Abstract
Immunostimulants are widely applied in aquaculture practice and may have beneficial effects on the immune system and physical functions allowing higher tolerance to stress. In the current study, the impact of four (i–iv) dietary active ingredients on the immune and stress response of turbot was examined in two experiments (I and II). A basal low fish meal (FM; 32%) diet was formulated and supplemented with (i) yeast β-glucan and mannan oligosaccharide (GM), (ii) alginic acid (AC), (iii) yeast nucleotides and RNA (NR), or (iv) Bacillus strains (BS). The basal diet (C-LF) and a high FM (59%) control (C-HF) were maintained. All six diets were fed to juvenile turbots for 84 days in experiment I and for additional 28 days prior to experiment II. Immunological and hematological parameters were determined in experiment I. In experiment II, physical stress response to a typical short-term (<1 day) aquaculture handling procedure (combination of capture, netting/transfer, and crowding) was investigated. For this, turbot blood was sampled before and at 0.5, 1, 4, and 24 h post stress. Plasma lysozyme activity, neutrophil reactive oxygen species (ROS) production, and total plasma protein levels did not significantly differ between treatment groups; however, plasma cholesterol increased significantly in fish fed GM, AC, NR, and C-HF compared to C-LF (I). A significant increase in plasma glucose and triglyceride was observed in GM and NR treatments, while glucose levels were significantly higher in C-HF compared to C-LF. Moreover, the immunostimulant-supplemented diets exhibited significantly lower cortisol levels compared to controls C-LF (at 0.5 h) and C-HF (at 1 h) post stress, respectively (II). According to our findings, FM substitution did not modulate the innate immune response but was associated with reduced levels of cholesterol. Dietary immunostimulants were not effective enough to boost the immune response, but we believe they might be helpful to trigger metabolic advantages during stressful handling events on fish farms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish health and welfare in aquaculture is a key issue in the provision of high quality, consumer-orientated, and sustainable fish products (Naylor et al. 2000; Focardi et al. 2005; Huntingford et al. 2006; Ashley 2007; Kiron 2012). Handling, feeding, transport, vaccination, water quality, and high stocking densities in aquaculture are potential stressors affecting the physiology and health condition of fish (Barton 2002; Ashley 2007; Segner et al. 2012). Severe or long-lasting stress is found to be detrimental to animal health and welfare, affecting the physiology and performance in fish such as energy metabolism, oxygen uptake, immune function, growth, disease resistance, and survival (Mugnier et al. 1998; Gregory and Wood 1999; Barton 2002; Laiz-Carrión et al. 2003; Verburg-Van Kemenade et al. 2009). The organism has to deal with an energy-demanding process by synthesizing stress-related proteins and other compounds to fuel cellular processes (Tort 2011; Segner et al. 2012).
Assuring an optimal nutritional status and efficient immune system in farmed fish can help to reduce susceptibility to stress and diseases. At present, the use of preventative measures is central to disease control in aquaculture production. Vaccinations are known to be effective prophylactic methods but, as earlier mentioned, can be stressful for fish and remain costly and labor-intensive for the producers (Lillehaug 1989; Horne 1997; Gudding et al. 1999; Thorarinsson and Powell 2006). The application of immunostimulants to counter stress and diseases has been increasingly promoted in aquaculture (Anderson 1992; Sakai 1999; Merrifield et al. 2010). Injection of immunostimulating compounds seemed to be an effective method; however, bath immunization and oral administration showed to be less invasive and stressful for fish; in particular, the dietary uptake is a simple and time-saving way (Anderson 1992; Sakai et al. 2001; Peddie et al. 2002; Selvaraj et al. 2005).
Immunostimulants can be synthetic compounds or biological extracts derived from bacteria, fungi, or plants commonly in the form of polysaccharides. Alginic acids, β-glucans, mannan oligosaccharides (MOSs), nucleotides, and probiotic bacteria have essential physiological and biochemical functions in mediating energy metabolism, cell signaling, encoding and deciphering genetic information, or modifying intestinal microbial communities (Carver and Walker 1995; Dalmo and Bøgwald 2008; Kesarcodi-Watson et al. 2008; Holdt and Kraan 2011; Jung-Schroers et al. 2015). They are reported to strengthen the immune system, protecting fish against physiological stress and susceptibility to infections (Bagni et al. 2005; Staykov et al. 2007; Yoo et al. 2007; Ai et al. 2011; Peng et al. 2013). The fish’s innate immune system is the first line of defense against disease vectors and is considered a fundamental mechanism in fighting all kinds of pathogens (Magnadóttir 2006; Whyte 2007).
Dietary inclusion of β-glucans, MOSs, algal derivates, nucleic acids, and probiotics has been shown to modulate immune defense, stress response, and survival in various fish species (Balcázar et al. 2006; Ringø et al. 2010, 2012). Immunostimulants can promote the production of antibacterial peptides, such as lysozyme, and the phagocytic activity of macrophages. Elevation in serum lysozyme activity has been found in various fish species, such as sea bass, Dicentrarchus labrax (Bagni et al. 2005); Nile tilapia, Oreochromis niloticus (El-Boshy et al. 2010); sturgeon, Huso huso (Heidarieh et al. 2011); and rainbow trout, Oncorhynchus mykiss (Staykov et al. 2007), which were fed on β-glucan-, MOS-, or alginic acid-supplemented diets. Dietary uptake of β-glucan in combination with a feed stimulant (BAISM) was able to improve the lysozyme activity in juvenile olive flounder, Paralichthys olivaceus (Yoo et al. 2007). Phagocyte respiratory burst was enhanced with dietary ribonucleic acid in rohu (Labeo rohita; Choudhury et al. 2005) and β-glucan in sea bass (Bonaldo et al. 2007). Dietary supplementation of β-glucan and MOS enhanced respiratory burst and survival of juvenile turbot, Scophthalmus maximus (Li et al. 2008). However, Ogier de Baulny et al. (1996) observed no effect of β-glucan on the lysozyme activity in turbot.
Information on the effect of immunostimulants on stress response in fish is scarce. Alginic acid-enriched (Gioacchini et al. 2008) and nucleic acid-enriched (Leonardi et al. 2003; Tahmasebi-Kohyani et al. 2012; Palermo et al. 2013) diets can suppress stress response (expressed as plasma cortisol levels) in rainbow trout and sole (Solea solea) exposed to different stressors (vaccination, virus disease, handling, and crowding). Recent attempts to minimize the fish meal (FM) content in formulated finfish feeds due to economic and environmental concerns may increase susceptibility to stress and infections potentially as a result of the reduction of high-quality animal proteins and lipids in diets (Burrells et al. 1999; Urán et al. 2008; Bonaldo et al. 2014; Khosravi et al. 2015).
Diets for carnivorous fish species, such as turbot (S. maximus), are yet to be optimized. In particular, the adverse effects of dietary plant substitutes and stressful husbandry conditions must be overcome. The interaction of immunostimulants and plant proteins acting as FM substitutes as regards immune and stress response remains, however, poorly studied. The influence of immunostimulant supplementation in FM-reduced diets on plasma cortisol levels after an acute stress challenge remains unstudied for turbot. Therefore, the current study aims to evaluate the effect of commercially applied and piloted immunostimulants with the following active ingredients: (i) yeast β-glucan and mannan oligosaccharide (GM), (ii) alginic acid from brown algal extracts (AC), (iii) purified yeast nucleotides and ribosomal RNA (NR), and (iv) probiotic bacteria strains Bacillus subtilis and Bacillus licheniformis (BS), on the immune and stress responses in juvenile turbot fed low fish meal content diets.
Materials and methods
Fish species and experimental setup
The feeding experiment was conducted at the aquaculture facilities Zentrum für Aquakulturforschung (ZAF) at the Alfred Wegener Institute Helmholtz Center for Polar and Marine Research (AWI) in Bremerhaven, Germany. Juvenile turbots were obtained from Maximus A/S (Bedsted Thy, Denmark). Fish were examined for infectious diseases before and at the end of the experiment to monitor health conditions of experimental animals. The experiment was performed under the guidelines of the local authority (Department of Food Safety, Veterinary Affairs and Plant Protection) in Bremen with the permission to carry out animal experiments (522-27-11/02-00(112)).
The rearing system consisted of 36 tanks (0.8 m2 bottom surface, 500 l total water volume). Tanks were connected to a recirculating aquaculture system (RAS; total water volume 40 m3) and equipped with a drum filter, protein skimmer, moving bed biofilter, and disinfection unit (ozone generator; Sander Aquatec GmbH, Uetze-Eltze, Germany). The photoperiod was maintained at a 12 h light/12 h dark cycle throughout. Physical water parameters were monitored constantly (temperature 17.3 ± 0.5 °C, salinity 28.6 ± 1.4 g l−1, dissolved oxygen 9.3 ± 0.5 mg l−1; SC 1000 Multi-parameter Universal Controller, Hach Lange GmbH, Düsseldorf, Germany). Chemical water parameters ammonia, nitrite, and nitrate were determined in a 3-day interval before feeding (NH4-N 0.01 ± 0.02 mg l−1, NO2-N 0.04 ± 0.03 mg l−1, NO3-N 80.6 ± 16.7 mg l−1; photometer DR 2800; Hach Lange GmbH, Germany).
Experimental diets
Six diets were formulated with regard to an isonitrogenous (565 ± 7 g CP kg−1) and isocaloric (22 ± 0.5 g MJ kg−1 DM) content (Table 1). Four experimental diets were supplemented with active ingredients of commercially applied or piloted feed additives: (1) a yeast (Saccharomyces cerevisiae) product consisting of 20% beta-1,3/1,6 glucan and 17% mannan oligosaccharide (ProEnMune, ProEn Protein and Energie GmbH, Soltau, Germany) (GM), (2) an alginic acid product of brown algal extracts containing 99% Laminaria digitata and 1% Ascophyllum nodosum (Ergosan®, Intervet/Schering-Plough Aquaculture, Saffron Walden, UK) (AC), (3) a product of purified yeast nucleotides (cytidine-5V-monophosphate (CMP), disodium uridine-5V-monophosphate (UMP), adenosine-5V-monophosphate (AMP), disodium inosine-5V-monophosphate (IMP), disodium guanidine-5V-monophosphate (GMP)) and ribosomal RNA (Vannagen®, Chemoforma Ltd., Augst, Switzerland) (NR), and (4) a probiotic product of bacteria strains B. subtilis and B. licheniformis (Probiotic-plus.ru, Russia) (BS). Two control diets, a FM-based diet (C-HF; 585 g FM protein kg−1 feed) and a FM-reduced diet (C-LF; 320 g FM kg−1 feed), were additionally investigated. The protein content in C-LF and the supplemented diets (GM, AC, NR, and BS) was partly replaced with soy protein concentrate (SPC) and wheat gluten (WG) including 56% protein from plants. All diets were extruded to floating pellets of 5 mm in diameter, manufactured by the Institute of Food Technology and Bioprocess Engineering (BILB-ttz Bremerhaven, Germany).
Experiment I: feeding trials
During the acclimatization period, fish were fed with a commercial dry feed with 55% crude protein and 16% crude fat (R Europa 15, 2 mm diameter; Skretting ARC, Stavanger, Norway). For the experiment, 900 turbot individuals were weighed (initial mean body weight 95.8 g ± 17.7 g) and measured in total body length (initial mean length 18.0 cm ± 1.1 cm) and randomly stocked in the 36 experimental tanks (25 individuals tank−1; 3.0 kg m−2 stocking density). Fish were starved 24 h prior to weighing. The feeding trial was designed to contain six fish groups consisting of six replicates each. The turbots were hand-fed until apparent satiation twice a day (10:00 a.m. and 2:00 p.m.) over an 84-day period. The effect of fish meal substitution and feed additives on feed conversion and growth performance was reported in a previous communication (Fuchs et al. 2015).
At the end of the experiment, 108 individual fish (3 fish tank−1) were selected randomly and placed into a 500 mg l−1 solution of tricaine methanesulfonate (MS 222; Sigma-Aldrich Co. LLC., Munich, Germany) until death (Neiffer and Stamper 2009). Subsequently, blood was drawn from the caudal vein into disposable syringes pre-filled with a lithium-heparin bead (Sarstedt AG & Co. KG, Nümbrecht, Germany), centrifuged at 2.000g for 15 min to collect the supernatant plasma. Plasma was stored at −80 °C for biochemical analysis. Furthermore, head kidneys of another fish per tank (6 fish treatment−1, 36 fish in total) were isolated and placed in centrifuge tubes (50 ml; Sarstedt AG & Co. KG, Germany) filled with 15 ml of wash medium (RPMI medium mixed with 10,000 IU sodium heparin; Sigma-Aldrich Co. LLC., Germany). Head kidney samples were processed immediately to measure the production of the reactive oxygen species (ROS).
Experiment II: handling simulation (capture, netting/transfer, crowding)
Prior to experiment II, the remaining turbots from all six tanks of the six treatment groups were restocked in three replicate tanks (35 fish tank−1, 630 in total). Turbots were kept in 500-l tanks, and feeding (twice a day) was continued for additional 28 days with the before-mentioned experimental diets. In this experiment, the influence of a typical short-term (<1 day) aquaculture handling procedure (combination of capture, netting/transfer, and crowding) on physical stress response in turbot was determined. After 28 days, the mean initial stocking density of fish was 16.1 kg m−2 (C-HF), 13.5 kg m−2 (C-LF), 13.3 kg m−2 (GM), 13.3 kg m−2 (AC), 12.6 kg m−2 (NR), and 13.3 kg m−2 (BS), respectively. Fish were starved 24 h prior to sampling as feeding has shown to influence plasma cortisol levels (Arends et al. 1999). As a pre-treatment control, three fish were simultaneously taken from each tank (nine fish per treatment), immediately anesthetized with a lethal dose of MS 222 and bled using lithium-heparinized syringes (Sarstedt, Germany). Subsequently, all other fish were subjected to handling treatment, which consisted of netting from the rearing tanks, and transferred into aerated 90-l tanks (0.3 m−2 bottom surface, 72 cm length × 42 cm width × 30 cm depth) at a stocking density of 39.4 kg m−2 (C-HF), 32.9 kg m−2 (C-LF), 32.4 kg m−2 (GM), 32.4 kg m−2 (AC), 30.8 kg m−2 (NR), and 32.5 kg m−2 (BS). Fish were kept there in crowded conditions for 5 min before relocating them into the rearing tanks. At 0.5 h, 1 h, 4 h (11:30 a.m., 12:00 a.m., 3:00 p.m.), and 24 h after the end of the handling simulation, three fish per tank (nine fish per treatment and time point) were simultaneously captured, while ensuring minimal disturbance to other tank occupants, placed into anesthetic, and bled as described above. Only three treatment groups (C-HF, GM, and AC) were subjected to handling stress on the same day to allow equal timing for blood sampling (11:30 a.m., 12:00 a.m., 3:00 p.m.) during this narrow time frame. All samples (pre-treatment control and post stress) of the three treatments were taken directly before stress and within 24 h. The following day, the same procedure was applied to the other three groups (C-LF, NR, and BS). Blood samples were stored on ice and centrifuged at 2.000g for 15 min at 4 °C. The supernatant plasma was frozen for storage at −80 °C to determine the cortisol and glucose concentrations in fish at resting condition and after handling.
Immunological analysis
Generation of ROS by head kidney leucocytes (HKLs) was measured by a nitro blue tetrazolium salt (NBT) reduction assay (Pick et al. 1981; Verburg-van Kemenade et al. 1996) as described before by Skouras and Steinhagen (2003). Leucocytes were collected by pressing and washing the HK tissue through a 100-μm nylon mesh with three times 5 ml of wash medium (RPMI medium with 10,000 IU l−1 sodium heparin; Sigma-Aldrich Co. LLC., Germany). The isolated cells in medium were centrifuged at 580g for 10 min at 4 °C. After decanting the supernatant, cell pellets were resuspended with 15 ml of wash medium and centrifuged a second time. Subsequently, pellets were resuspended with culture medium (RPMI medium supplemented with 10% fetal bovine serum; Sigma-Aldrich Co. LLC., Germany). The viable cell concentration was determined by trypan blue staining and cell counting using a hemocytometer. Cells were adjusted to 106 cells ml−1 in the culture medium. To measure ROS generation, cell suspensions were incubated in 96-well flat-bottom microtiter plates (106 cells per well in a final volume of 175 μl medium) in triplicate with and without a stimulator. Therefore, the culture medium, the indicator NBT (1 g l−1; Sigma-Aldrich), the stimulator phorbol myristate acetate (PMA, 0.15 mg l−1; Sigma-Aldrich), and cell suspension were added per well to induce ROS production. Additionally, cells were incubated without a stimulator to determine spontaneous ROS generation of cells. The solutions were discarded after 2 h of incubation at 22 °C. Cells were fixed for 10 min with 125 μl of 100% methanol (Carl Roth GmbH & Co. KG, Karlsruhe, Germany), and subsequently, wells were washed twice with 125 μl of 70% methanol. Plates were stored in the dark to allow air-drying of the fixed cells for 24 h at room temperature. On the following day, the reduced NBT (formazan) was solubilized in 125 μl of 2 M KOH and 150 μl DMSO (Carl Roth) per well (Rook et al. 1985). The optical densities were recorded immediately at 650 nm in a microplate reader (TriStar LB 941; Berthold Technologies, Germany) at 22 ± 0.5 °C.
Plasma biochemical analysis
Plasma lysozyme (LZY) activity was analyzed by a turbidimetric assay according to Parry et al. (1965), adapted for the measurement in microtiter plates (Skouras et al. 2003). A 0.2 g l−1 suspension of Micrococcus lysodeikticus was prepared with 0.05 M sodium phosphate buffer (pH 7.4) (chemicals: Sigma-Aldrich Co. LLC., Munich, Germany). The bacterial cell suspension was added to a 96-well microtiter plate and mixed with 25 μl plasma to receive a total volume of 200 μl per well. The optical density (OD) was measured after 0.5 and 4.5 min at 530 nm in a microplate reader (TriStar LB 941; Berthold Technologies GmbH & Co. KG, Bad Wildbach, Germany) at a temperature of 23 ± 1 °C. The lysozyme activity was calculated according to the decrease in absorbance, defining 1 unit of lysozyme activity as the amount of sample causing a decrease in absorbance of 0.001 OD min−1. As an external standard, hen egg-white lysozyme (Sigma-Aldrich Co. LLC., Germany) was used (Hutchinson and Manning 1996). Analysis of total protein (TP), cholesterol (CHO), glucose (GC), and triglyceride (TG) was performed using an automatic biochemical analyzer (Pentra 400, Horiba Medical, Kyoto, Japan).
Plasma cortisol levels were measured by solid-phase enzyme-linked immunosorbent assay (ELISA; RE52611, IBL International, Hamburg), which was already used for turbot (Reiser et al. 2010). Before ELISA, plasma samples were treated with heat denaturation; 200 μl of plasma was denatured at 80 °C for 1 h, subsequently vortexed for 20 s, diluted with phosphate-buffered saline (PBS), vortexed for 20 s, and centrifuged at 13,000g for 20 min for separation of the supernatant. Duplicate aliquots (50 μl) of diluted plasma were then used in the assay. The accuracy of heat treatment was evaluated by cold spiking with cortisol (40 ng ml−1) to determine the recovery (recovery = 94%).
Statistics
Data are presented as a mean ± standard deviation (SD) for each treatment. The SigmaPlot 11 for Windows (Systat Software, Inc., San Jose, CA, USA) software package was used for statistical evaluations. All data were tested for normal distribution by the Shapiro-Wilk test. If normality and homogeneity of variances were confirmed, multiple comparisons for the immune and hematological data were done by one-way analysis of variance (ANOVA). A non-parametric Kruskal-Wallis test was used when the normality assumption was not met. Data from acute stress measurements were analyzed by two-way ANOVA with two independent variables, treatment (diet) and time (hours), and cortisol or glucose concentration as dependent variables. Tukey’s post hoc (HSD) or Dunn tests were carried out to identify significantly different groups. Differences between sets of comparisons were considered significant at a probability of error at p < 0.05.
Results
Overall survivorship during the experimental period was high with a total of 0 and 0.7% mortalities in treatments C-HF, C-LF, AC, and BS and treatments GM and NR, respectively (p < 0.05; one-way ANOVA). No signs of infections were observed during the experiments. The final weight (299.5 ± 92.0 g) of fish fed the high FM diet (C-HF) was significantly higher (p < 0.01) compared to that of low FM groups (NR = 246.3 ± 71.1 g, GM = 251.9 ± 69.6 g, BS = 254.0 ± 72.0 g, C-LF = 254.2 ± 77.9 g, AC = 257.5 ± 70.4) (Fuchs et al. 2015). Feed additives had no significant influence (p > 0.05) on growth performance.
Experiment I: plasma biochemistry and immunology
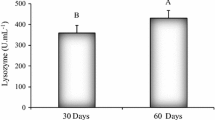
The mean lysozyme activity (units ml−1 plasma) did not differ significantly (p > 0.05) between treatments (Table 2). However, all fish treated with dietary immunostimulants or high FM (C-HF) showed a tendency towards increased lysozyme activities between 1006 ± 133 (BS) and 1108 ± 106 (AC), compared to an activity of 971 ± 118 in fish fed the low FM control diet (C-LF) (Table 2).
The values of the baseline reactive oxygen species production (OD), 0.02 ± 0.01, indicated no significant differences (p > 0.05) among all treatments (Table 2). Head kidney phagocytes from turbots could be stimulated by phorbol ester (PMA) and cells responded with a high NBT reduction. Stimulated ROS activities were measured between 0.53 ± 0.19 (NR) and 0.78 ± 0.38 (C-HF)/0.78 ± 0.53 (BS), while phagocytes in fish fed the low FM control diet (C-LF) showed a mean activity of 0.66 ± 0.34 (Table 2). However, upon cell stimulation, the mean production of ROS was not significantly different (p > 0.05) between all fish groups.
Mean concentrations of total plasma protein (mg ml−1) ranged between 29.0 ± 2.7 (C-LF) and 31.7 ± 3.3 (AC) and did not significantly differ between dietary treatments (Table 2). However, plasma cholesterol (mg dl−1) was significantly higher, between 43.7 ± 3.1 (AC) and 58.6 ± 7.4 (C-HF), in fish fed diets supplemented with GM, AC, NR, and the high FM control diet (C-HF) compared to the low FM control diet, 36.8 ± 2.8 (C-LF) (Table 2). Significant increases in plasma glucose (mg dl−1), 44.4 ± 3.8 and 43.7 ± 4.5, and triglyceride (mol dl−1), 105.5 ± 15.3 and 90.6 ± 19.2, were recorded under GM and NR supplementation compared to C-LF, 34.8 ± 2.4 and 38.3 ± 10.2, while glucose levels were significantly higher in C-HF, 45.4 ± 7.3.
Experiment II: stress indicators
Plasma cortisol concentrations (ng ml−1) in unstressed turbots ranged between 4.9 ± 3.6 (NR) and 16.5 ± 14.9 (C-HF) (mean 10.0 ± 5.4) and can be defined as baseline levels (Fig. 1). At experimental outset, cortisol levels were not significantly different (p > 0.05) among dietary treatments. Cortisol levels in fish rose rapidly after handling, peaked by 0.5 and 1 h, and returned to basal concentrations in fish plasma 4 h after handling (Fig. 1). Fish fed the low fish meal control diet C-LF showed significantly higher levels (60.1 ± 10.0; p < 0.05) at 0.5 h post handling compared to all other fish groups (Fig. 1). In contrast, fish of the high fish meal control group C-HF exhibited significantly higher levels (59.4 ± 7.8; p < 0.05) at 1 h post handling. All other groups treated with supplemented diets showed the highest plasma cortisol concentrations between 23.7 ± 8.1 (BS) and 32.6 ± 13.5 (NR) 0.5 and 1 h after the handling procedure (Fig. 1). At 4 and 24 h post handling, cortisol levels were not significantly different (p > 0.05) in turbot plasma among all fish groups. Plasma glucose concentrations (mg dl−1) ranged between 31.8 ± 2.3 (AC) and 38.4 ± 4.8 (C-HF) (mean 34.9 ± 1.4) under initial resting condition (Fig. 2). Glucose concentrations were significantly increased up to 50.0 ± 5.0 in fish group C-HF following handling stimuli at 1 h post stress (Fig. 2). Only a trend towards elevated glucose concentrations was visible in the fish of the other groups at 0.5, 1, and 4 h post stress, although changes were not significant.
Plasma cortisol concentrations in nanograms per milliliter after stress treatment collected from turbots of the six dietary treatments. Data are presented as mean ± SD (n = 6). Different letters denote significant differences within the same treatment, and asterisk and section sign denote significant differences between treatments at the same time point (p < 0.05)
Plasma glucose concentrations in nanograms per milliliter after stress treatment collected from turbots of the six dietary treatments. Data are presented as mean ± SD (n = 6). Different letters denote significant differences within the same treatment, and asterisk and section sign denote significant differences between treatments at the same time point (p < 0.05)
Discussion
The use of immunostimulating substances in fish and shellfish feeds has been widely accepted in the aquaculture industry despite their exact modes of action inside the organism remaining far from fully understood. Any prophylactic measures that have nutritional value and can support stress tolerance and defense mechanisms against pathogens are likely to be of commercial interest for producers of feed and fish. The current results provides new insight into the immunostimulant activity among common diet supplements and their effect on stress response in juvenile turbot maintained on reduced fish meal (FM) diets.
A FM reduction to 320 g kg−1 diet (44% protein derived from FM) did not affect innate immune activity in turbot, indicating that dietary SPC and WG do not compromise the potential of lysozyme and leucocyte ROS production to fight pathogens. Similar trends were observed in immune activities of Atlantic salmon (Salmo salar), gilthead sea bream (Sparus aurata), and turbot feeding on plant protein (PP)-rich diets (Bransden et al. 2001; Sitjà-Bobadilla et al. 2005; Kokou et al. 2012; Zheng et al. 2013). Stimulated respiratory burst of head kidney leucocytes did not show any changes in sea bream fed diets reduced to 350 and 110 g kg−1 FM, respectively (Sitjà-Bobadilla et al. 2005; Kokou et al. 2012). In general, plasma lysozyme activities between 971 and 1108 U ml−1 reveal relatively high values compared to activities between 8.8 and 9.4 U ml−1 (Zheng et al. 2013), between 500 and 900 U ml−1 (Sun et al. 2016), and between 900 and 1500 U ml−1 (Ogier de Baulny et al. 1996) in turbots, indicating an intact function of innate immune response in fish. No sign of inflammation was observed in the current study, unlike previous studies where high inclusion of PP in diets also increased immune responses in fish, an effect interpreted as either immunostimulating or inflammatory effects (Gabrielsen and Austreng 1998; Krogdahl et al. 2000).
Previous investigations proved capabilities of seaweed extracts and β-glucan products to enhance a respiratory burst activity of phagocytes in turbot, gilthead sea bream, Atlantic cod (Gadus morhua), and Atlantic salmon, after in vitro stimulation (Castro et al. 1999, 2004; Bridle et al. 2005; Caipang et al. 2011), although positive findings were not confirmed in vivo, feeding salmon β-glucan for 1 week. A short-term administration of dietary β-glucan and alginic acid (15 to 30 days) and that of oligonucleotides (42 to 56 days) improved some innate immune responses in fish (D. labrax, Morone chrysops × Morone saxatilis hybrid) (Li et al. 2004; Bagni et al. 2005). However, increased immune activities did not last for 112 weeks after long-term feeding of hybrid sea bass. Similarly, dietary supplementation with immunostimulating substances did not have any significant effects on lysozyme and ROS activities in turbot after an apparent long-term feeding of 84 days in this study, although a clear tendency towards increased activities is observable. In accordance with this finding, nucleotides in low fish meal diets (400 g kg−1 FM) did not affect activities of serum superoxide dismutase and catalase in juvenile turbot, although improved the antioxidative capacity after 60 days (Meng et al. 2016). Only tendencies of higher serum lysozyme activity and neutrophil oxidative radical anion production were found in red drum (Sciaenops ocellatus) with increasing nucleotide levels, 0.5 and 1%, in diets of 580 g kg−1 FM (Cheng et al. 2011). Similarly, dietary fructooligosaccharide (FOS) supplementation did not significantly improve turbot’s innate humoral parameters, like lysozyme, and hematology when fed at 50% PP diets for 63 days at 15 and 20 °C water temperature (Guerreiro et al. 2014). On the other hand, dietary supplementation with yeast (S. cerevisiae) did improve the immune response in RAS-reared turbot (151 g mean initial weight) after 72 days (Li et al. 2008). Recent investigations on the effect of nucleotides and B. subtilis supplementation, respectively, in FM-reduced diets revealed positive effects on some immune parameters, either lysozyme or respiratory burst activity, in turbot and yellow croaker (Larimichthys crocea) (Ai et al. 2011; Peng et al. 2013). However, turbot individuals of another age/size class (9.2 vs. 96 g mean initial weights in the present study) were used and fed diets with increasing soy bean meal content for 60 days. Stressful farming conditions, e.g., vaccination, can impair physiological conditions and immune response. As an example, a β-glucan administration showed no effect in turbot, but combined with a vaccine, adjuvants increased immune activities compared to single vaccination (Ogier de Baulny et al. 1996; Figueras et al. 1998). Burrells et al. (2001b) confirmed this finding for vaccinated salmon whose immunity and survival were supported by a combined inclusion of 0.03% nucleotides that significantly enhanced the efficacy of vaccination. Obviously, the value of certain feed supplements varies not only between fish species and between methods of application, but is also highly dependent on the nutritional and physiological status of fish. Therefore, the influence of immunostimulants on fish has to be interpreted carefully, because many factors can change physical reactions to stimulants. Researchers assume that active compounds of dietary immunostimulants, like β-glucans, act as a ligand and may bind to specific β-glucan receptors on macrophages and neutrophils in fish influencing the functional status of phagocytes (Engstad and Robertsen 1993; Ainsworth 1994). At present, however, an uptake of particulate compounds, e.g., yeast β-glucans, in intestinal cells and the blood system of fish to stimulate immune cells and components has not been proved yet (Dalmo and Bøgwald 2008).
A balance of essential amino acids (EAAs) in diets with high PP content, meeting the requirements of turbot, is essential for optimal growth and likely immune response in fish. Previous studies have shown high substitution with SPC and WG reduces growth performance in juvenile turbot (Bonaldo et al. 2011; Fuchs et al. 2015). However, these alternative ingredients did not affect turbot’s immune capacity in the current study. Therefore, turbots tolerate relatively high contents of dietary SPC and WG associated with a balanced EAA profile to maintain normal immune function. It can, however, be assumed that gross energy demand for maintenance increases with dietary PP inclusion as indicated by Dietz et al. (2012) and, hence, affects the capacity to regulate immune responses.
In contrast of the unchanged immune functions, turbot’s physiological status declined with FM replacement in terms of reduced plasma cholesterol levels. However, dietary supplementation of NR, GM, and AC showed a positive physiological change of juvenile turbot, as indicated by elevated cholesterol and triglyceride (NR, GM) concentrations. Plasma protein levels were not found to be modified with reduced FM content. Similar findings were reported for turbot and sea bream fed diets high in corn, wheat, or soy proteins (Regost et al. 1999; Sitjà-Bobadilla et al. 2005; Bonaldo et al. 2014).
At present, little has been published on the influence of dietary PP inclusion on stress response in fish (Bonaldo et al. 2014). High blood cortisol levels, above 50 ng ml−1, are accepted as indicators of stress in fish (Barton and Iwama 1991; Wendelaar Bonga 1997). Base cortisol levels (4.9–16.5 ng ml−1) of most turbots in the current study were similar to reported values for turbots at resting condition (Waring et al. 1996; Mugnier et al. 1998). Individual turbots in all replicate tanks of the six treatments exhibit higher base cortisol levels (>8 ng ml−1) resulting in high variability in tanks (coefficient of variation: C-HF = 11, 36, and 75%; C-LF = 71, 87, and 118%; GM = 65, 78, and 84%; AC = 40, 70, and 71%; NR = 33, 61, and 61%, BS = 73, 117, and 125%). Discrepancies in basal cortisol levels between different studies may be the result of differential methods of killing, capture, or differences in overall holding conditions. In other studies, turbots were either killed using ice water (Reiser et al. 2010) and a blow to the head (Van Ham et al. 2003) or kept alive and cannulated (Waring et al. 1996; Mugnier et al. 1998). Holding conditions, e.g., temperature, stocking rate, or feeding, can modify cortisol resting values in fish and incidences of a stress response (Van Ham et al. 2003; Davis 2004). It is also possible that a visual contact with the sampler elicited a slight cortisol elevation in some fish, perhaps in anticipation of feeding in the morning. Among dietary treatments, stocking density was highest in C-HF which might have caused higher base cortisol levels in fish and higher variances between tanks.
A primary stress reaction induced by capture, netting, and crowding was clearly evidenced by significantly elevated cortisol values at 0.5 and 1 h. These were comparable to post-stress cortisol levels in turbot and gilthead sea bream (Van Ham et al. 2003; Ganga et al. 2011; Tahmasebi-Kohyani et al. 2012). But, cortisol levels in turbots remained quite low, in particular for the immunostimulant- treated fish. Only unsupplemented fish fed the control diets reached cortisol levels (59 and 60 ng ml−1) above the 50 ng ml−1 threshold. During the repeated sampling at 0.5, 1, 4, and 24 h post stress, care was taken that all turbots from a particular tank were collected quickly to avoid further handling disturbance that may raise cortisol levels of the remaining tank occupants. According to Van Ham et al. (2003) and Mugnier et al. (1998), turbots are relatively insensitive to repeated handling disturbances. The peak stress responses, though with differences between the low fish meal groups C-LF and GM, AC, NR, and BS, were already recorded at 0.5 h post stress when no further disturbances had occurred. Cortisol levels returned to resting levels within 4 h, indicating an acute stress reaction after a short-term stress incidence. Post-exercise recovery was faster than the 24 h reported following capture and 9 min net confinement for turbot (Waring et al. 1996). However, concentrations of blood cortisol can vary between fish species, time of sampling depending on fish’s circadian rhythm, and type and severity of stress (Pickering and Pottinger 1989; Barton and Iwama 1991). To our knowledge, no studies evaluating the influence of daytime on cortisol and glucose levels in turbot have been published. Compared to circadian rhythms of fish species, our data detected no similar pattern of stress response affected by these rhythms (Cerdá-Reverter et al. 1998; Montoya et al. 2010; Oliveira et al. 2013). In general, fluctuations of cortisol values due to a circadian rhythm show to be marginal during the course of the day when fish were not fed. Ferrari et al. (2015) also observed differences in an individual behavior of fish held in groups identified as divergent coping styles that affect the intensity of stress response and release of corticosteroids.
Blood glucose level, a typical secondary stress response (Barton and Iwama 1991), increased parallel to those of cortisol in fish of the high FM control diet. In contrast, tendencies of increased blood glucose levels in low FM groups after 0.5, 1, and 4 h of handling exposure were apparent but did not reflect the pattern of cortisol release as a physiological stress response. Differences in magnitudes of the secondary stress response may be due to a higher stocking density of the high FM group due to higher individual weight at the end of the previous feeding trial. However, previous studies have detected that turbot did not exhibit a strong plasma glucose response when compared to other finfish, which may represent a fundamental difference in turbot stress physiology (Waring et al. 1992, 1996; Van Ham et al. 2003). Apparently, plasma cortisol is a more sensitive indicator of handling stress for turbot than plasma glucose changes.
All additives proved beneficial in suppressing stress response modifying the metabolism in turbot. Similarly, positive effects were noted in mannan oligosaccharide (MOS)-, Ergosan-, and nucleotide-treated European sea bass (D. labrax), rainbow trout, and sole (S. solea) subjected to stressors such as vaccination, bacterial or viral infection, and handling/crowding, respectively (Leonardi et al. 2003; Gioacchini et al. 2008; Tahmasebi-Kohyani et al. 2012; Torrecillas et al. 2012; Palermo et al. 2013).
Repeated stressors in routine husbandry procedures can affect strong physiological stress responses in turbot associated with impaired homeostasis and immunosuppression and changes in the energy metabolism (Mommsen et al. 1999; Costas et al. 2013). Particularly, stress response is associated with energetic costs to cope with stress, while consequently, less energy is available for other biological functions, including defense mechanisms (immune system) and physiological processes (e.g., growth) (Wendelaar Bonga 1997). Therefore, preventive methods and technologies have to be developed to ensure fish welfare and health to maintain profitable fish production.
In conclusion, juvenile turbot diets containing 320 g kg−1 FM and mixed PP (SPC and WG; 560 kg−1 diet) for juvenile turbots did not significantly affect the important innate immune mechanisms (lysozyme activity and reactive oxygen production of neutrophils). Dietary supplementation of immunostimulants (β-glucan/MOS, alginic acid, nucleotides/RNA, Bacillus strains) failed to enhance immunity; however, all effectively diminished an increase of blood cortisol levels in turbot subjected to handling stress. Further studies are required to determine an optimal inclusion level of PP that enables immunostimulants to activate immune responses in fish.
References
Ai Q, Xu H, Mai K, Xu W, Wang J, Zhang W (2011) Effects of dietary supplementation of Bacillus subtilis and fructooligosaccharide on growth performance, survival, non-specific immune response and disease resistance of juvenile large yellow croaker, Larimichthys crocea. Aquaculture 317:155–161
Ainsworth AJ (1994) A β-glucan inhibitable zymosan receptor on channel catfish neutrophils. Vet Immunol Immunopathol 41:141–152
Anderson DP (1992) Immunostimulants, adjuvants, and vaccine carriers in fish: applications to aquaculture. Annu Rev Fish Dis 2:281–307
Arends R, Mancera J, Munoz J, Wendelaar Bonga S, Flik G (1999) The stress response of the gilthead sea bream (Sparus aurata L.) to air exposure and confinement. J Endocrinol 163:149–157
Ashley PJ (2007) Fish welfare: current issues in aquaculture. Appl Anim Behav Sci 104:199–235
Bagni M, Romano N, Finoia MG, Abelli L, Scapigliati G, Tiscar PG, Sarti M, Marino G (2005) Short- and long-term effects of a dietary yeast β-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol 18:311–325
Balcázar JL, Blas I, Ruiz-Zarzuela I, Cunningham D, Vendrell D, Múzquiz JL (2006) The role of probiotics in aquaculture. Vet Microbiol 114:173–186
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26
Bonaldo A, Thompson KD, Manfrin A, Adams A, Murano E, Mordenti AL, Gatta PP (2007) The influence of dietary β-glucans on the adaptive and innate immune responses of European sea bass (Dicentrarchus labrax) vaccinated against vibriosis. Ital J Anim Sci 6:151–164
Bonaldo A, Parma L, Mandrioli L, Sirri R, Fontanillas R, Badiani A, Gatta PP (2011) Increasing dietary plant proteins affects growth performance and ammonia excretion but not digestibility and gut histology in turbot (Psetta maxima) juveniles. Aquaculture 318:101–108
Bonaldo A, Di Marco P, Petochi T, Marino G, Parma L, Fontanillas R, Koppe W, Mongile F, Finoia MG, Gatta PP (2014) Feeding turbot juveniles Psetta maxima L. with increasing dietary plant protein levels affects growth performance and fish welfare. Aquac Nutr 21:401–413
Bransden MP, Carter CG, Nowak BF (2001) Effects of dietary protein source on growth, immune function, blood chemistry and disease resistance of Atlantic salmon (Salmo salar L.) parr. Anim Sci 73:105–113
Bridle AR, Carter CG, Morrison RN, Nowak BF (2005) The effect of β-glucan administration on macrophage respiratory burst activity and Atlantic salmon, Salmo salar L., challenged with amoebic gill disease—evidence of inherent resistance. J Fish Dis 28:347–356
Burrells C, Williams PD, Southgate PJ, Crampton VO (1999) Immunological, physiological and pathological responses of rainbow trout (Oncorhynchus mykiss) to increasing dietary concentrations of soybean proteins. Vet Immunol Immunopathol 72:277–288
Burrells C, Williams PD, Forno PF (2001a) Dietary nucleotides: a novel supplement in fish feeds: 1. Effects on resistance to disease in salmonids. Aquaculture 199:159–169
Burrells C, Williams PD, Southgate PJ, Wadsworth SL (2001b) Dietary nucleotides: a novel supplement in fish feeds: 2. Effects on vaccination, salt water transfer, growth rates and physiology of Atlantic salmon (Salmo salar L.) Aquaculture 199:171–184
Caipang C, Lazado C, Berg I, Brinchmann M, Kiron V (2011) Influence of alginic acid and fucoidan on the immune responses of head kidney leukocytes in cod. Fish Physiol Biochem 37:603–612
Carver JD, Walker WA (1995) The role of nucleotides in human nutrition. J Nutr Biochem 6:58–72
Castro R, Couso N, Obach A, Lamas J (1999) Effect of different β-glucans on the respiratory burst of turbot (Psetta maxima) and gilthead seabream (Sparus aurata) phagocytes. Fish Shellfish Immunol 9:529–541
Castro R, Zarra I, Lamas J (2004) Water-soluble seaweed extracts modulate the respiratory burst activity of turbot phagocytes. Aquaculture 229:67–78
Cerdá-Reverter JM, Zanuy S, Carrillo M, Madrid JA (1998) Time-course studies on plasma glucose, insulin, and cortisol in sea bass (Dicentrarchus labrax) held under different photoperiodic regimes. Physiol Behav 64:245–250
Cheng Z, Buentello A, Gatlin Iii DM (2011) Dietary nucleotides influence immune responses and intestinal morphology of red drum Sciaenops ocellatus. Fish Shellfish Immunol 30:143–147
Choudhury D, Pal AK, Sahu NP, Kumar S, Das SS, Mukherjee SC (2005) Dietary yeast RNA supplementation reduces mortality by Aeromonas hydrophila in rohu (Labeo rohita L.) juveniles. Fish Shellfish Immunol 19:281–291
Costas B, Rêgo PCNP, Conceição LEC, Dias J, Afonso A (2013) Dietary arginine supplementation decreases plasma cortisol levels and modulates immune mechanisms in chronically stressed turbot (Scophthalmus maximus). Aquac Nutr 19:25–38
Dalmo RA, Bøgwald J (2008) β-Glucans as conductors of immune symphonies. Fish Shellfish Immunol 25:384–396
Davis KB (2004) Temperature affects physiological stress responses to acute confinement in sunshine bass (Morone chrysops×Morone saxatilis). Comp Biochem Physiol A Mol Integr Physiol 139:433–440
Dietz C, Kroeckel S, Schulz C, Susenbeth A (2012) Energy requirement for maintenance and efficiency of energy utilization for growth in juvenile turbot (Psetta maxima, L.): the effect of strain and replacement of dietary fish meal by wheat gluten. Aquaculture 358–359:98–107
El-Boshy ME, El-Ashram AM, AbdelHamid FM, Gadalla HA (2010) Immunomodulatory effect of dietary Saccharomyces cerevisiae, β-glucan and laminaran in mercuric chloride treated Nile tilapia (Oreochromis niloticus) and experimentally infected with Aeromonas hydrophila. Fish Shellfish Immunol 28:802–808
Engstad RE, Robertsen B (1993) Recognition of yeast cell wall glucan by Atlantic salmon (Salmo salar L.) macrophages. Dev Comp Immunol 17:319–330
Ferrari S, Millot S, Leguay D, Chatain B, Bégout M-L (2015) Consistency in European seabass coping styles: a life-history approach. Appl Anim Behav Sci 167:74–88
Figueras A, Santarém M, Novoa B (1998) Influence of the sequence of administration of β-glucans and a Vibrio damsela vaccine on the immune response of turbot (Scophthalmus maximus L.) Vet Immunol Immunopathol 64:59–68
Focardi S, Corsi I, Franchi E (2005) Safety issues and sustainable development of European aquaculture: new tools for environmentally sound aquaculture. Aquacult Int 13:3–17
Fuchs VI, Schmidt J, Slater MJ, Zentek J, Buck BH, Steinhagen D (2015) The effect of supplementation with polysaccharides, nucleotides, acidifiers and Bacillus strains in fish meal and soy bean based diets on growth performance in juvenile turbot (Scophthalmus maximus). Aquaculture 437:243–251
Gabrielsen BO, Austreng E (1998) Growth, product quality and immune status of Atlantic salmon, Salmo salar L., fed wet feed with alginate. Aquac Res 29:397–401
Ganga R, Montero D, Bell JG, Atalah E, Ganuza E, Vega-Orellana O, Tort L, Acerete L, Afonso JM, Benitez-Sanatana T, Fernández Vaquero A, Izquierdo M (2011) Stress response in sea bream (Sparus aurata) held under crowded conditions and fed diets containing linseed and/or soybean oil. Aquaculture 311:215–223
Gioacchini G, Smith P, Carnevali O (2008) Effects of Ergosan on the expression of cytokine genes in the liver of juvenile rainbow trout (Oncorhynchus mykiss) exposed to enteric red mouth vaccine. Vet Immunol Immunopathol 123:215–222
Gregory TR, Wood CM (1999) The effects of chronic plasma cortisol elevation on the feeding behaviour, growth, competitive ability, and swimming performance of juvenile rainbow trout. Physiol Biochem Zool 72:286–295
Gudding R, Lillehaug A, Evensen Ø (1999) Recent developments in fish vaccinology. Vet Immunol Immunopathol 72:203–212
Guerreiro I, Pérez-Jiménez A, Costas B, Oliva-Teles A (2014) Effect of temperature and short chain fructooligosaccharides supplementation on the hepatic oxidative status and immune response of turbot (Scophthalmus maximus). Fish Shellfish Immunol 40:570–576
Heidarieh M, Soltani M, Tamimi AH, Toluei MH (2011) Comparative effect of raw fiber (Vitacel) and alginic acid (Ergosan) on growth performance, immunocompetent cell population and plasma lysozyme content of giant sturgeon (Huso huso). Turkish J Fish Aquat Sci 11:445–450
Holdt SL, Kraan S (2011) Bioactive compounds in seaweed: functional food applications and legislation. J Appl Phycol 23:543–597
Horne MT (1997) Technical aspects of the administration of vaccines. Dev Biol Stand 90:79–89
Huntingford FA, Adams C, Braithwaite VA, Kadri S, Pottinger TG, Sandøe P, Turnbull JF (2006) Current issues in fish welfare. J Fish Biol 68:332–372
Hutchinson TH, Manning MJ (1996) Seasonal trends in serum lysozyme activity and total protein concentration in dab (Limanda limanda L.) sampled from Lyme Bay, UK. Fish Shellfish Immunol 6:473–482
Jung-Schroers V, Adamek M, Jung A, Harris S, Dóza ÖS, Baumer A, Steinhagen D (2015) Feeding of β-1,3/1,6-glucan increases the diversity of the intestinal microflora of carp (Cyprinus carpio). Aquac Nutr. doi:10.1111/anu.12320
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14
Khosravi S, Rahimnejad S, Herault M, Fournier V, Lee C-R, Dio Bui HT, Jeong J-B, Lee K-J (2015) Effects of protein hydrolysates supplementation in low fish meal diets on growth performance, innate immunity and disease resistance of red sea bream Pagrus major. Fish Shellfish Immunol 45:858–868
Kiron V (2012) Fish immune system and its nutritional modulation for preventive health care. Anim Feed Sci Technol 173:111–133
Kokou F, Rigos G, Henry M, Kentouri M, Alexis M (2012) Growth performance, feed utilization and non-specific immune response of gilthead sea bream (Sparus aurata L.) fed graded levels of a bioprocessed soybean meal. Aquaculture 364–365:74–81
Krogdahl Å, Bakke-Mckellep AM, RØed KH, Baeverfjord G (2000) Feeding Atlantic salmon Salmo salar L. soybean products: effects on disease resistance (furunculosis), and lysozyme and IgM levels in the intestinal mucosa. Aquac Nutr 6:77–84
Laiz-Carrión R, Martín Del Río MP, Miguez JM, Mancera JM, Soengas JL (2003) Influence of cortisol on osmoregulation and energy metabolism in gilthead seabream Sparus aurata. J Exp Zool Part A: Comp Exp Biol 298A:105–118
Leonardi M, Sandino AM, Klempau A (2003) Effect of a nucleotide-enriched diet on the immune system, plasma cortisol levels and resistance to infectious pancreatic necrosis (IPN) in juvenile rainbow trout (Oncorhynchus mykiss). Arch Bull Eur Assoc Fish Pathol 23:52–59
Li P, Lewis DH, Gatlin Iii DM (2004) Dietary oligonucleotides from yeast RNA influence immune responses and resistance of hybrid striped bass (Morone chrysops × Morone saxatilis) to Streptococcus iniae infection. Fish Shellfish Immunol 16:561–569
Li Y, Wang YJ, Wang L, Jiang KY (2008) Influence of several non-nutrient additives on nonspecific immunity and growth of juvenile turbot, Scophthalmus maximus L. Aquac Nutr 14:387–395
Lillehaug A (1989) A cost-effectiveness study of three different methods of vaccination against vibriosis in salmonids. Aquaculture 83:227–236
Magnadóttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Meng Y, Ma R, Ma J, Han D, Xu W, Zhang W, Mai K (2016) Dietary nucleotides improve the growth performance, antioxidative capacity and intestinal morphology of turbot (Scophthalmus maximus). Aquac Nutr. doi:10.1111/anu.12425:n/a-n/a
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bøgwald J, Castex M, Ringø E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18
Merrifield DL, Harper GM, Mustafa S, Carnevali O, Picchietti S, Davies SJ (2011) Effect of dietary alginic acid on juvenile tilapia (Oreochromis niloticus) intestinal microbial balance, intestinal histology and growth performance. Cell Tissue Res 344:135–146
Mommsen T, Vijayan M, Moon T (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Montoya A, López-Olmeda JF, Garayzar ABS, Sánchez-Vázquez FJ (2010) Synchronization of daily rhythms of locomotor activity and plasma glucose, cortisol and thyroid hormones to feeding in gilthead seabream (Sparus aurata) under a light–dark cycle. Physiol Behav 101:101–107
Mugnier C, Fostier A, Guezou S, Gaignon J-L, Quemener L (1998) Effect of some repetitive factors on turbot stress response. Aquacult Int 6:33–45
Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenco J, Mooney H, Troell M (2000) Effect of aquaculture on world fish supplies. Nature 405:1017–1024
Neiffer DL, Stamper MA (2009) Fish sedation, anesthesia, analgesia, and euthanasia: considerations, methods, and types of drugs. ILAR J 50:343–360
Ogier de Baulny M, Quentel C, Fournier V, Lamour F, Le Gouvello R (1996) Effect of long-term oral administration of β-glucan as an immunostimulant or an adjuvant on some non-specific parameters of the immune response of turbot Scophthalmus maximus. Dis Aquat Org 26:139–147
Oliveira CV, Aparício R, Blanco-Vives B, Chereguini O, Martín I, Javier Sánchez-Vazquez F (2013) Endocrine (plasma cortisol and glucose) and behavioral (locomotor and self-feeding activity) circadian rhythms in Senegalese sole (Solea senegalensis Kaup 1858) exposed to light/dark cycles or constant light. Fish Physiol Biochem 39:479–487
Palermo FA, Cardinaletti G, Cocci P, Tibaldi E, Polzonetti-Magni A, Mosconi G (2013) Effects of dietary nucleotides on acute stress response and cannabinoid receptor 1 mRNAs in sole, Solea solea. Comp Biochem Physiol A Mol Integr Physiol 164:477–482
Parry RM, Chandan RC, Shahani KM (1965) A rapid and sensitive assay of muramidase. Proc Soc Exp Biol Med 119:384–386
Peddie S, Zou J, Secombes CJ (2002) Immunostimulation in the rainbow trout (Oncorhynchus mykiss) following intraperitoneal administration of Ergosan. Vet Immunol Immunopathol 86:101–113
Peng M, Xu W, Ai Q, Mai K, Liufu Z, Zhang K (2013) Effects of nucleotide supplementation on growth, immune responses and intestinal morphology in juvenile turbot fed diets with graded levels of soybean meal (Scophthalmus maximus L.) Aquaculture 392–395:51–58
Pick E, Charon J, Mizel D (1981) A rapid densitometric microassay for nitroblue tetrazolium reduction and application of the microassay to macrophages. J Reticuloendothel Soc 30:581–593
Pickering AD, Pottinger TG (1989) Stress responses and disease resistance in salmonid fish: effects of chronic elevation of plasma cortisol. Fish Physiol Biochem 7:253–258
Regost C, Arzel J, Kaushik SJ (1999) Partial or total replacement of fish meal by corn gluten meal in diet for turbot (Psetta maxima). Aquaculture 180:99–117
Reiser S, Schroeder JP, Wuertz S, Kloas W, Hanel R (2010) Histological and physiological alterations in juvenile turbot (Psetta maxima, L.) exposed to sublethal concentrations of ozone-produced oxidants in ozonated seawater. Aquaculture 307:157–164
Ringø E, Olsen RE, Gifstad TØ, Dalmo RA, Amlund H, Hemre GI, Bakke AM (2010) Prebiotics in aquaculture: a review. Aquac Nutr 16:117–136
Ringø E, Olsen RE, Vecino JLG, Wadsworth S, Song SK (2012) Use of immunostimulants and nucleotides in aquaculture: a review. J Marine Sci Res Dev 1:104. doi:10.4172/2155-9910.1000104
Rook GAW, Steele J, Umar S, Dockrell HM (1985) A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by γ-interferon. J Immunol Methods 82:161–167
Sakai M (1999) Current research status of fish immunostimulants. Aquaculture 172:63–92
Sakai M, Taniguchi K, Mamoto K, Ogawa H, Tabata M (2001) Immunostimulant effects of nucleotide isolated from yeast RNA on carp, Cyprinus carpio L. J Fish Dis 24:433–438
Segner H, Sundh H, Buchmann K, Douxfils J, Sundell K, Mathieu C, Ruane N, Jutfelt F, Toften H, Vaughan L (2012) Health of farmed fish: its relation to fish welfare and its utility as welfare indicator. Fish Physiol Biochem 38:85–105
Selvaraj V, Sampath K, Sekar V (2005) Administration of yeast glucan enhances survival and some non-specific and specific immune parameters in carp (Cyprinus carpio) infected with Aeromonas hydrophila. Fish Shellfish Immunol 19:293–306
Sitjà-Bobadilla A, Peña-Llopis S, Gómez-Requeni P, Médale F, Kaushik S, Pérez-Sánchez J (2005) Effect of fish meal replacement by plant protein sources on non-specific defence mechanisms and oxidative stress in gilthead sea bream (Sparus aurata). Aquaculture 249:387–400
Skouras A, Steinhagen D (2003) Measuring some flounder (Platichthys flesus L.) innate immune responses to be incorporated in effect biomonitoring concepts. Helgol Mar Res 57:199–205
Skouras A, Broeg K, Dizer H, von Westernhagen H, Hansen P-D, Steinhagen D (2003) The use of innate immune responses as biomarkers in a programme of integrated biological effects monitoring on flounder (Platichthys flesus) from the southern North Sea. Helgol Mar Res 57:190–198
Staykov Y, Spring P, Denev S, Sweetman J (2007) Effect of a mannan oligosaccharide on the growth performance and immune status of rainbow trout (Oncorhynchus mykiss). Aquacult Int 15:153–161
Sun G, Li M, Wang J, Liu Y (2016) Effects of flow rate on growth performance and welfare of juvenile turbot (Scophthalmus maximus L.) in recirculating aquaculture systems. Aquac Res 47:1341–1352
Tahmasebi-Kohyani A, Keyvanshokooh S, Nematollahi A, Mahmoudi N, Pasha-Zanoosi H (2012) Effects of dietary nucleotides supplementation on rainbow trout (Oncorhynchus mykiss) performance and acute stress response. Fish Physiol Biochem 38:431–440
Thorarinsson R, Powell DB (2006) Effects of disease risk, vaccine efficacy, and market price on the economics of fish vaccination. Aquaculture 256:42–49
Torrecillas S, Makol A, Caballero MJ, Montero D, Dhanasiri AKS, Sweetman J, Izquierdo M (2012) Effects on mortality and stress response in European sea bass, Dicentrarchus labrax (L.), fed mannan oligosaccharides (MOS) after Vibrio anguillarum exposure. J Fish Dis 35:591–602
Tort L (2011) Stress and immune modulation in fish. Dev Comp Immunol 35:1366–1375
Urán PA, Schrama JW, Rombout JHWM, Obach A, Jensen L, Koppe W, Verreth JAJ (2008) Soybean meal-induced enteritis in Atlantic salmon (Salmo salar L.) at different temperatures. Aquac Nutr 14:324–330
Van Ham EH, Van Anholt RD, Kruitwagen G, Imsland AK, Foss A, Sveinsbø BO, FitzGerald R, Parpoura AC, Stefansson SO, Wendelaar Bonga SE (2003) Environment affects stress in exercised turbot. Comp Biochem Physiol A Mol Integr Physiol 136:525–538
Verburg-van Kemenade BML, Daly JG, Groeneveld A, Wiegertjes GF (1996) Multiple regulation of carp (Cyprinus carpio L.) macrophages and neutrophilic granulocytes by serum factors: influence of infection with atypical Aeromonas salmonicida. Vet Immunol Immunopathol 51:189–200
Verburg-Van Kemenade BML, Stolte EH, Metz JR, Chadzinska M (2009) Chapter 7: Neuroendocrine–immune interactions in teleost fish. In: Bernier NJ, Kraak GVD, Farrell AP, Colin JB (eds) Fish physiology. Academic, pp 313–364
Waring CP, Stagg RM, Poxton MG (1992) The effects of handling on flounder (Platichthys flesus L.) and Atlantic salmon (Salmo salar L.) J Fish Biol 41:131–144
Waring CP, Stagg RM, Poxton MG (1996) Physiological responses to handling in the turbot. J Fish Biol 48:161–173
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Whyte SK (2007) The innate immune response of finfish—a review of current knowledge. Fish Shellfish Immunol 23:1127–1151
Yoo G, Lee S, Kim YC, Okorie OE, Park GJ, Han YO, Choi S-M, Kang J-C, Sun M, Bai SC (2007) Effects of dietary β-1,3 glucan and feed stimulants in juvenile olive flounder, Paralichthys olivaceus. J World Aquacult Soc 38:138–145
Zheng K, Liang M, Yao H, Wang J, Chang Q (2013) Effect of size-fractionated fish protein hydrolysate on growth and feed utilization of turbot (Scophthalmus maximus L.) Aquac Res 44:895–902
Acknowledgements
Sincere thanks to Michael Lutz (Köster Marine Proteins) and Matthias Seidel (J. Müller Weser) for supporting this study. Furthermore, special thanks to the scientific and technical staff of AWI and TiHo for the helpful work during the project. The authors wish to thank the anonymous reviewers for their constructive help in improving the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Federal Ministry of Food and Agriculture (BMEL, “Programm Innovationsförderung”) via the Federal Office for Agriculture and Food (BLE; no. 2817304110).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Fuchs, V.I., Schmidt, J., Slater, M.J. et al. Influence of immunostimulant polysaccharides, nucleic acids, and Bacillus strains on the innate immune and acute stress response in turbots (Scophthalmus maximus) fed soy bean- and wheat-based diets. Fish Physiol Biochem 43, 1501–1515 (2017). https://doi.org/10.1007/s10695-017-0388-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0388-6