Abstract
Defense pathways and stress responses induced under Cd stress were illustrated in roots of hydroponically grown Medicago truncatula seedlings. Actually, the ascorbate–glutathione and antioxidative system, secondary metabolism events including peroxidases, phenolic compounds, and lignification launching, and developmental modifications were described. Cd (100 μM) initially increased reactive oxygen species, enhanced antioxidative (total SOD, CAT, and PRX) and ascorbate–glutathione-related metabolism enzymes (APX and MDAR), except in A17 and TN1.11. In agreement with peroxidase enhancement, physiological measurement and in situ observation illustrated soluble phenolic compound accumulation under Cd treatment. However, lignification was restricted to recently created protoxylem elements established in the root tip area, usually constituting the elongation zone. Cell death was increased. In the absence of necrotic reactions, developmental changes including lignin deposition, increase in cellulose and pectin contents, intercellular meatus, and condensed and deformed hairs were noticed in Cd-treated roots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium is recognized as one of the most phytotoxic pollutants found in air, water, and soil (Wagner 1993; Mendiola et al. 2011). Cadmium contamination has disastrous effects on plant productivity and both animal and human health. It can interrupt a wide range of plant physiological processes, including water relations, nitrogen metabolism, photosynthesis, respiration, and mineral nutrition (Hassan et al. 2009; Singh and Tamari 2003; Garmash and Gorlovka 2009), and result in visible symptoms of injury in plants such as chlorosis, growth inhibition, browning of root tips, and finally death (Kahle 1993).
In addition, Cd produces disturbances in the plant antioxidant defenses, producing an oxidative stress. Concerning the mechanism of reactive oxygen species (ROS) production, Cd does not participate in Fenton-type reactions, but can indirectly favor the production of different ROS, such as hydrogen peroxide (H2O2), superoxide (O2⋅2), and hydroxyl radical (⋅OH), giving rise to an oxidative burst (Olmos et al. 2003; Romero-Puertas et al. 2003).
Therefore, ROS overproduction is as important as the antioxidative defense mechanisms may be lacking or altered (Romero-Puertas et al. 2002a, b, 2007; Rodriguez-Serrano et al. 2006; Cho and Seo 2005; Chaoui and El Ferjani 2005; Weber et al. 2006). Likewise, when diffusing through cells, cadmium changes the redox status and polarity of neighboring cells and stimulates the antioxidant defense mechanisms (Alvarez et al. 1998). ROS deleterious effects can be alleviated by detoxification activities such as superoxide dismutase (SOD), peroxidases (PRXs), or glutathione–ascorbate cycle enzymes. SOD, as a first antioxidant defense, catalyzes the conversion of two molecules of singlet oxygen (O2) in hydrogen peroxide (H2O2) and dioxygen (O2) (Noctor and Foyer 1998). H2O2 had to be rapidly metabolized to guarantee the effective protection afforded by SOD; otherwise, its accumulation can be harmful (Michiels et al. 1994). Therefore, PRX is the subsequent antioxidative defense, using H2O2 to oxidize a wide variety of substrates. Moreover, peroxidases can be involved in various physiological and biochemical processes, such as control of plant growth and lignifications (Mansouri et al. 1999; Quiroga et al. 2000).
As antioxidants, antioxidative enzymes, in concert with glutathione, ascorbate and SODs (EC 1.15.1.1), ascorbate APXs (EC 1.11.1.11), and catalases (CAT; EC 1.11.1.6), regulate reactive oxygen species levels (Noctor and Foyer 1998). Ascorbate reprocess is realized by monodehydroascorbate reductase (MDAR; EC 1.6.5.4.), dehydroascorbate reductase (DAR; EC 1.8.5.1), and glutathione reductase (GR; EC 1.6.4.2).
Cd exposure changes antioxidative enzymes’ activities. Nevertheless, different results have been observed. For example, ascorbate–glutathione-related activities were decreased after Cd stress in Helianthus annuus leaves (Gallego et al. 1996). After Cd treatment, elevated APX activities were observed in Phaseolus vulgaris roots and leaves as well as in Nicotiana tabacum suspension culture cells (Chaoui et al. 1997; Piqueras et al. 1999). In Phaseolus aureus seedlings, Cd induced elevated guaiacol PRX, but decreased CAT activities (Shaw 1995).
However, under Cd exposure, common defense pathways are launched in plant cells likewise to other biotic or abiotic stresses. Acting as a signaling molecule, H2O2 accumulation is the common first event of these pathways. In plant–pathogen interactions, a coordinated succession of reactions relating peroxidase activation, secondary metabolism enhancement, structural changes such as lignin deposition, and, eventually, cell death are induced by H2O2 (Alvarez et al. 1998). In fact, Fojtova and Kovarik (2000) illustrated apoptotic alterations in suspension cultures of tobacco cells after Cd exposure, which were characterized by DNA fragmentation.
It must be recalled that cell death (CD) is a process that cannot be inverted. After cadmium exposure, unusual development leading to xylogenesis and ultimate lignification establishment was observed (Polle et al. 1997). All the same, lignified xylem elements were established too near to the root tip at the expense of the elongation zone (Polle et al. 1997; Schutzendubel et al. 2001; Durcekova et al. 2007).
In the case of heavy metal stress, development alterations may increase heavy metal potential to bind cell walls by increasing their cation exchange capacity. Thus, cell wall composition changes due to cadmium stress may be involved in cell response optimization (Song et al. 2013a, b).
It is important to consider that the first path of metal uptake is simple diffusion through the apoplast of the root cortex and the endoderm, and through pores in networks of cellulose, hemicellulose, and glycoproteins. However, some ions may be absorbed by the negative surface charges (polygalacturonic acids and pectins) which act as ion exchangers (Briat and Lebrun 1999).
Similarly, ROS accumulation and peroxidase stimulation are involved in defense polymer polymerization in the cell wall, forming a mechanical barrier to stress and ensuring cell wall rigidity (Ferrer et al. 1991; Velikova et al. 2000).
In order to investigate the effect of cadmium on germination and early seedling growth in legumes, Medicago truncatula Gaertn. was used as a model. This wild relative of alfalfa (Medicago sativa) has been adopted as a model plant for the study of legume biology and is being studied in a large number of laboratories. M. truncatula is easily grown and maintained under laboratory conditions, and numerous genetic and genomic tools are available. The biodiversity of this species should reveal potentially important characteristics during germination and heterotrophic growth.
Seeds are crucial organs for plant life dispersal and survival, and the germination time control is a strong advantage under adverse environmental conditions (Bewley 1997). Recognized explanations for the impact of Cd on seed germination are that it produces respiratory disturbances (Sharma et al. 2013) and limitation in nutriment (minerals and carbohydrates) availability (Rahoui et al. 2010a, b, 2015; Sfaxi-Bousbih et al. 2010), but not an osmotic effect delaying the seed tissue hydration capacity (Mihoub et al. 2005; Rahoui et al. 2008).
During the last years, few works were conducted to evaluate the impact of cadmium toxicity in M. truncatula using seed germination and early growth assays and focused on (1) cadmium interference with radicle growth, (2) some oxidative stress-enhanced responses, and (3) detoxification gene alterations (Rahoui et al. 2014, 2015).
Cd toxicity mechanisms on antioxidative systems and cytological differentiation in roots of contrasted M. truncatula have not yet been tightly recognized (Ghodratollah et al. 2012; Rahoui et al. 2014).
Therefore, the aim of the present work was to focus on (1) the sequence of physiological reactions, including changes in ascorbate–glutathione-related antioxidant systems, (2) secondary metabolism (phenolics and lignifications), and (3) developmental changes and CD occurring in roots after Cd exposure. The response to Cd stress was analyzed in five M. truncatula genotypes. The seedlings were exposed to 100 μM Cd in hydroponics and used to study physiological defense reactions and anatomical changes in root tips.
Cd-treated seedling showed root growth inhibition with a genotype-dependent pattern (Rahoui et al. 2014). Oxidative status alteration was described according to ROS (Rahoui et al. 2014) and phenolics in situ observation. CD was measured and membrane integrity was analyzed using conductivity. Antioxidative and detoxification capacity alterations were analyzed in some up-described (1) ROS-scavenging enzymes—superoxide dismutases (EC 1.15.1.1), catalase (EC: 1.11.1.6), and peroxidase (EC: 1.11.1.7) and (2) ascorbate–glutathione-related antioxidant systems—APX (EC 1.11.1.11) and MDAR (EC 1.6.5.4). Root differentiation was analyzed in situ with the observation of lignification and pectin and cellulose changes.
Materials and methods
Plant material and cadmium treatment
The M. truncatula genotypes used in this study were A17 (derived from Jemalong cultivar), TN1.11 (Tunisia), TN1.21 (Tunisia), F83005.5 (France), and DZA315.16 (Algeria). The same number of seeds (between 85 and 100) for each genotype was germinated at 25 °C on filter paper moistened with H2O. Twelve-hour-old germinating seeds were transferred into square Petri dishes containing filter paper moistened with H2O or 100 μM CdCl2 solution. They were incubated in the dark at 25 °C and a relative humidity of 65 % (±5) for 48 h. Root samples, freshly harvested, were stored at −80 °C for enzyme assay or directly used for the determination of total phenolics and CD.
Root growth and germination efficiency
Embryonic axis growth was measured as root length. Germination efficiency was estimated by germination percentage (GP = germinated seeds/total seeds), calculated as a standard of radical emergence and the number of hours required for 50 % of the total number of seeds to have germinated, regarded as T 50 (Orchard 1977; Table 1).
Enzyme assays and protein determination
Root tips were powdered in liquid nitrogen. Of the fresh root samples, 1 g was homogenized in 5 ml of 50 mM potassium phosphate buffer (K2HPO4/KH2PO4, pH 7.0), 5 mM sodium ascorbate, and 0.2 mM EDTA using a chilled mortar and pestle. The homogenate was centrifuged at 12,000×g for 10 min at 4 °C, and after dialysis the supernatant was used for enzyme assays. The enzyme activities were determined according to the following methods: total SOD (Polle et al. 1989), Cu–Zn SOD and Fe SOD (Sandalio et al. 1987), CAT (Aebi 1983), APX (Nakano and Asada 1981), MDAR (Hossain et al. 1984), and PRXs (Fielding and Hall 1978).
Detection of cell death
Cell death, indicated as loss of plasma membrane integrity, was measured spectrophotometrically as Evans Blue uptake (Turner and Novacky 1974). After Cd treatment, three roots were incubated in Evans Blue solution (0.025 %, w/v, Evans Blue in water) for 15 min. Then, the roots were decolored in boiling ethanol to develop the blue precipitates, which were quantified by solubilization with 1 % (w/v) sodium dodecyl sulfate in 50 % (v/v) methanol at 50 °C for 10 min. The optical density of the supernatant was determined at 600 nm and expressed on the basis of dry mass.
Plasma membrane integrity was also estimated by measuring ion leakage from seedlings. The remaining germination media was analyzed for electrolyte leakage. Special care was taken to remove most of the medium from the filter paper by folding the paper and applying pressure. Small volumes of H2O were used to wash the medium from the filter paper. This was repeated three times (Pretorius and Small 1993). Electrolyte leakage measurements were carried out using a conductivity meter (Multi-parameter 197i VTW). The electrical conductivity of the germination media was expressed on the basis of seed number (in microohm per centimeter per seed) (Powell and Raymond 1981). Blanks containing water or 5 mM of aqueous cadmium chloride solution were included in the experiments and their conductivity values were subtracted from those of the control or Cd treatment, respectively (Perry and Harrison 1970).
Determination of phenolics
Phenolics were determined according to Swain and Hillis (1959) (Table 2). Root tissues were homogenized with 80 % (v/v) ethanol. The homogenate was agitated for 30 min with a magnetic stirrer. The ethanol was evaporated and the residues redissolved in 10 ml of water. A 1-ml aliquot of aqueous solution was added to 7.5 ml water and 0.5 ml Folin reagent. After 3 min, 1 ml of saturated NaCO3 solution was added and incubated for 1 h. The absorbance was determined after 1 h at 725 nm. Of the total phenols, 1 U corresponds to the relative absorbance of 1 ml of crude extract.
Histochemical staining of roots for phenolics and developmental changes
Root tip alterations and cell wall modifications were assayed on fresh material included in 5 % low-melting agar (agarose type I; Sigma). Root tips were sectioned with a vibratom (Leica VT 1000S). The observation of phenolic compounds was based on their absorption of UV and the emitted fluorescence (Rispail et al. 2005).
Phloroglucinol (hydrochloric solution; VWR Prolabo) coloration (10 min, violet) and Auramine-O (VWR Prolabo) coloration (10 min) were used to detect lignin (yellow) deposits (Herth 1980). Ruthenium red (0.02 %; VWR Prolabo) coloration (10 min) was applied to detect pectin (Hou et al. 1999). Cellulose was detected using calcofluor (0.01 %; VWR Prolabo) coloration (10 min) absorbing UV wavelengths and emitting in the blue or green (Kilbey 1977).
Seven plants were sectioned for each biological replicate. All images were obtained using an inverted microscope (Leica DMIRBE), and images were acquired with a CCD camera (color-cooled view; Photonic Science). For the autofluorescence test, excitation range of 340–380 nm, dichroic mirror of 400 nm, and long-pass emission filter of 425 nm were used.
Statistics
The measurements were performed with three independent biological repeats, as indicated in the figure legends. ANOVA test was performed to evaluate significant treatment effects at a significance level of P ≤ 0.05.
Results
Responses of antioxidant systems to Cd exposure
As already described in a previous work, Cd treatment significantly affected root length—responses to the metal varied among genotypes—and induced the accumulation of ROS in roots (Rahoui et al. 2014). Hence, we investigated enzyme activities related to oxidative stress and antioxidant defense.
ROS-scavenging activities
Superoxide dismutases
-
1.
Total SOD activity changed in Cd-treated plants compared to the water-treated controls (Fig. 1a). Line F83005.5 showed strong induction of enzyme activity estimated to a factor of 21.5. Lines A17, DZA315.16, and TN1.21 presented a weak induction of total SOD levels, with induction factors of 1.8, 1.4, and 1.5, respectively. Finally, TN1.11 showed an insignificant alteration of enzyme activity. The enzyme levels in the control plants differed among genotypes, with higher levels in TN1.11 and TN1.21.
Fig. 1 ROS-scavenging enzymes. Total SOD (a), Fe/MnSOD (b), and Cu–ZnSOD (c) activities in M. truncatula 48-h-old seedlings (genotypes A17, DZA315.16, F83005.5, TN1.11, and TN1.21) after imbibing with H2O (white) or 100 μM CdCl2 (black). Data are the mean values of three independent experiments ± SE. Values are the means of three measures. Each measure was carried out with 12 seeds. Data with asterisk are not significantly different from respective controls at the 0.05 level of probability
-
2.
Fe/Mn SOD activities were not significantly altered in Cd-treated A17 and TN1.21 genotypes (Fig. 1b). TN1.11, F83005.5, and DZA315.16 lines showed an induction of these enzyme activities, estimated to a factor of about 1.5.
-
3.
Cu–Zn SOD activity changed in A17, F83005.5, and DZA315.16 Cd-treated plants compared to the water-treated controls, showing induction factors estimated at 3.9 in A17 and 1.2 in F83005.5 and DZA315.16 (Fig. 1c). Cu–Zn SOD activity was unchanged in TN1.21 and depressed in TN1.11 to a factor of 0.5.
Catalase
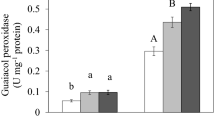
CAT activity was induced in all Cd-treated plants compared to the controls (Fig. 2a) and was not significantly modified in line A17. Line F83005.5 presented only a weak stress response, with an increase in CAT levels (2.98 times the control), whereas lines TN1.11, DZA315.16, and TN1.21 showed strong enhanced activity levels ranging from 4.6 to 7.73 times the control. The enzyme levels in the control plants differed among genotypes, with higher levels in TN1.21 and F83005.5.
CAT (a) and PRX (b) activities in M. truncatula 48-h-old seedlings (genotypes A17, DZA315.16, F83005.5, TN1.11, and TN1.21) after imbibing with H2O (white) or 100 μM CdCl2 (black). Data are the mean values of three independent experiments ± SE. Values are the means of three measures. Each measure was carried out with 12 seeds. Data with asterisk are not significantly different from respective controls at the 0.05 level of probability
Peroxidase
PRX activity levels were also increased in four genotypes by cadmium exposure (Fig. 2b). PRX activity increased in lines TN1.11, TN1.21, F83005.5, and DZA315.16, with induction factors of 2.61, 3.93, 3.16, and 18-fold, respectively, and was not significantly modified in line A17. The enzyme levels in the control plants differed among genotypes, with the highest levels in F83005.5.
Ascorbate–glutathione-related antioxidant system activities
Monodehydroascorbate reductase
MDAR activity was found to be enhanced in Cd-treated plants compared to the controls (Fig. 3a) and was not significantly modified in line A17. Lines DZA315.16, F83005.5, and TN1.11 showed enhanced activity levels ranging from 2.59, 3.92 to 6.91 times the control. Line TN1.21 showed a very strong response, with an increase of MDAR activity of 21.6 times the control.
Ascorbate–glutathione-involved activities. MDAR (a) and APX (b) in M. truncatula 48-h-old seedlings (genotypes A17, DZA315.16, F83005.5, TN1.11, and TN1.21) after imbibing with H2O (white) or 100 μM CdCl2 (black). Data are the mean values of three independent experiments ± SE. Values are the means of five measures. Each measure was carried out with 12 seeds. Data with asterisk are not significantly different from respective controls at the 0.05 level of probability
Ascorbate peroxidase
APX activity was altered in Cd-treated plants of four genotypes compared to their respective water-treated controls (Fig. 3b) and was not significantly modified in line TN1.11. Line A17 presented a slight decrease in enzyme activity. In contrast, lines DZA315.16, F83005.5, and TN1.21 exhibited enhanced APX activity levels, with induction factors ranging from 3.17 to 5.69.
Secondary metabolism and developmental changes
Phenolics
The levels of phenolic compounds significantly varied as a function of genotype and treatment (Table 2). Lines F83005.5, TN1.21, and DZA315.16 exhibited an induction of the phenolic compound levels ranging from 1.54, 2.65 to 3.60 times the control. Lines A17 and TN1.11 responded more strongly to metal treatment, with induction factors of 6.90 and 7.89 times the control, respectively.
UV fluorescence revealed a significantly increased presence of phenolic compounds in sections of Cd-treated roots, visible as a blue fluorescence, which seemed to be evenly distributed in the cell lumen (Fig. 4). This was observed in the different section levels (500, 1000, and 1500 μm from the apex). Blue fluorescence was abundant in the cortex of Cd-treated roots, but almost absent in the control roots. It was observed that line TN1.11 exhibited the strongest response, with intense fluorescence in many cells.
Phenolic compounds observed in 48-h-old M. truncatula roots. a F83005.5, control root (section level, 1500 μm). Scale bar, 200 μm. b F83005.5, treated root (section level, 500 μm). Scale bar, 200 μm. c TN1.21, control root (section level, 1000 μm). Scale bar, 100 μm. d TN1.21, treated root (section level, 1000 μm). Scale bar, 100 μm. e TN1.21, treated root (section level, 1500 μm). Scale bar, 200 μm. f TN1.11, treated root (section level, 1500 μm). Scale bar, 200 μm. g TN1.21, control root (section level, 1500 μm). Scale bar, 100 μm. h A17, treated root (section level, 1500 μm). Scale bar, 100 μm. i DZA315.16, control root (section level, 500 μm). Scale bar, 200 μm. j DZA315.16, treated root (section level, 500 μm). Scale bar, 200 μm. Phen phenolics, R.H. root hairs, Xyl xylogenesis
Lignification
Lignin deposition was first assessed by auramine O staining in the different root sections (500, 1000, and 1500 μm from the apex), in Cd-treated roots, and in water-treated controls. An intense yellow staining was observed in the epidermal cells of Cd-treated roots, but not in the controls, for all five genotypes (Fig. 5). Similarly, this coloration showed that lignin deposits were also increased in the xylem of treated roots. This staining highlighted a developed xylogenesis in treated roots in comparison with the controls (Fig. 5b, e, d).
Auramine O staining in 48-h-old M. truncatula roots. a F83005.5, control root (section level, 500 μm). Scale bar, 200 μm. b F83005.5, treated root (section level, 500 μm). Scale bar, 200 μm. c DZA315.16, control root (section level, 500 μm). Scale bar, 100 μm. d DZA315.16, treated root (section level, 1500 μm). Scale bar, 100 μm. e A17, treated root (section level, 1500 μm). Scale bar, 100 μm. f TN1.21, treated root (section level, 1500 μm). Scale bar, 100 μm. g TN1.11, treated root (section level, 500 μm). Scale bar, 100 μm. h DZA315.16, treated root (section level, 500 μm). Scale bar, 100 μm. Lig lignin, R.H. root hairs, Xyl xylogenesis
Phloroglucinol coloration exemplified lignin in the cell walls of both control and treated roots. However, through this coloration, it was possible to observe the presence of intercellular meatus, appearing as black spots on sections of Cd-treated roots only (Fig. 6).
Cell death
The induction of cell death by cadmium was studied using two approaches. Staining with Evans Blue dye was used as a marker of CD. The results obtained after extracting this dye from the roots are shown in Fig. 6a. Roots treated with cadmium showed a higher penetration by the dye in comparison with the control plantlets, except in line DZA315.16. The measurement of Evans Blue extracted from the roots showed that lines A17, TN1.11, F83005.5, and TN1.11 responded, with increases of 1.25, 1.84, 2.08, and 3.21 times the control. Considering ion leakage as a CD marker, it could be observed that cadmium increased conductivity in the treated samples (Fig. 7b) of four genotypes. Line F83005.5 did not show significant variation of conductivity after exposure to Cd. It should be noted that the levels of cell death in the controls differed strongly depending on the genotype and the marker.
a Estimated cell death as Evans Blue penetration in M. truncatula 48-h-old seedlings (genotypes A17, DZA315.16, F83005.5, TN1.11, and TN1.21) after imbibing with H2O or 100 μM CdCl2. Data are the mean values of three independent experiments ± SE. Values are the means of three measures. Each measure was carried out with 12 seeds. b Electrical conductivity of germination media (genotypes A17, DZA315.16, F83005.5, TN1.11, and TN1.21) after imbibing with H2O (0 μM) or Cd (100 μM). Values are the means of four measures ± SE. Each measure was carried out with an imbibed medium of 60 seeds. Data with asterisk are not significantly different from respective controls at the 0.05 level of probability
These results demonstrated that cadmium-induced damage in root cells could produce CD.
Secondary cell wall
Cellulose
Calcofluor staining of root sections showed a thickening of the phloem and xylem in Cd-treated roots (Fig. 8). Cellulose fluorescence in Cd-treated roots was more intense than in the controls, particularly in the xylem and phloem, suggesting a modification of these tissues which are developed close to the apex under cadmium stress in detriment of the elongation zone in the treated roots (Fig. 8). These observations were made in the different sections (500, 1000, and 1500 μm from the apex). Similarly, cellulose staining revealed cells with irregular shapes and well-developed intercellular meatus.
Calcofluor staining in 48-h-old M. truncatula roots. a F83005.5, control root (section level, 500 μm). Scale bar, 200 μm. b F83005.5, treated root (section level, 500 μm). Scale bar, 200 μm. c A17, control root (section level, 1500 μm). Scale bar, 200 μm. d A17, treated root (section level, 1500 μm). Scale bar, 200 μm. e A17, control root (section level, 1500 μm). Scale bar, 100 μm. f A17, treated root (section level, 1500 μm). Scale bar, 100 μm. g A17, treated root (section level, 1500 μm). Scale bar, 100 μm. h A17, treated root (section level, 1000 μm). Scale bar, 100 μm. R.H. root hairs, Xyl xylogenesis, Mea meatus
Pectin
As with cellulose, pectin staining by Ruthenium red showed an increase of stain intensity in Cd-treated roots, particularly in the xylem and phloem cells which were developed close to the apex in the detriment of the elongation area, as we have shown above (Fig. 9). Moreover, we noticed that the treated roots were drastically larger than the controls at the same observation level. These results clearly showed that cell wall development was modified due to cadmium stress. In addition, pectin coloration highlighted the particular form of hairs, numerous, condensed, enlarged, and inflated in the treated samples. Finally, as revealed with cellulose staining, we noticed intercellular meatus.
Ruthenium red staining in 48-h-old M. truncatula roots. a A17, control root (section level, 1000 μm). Scale bar, 200 μm. b A17, treated root (section level, 500 μm). Scale bar, 200 μm. c TN1.11, control root (section level, 1000 μm). Scale bar, 200 μm. d TN1.11, treated root (section level, 1000 μm). Scale bar, 200 μm. e F83005.5, control root (section level, 1500 μm). Scale bar, 200 μm. f F83005.5, treated root (section level, 500 μm). Scale bar, 100 μm. R.H. root hairs, Xyl xylogenesis, Mea meatus
Discussion
It is well known that Cd inhibits growth (Rahoui et al. 2008, 2010a, b; Sfaxi-Bousbih et al. 2010) and affects antioxidative metabolism (Rauser 1995; Zenk 1996; Xiang and Oliver 1998; Arisi et al. 2000; Rahoui et al. 2014). In previous works on Cd stress in M. truncatula (Rahoui et al. 2014, 2015), reduction of seed germination and early seedling growth were reported and focused on the mechanisms of heavy metal toxicity (capacity of moisture, reserve mobilization, nutrient availability, and oxidative status) in 6-day-old seedling of the same genotypes as used in the present work. Root growth inhibition (percent of the control) values under treatment with 100 μM of CdCl2 in the genotypes were as follows: A17, 69 %; TN1.11, 76 %; TN1.21, 68 %; F83005.5, 61 %; and DZA315.16, 55 % (Rahoui et al. 2014).
It was proposed that seedlings may survive in Cd-contaminated medium through the efficiency of some components of the antioxidative system to avoid an oxidative burst, through reduced metal uptake by cadmium chelating within roots and the induction of antioxidant defenses during germination. Differential responses among accessions suggested that either there are different tolerance mechanisms that are not shared by all accessions or that tolerance mechanisms are common to all accessions, but differ in their levels or intensities. In the present study, biochemical measurements of antioxidative system and developmental changes were performed, thus enabling in-depth investigation on the control of Cd tolerance in the model legume M. truncatula.
Cd induces oxidative stress
ROS accumulation, as reported in a previous work (Rahoui et al. 2014), might be due to the activation of enzymes such as SOD (Karlsson et al. 2005) and cell wall NADH oxidase, which could lead, in part, to H2O2 accumulation at the apoplasmic level (Bolwell et al. 2002; Ranieri et al. 2003; Chaoui and El Ferjani 2005). Likewise, H2O2 accumulation could result in the oxidative polymerization of some defense polymers in the cell wall, forming a mechanical barrier to counteract stresses (Velikova et al. 2000).
Antioxidant system alterations have been widely reported in seedlings following metal exposure. The deleterious effects resulting from ROS accumulation and altered cellular oxidative state may be alleviated by detoxifying enzymes, such as SOD, CAT, and enzymes of glutathione (GSH)–ascorbate (Sandalio et al. 2001; Schutzendubel et al. 2002).
The analysis of enzyme activity in the present study showed that Cd induced an enhancement of total SOD in four out of five genotypes and a decrease in line TN1.11. The changes observed for enzyme activity are in agreement with a previous study on gene expression (Rahoui et al. 2014). Downregulation in line TN1.11 result might be related to the fact that this line is the least vital in stress conditions. Nevertheless, Cd caused alterations of the activity of Fe/MnSOD and Cu–ZnSOD isoforms in various response patterns depending on the genotypes. In fact, Fe/MnSODs were enhanced only in tolerant lines, namely, F83005.5 and DZA315.16 (Fig. 1b), while Cu–ZnSOD was significantly enhanced only in A17 after Cd treatment (Fig. 1c). This suggests a differential sensitivity of the SOD isoforms to Cd stress in M. truncatula seedlings. In concert with our results, Cd stress induced upregulated expression of Fe/MnSOD genes in Cd-treated perennial ryegrass plants (Luo et al. 2011). Overexpression of the MnSOD isoform in N. tabacum led to a higher tolerance to abiotic stresses such as the herbicide parquet (Bowler et al. 1992) and ozone damage (Van Camp et al. 1994). Nevertheless, solely higher total SOD activity was detected in Cd-tolerant transgenic N. tabacum, while MnSOD activity and the gene expression levels did not differ in both the control and Cd-treated plants (Ortega-Villasante et al. 2011).
Inhibition of Cu–ZnSOD activity was observed in leaves and roots after heavy metal stress and was attributed to a metabolic disorder caused by the accumulation of hydrogen peroxide, which acts as an inhibitor of this isoform (Asada et al. 1974; Sandalio et al. 2001; Dixit et al. 2001). The inhibition of Cu–ZnSOD activity can also be attributed to its sensitivity to zinc deficiency and its possible substitution by Cd (Van Assche and Clijsters 1990; Aravind and Prasad 2003). Indeed, Bauer et al. (1980) reported that Cd can interfere with Cu–ZnSOD taking the place of Zn in the molecule and, thus, forming eventually an inactive enzyme Cu111Cd SOD. Thereby, ROS accumulation and zinc substitution seem to be the causes of the decrease in the activity of Cu–ZnSOD. Moreover, Romero-Puertas et al. (2002a, b) suggested that the decreased activity of the isoform Cu–ZnSOD under Cd exposure should be related to the change of its biosynthesis at the transcriptional and translational levels.
H2O2 produced by SODs has to be rapidly metabolized to guarantee the effective protection afforded by these activities; otherwise, its accumulation can be harmful (Michiels et al. 1994). Under cadmium exposure, PRX and CAT activities were enhanced in four out of five lines. A17 did not respond with a significant increase. In contrast to its SOD response, line TN1.11 showed induction of these two enzymes. These results are in agreement with the reported induction of PRX gene expression by Cd treatment in all genotypes (Rahoui et al. 2014).
Our results show that Cd stress induces an upregulation of PRX, CAT, and total SOD activities to counteract ROS and oxidative burst (Rahoui et al. 2014). It has been reported that SOD activity increased during wheat germination as a response to arsenic (Li et al. 2007). Likewise, SOD gene expression was upregulated in maize seedlings by chrome treatment (Labra et al. 2006), and SOD and PRX activities increased in response to lead stress in rice seedlings (Verma and Dubey 2003).
In contrast, rice seedlings showed PRX activity induction after Cd treatment, while SOD activity was not affected (Guo-ying et al. 2000). Moreover, PRX upregulation seems to be correlated to root growth alterations and cell wall strengthening (Passardi et al. 2006). Furthermore, it has been suggested that peroxide overproduction could directly injure SOD and CAT macromolecules and, thus, triggering oxidative injury and illustrating heavy metal toxicity (Sandalio et al. 2001; Romero-Puertas et al. 2007).
Peroxide accumulation has been observed after Cd exposure in the roots of Pisum sativum and M. sativa (Ortega-Villasante et al. 2005; Rodriguez-Serrano et al. 2006). After cadmium exposure, ROS overproduction was also observed in Alyssum plants, radish roots, and Coffea arabica cells (Schickler and Caspi 1999; Vitoria et al. 2001; Gomes-Junior et al. 2006). These divergences are dependent on the metal type, concentration, and treatment period, in addition to the plant tissue and species. For instance, SOD increased under short Cd treatment, but decreased after long-term exposure in Allium sativum plants (Zhang et al. 2005).
Antioxidative enzymes (SOD and CAT), together with ascorbate APX and glutathione, control the cellular concentrations of H2O2 and O2 (Noctor and Foyer 1998). Recycling of ascorbate and GSH is achieved by monodehydroascorbate reductase (MDAR), DAR, and GR.
In the present study, Cd treatment affected APX and MDAR; contrasting results were obtained in different genotypes. APX was slightly inhibited in A17, not significantly affected in TN.11 under cadmium exposure, but enhanced in the other genotypes. In contrast, Cd enhanced MDAR activity in all the tested genotypes. Similar results were reported for various other plants. For example, ascorbate–glutathione-associated defense enzymes were reduced in the leaves of Cd-exposed H. annuus plants (Gallego et al. 1996). Elevated APX activities were reported in the roots and leaves of P. vulgaris, as well as in suspension cultures of N. tabacum cells after Cd treatment (Chaoui et al. 1997; Piqueras et al. 1999). MDAR was upregulated in plants with reduced CAT and APX levels (Chamnongpol et al. 1998). Nevertheless, Cd repressed H2O2 detoxication systems such as GR, CAT, and APX activities and improved SOD activities in Scots pine roots (Schutzendubel et al. 2001).
Cd initiates secondary metabolism and differentiation
The amount of total phenolic compounds and their induction by Cd treatment varied in the different accessions and were particularly enhanced in the more sensitive lines (A17 and TN1.11; Table 2). We noticed that Cd triggered cytosolic soluble phenolic accumulation, a response which was much earlier than lignification and extended over the entire root cross-section (Fig. 4).
Phenolics may protect from oxidative injury. In fact, in concert with ascorbate and APX, phenolics participate in H2O2 annihilation (Polle et al. 1997). Phenolic compounds are electron donors for NADH oxidase and coniferyl–alcohol–peroxidase enzymes. They are known for their protective role in plants and participate in several compound biosynthesis such as lignin, suberin, or flavonoids (Van Tunen and Mol 1990; Santiago et al. 2000).
After heavy metal exposure, cell wall lignification was revealed as strictly related to ROS levels and their oxidative injury (Chaoui and El Ferjani 2005; Ali et al. 2006; Bouazizi et al. 2010), exemplifying that ROS could be a switch signal that induces lignin metabolism activation as a defense mechanism under stress exposure to restore unbalanced cellular oxidative system (Schutzendubel et al. 2001).
During exposure of plantlets to Cd, metal toxic effects on cell viability were evidenced by Evans Blue staining and by measuring electrolyte leakage in root tips. These two parameters have been considered as indicators of CD in plantlets, although they only give information about plasma membrane integrity. Compared to the control roots, Cd-treated roots showed an increase of relative conductivity. This might be due to one or more of several factors, namely, Cd-mediated oxidative injury altering membrane integrity, lipid peroxidation, and loss of essential nutrients (Rahoui et al. 2010a, b). Taken together, the higher penetration of Evans Blue and the increase of ion leakage in cadmium-exposed plants could be an indicator of oxidative deterioration and accelerated senescence induced by cadmium (Schutzendubel et al. 2001; Romero-Puertas et al. 2002a, b). Although line TN1.21 responded to Cd stress by induction of cell death, as assessed by Evans Blue penetration, the absolute values were lowest in the controls and Cd-treated roots compared to the other genotypes. It should be noted that line TN1.21 was considered as tolerant based on root growth inhibition (Rahoui et al. 2014). While our data only show the toxic effects of Cd on cell viability, but are no proof of CD, other studies have demonstrated that heavy metals induced CD. Schutzendubel et al. (2001) reported that high Cd concentrations led to transient increase in CD. Fojtova and Kovarik (2000) reported that Cd activated apoptotic alterations in tobacco cell suspension cultures that were illustrated by DNA fragments observed 48 h after Cd treatment.
CD occurs as part of plants’ normal developmental program and is tightly controlled in committed cells all through xylogenesis (Teichmann 2001). H2O2 is an intermediate signal molecule in CD (Alvarez et al. 1998), and a rapid H2O2-mediated strengthening of the cell walls would explain the fast abolishment of growth due to Cd treatment, which was noticeable after less than 48 h (Rahoui et al. 2014).
Unlike necrosis, CD is severely controlled, and once cells are dedicated to CD, the process cannot be upturned (Schutzendubel et al. 2001). In our study (Fig. 7), we did not observe arbitrary injury in the cross-sections of roots (Figs. 4, 5, 6, 8, and 9) as an expectation for necrotic reactions, suggesting that Cd-induced loss of viability might correspond to CD rather than to necrosis. In contrast to its effect on cell viability, Cd induced xylogenesis since “normal” protoxylem elements were observed. Nevertheless, in contrast to normal growth suggesting that lignification is the final step in this process (Polle et al. 1997), lignified xylem elements were established here already at a very short distance from the root tip, usually corresponding to the elongation area.
Cell wall composition varied under cadmium exposure, as shown by observations of cellulose and pectin staining (Figs. 8 and 9). In addition to lignification, the deposition of proteins, glycoproteins, and polysaccharides can lead to cell wall reinforcement (Vorwerk et al. 2004; Passardi et al. 2004).
It should be kept in mind that the main mechanism of metal uptake relies on a simple diffusion through the root cortex apoplast and the endoderm, and, namely, through pores in the network of cellulose, hemicelluloses, and glycoproteins. Yet, some ions can be absorbed by negative charges at the cell wall surface, which acts as ion exchangers (Briat and Lebrun 1999). In response to heavy metal stress, cell wall modifications could increase its potential for entrapping metals by increasing, for example, the capacity of cationic exchange. Heavy metal binding to cell wall depends hugely on the negative charge density, which itself depends on pectin methylation. These charge densities and their distribution within the parietal compartment then determine the cell wall cationic exchange capacity (CEC; Haynes 1980).
Muschitz et al. (2009) have shown induced changes in cell wall CEC in response to Zn stress. In our study, slight increases in pectin content were observed. Increases in pectin levels were correlated with important aluminum levels in Zea mays cells (Schmohl and Horst 2000). In the same way, pectin increase in the walls of a tobacco cell suspension (Chang et al. 1999) or for As-treated wheat seedlings has been noted (Hossain et al. 2006). Studies relating the effect of abiotic stress on the organization and distribution of polymers in the cell wall are rather scarce. However, it seems plausible that the changes in the wall composition following stress brought about by heavy metals could be involved in optimizing the cellular response. The role of cell walls in the subcellular compartmentalization of Zn, Cd, and Cu was highlighted in many works (Giguère et al. 2006; Nyquist and Greger 2007), but the molecular bases of detoxification at the level of the wall are not well understood yet (Song et al. 2013a, b).
Cell wall lignification leads to growth inhibition. The increase of peroxidase activity and the decrease of root elongation were reported to be correlated (Jackson and Ricardo 1998). Heavy metals stimulate lignin polymerization, leading to an increase of cell wall mechanical resistance (Degenhardt and Gimmler 2000; Ghanati et al. 2005). The thickening of the Casparian band under metallic stress is a form of defense adopted by plants (Degenhardt and Gimmler 2000).
In addition to early development of the endoderm and ectoderm, many changes in root anatomy are recorded when it is exposed to high Cd concentrations (Seregin and Kozhevnikova 2008). These changes depend on the metal concentration, plant species, and tissue.
In agreement with our observations in this work (Figs. 4, 5, 6, 8, and 9), Cd has been described to reduce root growth and increase root hair production near the apex in maize, radish, barley, sorghum, and Rhodes grass, suggesting that Cd accelerates cell senescence (Durcekova et al. 2007; Kuriakose and Prasad 2008; Kopittke et al. 2010). Cd treatment also disintegrated the rhizodermis and external cortical cell layers (Kuriakose and Prasad 2008; Kopittke et al. 2010; Gratao et al. 2009), which was accompanied by loss of cell turgor and formation of intercellular air-carrying meatus and cortical cells whose epidermis was irregularly shaped (Lunackova et al. 2003; Vitoria et al. 2003).
In line with our results, several studies have shown that the root becomes shorter and thicker without setting off necrotic reactions under cadmium stress (Lunackova et al. 2003; Maksimovic et al. 2007). Maksimovic et al. (2007) explained this by an increase of the size of the parenchyma cells and suggest that the expansion of the cortical tissue has a functional role in increasing the resistance to radial flow of water and solutes.
Vitoria et al. (2003) observed the proliferation of cambium cells followed by loss of the organization of the cambial region in radish roots exposed to 0.5 mM Cd. These authors suggested that Cd accelerates the maturation of the root with the development of the xylem element in the central cylinder. Consistent with this interpretation, Schutzendubel et al. (2001) found that exposure to 50 μM Cd causes accelerated protoxylemic lignification elements near the radical apex in Scots pine (Pinus sylvestris L.), and Durcekova et al. (2007) observed a premature xylogenesis in barley roots exposed to Cd. The role of these developmental changes in heavy metal tolerance remains to be further investigated.
Abbreviations
- APX:
-
Ascorbate peroxidase
- Cd:
-
Cadmium
- CAT:
-
Catalase
- FW:
-
Fresh weight
- MDAR:
-
Monodehydroascorbate reductase
- CD:
-
Cell death
- PRX:
-
Peroxidase
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Aebi H (1983) Catalase. In: Bergmeyer H (ed) Methods of enzymatic analysis 3. Verlag Chemie, Weinheim, pp 273–277
Ali MB, Singh N, Shohael AM, Hahn EJ, Paek KY (2006) Phenolics metabolism and lignin synthesis in root suspension cultures of Panax ginseng in response to copper stress. Plant Sci 171:147–154
Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Aravind P, Prasad MNV (2003) Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L.: a free floating freshwater macrophyte. Plant Physiol Biochem 41:391–397
Arisi ACM, Mocquot B, Lagriffoul A, Mench M, Foyer CH, Jouanin L (2000) Responses to cadmium in leaves of transformed poplars overexpressing gamma-glutamylcysteine synthetase. Physiol Plant 109:143–149
Asada K, Kiso K, Yoshikawa K (1974) Univalent reduction on molecular oxygen by spinash chloroplasts on illumination. J Biol Chem 249:2175–2181
Bauer R, Demeter I, Hasemann V, Johansen JT (1980) Structural properties of the zinc site in Cu,Zn-superoxide dismutase; perturbed angular correlation of gamma ray spectroscopy on the Cu,111Cd-superoxide dismutase derivative. Biochem Biophys Res Commun 94:1296–1302
Bewley DJ (1997) Seed germination and dormancy. Plant Cell 9:1055–1066
Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three component system. J Exp Bot 53:1367–1376
Bouazizi H, Jouili H, Geitmann A, El Ferjani E (2010) Structural changes of cell wall and lignifying enzymes modulations in bean roots in response to copper stress. Biol Trace Elem Res 136:232–40
Bowler C, Montagu MV, Inze D (1992) Superoxide dismutase and stress tolerance. Annu Rev Plant Physiol Plant Mol Biol 43:83–116
Briat JF, Lebrun M (1999) Plant responses to metal toxicity. Comptes-Rendus de l’Académie des Sciences III43-54
Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Van Montagu M, Inzé D, Van Camp W (1998) Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci U S A 95:5818–5823
Chang YC, Yamamoto Y, Matsumoto H (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22:1009–1017
Chaoui A, El Ferjani E (2005) Effects of cadmium and copper on antioxidant capacities, lignification and auxin degradation in leaves of pea (Pisum sativum L.) seedlings. C R Biol 328:23–31
Chaoui A, Mazhoudi S, Ghorbal MH, El Ferjani E (1997) Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci 127:139–147
Cho UH, Seo NH (2005) Oxidative stress in Arabidopsis thaliana exposed to cadmium is due to hydrogen peroxide accumulation. Plant Sci 168:113–120
Degenhardt B, Gimmler H (2000) Structural adaptations of corn roots (Zea mays) to environmental stress. J Exp Bot 51:595–603
Dixit V, Pandey V, Shyam R (2001) Differential antioxidative responses to cadmium in roots and leaves of pea. J Exp Bot 52:1101--1109
Durcekova K, Huttova J, Mistrik I, Olle M, Tamas L (2007) Cadmium induces premature xylogenesis in barley roots. Plant Soil 290:61–68
Ferrer MA, Muñoz R, Barcelö AR (1991) A biochemical and cytochemical study of cuticle-associated peroxidases in Lupinus. Ann Bot 67:561–568
Fielding JL, Hall JL (1978) A biochemical and cytochemical study of peroxidise activity in roots of Pisum sativum: I. A comparison of dab-peroxidase and guaiacol peroxidase with particular emphasis on the properties of cell wall activity. J Exp Bot 29:969–981
Fojtova M, Kovarik A (2000) Genotoxic effect of cadmium is associated with apoptotic changes in tobacco cells. Plant Cell Environ 23:531–537
Gallego SM, Benavides MP, Tomaro ML (1996) Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Sci 121:151–159
Garmash EV, Gorlovka TK (2009) Effect of cadmium on growth and respiration of barley plants grown under two temperature regimes. Russ J Plant Physiol 56:343–347
Ghanati F, Morita A, Yokota H (2005) Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil 276:133–41
Ghodratollah S, Rickauer M, Gentzbittel L (2012) Tolerance for cadmium pollution in a core-collection of the model legume, Medicago truncatula L. at seedling stage. Aust J Crop Sci 6:641–648
Giguère A, Campbell PG, Hare L, Couture P (2006) Sub-cellular partitioning of cadmium, copper, nickel and zinc in indigenous yellow perch (Perca flavescens) sampled along a polymetallic gradient. Aquat Toxicol 77:178–189
Gomes-Junior RA, Moldes CA, Delite FS, Pompeu GB, Gratao PL, Mazzafera P, Lea PJ, Azevedo RA (2006) Antioxidant metabolism of coffee cell suspension cultures in response to cadmium. Chemosphere 65:1330–1337
Gratao PL, Monteiro CC, Rossi ML, Martinelli AP, Peres LEP, Medici LO, Lea PJ, Azevedo RA (2009) Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ Exp Bot 67:387–394
Guo-ying Y, Guo-ping W, Chi-quan H (2000) Comparison of physiological responses to oxidative and heavy metal stress in seedlings of rice paddy, Oryza sativa L. J Environ Sci 12:458–462
Hassan SA, Fariduddin Q, Ali B, Hayat S, Ahmad A (2009) Cadmium: toxicity and tolerance in plants. J Environ Biol 30:165–174
Haynes RJ (1980) Ion exchange properties of roots and ionic interactions within the root apoplasm: their role in ion accumulation by plants. Bot Rev 46:75–99
Herth W (1980) Calcofluor White and Congo Red inhibit chitin microfibril assembly of Poterioochromonas: evidence for a gap between polymerization and microfibril formation. J Cell Biol 87:442–450
Hossain MA, Nakano Y, Asada K (1984) Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol 25:385–395
Hossain MZ, Hossain MD, Fujita M (2006) Induction of pumpkin glutathione S-transferase by different stresses and its possible mechanisms. Biol Plant 50:210–218
Hou WC, Chang WH, Jiang CM (1999) Carboxyl group distributions in pectins. Bot Bull Acad Sin 40:115–119
Jackson P, Ricardo CPP (1998) The changing peroxidase polymorphism in Lupinus albus during vegetative development. Aust J Plant Physiol 25:261–269
Kahle H (1993) Response of roots of trees to heavy metals. Environ Exp Bot 33:99–119
Karlsson M, Melzer M, Prpkhorenko I, Johansson T, Wingsle G (2005) Hydrogen peroxide and expression of hipl-superoxide dismutase are associated with the development of secondary cell walls in Zinnia elegans. J Exp Bot 56:2085–2093
Kilbey BJ (1977) A review of genetic studies with fluorescent whitening agents using bacteria, fungi and mammals. Mutat Res 39:177–188
Kopittke PM, Blamey FPC, Asher CJ, Menzies NW (2010) Trace metal phytotoxicity in solution culture: a review. J Exp Bot 61:945–954
Kuriakose SV, Prasad MNV (2008) Cadmium stress affects seed germination and seedling growth in Sorghum bicolor L. Moench by changing the activities of hydrolyzing enzymes. Plant Growth Regul 54:143–156
Labra M, Gianazza E, Waitt R, Eberini I, Sozzi A, Regondi S, Grassi F, Agradi E (2006) Zea mays L. protein changes in response to potassium dichromate treatments. Chemosphere 62:1234–1244
Li CX, Feng SL, Shao Y, Jiang LN, Lu XY, Hou XL (2007) Effects of arsenic on seed germination and physiological activities of wheat seedlings. J Environ Sci 19:725–732
Lunackova L, Sottnikova A, Masarovicova E, Lux A, Stresko V (2003) Comparison of cadmium effect on willow and poplar in response to different cultivation conditions. Biol Plant 47:03–411
Luo H, Li H, Zhang X, Fu J (2011) Antioxidant responses and gene expression in perennial ryegrass (Lolium perenne L.) under cadmium stress. Ecotoxicology 20:770–778
Maksimovic M, Vidic D, Milos M, Solic ME, Abadzic S, Siljak-Yakovlev S (2007) Effect of the environmental conditions on essential oil profile in two Dinaric Salvia species: S. brachyodon Vandas and S. officinalis L. Biochem Syst Ecol 35:473–478
Mansouri I, Mercado JA, Santiago-Domenech N, Pliego-Alfaro F, Valpuesta V, Quesada MA (1999) Biochemical and phenotypical characterization of transgenic tomato plants overexpressing a basic peroxidase. Physiol Plant 106:355–362
Mendiola J, Moreno JM, Roca M, Vergara-Juárez N, Martínez-García MJ, García-Sánchez A, Elvira-Rendueles B, Moreno-Grau S, López-Espín JJ, Ten J, Bernabeu R, Torres-Cantero AM (2011) Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: a pilot study. Environ Health 10:6
Michiels C, Raes M, Toussaint O, Remacle J (1994) Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17:235–248
Mihoub A, Chaoui A, El Ferjani E (2005) Biochemical changes associated with cadmium and copper stress in germinating pea seeds (Pisum sativum L.). C R Biol 328:33–41
Muschitz A, Faugeron C, Morvan H (2009) Response of cultured tomato cells subjected to excess zinc: role of cell wall in zinc compartmentation. Acta Physiol Plant 31:1197–1204
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:860–867
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Nyquist J, Greger M (2007) Uptake of Zn, Cu, and Cd in metal loaded Elodea canadensis. Environ Exp Bot 60:219–226
Olmos E, Martinez-Solano JR, Piqueras A, Hellin E (2003) Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot 54:291–301
Orchard T (1977) Estimating the parameters of plant seedling emergence. Seed Sci Technol 5:61--69
Ortega-Villasante C, Rellán-Álvarez R, del Campo FF, Carpena-Ruiz RO, Hernández LE (2005) Cellular damage induced by cadmium and mercury in Medicago sativa. J Exp Bot 56:2239–2251
Ortega-Villasante C, Sobrino-Plata J, Carpena R, Verbruggen N, Escobar C, Hernandez L (2011) Oxidative stress induced by cadmium in transgenic Nicotiana tabacum over-expressing a plastidial Mn-superoxide dismutase. Funct Plant Sci Biotech 5:62–67
Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9:534–540
Passardi F, Tognolli M, De Meyer M, Penel C, Dunand C (2006) Two cell wall associated peroxidases of Arabidopsis influence root elongation. Planta 223:965–974
Perry DA, Harrison JG (1970) The deleterious effect of water and low temperature on germination of pea seed. J Exp Bot 67:504–512
Piqueras A, Olmos E, Martinez-Solano JR, Hellin E (1999) Cd-induced oxidative burst in tobacco BY2 cells: time course, subcellular location and antioxidant response. Free Radic Res 31:33–38
Polle A, Krings B, Rennenberg H (1989) Superoxide dismutase activity in needles of Norwegian spruce trees (Picea abies L.). Plant Physiol l90:1310–1315
Polle A, Otter T, Sandermann H Jr (1997) Biochemistry and physiology of lignin synthesis. In: Rennenberg H, Escherich W, Ziegler H (eds) Trees: contributions to modern tree physiology. Backhuys Publishers, Leiden, pp 455–477
Powell WW, Raymond BT (1981) Soaking injury and its reversal with polyethylene glycol in relation to respiratory metabolism in high and low vigour soybean seeds. Physiol Plant 53:263–268
Pretorius JC, Small JGC (1993) The effect of soaking injury in bean seeds on carbohydratelevels and sucrose phosphate synthase activity during germination. Plant Physiol Biochem 31:25–34
Quiroga M, Guerrero C, Botella MA, Ros Barceló A, Amaya I, Medina MI, Alonso FJ, de Forchetti SM, Tigier H, Valpuesta V (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol 122:1119–1128
Rahoui S, Chaoui A, El Ferjani E (2008) Differential sensitivity to cadmium in germinating seeds of three cultivars of faba bean (Vicia faba L.). Acta Physiol Plant 30:451–456
Rahoui S, Chaoui A, El Ferjani E (2010a) Reserve mobilization disorder in germinating seeds of Vicia faba exposed to cadmium. J Plant Nutr 33:809–817
Rahoui S, Chaoui A, El Ferjani E (2010b) Membrane damage and solute leakage from germinating pea seed under cadmium stress. J Hazard Mater 178:1128–1131
Rahoui S, Ben C, Chaoui A, Martinez Y, Yamchi A, Rickauer M, Gentzbittel L, El Ferjani E (2014) Oxidative injury and antioxidant genes regulation in roots of six Medicago truncatula genotypes in response to cadmium treatment. Environ Sci Pollut Res 21:8070–8083
Rahoui S, Chaoui A, Ben C, Rickauer M, Gentzbittel L, El Ferjani E (2015) Effect of cadmium pollution on mobilization of embryo reserves in seedlings of six contrasted Medicago truncatula lines. Phytochemistry 111:98–106
Ranieri A, Castagna A, Pacini J, Baldan B, Mensuali S, Soldatin GF (2003) Early production and scavenging of hydrogen peroxide in the apoplast of sunflower plants exposed to ozone. J Exp Bot 54:2529–2540
Rauser WE (1995) Phytochelatins and related peptides: structure, biosynthesis, and function. Plant Physiol 109:1149
Rispail N, Nash RJ, Webb KJ (2005) Secondary metabolite profiling. In: Márquez AJ, Stougaard J, Udvardi MK, Parniske M, Spaink HP, Saalbach G, Webb KJ, Chiurazzi Mand Márquez AJ (eds) Lotus japonicus Handbook. Springer, Dordrecht, The 20 Netherlands, pp 341–348
Rodriguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots: imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Romero-Puertas MC, Palma JM, Gomez M, del Rio IA, Sandalio LM (2002) Cadmium causes the oxidative modification of protein in pea plants. In: Drązkiewicz M (ed) The redox state and activity of superoxide dismutase classes in Arabidopsis thaliana under cadmium or copper stress. Chemosphere 67:188–193
Romero-Puertas MC, Palma JM, Gómez M, del Río LA, Sandalio LM (2002b) Cadmium causes the oxidative modification of proteins in pea plants. Plant Cell 25:677–686
Romero-Puertas MC, Zabalza A, Rodríguez-Serrano M, Gómez M, del Río LA, Sandalio LM (2003) Antioxidative response to cadmium in pea roots. Free Radic Res 37:44
Romero-Puertas MC, Corpas FJ, Rodriguez-Serrano M, Gomez M, del Río LA, Sandalio LM (2007) Differential expression and regulation of antioxidative enzymes by Cd in pea plants. J Plant Physiol 164:346–1357
Sandalio LM, Palma JM, Del Río LA (1987) Localization of manganese superoxide dismutase in peroxisomes isolated from Pisum sativum L. Plant Sci 51:1–8
Sandalio LM, Dalurzo HC, Gomez M, Romero-Puertas MC, Del Río LA (2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52:2115–2126
Santiago LMS, Louro RP, Dulce E (2000) Compartmentation of phenolic compounds and phenylalanine ammonia-lyase in leaves of Phyllanthus tenellus Roxb. and their induction by copper sulfate. Ann Bot 86:1023–1032
Schickler H, Caspi H (1999) Response of antioxidative enzymes to nickel and cadmium stress in hyperaccumulator plants of the genus Alyssum. Physiol Plant 105:39–44
Schmohl N, Horst WJ (2000) Cell wall pectin content modulates aluminum sensitivity of Zea mays (L.) cell grown in suspension culture. Plant Cell Environ 23:735–742
Schutzendubel A, Schwanz P, Terchmann T, Grossk Langeenfeld-Heyger R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 75:887–898
Schutzendubel A, Nikolova P, Rudolf C, Polle A (2002) Cadmium and H2O2-induced oxidative stress in Populus x canescens roots. Plant Physiol Biochem 40:577–584
Seregin I, Kozhevnikova A (2008) Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ J Plant Physiol 55:1–22
Sfaxi-Bousbih A, Chaoui A, EL Ferjani E (2010) Unsuitable availability of nutrients in germinating bean embryos exposed to copper excess. Biol Trace Elem Res 135:295–303
Sharma S, Kaur A, Bansal A, Gill BS (2013) Positional effects on soybean seed composition during storage. J Food Sci Technol 50(2):353–359
Shaw BP (1995) Effects of mercury and cadmium on the activities of antioxidative enzymes in the seedling of Phaseolus aureus. Biol Plant 37:587–596
Singh PK, Tamari RK (2003) Cadmium toxicity induced changes in plant water relations and oxidative metabolism of Brassica juncea L. plants. J Environ Biol 24:107–112
Song XQ, Liu LF, Jiang YJ, Zhang BC, Gao YP, Liu XL, Lin QS, Ling HQ, Zhou YH (2013a) Disruption of secondary wall cellulose biosynthesis alters cadmium translocation and tolerance in rice plants. Mol Plant 6:768–780
Song YF, Luo Z, Chen QL, Liu X, Liu CX, Zheng JL (2013b) Protective effects of calcium preexposure against waterborne cadmium toxicity in Synechogobius hasta. Arch Environ Contam Toxicol 65:105–121
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J Sci Food Agric 10:63–68
Teichmann T (2001) The biology of wood formation: scientific challenges and biotechnological perspectives. In: Panadalai SG (ed) Recent research developments in plant physiology. Research Signpost, Trivandrum, pp 269–284
Turner JG, Novacky A (1974) The quantitative relation between plant and bacterial cells involved in the hypersensitive reaction. Phytopathology 64:885–890
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Van Camp W, Willekens H, Bowler C, Van Montagu M, Inzé D, Reupold-Popp P, Sandermann H Jr, Langebartels C (1994) Elevated levels of superoxide dismutase protect transgenic plants against ozone damage. Biotechnology 12:165–168
Van Tunen AJ, Mol JNM (1990) Control of flavonoid synthesis and manipulation of flower colour. In: Grierson D (ed) Plant biotechnology series. Blacky and Son, Glasgow, pp 94–130
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci 151:59–66
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Vitoria AP, Lea PJ, Azevedo RA (2001) Antioxidant enzymes responses to cadmium in radish tissues. Phytochemistry 57:701–710
Vitoria AP, Rodriguez APM, Cunha M, Lea PJ, Azevedo RA (2003) Structural changes in radish seedlings exposed to cadmium. Biol Plant 47:561–568
Vorwerk S, Somerville S, Somerville C (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends Plant Sci 9:203–209
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 51:173–212
Weber M, Trampczynska A, Clemens S (2006) Comparative transcriptome analysis of toxic metal responses in Arabidopsis thaliana and the Cd2+ hypertolerant facultative metallophyte Arabidopsis halleri. Plant Cell Environ 29:950–963
Xiang C, Oliver DJ (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Zenk MH (1996) Heavy metal detoxification in higher plants: a review. Gene 179:21–30
Zhang HY, Jiang YN, He ZY (2005) Cadmium accumulation and oxidative burst in garlic (Allium sativum). J Plant Physiol 162:977–984
Acknowledgments
This work was financially supported by the Tunisian Ministry of High Education and Scientific Research. The authors wish to thank Mr. Bechir Azib for technical assistance and the IFR40-RIO Imaging Platform of Toulouse.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Néstor Carrillo
Rights and permissions
About this article
Cite this article
Rahoui, S., Martinez, Y., Sakouhi, L. et al. Cadmium-induced changes in antioxidative systems and differentiation in roots of contrasted Medicago truncatula lines. Protoplasma 254, 473–489 (2017). https://doi.org/10.1007/s00709-016-0968-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-0968-9