the total carotenoids (Ranga et al. 2009). The basic astaxanthin structure has two rings in terminal linked by a chain of polyene. It possesses keto (C = O) and hydroxyl groups on each ring of ionone that are responsible for its unique esterification ability and other exclusive properties (Ma and Chen 2001). Likewise, conjugated double bonds (hydroxyl and keto group) are accountable for the red astaxanthin color. These bonds make this pigment a better antioxidant as it donates electrons and lead to free radical and stable product formation, thus terminating the free radical chain reactions in living organisms. Closed tubular bioreactors are industrially explored for astaxanthin production from H. pluvialis (Lopez et al. 2006). Actively growing cells of haematococcus biomass are fragile and breaks easily, therefore the recovery of astaxanthin from hard walls of haematocysts is studied by (Mendes-Pinto et al. 2001). Settling and centrifugation are the steps involved in the biomass harvesting of microalgae. Finally, the biomass are broken to improve astaxanthin bioavailability and then dried. The biomass product, that are dried, is either encapsulated directly or used for extraction of astaxanthin (Olaizola and Huntley 2003).
14.3.5.1 Applications of Astaxanthins
Astaxanthin serve as the superior vitamin E, having a strong antioxidative activity (10X), compared to β-carotene (Jyonouchi et al. 1995). Higher concentration of various antioxidative enzymes, including peroxidases are found to be present in plasma and liver of rat fed with Haematococcus biomass as an astaxanthin source (Ranga Rao et al. 2010). The levels of these antioxidant enzymes were found to be increased, when model animals (rabbits and ethanol-induced gastric ulcer rats) were supplemented with astaxanthin (Augusti et al. 2012; Kamath et al. 2008). Likewise, astaxanthin is a superior antioxidant agent, compared to other carotenoids as seen in the dermal region of human fibroblasts and hence used as theranostic agents for diseases related to skin (Camera et al. 2009). In deep burn model, early progression of burn wounds is prevented by astaxanthin by reducing ROS production and oxidative stress (Fang et al. 2017). The exclusive molecular astaxanthin structure permits it to remain stable in both inside and outside the cell membrane and hence, it can aflatoxin carcinogenicity (Rao et al. 2013).
Astaxanthin is an antioxidant that acts against inflammation in biological systems. Haematococcus extract containing astaxanthin, reduced the H. pylori growth in gastric inflammation infected mice (Bennedsen et al. 1999; Park et al. 2010). The lower rate of respiratory inflammation enhances secondary messenger cGMP and cAMP levels, which were found in extracts of Ginkgo biloba, astaxanthin, and Vitamin C supplemented lung tissues (Haines et al. 2011). Another study demonstrated that Haematococcus sp. extracted astaxanthin from preventing gastric ulcers by inhibiting H1K1ATPase activity, elevating mucin content and antioxidative activity (Kamath et al. 2008). Further, astaxanthin are proved to be useful for the ocular inflammation treatment in rats as it inhibits the NFkB signaling pathway (Suzuki et al. 2006). The mechanism of continuous stress via singlet oxygen leading to inflammation is responsible for neurodegeneration, cancers, and skin damage is well known. Astaxanthin treatment has been proved to prevent the UV induced skin damage by reducing the levels of prostaglandin E2 after UV exposure (Yoshihisa et al. 2014). This inhibitory astaxanthin effect is important for the anti-inflammatory drug development, for skin infections including psoriasis. Astaxanthin also prevents skin thickening and inhibits collagenase, inflammatory mediators, and thus, responsible for the anti-wrinkle effects (Davinelli et al. 2018).
Numerous studies have demonstrated the purpose of astaxanthin in oxidative stress reduction, damages renal cells and thus prevents diabetic nephropathy in diabetic mice (Naito et al. 2004; Kim et al. 2009; Manabe et al. 2008). Astaxanthin, being an antioxidant, also serves as an immune modulator by providing protection from free radical damage. This pigment significantly alters immune functions in several studies using live models (Lin et al. 2015). In murine models, astaxanthin showed a higher immune modulating effect, compared to β-carotene (Jyonouchi et al. 1991). A human lymphocyte experiment showed enhanced responsorial production of immunoglobulins to T-cell dependent stimuli (Jyonouchi et al. 1995). In humans, astaxanthin supplementation for 8 weeks, improved the activity of NK cells that targets and destroys virus infected cells was observed (Park et al. 2010). Along with cell mediated immunity, astaxanthin has stimulated humoral immunity in murine model by inducing production of polyclonal IgG and IgM antibodies (Jyonouchi et al. 1995; Okai and Higashi-Okai 1996). Main pathological features of cardiovascular diseases are reduced by the antioxidative properties of astaxanthin in experimental model systems and humans. Thus, this pigment can be considered as latent agents for cardiovascular atherosclerotic disease treatments (Fassett and Coombes 2011).
Antioxidants by inhibiting oxidative damage of the cells decrease their rate of mutagenesis and carcinogenesis. In human tumors, cell to cell contact through gap junction is decreased. Gap restoration junctions by elevating the connexin-43 protein levels tend to decrease tumor cell progression. Natural carotenoids, specifically canthaxanthin and astaxanthin, increased gap junctions in mouse embryo and human skin fibroblasts (Hanusch et al. 1995; Daubrawa et al. 2005; Hix et al. 2004). Astaxanthin showed better anti-tumor activity by inhibiting the growth several tumor cells (Palozza et al. 2009). Further studies showed that astaxanthin lead to cell death and inhibited cell proliferation of mammary tumors in models of chemically induced murine (Tanaka et al. 1995; Jyonouchi et al. 2000; Nakao et al. 2010). The peroxynitrite reaction with lutein and astaxanthin leads to the formation of Nitrolutein and Nitroastaxanthin which are proved to possess enhanced anticancer properties as they inhibited mouse skin papillomas (Maoka et al. 2012).
H. pluvialis extracts containing astaxanthin have been accepted as a supplement in humans or animal diets and reduces the risk of various disorders (Kidd 2011; Yuan et al. 2011). Dietary astaxanthin enhances the fish and shrimp immunity system which eventually elevates their survival and growth. Additionally, it plays a crucial part in the markets of feeds for aquaculture and livestock (Dufosse et al. 2005; Cysewski and Todd 2004; Guerin et al. 2003). Further, Astaxanthin is utilized in food, feed, nutraceutical, medical and cosmetic industries. In addition, market availability of astaxanthin in distinct powder and capsule forms reveals their enhanced applications in food industries. The patented benefits of this algal pigment include its use in preventing inflammation, cancer, bacterial infections, cardiovascular diseases, improving brain functioning, and skin thickness.
14.3.6 Secondary Pigments, Essential Lipids, and Other Pigments
The secondary pigments in algae include the secondary carotenoids such as fucoxanthin, canxanthin, cryptoxanthin, violaxanthin, and echinone (Lemoine and Schoefs 2010). Fucoxanthin is the primary light harvesting carotenoid that efficiently performs the energy transfer and plays a significant role in photoprotection and antioxidation in algae. Fucoxanthin has shown activities of anti-proliferation and prevention of cancer by inhibiting the cell cycle process (Kumar et al. 2013). Anti-angiogenic effect of fucoxanthin has proven to prevent the development of cancer in endothelial cells (HUVECs). The anticancer activities of fucoxanthin with astaxanthin and β-carotene are reported towards HL-60 cancer cells (Kim et al. 2012). The role of several secondary pigments including zeaxanthin on cancer cell lines of prostate region is documented in the experiment by (Kotake-Nara et al. 2001) and fucoxanthin exhibited the highest growth inhibition rates. It is noteworthy that the effects of fucoxanthin and its metabolites varies depending upon the type of cancer cells. Moreover, Fucoxanthin also possesses antidiabetic activity. Obese mice treated with fucoxanthin restored normal blood glucose and insulin levels by elevating glucose transporter 4 (GLUT4) levels in skeletal muscle cells (Maeda et al. 2009). A carotenoid cryptoxanthin also inhibited colon cancer cell proliferation, whereas combinations with oxaliplatin induced cancer cells apoptosis (Sathasivam and Ki 2018). These are important applications of some of the secondary pigments of microalgae.
The essential lipid fraction of microalgae includes fatty acids, waxes, and sterols (Spolaore et al. 2006a). Based on head group polarity, fatty acids are divided into two groups, namely, neutral and amphipathic lipids (includes phospholipids and glycolipids) (Cuellar-Bermudez et al. 2015). Acylglycerols is characterized into monoacylglycerols, diacylglycerols and triacylglycerols depending on the number of fatty acids (Halim et al. 2012). Production of microalgal lipids is based on its species and the culture conditions, including pH and salinity (Guschina and Harwood 2006). The content of neutral lipids in microalgae is efficiently increased by nitrogen starvation (Breuer et al. 2012; Yeesang and Cheirsilp 2011; Cuellar-Bermudez et al. 2015). Important commercial source of DHA includes dinoflagellate Crypthecodinium cohnii, and Schizochytrium and can be extracted algal oil extraction methods includes mechanical pressing, homogenization, milling, and solvent extraction (Palmquist and Jenkins 2003).
Among the fatty acids of polyunsaturated nature, omega-3 PUFA is considered as a fish oil substitution (Ryckebosch et al. 2014). Microalgal fatty acids of omega-3 type have several medicinal benefits in reducing high blood pressure, stroke, myocardial infarction, and cardiac arrhythmia. Adequate intake of major omega-3 PUFA, namely, Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) by pregnant women is highly essential for the enhanced fetal brain growth (Adarme-Vega et al. 2012). Algal classes producing EPA include Bacillariophyeae and several other microalgal species (Cuellar-Bermudez et al. 2015). Thus, algal lipids are used as an alternate source of nutritionally significant PUFA and are composed of functional food formulations (Cuellar-Bermudez et al. 2015). Another important application of algal lipid is their benefits in aquaculture as documented in various studies (Gladue and Maxey 1994; Birkou et al. 2012).
14.4 Microalgal Pigments as a Potential Economic Booster
The evolution of biotechnology commenced to flourish during the last century, with a steep increase in the microalgal production for aquaculture, human as well as animal food and fodder, and cosmetics. This section deals with the application of various algal pigments with a great prospect of being an economic booster. This has resulted in the enhanced methodology in production and genetic microalgal strain modification. Increased application of pigments as natural dyes and polyunsaturated fatty acid oil in food, increase the need of diversifying the quest of microalgal biome for their potential economic implication (Spolaore et al. 2006b).
14.4.1 Commerce Behind Bioindustrialization
Commercial microalgae production started in the early 1970s in Japan, with Dunaliella salina, as a β-carotene source which later became a rapidly growing bioproduct industry, when Australia pioneered in mass outputs. The species showing higher growth rates, invasion resistance, and the presence of optimal lipids are the triad of selecting the best seeding culture. The biofuel production and utilization of bioactive compounds from microalgae is hindered by the increased industrial slab. Improvement of extraction techniques is mandatory for the efficient usage; this can be achieved by alternate methods of extraction such as microwave irradiation methods (Kapoore et al. 2018). Microalgal cells pose a challenge while disrupting the intracellular polymers such as sporopollenin and algaenan. In a few species such as Dunaliella tertiolecta, the absence of frustules led to rapid extraction of phycopigments, in contrast, the presence of frustules in Cylindrotheca closterium renders as a mechanical barrier to pigment extraction. Solvent-based disruption techniques require massive protocols to obtain products of interest. Conventional techniques such as cold and hot soaking technique, ultrasound assisted technique, are often very expensive and contribute to low efficiency. MAE is found to be a better alternative as it is a combination of rapidity, better reproducibility, uniform heating, and increased yields (Pasquet et al. 2011). The process of harvesting involves enormous energy and finance; the techniques include centrifugation, foam fractioning, chemo-flocculation, electro-flocculation, osmotic separation, and ultrasonic separation. Whereas, certain low energy and low budget harvesting process like gravity sedimentation are not economically feasible. Scientists are expected to come up with a solution to address the problems affixed to harvesting microalgae for the commercial isolation of valuable metabolites (Ghosh et al. 2016).
14.4.2 Potential of Various Microalgal Pigments in Industries
The markets were seen inviting microalgal components in the past, in this backdrop, a marked increase in research is expected to expand the horizon for various microalgal products. The quality of such products is maintained by the downstream processing technique.
14.4.2.1 β-Carotenoids
Fat soluble of β-carotenoid pigments obtained from certain photosynthetic bacteria, algae, and plants, they perform as an important photosynthetic component. It is predominantly isolated from the stenohaline species Dunaliella salina, the dried biomass of these algae composes of almost 10% of dried biomass, this is the highest among all species (Prieto et al. 2011b). After a successful pilot study in culturing these organisms by the USSR in 1960, many nations followed the lead in successful commercialization of Dunaliella salina. Annual production rates across the globe ranges around 1200 tons, with Australia as the leading producer. The worldwide market for this species is expected to be 1.8 billion dollars in this current year, this is a leap of 0.3 billion dollars from the levels in past demi-decade. The global demand of cyanobacteria-based pigments is predicted to increase in the next fiscal year and it is expected to shoot in the future to meet 1-billion-pound mark.
14.4.2.2 Chlorophyll and Astaxanthin
Chlorella microalgal species are considered as the Emerald food due to its richness in chlorophyll along with 7% of the biomass, which is roughly five times of the Spirulina chlorophyll content. It is an organic colorant of food that contains rich antioxidant content and acts as an antimutagenic agent. The Weiwang company in Taiwan was the first to commercially produce Chlorella at diverse scales in industries (Bewicke and Potter 2009b) and they are grown in large plastic tubes to harvest more solar radiation for efficient growth.
Astaxanthin is considered an efficient medicinal agent that can cure neurodegenerative diseases, high-cholesterol, hepatic dysfunctions, geriatric muscular atrophy, and for the prevention of malignancy. It is also used as a therapeutic agent for metabolic syndromes. In certain conditions, it is given as a supplement for increasing muscle fitness, endurance, increasing the exercise performance, decreasing muscle soreness after exercise. Oral intake of astaxanthin prevents sunburn, reduces sleep apnea, insomnia, dyspepsia, infertility disorders, carpal tunnel syndrome, rheumatoid arthritis, and menopausal syndrome. Direct application on the skin, reduces sunburn and wrinkles; it is also used as a colorant in few other cosmetics. It is beneficial as a supplement in food for egg laying chicken in the poultry industry. It works as an antioxidant and prevents cell damage. The investment return for distinct astaxanthin market prices are assessed to understand the economic viability of these phycopigments. Market astaxanthin value varies between $2500–7000/kg with a global market value of 280 metric tons/year (Panis and Carreon 2016).
14.4.2.3 Xanthophyll
Xanthophyll is a form of oxygenated carotenoids found in a plethora of photosynthetic organisms, there has been major advancements in molecular biology for the last few years, which has paved way for efficient isolation and production and bio-utilization of xanthophylls. Recently, molecular biology developments have paved way for an effective genetic microalgae manipulation to increase xanthophyll yield and helps in their commercial production (Jin et al. 2003). A commercially significant fucoxanthin- xanthophyll type is obtained from Fucus sp. and Phaeodactylum tricornutum, it is a protein bound component associated with chlorophyll. Antioxidant assay of Chaetoceros calcitrans and Isochrysia galbana showed an amazingly high number of antioxidants, primarily bound to the pigment (Foo et al. 2017).
14.4.3 Economic Boosters of Bioeconomy
The pigments derived from microalgae and microalgae themselves have unique vital characteristics that can be translated into advanced technical and commercial products. This contributes to the production of a variety of beneficial lipids, carbohydrates, bioactive compounds, etc. At a very cost-reducing way, they can incorporate the stable isotopes of 13C, 15 N, and 2H into their biomass and produce various other components they produce. They comprise huge unexplored taxa of organisms, providing a virtually unexplored source of products with potential implicational significance.
Industrial and scientific advancement in effectual microalgal product fabrication plays a vital role in influencing the monetary feasibility of microalgal products (Roux et al. 2017). However, limited articles focused on the large-scale microalgal utilization to obtain valuable compounds, especially pigments. It is inevitable to understand a few aspects of microalgae before starting a commercial spin-off using microalgae. Factors include the analysis of microalgae-based product production, market expectancy, demand, and production scale. The analysis must include a pilot study on the technological advances in the downstream processing of microalgal products, search on patented and protected inventions in the specific field, and a global screening on the present situation of a global market that depends on microalgal biomass with future outlooks.
During the last decade, the research focusing on microalgal pigments has made the noteworthy advancements. Novel methodology for purification permits large-scale harvesting of pigments with high purity. Microalgal PBP pigment generates extra demand in the pharmaceuticals and nutraceutical markets. It is essential to develop novel approaches for heterotrophic and mixotrophic production to meet the growing demand of such algal pigments. More widespread research in microalgal application is still needed to explicate the complete action of microalgal pigments using various model organisms. Studies focusing on the productivity involving various species producing a particular pigment have to be explored thoroughly to choose the best source of culture for effective productivity and increased financial output. The market for the pigments derived from various sources are kept at a high due to the increased awareness about the benefits of bio-products, the demand is rather satisfied with the alternative and synthetic substitutes. This bottleneck is mainly due to the difficulties associated with the mass production of microalgae, resource cost and comparative cheaper production cost in the case of synthetic pigments (Ambati et al. 2018).
Although there is a wide demand for plant pigments in the International market, 95% of this is satisfied by the synthetic substitutes. Synthetic pigments are often derived from petrochemical products and often raise alarming food safety issues with a potential toxicity in the end product. Besides, the hazard of environmental distress and sustainability issues also arise with the synthetic production. In fact, to date, the usage of synthetic pigments is restricted to its usage in aquaculture and are not permitted to consume by humans. Increasing awareness has spearheaded the market improvement for the naturally derived pigments due to the transition towards “green alternatives” and organic products, the market is expected to hit a $1.5 billion by the end of 2020 with all the natural pigments gaining an upper hand in the market (Bhat and Madyastha 2000; Begum et al. 2016).
14.5 Current and Future Perspective
The current status of microalgal pigments is concisely elaborated in this chapter. It is noteworthy that the microalgal pigments are extensively beneficial in improving the bioeconomy of algae-based companies and as a potential economic booster. The market size of microalgal products is increasing rapidly, especially the products with microalgal pigments are easily reaching the break-even point in a short time, due to low production cost and high yield (Singh et al. 2017). Among the microalgal/phycopigments, carotenoids are widely in several companies for the production of natural pigments and use them in finished products. In the US, Astaxanthin has been used by Mera Pharmaceuticals Inc., and Valensa International, β-carotene by aquacarotene Ltd., Spirulina by Nutrex Hawaii Inc., Chlorella by Maypro Industries Inc. for large quantities of organic pigment production. In addition, Cyanotech Corporation in US is the producing large quantities of astaxanthin and β-carotene for commercial applications that are extracted from Spirulina and Chlorella. Likewise, Fuji Chemicals Industry Co., Ltd., in Japan and Sweden extracted astaxanthin pigments from microalgal species for various applications. Other Japanese companies such as Nikken Sohonsha Corporation extracted β-carotene, Sun Chlorella Corporation and Yaeyama Shokusan Co., Ltd., used Chlorella to extract phycopigments to produce commercial products. In India, astaxanthin and β-carotene is produced in large quantities by E.I.D Parry Ltd., and the same company along with Hydrolina Biotech Pvt., Ltd., produced phycopigments with Spirulina for medicinal applications. Other countries such as China, Australia, Israel, Taiwan, and Germany also produces large quantities of phycopigments for unique and valuable commercial products (Ambati et al. 2018). Recently, BlueBioTech, a company from Germany, has been producing a wide variety of natural, pharmaceutical and dietary supplements via microalgal extracts, especially using their pigments (Misra et al. 2016).
In recent times, the association of nanotechnology with microalgal products to enhance their value and properties is the trend in product formulations. The emergence of nanoparticles in the formulation of phycopigments has led to reduced usage and elevates their value, compared to standalone phycopigments. Recently, biocompatible nanosized ovalbumin has been functionalized using microalgal phycocyanin proteins as functional groups and these nanoparticles are used to formulate cefpirome which is a derivative of cephalosporin. The results revealed that complex nanoformulated cefpirome with microalgal pigment possess potential to inhibit bacterial pathogens with high biocompatibility, compared to the non-formulated cefpirome, ovalbumin, and phycocyanin (Namasivayam 2017). Other than nanoformulations, several studies showed ample evidences that the addition of nanoparticles improves the production of bioactive contents in microalgae, especially bioactive pigments (Miazek et al. 2015; Lee et al. 2015; Hazeem et al. 2015). Moreover, the phycopigments along with microalgal bioactive molecules possess ability to synthesize biocompatible nanoparticles that can be utilized for several applications (Patel et al. 2015). Silver nanoparticles have recently been synthesized using the microalgal pigments of diatom Amphora sp. as the biogenic synthesis agent and the result showed that the obtained silver nanoparticles possess enhanced antimicrobial activity against a wide range of microbes (Jena et al. 2015). Silver, copper, gold and iron oxide nanoparticles are synthesized using extracts of microalgae with phycopigments in recent times (Salas-Herrera et al. 2019; Dahoumane et al. 2016; Siddiqi and Husen 2016; Shankar et al. 2016). Sometimes, the nanoparticles also may cause adverse effect in microalgae and may affect the ecosystem dynamics, if nanoparticles are exposed directly to the growth site of microalgae (Amanda et al. 2017). Nanoformulation of phycopigments into liposomes, micelles, polymers, dendrimers, and biocompatible encapsulations will further enhance their properties and can be used for controlled release of these phycopigments at the target site to elevate their pharmaceutical and nutraceutical purposes (Jeevanandam et al. 2016). Thus, nanoformulated phycopigments will help to boost the bioeconomy that depends on microalgal production, especially the pharma and nutraceutical industries in future.
14.6 Conclusion
This chapter shows the wide industrial applications of microalgae and the valuable bioactive compounds, especially pigments that are extracted from microalgae. These phycopigments are usually termed to be better in lab-scale production and applications. However, the list of companies that uses microalgal pigments in the large-scale production of commercial products revealed that the phycopigments slowly moving from lab to large-scale phase. The advancements in the microalgal biotechnology are the factors that contribute to the large-scale extraction of microalgal pigments and use them to produce highly valuable commercial products. In addition, the market size of the microalgal products also increased gradually over the years as the quest and marker demand for natural products has globally increased. In future, it is expected that the bioeconomy that depends on the plant products will eventually replace and increased by microalgae, provided the proper funding by governments, approval of products by regulatory bodies and increased acceptance from the public.
References
Abe, K., Hattori, H., & Hirano, M. (2007). Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga coelastrella striolata var. Multistriata Food Chem, 100, 656–661.
Acurio, L. P., Salazar, D. M., Valencia, A. F., Robalino, D. R., Barona, A. C., Alvarez, F. C., & Rodriguez, C. A. (2018). Antimicrobial potential of chlorella algae isolated from stacked waters of the andean region of ecuador. Iop Publishing, 012040.
Adarme-Vega, T. C., Lim, D. K., Timmins, M., Vernen, F., Li, Y., & Schenk, P. M. (2012). Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microbial Cell Factories, 11, 96.
Adenan, N. S., Yusoff, F. M., & Shariff, M. (2013). Effect of salinity and temperature on the growth of diatoms and green algae. Journal of Fisheries and Aquatic Science, 8, 397–404.
Agarwal, S., & Rao, A. V. (2000). Tomato lycopene and its role in human health and chronic diseases. CMAJ, 163, 739–744.
Albanes, D., Virtamo, J., Taylor, P. R., Rautalahti, M., Pietinen, P., & Heinonen, O. P. (1997). Effects of supplemental beta-carotene, cigarette smoking, and alcohol consumption on serum carotenoids in the alpha-tocopherol, beta-carotene cancer prevention study. American Journal of Clinical Nutrition, 66, 366–372.
Allen, J. W., Dirusso, C. C., & Black, P. N. (2017). Carbon and acyl chain flux during stress-induced triglyceride accumulation by stable isotopic labeling of the polar microalga coccomyxa subellipsoidea C169. Journal of Biological Chemistry, 292, 361–374.
Amanda, F., Cunha, R. L. D., Salomon, P. S., Júnior, E. R., & Valença, S. S. (2017). Nanoecotoxicological effects of a sunscreen formulation based on Tio2 nanoparticles on microalgae from Guanabara Bay (Rio De Janeiro, Brazil). Moj Poly Sci, 1, 00014.
Ambati, R. R., Gogisetty, D., Aswathanarayana, R. G., Ravi, S., Bikkina, P. N., Bo, L., & Yuepeng, S. (2018). Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Critical Reviews in Food Science and Nutrition, 1–22.
Appel, K., Munoz, E., Navarrete, C., Cruz-Teno, C., Biller, A., & Thiemann, E. (2018). Immunomodulatory and inhibitory effect of immulina®, and immunloges® in the Ig-E mediated activation of Rbl-2h3 cells. A new role in allergic inflammatory responses. Plants, 7, 13.
Augusti, P. R., Quatrin, A., Somacal, S., Conterato, G. M., Sobieski, R., Ruviaro, A. R., et al. (2012). Astaxanthin prevents changes in the activities of thioredoxin reductase and paraoxonase in hypercholesterolemic rabbits. Journal of Clinical Biochemistry and Nutrition, 51, 42–49.
Ausich, R. L. (1992). Commercial opportunities for carotenoid production by biotechnology. Pure and Applied Chemistry, 69, 2169–2173.
Balder, H. F., Vogel, J., Jansen, M. C., Weijenberg, M. P., Van Den Brandt, P. A., Westenbrink, S., et al. (2006). Heme and chlorophyll intake and risk of colorectal cancer in The Netherlands cohort study. Cancer Epidemiology, Biomarkers & Prevention, 15, 717–725.
Basha, O. M., Hafez, R. A., El-Ayouty, Y. M., Mahrous, K. F., Bareedy, M. H., & Salama, A. M. (2008). C-Phycocyanin inhibits cell proliferation and may induce apoptosis in human Hepg2 cells. Egyptian Journal of Immunology, 15, 161–167.
Begum, H., Yusoff, F. M., Banerjee, S., Khatoon, H., & Shariff, M. (2015). Availability and utilization of pigments from microalgae. Critical Reviews in Food Science and Nutrition, 56, 2209–2222.
Begum, H., Yusoff, F. M. D., Banerjee, S., Khatoon, H., & Shariff, M. (2016). Availability and utilization of pigments from microalgae. Critical Reviews in Food Science and Nutrition, 56, 2209–2222.
Benedetti, S., Benvenuti, F., Pagliarani, S., Francogli, S., Scoglio, S., & Canestrari, F. (2004). Antioxidant properties of a novel phycocyanin extract from the blue-green alga aphanizomenon flos-aquae. Life Sciences, 75, 2353–2362.
Bennedsen, M., Wang, X., Willen, R., Wadstrom, T., & Andersen, L. P. (1999). Treatment of H. Pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunology Letters, 70, 185–189.
Bermejo Roman, R., Alvarez-Pez, J. M., Acien Fernandez, F. G., & Molina Grima, E. (2002). Recovery of pure B-Phycoerythrin from the microalga porphyridium cruentum. Journal of Biotechnology, 93, 73–85.
Bewicke, D., & Potter, B. A. (2009a). Chlorella: The emerald food. Ronin Publishing.
Bewicke, D., & Potter, B. A. (2009b). Chlorella: The emerald food. Ronin Publishing.
Bharathiraja, B., Chakravarthy, M., Kumar, R. R., Yogendran, D., Yuvaraj, D., Jayamuthunagai, J., et al. (2015). Aquatic biomass (Algae) as a future feed stock for bio-refineries: A review on cultivation, processing and products. Renewable and Sustainable Energy Reviews, 47, 634–653.
Bhat, V. B., & Madyastha, K. M. (2000). C-Phycocyanin: A potent peroxyl radical scavenger in vivo and in vitro. Biochemical and Biophysical Research Communications, 275, 20–25.
Bhosale, P., & Bernstein, P. S. (2005). Microbial xanthophylls. Applied Microbiology and Biotechnology, 68, 445–455.
Birkou, M., Bokas, D., & Aggelis, G. (2012). Improving fatty acid composition of lipids synthesized by brachionus plicatilis in large scale experiments. Journal of the American Oil Chemists Society, 89, 2047–2055.
Bito, T., Bito, M., Asai, Y., Takenaka, S., Yabuta, Y., Tago, K., et al. (2016). Characterization and quantitation of vitamin B12 compounds in various chlorella supplements. Journal of Agricultural and Food Chemistry, 64, 8516–8524.
Borowitzka, M. A. (2018). Chapter 9—Microalgae in medicine and human health: A historical perspective. In I. A. Levine & J. Fleurence (Eds.), Microalgae in health and disease prevention. Academic Press.
Breuer, G., Lamers, P. P., Martens, D. E., Draaisma, R. B., & Wijffels, R. H. (2012). The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresource Technology, 124, 217–226.
Camera, E., Mastrofrancesco, A., Fabbri, C., Daubrawa, F., Picardo, M., Sies, H., et al. (2009). Astaxanthin, canthaxanthin and beta-carotene differently affect uva-induced oxidative damage and expression of oxidative stress-responsive enzymes. Experimental Dermatology, 18, 222–231.
Cantrell, A., Mcgarvey, D. J., Truscott, T. G., Rancan, F., & Bohm, F. (2003). Singlet oxygen quenching by dietary carotenoids in a model membrane environment. Archives of Biochemistry and Biophysics, 412, 47–54.
Careri, M., Furlattini, L., Mangia, A., Musc, M., Anklam, E., Theobald, A., et al. (2001). Supercritical fluid extraction for liquid chromatographic determination of carotenoids in spirulina pacifica algae: A chemometric approach. Journal of Chromatography A, 912, 61–71.
Cha, K. H., Koo, S. Y., & Lee, D. U. (2008). Antiproliferative effects of carotenoids extracted from chlorella ellipsoidea and chlorella vulgaris on human colon cancer cells. Journal of Agriculture and Food Chemistry, 56, 10521–10526.
Chew, K. W., Yap, J. Y., Show, P. L., Suan, N. H., Juan, J. C., Ling, T. C., et al. (2017). Microalgae biorefinery: High value products perspectives. Bioresource Technology, 229, 53–62.
Chidley, C., & Davison, G. (2018). The effect of chlorella pyrenoidosa supplementation on immune responses to 2 days of intensified training. European Journal of Nutrition, 57, 2529–2536.
Christaki, E., Bonos, E., Giannenas, I., & Florou-Paneri, P. (2013). Functional properties of carotenoids originating from algae. Journal of the Science of Food and Agriculture, 93, 5–11.
Ciccone, M. M., Cortese, F., Gesualdo, M., Carbonara, S., Zito, A., Ricci, G., et al. (2013). Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediators of Inflammation, 2013, 782137.
Cokrowati, N., Amir, S., Abidin, Z., Setyono, B. D. H., & Damayanti, A. A. (2014). Abundance and composition of phytoplankton in Kodek Bay Pemenang Lombok Utara. Depik, 3, 21–26.
Cuellar-Bermudez, S. P., Aguilar-Hernandez, I., Cardenas-Chavez, D. L., Ornelas-Soto, N., Romero-Ogawa, M. A., & Parra-Saldivar, R. (2015). extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microbial Biotechnology, 8, 190–209.
Cunningham, F. X., & Schiff, J. A. (1986). Chlorophyll-protein complexes from euglena gracilis and mutants deficient in chlorophyll B: I. Pigment Composition Plant Physiol, 80, 223–230.
Cysewski, G. R., & Todd, L. R. (2004). Industrial production of microalgal cell mass and secondary products-species of high potential haematococcus. In A. Richmond (Ed.), Handbook of microalgal culture. Biotechnology and applied phycology (pp. 281-288). Oxford, UK: Blckwell Science.
Da Silva Ferreira, V., & Sant’anna, C. (2017). Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World Journal of Microbiology and Biotechnology, 33, 20.
Dahoumane, S. A., Yéprémian, C., Djédiat, C., Couté, A., Fiévet, F., Coradin, T., et al. (2016). Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of euglena gracilis microalga. Journal of Nanoparticle Research, 18, 79.
Danaee, S., Yazdanbakhsh, N., Naghoosi, H., & Sheykhinejad, A. (2018). Assessment of phosphorescent paint effects on microalgae cultivation. Korean Journal of Chemical Engineering, 35, 1144–1150.
Daubrawa, F., Sies, H., & Stahl, W. (2005). Astaxanthin diminishes gap junctional intercellular communication in primary human fibroblasts. Journal of Nutrition, 135, 2507–2511.
Davinelli, S., Nielsen, M. E., & Scapagnini, G. (2018). Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients, 10.
De La Jara, A., Ruano-Rodriguez, C., Polifrone, M., Assunçao, P., Brito-Casillas, Y., Wägner, A. M., et al. (2018). Impact of dietary arthrospira (Spirulina) biomass consumption on human health: Main health targets and systematic review. Journal of Applied Phycology, 30, 2403–2423.
Del Campo, J. A., Garcia-Gonzalez, M., & Guerrero, M. G. (2007). Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Applied Microbiology and Biotechnology, 74, 1163–1174.
Del Campo, J. A., Moreno, J., Rodriguez, H., Vargas, M. A., Rivas, J., & Guerrero, M. G. (2000). Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in muriellopsis Sp. (Chlorophyta). Journal of Biotechnology, 76, 51–59.
Del Campo, J. A., Rodriguez, H., Moreno, J., Vargas, M. A., Rivas, J., & Guerrero, M. G. (2004). Accumulation of astaxanthin and lutein in chlorella zofingiensis (Chlorophyta). Applied Microbiology and Biotechnology, 64, 848–854.
Della Rosa, G., Vona, D., Aloisi, A., Ragni, R., Di Corato, R., Lo Presti, M., et al. (2018). Luminescent silica-based nanostructures from in vivo iridium-doped diatoms microalgae. Acs Sustainable Chemistry & Engineering, 7, 2207–2215.
Diwu, Z., Zhang, J., Tang, Y., & Guobing, X. (2012). Fluorometric analysis kit. Washington, DC: U.S. Patent No. 8,105,829.U.S. patent and trademark office.
Dufossé, L. (2016). Current and potential natural pigments from microorganisms (Bacteria, Yeasts, Fungi, Microalgae). Handbook on Natural Pigments in Food and Beverages: Elsevier.
Dufossé, L. (2018). Microbial pigments from bacteria, yeasts, fungi, and microalgae for the food and feed industries. Natural and artificial flavoring agents and food dyes. Elsevier.
Dufosse, L., Galaup, P., Yaron, A., Arad, S. M., Blanc, P., Murthy, K. N. C., et al. (2005). Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends in Food Science & Technology, 16, 389–406.
Durmaz, Y., Donato, M., Monteiro, M., Gouveia, L., Nunes, M. L., Pereira, T. G., et al. (2009). Effect of temperature on Α-Tocopherol, fatty acid profile, and pigments of diacronema vlkianum (Haptophyceae). Aquac Int, 17, 391–399.
Dutta, D., Chaudhuri, U. R., & Chakraborty, R. (2005). Structure, health benefits, antioxidant property and processing and storage of carotenoids. African Journal of Biotechnology, 4, 1510–1520.
Efferth, T., Banerjee, M., Paul, N. W., Abdelfatah, S., Arend, J., Elhassan, G., et al. (2016). Biopiracy of natural products and good bioprospecting practice. Phytomedicine, 23, 166–173.
Eid, F. A., Eldahshan, A. A. M., & Hamid, S. M. A. (2018). The possible radio protective role of aphanizomenon flos-aquae (Afa) on heart of the adult male albino rats. The Egyptian Journal Of Hospital Medicine, 71, 3559–3572.
Enzing, C., Ploeg, M., Barbosa, M., & Sijtsma, L. 2014. Microalgae-based products for the food and feed sector: An outlook for Europe. Jrc Scientific And Policy Reports, 19–37.
Eonseon, J., Polle, J. E. W., Lee, H. K., Hyund, S. M., & Chang, M. (2003). Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. Microbial Biotechnology, 13, 165–174.
Eriksen, N. T. (2016). Research trends in the dominating microalgal pigments, Β-Carotene, astaxanthin, and phycocyanin used in feed, in foods, and in health applications. Journal of Nutrition and Food Sciences, 6.
Fallah, A. A., Sarmast, E., Dehkordi, S. H., Engardeh, J., Mahmoodnia, L., Khaledifar, A., et al. (2018). Effect of chlorella supplementation on cardiovascular risk factors: A meta-analysis of randomized controlled trials. Clinical Nutrition, 37, 1892–1901.
Fang, Q., Guo, S., Zhou, H., Han, R., Wu, P., & Han, C. (2017). Astaxanthin protects against early burn-wound progression in rats by attenuating oxidative stress-induced inflammation and mitochondria-related apoptosis. Scientific Reports, 7, 41440.
Faried, M., Samer, M., Abdelsalam, E., Yousef, R. S., Attia, Y. A., & Ali, A. S. (2017). Biodiesel production from microalgae: Processes, technologies and recent advancements. Renewable and Sustainable Energy Reviews, 79, 893–913.
Fassett, R. G., & Coombes, J. S. (2011). Astaxanthin: A Potential Therapeutic Agent In Cardiovascular Disease. Mar Drugs, 9, 447–465.
Ferruzzi, M. G., & Blakeslee, J. (2007). Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutrition Research, 27, 1–12.
Foo, S. C., Yusoff, F. M., Ismail, M., Basri, M., Yau, S. K., Khong, N. M. H., et al. (2017). Antioxidant capacities of fucoxanthin-producing algae as influenced by their carotenoid and phenolic contents. Journal of Biotechnology, 241, 175–183.
Freitas, H. R. (2017). Chlorella vulgaris as a source of essential fatty acids and micronutrients: A brief commentary. The Open Plant Science Journal, 10.
Galasso, C., Corinaldesi, C., & Sansone, C. (2017). Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants, 6, 96.
Gantar, M., Dhandayuthapani, S., & Rathinavelu, A. (2012). Phycocyanin induces apoptosis and enhances the effect of topotecan on prostate cell line Lncap. Journal of Medicinal Food, 15, 1091–1095.
Garcia-Gonzalez, J., & Sommerfeld, M. (2016). Biofertilizer and biostimulant properties of the microalga acutodesmus dimorphus. Journal of Applied Phycology, 28, 1051–1061.
García, J. L., De Vicente, M., & Galán, B. (2017). Microalgae, old sustainable food and fashion nutraceuticals. Microbial Biotechnology, 10, 1017–1024.
Ghosh, A., Khanra, S., Mondal, M., Halder, G., Tiwari, O. N., Saini, S., et al. (2016). Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: A review. Energy Conversion and Management, 113, 104–118.
Gladue, R. M., & Maxey, J. E. (1994). Microalgal feeds for aquaculture. Journal of Applied Phycology, 6, 131–141.
Glazer, A. N. (1994). Phycobiliproteins—A family of valuable widely used fluorophores. Journal of Applied Phycology, 6, 105–112.
Godoy Danesi, E. D., Oliveira Rangel-Yagui, C., Sato, S., & Monteiro De Carvalho, J. C. (2011). Growth and content of spirulina platensis biomass chlorophyll cultivated at different values of light intensity and temperature using different nitrogen sources. Brazilian Journal of Microbiology, 42, 362–373.
González, R., González, A., & Remirez, D. (2003). Protective effects of phycocyanin on galactosamine—Induced hepatitis in rats. Biotecnología Aplicada, 20, 107–110.
Graverholt, O. S., & Eriksen, N. T. (2007). Heterotrophic high-cell-density fed-batch and continuous-flow cultures of galdieria sulphuraria and production of phycocyanin. Applied Microbiology and Biotechnology, 77, 69–75.
Grossman, A. R., Bhaya, D., Apt, K. E., & Kehoe, D. M. (1995). Light-harvesting complexes in oxygenic photosynthesis: Diversity, control, and evolution. Annual Review of Genetics, 29, 231–288.
Grossman, A. R., Schaefer, M. R., Chiang, G. G., & Collier, J. L. (1993). Environmental effects on the light-harvesting complex of cyanobacteria. Journal of Bacteriology, 175, 575–582.
Guedes, A. C., Amaro, H. M., & Malcata, F. X. (2011). Microalgae as sources of high added-value compounds–A brief review of recent work. Biotechnology Progress, 27, 597–613.
Guerin, M., Huntley, M. E., & Olaizola, M. (2003). Haematococcus astaxanthin: Applications for human health and nutrition. Trends in Biotechnology, 21, 210–216.
Gupta, A., & Sainis, J. (2010). Isolation of C-Phycocyanin from synechococcus Sp., (Anacystis Nidulans Bd1). Journal of Applied Phycology, 22, 231–233.
Guschina, I. A., & Harwood, J. L. (2006). Lipids and lipid metabolism in eukaryotic algae. Progress in Lipid Research, 45, 160–186.
Haines, D. D., Varga, B., Bak, I., Juhasz, B., Mahmoud, F. F., Kalantari, H., et al. (2011). Summative interaction between astaxanthin, ginkgo biloba extract (Egb761) and vitamin C in suppression of respiratory inflammation: A comparison with ibuprofen. Phytotherapy Research, 25, 128–136.
Halim, R., Danquah, M. K., & Webley, P. A. (2012). Extraction of oil from microalgae for biodiesel production: A review. Biotechnology Advances, 30, 709–732.
Hanusch, M., Stahl, W., Schulz, W. A., & Sies, H. (1995). Induction of gap junctional communication by 4-oxoretinoic acid generated from its precursor canthaxanthin. Archives of Biochemistry and Biophysics, 317, 423–428.
Hazeem, L. J., Waheed, F. A., Rashdan, S., Bououdina, M., Brunet, L., Slomianny, C., et al. (2015). Effect of magnetic iron oxide (Fe 3 O 4) nanoparticles on the growth and photosynthetic pigment content of picochlorum Sp. Environmental Science and Pollution Research, 22, 11728–11739.
Hejazi, M. A., Andrysiewicz, E., Tramper, J., & Wijffels, R. H. (2003). Effect of mixing rate on beta-carotene production and extraction by dunaliella salina in two-phase bioreactors. Biotechnology and Bioengineering, 84, 591–596.
Hejazi, M. A., Kleinegris, D., & Wijffels, R. H. (2004). Mechanism of extraction of beta-carotene from microalga dunaliellea salina in two-phase bioreactors. Biotechnology and Bioengineering, 88, 593–600.
Hemlata, & Fatma, T. (2009). Screening of cyanobacteria for phycobiliproteins and effect of different environmental stress on its yield. Bull Environ Contam Toxicol, 83, 509–515.
Hix, L. M., Lockwood, S. F., & Bertram, J. S. (2004). Upregulation of connexin 43 protein expression and increased gap junctional communication by water soluble disodium disuccinate astaxanthin derivatives. Cancer Letters, 211, 25–37.
Hoffmann, C., Márquez, J. R. G., & Krueger, T. (2018). A local perspective on drivers and measures to slow deforestation in the andean-amazonian foothills of Colombia. Land Use Policy, 77, 379–391.
Huang, B., Wang, G. C., Zeng, C. K., & Li, Z. G. (2002). The Experimental Research Of R-Phycoerythrin Subunits On Cancer Treatment: A New Photosensitizer In Pdt. Cancer Biother Radiopharm, 17, 35–42.
Humphrey, A. M. (1980). Chlorophyll. Food Chemistry, 5, 57–67.
Humphrey, A. M. (2004). Chlorophyll as a color and functional ingredient. Journal of Food Science, 69, 422–425.
Hyun, T. K., Kim, H.-C., Ko, Y.-J., & Kim, J.-S. (2016). Antioxidant, Α-Glucosidase inhibitory and anti-inflammatory effects of aerial parts extract from korean crowberry (Empetrum Nigrum Var. Japonicum). Saudi Journal of Biological Sciences, 23, 181–188.
Ismail, A., Marzuki, S., Mohd Yusof, N., Buyong, F., Mohd Said, M., Sigh, H., & Zulkifli, A. (2017). Epiphytic terrestrial algae (Trebouxia Sp.) as a biomarker using the free-air-carbon dioxide-enrichment (Face) system. Biology, 6, 19.
Jeevanandam, J., San Chan, Y., & Danquah, M. K. (2016). Nano-formulations of drugs: Recent developments, impact and challenges. Biochimie, 128, 99–112.
Jena, J., Pradhan, N., Dash, B. P., Panda, P. K., & Mishra, B. K. (2015). Pigment mediated biogenic synthesis of silver nanoparticles using diatom amphora Sp. and its antimicrobial activity. Journal of Saudi Chemical Society, 19, 661–666.
Jena, J., & Subudhi, E. (2019). Microalgae: An untapped resource for natural antimicrobials. The Role of Microalgae in Wastewater Treatment. Springer.
Jensen, G. S., Attridge, V. L., Beaman, J. L., Guthrie, J., Ehmann, A., & Benson, K. F. (2015). Antioxidant And anti-inflammatory properties of an aqueous cyanophyta extract derived from arthrospira platensis: Contribution to bioactivities by the non-phycocyanin aqueous fraction. Journal of Medicinal Food, 18, 535–541.
Jin, E.-S., Polle, Juergen Ew, Lee, Hong-Kum, Hyun, Sang-Min, & Chang, M. (2003). Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. Journal of Microbiology and Biotechnology, 13, 165–174.
Jyonouchi, H., Hill, R. J., Tomita, Y., & Good, R. A. (1991). Studies of immunomodulating actions of carotenoids. I. effects of beta-carotene and astaxanthin on murine lymphocyte functions and cell surface marker expression in in vitro culture system. Nutrition and Cancer, 16, 93–105.
Jyonouchi, H., Sun, S., & Gross, M. (1995). Effect of carotenoids on in vitro immunoglobulin production by human peripheral blood mononuclear cells: Astaxanthin, a carotenoid without vitamin A activity, enhances in vitro immunoglobulin production in response to a t-dependent stimulant and antigen. Nutrition and Cancer, 23, 171–183.
Jyonouchi, H., Sun, S., Iijima, K., & Gross, M. D. (2000). Antitumor activity of astaxanthin and its mode of action. Nutrition and Cancer, 36, 59–65.
Kadim, M. K., & Arsad, S. (2016). Distribution and abundance of microalgae based on coastal characteristic and ecology in bone bolango coastal region, indonesia. Asian Journal of Microbiology, Biotechnology & Environmental Sciences, 18, 395–401.
Kamath, B. S., Srikanta, B. M., Dharmesh, S. M., Sarada, R., & Ravishankar, G. A. (2008). Ulcer preventive and antioxidative properties of astaxanthin from haematococcus pluvialis. European Journal of Pharmacology, 590, 387–395.
Kanda, H., Kamo, Y., Machmudah, S., Wahyudiono, E. Y., & Goto, M. (2014). Extraction of fucoxanthin from raw macroalgae excluding drying and cell wall disruption by liquefied dimethyl ether. Mar Drugs, 12, 2383–2396.
Kapoore, R., Butler, T., Pandhal, J., & Vaidyanathan, S. (2018). Microwave-assisted extraction for microalgae: From biofuels to biorefinery. Biology, 7, 18.
Kaur, G., Khattar, J. I. S., D. P. Singh, D. P., Singh, Y., & Nadda, J. (2009). Microalgae: A source of natural colours. Algal Biology and Biotechnology.
Kaushik, B. D. (2000). Screening of cyanobacterial strains for natural colours. Indian Hydrobiology, 3, 81–84.
Keimes, J., & Gunther, P. (2016). Process for the preparation of a pharmaceutically effective extract from arthrospira Sp. Google Patents.
Khan, M. I., Shin, J. H., & Kim, J. D. (2018). The promising future of microalgae: Current Status, Challenges, And Optimization Of A Sustainable And Renewable Industry For Biofuels, Feed, And Other Products. Microbial Cell Factories, 17, 36.
Khanra, S., Mondal, M., Halder, G., Tiwari, O. N., Gayen, K., & Bhowmick, T. K. (2018). Downstream processing of microalgae for pigments, protein and carbohydrate in industrial application: A review. Food and Bioproducts Processing, 110, 60–84.
Kidd, P. (2011). Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Alternative Medicine Review, 16, 355–364.
Kim, S. M., Jung, Y. J., Kwon, O. N., Cha, K. H., Um, B. H., Chung, D., et al. (2012). A potential commercial source of fucoxanthin extracted from the microalga phaeodactylum tricornutum. Applied Biochemistry and Biotechnology, 166, 1843–1855.
Kim, Y. J., Kim, Y. A., & Yokozawa, T. (2009). Protection against oxidative stress, inflammation, and apoptosis of high-glucose-exposed proximal tubular epithelial cells by astaxanthin. Journal of Agriculture and Food Chemistry, 57, 8793–8797.
Kleinegris, D. M., Janssen, M., Brandenburg, W. A., & Wijffels, R. H. (2011). Continuous production of carotenoids from dunaliella salina. Enyzme and Microbial Technology, 48, 253–259.
Kotake-Nara, E., Kushiro, M., Zhang, H., Sugawara, T., Miyashita, K., & Nagao, A. (2001). Carotenoids affect proliferation of human prostate cancer cells. Journal of Nutrition, 131, 3303–3306.
Krishnaswamy, S. (2018). Bioprospecting, botany, biodiversity, and their impact on botanical drug development. Botanical Drug Products: Recent Developments and Market Trends, 39.
Kronick, M. N., & Grossman, P. D. (1983). Immunoassay techniques with fluorescent phycobiliprotein conjugates. Clinical Chemistry, 29, 1582–1586.
Kuddus, M., Singh, P., Thomas, G., & Al-Hazimi, A. (2013). Recent developments in production and biotechnological applications of C-phycocyanin. BioMed Research International, 2013, 742859.
Kumar, S. R., Hosokawa, M., & Miyashita, K. (2013). Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar Drugs, 11, 5130–5147.
Lee, K., Eisterhold, M. L., Rindi, F., Palanisami, S., & Nam, P. K. (2014). isolation and screening of microalgae from natural habitats in the midwestern United States of America for biomass and biodiesel sources. Journal of Natural Science, Biology, and Medicine, 5, 333.
Lee, Y.-C., Lee, K., & Oh, Y.-K. (2015). Recent nanoparticle engineering advances in microalgal cultivation and harvesting processes of biodiesel production: A review. Bioresource Technology, 184, 63–72.
Lemoine, Y., & Schoefs, B. (2010). Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynthesis Research, 106, 155–177.
Li, Z., Ma, X., Li, A., & Zhang, C. (2012a). A novel potential source of beta-carotene: Eustigmatos Cf. Polyphem (Eustigmatophyceae) and pilot beta-carotene production in bubble column and flat panel photobioreactors. Bioresource Technology, 117, 257–263.
Li, Z., Sun, M., Li, Q., Li, A., & Zhang, C. (2012b). Profiling Of Carotenoids In Six Microalgae (Eustigmatophyceae) and assessment of their beta-carotene productions in bubble column photobioreactor. Biotechnology Letters, 34, 2049–2053.
Lin, K. H., Lin, K. C., Lu, W. J., Thomas, P. A., Jayakumar, T., & Sheu, J. R. (2015). Astaxanthin, a carotenoid, stimulates immune responses by enhancing ifn-gamma and Il-2 secretion in primary cultured lymphocytes in vitro and ex vivo. International Journal of Molecular Sciences, 17.
Lopez, M. C., Sanchez Edel, R., Lopez, J. L., Fernandez, F. G., Sevilla, J. M., Rivas, J., et al. (2006). Comparative analysis of the outdoor culture of haematococcus pluvialis in tubular and bubble column photobioreactors. Journal of Biotechnology, 123, 329–342.
Ma, R. Y. N., & Chen, F. (2001). Enhanced production of free trans-astaxanthin by oxidative stress in the cultures of the green microalga chlorococcum Sp. Process Biochemistry, 36, 1175–1179.
Macias-Sanchez, M. D., Mantell, C., Rodriguez, M., Martinez De La Ossa, E., Lubian, L. M., & Montero, O. (2009). Comparison of supercritical fluid and ultrasound-assisted extraction of carotenoids and chlorophyll a from dunaliella salina. Talanta, 77, 948–952.
Madhyastha, H. K., Radha, K. S., Sugiki, M., Omura, S., & Maruyama, M. (2006). Purification of C-phycocyanin from spirulina fusiformis and its effect on the induction of urokinase-type plasminogen activator from calf pulmonary endothelial cells. Phytomedicine, 13, 564–569.
Maeda, H., Hosokawa, M., Sashima, T., Murakami-Funayama, K., & Miyashita, K. (2009). Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol Med Rep, 2, 897–902.
Manabe, E., Handa, O., Naito, Y., Mizushima, K., Akagiri, S., Adachi, S., et al. (2008). Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. Journal of Cellular Biochemistry, 103, 1925–1937.
Maoka, T., Tokuda, H., Suzuki, N., Kato, H., & Etoh, H. (2012). Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar Drugs, 10, 1391–1399.
Marchetti, M., Vizzarri, M., Lasserre, B., Sallustio, L., & Tavone, A. (2014). Natural capital and bioeconomy: challenges and opportunities for forestry. Annals of Silvicultural Research, 38, 62–73.
Markou, G., Iconomou, D., & Muylaert, K. (2016). Applying Raw Poultry Litter Leachate For The Cultivation Of Arthrospira Platensis And Chlorella Vulgaris. Algal Research, 13, 79–84.
Matamoros, V., Gutiérrez, R., Ferrer, I., García, J., & Bayona, J. M. (2015). Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. Journal of Hazardous Materials, 288, 34–42.
Mattson, M. P. (2004). Pathways towards and away from alzheimer’s disease. Nature, 430, 631–639.
Mendes-Pinto, M. M., Rapso, M. F. J., Bowen, J., Young, A. J., & Morais, R. (2001). Evaluation of different cell disruption processes on encysted cells of haematococcus pluvialis: Effects on astaxanthin recovery and implications on bioavailability. Journal of Applied Phycology, 13, 19–24.
Miazek, K., Iwanek, W., Remacle, C., Richel, A., & Goffin, D. (2015). Effect of metals, metalloids and metallic nanoparticles on microalgae growth and industrial product biosynthesis: A review. International Journal of Molecular Sciences, 16, 23929–23969.
Mishra, S. K., Shrivastav, A., & Mishra, S. (2011). Preparation of highly purified C-Phycoerythrin from marine cyanobacterium pseudanabaena Sp. Protein Expression and Purification, 80, 234–238.
Misra, N., Panda, P. K., Parida, B. K., & Mishra, B. K. (2016). Way forward to achieve sustainable and cost-effective biofuel production from microalgae: A review. International Journal of Environmental Science and Technology, 13, 2735–2756.
Mourelle, M., Gómez, C., & Legido, J. (2017). The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics, 4, 46.
Mubarak, M., Shaija, A., & Suchithra, T. V. (2015). A review on the extraction of lipid from microalgae for biodiesel production. Algal Research, 7, 117–123.
Naito, Y., Uchiyama, K., Aoi, W., Hasegawa, G., Nakamura, N., Yoshida, N., et al. (2004). Prevention of diabetic nephropathy by treatment with astaxanthin in diabetic Db/Db mice. BioFactors, 20, 49–59.
Nakao, R., Nelson, O. L., Park, J. S., Mathison, B. D., Thompson, P. A., & Chew, B. P. (2010). Effect of dietary astaxanthin at different stages of mammary tumor initiation in balb/C mice. Anticancer Research, 30, 2171–2175.
Nakashima, Y., Ohsawa, I., Konishi, F., Hasegawa, T., Kumamoto, S., Suzuki, Y., et al. (2009). Preventive effects of chlorella on cognitive decline in age-dependent dementia model mice. Neuroscience Letters, 464, 193–198.
Namasivayam, S. K. R. (2017). Nanoformulation of antibacterial antibiotics cefpirome with biocompatible polymeric nanoparticles and evaluation for the improved antibacterial activity and nontarget toxicity studies. Asian Journal of Pharmaceutics (Ajp): Free Full Text Articles From Asian J Pharm, 11.
Nuzzo, D., Presti, G., Picone, P., Galizzi, G., Gulotta, E., Giuliano, S., Mannino, C., Gambino, V., Scoglio, S., & Di Carlo, M. (2018). Effects of the aphanizomenon flos-aquae extract (Klamin®) on a neurodegeneration cellular model. Oxidative medicine and cellular longevity.
Okai, Y., & Higashi-Okai, K. (1996). Possible immunomodulating activities of carotenoids in in vitro cell culture experiments. International Journal of Immunopharmacology, 18, 753–758.
Olaizola, M., & Huntley, M. E. (2003). Recent advances in commercial production of astaxanthin from microalgae. In M. Fingerman & R. Nagabhushanam (Eds.), Biomaterials and bioprocessing. enfield science publ (pp. 143–164).
Palmquist, D. L., & Jenkins, T. C. (2003). Challenges with fats and fatty acid methods. Journal of Animal Science, 81, 3250–3254.
Palozza, P., Torelli, C., Boninsegna, A., Simone, R., Catalano, A., Mele, M. C., et al. (2009). Growth-inhibitory effects of the astaxanthin-rich alga haematococcus pluvialis in human colon cancer cells. Cancer Letters, 283, 108–117.
Panahi, Y., Darvishi, B., Jowzi, N., Beiraghdar, F., & Sahebkar, A. (2016). Chlorella vulgaris: A multifunctional dietary supplement with diverse medicinal properties. Current Pharmaceutical Design, 22, 164–173.
Panis, G., & Carreon, J. R. (2016). Commercial astaxanthin production derived by green alga haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Research, 18, 175–190.
Park, J. S., Chyun, J. H., Kim, Y. K., Line, L. L., & Chew, B. P. (2010). Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutrition and Metabolis (Lond), 7, 18.
Parmar, T. K., Rawtani, D., & Agrawal, Y. K. (2016). Bioindicators: The natural indicator of environmental pollution. Frontiers in Life Science, 9, 110–118.
Parveen, S. N. (2016). Antidiabetic and antioxidant properties of Spirulina-A review. Research Journal of Pharmacy and Technology, 9, 2034.
Pasquet, V., Chérouvrier, J.-R., Farhat, F., Thiéry, V., Piot, J.-M., Bérard, J.-B., et al. (2011). Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochemistry, 46, 59–67.
Patel, V., Berthold, D., Puranik, P., & Gantar, M. (2015). Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnology Reports, 5, 112–119.
Patridge, E., Gareiss, P., Kinch, M. S., & Hoyer, D. (2016). An analysis of fda-approved drugs: Natural products and their derivatives. Drug Discovery Today, 21, 204–207.
Penton-Rol, G., Marin-Prida, J., Pardo-Andreu, G., Martinez-Sanchez, G., Acosta-Medina, E. F., Valdivia-Acosta, A., et al. (2011). C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in Gerbils. Brain Research Bulletin, 86, 42–52.
Perumal, P., Prasath, B. B., Santhanam, P., Devi, A. S., Dineshkumar, S., & Jeyanthi, S. (2015). Isolation and intensive culture of marine microalgae. Advances in Marine and Brackishwater Aquaculture, 1–15.
Peters, R. K., Pike, M. C., Garabrant, D., & Mack, T. M. (1992). Diet and colon cancer in los angeles county, California. Cancer Causes and Control, 3, 457–473.
Pisal, D. S., & Lele, S. S. (2005). Carotenoid production from microalgae, Dunaliella Salina. Indian Journal of Biotechnology, 4, 476–483.
Plaza, M., Herrero, M., Cifuentes, A., & Ibanez, E. (2009). Innovative natural functional ingredients from microalgae. Journal of Agriculture and Food Chemistry, 57, 7159–7170.
Posten, C., & Chen, S. F. (2016). Microalgae biotechnology.
Prasanna, R., Prasanna, B. M., Mohammadi, S. A., & Singh, P. K. (2003). Analysis of phycobiliprotein content in tolypothrix germplasm. Folia Microbiologica, 48, 59–64.
Prasanna, R., Sood, A., Suresh, A., Nayak, S. & B., K. 2007. Potentials and applications of algal pigments in biology and industry. Acta Botanica Hungarica, 49, 131–156.
Prieto, A., Pedro Canavate, J., & Garcia-Gonzalez, M. (2011). Assessment of carotenoid production by dunaliella salina in different culture systems and operation regimes. Journal of Biotechnology, 151, 180–185.
Priyadarshani, I., & Rath, B. (2012). Commercial and industrial applications of micro algae–A review. Journal of Algal Biomass Utilization, 3, 89–100.
Pumas, C., Vacharapiyasophon, P., Peerapornpisal, Y., Leelapornpisid, P., Boonchum, W., Ishii, M., et al. (2011). Thermostability of phycobiliproteins and antioxidant activity from four thermotolerant cyanobacteria. Phycological Research, 59, 166–174.
Puyol, D., Batstone, D. J., Hülsen, T., Astals, S., Peces, M., & Krömer, J. O. (2017). Resource recovery from wastewater by biological technologies: Opportunities, challenges, and prospects. Frontiers in Microbiology, 7, 2106.
Raja, R., Anbazhagan, C., Ganesan, V., & Rengaswamy, R. (2004). Efficacy of dunaliella salina (Volvocales Chlorophyta) in salt refinery effluent treatment. Asian Journal of Chemistry, 16, 1081–1088.
Ramoz, A., Acien, F. G., Fernandez-Sevilla, J. M., Gonzalez, C. V., & Bermejo, R. (2010). Large-scale isolation and purification of C-Phycocyanin from the cyanobacteria anabaena marina using expanded bed adsorption chromatography. Journal of Chemical Technology & Biotechnology, 85, 783–792.
Ranga, R., Sarada, A. R., Baskaran, V., & Ravishankar, G. A. (2009). Identification of carotenoids from green alga haematococcus pluvialis by Hplc And Lc-Ms (Apci) and their antioxidant properties. Journal of Microbiology and Biotechnology, 19, 1333–1341.
Ranga Rao, A., Raghunath Reddy, R. L., Baskaran, V., Sarada, R., & Ravishankar, G. A. (2010). Characterization of microalgal carotenoids by mass spectrometry and their bioavailability and antioxidant properties elucidated in rat model. Journal of Agriculture and Food Chemistry, 58, 8553–8559.
Rao, A. R., Sindhuja, H. N., Dharmesh, S. M., Sankar, K. U., Sarada, R., & Ravishankar, G. A. (2013). Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga haematococcus pluvialis. Journal of Agriculture and Food Chemistry, 61, 3842–3851.
Reichert, M., Bergmann, S. M., Hwang, J., Buchholz, R., & Lindenberger, C. (2017). Antiviral activity of exopolysaccharides from arthrospira platensis against koi herpesvirus. Journal of Fish Diseases, 40, 1441–1450.
Remirez, D., Fernandez, V., Tapia, G., Gonzalez, R., & Videla, L. A. (2002). Influence of C-phycocyanin on hepatocellular parameters related to liver oxidative stress and kupffer cell functioning. Inflammation Research, 51, 351–356.
Rizwan, M., Mujtaba, G., Memon, S. A., Lee, K., & Rashid, N. (2018). Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renewable and Sustainable Energy Reviews, 92, 394–404.
Rock, C. L. (1997). Carotenoids: Biology and treatment. Pharmacology & Therapeutics, 75, 185–197.
Rodriguez, H., Rivas, J., Guerrero, M. G., & Losada, M. (1991). Enhancement of phycobiliprotein production in nitrogen fixing cyanobacteria. Journal of Biotechnology, 20, 263–270.
Romay, C., Gonzalez, R., Ledon, N., Remirez, D., & Rimbau, V. (2003). C-Phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Current Protein and Peptide Science, 4, 207–216.
Roodenburg, A. J., Leenen, R., Van Het Hof, K. H., Weststrate, J. A., & Tijburg, L. B. (2000). Amount of fat in the diet affects bioavailability of lutein esters but not of alpha-carotene, beta-carotene, and vitamin E in humans. American Journal of Clinical Nutrition, 71, 1187–1193.
Roux, J.-M., Lamotte, H., & Achard, J.-L. (2017). An overview of microalgae lipid extraction in a biorefinery framework. Energy Procedia, 112, 680–688.
Ryckebosch, E., Bruneel, C., Termote-Verhalle, R., Goiris, K., Muylaert, K., & Foubert, I. (2014). Nutritional evaluation of microalgae oils rich in omega-3 long chain polyunsaturated fatty acids as an alternative for fish oil. Food Chemistry, 160, 393–400.
Salam, K. A., Velasquez-Orta, S. B., & Harvey, A. P. (2016). A sustainable integrated in situ transesterification of microalgae for biodiesel production and associated co-product-a review. Renewable and Sustainable Energy Reviews, 65, 1179–1198.
Salas-Herrera, G., González-Morales, S., Benavides-Mendoza, A., Castañeda-Facio, A. O., Fernández-Luqueño, F., & Robledo-Olivo, A. (2019). Impact of microalgae culture conditions over the capacity of copper nanoparticle biosynthesis. Journal of Applied Phycology, 1–11.
Sandmann, G. (2001). Genetic manipulation of carotenoid biosynthesis: Strategies, problems and achievements. Trends in Plant Science, 6, 14–17.
Santiago-Santos, M., Ponce-Noyola, T., Olvera-Ram´Irez, R., Ortega-López, J., & Cañizares-Villanueva, R. O. (2004). Extraction and purification of phycocyanin from calothrix Sp. Process. Biochem, 39, 2047–2052.
Sathasivam, R., & Ki, J. S. (2018). A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar Drugs, 16, 26.
Scheer, H., William, J. L., & Lane, M. D. (2004). Chlorophylls and carotenoids in encyclopedia of biological Chemistry Elsevier, Ny.
Schumann, R., Häubner, N., Klausch, S., & Karsten, U. (2005). Chlorophyll extraction methods for the quantification of green microalgae colonizing building facades. International Biodeterioration and Biodegradation, 55, 213–222.
Sekar, S., & Chandramohan, M. (2008). Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. Journal of Applied Phycology, 20, 113–136.
Sengupta, S., Koley, H., Dutta, S., & Bhowal, J. (2018). Hypocholesterolemic effect of spirulina platensis (Sp) fortified functional soy yogurts on diet-induced hypercholesterolemia. Journal of Functional Foods, 48, 54–64.
Seo, Y. C., Choi, W. S., Park, J. H., Park, J. O., Jung, K. H., & Lee, H. Y. (2013). Stable isolation of phycocyanin from spirulina platensis associated with high-pressure extraction process. International Journal of Molecular Sciences, 14, 1778–1787.
Seyidoglu, N., Galip, N., Budak, F., & Uzabaci, E. (2017). The effects of spirulina platensis (Arthrospira Platensis) and saccharomyces cerevisiae on the distribution and cytokine production of Cd4 + And Cd8 + T-Lymphocytes in rabbits. Austral Journal of Veterinary Sciences, 49, 185–190.
Shankar, P. D., Shobana, S., Karuppusamy, I., Pugazhendhi, A., Ramkumar, V. S., Arvindnarayan, S., et al. (2016). A review on the biosynthesis of metallic nanoparticles (Gold And Silver) using bio-components of microalgae: formation mechanism and applications. Enzyme and Microbial Technology, 95, 28–44.
Shih, S. R., Tsai, K. N., Li, Y. S., Chueh, C. C., & Chan, E. C. (2003). Inhibition of enterovirus 71-Induced apoptosis by allophycocyanin isolated from a blue-green alga spirulina platensis. Journal of Medical Virology, 70, 119–125.
Siddiqi, K. S., & Husen, A. (2016). Fabrication of metal and metal oxide nanoparticles by algae and their toxic effects. Nanoscale Research Letters, 11, 363.
Singh, R., Parihar, P., Singh, M., Bajguz, A., Kumar, J., Singh, S., et al. (2017). Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Frontiers in Microbiology, 8, 515.
Solymosi, K., & Mysliwa-Kurdziel, B. (2017). Chlorophylls and their derivatives used in food industry and medicine. Mini Reviews in Medicinal Chemistry, 17, 1194–1222.
Spolaore, P., Joannis-Cassan, C., Duran, E., & Isambert, A. (2006). Commercial applications of microalgae. Journal of bioscience and bioengineering, 101, 87–96.
Srivastava, A. (2015). Freshwater cyanobacteria geitlerinema Ccc728 and Arthrospira Ccc729 as the anticancer and antimicrobial drug resource.
Srivastava, A., Tiwari, R., Srivastava, V., Singh, T. B., & Asthana, R. K. (2015). Fresh water cyanobacteria geitlerinema Sp. Ccc728 and Arthrospira Sp. Ccc729 as an anticancer drug resource. Plos One, 10, E0136838.
Subhashini, J., Mahipal, S. V., Reddy, M. C., Mallikarjuna Reddy, M., Rachamallu, A., & Reddanna, P. (2004). Molecular mechanisms in C-phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. Biochemical Pharmacology, 68, 453–462.
Suzuki, Y., Ohgami, K., Shiratori, K., Jin, X. H., Ilieva, I., Koyama, Y., et al. (2006). Suppressive effects of astaxanthin against rat endotoxin-induced uveitis by inhibiting the Nf-Kappab signaling pathway. Experimental Eye Research, 82, 275–281.
Tanaka, T., Makita, H., Ohnishi, M., Mori, H., Satoh, K., & Hara, A. (1995). Chemoprevention of rat oral carcinogenesis by naturally occurring xanthophylls, astaxanthin and canthaxanthin. Cancer Research, 55, 4059–4064.
Thaakur, S., & Sravanthi, R. (2010). Neuroprotective effect of spirulina in cerebral ischemia-reperfusion injury in rats. Journal of Neural Transmission (Vienna), 117, 1083–1091.
Tonegawa, I., Okada, S., Murakami, M., & Yamagushi, K. (1998). Pigment composition of the green microalga botryococcus Braunii Kawagushi-1. Fisheries Science, 64, 305–308.
Tornwall, M. E., Virtamo, J., Korhonen, P. A., Virtanen, M. J., Taylor, P. R., Albanes, D., et al. (2004). Effect of alpha-tocopherol and beta-carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the Atbc study. European Heart Journal, 25, 1171–1178.
Trantas, E. A., Koffas, M. A. G., Xu, P., & Ververidis, F. (2015). When plants produce not enough or at all: Metabolic engineering of flavonoids in microbial hosts. Frontiers in Plant Science, 6, 7.
Vanthoor-Koopmans, M., Wijffels, R. H., Barbosa, M. J., & Eppink, M. H. M. (2013). Biorefinery of microalgae for food and fuel. Bioresource Technology, 135, 142–149.
Verawaty, M., Melwita, E., Apsari, P., & Mayumi, M. (2017). Cultivation strategy for freshwater macro-and micro-algae as biomass stock for lipid production. Journal of Engineering and Technological Sciences, 49, 261–274.
Vidyashankar, S., & Ravishankar, G. A. (2016). Algae-based bioremediation: Bioproducts and biofuels for biobusiness. Bioremediation and bioeconomy. Elsevier.
Vigani, M., Parisi, C., Rodríguez-Cerezo, E., Barbosa, M. J., Sijtsma, L., Ploeg, M., et al. (2015). Food and feed products from micro-algae: Market opportunities and challenges for the Eu. Trends in Food Science & Technology, 42, 81–92.
Vilchez, C., Forjan, E., Cuaresma, M., Bedmar, F., Garbayo, I., & Vega, J. M. (2011). Marine carotenoids: Biological functions and commercial applications. Mar Drugs, 9, 319–333.
Viskari, P. J., & Colyer, C. L. (2003). Rapid extraction of phycobiliproteins from cultured cyanobacteria samples. Analytical Biochemistry, 319, 263–271.
Wells, M. L., Potin, P., Craigie, J. S., Raven, J. A., Merchant, S. S., Helliwell, K. E., et al. (2017). Algae as nutritional and functional food sources: Revisiting our understanding. Journal of Applied Phycology, 29, 949–982.
Yeesang, C., & Cheirsilp, B. (2011). effect of nitrogen, salt, and iron content in the growth medium and light intensity on lipid production by microalgae isolated from freshwater sources in Thailand. Bioresource Technology, 102, 3034–3040.
Yoshihisa, Y., Rehman, M. U., & Shimizu, T. (2014). Astaxanthin, a xanthophyll carotenoid, inhibits ultraviolet-induced apoptosis in keratinocytes. Experimental Dermatology, 23, 178–183.
Yu, Y., Yao, L., Bai, M., Ji, Z., Li, J., & Huang, X. (2019). Oh mineralization of norfloxacin in the process of algae bloom water treatment in a drinking water treatment system Of 12,000 M3 Per Day. Chemical Engineering Journal, 360, 1355–1362.
Yuan, J. P., Peng, J., Yin, K., & Wang, J. H. (2011). Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Molecular Nutrition & Food Research, 55, 150–165.
Zhang, D. H., & Lee, Y. K. (1997). Enhanced accumulation of secondary carotenoids in a mutant of the green alga, chlorococcum Sp. Journal of Applied Phycology, 9, 459–463.
Zhou, W., Wang, J., Chen, P., Ji, C., Kang, Q., Lu, B., et al. (2017). Bio-mitigation of carbon dioxide using microalgal systems: Advances and perspectives. Renewable and Sustainable Energy Reviews, 76, 1163–1175.
Zhu, Y., Chen, X. B., Wang, K. B., Li, Y. X., Bai, K. Z., Kuang, T. Y., et al. (2007). A Simple Method For Extracting C-Phycocyanin From Spirulina Platensis Using Klebsiella Pneumoniae. Applied Microbiology and Biotechnology, 74, 244–248.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jeevanandam, J., Choudhary, V., Selvam, J.D., Danquah, M.K. (2020). The Bioeconomy of Production of Microalgal Pigments. In: Jacob-Lopes, E., Queiroz, M., Zepka, L. (eds) Pigments from Microalgae Handbook. Springer, Cham. https://doi.org/10.1007/978-3-030-50971-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-50971-2_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-50970-5
Online ISBN: 978-3-030-50971-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)