Abstract
Probiotics have been widely applied in aquaculture industry as sustainable and environmentally friendly tools to sustain host’s health and the well-being. Among probiotics, Bacillus species have great potential applications in aquaculture because they can form the spores that makes them able to survive in the harsh environmental conditions. Moreover, they are nonpathogenic and nontoxic to aquacultural environments and animals. In addition, Bacillus species are able to produce antimicrobial substances making them more suitable candidates compared to other probiotics. In this chapter, we discussed the role of Bacillus in sustainable aquaculture as alternative strategies to enhance growth performance, disease resistance, and immune response of different aquaculture farmed animals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Aquaculture is one of the world’s fastest growing food sectors (Willer and Aldridge 2019). It is necessary to meet the global seafood demand, which is being accountable for 50% of the world’s seafood consumption (Gómez et al. 2019). However, sustainable development of aquaculture industry is constantly defeated by the outbreak of diseases, which is considered as main obstacles to the economical profitability of the industry (FAO 2020). The outbreak of diseases is also linked to application of antibiotics, posing a significant danger to the public health (World Health Organization 2014). Thus, new and natural alternatives that prohibit the incidence of diseases and improve human and animal health are urgently needed. The use of probiotics, “live organisms that can give a health benefit to the host when administered in the appropriate amounts,” is a potential alternative to boost the global health (FAO/WHO 2001). The scientific community has been searching for the environmentally friendly solutions to prevent aquacultural disease, where probiotics emerged as crucial alternative to antibiotics due to advert effects of antibiotics, such as the modulation of microbiota in the aquaculture systems and the development of resistance bacteria (Kuebutornye et al. 2019; Resende et al. 2012; Ringø 2020; Wang et al. 2019a). Consequently, wide range of probiotics, such as Bacillus, Enterococcus, Lactobacillus, Lactococcus, Micrococcus, Pediococcus, Enterobacter, Vibrio, Pseudomonas, Rhodopseudomonas, Roseobacter, and Shewanella, have been found and applied to improve growth performance, immune response, and disease resistance of farmed fish and shellfish (Abd El-Rhman et al. 2009; Adel et al. 2017; Feng et al. 2019; Kuebutornye et al. 2019; Li et al. 2006; Li et al. 2020; Ringø 2020; Yang et al. 2019). In aquaculture, probiotics have been applied as functional feed additives to boost host’s health and well-being via increasing growth, supplying nutrient, modulation gut microbiota, enhancing immunity, improving feed efficiency, increasing digestive enzyme activities and digestibility, and controlling diseases (Kuebutornye et al. 2019; Ringø 2020; Selim and Reda 2015).
Bacillus species are one of the most commonly used probiotics in the aquaculture industry because of their ability to form endospores, which is a benefit for industrial applications without losing their characteristics (Hong et al. 2005; Kuebutornye et al. 2019; Cutting 2011; Hai 2015). In addition, Bacillus is known to generate natural antimicrobial compounds, which are able to prohibit the proliferation of harmful bacteria in the aquaculture systems and host’s intestines (Abriouel et al. 2011; Caulier et al. 2019; Sumi et al. 2015). Similarly, Bacillus species are known to stimulate the digestive enzymes, antioxidant enzymes, relative immune gene expression, and stress-related genes, which in turn improve disease resistance of the host against pathogenic bacteria (Elshaghabee et al. 2017; Nayak 2010; Soltani et al. 2019). Bacillus species also increase the use of feed in fish, contributing to better growth rates (Mukherjee et al. 2019; Nair et al. 2020; Xia et al. 2020). Therefore, these chapters gather recent data on the role of Bacillus species in promoting growth performance, disease resistance, and immune response in aquaculture.
1.2 Mode of Action of Probiotics in Aquaculture
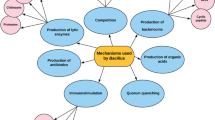
Probiotics can affect the host’s immune responses, as well as the interrelationship between these responses and their gastrointestinal microflora (Hemarajata and Versalovic 2013; La Fata et al. 2018; Yan and Polk 2011). Over the past decades, extensive researches on probiotics have provided insight into the significance of probiotics and their modes of action and numerous mechanisms have been suggested (Santacroce et al. 2019; Shi et al. 2016).
-
1.
Probiotics improve feed efficiency and growth rate of farmed fish and shellfish (Ringø 2020; Romano 2021). They also enhance the host’s appetite and feed digestion via decomposition of indigestible components, enhance vitamin productions, and detox diet’s substances (Ashaolu 2020; Cencic and Chingwaru 2010; Hoseinifar et al. 2018).
-
2.
Probiotics could compete the exclusion of gastrointestinal harmful bacteria via the secretion of peroxide, bacteriocin, siderophore, and lysozyme enzymes (Vieco-Saiz et al. 2019; Yang et al. 2014). The physiological and immunological effects are considered as one of the most essential modes of action of probiotics (Klaenhammer et al. 2012; Plaza-Diaz et al. 2019; Vieco-Saiz et al. 2019).
-
3.
Probiotics could enhance aquaculture animal’s disease resistance to stress caused by various environmental threats during aquaculture activities (Hlordzi et al. 2020; Mohapatra et al. 2013; Reverter et al. 2020).
These mechanisms display the favorable impacts of probiotics in farmed fish and shellfish. Future studies, however, on the relationship between probiotics and hosts, including metagenomics and proteomic studies, is important to clarify mode of action of probiotics.
1.3 Bacillus Applications in Aquaculture
1.3.1 Improve Growth Performance
The utmost target of aquaculture practice is to acquire the rapidest growth and lowest production cost. To achieve this goal, several means have been established to boost growth rate and feed consumption by adding functional feed additives and growth natural growth promoters (Hernández et al. 2016; Katya et al. 2014). Probiotics are potential tools to maintain the normal growth, health, and well-being of farmed fish and shellfish because they serve as nutrients source, vitamins, and digestive enzymes. These substances for their part will contribute significantly on feed consumption, nutrients uptake, and host’s growth rate (Lauriano et al. 2016; Nath et al. 2019). Probiotics consumption have been speculated to improve the host’s appetite or boost organisms’ digestibility (Irianto and Austin 2002). Probiotics can improve feed efficiency of fish and shellfish by stimulating the excretion of digestive enzymes and maintaining the balance of intestinal microbes, which lead to the improvement of nutrients absorption and utilization, as well as the survival and growth of the host (Ibrahem 2015; Irianto and Austin 2002). Studies on diets containing probiotics revealed the possible involvement of these probiotics on the improvement of intestinal microflora balance and the production of extracellular enzymes to elevate the feed efficiency and growth of cultured species as growth promoters (Giri et al. 2013; Ringø et al. 2018). Most of the studies using Bacillus in aquaculture focus on growth performance and survival rate (Table 1.1).
1.3.1.1 Tilapias
During past decades, Bacillus spp. have been intensively applied in Nile tilapia aquaculture. Han et al. (2015) indicated that 10 weeks feeding trial with B. licheniformis significantly enhanced growth performance. However, there were no significant discrepancies in survival rate and feed conversion ratio (FCR) and in villi length and muscular layer thickness of anterior intestine among the treatments. In contrast, Iwashita et al. (2015) reveal that administration of the probiotic had no significant effect on the growth rates of Nile tilapias, although the fish fed probiotics had better feed conversion. Likewise, no significant difference in growth performance and FCR was observed in Nile tilapia fed Bacillus amyloliquefaciens (Silva et al. 2015). This can be explained due to the low temperatures during experimental period. Marcusso et al. (2015) reported that the homeostasis of Nile tilapia rearing at temperatures below 24 °C could be affected, enhancing the susceptibility to bacterial infections and impairing the growth performance. No effects were observed on the growth performance of Nile tilapia fed Bacillus subtilis (Aqua NZ and AP193) and Bacillus subtilis strains (Addo et al. 2017a, 2017b). These results are not unexpected given the short duration of this trial. This statement agrees with Apún-Molina et al. (2009) who observed a tendency toward improved growth in Nile tilapia fry (0.14 g) only after 75 d of feeding with diets composed of Bacillus or Lactobacillus probiotics. On the contrary, dietary inclusion of Bacillus subtilis significantly improved body weight, percent weight gain, specific growth rate, and feed conversion ratio (Liu et al. 2017). It is well documented that Bacillus exoenzymes are very efficient at metabolizing a large variety of carbohydrate, lipids, and proteins (Liu et al. 2009). The exoenzymatic activity of Bacillus spp. is one of the main reasons for its ability to improve digestive enzyme activities (Han et al. 2015). Higher enzyme activities in the digestive tract enhance digestive capability and growth performance of the host. It is widely accepted that the level of digestive enzyme activity is a useful comparative indicator of food utilization rate, digestive capacity, and growth performance of the host (Suzer et al. 2008; Ueberschär 1995). Liu et al. (2017) also reported that 4-week B. subtilis HAINUP40 diet supplementation significantly increased protease and amylase activities of tilapia. This is because B. subtilis HAINUP40 could secrete exoenzymes; the improvement of indigestive tract enzyme activities may be partially due to enzymes synthesized by the bacteria. However, the proportion of enzymes contributed by bacteria cannot be assessed since the probiotic may also stimulate the production of endogenous enzymes in the fish (Dawood et al. 2016; Suzer et al. 2008; Wu et al. 2012; Ziaei-Nejad et al. 2006). In the same trend, supplementation of B. subtilis and B. licheniformis or B. subtilis and Bacillus licheniformis (BS) combined with traditional Chinese medicine (TCM) significantly enhanced weight gain and specific growth rate of Nile tilapia and Mozambique tilapia (Abarike et al. 2018b; Abarike et al. 2018a; Gobi et al. 2018). It is known that an increase in the body weight gain in fish fed with probiotic supplemented diets, could contribute to the increase in digestive enzyme activity, increase in appetite, increase in the production of vitamin, breakdown of indigestible components, as well as possible improvement of intestine morphology (Irianto and Austin 2002). In addition, Bacillus spp. could secret several digestive enzymes like protease, amylase, and lipase (Cai et al. 2019; Caulier et al. 2019).
1.3.1.2 Shrimps
Shrimp is a commercially important aquatic species with high economic value and good flavor, which has been widely farmed in the world, particularly in some Asia countries (Chen et al. 2020a). However, the shrimp industry has suffered severe economic losses because of the frequent outbreaks of diseases such as early mortality syndrome (EMS) and white spot syndrome virus (WSSV) (Alavandi et al. 2019; Castex et al. 2009; Chang et al. 2012). Chemotherapeutant and antibiotics are usually applied to settle this problem. Unfortunately, prolonged use of chemotherapeutant and antibiotics could lead to severe outcomes such as resistant bacteria, drug residues, and toxins, which pose a substantial threat to human beings and environment (Dash et al. 2015). Therefore, to seek an alternative way to solve this threat has caused increasing concern (Huynh et al. 2018). Probiotics have been widely applied in shrimp aquaculture. Jamali et al. (2015) revealed that dietary enrichment with B. licheniformis and B. subtilis significantly enhanced growth performance and survival rate of Pacific white shrimp, Litopenaeus vannamei. Elevation of growth performance has been demonstrated as the Bacillus could colonize shrimp digestive tract. In P. monodon, Bacillus, when used as a probiotic, was able to colonize both the culture water and the shrimp digestive tract; the Bacillus also was able to replace Vibrio spp. in the gut of the shrimp, thereby increasing shrimp survival (Rengpipat et al. 1998), via out-competing other bacteria for nutrients and space by producing antibiotics (Moriarty 1998; Verschuere et al. 2000). Similarly, significant improvement in growth performance of L. vannamei supplemented with Bacillus spp. has been reported in previous studies (Sadat Hoseini Madani et al. 2018; Sánchez-Ortiz et al. 2016; Swapna et al. 2015). Also, Amoah et al. (2019) indicated that dietary inclusion of B. coagulans significantly improved growth performance and feed utilization of Pacific white shrimp. The nutritive values as reported by Vijayavel and Balasubramanian (2006) is highly dependent on their biochemical constituents such as crude protein, crude lipid, ash content, and moisture, which also is noted to be an indication of improved meat quality. In addition, higher inclusion levels of probiotic BC at 1 × 108 CFU g−1 feed in diets could modulate gut microflora of L. vannamei (Amoah et al. 2019), which play an ardent role in the digestive enzyme activities and the intestinal health. It has been well documented that digestive enzymes are known to break down food and absorb nutrients (Gobi et al. 2018). The digestive enzymes including amylase, lipase, and trypsin (Rawlings and Barrett 1994; Svendsen 2000) in this study significantly increased in the treated group compared to the untreated. Similar results of improved digestive enzyme activities in Litopenaeus vannamei (Zokaeifar et al. 2012) and Fenneropenaeus indicus (Ziaei-Nejad et al. 2006) have been established. Verschuere et al. (2000) in their work also noted that, Bacillus genus secrets a wide range of exoenzymes which aid in the nutritional enhancement of the host. More recently, significant increase in growth performance, villus number, villus height, thicker submucosa, and propionic acid content has been reported in L. vannamei fed different Bacillus species (B. licheniformis, B. cereus, and B. subtilis) singularly or combined with other probiotics (Pediococcus acidilactici, P. pentosaceus, and Lactococcus lactis) (Chen et al. 2020a; Chen et al. 2020b; Khademzade et al. 2020; Won et al. 2020a).
Dietary supplementation of Bacillus coagulans on growth and feed utilization of freshwater prawn Macrobrachium rosenbergii showed that growth performance and feed utilization were found to be significantly higher (P < 0.05) in prawn fed 109 cfu g−1 diet. In addition, the specific activities of protease, amylase, and lipase digestive enzymes were significantly higher (P < 0.05) for 109 cfu g−1 diet (Gupta et al. 2016).
In Marron (Cherax cainii), Ambas et al. (2017) found that synbiotic use of B. mycoides and organic selenium (OS) significantly improved some immune parameters of marron, particularly the glutathione peroxidase, and to some extent total hemocyte counts. However, the synbiotic feed did not synergistically improve marron growth; in fact, the use of B. mycoides-supplemented diet alone demonstrated significantly higher growth in marron compared with the growth of marron fed on other test diets. A study conducted by Ock Kim et al. (2020), it was indicated that strain Bacillus subtilis isolated from the gut of Penaeus indicus and added at 2 × 102 CFU 100 g−1 as probiotics in feed, resulted in weight gain of the juvenile shrimp (16.8 ± 0.11 g) after 40 days. The weight gain was 16.8 ± 0.11 CFU 100 g−1 at 10 × 102 CFU 100 g−1 probiotic concentration.
1.3.1.3 Catfish
To the best of our knowledge, there were few studies regarding the use of Bacillus spp. on this fish. Afrilasari and Meryandini (2016) reported that Bacillus megaterium PTB 1.4 increased the activity of digestive enzymes and the growth of catfish. It is known that isolate PTB 1.4 is B. megaterium, where Bacillus spp. group is known to have ability to produce extracellular enzymes (Moriarty 1998). Probiotic bacteria are capable of producing digestive enzymes that help fish use feed nutrients and digest (Bairagi et al. 2002). Generally, endogenous enzyme can be produced by fish, but the presence of probiotics can improve digestive enzyme. Probiotics improve digestive enzyme activity by stimulating the synthesis of endogenous enzyme in the digestive tract (Mohapatra et al. 2012). Similarly, combination of B. subtilis, B. amyloliquefaciens, B. cereus, and a commercial B. amyloliquefaciens significantly improved growth performance of C. gariepinus (Reda et al. 2018). This improvement could be attributed to the production of amylase and protease by the same strain (Selim et al. 2019). In addition, Bacillus sp. are capable to detoxify the harmful substance in feed, produce essential vitamins such as vitamin B12 and biotin, and increase the intestinal villus heights (Ramirez and Dixon 2003; Reda and Selim 2015; Sugita et al. 1992).
In hybrid catfish (C. macrocephalus × C. gariepinus), Meidong et al. (2017) indicated that Bacillus siamensis strain B44v, selectively isolated from Thai pickled vegetables (Phak-dong), displayed a high potential as a probiotic in catfish culture. Fish fed diet containing strain B44v (107 CFU g−1 feed) displayed not only no mortality but also growth improvement. The potential probiotic B. siamensis strain B44v could produce cellulase and protease, whereas the Bacillus sp. strain B51f produced protease and amylase enzymes. Ability to produce some hydrolytic enzymes is beneficial to the host. Enzymes increase the digestion of macromolecules in animal feed and improve feed intake by reducing digesta viscosity and increasing nutrient absorption in host animals (Ray et al. 2012).
For striped catfish, Pangasianodon hypophthalmus, the mixture of probiotics (B. amyloliquefaciens 54A and B. pumilus 47B) isolated from striped catfish at concentrations of 1 × 108, 3 × 108, and 5 × 108 CFU g−1 was added to the fish feed and conducted for 90 days. Truong Thy et al. (2017) reported that AWG (476.6 ± 7.81 g fish−1) of fish fed probiotics at 5 × 108 CFU g−1 was significantly higher than the control (390 ± 25.7 g fish−1) after 90 days of feeding, but there was no significant (P > 0.05) effect of probiotics on FCR and SGR. However, in basa fish, Pangasius bocourti (Meidong et al. 2018) reported that the administration of strain B81e isolated from the fish’s gut (1 × 107 CFU g−1) for 60 days had significant effects (p < 0.05) on weight gain, specific growth rate, and feed utilization efficiency of P. bocourti. This growth improvement might be related to the capability of the putative probiotics in producing extracellular protease and lipase within fish gut and thus exert beneficial effects to the digestive processes of the host fish as bacterial enzymes can help degrade the proteinaceous and lipid substrates (Ramesh et al. 2015; Ray et al. 2012). The significant reduction in FCR indicated that the fish utilized dietary nutrients more efficiently when feed was supplemented with strain B81e.
1.3.1.4 Japanese eel (Anguilla japonica)
Bacillus spp. supplementations have been recently applied in Japanese eel. Lee et al. (2017) indicated that dietary supplementation of Bacillus subtilis WB60 at 108 CFU g−1 in diet of Japanese eel (Anguilla japonica) resulted in better weight gain, feed efficiency, and protein efficiency ratio compared to the control and Lactobacillus plantarum diets. Similar results were observed in Japanese eel fed Bacillus subtilis WB60 and mannanoligosaccharide (MOS), as well as (Bacillus subtilis or licheniformis) and (mannan or fructooligosaccharide) (Lee et al. 2018; Park et al. 2020). There is growing evidence that gastrointestinal bacteria facilitate the decomposition of nutrients in the host organism and provide physiologically active materials, such as enzymes, amino acids, and vitamins (Cencic and Chingwaru 2010; Morowitz et al. 2011; Wang et al. 2020a). These materials can positively influence the digestive tract and improve feed digestion and utilization (Bairagi et al. 2004; Dawood et al. 2019; Ramirez and Dixon 2003; Wang et al. 2020b).
1.3.1.5 Sea Cucumber (Apostichopus japonicus)
Supplementation of Bacillus cereus EN25 at 0 (control), 105, 107, and 109 CFU g−1 for 30 days showed no significant effects on growth of sea cucumbers A. japonicus (Zhao et al. 2016). Growth performance of sea cucumbers was one of the important indices to evaluate the effects of potential Bacillus spp. on culturing of sea cucumbers. Previous studies had proved that dietary Bacillus spp., such as indigenous B. subtilis T13 (Zhao et al. 2012), indigenous B. cereus (Yang et al. 2015), and commercial B. subtilis (Zhang et al. 2010), could improve the growth performance of sea cucumbers at suitable doses. This difference could be attributed to the differences in Bacillus strains, sizes of sea cucumbers, sources of sea cucumbers, experimental period, and experimental conditions. The present study was conducted with the same source of sea cucumbers at the same experimental period and conditions with Zhao et al. (2012), except Bacillus strain and initial sizes of sea cucumbers. Recently, Liu et al. (2020) indicated that dietary supplementation of B. baekryungensis MS1 at 107 cfu g−1 for a total of 60 days significantly improved the growth performance of the sea cucumber cultured under low temperature. This is related to the mode of action of probiotics, including the production of digestive enzymes, the production of antibacterial substances, immune stimulation, and interference of quorum sensing, all of which depend on the long-term growth and reproduction of probiotics. Studies have also shown that probiotics work by managing community assembly of the water and gut microbiota (Selim and Reda 2015; Wang et al. 2017a).
1.3.1.6 Tambaqui (Colossoma macropomum)
Dietary inclusion of Bacillus subtilis (109 UFC g−1) and Saccharomyces cerevisiae (109 UFC g−1) showed that no differences were found for the growth parameters between the treatments with probiotics (da Paixão et al. 2017). Although probiotics are supposed to be beneficial, the literature mentions possible synergistic effects. The total replacement of indigenous populations with probiotics may not be desirable to improve growth performance (Merrifield et al. 2010). The control of the endogenous balance between pathogenic and beneficial bacteria is still the target of many studies. According to Merrifield et al. (2010), the lack of improvements regarding growth and feed use may be explained by the level of gastrointestinal colonization that could be too high and any possible synergistic effect with the normal gut microbiota was negated. Thus, it is expected that the beneficial effects of probiotics for tambaquis are not on its performance but on its health and welfare. However, in another study with tambaqui, Dias et al. (2018) indicated that the use of the autochthonous bacteria B. cereus improves the growth performance, productivity, hematological profile, and survival of tambaqui juveniles. This enhanced growth performance of fish supplemented with probiotics is probably due to an improvement in digestion as well as an increase in the synthesis and absorption of nutrients (Hoseinifar et al. 2017). Similar results were obtained by El-Haroun et al. (2006) reporting increased growth performance and feed efficiency in tilapia fed the probiotics Bacillus licheniformis and Bacillus subtilis. According to these authors, the added probiotics improved digestibility, dietary protein, and energy utilization. These positive effects can be attributed to the capacity of the probiotics to promote an increase in the gut absorbent surface area, and stimulate and/or produce several enzymes on the intestinal tract, which improve digestibility and nutrient retention, leading to higher growth rates (El-Haroun et al. 2006; Ibrahem 2015).
1.3.1.7 Carp Species
Dietary administration of BioPlus 2B, a probiotic containing Bacillus licheniformis and B. subtilis, and Ferroin solution indicate that the combination of probiotic and Ferroin solution represents an effective dietary supplement for improving carcass quality, growth performance, and hematological parameters in kutum fry (Azarin et al. 2015). In mrigal fingerlings, Cirrhinus mrigala (avg.wt. 2.5 ± 0.20 g) were fed with three different doses (2 × 104, 2 × 105, and 2 × 106 CFU) of Bacillus sp. PP9 admixed with 100 g feed for a period of 60 days. It was found that the feed with Bacillus concentration of 2 × 104 CFU exhibited significantly higher growth and lower food conversion ratio compared to the control and other supplemented diets (Bandyopadhyay et al. 2015). More recently, Qin et al. (2020) found that dietary inclusion of B. licheniformis at the low-dose 1 × 105 cfu g−1 and the high-dose (HD) group with 1 × 106 cfu g−1 led to significantly (p < 0.05) improved percent weight gain (PWG) and specific growth rate (SGR) parameters. The improvement of growth performance parameters such as PWG and SGR with increasing concentrations of supplemented B. licheniformis FA6 observed in this study is in agreement with Han et al. (2015) observed a significant increase in the growth performance of tilapia fed with B. licheniformis. The increase in the growth performance of grass carp may due to the secretion of digestive enzymes by B. licheniformis, which improves feed digestibility (Kuebutornye et al. 2019).
In Pengze crucian carp, Carassius auratus, dietary supplementation with prebiotics β-glucan (BG group) and probiotics Bacillus subtilis (BS group) resulted in better growth performance than other groups whereas feed efficiency was unaffected by dietary treatments. The textures of muscle in terms of hardness, springiness, cohesiveness, gumminess, chewiness, and resilience were higher in BG and BS groups than the control group. Supplementation of β-glucan and B. subtilis acted as a hypolipidemic in terms of decreasing the total cholesterol, high-density lipoprotein, and low-density lipoprotein, whereas increased the immune responses in serum measured by acid phosphatase, alkaline phosphatase, and catalase activities. Dietary supplementation of β-glucan and B. subtilis significantly improved the fold height and microvillus height in contrast to basal diet. Moreover, β-glucan could significantly increase digestive capacity observed in terms of an increase in amylase and trypsase activities, and B. subtilis significantly increased amylase and lipase activities in intestine (Cao et al. 2019).
1.3.1.8 Trout
A commercial probiotic (4.2 × 109 CFU g−1 of additive) was supplemented to the experimental diets at 0% (control), 0.03% (P1; 6 × 103 CFU g−1of diet), or 0.06% (P2; 1.5 × 106 CFU g−1 of diet) and fed to brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss) for 9 and 20 weeks, respectively. Rainbow trout showed significantly better growth performance than brown trout, regardless of the dietary treatment. No effect of dietary probiotic supplementation was detected on growth performance and body composition (Ramos et al. 2017). However, in Caspian Brown Trout (Salmo trutta caspius)Aftabgard et al. (2019) found that the combined effects of IMOS, a prebiotic, and BetaPlus®, a probiotic containing B. subtilis and B. licheniformis, demonstrated a better performance of select growth indices, including BWI and FCR, than fish that were fed the control diet; these results were probably due to improved nutrition and digestive processes (Cerezuela et al. 2011).
1.3.1.9 Other Aquacultured Species
Two probiotics (Virgibacillus proomii and Bacillus mojavensis) were used to study their effects on the digestive enzyme activity, survival, and growth of sea bass, Dicentrarchus labrax at various ontogenetic stages in three separate experiments (Hamza et al. 2016). The results indicated that the two probiotics V. proomii and B. mojavensis were adequate for improved growth performance and survival and for healthy gut microenvironment of the host (Hamza et al. 2016).
In the study of Hauville et al. (2016) Florida pompano (Trachinotus carolinus) larvae were fed either live feed enriched with Algamac 3050 (Control), Algamac 3050, and probiotics (PB), or the previous diet combined with a daily addition of probiotics to the tank water (PB+). The results indicated that a mix of Bacillus sp. can promote growth through an early maturation of the digestive system during the early larval stages of pompano and snook.
In grouper Epinephelus coioides (Yan et al. 2016, juveniles (14.6 ± 0.2 g) were fed either a basal control diet (without probiotic) or the basal diet supplemented with 1.0 × 108 CFU g−1 live (T1) and heat-inactivated B. pumilus SE5 (T2). The results indicated that the heat-inactivated probiotic significantly improved the final weight, weight gain (WG), and specific growth rate (SGR) at day 60 and significantly decreased the feed conversion ratio (FCR) at day 30 and 60, while the viable probiotic significantly decreased the FCR at day 60 (P < 0.05). This suggested that live and heat-inactivated B. pumilus could promote the efficient utilization of dietary nutrients. Interestingly, significant increased growth was only observed in fish fed the heat-inactivated B. pumilus containing diet for 60 days, but not in fish fed the live B. pumilus containing diet. Likewise, Hoseinifar et al. (2011) observed that dietary supplementation of 20 g kg−1 inactive brewer’s yeast Saccharomyces cerevisiae var. ellipsoideus significantly improved the growth performance in juvenile beluga sturgeon (Huso huso). In rock bream, Oplegnathus fasciatus, Kim et al. (2017), revealed that supplementation of B. amyloliquefaciens spores at a concentration of 1.4 × 106 colony-forming units per gram (CFU g−1) of feed for 90 days resulted in significant improvements in body weight (BW), weight gain (WG), specific growth rate (SGR), and food conversion ratio (FCR) when compared with control group fish.
In hybrid sturgeon, Acipenser schrenckii ♂ and Acipenser baerii ♀, fish were fed with Bacillus amyloliquefaciens (GB-9) and Yarrowia lipolytica lipase2 (YLL2): Diet 1 (0-control), Diet 2 (5.0 g kg−1 GB-9), Diet 3 (4.0 g kg−1 YLL2), and Diet 4 (5.0 g kg−1 GB-9 + 4.0 g kg−1 YLL2), respectively (Fei et al. 2018). The results indicated that supplementations of GB-9 + YLL2 resulted in a significant increase in final weight, Docosahexaenoic acid (DHA) and Eicosapentaenoic acid (EPA) concentration, compared with that of control (p < 0.05). This might be because the DHA and EPA hydrolyzed by YYL2 improved the poor establishment of the GB-9 in the gastrointestinal tract of hybrid sturgeon and might have promoted the growth of GB-9 (Menni et al. 2017). Similarly, combination of B. licheniformis and B. amyloliquefaciens indicated up to 2.5 times higher survival with probiotic addition, as well as 20% higher survival 7 days following a transport event. These benefits could not be explained by faster larval growth. In fact, CONT larvae were significantly longer than probiotic-treated larvae, likely due to decreased competition for food in CONT tanks which exhibited significantly lower survival. The other differing morphometric in this study was oil globule volume which was lowest in CONT larvae, suggesting that CONT larvae were consuming their endogenous reserves more quickly than probiotic-treated larvae. Retention of oil globules allows for a longer transition time to exogenous feeding, and studies indicate larvae that retain their endogenous reserves longer demonstrate increased survival (Avila and Juario 1987; Berkeley et al. 2004). The probiotic may alter development of the digestive tract and thus the start of exogenous feeding, as has been demonstrated in previous studies involving Bacillus probiotics and common snook (Hauville et al. 2016).
Dietary supplementation of B. subtilis has been reported to improve the growth performance, feed utilization, amylase, protease, and lipase enzymes of parrotfish (Oplegnathus fasciatus) and red sea bream (Pagrus major) (Liu et al. 2018; Zaineldin et al. 2018). The observed improvement in growth performance might be ascribed to the enhanced intestinal digestive enzyme activity and beneficial intestinal microbiota (Dawood et al. 2014; Liu et al. 2009; Sun et al. 2010). Bacillus sp. can produce certain essential micronutrients to promote better growth and feed utilization of hosts (Sanders et al. 2003). Further, Bacillus species may participate in digestion processes to break down nutrients such as carbohydrates, proteins, and lipids by producing extracellular enzymes (Liu et al. 2009; Sun et al. 2010). In abalone, Haliotis discus hannai, Gao et al. (2018) indicated that the food containing 105 cfu mL−1 Bacillus licheniformis promoted food intake and growth of abalones. Bacillus licheniformis is an aerobic nonpathogenic bacterium that inhabits the intestinal microbial community in the form of spores, which can reduce intestinal pH, reduce ammonia concentration, and promote decomposition of starch and cellulose. Thus, it is generally considered to be a relatively stable probiotic (Hong et al. 2005; Vine et al. 2006).
1.3.2 Increase Disease Resistance
Probiotics have been proven as an effective tool for disease prevention in aquaculture (Hoseinifar et al. 2018). Probiotics can interact with or antagonize other enteric bacteria by resisting colonization or by directly inhibiting and reducing the incidence of opportunistic pathogens (Chiu et al. 2017). They can also improve host’s health and well-being via physiological or immune modulation (Butt and Volkoff 2019). Probiotics can produce effective molecules that have bactericidal activity on intestinal pathogenic bacteria of the host, providing a barrier against the proliferation of opportunistic pathogens (Martínez Cruz et al. 2012; Seghouani et al. 2017). The functional molecules produced during the bactericidal activity are antibiotics, bacteriocins, siderophores, enzymes and/or hydrogen peroxide as well as the alteration of the intestinal pH due to the generation of organic acids (Verschuere et al. 2000). The inhibition of intestinal related diseases has been reported in several cultured species by probiotic incorporation in aquafeeds (Ringø et al. 2018; Serra et al. 2019; Wanka et al. 2018). Thus, it can be confirmed that the ability of aquatic animals to avoid the infectious diseases mainly depends on the immunomodulatory effect that happened due to the administration of beneficial bacterial cells.
1.3.2.1 Tilapias
Dietary inclusion of B. licheniformis at 0%, 0.02%, 0.04%, 0.06%, 0.08%, and 0.1% containing live germ 2 × 1010 CFU/g for 10 weeks significantly increased disease resistance of Nile tilapia, Oreochromis niloticus against Streptococcus iniae (Han et al. 2015). Bacillus strains supplementation in diet could increase disease resistance in fish through the stimulation of both the cellular and humoral immune function, such as phagocytic activity, lysozyme activity, and complement activity (Arena et al. 2006; Queiroz and Boyd 1998; Sookchaiyaporn et al. 2020; Zhou et al. 2010). It was reported that Bacillus bacteria are able to outcompete other bacteria for nutrients and space and can exclude other bacteria through the production of antibiotics, and as usually lead to the enhanced immunity of fish (Cha et al. 2013). Similarly, dietary inclusion of B. licheniformis Dahb1 at 107 cfu g−1 could improve disease resistance of Mozambique tilapia (Oreochromis mossambicus) against A. hydrophila (Gobi et al. 2018). In terms of Bacillus subtilis HAINUP40, H. Liu et al. (2017) reported that dietary supplement of B. subtilis HAINUP40 at 108 cfu g−1 can effectively enhance disease resistance of Nile tilapia against Streptococcus agalactiae. In addition, combination of B. subtilis with S. cerevisiae and A. oryzae; Bacillus subtilis with Aqua NZ and AP193; Bacillus subtilis strains SB3086, SB3295, SB3615 with AP193; B. subtilis and B. licheniformis, and Bacillus subtilis and Bacillus licheniformis (BS) combined with traditional Chinese medicine (TCM) A. hydrophila and S. iniae. Higher intestinal Bacillus spp. counts can regulate the gut microbiota of fish, selectively stimulate other beneficial probiotic bacteria, and depress some potential harmful bacteria (Yang et al. 2012).
1.3.2.2 Shrimps
The efficiency of these isolates in controlling pathogens, which is a key factor in selecting appropriate bacteria as probiotics, was evaluated (Kesarcodi-Watson et al. 2008). Based on in vitro laboratory results, B4, B6, and B12 inhibited V. parahaemolyticus; however, only B. subtilis AQHPS001 (B12) showed the highest antagonistic property against VPAHPND strains. However, among the VPAHPND strains, there were different sizes of the inhibitory clear zone, and VPAHPNDAQH3.2 was the only strain that resisted B12. This suggests that there are varieties of VPAHPND and that each strain may employ different mechanisms in response to the target B12 (Kewcharoen and Srisapoome 2019). Previous reports found that Bacillus spp. could produce many kinds of bacteriocins, such as subtilin, subtilosin, coagulin, megacin, bacillin, bacillomycin, mycosubtilin, toximycin, and xanthobacidin, which could reduce pathogen colonization by directly inhibiting pathogens while having no resulting effects on the virulence resistance genes of pathogenic bacteria (Desriac et al. 2010; Hammami et al. 2012; Joseph et al. 2013). Zhao et al. (2015) also reported that Bacillus spp. could secrete quorum-quenching enzymes, which are expected to be quorum-sensing blockers to reduce disease infection. These results suggest that B. subtilis AQAHBS001 possesses more effective characteristics that are important for controlling the various harmful VPAHPND strains than other candidates. For these reasons, it was further chosen to study its application on a laboratory scale. Similarly, dietary inclusion of Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis at 108 CFU g−1 could improve disease resistance of whiteleg shrimp Litopenaeus vannamei against Vibrio parahaemolyticus (Won et al. 2020b). Generally, administration of probiotics in the shrimp diet was shown to decrease mortality rates compared to the CON diet (Balcázar et al. 2007; Sapcharoen and Rengpipat 2013; Zhang et al. 2009). Previous studies demonstrated that probiotic supplementation can be used for modulating fish health and disease resistance (Wang et al. 2018; Zuo et al. 2019). Indeed, probiotics can beneficially influence the disease resistance of fish to pathogen bacteria by producing antimicrobial substances and competing with pathogens for physical occupation of space (Lim et al. 2020). As a result, the enhanced survival and cumulative survival rates could be due to probiotic supplementation. Chen et al. (2020a) recently indicated that dietary MOS and/or B. licheniformis supplementation could positively increase ammonia resistance of Litopenaeus vannamei. According to Chen et al. (2012), immune parameters decrease after ammonia stress, yet these parameters recover faster when they were initially stimulated by a probiotic. Faster recovery of immune parameters might have contributed to the increased survival after ammonia stress for the Rps. palustris fed shrimp.
1.3.2.3 Catfish
Meidong et al. (2017) revealed that Bacillus siamensis strain B44v and Bacillus sp. strain B51f, derived from indigenous fermented foods, displayed strongly antagonistic activity against the bacterial fish pathogens, A. hydrophila and S. agalactiae. Both strains effectively inhibited Gram-positive and Gram-negative bacteria, indicating their broad spectrum as a useful antagonistic property as the two most striking bacterial fish pathogens in aquaculture in Thailand belong to the genera the Aeromonas and Streptococcus (Maisak et al. 2013). Besides fish pathogens, the bacteriocin-like substance from B. siamensis strain B44v inhibited several foodborne pathogens suggesting potential applications in human foods (Sivamaruthi et al. 2018). Likewise, Reda et al. (2018) showed that supplementation of three autochthonous Bacillus strains (B. subtilis, B. amyloliquefaciens, and B. cereus) and a commercial B. amyloliquefaciens at a dose of 1 × 1010 CFU kg−1 significantly increased disease resistance of African catfish against Aeromonas sobria. This may be returned to the ability of Bacillus spore to resist gastrointestinal conditions, survive and transit cross gastrointestinal tract, germinate and vegetate with heterologous antigen expression before being excreted (Duc et al. 2003). In striped catfish, Truong Thy et al. (2017) indicated that the mixed probiotics of Bacillus amyloliquefaciens 54A and B. pumilus 47B isolated from striped catfish (Pangasianodon hypophthalmus) intestine significantly enhanced disease resistance of the fish against Edwardsiella ictaluri and ammonia tolerance. Antimicrobial activity of probiotics has been demonstrated on many in vitro and in vivo studies in animals. The study of Corr et al. (2007) reported trial mice received protection from Lactobacillus salivarius against Listeria monocytogenes involved bacteriocin produced by L. salivariusUCC118. Additionally, antimicrobial activities of probiotics against pathogens include secretion of hydrogen peroxide (Pridmore et al. 2008), lactic acid (Fayol-Messaoudi et al. 2005), competitive exclusion (Lee et al. 2003), and stimulation of immune system (Ryan et al. 2009). The positive effect on barrier function of probiotics is to protect the host intestine by prevention of pathogen attachment to epithelial cells on gut surface (Mennigen et al. 2009). In basa fish, Pangasius bocourti, Meidong et al. (2018) found that B. aerius B81e has beneficial effects on growth performance, innate immunity, and disease resistance of P. bocourti against Aeromonas hydrophila and Streptococcus agalactiae. Bacterial co-aggregation has considerable significance in the host gut as co-aggregation ability of bacterial probiotics might interfere with the ability of pathogenic bacteria to infect the host and can prevent colonization of the pathogens (Spencer and Chesson 1994). In addition, B. aerius B81e has an absence of hemolysin and is susceptible to most of the common antibiotics tested which demonstrated that it is likely a nonpathogen and has an inability to transfer antibiotic-resistant genes to recipient bacteria in the host gut, thus preventing the development of antibiotic-resistant pathogens (Meidong et al. 2018).
1.3.2.4 Japanese eel (Anguilla japonica)
The combination of Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 or Bacillus subtilis WB60 and mannanoligosaccharide (MOS) significantly improved disease resistance of Japanese eel against V. anguillarum (Lee et al. 2017, 2018). Similarly, Park et al. (2020) reported that dietary inclusion of B. subtilis with FOS (BSF) and B. licheniformis significantly increased disease resistance against Aeromonas hydrophila. Significant increase in disease resistance in these works may be attributable to the stimulation of cellular and humoral immune function.
1.3.3 Sea Cucumber (Apostichopus japonicus)
Zhao et al. (2016) indicated that the cumulative mortality after V. splendidus challenge decreased significantly in sea cucumbers fed with EN25 at 107 CFU g−1 (P < 0.05). The present study confirmed dietary B. cereus EN25 at 107 CFU g−1 could significantly improve disease resistance in juvenile A. japonicus. Recently, Liu et al. (2020) showed that B. baekryungensis MS1 significantly reduced the mortality of sea cucumbers infected with Vibrio splendidus. By regulating the expression of immune-related genes and signaling pathways, B. baekryungensis MS1 improved the immunity of sea cucumber in winter and effectively controlled the infection of pathogenic bacteria such as V. splendidus.
1.3.4 Tambaqui (Colossoma macropomum)
da Paixão et al. (2017) indicated that supplementation of two probiotics Bacillus subtilis and Saccharomyces cerevisiae at 109 UFC g−1 significantly increased disease resistance of tambaqui, Colossoma macropomum, against Streptococcus agalactiae. Similarly, Dias et al. (2018) reported that B. cereus (4.2 × 104, 3.9 × 106 and 3.3 × 108 CFU g−1) supplemented as probiotics to C. macropomum for 120 days significantly increased disease resistance against Aeromonas hydrophila. The probiotic promoted a nonspecific response against bacterial infection, increasing fish survival after challenge with A. hydrophila.
1.3.5 Other Species
In rock bream, Oplegnathus fasciatus, Kim et al. (2017) demonstrated the benefit of incorporation of B. amyloliquefaciens as a feed supplement to improve the health status of Oplegnathus fasciatus challenged with Streptococcus iniae. The enhancement of the innate immune response with a B. amyloliquefaciens enriched probiotic diet and decreased mortality rate, thereby protecting the fish against S. iniae. Similarly, dietary inclusion of B. subtilis at 108 CFU kg−1 significantly increased disease resistance of parrotfish, Oplegnathus fasciatus, against Vibrio alginolyticus (Liu et al. 2018). The growth performance and health status improvement of aquatic animal might be involved with the gut microbiota change after probiotic administration. The previous study has also demonstrated the positive effects of B. subtilis E20 in terms of intestinal presence and subsequent health benefits for L. vannamei (Liu et al. 2009; Tseng et al. 2009) and E. coioides (Liu et al. 2010). In the same trend, dietary inclusion of Bacillus licheniformis significantly improved disease resistance of abalone, Haliotis discus hannai Ino., against V. parahaemolyticus and grass carp, Ctenopharyngodon idella, against A. hydrophila (Gao et al. 2018; Qin et al. 2020).
1.4 Immune Effects of Bacillus
Enhancement of host immunity is one important benefit of probiotic diet supplementation (Kuebutornye et al. 2019). As stated by Verschuere et al. (2000), probiotics can modulate innate immunity through the modulation of humoral immune responses and expression of immune-related genes. Effects of Bacillus on immune response of different fish and shellfish are displayed in Table 1.2.
1.4.1 Tilapias
Selim and Reda (2015) found that Bacillus amyloliquefaciens spores supplementation at concentrations of 1 × 106 (G3) and 1 × 104 (G2) colony-forming units per gram (CFU g−1) of feed significantly enhanced serum killing, serum nitric oxide, serum lysozyme activities, as well as IL-1 and TNF α mRNA levels in the kidneys of Nile tilapia, O. niloticus. The cell wall components of both Gram-positive and Gram-negative bacteria are able to stimulate cytokine production (Henderson et al. 1999). Probiotic bacteria colonize in the gut and are involved with the gut-associated lymphoid tissue to stimulate systemic signals that end with cytokine production (Kesarcodi-Watson et al. 2008; Rangavajhyala et al. 1997; Rescigno et al. 2001; Ringø 2011). Similarly, dietary inclusion of B. subtilis singularly or B. subtilis combined with S. cerevisiae and A. oryzae; B. subtilis with B. licheniformis; B. subtilis and Bacillus licheniformis (BS) combined with traditional Chinese medicine (TCM), and B. subtilis with Aqua NZ and AP193 significantly enhanced innate immune response, growth, relative immune, and antioxidant gene expressions of Nile tilapia (Abarike et al. 2018a; Abarike et al. 2018b; Addo et al. 2017a, 2017b; Iwashita et al. 2015; Liu et al. 2017; Wang et al. 2020a). Dietary inclusion of B. licheniformis has been found to increase alkaline phosphatase, myeloperoxidase, lysozyme, reactive oxygen species, reactive nitrogen species, superoxide dismutase, and glutathione peroxidase of Mozambique tilapia (Oreochromis mossambicus) (Gobi et al. 2018). Also, supplementation of Bacillus licheniformis HGA8B significantly improved lysozyme activity and content of complement C3 (Han et al. 2015). It is well documented that, the immune system can be nonspecifically modulated by probiotics (Hoseinifar et al. 2015; Lazado and Caipang 2014; Nayak 2010). Moreover, colony formation and adhesion of probiotics in the intestine of fish are necessary to enhance the immune responses (Ausubel 2005). Interaction between probiotic cells and immune system are through microbe associated molecular patterns (MAMPs) consisting of specific cell wall polysaccharides, peptidoglycan, lipoprotein anchors, and lipoteichoic acids (Hosoi et al. 2003). Cells or components of immune system can interact with MAMPs by pattern recognition receptor such as toll-like receptors, C-type receptor, and nucleotide oligomerization domain-like receptors (Bron et al. 2012; Kleerebezem et al. 2010). This fact may indicate that, addition of fresh culture of B. licheniformis to the diet maintains a high level of probiotics in the diet and improve the immune responses in fish. Similar results have been reported in Nile tilapia fed B. cereus and B. pumilus (Srisapoome and Areechon 2017; Wang et al. 2017b).
1.4.2 Shrimps
In shrimp, B. licheniformis has been intensively applied in Pacific white shrimp (Litopenaeus vannamei). Amoah et al. (2019) indicated that dietary inclusion of 1 × 108 CFU g−1 feed significantly enhanced activity of lysozyme (LYZ), acid phosphatase (ACP), superoxide dismutase (SOD), total protein (TP), albumin (ALB) in serum, glutathione peroxidase (GSH-Px) in serum and liver of Nile tilapia. Similarly, dietary administration of B. licheniformis significantly upregulated the expression of catalase, glutathione peroxidase, superoxide dismutase (SOD), penaeidin-3a (Pen-3a), and heat shock protein (Hsp-70) genes of Pacific white shrimp, Litopenaeus vannamei (Chen et al. 2020a, 2020b). In addition, the combination of B. licheniformis with B. subtilis significantly enhanced lysozyme and hemocyte cell count and upregulated the expression of proPO, LvToll1 and SOD, Hsp70, and TGase genes (Sadat Hoseini Madani et al. 2018; Sánchez-Ortiz et al. 2016). Likewise, dietary inclusion of B. subtilis E20 singularly or combined with other probiotics significantly innate immune response and related immune gene expression of Pacific white shrimp, Litopenaeus vannamei (Chien et al. 2020; Won et al. 2020a). Also, Khademzade et al. (2020) reported that dietary inclusion of Bacillus cereus and Pediococcus acidilactici significantly enhanced total hemocyte count, total protein, and lysozyme activities of L. vannamei. Similar results were found in tiger shrimp and freshwater pawn fed Bacillus sp. and Bacillus coagulant where significant increase in total heterotrophic count, amylolytic, cellulolytic, and proteolytic bacterial counts, phagocytic, lysozyme, and respiratory burst activities was recorded (De et al. 2018; Gupta et al. 2016). At molecular levels, Sánchez-Ortiz et al. (2016) indicated that dietary supplementation of Bacillus spp. resulted in upregulation of proPO, LvToll1, SOD genes, except the TGase gene expression. Similarly, Tepaamorndech et al. (2019) revealed that dietary inclusion of Bacillus aryabhattai TBRC8450 significantly upregulated C-type lectin, penaeidin-3, and heat shock protein 60 genes, as well as enhanced thioredoxin, ferritin, phenoloxidase, and total antioxidant activities of Pacific white shrimp, Litopenaeus vannamei. However, no significant increase in total hemocyte count, and superoxide dismutase were observed (Tepaamorndech et al. 2019).
1.4.3 Carps
In mrigal, Cirrhinus mrigala, Bandyopadhyay et al. (2015) indicated that dietary inclusion of Bacillus sp. PP9 significantly improved hemoglobin percentage, total erythrocyte count, total leukocyte count, corpuscular hemoglobin, total serum protein, albumin globulin ratio, and serum bactericidal activity. Similarly, dietary supplementation of B. subtilis singularly or combined with other Bacillus sp. and prebiotics significantly stimulated hematological, antioxidant, and immunological parameters of Labeo rohita (Ramesh and Souissi 2018); Labeo catla, Catla catla (Sangma and Kamilya 2015); common carp, Cyprinus carpio (Wang et al. 2017a); grass carp, Ctenopharyngodon idellus (Zhao et al. 2020), and Pengze crucian carp, Carassius auratus var. Pengze (Cao et al. 2019). At gene level, Yin et al. (2018) found that supplementation of B. subtilis resulted in higher protective effects against lead toxicity, superoxide dismutase, catalase and glutathione, lysozyme and IgM levels, as well as immune-related genes of gibel carp, Carassius auratus gibelio. Likewise, dietary inclusion of B. amyloliquefaciens significantly stimulated innate immune response, antioxidant, and relative immune gene expressions of roho labeo, Labeo rohita (Nandi et al. 2018); Indian major carp, Catla catla Singh et al. (2017), and grass carp, Ctenopharyngodon idella (Qin et al. 2020).
1.4.4 Sea Cucumber (Apostichopus japonicus)
Supplementation of B. cereus singularly or combined with B. subtilis significantly enhanced total coelomocytes count, acid phosphatase, phagocytosis, respiratory burst, total nitric oxide synthase, catalase, phenoloxidase, and superoxide dismutase activities (Li et al. 2015). Recently, Liu et al. (2020) indicated that dietary administration of B. baekryungensis significantly enhanced superoxide dismutase, catalase, alkaline phosphatase, acid phosphatase, nitric oxide synthetase, phagocytosis, respiratory burst activities, and ubiquitin-mediated proteolysis pathway. Ubiquitin-mediated proteolysis plays an important role in the dynamic regulation of host defense against pathogen infection. It has been reported that a number of key joint molecules in the natural immune and antiviral signaling pathways can be modified by ubiquitination to regulate the antiviral immune response of the body (Chuang and Ulevitch 2004; Liu and Chen 2011). Ubiquitination plays an important role in the Toll-like receptor (TLR) signaling pathway. The activation of this pathway leads to the upregulated expression of Toll-like receptors and enhances nonspecific immunity (Bhoj and Chen 2009). The upregulation of TLR in this study is consistent with the above theory. In the immune system, mTOR signaling plays an important role in maintaining immune homeostasis, for example, the survival and migration of natural immune cells and the secretion of inflammatory factors (Katholnig et al. 2013; Weichhart et al. 2008). Studies have found that the mTOR signaling pathway negatively regulates nonspecific immune responses (Weichhart et al. 2008). Therefore, the downregulation of the mTOR pathway in sea cucumber is beneficial to improve sea cucumber immunity.
1.4.5 Catfish
In striped catfish, Pangasianodon hypophthalmus, Truong Thy et al. (2017) reported that dietary inclusion of B. amyloliquefaciens and B. pumilus significantly enhanced phagocytic, respiratory bursts, and lysozyme activities. Similar results were observed in basa fish, Pangasius bocourti fed B. aerius (Meidong et al. 2018). Likewise, combination of B. subtilis, B. amyloliquefaciens, B. cereus, and B. amyloliquefaciens (Reda et al. 2018).
1.4.6 Japanese eel
Dietary inclusion of B. subtilis and Lactobacillus plantarum significantly enhanced lysozyme, superoxide dismutase (SOD), myeloperoxidase (MPO), level of intestine glyceraldehyde-3-phosphate dehydrogenase (GAPDH), heat shock protein 70, 90, and immunoglobulin (IgM). Similarly, dietary inclusion of B. subtilis and mannanoligosaccharide (MOS) significantly improved nonspecific enzymatic activities, heat shock protein 70 mRNA levels, and immunoglobulin M expressions (Lee et al. 2018). More recently, Park et al. (2020) indicated that dietary inclusion of B. subtilis or B. licheniformis and mannan or fructo oligosaccharide upregulated heat shock protein 70 and immunoglobulin M genes.
1.4.7 Other Species
Dietary inclusion of B. subtilis singularly or combined with B. licheniformis, Bacillus cereus toyoi, and isomaltooligosaccharides significantly stimulated hematological, innate immune response, antioxidant, and gene expression of parrotfish, Oplegnathus fasciatus (Liu et al. 2018); red sea bream, Pagrus major (Zaineldin et al. 2018); turbots, Scophthalmus maximus (Fuchs et al. 2017); rainbow trout, Oncorhynchus mykiss and brown trout, Salmo trutta (Ramos et al. 2017), and Caspian brown trout, Salmo trutta caspicus (Aftabgard et al. 2019). Regarding B. amyloliquefaciens, dietary inclusion of B. amyloliquefaciens singularly or combined with Yarrowia lipolytica lipase 2 (YLL2), B. licheniformis significantly enhanced innate immune response, antioxidant, and gene expression of rock bream, Oplegnathus fasciatus (Kim et al. 2017); hybrid sturgeon, Acipenser schrenckii ♂and Acipenser baerii ♀ (Fei et al. 2018), and zebrafish, Danio rerio (Lin et al. 2019).
In juvenile Atlantic salmon (Salmo salar L.), Wang et al. (2019a) reported that B. velezensis V4 and Rhodotorula mucilaginosa compound led to an increase in acid phosphatase, IgM, nitric oxide, glutamic pyruvic transaminase, glutamic oxalacetic transaminase, lysozyme, total superoxide dismutase malondialdehyde, glutathione, glutathione peroxide, total antioxidant capacity, and malondialdehyde. Similarly, dietary inclusion of Bacillus licheniformis significantly enhanced hematological, innate immune response, and Mn-SOD gene expression (Gao et al. 2018). Also, significant increase in innate immune response and relative immune gene expressions were observed in grouper, Epinephelus coioides, fed Bacillus pumilus (Yan et al. 2016) and in olive flounder, Paralichthys olivaceus, fed Bacillus sp. SJ-10 plus β-glucooligosaccharides (Hasan et al. 2018).
1.5 Conclusion
This chapter addressed the role of Bacillus probiotics in sustainable aquaculture. Although a wide range of researches have indicated beneficial effects of Bacillus species on grow rate, immunity, and disease resistance of farmed fish and shellfish, the investigated effects were species specific. In order to evaluate in vivo adherence and colonization of Bacillus bacteria within the complex microbial ecosystem of the intestine, detection of green fluorescence protein (GFP) tagged strains or fluorescence in situ hybridization (FISH) targeting 16S rRNA to identify the probiotics on the mucus surface must be carried out. Furthermore, mucus-associated (autochthonous) microbiome must be investigated by next-generation sequencing (NGS), transcriptomic, metagenomics or proteomic profiling, and not the allochthonous microbiome; mostly investigated per sc. In addition, we recommend that gnotobiotic approaches are used in future studies, as the gnotobiotic approaches have been reported to have important roles to understand the function of gut microbiota on numerous biological processes of the host. Moreover, data is needed to understand the mechanisms by which the immune system of the intestinal mucosa discriminates between pathogenic, probiotics, and commensal microorganisms.
References
Abarike ED, Cai J, Lu Y, Yu H, Chen L, Jian J et al (2018a) Effects of a commercial probiotic BS containing Bacillus subtilis and Bacillus licheniformis on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 82:229–238
Abarike ED, Jian J, Tang J, Cai J, Yu H, Lihua C, Jun L (2018b) Influence of traditional Chinese medicine and Bacillus species (TCMBS) on growth, immune response and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac Res 49(7):2366–2375
Abd El-Rhman AM, Khattab YAE, Shalaby AME (2009) Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 27(2):175–180. https://doi.org/10.1016/j.fsi.2009.03.020
Abriouel H, Franz CM, Omar NB, Gálvez A (2011) Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev 35(1):201–232
Addo S, Carrias AA, Williams MA, Liles MR, Terhune JS, Davis DA (2017a) Effects of Bacillus subtilis strains and the prebiotic Previda® on growth, immune parameters and susceptibility to Aeromonas hydrophila infection in Nile tilapia, Oreochromis niloticus. Aquac Res 48(9):4798–4810
Addo S, Carrias AA, Williams MA, Liles MR, Terhune JS, Davis DA (2017b) Effects of Bacillus subtilis strains on growth, immune parameters, and Streptococcus iniae susceptibility in Nile tilapia, Oreochromis niloticus. J World Aquac Soc 48(2):257–267
Adel M, Yeganeh S, Dawood MAO, Safari R, Radhakrishnan S (2017) Effects of Pediococcus pentosaceus supplementation on growth performance, intestinal microflora and disease resistance of white shrimp, Litopenaeus vannamei. Aquac Nutr 23(6):1401–1409. https://doi.org/10.1111/anu.12515
Afrilasari W, Meryandini A (2016) Effect of probiotic Bacillus megaterium PTB 1.4 on the population of intestinal microflora, digestive enzyme activity and the growth of catfish (Clarias sp.). HAYATI J Biosci 23(4):168–172. https://doi.org/10.1016/j.hjb.2016.12.005
Aftabgard M, Salarzadeh A, Mohseni M, Bahri Shabanipour AH, Zorriehzahra MEJ (2019) The combined efficiency of dietary isomaltooligosaccharides and Bacillus spp. on the growth, hemato-serological, and intestinal microbiota indices of caspian brown Trout (Salmo trutta caspius Kessler, 1877). Probiotics Antimicrob Proteins 11(1):198–206. https://doi.org/10.1007/s12602-017-9361-z
Alavandi SV, Muralidhar M, Syama Dayal J, Rajan JS, Ezhil Praveena P, Bhuvaneswari T et al (2019) Investigation on the infectious nature of running mortality syndrome (RMS) of farmed Pacific white leg shrimp, Penaeus vannamei in shrimp farms of India. Aquaculture 500:278–289. https://doi.org/10.1016/j.aquaculture.2018.10.027
Ambas I, Fotedar R, Buller N (2017) Synbiotic effect of Bacillus mycoides and organic selenium on immunity and growth of marron, Cherax cainii (Austin, 2002). Aquac Res 48(6):2729–2740. https://doi.org/10.1111/are.13105
Amoah K, Huang Q-C, Tan B-P, Zhang S, Chi S-Y, Yang Q-H et al (2019) Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 87:796–808. https://doi.org/10.1016/j.fsi.2019.02.029
Apún-Molina JP, Santamaría-Miranda A, Luna-González A, Martínez-Díaz SF, Rojas-Contreras M (2009) Effect of potential probiotic bacteria on growth and survival of tilapia Oreochromis niloticus L., cultured in the laboratory under high density and suboptimum temperature. Aquac Res 40(8):887–894
Arena A, Maugeri TL, Pavone B, Iannello D, Gugliandolo C, Bisignano G (2006) Antiviral and immunoregulatory effect of a novel exopolysaccharide from a marine thermotolerant Bacillus licheniformis. Int Immunopharmacol 6(1):8–13
Ashaolu TJ (2020) Immune boosting functional foods and their mechanisms: a critical evaluation of probiotics and prebiotics. Biomed Pharmacother 130:110625. https://doi.org/10.1016/j.biopha.2020.110625
Ausubel FM (2005) Are innate immune signaling pathways in plants and animals conserved? Nat Immunol 6(10):973–979. https://doi.org/10.1038/ni1253
Avila EM, Juario JV (1987) Yolk and oil globule utilization and developmental morphology of the digestive tract epithelium in larval rabbitfish, Siganus guttatus (Bloch). Aquaculture 65(3–4):319–331
Azarin H, Aramli MS, Imanpour MR, Rajabpour M (2015) Effect of a probiotic containing Bacillus licheniformis and Bacillus subtilis and ferroin solution on growth performance, body composition and haematological parameters in kutum (Rutilus frisii kutum) fry. Probioticsand AntimicrobProteins 7(1):31–37. https://doi.org/10.1007/s12602-014-9180-4
Bairagi A, Ghosh KS, Sen SK, Ray AK (2002) Enzyme producing bacterial flora isolated from fish digestive tracts. Aquac Int 10(2):109–121. https://doi.org/10.1023/a:1021355406412
Bairagi A, Sarkar Ghosh K, Sen S, Ray A (2004) Evaluation of the nutritive value of Leucaena leucocephala leaf meal, inoculated with fish intestinal bacteria Bacillus subtilis and Bacillus circulans in formulated diets for rohu, Labeo rohita (Hamilton) fingerlings. Aquac Res 35(5):436–446
Balcázar JL, Rojas-Luna T, Cunningham DP (2007) Effect of the addition of four potential probiotic strains on the survival of pacific white shrimp (Litopenaeus vannamei) following immersion challenge with Vibrio parahaemolyticus. J Invertebr Pathol 96(2):147–150
Bandyopadhyay P, Sarkar B, Mahanty A, Rathore RM, Patra BC (2015) Dietary administered Bacillus sp. PP9 enhances growth, nutrition and immunity in Cirrhinus mrigala (Hamilton). Proc Natl Acad Sci India Sect B 85(3):759–766. https://doi.org/10.1007/s40011-015-0561-6
Berkeley SA, Chapman C, Sogard SM (2004) Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology 85(5):1258–1264
Bhoj VG, Chen ZJ (2009) Ubiquitylation in innate and adaptive immunity. Nature 458(7237):430–437
Bron PA, van Baarlen P, Kleerebezem M (2012) Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10(1):66–78
Butt RL, Volkoff H (2019) Gut microbiota and energy homeostasis in fish. Front Endocrinol 10(9). https://doi.org/10.3389/fendo.2019.00009
Cai D, Rao Y, Zhan Y, Wang Q, Chen S (2019) Engineering Bacillus for efficient production of heterologous protein: current progress, challenge and prospect. J Appl Microbiol 126(6):1632–1642. https://doi.org/10.1111/jam.14192
Cao H, Yu R, Zhang Y, Hu B, Jian S, Wen C et al (2019) Effects of dietary supplementation with β-glucan and Bacillus subtilis on growth, fillet quality, immune capacity, and antioxidant status of Pengze crucian carp (Carassius auratus var. Pengze). Aquaculture 508:106–112. https://doi.org/10.1016/j.aquaculture.2019.04.064
Castex M, Lemaire P, Wabete N, Chim L (2009) Effect of dietary probiotic Pediococcus acidilactici on antioxidant defences and oxidative stress status of shrimp Litopenaeus stylirostris. Aquaculture 294(3–4):306–313
Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J (2019) Overview of the antimicrobial compounds produced by members of the Bacillus subtilis group. Front Microbiol 10:302–302. https://doi.org/10.3389/fmicb.2019.00302
Cencic A, Chingwaru W (2010) The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients 2(6):611–625. https://doi.org/10.3390/nu2060611
Cerezuela R, Meseguer J, Esteban MA (2011) Current knowledge in synbiotic use for fish aquaculture: a review. Res Aquac Res Dev S1(008). https://doi.org/10.4172/2155-9546.S1-008
Cha J-H, Rahimnejad S, Yang S-Y, Kim K-W, Lee K-J (2013) Evaluations of Bacillus spp. as dietary additives on growth performance, innate immunity and disease resistance of olive flounder (Paralichthys olivaceus) against Streptococcus iniae and as water additives. Aquaculture 402–403:50–57. https://doi.org/10.1016/j.aquaculture.2013.03.030
Chang Y-P, Liu C-H, Wu C-C, Chiang C-M, Lian J-L, Hsieh S-L (2012) Dietary administration of zingerone to enhance growth, non-specific immune response, and resistance to Vibrio alginolyticus in Pacific white shrimp (Litopenaeus vannamei) juveniles. Fish Shellfish Immunol 32(2):284–290
Chen Y-Y, Sim SS, Chiew SL, Yeh S-T, Liou C-H, Chen J-C (2012) Dietary administration of a Gracilaria tenuistipitata extract produces protective immunity of white shrimp Litopenaeus vannamei in response to ammonia stress. Aquaculture 370:26–31
Chen M, Chen X-Q, Tian L-X, Liu Y-J, Niu J (2020a) Beneficial impacts on growth, intestinal health, immune responses and ammonia resistance of pacific white shrimp (Litopenaeus vannamei) fed dietary synbiotic (mannan oligosaccharide and Bacillus licheniformis). Aquac Rep 17:100408. https://doi.org/10.1016/j.aqrep.2020.100408
Chen M, Chen X-Q, Tian L-X, Liu Y-J, Niu J (2020b) Enhanced intestinal health, immune responses and ammonia resistance in Pacific white shrimp (Litopenaeus vannamei) fed dietary hydrolyzed yeast (Rhodotorula mucilaginosa) and Bacillus licheniformis. Aquac Rep 17:100385. https://doi.org/10.1016/j.aqrep.2020.100385
Chien C-C, Lin T-Y, Chi C-C, Liu C-H (2020) Probiotic, Bacillus subtilis E20 alters the immunity of white shrimp, Litopenaeus vannamei via glutamine metabolism and hexosamine biosynthetic pathway. Fish Shellfish Immunol 98:176–185. https://doi.org/10.1016/j.fsi.2020.01.014
Chiu L, Bazin T, Truchetet M-E, Schaeverbeke T, Delhaes L, Pradeu T (2017) Protective microbiota: from localized to long-reaching co-immunity. Front Immunol 8:1678. https://doi.org/10.3389/fimmu.2017.01678
Chuang T-H, Ulevitch RJ (2004) Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat Immunol 5(5):495–502
Corr SC, Li Y, Riedel CU, O'Toole PW, Hill C, Gahan CG (2007) Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci 104(18):7617–7621
Cutting SM (2011) Bacillus probiotics. Food Microbiol 28(2):214–220
da Paixão AEM, dos Santos JC, Pinto MS, Pereira DSP, de Oliveira Ramos CEC, Cerqueira RB et al (2017) Effect of commercial probiotics (Bacillus subtilis and Saccharomyces cerevisiae) on growth performance, body composition, hematology parameters, and disease resistance against Streptococcus agalactiae in tambaqui (Colossoma macropomum). Aquac Int 25(6):2035–2045. https://doi.org/10.1007/s10499-017-0173-7
Dash G, Raman RP, Prasad KP, Makesh M, Pradeep M, Sen S (2015) Evaluation of paraprobiotic applicability of Lactobacillus plantarum in improving the immune response and disease protection in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Fish Shellfish Immunol 43(1):167–174
Dawood MA, El-Dakar A, Mohsen M, Abdelraouf E, Koshio S, Ishikawa M, Yokoyama S (2014) Effects of using exogenous digestive enzymes or natural enhancer mixture on growth, feed utilization, and body composition of rabbitfish, Siganus rivulatus. J Agric Sci Technol B 4(3B)
Dawood MAO, Koshio S, Ishikawa M, El-Sabagh M, Esteban MA, Zaineldin AI (2016) Probiotics as an environment-friendly approach to enhance red sea bream, Pagrus major growth, immune response and oxidative status. Fish Shellfish Immunol 57:170–178. https://doi.org/10.1016/j.fsi.2016.08.038
Dawood MAO, Koshio S, Abdel-Daim MM, Hien DV (2019) Probiotic application for sustainable aquaculture. Rev Aquac 11(3):907–924. https://doi.org/10.1111/raq.12272
De D, Ananda Raja R, Ghoshal TK, Mukherjee S, Vijayan KK (2018) Evaluation of growth, feed utilization efficiency and immune parameters in tiger shrimp (Penaeus monodon) fed diets supplemented with or diet fermented with gut bacterium Bacillus sp. DDKRC1. isolated from gut of Asian seabass (Lates calcarifer). Aquac Res 49(6):2147–2155. https://doi.org/10.1111/are.13669
Desriac F, Defer D, Bourgougnon N, Brillet B, Le Chevalier P, Fleury Y (2010) Bacteriocin as weapons in the marine animal-associated bacteria warfare: inventory and potential applications as an aquaculture probiotic. Mar Drugs 8(4):1153–1177
Dias JAR, Abe HA, Sousa NC, Couto MVS, Cordeiro CAM, Meneses JO et al (2018) Dietary supplementation with autochthonous Bacillus cereus improves growth performance and survival in tambaqui Colossoma macropomum. Aquac Res 49(9):3063–3070. https://doi.org/10.1111/are.13767
Duc LH, Hong HA, Cutting SM (2003) Germination of the spore in the gastrointestinal tract provides a novel route for heterologous antigen delivery. Vaccine 21(27–30):4215–4224
El-Haroun E, Goda AS, Kabir Chowdhury M (2006) Effect of dietary probiotic Biogen® supplementation as a growth promoter on growth performance and feed utilization of Nile tilapia Oreochromis niloticus (L.). Aquac Res 37(14):1473–1480
Elshaghabee FM, Rokana N, Gulhane RD, Sharma C, Panwar H (2017) Bacillus as potential probiotics: status, concerns, and future perspectives. Front Microbiol 8:1490
FAO (2020) The State of World Fisheries and Aquaculture (SOFIA). FAO, Rome
FAO/WHO (2001) Health and nutritional properties of probiotics in food including powder milk with liver lactic acid bacteria
Fayol-Messaoudi D, Berger CN, Coconnier-Polter M-H, Lievin-Le Moal V, Servin AL (2005) pH-, Lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl Environ Microbiol 71(10):6008–6013
Fei H, Lin G-D, Zheng C-C, Huang M-M, Qian S-C, Wu Z-J et al (2018) Effects of Bacillus amyloliquefaciens and Yarrowia lipolytica lipase 2 on immunology and growth performance of Hybrid sturgeon. Fish Shellfish Immunol 82:250–257. https://doi.org/10.1016/j.fsi.2018.08.031
Feng J, Chang X, Zhang Y, Yan X, Zhang J, Nie G (2019) Effects of Lactococcus lactis from Cyprinus carpio L. as probiotics on growth performance, innate immune response and disease resistance against Aeromonas hydrophila. Fish Shellfish Immunol 93:73–81. https://doi.org/10.1016/j.fsi.2019.07.028
Fuchs VI, Schmidt J, Slater MJ, Buck BH, Steinhagen D (2017) Influence of immunostimulant polysaccharides, nucleic acids, and Bacillus strains on the innate immune and acute stress response in turbots (Scophthalmus maximus) fed soy bean- and wheat-based diets. Fish Physiol Biochem 43(6):1501–1515. https://doi.org/10.1007/s10695-017-0388-6
Gao X, Zhang M, Li X, Han Y, Wu F, Liu Y (2018) Effects of a probiotic (Bacillus licheniformis) on the growth, immunity, and disease resistance of Haliotis discus hannai Ino. Fish Shellfish Immunol 76:143–152. https://doi.org/10.1016/j.fsi.2018.02.028
Giri SS, Sukumaran V, Oviya M (2013) Potential probiotic Lactobacillus plantarum VSG3 improves the growth, immunity, and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 34(2):660–666. https://doi.org/10.1016/j.fsi.2012.12.008
Gobi N, Vaseeharan B, Chen J-C, Rekha R, Vijayakumar S, Anjugam M, Iswarya A (2018) Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol 74:501–508. https://doi.org/10.1016/j.fsi.2017.12.066
Gómez B, Munekata PES, Zhu Z, Barba FJ, Toldrá F, Putnik P et al (2019) Chapter 7: Challenges and opportunities regarding the use of alternative protein sources: aquaculture and insects. In: Toldrá F (ed) Advances in food and nutrition research, vol 89. Academic Press, San Diego, CA, pp 259–295
Gupta A, Verma G, Gupta P (2016) Growth performance, feed utilization, digestive enzyme activity, innate immunity and protection against Vibrio harveyi of freshwater prawn, Macrobrachium rosenbergii fed diets supplemented with Bacillus coagulans. Aquac Int 24(5):1379–1392
Hai NV (2015) The use of probiotics in aquaculture. J Appl Microbiol 119(4):917–935
Hammami I, Jaouadi B, Bacha AB, Rebai A, Bejar S, Nesme X, Rhouma A (2012) Bacillus subtilis bacteriocin Bac 14B with a broad inhibitory spectrum: purification, amino acid sequence analysis, and physicochemical characterization. Biotechnol Bioprocess Eng 17(1):41–49
Hamza A, Fdhila K, Zouiten D, Masmoudi AS (2016) Virgibacillus proomii and Bacillus mojavensis as probiotics in sea bass (Dicentrarchus labrax) larvae: effects on growth performance and digestive enzyme activities. Fish Physiol Biochem 42(2):495–507. https://doi.org/10.1007/s10695-015-0154-6
Han B, Long W-Q, He J-Y, Liu Y-J, Si Y-Q, Tian L-X (2015) Effects of dietary Bacillus licheniformis on growth performance, immunological parameters, intestinal morphology and resistance of juvenile Nile tilapia (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 46(2):225–231
Hasan MT, Jang WJ, Kim H, Lee B-J, Kim KW, Hur SW et al (2018) Synergistic effects of dietary Bacillus sp. SJ-10 plus β-glucooligosaccharides as a synbiotic on growth performance, innate immunity and streptococcosis resistance in olive flounder (Paralichthys olivaceus). Fish Shellfish Immunol 82:544–553. https://doi.org/10.1016/j.fsi.2018.09.002
Hauville MR, Zambonino-Infante JL, Gordon Bell J, Migaud H, Main KL (2016) Effects of a mix of Bacillus sp. as a potential probiotic for Florida pompano, common snook and red drum larvae performances and digestive enzyme activities. Aquac Nutr 22(1):51–60. https://doi.org/10.1111/anu.12226
Hemarajata P, Versalovic J (2013) Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 6(1):39–51. https://doi.org/10.1177/1756283X12459294
Henderson B, Wilson M, McNab R, Lax A (1999) The innate immune response. B. Henderson, M. Wilson, M, 311–353
Hernández AJ, Romero A, Gonzalez-Stegmaier R, Dantagnan P (2016) The effects of supplemented diets with a phytopharmaceutical preparation from herbal and macroalgal origin on disease resistance in rainbow trout against Piscirickettsia salmonis. Aquaculture 454(Supplement C):109–117. https://doi.org/10.1016/j.aquaculture.2015.12.016
Hlordzi V, Kuebutornye FKA, Afriyie G, Abarike ED, Lu Y, Chi S, Anokyewaa MA (2020) The use of Bacillus species in maintenance of water quality in aquaculture: a review. Aquac Rep 18:100503. https://doi.org/10.1016/j.aqrep.2020.100503
Hong HA, Duc LH, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29(4):813–835
Hoseinifar SH, Mirvaghefi A, Merrifield DL (2011) The effects of dietary inactive brewer's yeast Saccharomyces cerevisiae var. ellipsoideus on the growth, physiological responses and gut microbiota of juvenile beluga (Huso huso). Aquaculture 318(1–2):90–94
Hoseinifar SH, Esteban MÁ, Cuesta A, Sun Y-Z (2015) Prebiotics and fish immune response: a review of current knowledge and future perspectives. Rev Fisher Sci Aquac 23(4):315–328
Hoseinifar SH, Dadar M, Ringø E (2017) Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: the functional feed additives scenario. Aquac Res 48(8):3987–4000
Hoseinifar SH, Sun Y-Z, Wang A, Zhou Z (2018) Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol 9:2429. https://doi.org/10.3389/fmicb.2018.02429
Hosoi T, Hirose R, Saegusa S, Ametani A, Kiuchi K, Kaminogawa S (2003) Cytokine responses of human intestinal epithelial-like Caco-2 cells to the nonpathogenic bacterium Bacillus subtilis (natto). Int J Food Microbiol 82(3):255–264. https://doi.org/10.1016/S0168-1605(02)00311-2
Huynh T-G, Cheng A-C, Chi C-C, Chiu K-H, Liu C-H (2018) A synbiotic improves the immunity of white shrimp, Litopenaeus vannamei: Metabolomic analysis reveal compelling evidence. Fish Shellfish Immunol 79:284–293
Ibrahem MD (2015) Evolution of probiotics in aquatic world: Potential effects, the current status in Egypt and recent prospectives. J Adv Res 6(6):765–791. https://doi.org/10.1016/j.jare.2013.12.004
Irianto A, Austin B (2002) Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 25(6):333–342. https://doi.org/10.1046/j.1365-2761.2002.00375.x
Iwashita MKP, Nakandakare IB, Terhune JS, Wood T, Ranzani-Paiva MJT (2015) Dietary supplementation with Bacillus subtilis, Saccharomyces cerevisiae and Aspergillus oryzae enhance immunity and disease resistance against Aeromonas hydrophila and Streptococcus iniae infection in juvenile tilapia Oreochromis niloticus. Fish Shellfish Immunol 43(1):60–66. https://doi.org/10.1016/j.fsi.2014.12.008
Jafariyan H, Sahandi J, Taati M, Eslamloo K (2015) The use of Bacillus probiotics in-feed improved stress resistance of Trichopodus trichopterus (Pallas, 1770) larvae. J Coast Life Med 3(10):757–760
Jamali H, Imani A, Abdollahi D, Roozbehfar R, Isari A (2015) Use of probiotic Bacillus spp. in rotifer (Brachionus plicatilis) and artemia (Artemia urmiana) enrichment: effects on growth and survival of Pacific White Shrimp, Litopenaeus vannamei, Larvae. Probiotics Antimicrob Proteins 7(2):118–125. https://doi.org/10.1007/s12602-015-9189-3
Joseph B, Dhas B, Hena V, Raj J (2013) Bacteriocin from Bacillus subtilis as a novel drug against diabetic foot ulcer bacterial pathogens. Asian Pac J Trop Biomed 3(12):942–946
Katholnig K, Linke M, Pham H, Hengstschläger M, Weichhart T (2013) Immune responses of macrophages and dendritic cells regulated by mTOR signalling. Biochem Soc Trans 41(4):927–933. https://doi.org/10.1042/BST20130032
Katya K, Yun Y-h, Park G, Lee J-Y, Yoo G, Bai SC (2014) Evaluation of the efficacy of fermented by-product of mushroom, pleurotus ostreatus, as a fish meal replacer in Juvenile Amur Catfish, Silurus asotus: effects on growth, serological characteristics and immune responses. Asian Australas J Anim Sci 27(10):1478–1486. https://doi.org/10.5713/ajas.2014.14038
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274(1):1–14. https://doi.org/10.1016/j.aquaculture.2007.11.019
Kewcharoen W, Srisapoome P (2019) Probiotic effects of Bacillus spp. from Pacific white shrimp (Litopenaeus vannamei) on water quality and shrimp growth, immune responses, and resistance to Vibrio parahaemolyticus (AHPND strains). Fish Shellfish Immunol 94:175–189. https://doi.org/10.1016/j.fsi.2019.09.013
Khademzade O, Zakeri M, Haghi M, Mousavi SM (2020) The effects of water additive Bacillus cereus and Pediococcus acidilactici on water quality, growth performances, economic benefits, immunohematology and bacterial flora of whiteleg shrimp (Penaeus vannamei Boone, 1931) reared in earthen ponds. Aquac Res 51(5):1759–1770
Kim D-H, Subramanian D, Heo M-S (2017) Dietary effect of probiotic bacteria, Bacillus amyloliquefaciens-JFP2 on growth and innate immune response in rock bream Oplegnathus fasciatus, challenged with Streptococcus iniae
Klaenhammer TR, Kleerebezem M, Kopp MV, Rescigno M (2012) The impact of probiotics and prebiotics on the immune system. Nat Rev Immunol 12(10):728–734. https://doi.org/10.1038/nri3312
Kleerebezem M, Hols P, Bernard E, Rolain T, Zhou M, Siezen R, Bron P (2010) The extracellular biology of the lactobacilli. FEMS Microbiol Rev 34(2):199–230
Kuebutornye FK, Abarike ED, Lu Y (2019) A review on the application of Bacillus as probiotics in aquaculture. Fish Shellfish Immunol 87:820–828
La Fata G, Weber P, Mohajeri MH (2018) Probiotics and the gut immune system: indirect regulation. Probiotics Antimicrob Proteins 10(1):11–21. https://doi.org/10.1007/s12602-017-9322-6
Lauriano ER, Pergolizzi S, Capillo G, Kuciel M, Alesci A, Faggio C (2016) Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish Shellfish Immunol 59:250–255. https://doi.org/10.1016/j.fsi.2016.11.003
Lazado CC, Caipang CMA (2014) Mucosal immunity and probiotics in fish. Fish Shellfish Immunol 39(1):78–89. https://doi.org/10.1016/j.fsi.2014.04.015
Lee Y-K, Puong K-Y, Ouwehand AC, Salminen S (2003) Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J Med Microbiol 52(10):925–930
Lee S, Katya K, Park Y, Won S, Seong M, Hamidoghli A, Bai SC (2017) Comparative evaluation of dietary probiotics Bacillus subtilis WB60 and Lactobacillus plantarum KCTC3928 on the growth performance, immunological parameters, gut morphology and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol 61:201–210. https://doi.org/10.1016/j.fsi.2016.12.035
Lee S, Katya K, Hamidoghli A, Hong J, Kim D-J, Bai SC (2018) Synergistic effects of dietary supplementation of Bacillus subtilis WB60 and mannanoligosaccharide (MOS) on growth performance, immunity and disease resistance in Japanese eel, Anguilla japonica. Fish Shellfish Immunol 83:283–291. https://doi.org/10.1016/j.fsi.2018.09.031
Li J, Tan B, Mai K, Ai Q, Zhang W, Xu W et al (2006) Comparative study between probiotic bacterium Arthrobacter XE-7 and chloramphenicol on protection of Penaeus chinensis post-larvae from pathogenic vibrios. Aquaculture 253(1):140–147. https://doi.org/10.1016/j.aquaculture.2005.07.040
Li J, Xu Y, Jin L, Li X (2015) Effects of a probiotic mixture (Bacillus subtilis YB-1 and Bacillus cereus YB-2) on disease resistance and non-specific immunity of sea cucumber, Apostichopus japonicus (Selenka). Aquac Res 46(12):3008–3019. https://doi.org/10.1111/are.12453
Li C, Zhang B, Liu C, Zhou H, Wang X, Mai K, He G (2020) Effects of dietary raw or Enterococcus faecium fermented soybean meal on growth, antioxidant status, intestinal microbiota, morphology, and inflammatory responses in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol 100:261–271. https://doi.org/10.1016/j.fsi.2020.02.070
Lim S-Y, Loo KW, Wong W-L (2020) Synergistic antimicrobial effect of a seaweed-probiotic blend against acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Probiotics Antimicrob Proteins 12(3):906–917
Lin Y-S, Saputra F, Chen Y-C, Hu S-Y (2019) Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish Immunol 86:410–419. https://doi.org/10.1016/j.fsi.2018.11.047
Liu S, Chen ZJ (2011) Expanding role of ubiquitination in NF-κB signaling. Cell Res 21(1):6–21
Liu CH, Chiu CS, Ho PL, Wang SW (2009) Improvement in the growth performance of white shrimp, Litopenaeus vannamei, by a protease-producing probiotic, Bacillus subtilis E20, from natto. J Appl Microbiol 107(3):1031–1041. https://doi.org/10.1111/j.1365-2672.2009.04284.x
Liu K-F, Chiu C-H, Shiu Y-L, Cheng W, Liu C-H (2010) Effects of the probiotic, Bacillus subtilis E20, on the survival, development, stress tolerance, and immune status of white shrimp, Litopenaeus vannamei larvae. Fish Shellfish Immunol 28(5–6):837–844
Liu H, Wang S, Cai Y, Guo X, Cao Z, Zhang Y et al (2017) Dietary administration of Bacillus subtilis HAINUP40 enhances growth, digestive enzyme activities, innate immune responses and disease resistance of tilapia, Oreochromis niloticus. Fish Shellfish Immunol 60:326–333. https://doi.org/10.1016/j.fsi.2016.12.003
Liu C-H, Wu K, Chu T-W, Wu T-M (2018) Dietary supplementation of probiotic, Bacillus subtilis E20, enhances the growth performance and disease resistance against Vibrio alginolyticus in parrot fish (Oplegnathus fasciatus). Aquac Int 26(1):63–74. https://doi.org/10.1007/s10499-017-0189-z
Liu B, Zhou W, Wang H, Li C, Wang L, Li Y, Wang J (2020) Bacillus baekryungensis MS1 regulates the growth, non-specific immune parameters and gut microbiota of the sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 102:133–139. https://doi.org/10.1016/j.fsi.2020.04.023
Maisak H, Jantrakajorn S, Lukkana M, Wongtavatchai J (2013) Antibacterial activity of tannin from sweet chestnut wood against aeromonas and streptococcal pathogens of tilapia (Oreochromis niloticus). Thai J Vet Med 43(1):105
Marcusso PF, Aguinaga JY, da Silva Claudiano G, Eto SF, Fernandes DC, Mello H et al (2015) Influence of temperature on Streptococcus agalactiae infection in Nile tilapia. Braz J Vet Res Anim Sci 52(1):57–62
Martínez Cruz P, Ibáñez AL, Monroy Hermosillo OA, Ramírez Saad HC (2012) Use of probiotics in aquaculture. ISRN Microbiol 2012:916845. https://doi.org/10.5402/2012/916845
Meidong R, Doolgindachbaporn S, Jamjan W, Sakai K, Tashiro Y, Okugawa Y et al (2017) A novel probiotic Bacillus siamensis B44v isolated from Thai pickled vegetables (Phak-dong) for potential use as a feed supplement in aquaculture. J Gen Appl Microbiol 63(4):246–253
Meidong R, Khotchanalekha K, Doolgindachbaporn S, Nagasawa T, Nakao M, Sakai K, Tongpim S (2018) Evaluation of probiotic Bacillus aerius B81e isolated from healthy hybrid catfish on growth, disease resistance and innate immunity of Pla-mong Pangasius bocourti. Fish Shellfish Immunol 73:1–10. https://doi.org/10.1016/j.fsi.2017.11.032
Menni C, Zierer J, Pallister T, Jackson MA, Long T, Mohney RP et al (2017) Omega-3 fatty acids correlate with gut microbiome diversity and production of N-carbamylglutamate in middle aged and elderly women. Sci Rep 7(1):1–11
Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M (2009) Probiotic mixture VSL# 3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol
Merrifield D, Bradley G, Baker R, Davies S (2010) Probiotic applications for rainbow trout (Oncorhynchus mykiss Walbaum) II. Effects on growth performance, feed utilization, intestinal microbiota and related health criteria postantibiotic treatment. Aquac Nutr 16(5):496–503
Mohapatra S, Chakraborty T, Prusty A, Das P, Paniprasad K, Mohanta K (2012) Use of different microbial probiotics in the diet of rohu, Labeo rohita fingerlings: effects on growth, nutrient digestibility and retention, digestive enzyme activities and intestinal microflora. Aquac Nutr 18(1):1–11
Mohapatra S, Chakraborty T, Kumar V, DeBoeck G, Mohanta KN (2013) Aquaculture and stress management: a review of probiotic intervention. J Anim Physiol Anim Nutr (Berl) 97(3):405–430. https://doi.org/10.1111/j.1439-0396.2012.01301.x
Moriarty DJW (1998) Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 164(1–4):351–358. https://doi.org/10.1016/S0044-8486(98)00199-9
Morowitz MJ, Carlisle EM, Alverdy JC (2011) Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg Clin North Am 91(4):771–778. https://doi.org/10.1016/j.suc.2011.05.001
Mukherjee A, Chandra G, Ghosh K (2019) Single or conjoint application of autochthonous Bacillus strains as potential probiotics: effects on growth, feed utilization, immunity and disease resistance in Rohu, Labeo rohita (Hamilton). Aquaculture 512:734302. https://doi.org/10.1016/j.aquaculture.2019.734302
Nair AV, Leo Antony M, Praveen NK, Sayooj P, Raja Swaminathan T, Vijayan KK (2020) Evaluation of in vitro and in vivo potential of Bacillus subtilis MBTDCMFRI Ba37 as a candidate probiont in fish health management. Microb Pathog:104610. https://doi.org/10.1016/j.micpath.2020.104610
Nandi A, Banerjee G, Dan SK, Ghosh K, Ray AK (2018) Evaluation of in vivo probiotic efficiency of Bacillus amyloliquefaciens in Labeo rohita challenged by pathogenic strain of Aeromonas hydrophila MTCC 1739. Probiotics Antimicrob Proteins 10(2):391–398
Nath S, Matozzo V, Bhandari D, Faggio C (2019) Growth and liver histology of Channa punctatus exposed to a common biofertilizer. Nat Prod Res 33(11):1591–1598. https://doi.org/10.1080/14786419.2018.1428586
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29(1):2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Ock Kim Y, Mahboob S, Viayaraghavan P, Biji D, Abdullah Al-Ghanim K, Al-Misned F et al (2020) Growth promoting activity of Penaeus indicus by secondary metabolite producing probiotic bacterium Bacillus subtilis isolated from the shrimp gut. J King Saud Univ Sci 32(2):1641–1646. https://doi.org/10.1016/j.jksus.2019.12.023
Park Y, Kim H, Won S, Hamidoghli A, Hasan MT, Kong I-S, Bai SC (2020) Effects of two dietary probiotics (Bacillus subtilis or licheniformis) with two prebiotics (mannan or fructo oligosaccharide) in Japanese eel, Anguilla japonica. Aquac Nutr 26(2):316–327. https://doi.org/10.1111/anu.12993
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A (2019) Mechanisms of action of probiotics. Adv Nutr (Bethesda, Md) 10(Suppl_1):S49–S66. https://doi.org/10.1093/advances/nmy063
Pridmore RD, Pittet A-C, Praplan F, Cavadini C (2008) Hydrogen peroxide production by Lactobacillus johnsonii NCC 533 and its role in anti-Salmonella activity. FEMS Microbiol Lett 283(2):210–215
Qin L, Xiang J, Xiong F, Wang G, Zou H, Li W et al (2020) Effects of Bacillus licheniformis on the growth, antioxidant capacity, intestinal barrier and disease resistance of grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol 97:344–350. https://doi.org/10.1016/j.fsi.2019.12.040
Queiroz JF, Boyd CE (1998) Effects of a bacterial inoculum in channel catfish ponds. J World Aquac Soc 29(1):67–73
Ramesh D, Souissi S (2018) Effects of potential probiotic Bacillus subtilis KADR1 and its subcellular components on immune responses and disease resistance in Labeo rohita. Aquac Res 49(1):367–377. https://doi.org/10.1111/are.13467
Ramesh D, Vinothkanna A, Rai AK, Vignesh VS (2015) Isolation of potential probiotic Bacillus spp. and assessment of their subcellular components to induce immune responses in Labeo rohita against Aeromonas hydrophila. Fish Shellfish Immunol 45(2):268–276. https://doi.org/10.1016/j.fsi.2015.04.018
Ramirez RF, Dixon BA (2003) Enzyme production by obligate intestinal anaerobic bacteria isolated from oscars (Astronotus ocellatus), angelfish (Pterophyllum scalare) and southern flounder (Paralichthys lethostigma). Aquaculture 227(1–4):417–426
Ramos MA, Gonçalves JFM, Costas B, Batista S, Lochmann R, Pires MA et al (2017) Commercial Bacillus probiotic supplementation of rainbow trout (Oncorhynchys mykiss) and brown trout (Salmo trutta): growth, immune responses and intestinal morphology. Aquac Res 48(5):2538–2549. https://doi.org/10.1111/are.13090
Rangavajhyala N, Shahani K, Sridevi G, Srikumaran S (1997) Nonlipopolysaccharide components) of Lactobacillus addophilus stimulate (s) the production of interleukin-1α and tumor necrosis factor-α by murine macrophages
Rawlings ND, Barrett AJ (1994) [2] Families of serine peptidases. Methods Enzymol:244, 19–261
Ray A, Ghosh K, Ringø E (2012) Enzyme-producing bacteria isolated from fish gut: a review. Aquac Nutr 18(5):465–492
Reda RM, Selim KM (2015) Evaluation of Bacillus amyloliquefaciens on the growth performance, intestinal morphology, hematology and body composition of Nile tilapia, Oreochromis niloticus. Aquac Int 23(1):203–217
Reda RM, El-Hady MA, Selim KM, El-Sayed HM (2018) Comparative study of three predominant gut Bacillus strains and a commercial B. amyloliquefaciens as probiotics on the performance of Clarias gariepinus. Fish Shellfish Immunol 80:416–425. https://doi.org/10.1016/j.fsi.2018.06.031
Rengpipat S, Phianphak W, Piyatiratitivorakul S, Menasveta P (1998) Effects of a probiotic bacterium on black tiger shrimp Penaeus monodon survival and growth. Aquaculture 167(3):301–313. https://doi.org/10.1016/S0044-8486(98)00305-6
Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R et al (2001) Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2(4):361–367
Resende JA, Silva VL, Fontes CO, Souza-Filho JA, de Oliveira TLR, Coelho CM et al (2012) Multidrug-resistance and toxic metal tolerance of medically important bacteria isolated from an aquaculture system. Microbes Environ:ME12049
Reverter M, Sarter S, Caruso D, Avarre J-C, Combe M, Pepey E et al (2020) Aquaculture at the crossroads of global warming and antimicrobial resistance. Nat Commun 11(1):1870. https://doi.org/10.1038/s41467-020-15735-6
Ringø E (2011) Evaluation of probiotic strain Bacillus subtilis C-3102 as a feed supplement for koi carp (Cyprinus carpio)
Ringø E (2020) Probiotics in shellfish aquaculture. Aquac Fisher 5(1):1–27. https://doi.org/10.1016/j.aaf.2019.12.001
Ringø E, Hoseinifar SH, Ghosh K, Doan HV, Beck BR, Song SK (2018) Lactic acid Bacteria in finfish—An update. Front Microbiol 9(1818). https://doi.org/10.3389/fmicb.2018.01818
Romano N (2021) Chapter 5: Probiotics, prebiotics, biofloc systems, and other biocontrol regimens in fish and shellfish aquaculture. In: Kibenge FSB, Baldisserotto B, Chong RS-M (eds) Aquaculture pharmacology. Academic Press, San Diego, CA, pp 219–242
Ryan KA, O'Hara AM, van Pijkeren J-P, Douillard FP, O'Toole PW (2009) Lactobacillus salivarius modulates cytokine induction and virulence factor gene expression in Helicobacter pylori. J Med Microbiol 58(8):996–1005
Sadat Hoseini Madani N, Adorian TJ, Ghafari Farsani H, Hoseinifar SH (2018) The effects of dietary probiotic Bacilli (Bacillus subtilis and Bacillus licheniformis) on growth performance, feed efficiency, body composition and immune parameters of whiteleg shrimp (Litopenaeus vannamei) postlarvae. Aquac Res 49(5):1926–1933
Sánchez-Ortiz AC, Angulo C, Luna-González A, Álvarez-Ruiz P, Mazón-Suástegui JM, Campa-Córdova ÁI (2016) Effect of mixed-Bacillus spp isolated from pustulose ark Anadara tuberculosa on growth, survival, viral prevalence and immune-related gene expression in shrimp Litopenaeus vannamei. Fish Shellfish Immunol 59:95–102. https://doi.org/10.1016/j.fsi.2016.10.022
Sanders ME, Morelli L, Tompkins T (2003) Sporeformers as human probiotics: Bacillus, Sporolactobacillus, and Brevibacillus. Compr Rev Food Sci Food Saf 2(3):101–110
Sangma T, Kamilya D (2015) Dietary Bacillus subtilis FPTB13 and chitin, single or combined, modulate systemic and cutaneous mucosal immunity and resistance of catla, Catla catla (Hamilton) against edwardsiellosis. Comp Immunol Microbiol Infect Dis 43:8–15. https://doi.org/10.1016/j.cimid.2015.09.003
Santacroce L, Charitos IA, Bottalico L (2019) A successful history: probiotics and their potential as antimicrobials. Expert Rev Anti Infect Ther 17(8):635–645. https://doi.org/10.1080/14787210.2019.1645597
Sapcharoen P, Rengpipat S (2013) Effects of the probiotic Bacillus subtilis (BP 11 and BS 11) on the growth and survival of Pacific white shrimp, Litopenaeus vannamei. Aquac Nutr 19(6):946–954
Seghouani H, Garcia-Rangel C-E, Füller J, Gauthier J, Derome N (2017) Walleye autochthonous Bacteria as promising probiotic candidates against Flavobacterium columnare. Front Microbiol 8:1349. https://doi.org/10.3389/fmicb.2017.01349
Selim KM, Reda RM (2015) Improvement of immunity and disease resistance in the Nile tilapia, Oreochromis niloticus, by dietary supplementation with Bacillus amyloliquefaciens. Fish Shellfish Immunol 44(2):496–503. https://doi.org/10.1016/j.fsi.2015.03.004
Selim KM, El-Sayed HM, El-Hady M, Reda RM (2019) In vitro evaluation of the probiotic candidates isolated from the gut of Clarias gariepinus with special reference to the in vivo assessment of live and heat-inactivated Leuconostoc mesenteroides and Edwardsiella sp. Aquac Int 27(1):33–51
Serra CR, Almeida EM, Guerreiro I, Santos R, Merrifield DL, Tavares F et al (2019) Selection of carbohydrate-active probiotics from the gut of carnivorous fish fed plant-based diets. Sci Rep 9(1):6384. https://doi.org/10.1038/s41598-019-42716-7
Shi LH, Balakrishnan K, Thiagarajah K, Mohd Ismail NI, Yin OS (2016) Beneficial properties of probiotics. Tropical Life Sci Res 27(2):73–90. https://doi.org/10.21315/tlsr2016.27.2.6
Silva TFA, Petrillo TR, Yunis-Aguinaga J, Marcusso PF, da Silva Claudiano G, de Moraes FR, de Moraes JR (2015) Effects of the probiotic Bacillus amyloliquefaciens on growth performance, hematology and intestinal morphometry in cage-reared Nile tilapia. Lat Am J Aquat Res 43(5):963–971
Singh ST, Kamilya D, Kheti B, Bordoloi B, Parhi J (2017) Paraprobiotic preparation from Bacillus amyloliquefaciens FPTB16 modulates immune response and immune relevant gene expression in Catla catla (Hamilton, 1822). Fish Shellfish Immunol 66:35–42. https://doi.org/10.1016/j.fsi.2017.05.005
Sivamaruthi BS, Kesika P, Chaiyasut C (2018) Thai fermented foods as a versatile source of bioactive microorganisms—A comprehensive review. Sci Pharm 86(3):37
Soltani M, Ghosh K, Hoseinifar SH, Kumar V, Lymbery AJ, Roy S, Ringø E (2019) Genus bacillus, promising probiotics in aquaculture: aquatic animal origin, bio-active components, bioremediation and efficacy in fish and shellfish. Rev Fisher Sci Aquac 27(3):331–379. https://doi.org/10.1080/23308249.2019.1597010
Sookchaiyaporn N, Srisapoome P, Unajak S, Areechon N (2020) Efficacy of Bacillus spp. isolated from Nile tilapia Oreochromis niloticus Linn. on its growth and immunity, and control of pathogenic bacteria. Fisher Sci:1–13
Spencer R, Chesson A (1994) The effect of Lactobacillus spp. on the attachment of enterotoxigenic Escherichia coli to isolated porcine enterocytes. J Appl Bacteriol 77(2):215–220
Srisapoome P, Areechon N (2017) Efficacy of viable Bacillus pumilus isolated from farmed fish on immune responses and increased disease resistance in Nile tilapia (Oreochromis niloticus): Laboratory and on-farm trials. Fish Shellfish Immunol 67:199–210. https://doi.org/10.1016/j.fsi.2017.06.018
Sugita H, Takahashi J, Deguchi Y (1992) Production and consumption of biotin by the intestinal microflora of cultured freshwater fishes. Biosci Biotechnol Biochem 56(10):1678–1679
Sumi CD, Yang BW, Yeo I-C, Hahm YT (2015) Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can J Microbiol 61(2):93–103
Sun Y-Z, Yang H-L, Ma R-L, Lin W-Y (2010) Probiotic applications of two dominant gut Bacillus strains with antagonistic activity improved the growth performance and immune responses of grouper Epinephelus coioides. Fish Shellfish Immunol 29(5):803–809. https://doi.org/10.1016/j.fsi.2010.07.018
Suzer C, Çoban D, Kamaci HO, Saka Ş, Firat K, Otgucuoğlu Ö, Küçüksari H (2008) Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: effects on growth performance and digestive enzyme activities. Aquaculture 280(1–4):140–145
Svendsen A (2000) Lipase protein engineering. Biochim Biophys Acta/Protein Struct Mol Enzymol 1543(2):223–238
Swapna B, Venkatrayulu C, Swathi AJEJOEB (2015) Effect of probiotic bacteria Bacillus licheniformis and Lactobacillus rhamnosus on growth of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931). Eur J Exp Biol 5(11):31–36
Tarnecki AM, Wafapoor M, Phillips RN, Rhody NR (2019) Benefits of a Bacillus probiotic to larval fish survival and transport stress resistance. Sci Rep 9(1):1–11
Tepaamorndech S, Chantarasakha K, Kingcha Y, Chaiyapechara S, Phromson M, Sriariyanun M et al (2019) Effects of Bacillus aryabhattai TBRC8450 on vibriosis resistance and immune enhancement in Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 86:4–13. https://doi.org/10.1016/j.fsi.2018.11.010
Truong Thy HT, Tri NN, Quy OM, Fotedar R, Kannika K, Unajak S, Areechon N (2017) Effects of the dietary supplementation of mixed probiotic spores of Bacillus amyloliquefaciens 54A, and Bacillus pumilus 47B on growth, innate immunity and stress responses of striped catfish (Pangasianodon hypophthalmus). Fish Shellfish Immunol 60:391–399. https://doi.org/10.1016/j.fsi.2016.11.016
Tseng D-Y, Ho P-L, Huang S-Y, Cheng S-C, Shiu Y-L, Chiu C-S, Liu C-H (2009) Enhancement of immunity and disease resistance in the white shrimp, Litopenaeus vannamei, by the probiotic, Bacillus subtilis E20. Fish Shellfish Immunol 26(2):339–344
Ueberschär B (1995) The use of tryptic enzyme activity measurement as a nutritional condition index: laboratory calibration data and field application. Paper presented at the ICES Marine Science Symposia
Utami D, Suprayudi MA (2015) Quality of dried Bacillus NP5 and its effect on growth performance of Tilapia (Oreochromis niloticus). Pak J Biol Sci: PJBS 18(2):88–93
Verschuere L, Rombaut G, Sorgeloos P, Verstraete W (2000) Probiotic bacteria as biological control agents in aquaculture. Microbiol Mol Biol Rev 64(4):655–671
Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D (2019) Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol 10(57). https://doi.org/10.3389/fmicb.2019.00057
Vijayavel K, Balasubramanian MP (2006) Fluctuations of biochemical constituents and marker enzymes as a consequence of naphthalene toxicity in the edible estuarine crab Scylla serrata. Ecotoxicol Environ Saf 63(1):141–147
Vine NG, Leukes WD, Kaiser H (2006) Probiotics in marine larviculture. FEMS Microbiol Rev 30(3):404–427. https://doi.org/10.1111/j.1574-6976.2006.00017.x
Wang L, Ge C, Wang J, Dai J, Zhang P, Li Y (2017a) Effects of different combinations of Bacillus on immunity and antioxidant activities in common carp. Aquac Int 25(6):2091–2099. https://doi.org/10.1007/s10499-017-0175-5
Wang M, Liu G, Lu M, Ke X, Liu Z, Gao F et al (2017b) Effect of Bacillus cereus as a water or feed additive on the gut microbiota and immunological parameters of Nile tilapia. Aquac Res 48(6):3163–3173
Wang H, Wang C, Tang Y, Sun B, Huang J, Song X (2018) Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture 494:30–36
Wang C, Liu Y, Sun G, Li X, Liu Z (2019a) Growth, immune response, antioxidant capability, and disease resistance of juvenile Atlantic salmon (Salmo salar L.) fed Bacillus velezensis V4 and Rhodotorula mucilaginosa compound. Aquaculture 500:65–74. https://doi.org/10.1016/j.aquaculture.2018.09.052
Wang A, Ran C, Wang Y, Zhang Z, Ding Q, Yang Y et al (2019b) Use of probiotics in aquaculture of China—a review of the past decade. Fish Shellfish Immunol 86:734–755. https://doi.org/10.1016/j.fsi.2018.12.026
Wang C, Chuprom J, Wang Y, Fu L (2020a) Beneficial bacteria for aquaculture: nutrition, bacteriostasis and immunoregulation. J Appl Microbiol 128(1):28–40. https://doi.org/10.1111/jam.14383
Wang M, Yi M, Lu M, Gao F, Liu Z, Huang Q et al (2020b) Effects of probiotics Bacillus cereus NY5 and Alcaligenes faecalis Y311 used as water additives on the microbiota and immune enzyme activities in three mucosal tissues in Nile tilapia Oreochromis niloticus reared in outdoor tanks. Aquac Rep 17:100309. https://doi.org/10.1016/j.aqrep.2020.100309
Wanka KM, Damerau T, Costas B, Krueger A, Schulz C, Wuertz S (2018) Isolation and characterization of native probiotics for fish farming. BMC Microbiol 18(1):119. https://doi.org/10.1186/s12866-018-1260-2
Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM et al (2008) The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29(4):565–577
Willer DF, Aldridge DC (2019) Microencapsulated diets to improve bivalve shellfish aquaculture for global food security. Glob Food Sec 23:64–73. https://doi.org/10.1016/j.gfs.2019.04.007
Won S, Hamidoghli A, Choi W, Bae J, Jang WJ, Lee S, Bai SC (2020a) Evaluation of potential probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on growth performance, immune response, gut histology and immune-related genes in Whiteleg Shrimp, Litopenaeus vannamei. Microorganisms 8(2):281
Won S, Hamidoghli A, Choi W, Bae J, Jang WJ, Lee S, Bai SCJM (2020b) Evaluation of potential probiotics Bacillus subtilis WB60, Pediococcus pentosaceus, and Lactococcus lactis on growth performance, immune response, gut histology and immune-related genes in Whiteleg Shrimp. Litopenaeus vannamei 8(2):281
World Health Organization (2014) Antimicrobial resistance: global report on surveillance. World Health Organization, Geneva
Wu ZX, Feng X, Xie LL, Peng XY, Yuan J, Chen XX (2012) Effect of probiotic Bacillus subtilis Ch9 for grass carp, Ctenopharyngodon idella (Valenciennes, 1844), on growth performance, digestive enzyme activities and intestinal microflora. J Appl Ichthyol 28(5):721–727. https://doi.org/10.1111/j.1439-0426.2012.01968.x
Xia Y, Wang M, Gao F, Lu M, Chen G (2020) Effects of dietary probiotic supplementation on the growth, gut health and disease resistance of juvenile Nile tilapia (Oreochromis niloticus). Anim Nutr (Zhongguo xu mu shou yi xue hui) 6(1):69–79. https://doi.org/10.1016/j.aninu.2019.07.002
Yan F, Polk DB (2011) Probiotics and immune health. Curr Opin Gastroenterol 27(6):496–501. https://doi.org/10.1097/MOG.0b013e32834baa4d
Yan YY, Xia HQ, Yang HL, Hoseinifar SH, Sun YZ (2016) Effects of dietary live or heat-inactivated autochthonous Bacillus pumilus SE5 on growth performance, immune responses and immune gene expression in grouper Epinephelus coioides. Aquac Nutr 22(3):698–707. https://doi.org/10.1111/anu.12297
Yang HL, Sun YZ, Ma RL, Ye JD (2012) PCR-DGGE analysis of the autochthonous gut microbiota of grouper Epinephelus coioides following probiotic Bacillus clausii administration. Aquac Res 43(4):489–497
Yang S-C, Lin C-H, Sung CT, Fang J-Y (2014) Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol 5:241–241. https://doi.org/10.3389/fmicb.2014.00241
Yang G, Tian X, Dong S, Peng M, Wang D (2015) Effects of dietary Bacillus cereus G19, B. cereus BC-01, and Paracoccus marcusii DB11 supplementation on the growth, immune response, and expression of immune-related genes in coelomocytes and intestine of the sea cucumber (Apostichopus japonicus Selenka). Fish Shellfish Immunol 45(2):800–807. https://doi.org/10.1016/j.fsi.2015.05.032
Yang Q, Lü Y, Zhang M, Gong Y, Li Z, Tran NT et al (2019) Lactic acid bacteria, Enterococcus faecalis Y17 and Pediococcus pentosaceus G11, improved growth performance, and immunity of mud crab (Scylla paramamosain). Fish Shellfish Immunol 93:135–143. https://doi.org/10.1016/j.fsi.2019.07.050
Yin Y, Zhang P, Yue X, Du X, Li W, Yin Y et al (2018) Effect of sub-chronic exposure to lead (Pb) and Bacillus subtilis on Carassius auratus gibelio: bioaccumulation, antioxidant responses and immune responses. Ecotoxicol Environ Saf 161:755–762. https://doi.org/10.1016/j.ecoenv.2018.06.056
Zaineldin AI, Hegazi S, Koshio S, Ishikawa M, Bakr A, El-Keredy AM et al (2018) Bacillus subtilis as probiotic candidate for red sea bream: Growth performance, oxidative status, and immune response traits. Fish Shellfish Immunol 79:303–312
Zhang L, Mai K, Tan B, Ai Q, Qi C, Xu W et al (2009) Effects of dietary administration of probiotic Halomonas sp. B12 on the intestinal microflora, immunological parameters, and midgut histological structure of shrimp. J World Aquac Soc 40(1):58–66
Zhang Q, Ma H, Mai K, Zhang W, Liufu Z, Xu W (2010) Interaction of dietary Bacillus subtilis and fructooligosaccharide on the growth performance, non-specific immunity of sea cucumber, Apostichopus japonicus. Fish Shellfish Immunol 29(2):204–211
Zhao Y, Zhang W, Xu W, Mai K, Zhang Y, Liufu Z (2012) Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 32(5):750–755. https://doi.org/10.1016/j.fsi.2012.01.027
Zhao J, Chen M, Quan C, Fan S (2015) Mechanisms of quorum sensing and strategies for quorum sensing disruption in aquaculture pathogens. J Fish Dis 38(9):771–786
Zhao Y, Yuan L, Wan J, Sun Z, Wang Y, Sun H (2016) Effects of potential probiotic Bacillus cereus EN25 on growth, immunity and disease resistance of juvenile sea cucumber Apostichopus japonicus. Fish Shellfish Immunol 49:237–242. https://doi.org/10.1016/j.fsi.2015.12.035
Zhao H, Luo Y e, Zhang Y, Chen X, Wang H, Guo D, Wu Z (2020) Effects of Bacillus subtilis on hepatic lipid metabolism and oxidative stress response in grass carp (Ctenopharyngodon idellus) fed a high-fat diet. Marine Life Sci Technol 2(1):50–59. https://doi.org/10.1007/s42995-019-00005-2
Zhou X, Tian Z, Wang Y, Li W (2010) Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol Biochem 36(3):501–509
Ziaei-Nejad S, Rezaei MH, Takami GA, Lovett DL, Mirvaghefi A-R, Shakouri M (2006) The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252(2):516–524. https://doi.org/10.1016/j.aquaculture.2005.07.021
Zokaeifar H, Balcázar JL, Saad CR, Kamarudin MS, Sijam K, Arshad A, Nejat N (2012) Effects of Bacillus subtilis on the growth performance, digestive enzymes, immune gene expression and disease resistance of white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 33(4):683–689
Zuo Z-H, Shang B-J, Shao Y-C, Li W-Y, Sun J-S (2019) Screening of intestinal probiotics and the effects of feeding probiotics on the growth, immune, digestive enzyme activity and intestinal flora of Litopenaeus vannamei. Fish Shellfish Immunol 86:160–168. https://doi.org/10.1016/j.fsi.2018.11.003
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Van Doan, H. (2021). Bacillus spp. in Aquaculture - Mechanisms and Applications: An Update View. In: Mojgani, N., Dadar, M. (eds) Probiotic Bacteria and Postbiotic Metabolites: Role in Animal and Human Health. Microorganisms for Sustainability, vol 2. Springer, Singapore. https://doi.org/10.1007/978-981-16-0223-8_1
Download citation
DOI: https://doi.org/10.1007/978-981-16-0223-8_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-16-0222-1
Online ISBN: 978-981-16-0223-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)