Abstract
Wastewater generation has concomitantly increased with the growth of world human population in the last century. The uncontrolled discharge of wastewater may result in serious social, environmental and health problems. At the same time, the use of microalgal-based systems has been widely studied for a variety of residual effluents treatment since the early 1950s. In this context, different technologies have been developed, and new strategies to cope with specific needs have been investigated worldwide. There are several advantages of microalgal-based systems compared to traditional wastewater treatment technologies, namely, (1) pollutants and pathogen decrease, (2) nutrient recovery in the form of valuable biomass, (3) energy savings and (4) CO2 emissions reduction. In spite of all these advantages, there are still many challenges to overcome before attaining the real implementation of this technology. Those challenges include (1) land requirement, (2) effect of wastewater characteristics, (3) environmental and operational condition influence and (4) biomass harvesting and valorization. This chapter will explore and discuss the main advantages and limitations of using this green technology for wastewater treatment based on our expertise and the latest insights on this topic.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Microalgae are a group of photosynthetic microorganisms mostly developed in aquatic habitats and capable of converting light energy and inorganic carbon sources (carbonate and CO2) into biomass while releasing O2 to the atmosphere. The term microalgae is generally considered as a general term and often includes cyanobacteria (blue-green algae) as both, microalgae and cyanobacteria, are commonly found in microalgal-based systems for wastewater treatment. However, it is important to underline that cyanobacteria are photosynthetic prokaryotes and microalgae are photosynthetic eukaryotes. In this chapter, the term microalgae will be referred to both groups.
Microalgae biotechnology has been developed for different commercial applications, but in recent years, development of microalgae-bacteria consortia for wastewater treatment has received more attention as an efficient alternative to conventional wastewater treatment plants, based on the avoidance of external oxygen supplementation for heterotrophic bacteria, decreasing energy costs and recovering nutrients as valuable biomass (Hernández et al. 2016). During photosynthesis, microalgae capture light using pigments (chlorophylls and carotenoids) as electromagnetic energy source to break down H2O (light phase) and to reduce inorganic carbon to glucose (dark phase) through the Calvin cycle, releasing O2. The photosynthesis-respiration process can be represented by the following equation (Eq. 20.1):
The presence of inorganic carbon forms (CO2, H2CO3, HCO3− and CO3−2) in wastewater is governed by the following equations (Eqs. 20.2, 20.3 and 20.4) (Andersen 2002):
During the photosynthesis process, algae capture high amounts of CO2, causing a gradual increment of pH. When microalgal biomass concentration is high, CO2 concentration decreases, and carbonate/bicarbonate species dissociate into CO2 with the subsequent alkalinity drop, so that culture medium losses stability. Therefore, the lack of CO2 triggers carbon sequestration from atmosphere to the water. In this manner, microalgal biomass culture shows to be a suitable tool to capture carbon, fixing it in the form of valuable biomass.
As previously mentioned, mitigation of pollutants in photobioreactors is usually made by consortia of microalgae and bacteria. Interactions between both groups of microorganisms can support an efficient removal of organic and inorganic carbon, nitrogen, phosphorus, heavy metals and other pollutant compounds, as they play a complementary or competitive role in the consortia. Due to this interaction, organic matter mineralization by aerobic bacteria produces the inorganic carbon needed by the microalgae. In turn, the O2 required for bacterial degradation is produced photosynthetically by microalgae (González-Fernández et al. 2011). In the case of nutrient removal, nitrogen assimilation into microalgal-bacteria biomass is the most common removal mechanism observed in microalgae-bacteria cultures. The preferred N source in microalgal cultures is NH4+ as it is easily assimilated into amino acids to produce microalgae-bacteria biomass. However, NH3 can result toxic and inhibit photosynthetic activity in some microalgal species (Park and Craggs 2010).
Microalgal species growing on wastewater treatment systems are especially tolerant to pollution. It has been reported that Chlorella, Nitzschia and Scenedesmus are the most tolerant genera with a high presence in wastewater systems (Muñoz and Guieysse 2006; de Godos et al. 2009). Other species such as Ankira, Microspora, Chroococcus limneticus, Cyanophyta cocal, Dactylococcopsis sp., Phormidium sp. and Stigeoclonium sp. have been reported in microalgal-based systems treating fish processing wastewater (Riaño et al. 2011); and Chlamydomonas subcaudata, Teilingia sp., Anabaena sp., Phormidium tergestinum, Pinnularia sp. and Nitzschia sp. have been reported in open ponds for slaughterhouse wastewater (Hernández et al. 2016) (Table 20.1).
As in other wastewater treatment systems, microalgae community composition is influenced by different variables that act as a key selection pressure. These variables produce changes in the community composition from the initially inoculated microalgae to the steady-state period, changing microalgae species diversity and its abundance. The main factors responsible for microalgae community structure are related to wastewater characteristics, species interaction, variations in the environmental conditions, photobioreactor configuration and operational conditions. Diverse species with differential interactions/competition also contribute to the system stability with enhanced biomass growth and efficient removal of nutrients (Hernández et al. 2016).
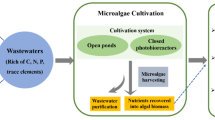
Microalgal cultivation can be carried out in fully contained photobioreactors or in open ponds and channels (Molina Grima et al. 2003). Open ponds, namely, raceway ponds or high-rate algae ponds (HRAPs), are the most widespread systems for microalgal cultivation. They consist of rectangular basins or channels where the wastewater is kept in constant motion with a powered paddle wheel. Closed systems are mainly designed as a column or with tubular shape, although there are different designs and configurations seeking to combine high productivity and low-energy consumption for large-scale application (Gouveia 2011). Open and closed reactors present pros and constrains; some of them are the following, according to Gouveia (2011): open systems need more area-to-volume ratio, water loss through evaporation is high, gas transfer and light utilization efficiency are poor, harvesting cost is high and contamination by other microorganisms is high. In a closed system, the control of growth conditions is easy, capital investment and operating cost are high but harvesting cost is lower and scale-up technology for commercial level is more difficult than in open systems. As pointed out, in open systems, maintenance of microalgae population is complicated due to external contamination of small and rapidly growing microalgae; therefore, some authors have proposed the use of enclosed photobioreactors since they support more effective species control (Tredici 1999). In the case of wastewater treatment, mixed open ponds are the only large-scale implemented technology, probably due to the high-energy costs to operate closed photobioreactors.
Regarding to the scale-up of this technology, the main challenge is the recovery of the produced biomass, called the harvesting process. Biomass concentration in photobioreactors is usually low, between 0.5 and 5 g/L dry weight (Gouveia 2011); consequently it is necessary to remove water to concentrate and harvest the biomass for its further valorization. Harvesting is still considered one of the bottlenecks of wastewater treatment with microalgae, as these microorganisms’ cells have a small size (5–20 μm) and they are very stable in colloidal suspension. Different harvesting methods have been explored, which include gravity sedimentation, centrifugation, filtration, flotation, coagulation and flocculation, as well as several combinations of them. The harvesting process is considered the major limiting factor for wastewater treatment development by microalgae (Molina Grima et al. 2003); for that reason, the choice of the harvesting method is of vital importance for the economic feasibility of microalgal-based wastewater treatment systems. The harvested biomass from wastewater treatment systems is mainly used for energy, biofertilizers and animal food production (Acien et al. 2017). Specifically, microalgae biomass grown in wastewater is characterized by high-protein content, being successfully used as protein source for rainbow trout feed (Tomás-Almenar et al. 2017; Tomás-Almenar et al. 2018; Larrán et al. 2017). This chapter will explore and discuss the main advantages and limitations of using microalgae-based technology for wastewater treatment, from the recovery of nutrients for biomass production, considered one of the main advantages of these systems, to the biomass harvesting and valorization processes; all are based on our expertise and the latest insights on this topic.
2 Advantages of Microalgae for Wastewater Treatment
2.1 Pollutants and Pathogen Decrease
2.1.1 Nutrient Removal During Wastewater Treatment by Microalgae-Bacteria Consortia
Most wastewaters are rich in ammonium, nitrates and phosphorus, and treatments are usually aimed at removing them. In conventional activated sludge treatment plants, carbon is oxidized to CO2, nitrogen (N) is stripped to the atmosphere in the form of N2 and phosphorus (P) is usually precipitated, avoiding nutrient valorization (Adav et al. 2008). Microalgae-bacteria consortia are capable to remove nutrients while producing valuable biomass, so that their use for wastewater treatment has been widely studied during the last two decades. During photosynthesis, microalgae liberate O2 to the medium, which is used by the aerobic bacteria to degrade organic matter into CO2, soluble phosphorus and different inorganic N sources (NH4+, NO3− and NO2−). Then, microalgae uptake inorganic carbon (CO2) and solubilized macro- and micronutrients to grow, resulting in a clean effluent and a valuable biomass. Therefore, if compared to conventional wastewater treatment plants, the use of microalgae-bacteria consortia for wastewater treatment presents the advantage of nutrient recycling (Adav et al. 2008; Hernández et al. 2016).
Table 20.2 shows total nitrogen (TN) and total phosphorus (TP) removal efficiencies in microalgal-based systems treating different wastewaters. Wastewater characteristics and microalgae species determine nutrient removal efficiency in microalgal-based systems. The overall wastewater composition affects nutrient uptake, existing an optimal C:N:P ratio, which differs between microalgae species. Moreover, nutrient uptake also depends on environmental factors that affect microalgae growth such as pH, temperature, light intensity, turbidity and watercolour, among others. Therefore, a wide variation in TN and TP removal rates, in ranges of 22–100% and 20–98% of the initial TN and TP in the wastewater, respectively, has been reported (Table 20.2). According to the results presented in Table 20.2, the main genera present in photobioreactors used for wastewater bioremediation are Chlorella and Scenedesmus. In many occasions, a consortium of different microalgae (i.e. freshwater algae) is used to treat wastewater. Freshwater algae are composed of several species, being most of them unable to acclimate to wastewater polluting conditions and, subsequently, dying. On the contrary, depending on the particular characteristics of each wastewater, some genera grow and become the main species (Hernández et al. 2016).
2.1.2 Organic Pollutants Removal During Wastewater Treatment by Microalgae-Bacteria Consortia
Microalgae and bacteria symbiotically carry out organic pollutants’ elimination. In the presence of light, microalgae (autotrophs) produce the O2 required by heterotrophs to oxidize the organic pollutants. In this way, high organic matter removal rates have been reported for different wastewaters treated by microalgae-bacteria consortia. For instance, 62, 85 and up to 92% of total chemical oxygen demand (TCOD) was removed when treating piggery effluents, potato processing waste and slaughterhouse wastewater, respectively. The initial TCOD concentrations in these wastewaters corresponded to 616, 1536 and 1621 mg TCOD/L, respectively. Different removal rates were attributed to variations in the biodegradability of the different wastewaters (Hernández et al. 2013, 2016). Moreover, the symbiotic relationship between bacteria and microalgae has resulted in an efficient bioremediation of oil spills in marine environments or in the increase water quality in aquaculture hatcheries (Paniagua-Michel 2017). Even though heterotrophic activity has been traditionally associated to bacteria, the occurrence of a mixotrophic algal metabolism and a key role of microalgae during organic matter removal have been recently highlighted (Olguín et al. 2015).
2.1.3 Pathogen Removal During Wastewater Treatment by Microalgae-Bacteria Consortia
Wastewaters often contain a variety of microorganisms such as Escherichia coli, which can potentially contribute to disease transmission. In fact, the absence of E. coli and faecal coliforms after wastewater treatment is included as an indicator parameter for effluent discharge to public water bodies. Pathogen removal in microalgae-bacteria systems is mainly determined by dissolved oxygen (DO) and pH in the culture broth (Posadas et al. 2015, 2018). The photosynthetic activity of microalgae results in an increase in the DO and pH of the cultivation broth. High temperature and pH reduce pathogen survival, while high DO concentrations promoted photo-oxidative damage of cells, resulting in pathogen removal. Moreover, sunlight may inactivate bacteria cells due to both the UV-B radiation, which causes damage on the bacterial DNA structure, and the UV-A radiation, which results in the damage of cell organelles (Al-Geethi et al. 2017), boosting pathogen removal. Finally, the excretion of inhibitory metabolites by microalgae to compete with bacteria also contributes to pathogen removal. For example, Mezrioui et al. (1994) reported high removal efficiency of Vibrio cholerae due to the toxic products secreted by Chlorella sp. when treating domestic wastewater. Although pathogen removal during wastewater treatment by microalgae-bacteria systems has been widely reported in literature, there is little information about the removal mechanisms and the survival of pathogens such as viruses or intestinal parasites.
2.1.4 Heavy Metals and Organic Pollutant Removal During Wastewater Treatment by Microalgae-Bacteria Consortia
During wastewater treatment by microalgae-bacteria consortia, heavy metals are removed from wastewater, being assimilated into the biomass. However, the efficiency of this process is determined by the wastewater characteristics, since the activity of microorganisms can be diminished due to the presence of certain heavy metals. Bacteria are often much more tolerant to these toxic compounds than microalgae, which are severely inhibited in the presence of a few milligrammes per litre of toxicants. More specifically, heavy metals in wastewater may inhibit photosynthesis of microalgae, since metals are able to substitute the metal atoms in the prosthetic groups for specific photosynthetic enzymes.
Microalgae and bacteria are able to accumulate heavy metals from polluted effluents, being a cost-effective and sustainable wastewater treatment alternative to traditional methods (Paniagua-Michel 2017). The main heavy metals in wastewater are Cu, Cd, Cr, Hg, Zn, Pb and Ni. The principle of metal removal from wastewater is mainly based on the relationship between heavy metals and negatively charged groups contained in the carbohydrates and exopolysaccharides of the bacteria and microalgae cell surface (Subashchandrabose et al. 2011). The microorganisms carry out these removal mechanisms as a response of the presence of heavy metals in their growth media (i.e. wastewater). Different mechanisms including adsorption, ion exchange, covalent bonding or heavy metal precipitation have been reported for heavy metal removal in wastewaters (Ozturk et al. 2014; Chojnacka et al. 2005; Posadas et al. 2018). Simultaneously, those metals are bioaccumulated (i.e. bioabsorpted) in cell vacuoles, by a metabolically active biological process of diffusion (Pereira et al. 2013; González et al. 2017). In this way, several commercial biofilms have been developed based on the microalgae capacity for accumulating heavy metals. Some examples are (1) ALGASORB™, produced by Bio-Recovery System, Inc. (USA). It consists in Chlorella vulgaris immobilized in silica gel polymer matrix. It can be used for a wide range of heavy metal concentrations (1–100 mg g−1) and (2) BV-SORBEX™, produced by BV Sorbex, Inc. (Canada). This adsorbent contains Sphaerotilus natans, Ascophyllum nodosum, Halimeda opuntia, Palmyra pomata, Chondrus crispus and Chlorella vulgaris, and it is able to recover up to 99% of metal in the solution.
2.2 Nutrient Recovery in the Form of Valuable Biomass
Microalgae need a great amount of nutrients (N and P) to successfully grow. According to Oswald (1988), microalgal biomass is composed by CO0.48H1.83N0.11P0.01. Thus, N and P are essential elements for their growth. The use of commercial fertilizers as nutrient source for microalgae growth would lead to an increase in cost production, making food market unstable (Chisti 2008). In this context, the use of agro-industrial wastewaters, rich in nutrients, is an interesting alternative as nutrient source to produce microalgal biomass according to life-cycle analysis studies (Christenson and Sims 2011). Removal efficiency is mainly related to microalgal productivity; thus, the higher amount of N and P removed from the wastewater, the higher biomass productivity. This biomass has been proposed for different applications like biofuel production, biofertilizer and feed additive in the commercial rearing of many aquatic animals, both freshwater and marine (Mata et al. 2010; Larrán et al. 2017; Tomás-Almenar et al. 2017).
2.2.1 Nitrogen Recovery
Nitrogen has a key role in amino acids, nucleic acids and pigment synthesis (Richmond 2008). During the last years, many studies have evidenced that when using microalgal-based systems, the assimilation of N is the main mechanism to remove this nutrient from wastewater. Other mechanisms, such as ammonia stripping or denitrification, have less importance in the nitrogen balance in microalgal-based wastewater systems (Cai et al. 2013). The preferred inorganic nitrogen source for microalgae is NH4+, although they are also able to use NO3− and, in a lesser extent, NO2− (Jia and Yuan 2016). The assimilation processes need active transport to incorporate N forms to the cell, but since a reduction to N3− state is required before assimilation, the energetic cost for NO3− and NO2− assimilation is higher than the one for NH4+ (Cai et al. 2013). The assimilation of inorganic nitrogen is governed by the equation represented in Fig. 20.1, where “Fd” corresponds to the enzyme ferredoxin (Richmond 2008).
The presence of bacteria in the wastewater presents several advantages for nitrogen assimilation by microalgae. When microalgae are used for agro-industrial wastewater bioremediation, aerobic bacteria oxidize proteins and nucleic acids to NH4+, which is assimilated by microalgae. However, when NH4+ concentration is higher than 100 mg/L and pH is higher than 8, the proportion of NH4+ that turns to NH3 could result toxic for algae inhibiting their growth (Park and Craggs 2010). Moreover, under these conditions, an important part of NH3 could volatilize to the atmosphere, diminishing nitrogen recovery. On the other hand, nitrifying bacteria helps to avoid NH3 stripping and therefore contribute to mitigate nitrogen losses by volatilization. Nitrifying bacteria comprise ammonium-oxidizing bacteria (AOB) and nonoxidizing bacteria (NOB) (Eqs. 20.5, 20.6 and 20.7). They grow slower than microalgae and aerobic bacteria present in the wastewater; thus, they require higher HRT than typical operational conditions in microalgal-based systems, which usually ranges between 2 and 10 days. According to de Godos et al. (2014), when the hydraulic retention time (HRT) ranges from 2 to 10 days, most AOB and NOB are washed out, and the nitrification process does not take place. In order to enhance AOB and NOB growth, and help aerobic bacteria to avoid NH3 stripping, microalgae-bacteria sludge retention time should be controlled and the settled biomass continuously pumped to the HRAP to increase retention time, allowing to grow and to nitrify to the AOB and NOB organisms, so that nitrification process only occurs when hydraulic retention time (HRT) is higher than 10 days or when settled biomass is recirculated into the system (de Godos et al. 2014).
Finally, the activity of denitrification bacteria (Eq. 20.8) is avoided by the presence of oxygen in the media, since it is an anoxic process. The concentration of dissolved oxygen is usually higher than 1 mg/L, as microalgae release oxygen during photosynthesis. In this way, nitrogen recovery by microalgae is favoured.
2.2.2 Phosphorus Recovery
Phosphorus is an essential element for microalgae, necessary for metabolic activities, energy transfer and phospholipid and DNA synthesis (Richmond 2008). In conventional wastewater treatment plants, P is chemically removed through precipitation. In microalgal-based systems, P removal occurs simultaneously to nitrogen assimilation, so that P is recovered within the biomass. Moreover, under specific operational conditions, microalgae can be induced to further accumulate polyphosphates inside the cell structure independently of the biomass productivity. Thus, when microalgae are exposed to “P excess – P starvation – P excess” conditions or under certain light supply and temperature conditions, the accumulation of polyphosphates inside the cell (luxury uptake) allows high P removal efficiencies (Brown and Shilton 2014). On the other hand, microalgae growth under P limitation results in a build-up of carbohydrates and/or lipids. The accumulation of one or another macromolecular compound depends more on the microalgae species than on the operational conditions. Most species including Chlorella, Scenedesmus, Chlamydomonas or Spirulina accumulate lipids inside the cell (Wang et al. 2010). Carbohydrate accumulation is frequent in Spirogyra, Ulva, Gelidium, Laminaria or Saccharina, among others (Buck and Buchholz 2004; Kraan 2013).
2.2.3 Nutrient Uptake Efficiencies for Different Wastewaters
Nitrogen uptake rates found in literature vary from 0.1 to 65 mg of total nitrogen per litre of photobioreactor per day for different microalgae like chlorophyte (mainly composed of Chlorella and Scenedesmus), cyanobacteria (Arthrospira and Oscillatoria), diatom and haptophyte (Cai et al. 2013). Phosphorus uptake rates can reach up to 40 g of soluble P per kg of produced biomass under luxury uptake. However, the general demand of P is in the range of 10–15 g per kg of microalgae (Powell et al. 2009). The optimization of the configuration of the reactors and the operational conditions result essential to maximize nutrient recovery during wastewater treatment by microalgae-bacteria consortia. In this manner, diverse studies have reported final biomass with very variable concentrations ranging, 0.11–0.70 g/L and 5.5–35 g/m2 day.
2.3 Energy Savings
Microalgae biomass is often associated with the production of feedstock for biofuel production or high added-value products, and therefore, their energy consumption and production costs are associated with these processes. There are few works exploring and assessing microalgae energy consumption and production costs from a global point of view; this means considering the sum of energy used for cultivation, harvesting and drying. In the same way, the data for LCA (life-cycle assessment) studies must be extrapolated from laboratory-scale systems, as no industrial-scale process (for biofuel production) exists yet (Slade and Bauen 2013). Some other studies pointed out that energy production (i.e. biofuels production) from microalgal biomass will only be commercially feasible if it is coupled with an algae-based wastewater treatment system (Mulbry et al. 2008; Gouveia 2011), which is related to input energy cost. Therefore, recycling of N and P from wastewater will provide some of the nutrients for microalgae growth in combination with wastewater remediation, hence contributing to reduce energy cost.
Microalgae-based system feasibility is highly related to its energy demand, and it is mostly focused on biomass production for its further valorization. According to Slade and Bauen (2013), in raceway ponds, the most important contributions to the energy demand come from the electricity, required to circulate the culture, and the embodied energy in pond construction, as well as the energy embodied in the nitrogen fertilizer. However, microalgae-based systems for wastewater remediation can compete with activated sludge processes, as energy demand for these systems is approximately 500 Wh per m3 of wastewater treated and the energy required for mixing conventional HRAPs ranges from 1.5 to 8 Wh per m3 (Mendoza et al. 2013). In these order to evaluate energy demand of microalgae-based systems for wastewater treatment, empirical data of the performance of these specific systems is necessary.
2.4 CO2 Emission Reduction
Current global warming has triggered international awareness concerning greenhouse gas emissions. Greenhouse gas emissions were 5.25 × 107 kt CO2eq/year in 2014 (FAO 2014). Different techniques have been studied for CO2 capture, which may be divided in geological sequestration, chemical processing or absorption and bioprocessing from photosynthetic organisms (Morrissey and Justus 2000; Chisti 2007). The geological sequestration consists of the storage of liquid or gaseous CO2 underground in a geological formation or in deep ocean storages (Lal 2004). There is a major concern about the possibility of the escape of huge amounts of CO2 towards surface waters that can lead to their acidification and the subsequent disturbance to aquatic ecosystems. Chemical processing/absorption processes require alkaline reagents, which are energetic intensive and expensive. Meanwhile, natural bioprocesses remove close to 50% of anthropogenic CO2 emissions per year from atmosphere (Benemann 1993). Moreover, photosynthetic organisms have a double-positive environmental impact as they capture high amounts of inorganic carbon and release O2 to the atmosphere. Microalgae present several advantages compared with other photosynthetic organisms for carbon capture (i.e. fixation), namely, (1) they are able to grow tenfold higher than terrestrial plants; (2) their growth is independent from arable lands, attenuating thus food and feed competition; and (3) they may not require nutrient supplementation when they grow in agro-industrial wastewaters, especially those rich in N and P (Rittman 2008; Stephens et al. 2010). Moreover, photosynthesis conversion efficiency in microalgal cells is remarkably higher than in superior plants due to the absence of structures, differentiation and lining structures, among others. When agro-industrial wastewater is treated using algae-bacteria consortia, microalgae capture the CO2 produced after organic matter degradation by aerobic bacteria, thus reducing CO2 emissions when compared with other aerobic wastewater treatments. The resulting biomass may also be valorized in the form of a carbon neutral renewable fuel like biodiesel, bioethanol or biogas (Chisti 2008; Hernández et al. 2015, 2016). Hence, the use of photosynthetic biomass for energy production would diminish the dependence of oil-based fuels.
Carbon capture with microalgae has been widely studied during the last decades (Benemann 1993; Aresta et al. 2005; Park and Craggs. 2010). During the photosynthesis process, algae capture high amounts of CO2 to produce organic molecules. When microalgae productivity is high, the lack of CO2 triggers carbon sequestration from atmosphere to the water, and O2 is released to the atmosphere. In this manner, microalgal biomass culture shows to be a suitable tool to capture carbon, fixing it in the form of valuable biomass. However, when light supply is scarce, an important amount of mixotrophic microalgae turns from autotrophic to heterotrophic metabolism. Microalgae are thus able to oxidize organic matter from wastewater releasing CO2 that may be subsequently captured by photosynthetic algae. In this vein, although photosynthesis is more efficient from a carbon capture point of view, when heterotrophic behaviour occurs, most of the carbon is still captured in the form of valuable algal biomass.
Emissions of CO2 in a microalgae-bacteria wastewater treatment plant are remarkably lower than those produced in conventional aerated activated sludge processes, and a negative balance can also occur when flue gas rich in CO2 is injected into the process. Thus, from the CO2 emissions point of view, the use of microalgal-based systems for wastewater treatment is always a better alternative than conventional active sludge treatment, since microalgae assimilate 1.8 tonnes of CO2 per ton of algal biomass, while in activated sludge treatments, all the sludge produced must be managed and may not be valorized. When industrial CO2-enriched air is efficiently injected to the photobioreactor and captured by microalgae, biomass productivity remarkably increases. The injection of CO2 leads to a decrease in culture medium pH, and therefore, injection rate must be controlled in the ponds. Scientific literature has already presented many different optimal CO2 concentrations and injection rates to maximize biomass production, but differences between them are high due to the great heterogeneity of microalgal species, culture mediums used, types of photobioreactors employed and operational conditions tested (light intensity, salinity and temperature). In this manner, it should be kept in mind that CO2 feed rate must be optimized to the previously selected algae and to the operational conditions. The optimization of these parameters will maximize biomass production and thus carbon sequestration.
3 Challenges of Microalgae for Wastewater Treatment
3.1 Land Use
Most studies indicate that replacing conventional activated sludge wastewater treatments by high-rate algal ponds (HRAP) with microalgae would reduce GHG emissions and operational costs. However, the installation of these ponds would require enormous land areas, and the impacts derived of the land use change (LUC) should be addressed, due to its potential environmental damage and the changes in soil carbon stocks. In most of the cases, LUC and suitability of land close to the wastewater producers are not taking into account for the studies of wastewater treatment by HRAP (Searchinger et al. 2008). In fact, many studies indicate that nonarable or marginal lands may be used to place enormous HRAP. As an example, a recent study comparing the potential land competition between microalgae and terrestrial feedstock production in the USA reported that there is little conflict with each other. In this way, microalgae production in HRAP in the USA could be located in areas outside the key agricultural-producing regions (Langholtz et al. 2016). However, the majority of the wastewater is produced close to urban centres where the price of land is expensive and wastewater transport to a further place is not an option, due to high costs.
Furthermore, if most of wastewater is treated using microalgae, most of the existing biomass in the landscape would be removed, including grasslands, croplands and forestlands. The impacts that are considered for life-cycle analyses during the installation of the ponds of microalgae systems are (1) loss of soil carbon, (2) removal of carbon below the original vegetation, (3) change in the surface albedo and (4) change in GHG emissions from the original vegetation in comparison to the microalgae ponds (Fortier et al. 2017). However, there is little information concerning these impacts and therefore the GHG emissions related to LUC in large-scale microalgae cultivation. Hander et al. (2017) reported that forests and grasslands/croplands also forego ongoing carbon sequestration and a significant CO2 penalty is related to LUC in microalgae cultivation for energy production. More specifically, this penalty is estimated in 4–8 and over 40 g CO2eq/MJ for grassland/croplands and forest, respectively. Moreover, terrestrial feedstock and microalgae production may compete for the same land. In this context, the LUC only results convenient when it increases carbon capture compared to conventional use. Hence, carbon sequestration accounting must reflect the net impact on the carbon benefit and not only the assimilation by microalgae. In this vein, many studies have failed in accounting emissions related to LUC. However, to substitute conventional treatments to implement large-scale HRAP for wastewater treatments, a deep economic and environmental impact is required, even with generous government subsides.
Previous studies have shown that LUC may result in enormous differences between different locations (e.g. Everglades, Florida and Tamaulipas, Mexico), despite these locations were selected due to their similar irradiance and temperature during the year (Fortier et al. 2017). Hence, in order to evaluate this impact, an analysis focused in each particular place must be previously performed. There is no doubt that a good progress in wastewater treatment with microalgae-bacteria using pilot-scale HRAP has been made during the last years (Park and Craggs 2010; Hernández et al. 2016). There is also a better understanding by the industry of the need to use a more sustainable and eco-friendly technology, and at the same time, the resulting biomass may result an income source. However, higher effort must be made to determine the economic and the GHG emission impact due to the LUC in order to obtain a deeper view of this key aspect.
3.2 Culture Conditions: Influence of Wastewater Characteristics
The characteristics of wastewater highly determine the efficiency of microalgal-based systems for wastewater treatment. These characteristics include pollutant and nutrient concentration, turbidity, colour and wastewater pH.
3.2.1 Pollutant and Nutrient Concentration
The ratio C:N:P in wastewater is an important factor affecting the overall efficiency of the system. In Table 20.3, a summary of the general characterization of municipal and industrial wastewater is presented. The lack of some essential nutrients or their low bioavailability in some types of wastewaters could negatively affect the performance of microalgal-based systems in terms of pollutant removal efficiencies and biomass production (Posadas et al. 2013; Markou et al. 2014). In addition, some compounds present in wastewater or produced during its treatment could cause inhibition in microalgal activity. The most usual toxic compound during wastewater treatment is NH3, whose concentration concomitantly rises when increasing the pH values in the photobioreactor because of photosynthesis. Ammonia concentration that results inhibitory is species dependent. Free ammonia concentrations of up to 51 g/m3 have been proved to diminish photosynthetic rates by 90% in dense culture of the microalgal species Scenedesmus obliquus, Phaeodactylum tricornutum and Dunaliella tertiolecta (Azov and Goldman 1982; Sutherland et al. 2015). Ammonia toxicity should be taken into consideration when treating wastewater with high nitrogen concentration such as livestock wastes or anaerobically digested agro-industrial effluents. For this type of wastewaters, a strategy to avoid microalgal inhibition, such as pH control, a previous dilution step or the operation at low loading rates, is required (Markou et al. 2014). Toxic effects of nitrite, an intermediate product from the oxidation of ammonium to nitrate, have been also reported at high concentrations (Markou et al. 2014). In addition, some heavy metal ions that can be present in industrial wastewater affect the survival of specific microalgal strains (Zeraatkar et al. 2016). The presence of organic acids, phenols and pesticides also decreases microalgal activity (Guldhe et al. 2017). Nevertheless, some microalgae species such as Chlorella, Nitzschia and Chlamydomonas present a high tolerance to pollutant concentration, which can explain its predominance in microalgal-based wastewater treatment systems (Muñoz and Guieysse 2006; de Godos et al. 2009).
3.2.2 Turbidity, Colour and pH
Light availability is a major factor affecting microalgal performance and, therefore, the overall wastewater treatment efficiency. In this sense, both industrial and domestic wastewaters usually present high concentrations of suspended solids that result in wastewater turbidity. These solids, together with the presence of coloured dissolved compounds in wastewaters, can absorb light and reduce its penetration in culture broth. Consequently, biomass production in microalgal-based systems could diminish. A physical-chemical pretreatment of the wastewater before feeding the photobioreactors would decrease turbidity and the concentration of coloured compounds. Dilution of feeding stream is also required in some cases for highly polluted wastewaters. Nevertheless, this issue might not be very important if the wastewater contains organic carbon and the microalgae species can grow in heterotrophic or mixotrophic mode (Markou et al. 2014). Finally, the pH of wastewater highly influences on the microalgae-bacteria systems. In this way, wastewaters that present a pH outside of the optimal range for their treatment in microalgal-based systems are hardly biodegraded without any pretreatment (Posadas et al. 2018).
3.3 Culture Conditions: Environmental and Operational Aspects
The efficiency of microalgal-based wastewater treatment will depend on a combination of factors including environmental and operational conditions. In this section, the main environmental and operational aspects affecting the performance of microalgal systems are briefly described. Temperature, light availability, pH, concentration of O2 and CO2 in the culture medium and hydraulic retention time (HRT) are described as the main factors affecting microalgal-based systems.
3.3.1 Temperature
The effect of temperature on microalgae growth is well known. Increasing temperature enhances microalgal growth until achieving an optimum value. Once this optimum value is reached, biomass productivity dramatically declines with the increment in temperature (Larsdotter 2006). Although the optimum value of temperature for microalgal growth is genera and strain dependent, it usually lies between 20 and 30 °C (Umamaheswari and Shanthakumar 2016; de Godos et al. 2017). Operating at favourable temperature conditions led to greater nutrient removal efficiencies as well as higher biomass productivities (Molinuevo-Salces et al. 2016). Under lower temperatures, metabolic rates of microalgae diminish, attaining lower growth rates and diminishing nutrient removal efficiency. As an example, Cho et al. (2015) reported a biomass productivity reduction of approximately tenfold when treating raw municipal wastewater, from summer (temperatures up to 30 °C) to winter (temperatures near 5 °C). On the contrary, higher temperatures above the optimum range can cause oxidative stress and a decrease of photosynthetic activity (Posadas et al. 2018). Reactor overheating is thus a problem, especially in humid climates where evaporation is inhibited (Larsdotter 2006). Supplying cooling water on the surface of the reactor and the regulation of air temperature by refrigerated air conditions units are two of the strategies that can be applied to prevent overheating (Lavens and Sorgeloos 1996; Umamaheswari and Shantakumar 2016), but both alternatives significantly increase operational costs.
3.3.2 Light Availability
In a similar way as described for temperature, a positive correlation between light availability and biomass productivity occurs. Microalgae growth increases when increasing light intensity until reaching an optimum value. Above this optimum value, too much light may decrease photosynthesis rate (Park et al. 2011). Photosynthetic activity is saturated at relatively low irradiances ranging from 100 to 200 μE/m2 day (Acien et al. 2017). Since solar radiation is several times higher than this saturation level, an excess of solar radiation can lead to microalgal photoinhibition. A strategy to overcome the influence of the excess of irradiance is to operate under higher culture densities and a proper mixing regime (de Godos et al. 2017). In fact, mixing is one of the most important parameters during microalgal-based system operation, providing turbulence and a degree of vertical mixing through the pond depth that ensures that microalgae are intermittently exposed to light (Park et al. 2011; Posadas et al. 2018). Strong mixing could result in shear stress and in cell rupture (mainly in cyanobacteria), negatively affecting microalgae growth.
3.3.3 pH
Because of microalgal photosynthesis and respiration, pH oscillates over the day in microalgal-based systems (Sutherland et al. 2015), as seen in Fig. 20.2. pH values above 9 can be commonly achieved in photobioreactors, especially when operating at low organic and nutrient loading rates and at favourable conditions (Riaño et al. 2012; Hernández et al. 2016). Most microalgae usually tolerate wide pH intervals, but out of this interval, the growth is greatly reduced. More specifically, pH values higher than 9 negatively limit microalgal activity, since the capacity to absorb CO2 is dramatically reduced and the cell’s ability to maintain the activity of the RuBisCO enzyme is interfered (Sutherland et al. 2015). Moreover, high pH in the culture broth also causes the dissociation of NH4+ ion, increasing free NH3 concentration that can significantly inhibit microalgal growth and increase nitrogen losses by volatilization. The pH control allows reducing NH3 volatilization and enabling greater nitrogen recovery into microalgae-bacteria biomass. Injection of CO2 into the culture broth is the most common strategy to reduce pond culture pH; however, nowadays few studies have evaluated the performance of wastewater supplied by CO2 addition at semi-industrial or industrial scale.
3.3.4 O2 Concentration
The evolution of dissolved oxygen concentration in photobioreactors is characterized by an increase during the day and a decline during the night, according to the cycle of photosynthesis and respiration of microalgae (Fig. 20.2). Although more research is required regarding toxicity of O2 for microalgae, O2 concentrations above 20 mg/L are believed to negatively affect microalgae growth, favouring photorespiration and O2 radical formation and causing thus partial inhibition of photosynthesis (de Godos et al. 2017). Bacteria activity such as the organic matter oxidation and the nitrification during wastewater treatment involves a decrease in the oxygen concentration, preventing microalgae inhibition. For instance, reported O2 concentrations in microalgal-based systems for several agro-industrial wastewater treatments were lower than 14 mg/L (Riaño et al. 2011; Hernández et al. 2013, 2016). Nevertheless, CO2-enriched air supply has been again defined as a strategy for degassing the culture broth in high-rate algal ponds to enhance nutrient assimilation while decreasing the dissolved oxygen concentrations (de Godos et al. 2017).
3.3.5 CO2 Concentration
During microalgal-based wastewater treatment, non-photosynthetic microorganisms produce the CO2 needed for microalgae growth during organic matter degradation (Molinuevo-Salces et al. 2010). However, C:N ratios in most agro-industrial wastewaters are lower than the optimal reported ratio of 100:18 (Posadas et al. 2018). These lower C:N ratios are correlated with lower biodegradability, resulting in a reduction of removal efficiencies and biomass production (Posadas et al. 2014). The injection of inorganic carbon (via flue gas or biogas resulting from anaerobic digestion) has been proposed as an alternative to enhance wastewater treatment, preventing CO2 competition between the autotrophic communities present in the culture broth (Alcántara et al. 2015) and also a change from autotrophic to heterotrophic metabolism that will suppose a decrease on productivity. Nevertheless, it is worth mentioning that the presence of volatile fatty acids in wastewater can be also used as carbon source in mixotrophic cultures (Olguín et al. 2015).
3.3.6 Hydraulic Retention Time (HRT)
HRT (defined as the volume of the photobioreactor divided by the flow rate) is a key design parameter that affects the performance of microalgal-based systems for wastewater treatment. It has been already demonstrated that organic and inorganic contaminant removal from wastewaters in natural treatment systems increases at longer HRTs due to the enhancement of biodegradation, photodegradation and sorption processes (Matamoros et al. 2015). The operation at longer or shorter HRTs also allows or prevents biomass accumulation (Sutherland et al. 2015). Most typical values of HRTs are in the range between 2 and 10 days (Muñoz and Guieysse 2006; Posadas et al. 2018). Longer HRTs are required in colder seasons due to the decrease in the metabolic activity and to the low microalgal growth rates.
3.4 Biomass Harvesting and Valorization
3.4.1 Biomass Harvesting
Biomass harvesting is one of the major challenges for large-scale production of microalgae in wastewater treatment systems, accounting for 20–30% of the total operational costs (Molina-Grima et al. 2003; Vandamme et al. 2013). For most of the microalgae species (exception made for Spirulina, due to its filamentous nature), biomass is suspended in water due to the small size of microalgae cells (5–20 μm) coupled to their colloidal stability in suspension. In this way, biomass concentrations in the range of 0.5 g microalgae/L have been reported for HRAPs, which are mostly used to treat wastewater (Benemann 1993). Biomass harvesting consists of separating microalgae biomass from water. The low biomass content needs to be concentrated to final values of 150–250 g microalgae/L. Prior to the final concentration values (i.e. biomass thickening), a harvesting step, where initial biomass concentration is increased to 10–50 g microalgae/L, is usually carried out (Muylaert et al. 2018). Since microalgae are a very heterogeneous group, the harvesting process should be adjusted to the microalgae species and the culture conditions (both wastewater characteristics and operational parameters). Moreover, the choice of the harvesting technology is also determined by the valorization strategy of the final biomass, which should not compromise both biomass and final effluent qualities (Muylaert et al. 2018). In this way, low-cost technologies are preferred for microalgae harvesting in the case of biomass obtained from wastewater systems.
Table 20.4 shows the main advantages and disadvantages of different microalgae biomass harvesting methods. These methods comprise sedimentation, flotation, centrifugation, filtration, coagulation-flocculation as well as several combinations of them. Sedimentation is easy to perform; it requires low-cost equipment and low-energy demand. For instance, for harvesting a biomass concentration of 1–15 g dry microalgae/L, the sedimentation process requires 0.1 kWh/m3 using lamella separators (Milledge and Heaven 2013). It is a slow process (retention times of 1–2 days) that can lead to some deterioration of the biomass. It may be a good harvesting alternative for filamentous microalgae as Spirulina or for algae-forming aggregates, like Scenedesmus. In the case of wastewater treatment, biomass sedimentation is favoured by the high amount of bacteria and nutrients in the media (Alam et al. 2017). This technology usually needs to be combined with another technology (e.g. coagulation-flocculation) to further concentrate the biomass. Opposite to sedimentation, when using flotation as harvesting technology, microalgae are collected from the surface of the tank. Air is bubbled into the microalgae solution, so that the bubbles are attached to the microalgae cells and both move to the surface. Flotation is a relatively fast separation method where biomass is not damaged. However, the aeration sometimes results in high-energy costs, and the interaction between cells and bubbles is not always good. Similar to sedimentation, chemical addition is often needed to increase flotation efficiency. In this way, it has been seen that the addition of surfactants; the use of ozone as gas carrier, instead of air; or the combined flotation-flocculation method may achieve promising results for microalgae biomass recovery in the floating cakes. However, the economic feasibility of flotation is still a challenge. The increase in the biomass recovery yield depends either on an expensive coagulant or on high-energy requirements (in the case of electrical-based systems), increasing in both cases the operational costs (Laamanen et al. 2016).
On the other hand, centrifugation is a fast and easy technology that produces a high-quality thick biomass paste. A microalgae biomass concentration up to 250 g TSS/L can be achieved in a single step. It is currently the most used technology both for harvesting of high-value biomass and as a second step for thickening of low-cost biomass. However, the high-energy consumption (50–75 kWh) (Milledge and Heaven 2013) coupled with the high investment costs still makes this technology economically unfeasible as a single step for harvesting microalgae biomass for low-cost purposes (low-cost biomass), which is the case of biomass obtained from wastewater treatment. In the case of filtration, water and microalgae biomass are separated by means of a selectively permeable medium. The high concentration efficiency together with the no need of chemical addition makes this technology an interesting option. However, some drawbacks such as membrane or filters clogging or high investment cost for membranes have been reported. In general, filtration results an economically unfeasible method for harvesting low-cost microalgae biomass. However, filtration by means of low-cost nylon screens has been demonstrated as an effective method for harvesting large-sized microalgae such as Spirulina (Toyoshima et al. 2015). Finally, by coagulation-flocculation, single cells are aggregated to form flocs, which are easy to harvest. It has been highly used due to the low-cost and high separation efficiency (Alam et al. 2017). Microalgae cells are negatively charged, and chemical addition to neutralize those charges is often required. Different chemicals have been successfully tested for microalgae harvesting, including metal salts, inorganic polymers and biopolymers. The high cost of the chemicals together with a possible decrease in the quality of the harvested biomass is the main drawback for not scaling up this technology. On the contrary, electrocoagulation and bio-flocculation are two of the most economically feasible methods for microalgae harvesting, due to the high quality of the resultant biomass. Bio-flocculation refers to a spontaneous microalgae flocculation due to the action of other microorganisms such as bacteria or fungi. In the case of wastewater treatment by microalgae, bio-flocculation is a promising alternative since it spontaneously occurs quite often. In some cases, auto-flocculation can be achieved when pH increases in the culture media. This is due to the precipitation of Ca and P salts, which act as flocculants and can be naturally present in wastewater. This strategy involves some advantages since these salts are not as toxic as metal salts. The separation efficiency of both bio-flocculation and auto-flocculation is strongly dependent on the media composition and the microalgae species, so that they cannot be applied to any kind of wastewater.
The development of a low-cost method for microalgae harvesting is still a challenge. According to recent studies (Barros et al. 2015; Alam et al. 2017; Muylaert et al. 2018), a two-stage process would be necessary to concentrate microalgae biomass. Flocculation followed by sedimentation is proposed, being bio-flocculation the best alternative, since it would reduce chemical costs while not compromising biomass quality. Although there is a wide variety of investigations at lab-scale reporting promising results, more large-scale studies are required in order to demonstrate the different harvesting technologies proposed. Finally, the harvesting method should be adapted to microalgae species and to the final use of the obtained biomass.
3.4.2 Biomass Valorization
Microalgae biomass can be used for different applications. These applications include energy production (i.e. biofuels), products for agriculture (biofertilizers, biopesticides etc.), animal feed and products for human consumption (foods and pharmaceuticals). When biomass is obtained as a by-product after wastewater treatment, low-cost applications are preferred, since only microalgae recognized as safe (GRAS) can be sold for human consumption. Therefore, the most common uses are energy, biofertilizer and animal food production (Acien et al. 2017).
The use of microalgae as feedstock for biofuels production, such as biodiesel and bioethanol, presents several advantages over other biomass feedstock such as corn or vegetable oil, but it is noteworthy that microalgae production, harvesting and conversion into biofuels are expensive, compromising the economic feasibility of the process. However, a recent bioeconomy study about biofuels production from microalgae biomass reported promising results. More specifically, positive net present values (NPV) were achieved both for economy studies for ethanol and for biodiesel production, meaning that these technologies are worth to invest in (Peng et al. 2018). In the case of biogas production, anaerobic hydrolysis of microalgae appears to be the limiting step to reach an economically feasible process. A deeper knowledge of anaerobic digestion, hydrolytic bacteria and microalgae cell wall composition are needed to overcome that bottleneck and increase methane yields (González-Fernández et al. 2015). Microalgae grown on wastewater present an enormous potential as biofertilizer. The ability of microalgae to uptake nutrients such as C, N and P from wastewater results in an enhancement of nutrient availability for plant systems. Microalgae present a chemical composition, including macronutrients such as N, P and K and micronutrients as Ca+, better than available organic fertilizers (Mahapatra et al. 2018). Even though microalgae are a source of proteins, lipids and carbohydrates, there are few studies regarding microalgae supplementation to animal diets. Microalgae biomass grown in wastewater is generally characterized by high-protein contents, so that a possible valorization way is its use as protein source for animal feed. For instance, microalgae were included in rainbow trout diets in percentages of 12.5%, 25% and 50%. The results evidenced that an inclusion higher than 12.5% resulted in nutritional deficiencies in trout (Dallaire et al. 2007).
4 Conclusions
The use of microalgae-based systems for wastewater treatment has been widely studied due to the enormous potential of this technology as an alternative to traditional wastewater treatment systems. This potential comprises the high bioremediation efficiency for a variety of wastewaters, the contribution to CO2 emission reduction and the remarkable energy savings (including the potential valorization of the produced biomass as energy). However, there are still some challenges to overcome before a real implementation of this technology. These issues include land use competition, wastewater variability, the influence of environmental conditions and high-cost biomass harvesting. Although there is little conflict for land competition between microalgae and terrestrial feedstock, GHG emissions related to land change use should be addressed when installing open ponds for large-scale microalgae cultivation. Secondly, wastewater characterization is of major importance for an efficient treatment. The lack of essential nutrients, the presence of toxic compounds or the low bioavailability in some wastewaters could negatively affect the performance of microalgal-based systems. Moreover, the proposed strategies as wastewater dilution to ensure light availability or the pretreatments to control wastewater pH are energy demanding, so that an economic study with different scenarios for each specific case should be carried out before large-scale implementation. In the third place, environmental and operational aspects as temperature, light availability, pH, O2 and CO2 concentration in the culture medium and hydraulic retention time (HRT) determine microalgae productivity and, consequently, wastewater treatment efficiency. For instance, pH control could be done by coupling a CO2 emission source with a microalgae-based wastewater treatment facility. Finally, low-cost technologies are preferred for microalgae harvesting in the case of biomass obtained from wastewater streams as culture media. A two-stage process would be necessary to concentrate microalgae biomass. Flocculation followed by sedimentation is proposed, being bio-flocculation the best alternative, since it would reduce chemical costs while not compromising biomass quality. The harvesting method should be adapted to microalgae species and to the final use of the obtained biomass, being energy, biofertilizer and animal food production the most common uses.
References
Acien FG, Fernández-Sevilla JM, Molina-Grima E. Microalgae: the basis of making sustainability. In: Case study of innovative projects – successful real cases. 2017. https://doi.org/10.5772/67930.
Adav SS, Lee DJ, Show KY, Tay JH. Aerobic granular sludge: recent advances. Biotechnol Adv. 2008;26:411–23.
Alam MA, Wang Z, Yuan Z. Generation and harvesting of microalgae biomass for biofuel production. In: Prospects and challenges in algal biotechnology. Singapore: Springer; 2017. p. 89–111.
Alcántara C, Domínguez J, García D, Blanco S, Pérez R, García-Encina PA, Muñoz R. Evaluation of wastewater treatment in a novel anoxic-aerobic algal-bacterial photobioreactor with biomass recycling through carbon and nitrogen mass balances. Bioresour Technol. 2015;191:173–86.
Al-Gheethi AA, Mohamed RM, Jais NM, Efaq AN, Halid AA, Wurochekke AA, Amir-Hashim MK. Influence of pathogenic bacterial activity on growth of Scenedesmus sp. and removal of nutrients from public market wastewater. J Water Health. 2017;15:741–56.
Andersen CB. Understanding carbonate equilibria by measuring alkalinity in experimental and natural systems. J Geosci Educ. 2002;50:389–403.
Aresta M, Dibenedetto A, Barberio G. Utilization of macro-algae for enhanced CO2 fixation and biofuels production: development of a computing software for an LCA study. Fuel Process Technol. 2005;86:1679–93.
Azov Y, Goldman JC. Free ammonia inhibition of algal photosynthesis in intensive culture. Appl Environ Microbiol. 1982;43(4):735–9.
Barros AI, Gonçalves AL, Simões M, Pires JC. Harvesting techniques applied to microalgae: a review. Renew Sust Energ Rev. 2015;41:1489–500.
Benemann JR. Utilization of carbon dioxide from fossil fuel-burning power plants with biological systems. Energy Convers Manag. 1993;34:999–1004.
Boelee NC, Temmink H, Janssen M, Buisman CJN, Wijffels RH. Nitrogen and phosphorus removal from municipal wastewater effluent using microalgal biofilms. Water Res. 2011;45:5925–33.
Brown N, Shilton A. Luxury uptake of phosphorus by microalgae in waste stabilisation ponds: current understanding and future direction. Rev Environ Sci Biotechnol. 2014;13:321–8.
Buck BH, Buchholz CM. The offshore-ring: a new system design for the open ocean aquaculture of macroalgae. J Appl Phycol. 2004;16:355–68.
Cai T, Park SY, Li Y. Nutrient recovery from wastewater streams by microalgae: status and prospects. Renew Sust Energ Rev. 2013;19:360–9.
Chinnasamy S, Bhatnagar A, Hunt RW, Das KC. Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol. 2010;101:3097–105.
Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25:294–306.
Chisti Y. Biodiesel from microalgae beats bioethanol. Trends Biotechnol. 2008;26:126–31.
Cho DH, Ramanan R, Heo J, Kang Z, Kim BH, Oh HM, Kim HS. Organic carbon, influent, microbial diversity and biomass in raceways ponds treating raw municipal. Bioresour Technol. 2015;191:481–7.
Chojnacka K, Chojnacki A, Gorecka H. Biosorption of Cr3+, Cd2+ and Cu2+ ions by blue-green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere. 2005;59:75–84.
Christenson L, Sims R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol Adv. 2011;29:686–702.
Dallaire V, Lessard P, Vandenberg G, de la Noü J. Effect of algal incorporation on growth, survival and carcass composition of rainbow trout (Oncorhynchus mykiss) fry. Bioresour Technol. 2007;98(7):1433–9.
De Godos I, Blanco S, García-Encina PA, Becares E, Muñoz R. Long-term operation of high rate algal ponds for the bioremediation of piggery wastewaters at high loading rates. Bioresour Technol. 2009;100:4332–9.
De Godos I, Vargas VA, Guzmán HO, Soto R, García B, García PA, Muñoz R. Assessing carbon and nitrogen removal in a novel anoxic–aerobic cyanobacterial–bacterial photobioreactor configuration with enhanced biomass sedimentation. Water Res. 2014;61:77–85.
De Godos I, Arbid Z, Lara E, Cano R, Muñoz R, Rogalla F. Wastewater treatment in algal systems. In: Innovative wastewater treatment and resource recovery technologies. Impacts on energy, economy and environment. London: IWA Publishing; 2017.
FAO. FAOSTAT online database. 2014. Available at http://faostat.fao.org/.Accessed Mar 2018.
Feng Y, Li C, Zhang D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour Technol. 2011;102:101–5.
Fortier MOP, Roberts GW, Stagg-Williams SM, Sturm BS. Determination of the life cycle climate change impacts of land use and albedo change in algal biofuel production. Algal Res. 2017;28:270–81.
González LE, Cañizares RO, Baena S. Efficiency of ammonia and phosphorus removal from a Colombian agroindustrial wastewater by the microalgae Chlorella vulgaris and Scenedesmus dimorphus. Bioresour Technol. 1997;60:259–62.
González AG, Oleg S, Pokrovsky J, Santana-Casiano M, González-Dávila M. Bioadsorption of heavy metals. In: Prospects and challenges in algal biotechnology. Singapore: Springer; 2017. p. 257–75.
González-Fernández C, Riaño-Irazábal B, Molinuevo-Salces B, García-González MC. Effect of operational conditions of the degradation of organic matter and develop-ment of microalgae-bacteria consortia when treating swine slurry. Appl Microbiol Biotechnol. 2011;90:1147–53.
Gonzalez-Fernandez C, Sialve B, Molinuevo-Salces B. Anaerobic digestion of microalgal biomass: challenges, opportunities and research needs. Bioresour Technol. 2015;198:896–906.
Gouveia L. Microalgae as a feedstock for biofuels. Springer Briefs in Microbiology. 2011. https://doi.org/10.1007/978-3-642-17997-6.
Guldhe A, Kumari S, Ramanna L, Ramsundar P, Singh P, Rawat I, Bux F. Prospects, recent advancements and challenges of different wastewater streams. J Environ Manag. 2017;203:299–315.
Handler RM, Shi R, Shonnard DR. Land use change implications for large-scale cultivation of algae feedstocks in the United States Gulf Coast. J Clean Prod. 2017;153:15–25.
Hernández D, Riaño B, Coca M, García-González MC. Treatment of agro-industrial wastewater using microalgae–bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour Technol. 2013;135:598–603.
Hernández D, Riaño B, Coca M, García-González MC. Saccharification of carbohydrates in microalgal biomass by physical, chemical and enzymatic pre-treatments as a previous step for bioethanol production. Chem Eng J. 2015;262:939–45.
Hernández D, Riaño B, Coca M, Solana M, Bertucco A, García-González MC. Microalgae cultivation in high rate algal ponds using slaughterhouse wastewater for biofuel applications. Chem Eng J. 2016;285:449–58.
Jia H, Yuan Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent Environ Sci. 2016;2:1275089.
Kraan S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig Adapt Strateg Glob. 2013;18:27–46.
Laamanen CA, Gregory MR, Scott JA. Flotation harvesting of microalgae. Renew Sust Energ Rev. 2016;58:75–86.
Lal R. Soil carbon sequestration impacts on global climate change and food security. Science. 2004;304:1623–7.
Langholtz MH, Coleman AM, Eaton LM, Wigmosta MS, Hellwinckel CM, Brandt CC. Potential land competition between open-pond microalgae production and terrestrial dedicated feedstock supply systems in the US. Renew Energy. 2016;93:201–14.
Larrán García MA, Tomás-Almenar C, de Mercado E, Hernández D, García-González MC. Valorización de la biomasa algal procedente del tratamiento de purines mediante la inclusión en piensos para trucha arco iris (Oncorhynchus mykiss). In Proc. XVI National Aquaculture Congress, Zaragoza (Spain); 2017. p. 217–218.
Larsdotter K. Wastewater treatment with microalgae: a literature review. Vatten. 2006;62:31–8.
Lavens P, Sorgeloos P. Manual on the production and use of live food for aquaculture. Rome: Food and Agriculture Organization (FAO); 1996.
Lee CS, Lee SA, Ko SR, Oh HM, Ahn CY. Effects of photoperiod on nutrient removal, biomass production, and algal-bacterial population dynamics in lab-scale photobioreactors treating municipal wastewater. Water Res. 2015;68:680–91.
Mahapatra DM, Chanakya HN, Joshi NV, Ramachandra TV, Murthy GS. Algae-based biofertilizers: a biorefinery approach. In: Microorganisms for green revolution. Singapore: Springer; 2018. p. 177–96.
Markou G, Vandamme D, Muylaert K. Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res. 2014;65:186–202.
Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev. 2010;14:217–32.
Matamoros V, Gutiérrez R, Ferrer I, García J, Bayona JM. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: a pilot-scale study. J Hazard Mater. 2015;288:34–42.
Mendoza JL, Granados MR, Godos I, Acien FG, Molina E, Banks C, Heaven S. Fluid-dynamic characterization of real-scale raceway reactors for microalgae production. Biomass Bioenerg. 2013;54:267–75.
Mezrioui N, Oudra B, Oufdou K, Hassani L, Loudiki M, Darley J. Effect of microalgae growing on wastewater batch culture on Escherichia coli and Vibrio cholerae survival. Water Sci Technol. 1994;30:295–302.
Milledge JJ, Heaven S. A review of the harvesting of micro-algae for biofuel production. Rev Environ Sci Biotechnol. 2013;12(2):165–78.
Molina Grima E, Belarbi EH, Acien Fernandez FG, Robles Medina A, Chisti Y. Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv. 2003;20:491–515.
Molinuevo-Salces B, García-González MC, González-Fernández C. Performance comparison of two photobioreactors configurations (open and closed to the atmosphere) treating anaerobically degraded swine slurry. Bioresour Technol. 2010;101:5144–9.
Molinuevo-Salces B, Mahdy A, Ballesteros M, González-Fernández C. From piggery wastewater nutrients to biogas: microalgae biomass revalorization through anaerobic digestion. Renew Energy. 2016;96:1103–10.
Morrissey WA, Justus JR. Global climate change. Congressional Research Service, Library of Congress; 2000.
Mulbry W, Kondrad S, Buyer J. Treatment of dairy and swine manure effluents using freshwater algae: fatty acid content and composition of algal biomass at different manure loading rates. J Appl Phycol. 2008;20:1079–85.
Muñoz R, Guieysse B. Algal-bacterial processes for the treatment of hazardous contaminants: a review. Water Res. 2006;40:2799–815.
Muylaert K, Bastiaens L, Vandamme D, Gouveia L. Harvesting of microalgae: overview of process options and their strengths and drawbacks. In Microalgae-based biofuels and bioproducts; 2018. p. 113–132.
Olguín EJ, Castillo SO, Mendoza A, Tapia K, González-Portela RE, Hernández-Landa VJ. Dual purpose system that treats anaerobic effluents from pig waste and produce Neochloris oleoabundans as lipid rich biomass. New Biotechnol. 2015;32:387–95.
Oswald WJ. Micro-algae and wastewater treatment. In: Borowitzka MA, Borowitzka LJ, editors. Micro-algal biotechnology. Cambridge, UK: Cambridge University Press; 1988. p. 305–28.
Ozturk S, Aslim B, Suludere Z, Tan S. Metal removal of cyanobacterial exopolysaccharides by uronic acid content and monosaccharide composition. Carbohydr Polym. 2014;101:265–71.
Paniagua-Michel J. Wastewater treatment using phototrophic–heterotrophic biofilms and microbial mats. In: Prospects and challenges in algal biotechnology. Singapore: Springer; 2017. p. 257–75.
Park JBK, Craggs RJ. Wastewater treatment and algal production in high rate algal ponds with carbon dioxide addition. Water Sci Technol. 2010;61:633–9.
Park JBK, Craggs RJ, Shilton AN. Wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol. 2011;102:35–42.
Peng K, Li J, Jiao K, Zeng X, Lin L, Pan S, Danquah MK. The bioeconomy of microalgal biofuels. In: Energy from microalgae. Cham: Springer; 2018. p. 157–69.
Pereira M, Bartolome MC, Sánchez-Fortum SS. Bioadsorption and bioaccumulation of chromium trivalent in Cr (III) tolerant microalgae: a mechanism for chromium resistance. Chemosphere. 2013;93:1057–63.
Posadas E, García-Encina PA, Soltau A, Domínguez A, Díaz I, Muñoz R. Carbon and nutrient removal from centrates and domestic wastewater using algal–bacterial biofilm bioreactors. Bioresour Technol. 2013;139:50–8.
Posadas E, Bochon S, Coca M, García-González MC, García-Encina PA, Muñoz R. Microalgae-based agro-industrial wastewater treatment: a preliminary screening of biodegradability. J Appl Phycol. 2014;26:2335–45.
Posadas E, Morales MM, Gómez C, Acién FG, Muñoz R. Influence of pH and CO2 source on the performance of microalgae-based secondary domestic wastewater treatment in outdoors pilot raceways. Chem Eng J. 2015;265:239–48.
Posadas E, Alcántara C, García-Encina PA, Gouveia L, Guieysse B, Norvill Z, Acién FG, Markou G, Congestri R, Koreiviene J, Muñoz R. Microalgae cultivation in wastewater. In Microalgae-based biofuels and bioproducts, from feedstock cultivation to end-products; 2018 p. 67–91. ISBN:978–0–08-101023-5.
Powell N, Shilton A, Chisti Y, Pratt S. Towards a luxury uptake process via microalgae-defining the polyphosphate dynamics. Water Res. 2009;43:4207–13.
Riaño B, Molinuevo B, García-González MC. Treatment of fish processing wastewater with microalgae-containing microbiota. Bioresour Technol. 2011;102:10829–33.
Riaño B, Hernández D, García-González MC. Microalgal-based systems for wastewater treatment: effect of applied organic and nutrient loading rate on biomass composition. Ecol Eng. 2012;49:112–7.
Richmond A. Handbook of microalgal culture: biotechnology and applied phycology. Hoboken: Wiley; 2008.
Rittmann BE. Opportunities for renewable bioenergy using microorganisms. Biotechnol Bioeng. 2008;100:203–12.
Ruiz-Marin A, Mendoza-Espinosa LG, Stephenson T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour Technol. 2010;101:58–64.
Searchinger T, Heimlich R, Houghton RA, Dong F, Elobeid A, Fabiosa J, Yu TH. Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science. 2008;319(5867):1238–40.
Slade R, Bauen A. Micro-algae cultivation for biofuels: cost, energy balance, environmental impacts and future prospects. Biomass Bioenerg. 2013;53:29–38.
Stephens E, Ross IL, King Z, Mussgnug JH, Kruse O, Posten C, Hankamer B. An economic and technical evaluation of microalgal biofuels. Nat Biotechnol. 2010;28:126.
Subashchandrabose SR, Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R. Consortia of cyanobacteria/microalgae and bacteria: biotechnological potential. Biotechnol Adv. 2011;29:896–907.
Sutherland DL, Turnbull MH, Craggs RJ. Increased pond depth improves algal productivity and nutrient removal in wastewater treatment high rate algal ponds. Water Res. 2014;53:271–81.
Sutherland DL, Howard-Willians C, Turnbull MH, Broady PA, Craggs RJ. Enhancing microalgal photosynthesis and productivity in wastewater treatment high rate algal ponds for biofuel production. Bioresour Technol. 2015;184:222–9.
Tomás-Almenar C, Larrán-García AM, de Mercado E, Sanz-Calvo MA, Hernández D, García-González MC. Microalgae as a protein recovery system for feed rainbow trout. In Proc. 47th Conference of the West European Fish Technologists Association, Dublin (Ireland); 2017. p. 130.
Tomás-Almenar C, Larrán-García AM, de Mercado E, Sanz-Calvo MA, Hernández D, García-González MC. Scenedesmus almeriensis from an integrated system waste-nutrient, as sustainable protein source for feed to rainbow trout (Oncorhynchus mykiss). Aquaculture. 2018;497:422–30.
Toyoshima M, Aikawa S, Yamagishi T, Kondo A, Kawai H. A pilot-scale floating closed culture system for the multicellular cyanobacterium Arthrospira platensis NIES-39. J Appl Phycol. 2015;27(6):2191–202.
Tredici M. Bioreactors, photo. In: Flickinger MC, Drew SW, editors. Encyclopedia of bioprocess technology: fermentation, Biocatal. Biosep. New York: Wiley; 1999.
Umamaheswari J, Shanthakumar S. Efficacy of microalgae for industrial wastewater treatment: a review on operating conditions, treatment efficiency and biomass productivity. Rev Environ Sci Biotechnol. 2016;15:265–84.
Vandamme D, Foubert I, Muylaert K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013;31:233–9.
Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, Ruan RR. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol. 2010;101:2623–8.
Wilkie AC, Mulbry WW. Recovery of dairy manure nutrients by benthic freshwater algae. Bioresour Technol. 2002;84:81–91.
Zeraatkar AH, Ahmadzadeh H, Talebi AF, Moheimani MR, McHenry MP. Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manag. 2016;181:817–31.
Acknowledgements
This work has been supported by Ministerio de Ciencia, Innovación y Universidades - Gobierno de España (grant number CTQ2017-84006-C3-1-R) and cofinanced by EU-FEDER.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Molinuevo-Salces, B., Riaño, B., Hernández, D., Cruz García-González, M. (2019). Microalgae and Wastewater Treatment: Advantages and Disadvantages. In: Alam, M., Wang, Z. (eds) Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment. Springer, Singapore. https://doi.org/10.1007/978-981-13-2264-8_20
Download citation
DOI: https://doi.org/10.1007/978-981-13-2264-8_20
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-2263-1
Online ISBN: 978-981-13-2264-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)