Abstract
Excessive cadmium concentrations in agricultural soils result in minimizing the soil fertility and health which leads to decrease in crop production. Plant growth promoting rhizobacteria (PGPR) are beneficial bacteria, which can protect the plants against many abiotic stresses. Current study aimed to identify important rhizobacterial strains by using enrichment technique and examine their inoculation effects on the growth and physiological parameters of wheat, under cadmium pollution. Cadmium was added to 3 kg soil in each pot (with 6 seeds/pot) using cadmium chloride at the rate of 0, 50, 100, 150, and 200 mg kg−1 with three replications in completely randomized design. Rhizobacterial isolates performed considerably better under all cadmium levels, i.e. 50–200 mg kg−1 soil, compared to control. Nevertheless, rhizobacterial isolates containing both abilities, i.e. deaminase and nitrogen fixing, e.g. SAN1 had the highest effect and caused a significant (P < 0.05) increase in the shoot (25.1-fold) and root length (30.2-fold), seedling fresh (17.1-fold) and dry weights (31.1-fold), chlorophyll a (13.1-fold), chlorophyll b (8.2-fold), carotenoids (5.1-fold), protein (50.1-fold), proline (18.8-fold), glutathione S-transferase (26.2-fold), peroxidase (26.8-fold) and catalase (30.5-fold), while lowest cadmium uptake in the shoot (10.1-fold) and root (8.7-fold), respectively, at the highest cadmium level, i.e. 200 mg kg−1 soil compared to control. Results revealed that PGPR significantly decreased the deleterious effects of cadmium pollution by chelating and influencing its bioavailability and increased the wheat growth. The PGPR with both deaminase and nitrogen fixing activities are more resilient against cadmium pollution than PGPR having either deaminase or nitrogen fixing activity alone. The enrichment technique is an efficient approach to select promising PGPR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among all the emerging environmental threats of twentieth century, the effect of heavy metal accumulation in agricultural soils has been considered one of the most disturbing and alarming threat in both developed and developing countries (Hassan and David 2014). Extreme accumulation of heavy metals in agricultural soils not only result in environmental contamination, but also lead to elevation in the uptake of heavy metals by crops and affect the food quality and safety (Hassan and David 2014). Heavy metals, such as cadmium (Cd), lead (Pb), chromium (Cr), zinc (Zn) and nickel (Ni) are important environmental pollutants, particularly in areas with high anthropogenic pressure (Hassan and David 2014). Among these heavy metals, Cd is the most abundant and ubiquitous metal, and plays a major role in the contamination of soil and atmosphere, due to its relatively high mobility in the soil–plant system (Hassan et al. 2013a). It has been reported that the average annual production of Cd throughout the world increased from 20 tons (in the 1920s) to about 20,000 tons due to emerging industrial activities (Wang and Yanli 2013). According to an estimate about 25,000 tons Cd year−1 is released into the environment and approximately 85–90 % of this Cd emission is due to anthropogenic activities, e.g. smelting and refining of nonferrous metals, fossil fuel combustion and municipal waste incineration (Azevedo et al. 2012). The Cd affected soils contain Cd in the range of 100–120 mg kg−1, whereas the industrialized areas contain Cd up to 160 mg kg−1 (Azevedo et al. 2012). Similarly, Cd toxicity affects the morphology, growth and photosynthetic processes of plants and causes inhibition of enzyme activities, water imbalance, alterations in membrane permeability and disturbs mineral nutrition (Azevedo et al. 2012).
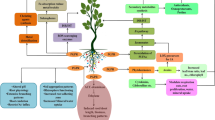
Recently, the use of microorganisms for the purpose of improving crop production and protection and to understand their mode of action is attracting considerable attention of microbiologists (Ahemad and Kibret 2014). Several species of bacteria present in the rhizosphere have great potential to promote the growth and protect the plants from many deleterious biotic and abiotic environmental stresses (Glick 2012), such bacteria are known as plant growth promoting rhizobacteria (PGPR). The PGPR are microorganisms which live freely in the rhizosphere and can promote the plant growth and protect them from many harmful biotic and abiotic stresses by a number of direct and indirect ways (Ahemad 2012). Directly PGPR facilitate the uptake of certain macro- and micro-nutrients and produced certain phytohormones, e.g. auxin, cytokinins, ethylene, abscisic acid and gibberellins (Ahemad 2012; Ahemad and Kibret 2014). Indirectly, PGPR diminish or avert the harmful effects of many abiotic and biotic stresses, e.g. heavy metals (Ahemad and Malik 2011).
To cope with the abiotic and biotic environmental stresses plants produce different antioxidant enzymes, phytohormones and antibiotics, e.g. superoxide dismutase, deaminase, peroxidase, catalase, glutathione reductase, cysteine, ascorbic acid, thiols, and proline as a defense mechanism (Glick 2012; Ahemad and Kibret 2014). This increase in the activity of such antioxidant enzymes and phytohormones positively correlated with resistance in many plant species, against biotic and abiotic environmental stresses (Ahemad and Kibret 2014). PGPR can prompt plant growth under many biotic and abiotic environmental stresses, e.g. heavy metals and pathogen attack by the synthesis of different antioxidant enzymes, e.g. peroxidase, catalase, glutathione reductase and deaminase and phytohormones and antibiotics, e.g. cysteine, ascorbic acid, thiols, and proline (Glick 2012; Ahemad and Kibret 2014).
The PGPR with the enzyme, 1-aminocyclopropane-1-carboxylate (ACC) deaminase, assist in plant growth and development by minimizing the harmful effects of biotic and abiotic environmental stresses (Ahemad and Kibret 2014). Singh et al. (2013) found that PGPR having ACC-deaminase activity were resistant against Cd, Cr, Pb and Cu toxicity, and increased the wheat and pigeon pea growth. Rathaur et al. (2012) investigated that certain PGPR with ACC-deaminase activity (e.g. Bacillus, Pseudomonas and Azotobacter) were resistant to heavy metals toxicity. Belimov et al. (2005) found that PGPR with ACC-deaminase activity showed significant tolerance against Cd pollution and promoted the growth of the Indian mustard. PGPR with enzyme ACC-deaminase, are capable of improving the plants growth and protect the plant against Cd pollution (Pishchik et al. 2002). Similarly, phytohormone auxin (indole-3-acetic acid/indole acetic acid/IAA) also played a crucial role in the development of plant defense mechanism (Glick 2012). The indole acetic acid (IAA) produced by rhizobacteria decreased the effects of environmental stresses on the growth, development and physiological processes of plants by shifting the plant auxin pool (Spaepen and Vanderleyden 2011). Auxin-producing PGPR immobilized the Cd and promoted the growth and nutrient uptake of barley plants in the presence of toxic Cd concentrations (Pishchik et al. 2002). Therefore, the current study was conducted with the objectives: (1) to find out and select important rhizobacterial strains, (2) to examine the role of ACC-deaminase and/or nitrogen fixing rhizobacteria, under Cd pollution, on the growth and development and Cd uptake of wheat plant.
Materials and methods
Physical and chemical analysis
Soil was taken at 0–5 cm depth and transported to the laboratory. After air-drying and sieving (2 mm) the soil was stored properly for further use in the incubation study. Soil texture, saturation percentage, pH, EC (electrical conductivity), available P, extractable K, Ca, Mg and Na, and organic matter (OM) were measured by the methods of Hassan et al. (2013b, c, 2014a, b, c, d, e, 2015); Hassan 2013; Hassan and David (2014). The basic physico-chemical properties of the experimental soil are given in Table 1.
Isolation of PGPR
Rhizobacteria were isolated from the sugarcane rhizosphere by dilution plate technique (Wollum II 1982) using Dworkin and Foster (DF) salt minimal media (Dworkin and Foster 1958) having ACC as a sole N source for PGPR having ACC-deaminase activity; whereas, modified mannitol agar media was used to isolate PGPR possessing N fixing activity (Enrichment Technique).
Preparation of inocula
Autoclaved flasks of 250 ml capacity were incubated for 2 days at 28 ± 1 °C temperature in the orbital shaking incubator at 100 rpm. The flasks contained autoclaved DF salt minimal media for the rhizobacterial isolates having ACC-deaminase activity and modified mannitol agar media for the isolates having N fixing activity. In order to keep the uniform population of bacteria in the broth at the time of inoculation, optical density was measured accordingly.
Characterization of isolates
Glass jars containing sand were sterilized by autoclaving at 121 °C for 20 min. After autoclaving, Hoagland solution (40 ml) was added into glass jars and stored at room temperature for at least 48 h to equilibrate. Inoculated seeds were sown in sand with sterile forceps, and the jars were kept in growth chamber (18 °C, 70 % relative humidity, 16 h daylight). After a week of germination, the roots were cut off, dipped in phosphate buffer, and were shaken strongly to isolate the bacteria. The bacterial suspension was diluted tenfold and 2 ml was plated on Petri dishes containing DF salt minimal media with agar and modified mannitol agar media. The number of colonies (CFU/cm) was calculated after 2 days of incubation at 28 °C temperature (Davies and Whitbread 1989).
In order to measure the phosphorus solubilizing activity, the bacterial strains were cultured in national botanical research institute’s phosphate (NBRIP) growth medium and a loop full of each culture was placed on the plates (5/plate) and the plates were incubated at 28 °C temperature for a week. A zone of clearing around the colonies after a week was scored as positive for phosphate solubilization; whereas, phosphate solubilizing index (PSI) was measured by the formula of Premono et al. (1996).
The ACC-deaminase activity of the selected isolates was determined by the modified method of Honma and Shimomura (1978). When enzyme ACC-deaminase cleaves ACC the amount of α-ketobutyrate produced, the number of µmol of α-ketobutyrate produced during this reaction was determined by comparing the absorbance at 540 nm to a standard curve of α-ketobutyrate. A stock solution of 100 mM α-ketobutyrate was prepared in 0.1 M Tris–HCl (pH 8.5) and stored at 4 °C. A series of known α-ketobutyrate concentration was prepared in a volume of 2 ml of the 2,4-dinitrophenyl-hydrazine reagent (0.2 %, 2,4-dinitrophenyl-hydrazine in 2 M HCl) and the contents were vortexed and incubated at 30 °C for 30 min, during this time the α-ketobutyrate was derivatized as a phenylhydrazine. The color of phenylhydrazine was developed by the addition of 2 ml 2 M NaOH; after mixing, the absorbance of the mixture was measured at 540 nm.
Auxin production as IAA equivalents by the selected isolates was measured in the presence and absence of l-tryptophan (i.e. an auxin precursor). The l-tryptophan was filter sterilized by passing through 0.2 μm membrane filter and added at concentration of 1 g l−1 to the liquid medium. The flask contents were inoculated with respective bacterial isolates and adjusted to optical density of 0.5 (108–109 CFU ml−1) that was measured at 550 nm by spectrophotometer. The flasks were plugged tightly and incubated at 28 ± 1 °C for 48 h. Non-inoculated/untreated control was kept for comparison. Auxin compounds (IAA-equivalents) were determined by spectrophotometer, using Salkowski coloring reagent; whereas, measuring IAA-equivalents, 3.0 ml of filtrate was taken in test tubes and 2.0 ml of Salkowski reagent (2.0 ml of 0.5 M FeCl3 + 98.0 ml of 35 % HClO4) was added to it. The contents in the test tubes were allowed to stand for half an hour for color development. Similarly, color was also developed in standard solutions of IAA. The intensity of color was measured at 535 nm wavelength by using spectrophotometer (Sarwar et al. 1992). Some important characteristics of the rhizobacterial isolates used in this experiment are given in Table 2.
Determination of plant physical parameters
After harvest, roots and shoots were separated from soil and shoot and root lengths and fresh and dry weights of seedlings were determined with the help of analytical balance and thereafter by oven drying at 70 °C to a constant dry weight.
Quantification of chlorophyll and carotenoid contents
Chlorophyll a, b and carotenoid contents were measured in the acetone extract (80 % v/v) by using spectrophotometer at 663, 645 and 480 nm wavelengths, respectively (Arnon 1949).
Determination of protein and proline contents
Bovine serum albumin (BSA) was used as a standard to measure the concentration of protein; whereas, proline contents were determined spectrophotometrically at 520 nm wavelength (Bradford 1976).
Plant enzymes activity
Frozen leaf material (0.5 g) was homogenized in 3 ml of cold solution containing 50 × 10−3 M Na phosphate buffer (pH 7.8), 1 × 10−3 M ethylenediaminetetraacetic acid and 2 % (w/v) polyvinylpolypyrrolidone for plant enzymes activity. The homogenate was centrifuged at 0 °C for 40 min at 13,000g. Glutathione S-transferase is measured by following the method of Habig et al. (1974). Briefly, the phosphate buffer with pH 6.5 and 100 mM concentration was used to homogenize the samples. Then the homogenized samples were centrifuged at 9000g for 30 min. The change in optical density was measured at 340 nm wave length. The methods of Nakano and Azada (1987) and Cakmak and Horst (1991) were followed for the spectrophotometric measurement of peroxidase and catalase activity. Briefly, the final solution (3 ml) was made by the addition of 100 μl of enzyme extract, 50 μl of 0.3 % H2O2 and 2850 μl phosphate buffer NaK-ascorbate (50 mM NaK, 0.5 mM ascorbate) with pH 7.2. The activity was measured spectrophotometrically at the wave length of 290 and 240 nm, respectively.
Cadmium accumulation and uptake in plant
Cadmium contents in plants were measured by dry ashing. 1 g of ground plant material was taken in porcelain crucibles (50 ml) and placed in a cool muffle furnace. The temperature of the muffle furnace was increased gradually to 550 °C, and the sample is kept in the muffle furnace for 5 h. After 5 h, muffle furnace was shut off and left for cooling and crucibles were taken out carefully. The sample, i.e. cooled ash was dissolved in 5 ml of 2 N HCl and mixed thoroughly with the help of a plastic rod, for 20 min. After mixing the volume was brought up to 50 ml, and was filtered by using Whatman no. 42 filter paper. The metal contents in the extract were determined by using the atomic absorption spectrophotometer (Page et al. 1982).
Experimental design
Three different rhizobacterial strains, strain 1 containing ACC-deaminase activity (SACC1, SACC2); strain 2 having nitrogen fixing ability (Azotobacter, SN1) and strain 3 having both ACC-deaminase activity and nitrogen fixing ability (SAN1, SAN2) were selected to test their comparative effectiveness under Cd pollution. The Cd was applied as a CdCl2 solution to a sterilized soil (3 kg/pot) in each pot maintaining the concentrations of 0, 50, 100, 150 and 200 mg Cd kg−1 of soil. Soils mixed with Cd were allowed to equilibrate for 15 days and then seeds of wheat (variety Uqab-2000) were sown at the rate of six seeds per pot which were thinned up to two seedlings in each pot after germination. For inoculation, the germinated seeds were dipped in broth having selected rhizobacterial isolates. For control treatments, the wheat seeds were treated with 0.03 M MgSO4. Sterilized ½ strength nitrogen-free Hoagland solution was used to provide nutrients to growing seedling. The experimental pots were organized in complete randomized design (CRD), with four replications, in a growth room under axenic conditions. After 35 days of germination, wheat plants were harvested and growth parameters, Cd uptake and antioxidant enzymes, i.e. GST, POX and Cata were recorded.
Statistical analysis
The Statistix 8.1 (Statistix, USA) was used for the statistically analysis of data. Parametric statistics of ANOVA analysis was conducted to estimate the effect of rhizobacterial-isolate treatment on plant growth, under Cd pollution. A least significant difference (LSD) test at P < 0.05 was done to measure the mean separations.
Results
Shoot and root lengths
Inoculation with rhizobacterial isolates caused a significant (P < 0.05) increase in the shoot and root lengths of wheat seedlings under all Cd levels, i.e. 50–200 mg kg−1 soil. Evaluating the effectiveness of rhizobacterial isolates under all Cd levels (50, 100, 150 and 200 mg Cd kg−1 soil), the rhizobacterial isolates with both ACC-deaminase and nitrogen fixing abilities, i.e. SAN1 and SAN2 produced highest increase in the shoot and root lengths, followed by rhizobacterial isolates having ACC-deaminase activity alone (SACC1 and SACC2), and then rhizobacterial isolates having N fixing ability, i.e. Azotobacter and SN1 (Fig. 1). The inoculation with rhizobacterial isolates, namely SAN1 and SAN2 increased the means of the shoot and root lengths by 13.7- and 5.7-fold and 11.4- and 4.5-fold, respectively, compared to control (Table 3). Conversely, the increase in the means of the shoot and root lengths due to the inoculation of SACC1 and SACC2 was 9.2- and 3.7-fold and 6.6- and 2.3-fold correspondingly compared to control (Table 3). The minimum increase in the means of the shoot and root lengths was observed in the treatments inoculated with Azotobacter and SN1 ranged from 4.0- and 1.5-fold to 2.9- and 1.0-fold (Table 3).
Fresh and dry weights of seedling
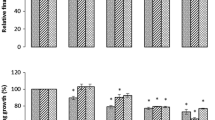
The effect of rhizobacterial inoculation on the fresh and dry weights of wheat seedlings, under Cd pollution is presented in Fig. 2. Rhizobacterial isolates inoculation produced significant (P < 0.05) improvement in the fresh and dry weights of the seedlings, under all Cd levels, i.e. 50–200 mg kg−1 soil. Generally the effective trend of the rhizobacterial isolates on the fresh and dry weights of wheat seedlings was in the order SAN1 > SAN2 > SACC1 > SACC2 > Azotobacter > SN1. Inoculation with rhizobacterial isolates SAN1 and SAN2 increased the means of the fresh and dry weights of wheat seedlings, i.e. 3.24 and 0.57 g and 2.40 and 0.47 g, respectively, compared to uninoculated control (Table 3). On the other hand, the increase in the means of the shoot and root fresh and dry weights of wheat seedlings due to the inoculation of SACC1 and SACC2 was 1.64 and 0.39 g and 1.01 and 0.26 g correspondingly compared to control (Table 3). The least increase in the means of the shoot and root fresh and dry weight was found in the treatments inoculated with Azotobacter and SN1, that ranged from 0.66 and 0.17 g to 0.46 and 0.12 g compared to control (Table 3).
Comparative effectiveness of ACC-deaminase and/or nitrogen fixing rhizobacteria on seedling fresh and dry weight, under Cd pollution. AB azotobacter, SFW seedling fresh weight, SDW seedling dry weight. Different letters a–e on bars indicate significant differences of mean values for SFW and SDW. Bars represent standard errors
Chlorophyll a, b and carotenoid contents
The treatment with ACC-deaminase and/or nitrogen fixing rhizobacteria considerably (P < 0.05) increased the chlorophyll a, b and carotenoid contents under all Cd levels, i.e., 50, 100, 150 and 200 mg Cd kg−1 soil (Fig. 3). Rhizobacterial isolate SAN1 was found to be the most effectual isolate that caused 18.1-, 21.2- and 38.2-fold increase in the means of the chlorophyll a, b and carotenoid contents over uninoculated control. The next effective rhizobacterial isolates were SAN2, SACC1, SACC2, Azotobacter and SN1 that resulted in about 15.3-, 19.2- and 32.9-fold, 13.4-, 17.2- and 27.8-fold, 10.3-, 14.1- and 24.0-fold, 7.18-, 11.2, and 19.7-fold, 5.38-, 9.34-, and 17.9-fold increase in the means of the chlorophyll a, b and carotenoid contents, respectively, compared to control (Table 3).
Comparative effectiveness of ACC-deaminase and/or nitrogen fixing rhizobacteria on chlorophyll a and b and carotenoid content, under Cd pollution. AB azotobacter, Chl a chlorophyll a, Chl b chlorophyll b, cart carotenoids. Different letters a–e on bars indicate significant differences of mean values for Chl a, Chl b and Cart. Bars represent standard errors
Protein and proline contents
Rhizobacterial isolates having either nitrogen fixing and/or ACC-deaminase activity, substantially (P < 0.05) increased the protein and proline contents of wheat seedlings under all Cd levels, i.e. 50–200 mg kg−1 soil compared to uninoculated control (Fig. 4). The most promising rhizobacterial isolate was SAN1 that caused an increase of 1.19 mg g−1 and 2.42 µmol g−1 in the means of the protein and proline contents of wheat seedlings over uninoculated control (Table 3). The rhizobacterial isolate SAN2 was the second efficient isolate that increased the means of the protein and proline contents from 0.90 mg g−1 and 1.67 µmol g−1 as compared to uninoculated control (Table 3). The significant (P < 0.05) increase in the means of the protein and proline contents was also observed when inoculated with SACC1, SACC2, Azotobacter, and SN1, that ranged from 0.65 mg g−1 and 1.26 µmol g−1, 0.47 mg g−1 and 0.89 µmol g−1, 0.33 mg g−1 and 0.55 µmol g−1, to 0.23 mg g−1 and 0.38 µmol g−1, respectively, as compared to control (Table 3).
Enzymes activity
The selected rhizobacteria’s inoculation considerably (P < 0.05) increased the antioxidant enzymes, i.e. GST, Pox and Cata activities, under all Cd levels, i.e. 50–200 mg kg−1 soil compared to control (Fig. 5). All rhizobacterial isolates caused increase in the GST, Pox and Cata activities and their means. Nevertheless, the isolate SAN1 was the best isolate and caused maximum increase in the means of GST, Pox and Cata activities, i.e. 13.2-, 9.22- and 10.2-fold, respectively, compared to control (Table 3). The SAN2, SACC1 and SACC2 were the subsequently best effective rhizobacterial isolates and caused an increase of 11.1-, 7.22- and 8.26-fold, 9.08-, 5.29- and 7.18-fold, and 6.10-, 3.65- and 5.25-fold increase in the means of the GST, Pox and Cata activities, respectively, compared to control (Table 3). Whereas, Azotobacter and SN1 caused a minimum increase in the means of the GST, Pox and Cata activities comparing to control, i.e. 3.72-, 2.54- and 3.65-fold, and 2.52-, 1.65- and 2.27-fold (Table 3).
Comparative effectiveness of ACC-deaminase and/or nitrogen fixing rhizobacteria on glutathione S-transferase, peroxidase and catalase enzyme activity, under Cd pollution. AB azotobacter, GST glutathione S-transferase, Pox peroxidase, cata catalase. Different letters a–e on bars indicate significant differences of mean values for GST, Pox and Cata. Bars represent standard errors
Cadmium uptake in shoot and root
The rhizobacterial inoculation significantly (P < 0.05) decreased Cd uptake in the shoot and root of the wheat seedlings, under all Cd levels, i.e. 50, 100, 150 and 200 mg Cd kg−1 soil compared to control (Fig. 6). The rhizobacterial isolate SAN1 was the most effectual isolate and caused least Cd uptake and minimum increase in the means of the Cd uptake in the shoot and root of the wheat seedlings compared to control, i.e. 0.47 and 0.38 mg Cd kg−1 (Table 3). The effectual trend of remaining rhizobacterial isolates was in the order SAN2, SACC1, SACC2, Azotobacter and SN5 that resulted in lowest Cd uptake, and means in the shoot and root of the wheat seedlings compared to control, that ranged from 0.63 and 0.53 mg Cd kg−1, 1.06 and 0.74 mg Cd kg−1, 1.60 and 1.04 mg Cd kg−1, 2.24 and 1.64 mg Cd kg−1, 2.82 and 2.25 mg Cd kg−1 in the plant shoot and roots, compared to control (Table 3).
Comparative effectiveness of ACC-deaminase and/or nitrogen fixing rhizobacteria on Cd uptake in shoot and root, under different Cd treatments. AB azotobacter. Different letters a–d on bars indicate significant differences of mean values for Cd uptake in shoot and root. Bars represent standard errors
Characteristics of isolates
Important characteristics of experimental rhizobacterial isolates are given in Table 2. It was observed that rhizobacterial isolate SAN1 had maximum root colonization activity, i.e. 6.8 × 107 and produced more indole acetic acid, i.e. 19.5 mg l−1 in the presence of L-TRP. On the other hand, rhizobacterial isolate SACC1 had highest ACC-deaminase activity, i.e. 15.6 μmol α-ketobutyrate g-biomass 1/2 h−1.
Discussion
Comparative effectiveness of rhizobacterial isolates showed that the isolates having both ACC-deaminase and/or nitrogen fixing activity (i.e. SAN1 and SAN2) caused highest increase in the root and shoot lengths, and fresh and dry weights of the wheat seedlings under Cd contamination than other rhizobacterial isolates (SACC1, SACC2, Azotobacter and SN1) and uninoculated control (CK). Overall the comparative effectiveness of rhizobacterial isolates was in the order SAN1 > SAN2 > SACC1 > SACC2 > Azotobacter > SN1 (Figs. 1, 2). Eva et al. (2012) investigated the effects of biofertilizers on plant production and nutrient uptake of maize and sunflower under Cd pollution at Debrecen, Hungary, and concluded that bacterium-containing biofertilizers alleviated the Cd stress and enhanced the root and shoot growth and fresh and dry weights. Sinha and Mukherjee (2008) observed the effects of PGPR (Pseudomonas aeruginosa) inoculation on the growth of mustard and pumpkin plants in Cd-added soil at West Bengal, India, and concluded that PGPR inoculation substantially improved the plants growth, e.g. root and shoot lengths, and fresh and dry biomass, and reduced the Cd uptake. Madhaiyan et al. (2007) set an experiment at Chungbuk, Korea, to examine the PGPR inoculation effects on the growth and development of tomato (Lycopersicon esculentum L.) under Cd and Ni pollution and found that PGPR strains (CBMB20 and CBMB40) reduced the toxicity of Cd in tomato and promoted plant growth, e.g. shoot and root lengths and biomass by decreasing the further Cd uptake.
The results of chlorophyll a, b and carotenoid contents showed that among all rhizobacterial isolates, the SAN1 isolate with both ACC-deaminase and nitrogen fixing activity, showed highest increase in the chlorophyll a, b and carotenoid contents, under all Cd levels, i.e. 50–200 mg Cd kg−1 soil, compared to other rhizobacterial isolates and uninoculated control (Fig. 3). The effectual trend of other rhizobacterial isolates was in the order SAN2 > SACC1 > SACC2 > Azotobacter > SN5. Stefan et al. (2013) observed the effect of PGPR inoculation on the growth of runner bean and found that PGPR inoculation considerably increased the chlorophyll and carotenoid contents of the runner bean. Azevedo et al. (2012) stated that inoculation of agricultural and horticultural crops with beneficial microorganisms (PGPR) could be used to improve the chlorophyll and photosynthesis and ultimately overall growth of the plants under biotic and abiotic stresses, i.e. heavy metal pollution.
Significant increase in the plant protein and proline was observed after inoculation with rhizobacterial isolates, under all Cd levels, i.e. 50–200 mg Cd kg−1 soil. However, rhizobacterial isolate SAN1, with both ACC-deaminase and nitrogen fixing abilities, was the best among the all experimental rhizobacterial isolates, and caused the highest increase in the plant protein and proline contents (Fig. 4). The comparative efficacy of other rhizobacterial isolates on the plant protein and proline contents was in the order SAN2 > SACC1 > SACC2 > Azotobacter > SN5 >. Mendoza-Cózatl et al. (2011) examined a high tolerance to metal toxicity upon inoculation with effective rhizosphere bacteria (PGPR) and concluded that this tolerance can be related to the production of more protein and proline and other phytohormones. Andrews et al. (2010) observed that microorganisms can interact positively with plants in agricultural systems in relation to their nutrition and ability to resist biotic and abiotic stresses (e.g. heavy metal pollution) by producing different antibiotics and phytohormones (e.g. protein, cysteine, ascorbic acid, thiols, and proline). Dell’Amico et al. (2008) observed, resistance and improvement in the growth of Brassica napus under Cd stress at Milan, Italy, and stated that PGPR having ACC-deaminase activity performed substantially better, and increased the plant resistance and biomass, by producing different phytohormones and antibiotics, e.g. protein, cysteine and proline.
Inoculation with rhizobacterial isolates had a positive effect on plant antioxidant enzymes activity under all Cd levels, i.e. 50–200 mg Cd kg−1 soil. However, the isolate SAN1 (with both ACC-deaminase and nitrogen fixing activities) was the best, among the all other experimental rhizobacterial isolates and uninoculated control (Fig. 5). The overall effect of other rhizobacterial isolates on the plant antioxidant enzymes activity was in the order SAN2 > SACC1 > SACC2 > Azotobacter > SN1. Ahemad and Kibret (2014) concluded that the PGPR played a vital role in the production of a wide variety of antioxidant enzymes, phytohormones and antibiotics in the inoculated plants under stressed conditions to improve the plant immunity. Sahran and Nehra (2011) reviewed that PGPR increased the plant vigor and decreased the effect of many biotic and abiotic stresses (e.g. heavy metals) by producing different proteins, antibiotics and enzymes. Saleem et al. (2007) concluded that PGPR produced some antioxidant enzymes and phytohormones, e.g. ACC-deaminase, to regulate the ethylene production by metabolizing ACC into α-ketobutyrate and NH3 and to protect the plants from many abiotic and biotic stresses.
Significant (P < 0.05) decrease in the Cd uptake in the shoot and root of wheat seedling, under all Cd levels, i.e. 50–200 mg Cd kg−1 soil, was observed upon inoculation with rhizobacterial isolates compared to control (Fig. 6). Once again rhizobacterial isolates having both ACC-deaminase and/or nitrogen fixing activity, i.e. SAN1 and SAN2 performed considerably better than rhizobacterial isolates possessing only ACC-deaminase (SACC1 and SACC2) and nitrogen fixing (Azotobacter and SN1) ability, respectively. The overall effect of rhizobacterial isolates on the Cd uptake in the shoot and root was in the order SAN1 > SAN2 > SACC1 > SACC2 > Azotobacter > SN1. Ahemad and Kibret (2014) stated that PGPR can facilitate plant growth and increase the plant resistance against heavy metal stress by directly influencing the metal solubility and speciation in the rhizosphere. Sousa et al. (2012) evaluated the response of Pinus pinaster seedlings to Cd exposure and found that inoculation with effective microbes has a significant impact on Cd immobilization and metal uptake in Pinus pinaster seedlings. Azevedo et al. (2012) examined the Cd-induced stress in plants and revealed that large population of bacteria inhabiting the rhizosphere can influence the heavy metals chelation, immobilization and uptake by plants.
The ultimate reason for the SAN1 being the best rhizobacterial isolate was, having both ACC-deaminase and nitrogen fixing abilities, maximum root colonization activity and ability to produced more IAA in the presence of L-TRP (Table 2). Ahemad and Kibret (2014) stated that IAA produced by certain rhizosphere bacteria engaged in many plant developmental processes and plant defense mechanisms. Glick (2012) reported that PGPR synthesizing IAA played a vital role in preventing the deleterious effects of biotic and abiotic environmental stresses. Bianco and Defez (2009) observed a high tolerance against abiotic environmental stresses in the Medicago truncatula plants, nodulated by the IAA-overproducing strain Sinorhizobium meliloti (DR-64 PGPR). The differences in plant growth promotion due to inoculation with different PGPR isolates is may be due to the differences in their characteristics, i.e. ability to produce IAA, auxin, different hormones, antioxidant enzymes, capability to hydrolyze the ACC in plant roots and ability to colonize plant roots (Ahemad and Kibret 2014; Ahemad 2012).
Conclusions
Treatment of agricultural crops with PGPR is a promising approach to utilize Cd affected soils, and hence increase the agricultural land and crop production. PGPR protect the plants from the deleterious effects of Cd pollution by producing phytohormones and antioxidant enzymes, and by decreasing the Cd release and bioavailability in soils. Rhizobacteria having both ACC-deaminase and nitrogen fixing activity are more effective and resilient under Cd pollution than rhizobacteria containing either ACC-deaminase or nitrogen fixing activity alone for growth promotion of agricultural crops. It is important to increase the working and productive efficiency of a specific PGPR under different heavy metals with the proper optimization and acclimatization. Further in-depth research is essential to find out promising PGPR and to test their functioning mechanisms and effectiveness under soils polluted with different heavy metals or their mixtures.
References
Ahemad M (2012) Implications of bacterial resistance against heavy metals in bioremediation: a review. Inst Integr Omics Appl Biotechnol 3:39–46
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J King Saud Univ Sci 26:1–20
Ahemad M, Malik A (2011) Bioaccumulation of heavy metals by zinc resistant bacteria isolated from agricultural soils irrigated with wastewater. Bacteriol J 2:12–21
Andrews M, Hodge S, Raven JA (2010) Positive plant microbial interactions. Ann Appl Biol 157:317–320
Arnon DI (1949) Copper induced enzyme in isolated chloroplasts; polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Azevedo RA, Gratão PL, Monteiro CC, Carvalho RF (2012) What is new in the research on cadmium-induced stress in plants?—Food energy. Security 1:133–140
Belimov AA, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR (2005) Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol Biochem 37:241–250
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxidase dismutase, catalase and peroxidase activities in root tips of soybean (glycine max). Physiol Plant 83:463–468
Davies KG, Whitbread RA (1989) Comparison of method for measuring the colonization of root system by fluorescent pseudomonads. Plant Soil 116:239–246
Dell’Amico E, Cavalca L, Andreoni V (2008) Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol Biochem 40:74–84
Dworkin M, Foster J (1958) Experiment with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:92–601
Eva G, Levai L, Szilvia V, Bela K (2012) Effects of biofertilizers on maize and sunflower seedlings under cadmium stress. Commun Soil Sci Plant Anal 43:272–279
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:15. doi:10.6064/2012/963401
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases, the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Hassan W (2013) C and N mineralization and dissolved organic matter potentials of two contrasting plant residues: effects of residue type, moisture and temperature. Acta Agric Scand Sect B Soil Plant Sci 63:642–652
Hassan W, Akmal M, Muhammad I, Younas M, Zahaid KR, Ali F (2013a) Response of soil microbial biomass and enzymes activity to cadmium (Cd) toxicity under different soil textures and incubation times. Aust J Crop Sci 7:674–680
Hassan W, Bano R, Bashir F, David J (2014a) Comparative effectiveness of ACC-deaminase and/or nitrogen fixing rhizobacteria in promotion of maize (Zea mays L.) growth under lead pollution. Environ Sci Pollut Res 21:10983–10996
Hassan W, Bano R, Khatak BU, Hussain I, Yousaf M, David J (2014b) Temperature sensitivity and soil organic carbon pools decomposition under different moisture regimes: effect on total microbial and enzymatic activity. Clean Soil Air Water. doi:10.1002/clen.201300727
Hassan W, Chen W, Cai P, Huang Q (2013b) Oxidative enzymes, the ultimate regulator: implications for factors affecting their efficiency. J Environ Qual 42:1779–1790
Hassan W, Chen W, Cai P, Huang Q (2014c) Estimation of enzymatic, microbial and chemical properties in Brown soil by microcalorimetry. J Therm Anal Calorim 116:969–988
Hassan W, Chen W, Huang Q, Mohamed I (2013c) Microcalorimetric evaluation of soil microbiological properties under plant residues and dogmatic water gradients in Red soil. Soil Sci Plant Nutr 59:858–870
Hassan W, David J (2014) Effect of lead pollution on soil microbiological index under spinach (Spinacia oleracea L.) cultivation. J Soils Sediments 14:44–59
Hassan W, David J, Abbas F (2014d) Effect of type and quality of two contrasting plant residues on CO2 emission potential of Ultisol soil: implications for indirect influence of temperature and moisture. Catena 114:90–96
Hassan W, David J, Bashir F (2014e) ACC-deaminase and/or nitrogen fixing rhizobacteria and growth response of tomato (Lycopersicon pimpinellfolium Mill.). J Plant Interact 14:869–882
Hassan W, Hussain M, Bashir S, Shah AN, Bano R, David J (2015) ACC-deaminase and/or nitrogen fixing rhizobacteria and growth of wheat (Triticum aestivum L.). J Soil Sci Plant Nutr 15:232–248
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42:1825–1831
Madhaiyan M, Poonguzhali S, Sa T (2007) Metal tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 69:220–228
Mendoza-Cózatl DG, Jobe TO, Hauser F, Schroeder JI (2011) Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic. Curr Opin Plant Biol 14:554–562
Nakano Y, Azada K (1987) Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol 28:131–140
Page AL, Miller RH, Keeney DR (1982) Methods of soil analysis-chemical and microbiological properties. Part 2. Am Soc Agron, 2nd edn. No. 9, Madison, Wisconsin, USA, pp 595–624
Pishchik VN, Vorobyev NI, Chernyaeva II, Timofeeva SV, Kozhemyakov AP, Alexeev YV, Lukin SM (2002) Experimental and mathematical simulation of plant growth promoting rhizobacteria and plant interaction under cadmium stress. Plant Soil 243:173–186
Premono HME, Moawad AM, Vlek PLG (1996) Effect of phosphate-solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indones J Crop Sci 11:13–23
Rathaur P, Ramteke PW, Raja W, John SA (2012) Isolation and characterization of nickel and cadmium tolerant plant growth promoting rhizobacteria from rhizosphere of Withania somnifera. J Biol Environ Sci 6:253–261
Sahran BS, Nehra V (2011) Plant growth promoting rhizobacteria: a critical review. Life Sci Med Res 21:1–30
Saleem M, Arshad M, Hussain S, Bhatti AS (2007) Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J Ind Microbiol Biotechnol 34:635–648
Sarwar M, Arshad M, Martins DA, Jr Frankenberger (1992) Tryptophan-dependent biosynthesis of auxins in soil. Plant Soil 147:207–215
Singh Y, Ramteke PW, Shukla PK (2013) Isolation and characterization of heavy metal resistant Pseudomonas spp. and their plant growth promoting activities. Adv Appl Sci Res 4:269–272
Sinha S, Mukherjee SK (2008) Cadmium-induced siderophore production by a high Cd-resistant bacterial strain relieved Cd toxicity in plants through root colonization. Curr Microbiol 56:55–60
Sousa NR, Ramos MA, Marques APGC, Castro PML (2012) The effect of ectomycorrhizal fungi forming symbiosis with Pinus pinaster seedlings exposed to cadmium. Sci Total Environ 414:63–67
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol 3:4. doi:10.1101/cshperspect.a001438 (pii: a001438)
Stefan MA, Munteanu N, Stoleru V, Mihasan M (2013) Effects of inoculation with plant growth promoting rhizobacteria on photosynthesis, antioxidant status and yield of runner bean. Rom Biotechnol Lett 18(2):8132–8143
Wang B, Yanli D (2013) Cadmium and its neurotoxic effects-review article. Oxid Med Cell Longev. doi:10.1155/2013/898034 (Article ID 898034)
Wollum II AG (1982) Cultural methods for soil microorganisms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties, 2nd edn. ASA-SSSA, Madison, Wisconsin, pp 781–802
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, W., Bashir, S., Ali, F. et al. Role of ACC-deaminase and/or nitrogen fixing rhizobacteria in growth promotion of wheat (Triticum aestivum L.) under cadmium pollution. Environ Earth Sci 75, 267 (2016). https://doi.org/10.1007/s12665-015-4902-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4902-9