Abstract
Heavy metals (HMs) are toxic, non-biodegradable elements, which causes oxidative stress in plant and microbes. Oxidative stress generates reactive oxygen species (ROS) that damage the cells of plants and microbes. Plant and microbes evolved a biological mechanism to protect themselves from reactive oxygen species. Antioxidants are the molecules that neutralize the effect of reactive oxygen species (ROS). Antioxidant defense system contains enzymatic antioxidants and non-enzymatic antioxidants. Enzymatic antioxidants include superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GRx), ascorbate peroxidase (APx), etc. Glutathione (GSH), ascorbic acid (AsA), phenolic acid, thiols, proline, etc., are non-enzymatic antioxidants. Bioremediation of heavy metals through phytoremediation and/or microbial remediation is eco-friendly approaches. Phytoremediation refers to the technique in which the use of the plant to remediate the contaminant from the contaminated sites. Microbial remediation involves the microorganisms to remediates the pollutants from the environment. Antioxidants play an important role in tolerance against heavy metal stress and provide the potential to plant and microbe to bio-remediates heavy metals. It this chapter, we explain the role of antioxidants in the remediation of heavy metals through phytoremediation or microbial remediation.
Arun Pratap Singh and Balendu Shekher Giri contributed equally to this work.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The natural and anthropogenic activities contribute to non-biodegradable pollution such as heavy metals pollution that are a major concern for environmental health and safety. Heavy metals (HMs) are those elements characterized by relatively high densities (>5 g cm−3) and are toxic to living beings at low concentrations (Alaraidh et al. 2018). Anthropological activities or natural processes such as mining, pesticides, metal industries, mineral fertilizers, and others caused heavy metal pollution in the environment in the present time (Bhadur and Fulekar 2012). HMs are non-biodegradable pollutants, accumulated in tissues cause deleterious effects on living beings through a different mechanism. Due to heavy metal toxicity, oxidative stress generates ROS like H2O2, OH−, singlet oxygen (1O2), superoxide radical (O2−), in plant cells (Rajkumar et al. 2012). HMs induce oxidative damage in plants, develop ROS which alters enzymatic activity, DNA damage, membrane permeability, respiratory and photosynthesis processes induce plant senescence, and leakage of ions (Quartacci et al. 2001; Monferran et al. 2009). Various bioremediation technologies such as phytoremediation, mycoremediation, and microbial remediation are applied to deal with heavy metals pollution. In a biological system, antioxidant defense systems are present to neutralize the effect of reactive oxygen species caused by oxidative stress. Antioxidant provides defense against the toxic effect of heavy metals and other pollutants that cause oxidative stress in a living being. Antioxidant defense systems present in cells can be enzymatic and non-enzymatic, develop against oxidative damage, and are those that prevent ROS occurrence and capture, block, free radicals that are formed in cells (Cheeseman and Slater 1993). The biological antioxidant (present at a lower concentration) refers to any compound that can either prevent or delay the oxidation of the substrate (Halliwell and Gutteridge 2015). The main feature of antioxidants is reversing the effect of free radicals (Prakash et al. 2012). In the environment, physical, chemical, and biological methods are used for the remediation of heavy metals. Bioremediation is involved in the biological mechanism of plant and microorganism to improve environments contaminated with heavy metals, which is a profit-making and eco-friendly method (Ojuederie and Babalola 2017).
Phytoremediation and microbial remediation is an efficient strategy for the removal of environmental pollution as well as sustainable to the environment. Phytoremediation is an alternative method as an environment friendly, profit-making to cope with the kind of pollutants from soil, water, and plant tolerant to pollutants require for this process (Wang et al. 2012). Phytoremediation includes several processes, namely phytostabilization, phytoextraction, and rhizofiltration, and accumulation of toxic compounds by plants (Jasrotia et al. 2017; Sarwar et al. 2017; da Silva et al. 2018). Due to the toxic effect of heavy metals, ROS accumulated in the cell cause disruption of cellular activity. To alleviate their deleterious effects and scavenge reactive oxygen species, plants have developed an enzymatic and non-enzymatic mechanism that protects from oxidative damage (Goswami and Das 2016). Hence, studying the antioxidant defense system in the phyto-accumulator plant may reveal the phytoremediation potential of such a plant. Antioxidants can be categorized into enzymatic antioxidants such as peroxidase (POD), superoxide dismutase (SOD), and catalase (CAT) and non-enzymatic antioxidants including ascorbic acid (AsA) and glutathione (GSH) which prevent cells against O2− and H2O2 (Halliwell and Gutteringe 2006). In plants, the ascorbate-glutathione pathway consists of the enzymes monodehydroascorbate reductase, ascorbate peroxidase, dehydroascorbate reductase, and glutathione reductase, and glutathione and ascorbate is a very efficient system to remove lipid peroxides and hydrogen peroxide (H2O2) (Foyer and Shigeoka 2011). Microorganisms induced different enzymatic and non-enzymatic antioxidants to alleviate the oxidative stress caused through HMs and lessen the radicals’ formations in plant cells under metal stress (Khanna et al. 2018). In wheat plants under Zn stress, Pseudomonas aeruginosa modulates the activity of enzymatic antioxidants such as CAT, POD, and SOD which scavenge ROS to prevent from H2O2 and malondialdehyde (MDA) level (Islam et al. 2014a, b). Pseudomonas aeruginosa up-regulated SOD, APX, CAT, and POD levels, whereas Solanum nigrum alleviated oxidative stress generated under stress (Shi et al. 2016). The remediation of HMs has been carried out using phytoremediation, mycoremediation, or microbial remediation or a combination of these techniques. Recent findings have reported the use of Genetically modified bacteria for Arsenic remediation (Mateos et al. 2017).
This chapter focuses on the importance of antioxidants in the plants and microbial defense system in the phytoremediation and bioremediation of heavy metals. Antioxidants play a crucial role in scavenging ROS generating during the oxidative stress of toxic compounds. Plant and microorganisms used different biological mechanisms for bioremediation purposes and able to tolerate with the help of an anti-oxidative defense system and accumulate and detoxification of heavy metals.
2 Classification of Antioxidant and Its Applications
An antioxidant is an enzyme or molecule capable of inhibiting or preventing the oxidation of other molecules (Fig. 9.1). Antioxidants may be able to donate or accepting electron (s) to neutralize free radicals (Lü et al. 2010). Antioxidants can protect the cells against oxidative stress through different mechanisms (Aziz et al. 2019). Antioxidants can be classified into enzymatic peroxidase (POD), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), etc., and non-enzymatic antioxidants ascorbic acid (AsA), thiols, glutathione (GSH), proline, and carotenoids on the bases of their activity. The production of POD, SOD, CAT, AsA, GSH, and carotenoids protects against oxidative stress (Hall 2002; Caregnato et al. 2008). The enzyme antioxidant glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase (CAT) are an essential unit of the antioxidant defense system, and they are responsible for scavenging free radical (Butnariu and Grozea 2012). Antioxidant enzymes are the crucial substances of the protection mechanisms, preventing ROS via transferring ROS into relatively stable compounds (Pandey and Rizvi 2010). Among non-enzymatic antioxidants tocopherol, glutathione and ascorbate play a major role in the defense mechanism of a plant to prevent oxidative stress (Mittler et al. 2004; Scandalios 2005; Halliwell 2006).
2.1 Antioxidant Enzymes and Its Applications
The application of antioxidant enzyme is to detoxify the deleterious effect of HM pollution in plants and microbes. Antioxidant provides potential to plant to tolerance against heavy metals toxicity. Antioxidant enzymes play a crucial role to alleviate metal stress during the bioremediation of heavy metals. The tolerance mechanisms of plant for heavy metal toxicity such as to alleviate the ROS effect through antioxidant enzyme can be assessed for phytoremedial potential of the plant (Bhadur and Fulekar 2012). The Plant possesses a complex system of enzymatic antioxidants; antioxidant enzymes are important substances that provide defense against oxidative stress and alleviate the toxic effect of oxidative stress (Bano and Ashfaq 2013). CAT, SOD, POD, APx, and GRx get activated in ROS detoxification (Gratao et al. 2008; Roychoudhury et al. 2012). SOD catalyzes the dismutation of superoxide ion (O2−) into either hydrogen peroxide (H2O2) or molecular oxygen (O2) (Rusin et al. 2020). Catalase enzyme involved in the reduction of H2O2 to H2O. Glutathione peroxidase (GPx), using glutathione as an essential cofactor to catalyze the reduction of lipid hydroperoxide, organic hydroperoxides, and H2O2 to H2O or corresponding alcohols (Kieliszek and Błażejak 2013; Pisoschi and Pop 2015). SOD-specific activity increases in leaves of Medicago sativa plants grown in the presence of sludge (Martí et al. 2009). Five different SOD isoenzymes, such as Mn-SOD, Fe-SOD, and three Cu-, Zn-SODs, were detected in leave extracts of the alfalfa plant (McKersie et al. 1993). The first line of defense toward metal stress is generally SOD enzyme. The breakdown of H2O2 into H2O and O2 in plant cell becomes oxidative stress which is protected by catalase enzyme (Chelikani et al. 2004). The POD, CAT, and APX encoding gene showed a significant increase in mRNA expression levels were observed in response to Pb, Cd, and Cr (Alaraidh et al. 2018). Goswami and Das (2016) observed that under Cu stress, SOD activity in root tissues was higher than that of leaves tissues in C. officinalis.
2.2 Non-Enzymatic Antioxidant and Its Applications
Various non-enzymatic antioxidants are involved in ROS-scavenging pathways, and HMs detoxification, produced in plants upon heavy metal exposure, antioxidants like phenolics, and non-protein thiol have a role in Cd detoxification (Mishra et al. 2014). Plant uses non-enzymatic antioxidants like glutathione (reduced form) to scavenge ROS generating during oxidative stress (Noctor and Foyer 1998; Chou et al. 2011). A small amount of glutathione presents in the fully oxidized form (GSSG), and glutathione is normally found in reduced form (GSH) (Pocsi et al. 2004). Reduced GSH (contain cysteine residue) is one of the important thiol compounds that alleviate HMs stress and protect plants (Deng et al. 2010; Sun et al. 2014; Mahawar et al. 2018). Glutathione functions as a non-enzymatic antioxidant by ROS scavenging in cells, as well as a cofactor for various enzymes, such as glutathione reductase, glutathione transferase, and glutathione peroxidase (Sun 2010; Skowyra 2014). Glutathione is a key antioxidant in HMs tolerance. Glutathione is also important for the synthesis of phytochelatins that are important in HMs detoxification (Jozefczak et al. 2012). An increase in proline level can play a crucial role against metal stress, most likely reduced loss due to oxidation and the effect of rise in metabolism (Dash and Panda 2001). Potamogeton pectinatus L. and Potamogeton crispus L. grown under Pb, Cr, Cu, and Zn stress showed increased non-enzymatic activity of proline and cysteine under Pb and Cr stress (Upadhyay et al. 2014). N-acetylcysteine can alleviate HMs stress and improved the growth of the wheat by coordinated induction of antioxidant defense system (Colak et al. 2019).
3 Effect of Metal Stress on Living Being
The contamination of heavy metal in soil, water, and air through anthropogenic activities causes harmful effect on a living being. Heavy metals such as cadmium, arsenic, chromium, lead, and mercury cause toxic effects (various disease) in human as well as plants and animals. The non-biodegradable nature of heavy metals is responsible for their poor elimination from tissues (Ayangbenro and Babalola 2017). The best-known indirect effects of heavy metals include elevated levels of ROS such as hydrogen peroxide (H2O2), superoxide anion (O2•¯), alkoxyl (RO•), hydroxyl radical (•OH), and development of oxidative stress (Colak et al. 2019). The ROS detoxification process in plants through the antioxidant defense system is important for protection against oxidative stress (Apel and Hirt 2004). The degree of toxicity of heavy metal is determined by absorbed dosage as well as the duration of exposure by the organism (Ojuederie and Babalola 2017). In humans, heavy metals like Pb, Hg, and As drastically affect the nervous system and kidney leading to mental disorders along with abdominal cramps, anemia, diarrhea, and headache (Sharma et al. 2014). The excessive exposure to Pb causes lead poisoning/intoxication. The exposure of human beings to the mercury may lead to nervous and renal disorder (Azimi and Moghaddam 2013). Various microbial processes such as enzymatic activity, respiration, and denitrification and hence retard bioremediation processes due to heavy metal toxicity (Zhuang et al. 2007; Sobolev and Begonia 2008). Heavy metals reduce the microbial populations that cause a shift in the structure of microbial communities (Saxena et al. 2019). It affects disrupting the cell membranes, morphology, and microbial growth by altering the nucleic acid (DNA and RNA) structure, metabolism, causing lipid peroxidation and inhibiting enzyme activity, protein denaturation, and cause functional disturbance (Fashola et al. 2016). The HMs toxicity varies in plants, depending on metal concentration, plant species, specific metal involved, the oxidation state of metal, and pH and composition of soil (Nagajyoti et al. 2010). To study the effects of stress on plants, cell membrane stability has been determined. The effect of accumulation of HMs in plant tissue is on growth inhibition and development, which is related to cell division (Kumar and Rai 2007). An accumulation of HMs in soil and aquatic environments can induce adverse toxic effects on plants, such as biomass decrease, growth inhibition, deficiency of nutrient uptake, and photosynthesis disturbance (Gavrilescu 2004; Pavel et al. 2013). In Brassica napus decline in seedling growth and seed germination due to the toxic effect of Cadmium (Cd) (Irfan et al. 2014) and enzyme activity inhibit in Brassica juncea (Bashir et al. 2015). Lead stress caused disturbed in metabolic function and inhibited plant growth in Brassica oleracea (Ashraf et al. 2011; Theriappan et al. 2011).
4 Role of Antioxidant Under Metal Stress
Heavy metals tolerant plant and microbes which possess antioxidant defense system (ADS) can be used for remediation of heavy metals through phytoremediation and microbial remediation techniques. Non-enzymatic activity of proline and cysteine and antioxidant enzymes (guaiacol peroxidase, superoxide dismutase, and ascorbate peroxidase) increased particularly under lead and chromium stress (Upadhyay et al. 2014). Due to the metal stress, the effect of increased proline level, an increase in plant metabolism and prevent to oxidative damage (Dash and Panda 2001). The antioxidant defenses of Arabidopsis thaliana to the Heavy metals altered by subtle change in glutathione (Sobrino-Plata et al. 2014). Plant produced sufficient amount of antioxidants such as phenolics, flavonoids, and polyphenolics, to prevent the oxidative damage (Garhwal 2010). The toxicity of heavy metals causes the formation of ROS thereby decreasing the antioxidant defense systems which protect cells (Ojuederie and Babalola 2017). To remove the oxidative stress produced by ROS, an active antioxidant defense system is found naturally in plants (Skórzyńska-Polit et al. 2010). Uraguchi et al. (2006) observed the increased activity of CAT, SOD, and GRx in Avena strigose under Cadmium stress. Enzyme participated in ascorbate-glutathione, and SOD, POD, and parallel to total homoglutathione showed increased activity allowing the plant to tolerate HMs and hydrocarbons stress (Martí et al. 2009). The non-enzymatic antioxidants, such as AsA, GSH, phenolic compounds, carotenoids, and tocopherol, are best known for their important role to chelate/bind HMs and/or scavenge the ROS in plant cells (Maleki et al. 2017). Th antioxidant defense system (ADS) includes enzymatic and non-enzymatic antioxidants prevent the cell from the toxic effect of ROS which caused by oxidative stress.
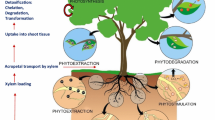
5 Role of Antioxidant System in Phytoremediation
The process of phytoremediation involves the use of plant to remediate hazardous materials from environment and applied to remediate contaminants present in water, soil, and air (Yanqun et al. 2005). The detoxification/decontamination processes through plants are commonly known as phytoremediation (Fig. 9.2). Phytoremediation includes several processes, namely phytostabilization, phytoextraction, and rhizofiltration, and accumulation of toxic compounds by plants (Jasrotia et al. 2017; Sarwar et al. 2017). Phytoremediation includes phytoextraction that involves the use of hyperaccumulators plants to detoxification of HMs from contaminated soil by concentrating them in plant tissue (Rajkumar et al. 2012). Phyto-stabilization is a process in which plant should have low mobility of HMs from root to shoots and broad plant root system (Islam et al. 2013). The success of phytoremediation as a means of HMs eradication from the polluted site using plants, depending upon the bioavailability of the metal impurity, the level of contaminated soil, as well as the accumulation of HMs as plant biomass (Tak et al. 2013). Arabidopsis thaliana and Pteris vittata have been widely utilized in the remediation of Arsenic polluted soil (Huang et al. 2016). The use of microorganisms can stimulate the phytoremediation process to provide tolerance against HMs stress, by altering the level of phytohormone, upregulation of antioxidant enzymes, modulation of protein related to defense, and modulation of metal transporters (Gallego et al. 2012). The application of plants alone or in combination with PGPB is an efficient method for the phytoremediation, prevention, and control of heavy metals (Saxena et al. 2019) (Table 9.1). Microorganism enhances plant survival and HMs stress, and stimulating the activity of reactive oxygen species-scavenging pathways and maintains homeostasis of ROS (Khanna et al. 2018). The tolerance mechanism in plant against oxidative stress induced by antioxidant enzymes that help in reduce the oxidation of molecules and inhibit the process of ROS formation and oxidative chain reaction (Bhadur and Fulekar 2012). Plants have developed an efficient antioxidant defense system by which ROS is scavenged by antioxidant enzymes such as GRx, POD, SOD, and CAT (Joseph and Jini 2010). Lead toxicity caused oxidative damage in plants, and the antioxidant enzymes include GRx, SOD, and POD play an important role in alleviating oxidative stress in plants (Verma and Dubey 2003). The mechanism of the ascorbate-glutathione cycle involved in controlling the cellular oxidation-reduction status especially due to HMs stress (Cuypers et al. 2000; Smeets et al. 2005). Akinyemi et al. (2017) suggested that non-enzymatic antioxidants like phenolic compounds and GSH have a crucial role in the cadmium detoxification process. Glutathione reductase, SOD, CAT, GSH, AsA, tocopherols, alkaloids, etc., have prevented the effect of oxidative damage of ROS (Rastgoo et al. 2011; Singh et al. 2016). To study the role of the antioxidant defense system provides a better understating of optimizing the efficient process of phytoremediation and selection of most appropriate plants.
Bioremediation techniques remove pollutant from environments includes phytoremediation and microbial remediation, phytoremediation involves, phytoextraction, phytostabilization, phytostimulation, and rhizofiltration while microbial remediation through biosorption, bioaccumulation, biotransformation, and biomineralization
6 Role of Antioxidant System in Microbial Remediation
The microbial remediation of heavy metals depends on microbial interaction with heavy metals and different factor such as microbial community, concentration, and toxicity of heavy metals. Microbes perform the oxidation, reduction, precipitation, and absorption of HMs in the soil (Su 2014). Microbial remediation includes bioaccumulation, biotransformation, biosorption, and biomineralization mechanism employed by microbes involved in the remediation of contaminated sites, and biosorption is the key process of microbes involved in metal sequestration (Ayangbenro and Babalola 2017). In modern technology, microorganisms are used to perform the function of bioremediation to remediate heavy metals. Multi-metal resistance Paenibacillus sp. isolated from Trida xprocumbens can be utilized as an appropriate candidate for the bioremediation from heavy metals (Govarthanan et al. 2016). Mechanism of tolerance in bacteria, by which they uptake and transform, mobilize and immobilize heavy metals. Bacteria employed the mechanisms are exclusion, physical sequestration, detoxification, and complexation to alleviate the toxicity of HMs (ul Hassan et al. 2017). Microorganism interacts with HMs through extracellular polymeric reactions with transformation, intracellular accumulation, cell wall-associated metals, production of siderophore, immobilization, or mobilization (Ahluwalia and Goyal 2007). The toxic character of hazardous waste influences the survival of microbes in incompatible environments resulting in the reduction of specific microbes in the environment and has led to evolved mechanisms by microbes that prevent to them by HMs contamination (Förstner and Wittmann 2012). Antioxidants enzymes neutralize the ROS and repair damage biomolecules (Poljsak et al. 2010). Superoxide dismutase mainly catalyzes the reaction of superoxide anion to hydrogen peroxide and oxygen. Catalase is responsible for the conversion of hydrogen peroxide to H2O and O2, thereby alleviate H2O2-induced oxidative stress (Medvedeva et al. 2017). Bacillus sp. improved antioxidant defense system in Triticum aestivum under Copper stress through increased activities of APX, POD, dehydroascorbate reductase (DHAR), and SOD by reduction of superoxide radicals and hydrogen peroxide in plants (Wang et al. 2013). The robust antioxidant defense system, based on the redox couples MSH/Mrx-1 and Trx/TrxR, suggests the potential of Corynebacterium glutamicum for bioremediation purposes (Mateos et al. 2017). Antioxidant defense system enhanced the tolerance capability of microorganisms against oxidative stress, hence increase in the potential of bioremediation of contaminants such as heavy metals.
7 Conclusion and Future Prospective
This chapter summarized the role of antioxidant defense system which are scavenging of ROS and reduce the oxidative stress under heavy metal. It also focused on the detoxification or decontamination of heavy metals through phytoremediation and microbial remediation. Heavy metal contamination in the environment and its related toxicity in living beings is a major concern for environment. The toxicity of heavy metal causes oxidative damage of organisms. Organism evolved the antioxidant defense system to protect against oxidative damage. Antioxidant enzyme plays a crucial role during the stress induced by HMs or uptake of HMS in the phytoremediation and microbial remediation process. Antioxidant provides potential to Plant and microorganism to remediate HMs in the environments. Antioxidant plays a crucial role in bioremediation process to alleviate the toxic effect of HMs. Plants possess a best-known antioxidant defense mechanism to reduce and neutralize the free radicals. The defensive biological mechanisms of Plants and microorganisms help to survive under HMs stress and remediate the metals from the environment.
However, future research is based on role of antioxidant and mechanism found in plant and microbes involved in remediation of pollutant in contaminated site. In bioremediation approaches, Detailed study is required at cellular and molecular level for comprehension the role of antioxidant. Characterization of antioxidants incriminate in oxidative stress management will involve in upcoming work.
References
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257
Akinyemi AJ, Faboya OL, Olayide I, Faboya OA, Ijabadeniyi T (2017) Effect of cadmium stress on non-enzymatic antioxidant and nitric oxide levels in two varieties of maize (Zea mays). Bull Environ Contam Toxicol 98(6):845–849
Alaraidh IA, Alsahli AA, Razik EA (2018) Alteration of antioxidant gene expression in response to heavy metal stress in Trigonellafoenum-graecum L. S Afr J Bot 115:90–93
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Ashraf MY, Azhar N, Ashraf M, Hussain M, Arshad M (2011) Influence of lead on growth and nutrient accumulation in canola (Brassica napus L.) cultivars. J Environ Biol 32(5):659
Ayangbenro AS, Babalola OO (2017) A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int J Environ Res Public Health 14(1):94
Azimi S, Moghaddam MS (2013) Effect of mercury pollution on the urban environment and human health. Environ Ecol Res 1(1):12–20
Aziz MA, Diab AS, Mohamme AA (2019) Antioxidant categories and mode of action. In: Antioxidants. IntechOpen. https://doi.org/10.5772/intechopen.83544
Bano SA, Ashfaq D (2013) Role of mycorrhiza to reduce heavy metal stress. Nat Sci. https://doi.org/10.4236/ns.2013.512A0032013
Bashir H, Ibrahim MM, Bagheri R, Ahmad J, Arif IA, Baig MA, Qureshi MI (2015) Influence of sulfur and cadmium on antioxidants, phytochelatins and growth in Indian mustard. AoB Plants 7. https://doi.org/10.1093/aobpla/plv001
Beladi M, Habibi D, Kashani A, Paknejad F, Nooralvandi T (2011) Phytoremediation of lead and copper by sainfoin (Onobrychisvicifolia): role of antioxidant enzymes and biochemical biomarkers. Am Euras J Agric Environ Sci 10(3):440–449
Bhadur AM, Fulekar MH (2012) Antioxidant enzyme responses of plants to heavy metal stress. Rev Environ Sci Biotechnol 11(1):55–69
Bhagyawant SS, Narvekar DT, Gupta N, Bhadkaria A, Koul KK, Srivastava N (2019) Variations in the antioxidant and free radical scavenging under induced heavy metal stress expressed as proline content in chickpea. Physiol Mol Biol Plants 25(3):683–696
Butnariu M, Grozea I (2012) Antioxidant (antiradical) compounds. J Bioequiv 4(6):17–19
Caregnato FF, Koller CE, MacFarlane GR, Moreira JC (2008) The glutathione antioxidant system as a biomarker suite for the assessment of heavy metal exposure and effect in the grey mangrove, Avicennia marina (Forsk.) Vierh. Mar Pollut Bull 56(6):1119–1127
Cheeseman KH, Slater TF (1993) An introduction to free radical biochemistry. Br Med Bull 49(3):481–493
Chelikani P, Fita I, Loewen PC (2004) Diversity of structures and properties among catalases. Cell Mol Life Sci 61(2):192–208
Chou TS, Chao YY, Huang WD, Hong CY, Kao CH (2011) Effect of magnesium deficiency on antioxidant status and cadmium toxicity in rice seedlings. J Plant Physiol 168(10):1021–1030
Colak N, Torun H, Gruz J, Strnad M, Ayaz FA (2019) Exogenous N-acetylcysteine alleviates heavy metal stress by promoting phenolic acids to support antioxidant defence systems in wheat roots. Ecotoxicol Environ Saf 181:49–59
Cuypers A, Vangronsveld J, Clijsters H (2000) Biphasic effect of copper on the ascorbate-glutathione pathway in primary leaves of Phaseolus during the early stages of metal assimilation. Physiol Plant 110:512–517
da Silva AA, de Oliveira JA, de Campos FV, Ribeiro C, dos Santos Farnese F, Costa AC (2018) Phytoremediation potential of Salviniamolesta for arsenite contaminated water: role of antioxidant enzymes. Theoret Exper Plant Physiol 30(4):275–286
Dash M, Panda SK (2001) Salt stress induced changes in growth and enzyme activities in germinating Phaseolusmungo seeds. Biologiaplantarum 44(4):587–589
Deng X, Xia Y, Hu W, Zhang H, Shen Z (2010) Cadmium-induced oxidative damage and protective effects of N-acetyl-l-cysteine against cadmium toxicity in Solanumnigrum L. J Hazard Mater 180(1–3):722–729
Fashola MO, Ngole-Jeme VM, Babalola OO (2016) Heavy metal pollution from gold mines: environmental effects and bacterial strategies for resistance. Int J Environ Res Public Health 13(11):1047
Förstner U, Wittmann GT (2012) Metal pollution in the aquatic environment. Springer
Foyer CH, Shigeoka S (2011) Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol 155(1):93–100
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Garhwal S (2010) Medicinal plants as a source of antioxidants. Res J Phytochem 4(4):213–224
Gavrilescu M (2004) Removal of heavy metals from the environment by biosorption. Eng Life Sci 4(3):219–232
Goswami S, Das S (2016) Copper phytoremediation potential of Calandula officinalis L. and the role of antioxidant enzymes in metal tolerance. Ecotoxicol Environ Saf 126:211–218
Govarthanan M, Mythili R, Selvankumar T, Kamala-Kannan S, Rajasekar A, Chang YC (2016) Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech 6(2):242
Gratao PL, Monteiro CC, Peres LE, Azevedo RA (2008) The isolation of antioxidant enzymes from mature tomato (cv. Micro-Tom) plants. HortScience 43(5):1608–1610
Hall JL (2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53(366):1–11
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141(2):312–322
Halliwell B, Gutteridge JM (2015) Free radicals in biology and medicine. Oxford University Press, New York
Halliwell B, Gutteringe JMC (2006) Free radicals in biology and medicine, 4nd edn. Clarendon Press, Oxford, UK
Hameed A, Qadri TN, Qadri TN, Iqbal M (2011) Differential activation of the enzymatic antioxidant system of Abelmoschus esculentus L. under CdCl2 and HgCl2 exposure. Braz J Plant Physiol 23(1):46–54
Hassan W, Bashir S, Ali F, Ijaz M, Hussain M, David J (2016) Role of ACC-deaminase and/or nitrogen fixing rhizobacteria in growth promotion of wheat (Triticum aestivum L.) under cadmium pollution. Environ Earth Sci 75(3):267
Huang Y, Miyauchi K, Inoue C, Endo G (2016) Development of suitable hydroponics system for phytoremediation of arsenic-contaminated water using an arsenic hyperaccumulator plant Pteris vittata. Biosci Biotechnol Biochem 80(3):614–618
Irfan M, Ahmad A, Hayat S (2014) Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21(2):125–131
Islam MS, Ueno Y, Sikder MT, Kurasaki M (2013) Phytofiltration of arsenic and cadmium from the water environment using Micranthemum umbrosum (jf GMEL) sf blake as a hyperaccumulator. Int J Phytoremediation 15:1010–1021
Islam F, Yasmeen T, Riaz M, Arif MS, Ali S, Raza SH (2014a) Proteus mirabilis alleviates zinc toxicity by preventing oxidative stress in maize (Zea mays) plants. Ecotoxicol Environ Saf 110:143–152
Islam F, Yasmeen T, Ali Q, Ali S, Arif MS, Hussain S, Rizvi H (2014b) Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol Environ Saf 10:285–293
Jasrotia S, Kansal A, Mehra A (2017) Performance of aquatic plant species for phytoremediation of arsenic-contaminated water. Appl Water Sci 7(2):889–896
Joseph B, Jini D (2010) Insight into the role of antioxidant enzymes for salt tolerance in plants. Int J Bot 6:456–464
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal-induced oxidative stress defenses. Int J Mol Sci 13(3):3145–3175
Khanna K, Kohli SK, Bali S, Kaur P, Saini P, Bakshi P, Ohri P, Mir BA, Bhardwaj R (2018) Role of micro-organisms in modulating antioxidant defence in plants exposed to metal toxicity. In: Plants under metal and metalloid stress. Springer, Singapore, pp 303–335
Kieliszek M, Błażejak S (2013) Selenium: significance, and outlook for supplementation. Nutrition 29(5):713–718
Kong Z, Glick BR, Duan J, Ding S, Tian J, McConkey BJ, Wei G (2015) Effects of 1-aminocyclopropane-1-carboxylate (ACC) deaminase-overproducing Sinorhizobium meliloti on plant growth and copper tolerance of Medicago lupulina. Plant Soil 391(1–2):383–398
Kumar G, Rai P (2007) Comparative genotoxic potential of mercury and cadmium in soybean. Turk J Biol 31(1):13–18
Leão GA, Oliveira JAD, Felipe RTA, Farnese FS (2017) Phytoremediation of arsenic-contaminated water: the role of antioxidant metabolism of Azolla caroliniana Willd.(Salviniales). Acta Bot Brasil 31(2):161–168
Lü JM, Lin PH, Yao Q, Chen C (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 14(4):840–860
Mahawar L, Kumar R, Shekhawat GS (2018) Evaluation of hemeoxygenase 1 (HO 1) in cd and Ni induced cytotoxicity and crosstalk with ROS quenching enzymes in two to four leaf stage seedlings of Vigna radiata. Protoplasma 255(2):527–545
Maleki M, Ghorbanpour M, Kariman K (2017) Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene 11:247–254
Martí MC, Camejo D, Fernández-García N, Rellán-Álvarez R, Marques S, Sevilla F, Jiménez A (2009) Effect of oil refinery sludges on the growth and antioxidant system of alfalfa plants. J Hazard Mater 171(1–3):879–885
Mateos LM, Villadangos AF, Alfonso G, Mourenza A, Marcos-Pascual L, Letek M, Pedre B, Messens J, Gil JA (2017) The arsenic detoxification system in corynebacteria: basis and application for bioremediation and redox control. In: Advances in applied microbiology, vol 99. Academic Press, pp 103–137
McKersie BD, Chen Y, de Beus M, Bowley SR, Bowler C, Inzé D, D’Halluin K, Botterman J (1993) Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant Physiol 103(4):1155–1163
Medvedeva N, Zaytseva T, Kuzikova I (2017) Cellular responses and bioremoval of nonylphenol by the bloom-forming cyanobacterium Planktothrix agardhii 1113. J Mar Syst 171:120–128
Mishra B, Sangwan RS, Mishra S, Jadaun JS, Sabir F, Sangwan NS (2014) Effect of cadmium stress on inductive enzymatic and nonenzymatic responses of ROS and sugar metabolism in multiple shoot cultures of Ashwagandha (Withania somnifera Dunal). Protoplasma 251(5):1031–1045
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Monferran MV, Aguado JA, Pignata ML, Wunderlin DA (2009) Copper induced response of physiological parameters and antioxidant enzymes in the aquatic macrophyte Potamogeton pusillus. Environ Pollut 157:257–276
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Biol 49(1):249–279
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14(12):1504
Pandey KB, Rizvi SI (2010) Markers of oxidative stress in erythrocytes and plasma during aging in humans. Oxidative Med Cell Longev 3:2–12
Pavel VL, Sobariu DL, Diaconu M, Stătescu F, Gavrilescu M (2013) Effects of heavy metals on Lepidium sativum germination and growth. Environ Eng Manag J 12(4):727–733
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74
Pocsi I, Prade RA, Penninckx MJ (2004) Glutathione,altruistic metabolite in fungi. Adv Microb Physiol 49(1):1–76
Poljsak B, Pócsi I, Raspor P, Pesti M (2010) Interference of chromium with biological systems in yeast and fungi: a review. J Basic Microbiol 50:21–36
Prakash D, Upadhyay G, Gupta C, Pushpangadan P, Singh KK (2012) Antioxidant and free radical scavenging activities of some promising wild edible fruits. Int Food Res J 19(3):1109
Quartacci MF, Cosi E, Navari-Izzo F (2001) Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J Exp Bot 52(354):77–84
Rahoui S, Martinez Y, Sakouhi L, Ben C, Rickauer M, El Ferjani E, Gentzbittel L, Chaoui A (2017) Cadmium-induced changes in antioxidative systems and differentiation in roots of contrasted Medicago truncatula lines. Protoplasma 254(1):473–489
Rajkumar M, Sandhya S, Prasad MNV, Freitas H (2012) Perspectives of plant-associated microbes in heavy metal phytoremediation. Biotechnol Adv 30(6):1562–1574
Rastgoo L, Alemzadeh A, Afsharifar A (2011) Isolation of two novel isoforms encoding zinc-and copper-transporting P1B-ATPase from Gouan (Aeluropus littoralis). Plant Omics J 4(7):377–383
Roychoudhury A, Basu S, Sengupta DN (2012) Antioxidants and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Acta Physiol Plant 34(3):835–847
Rusin M, Gospodarek J, Nadgórska-Socha A (2020) Soil pollution by petroleum-derived substances and its bioremediation: the effect on aphis fabae scop. infestation and antioxidant response in Vicia faba L. Agronomy 10(1):147
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Saxena G, Purchase D, Mulla SI, Saratale GD, Bharagava RN (2019) Phytoremediation of heavy metal-contaminated sites: eco-environmental concerns, field studies, sustainability issues, and future prospects. In: Reviews of environmental contamination and toxicology, vol 249. Springer, Cham, pp 71–131
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 38(7):995–1014
Sharma B, Singh S, Siddiqi NJ (2014) Biomedical implications of heavy metals induced imbalances in redox systems. BioMed. https://doi.org/10.1155/2014/640754
Shi P, Zhu K, Zhang Y, Chai T (2016) Growth and cadmium accumulation of Solanumnigrum L. seedling were enhanced by heavy metal-tolerant strains of Pseudomonas aeruginosa. Water Air Soil Pollut 227(12):459
Singh A, Prasad SM, Singh S, Singh M (2016) Phytoremediation potential of weed plants’ oxidative biomarker and antioxidant responses. Chem Ecol 32(7):684–706
Skórzyńska-Polit E, Drążkiewicz M, Krupa Z (2010) Lipid peroxidation and antioxidative response in Arabidopsis thaliana exposed to cadmium and copper. Acta Physiol Plant 32(1):169
Skowyra M (2014) Antioxidant properties of extracts from selected plant materials (Caesalpinia spinosa, Perilla frutescens, Artemisia annua and Viola wittrockiana) in vitro and in model food systems. http://hdl.handle.net/2117/95555
Smeets K, Cuypers A, Lambrechts B, Semane HP, Van Laere A, Vangronsveld J (2005) Induction of oxidative stress and antioxidative mechanisms in Phaseolus vulgaris after Cd. Plant Physiol Biochem 43:437–444
Sobolev D, Begonia M (2008) Effects of heavy metal contamination upon soil microbes: lead-induced changes in general and denitrifying microbial communities as evidenced by molecular markers. Int J Environ Res Public Health 5(5):450–456
Sobrino-Plata J, Meyssen D, Cuypers A, Escobar C, Hernández LE (2014) Glutathione is a key antioxidant metabolite to cope with mercury and cadmium stress. Plant Soil 377(1–2):369–381
Su C (2014) A review on heavy metal contamination in the soil worldwide: situation, impact and remediation techniques. Environ Skept Crit 3:24–38
Sun SY (2010) N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol Ther 9(2):109–110
Sun H, Zhang X, He X, Ahmed IM, Cao F, Zhang G, Wu F (2014) N-acetyl-cysteine alleviates Cd toxicity and reduces Cd uptake in the two barley genotypes differing in Cd tolerance. Plant Growth Regul 74(1):93–105
Tak HI, Ahmad F, Babalola OO (2013) Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals. In: Reviews of environmental contamination and toxicology, vol 223. Springer, New York, NY, pp 33–52
Theriappan P, Gupta AK, Dhasarrathan P (2011) Accumulation of proline under salinity and heavy metal stress in cauliflower seedlings. J Appl Sci Environ Manag 15(2):251–255
ul Hassan Z, Ali S, Rizwan M, Ibrahim M, Nafees M, Waseem M (2017) Role of bioremediation agents (bacteria, fungi, and algae) in alleviating heavy metal toxicity. In: Probiotics in agroecosystem. Springer, Singapore, pp 517–537
Upadhyay AK, Singh NK, Rai UN (2014) Comparative metal accumulation potential of Potamogeton pectinatus L. and Potamogeton crispus L.: role of enzymatic and non-enzymatic antioxidants in tolerance and detoxification of metals. Aquat Bot 117:27–32
Uraguchi S, Watanabe I, Yoshitomi A, Kiyono M, Kuno K (2006) Characteristics of cadmium accumulation and tolerance in novel Cd-accumulating crops, Avena strigosa and Crotalaria juncea. J Exp Bot 57(12):2955–2965
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Wan Y, Luo S, Chen J, Xiao X, Chen L, Zeng G, Liu C, He Y (2012) Effect of endophyte-infection on growth parameters and Cd-induced phytotoxicity of Cd-hyperaccumulator Solanum nigrum L. Chemosphere 89(6):743–750
Wang J, Feng X, Anderson CW, Xing Y, Shang L (2012) Remediation of mercury contaminated sites–a review. J Hazard Mater 221:1–18
Wang H, Xu R, You L, Zhong G (2013) Characterization of Cu-tolerant bacteria and definition of their role in promotion of growth, Cu accumulation and reduction of Cu toxicity in Triticum aestivum L. Ecotoxicol Environ Saf 94:1–7
Yanqun Z, Yuan L, Jianjun C, Haiyan C, Li Q, Schvartz C (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead-zinc mining area in Yunnan, China. Environ Int 31:755–762
Zhuang X, Chen J, Shim H, Bai Z (2007) New advances in plant growth-promoting rhizobacteria for bioremediation. Environ Int 33(3):406–413
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Singh, A.P., Giri, B.S., Singh, A., Chaturvedi, P. (2021). Role of Antioxidant in Plant- and Microbe-Based Remediation of Metal Stress. In: Singh, H.B., Vaishnav, A., Sayyed, R. (eds) Antioxidants in Plant-Microbe Interaction. Springer, Singapore. https://doi.org/10.1007/978-981-16-1350-0_9
Download citation
DOI: https://doi.org/10.1007/978-981-16-1350-0_9
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-1349-4
Online ISBN: 978-981-16-1350-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)