Abstract
The present study was conducted to investigate the effects of dietary supplementation of Bacillus coagulans on growth, feed utilization, digestive enzyme activity, innate immune response and disease resistance of freshwater prawn Macrobrachium rosenbergii. Three treatment groups (designated as T1, T2 and T3) and a control group (C), each in triplicates, were established. The prawn in the control were fed a basal diet and those in T1, T2 and T3 were fed basal diet containing B. coagulans at 105, 107 and 109 cfu g−1, respectively. After 60 days, growth performance and feed utilization were found significantly higher (P < 0.05) in prawn fed T3 diet. The specific activities of protease, amylase and lipase digestive enzymes were significantly higher (P < 0.05) for T3. Innate immunity in terms of lysozyme and respiratory burst activities were significantly elevated (P < 0.05) in all the probiotic treatment groups as compared to control. Challenge study with Vibrio harveyi revealed significant increase (P < 0.05) in disease resistance of freshwater prawn in T2 and T3 groups. The results collectively suggested that supplementation of B. coagulans as probiotic in the diet at approximately 109 cfu g−1 can improve the growth performance, feed utilization, digestive enzyme activity, innate immune response and disease resistance of freshwater prawn.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Macrobrachium rosenbergii, the giant freshwater prawn is considered as a candidate species among the crustaceans for aquaculture in different parts of tropical and subtropical regions. It has cultural importance due to its fast growth rate, high demand in national and international markets and tolerance to aquatic environmental condition (Ranjeet and Kurup 2002; New 2005; Gupta et al. 2007).
In large-scale production facilities, aquatic animals are exposed to stressful conditions. The increased intensity of aquaculture has led to a high number of disease outbreak with an increasing range of pathogens as a result in serious economic losses (Verma and Gupta 2015). The abuse of disinfectants, pesticides, and antimicrobial drugs has caused the evolution of resistant strains of bacteria and concern of the society (Esiobu et al. 2002). Probiotic is the use of microbial supplements to benefit their host (Fuller 1989). Thus, the use of microorganisms as probiotics in the culture of aquatic organisms is increasing with the demand for more environment-friendly aquaculture practice (Gatesoupe 1999).
The most commonly used probiotics in aquaculture belong to gram-positive spore-forming Bacillus spp. (Wang et al. 2008; Gupta and Dhawan 2011, 2013; Gupta et al. 2014). Bacillus preparations are resistant to the environment, have a long lasting shelf life and the beneficial roles of these bacterial species in the aquaculture field are well established (Gatesoupe 1999; Wang et al. 2008). Within the Bacillus genus, Bacillus coagulans has potent biocontrol ability against a number of fish pathogens which are administered by enrichment of live feeds, added to the diets or to culture water (Panigrahi et al. 2005; Kumar et al. 2006; Kim and Austin 2006; Gupta et al. 2014). The effects of probiotic B. coagulans have been linked to modulation of gut microbiota and establishment of the beneficial microorganisms, higher specific and total digestive enzyme activities in the brush-border membrane which increases the nutrient digestibility and feed utilization (Balcazar et al. 2006; Kesarcodi-Watson et al. 2008). The digestive enzymes in fish have been studied by several workers (Kawai and Ikeda 1972; Das and Tripathi 1991). Furthermore, the immunostimulatory effects of these probiotic bacteria against Aeromonas hydrophila infection in fish have been demonstrated (Aly et al. 2008; Kumar et al. 2008; Zhou et al. 2010; He et al. 2011). However, there is lack of studies regarding the use of spore-forming lactic acid-producing bacteria such as B. coagulans as a probiotic in freshwater prawn.
Keeping this in view, the principal objective of the current study was to evaluate the effects of dietary administration of B. coagulans on the changes in digestive enzyme activities and innate immune responses of M. rosenbergii. In addition, effect on water quality, growth performance and survival was also investigated. To the best of our information, the present study is the first to evaluate the potential of probiotic B. coagulans in relation to growth performance, feed utilization, digestive enzyme activity and immune response in commercially important crustacean M. rosenbergii.
Materials and methods
Probiotic bacteria
Bacillus coagulans strain (MTCC 9872) was procured from the Indian Institute of Microbial Technology, Chandigarh, India, and used as potential probiotic. The strain was revived in nutrient broth for 48 h at 37 °C. To confirm the purity of strain, colonies were identified on the basis of their morphological, Gram’s staining and biochemical characteristic using bacterial identification kit (HiMedia, India). Cell density was calculated using spectrophotometer (Hach DR2800, USA) at 600 nm and values were correlated with colony-forming unit (CFU) counts using serial dilution and spread plating on their respective agar. The quantified bacteria were centrifuged, and suspensions were made with phosphate-buffered saline (PBS, pH 7.5). The suspended form was used for feed preparation as required.
Antagonism study and evaluation of possible harmful effect of probiotic strain on prawn
Agar well diffusion test was conducted to study antagonistic activity of B. coagulans following a previously described method (Balcazar et al. 2007) with some modification (Gupta et al. 2014). The antagonistic activity was examined against indicator pathogenic bacteria like A. hydrophila (MTCC 1739) and Vibrio harveyi (MTCC 7771). For the determination of possible harmful effect of B. coagulans, the culture grown in broth was centrifuged at 1000g for 10 min at 4 °C, washing twice and re-suspending in PBS. Volume (20 µl) of each suspension containing approximately 105 cells ml−1, as determined by a haemocytometer slide at a magnification of 400 on a Leica semi-motorized research microscope (DM3000; Germany), was injected into the third abdominal segment into group of 20 prawns. Another group of twenty prawns were injected with normal saline solution (NSS), as controls, to observe the symptoms of diseases. After 10 days, survivors were examined for evidence of disease.
Experimental diets
The formulation and proximate analysis of the basal diet is presented in Table 1 and was prepared according to Gupta and Dhawan (2012) and Gupta et al. (2014). The basal diet was used as control (C). In the three experimental diets T1, T2, and T3, probiotic B. coagulans suspension was added at a final dose of 105, 107 and 109 cfu g−1, respectively. To achieve accurate final concentrations in the diet, the bacterial suspension was slowly added to dough, with gradual mixing in the laminar airflow chamber. Each diet was then passed through a mincer, and the resulting pellets were dried in an oven using an air blower at 37 °C until the moisture levels were around 10 %. After drying, the pellets were broken up and sieved into pellets of approximate size and stored at −20 °C in sealed plastic ziploc bags until used.
The survival of the supplemented bacteria in the diet was assessed following storage at 4 °C on weekly basis for two weeks. One gram of the diet was homogenized in 9.0 ml of sterile saline solution, and serial dilutions to 10−5 were prepared and 0.2 ml was spread onto duplicate plates of nutrient agar. The colonies were counted after incubation for 24–48 h at 30 °C. Based on the data of survival of probiotic, feeds were prepared on weekly basis to ensure high probiotic levels in the supplemented feed.
Experimental animal and conditions
Live specimens of healthy freshwater prawn were provided by the College of Fisheries, GADVASU, Ludhiana (Punjab), India, and the experiment was conducted at the same place. Twelve experimental fibreglass tanks of 500-l capacity were prepared before stocking of prawn. A 5-in.-thick layer of soil was spread on the bottom of each tank to facilitate breakdown of organic matter. Over the soil, lime was applied at the rate of 150 kg ha−1 seven days before the tanks were filled with water to a depth of 20 cm. The tanks were also provided with PVC pipes of 10 × 20 cm size as a substrate to increase the surface area for prawn. Two hundred and forty juvenile prawns were weighted, randomly distributed in tanks containing freshwater and acclimatized for seven days prior to the experiment.
One group served as the control (C) and was fed basal diet during the entire experimental period. The other three groups were fed B. coagulans-supplemented diets at three different doses 105 (T1), 107 (T2) and 109 (T3) cfu g−1 feed, until the end of the experiment. Experiment was conducted in triplicate for 60 days. Prawns were fed their specific diets twice a day at 5 % of the body weight. The mortality rate of prawn in each tank was recorded daily.
Water quality analysis
Temperature, pH, dissolved oxygen (DO), free carbon dioxide (FCO2), ammonium (NH4-N) and nitrite (NO2-N) as water quality parameters were measured fortnightly before water exchange by following the standard procedures (APHA 1989).
Biometry data
At the end of 60 day experiment, the final body weight, weight gain, specific growth rate, survival, feed conversion ratio (FCR) and protein efficiency ratio (PER) were calculated by using following formulae as described by Gupta et al. (2007):
Digestive enzyme assay
Five prawns from each tank were randomly collected for digestive enzyme assay after the termination of experimental trial. The gastrointestinal tract (GIT) of each prawn was removed aseptically and then immediately packed and stored in −20 °C until analysis. Enzymatic extracts were prepared according to Ding et al. (2004). Briefly, the prawns were rinsed with distilled water and deshelled. A brief cut was given to obtain intact GIT at 4 °C and rinsed with cold distilled water. The total GIT content was homogenized in PBS (phosphate-buffered saline, pH 7.5; 1:10) in a handheld homogenizer at 4 °C, and homogenates were centrifuged at 5000×g for 20 min at 4 °C. The supernatant was retained and stored at −20 °C to analyze different enzymes.
The total protein content was measured using in diluted homogenates following the Lowry method (Lowry et al. 1951) using bovine serum albumin as a standard. Protease activity was evaluated according to Anson (1938) using Folin phenol reagent, and amylase activity was measured according to Jiang (1982) and Worthington (1993) using iodine solution to reveal non-hydrolysed starch. Lipase activity was determined based on the measurement of fatty acid released due to enzymatic hydrolysis of triglycerides in stabilized emulsion of olive oil (Borlongan 1990; Jin 1995). Enzyme activities were measured as the change in absorbance using spectrophotometer and expressed as specific activity (U mg−1 protein).
Immune assay
For innate immune response, six prawns were randomly removed from each tank at the end of experiment. Haemolymph (50–100 µl) was withdrawn from the ventral sinus at the base of the first abdominal segment of each prawn into a 26-G needle and a 1-ml syringe containing an anticoagulant solution (13 mM tri-sodium citrate, 0.34 M sodium chloride and 10 mM EDTA-Na2, at a pH of 7.55 with osmolality adjusted to 780 mosM kg−1 using 0.115 M glucose) (Liu and Chen 2004). The anticoagulant–haemolymph of prawns was pooled and gently mixed in sterile eppendorf tube immediately. All assays of immune parameters were conducted in triplicates.
Lysozyme activity
The lysozyme activity level was measured using the turbidimetric assay following Sankaran and Gurnani (1972). Lysozyme activity was expressed as units/ml, where one unit was defined as the reduction in absorbance of 0.001/min. Lyophilized hen egg white lysozyme (HiMedia, India) was used to develop a standard curve.
Respiratory burst
The respiratory burst or super oxide generation (O2 −) assay of haemolymph was carried out following the protocol of Anderson and Siwicki (1995) using nitroblue tetrazolium (HiMedia, India) as standard. The optical density (OD) of the supernatant was measured at 540 nm in the microplate reader (Model: Infinite M200 PRO, Tecan, Switzerland). N,N-dimethyl formamide (Qualigens, Fisher Scientifics, India) was used as blank. The results were expressed as an increase in absorbance 100 µl HL−1.
Experimental infection/bacterial challenge
After 60 days of the feeding period, an experimental infection was induced in prawn with the pathogenic bacterium, V. harveyi (MTCC 7771). V. harveyi was grown overnight in nutrient broth, and the concentration was adjusted to 107 cfu ml−1 using NSS. A total of sixty prawn (five from each replicate) in the intermoult stage were collected from the treatment and control groups and injected with 20 µl of the bacterial suspension into the ventral sinus of the third abdominal segment resulting 106 cfu prawn−1. Immediately after injection, prawns were transferred into the 50-l glass aquaria. The experiment was conducted in duplicate. A group of untreated prawn with B. coagulans-supplemented diet, which was injected with NSS (20 µl), served as positive/unchallenged control (PC). During the experimental infection, prawns were fed their specific diets as previously described. The mortality was monitored daily for up to 7 days.
Statistical analysis
Data obtained from the present experiment were analysed using one-way analysis of variance (ANOVA) to determine if significant differences (P < 0.05) existed among treatment means. The percentage date were transformed to square-root arcsine values before the statistical analysis. Tukey’s test was used to compare means between individual treatments. Statistical analyses were performed using SPSS for Windows version 16.0.
Results
Safety of probiotic strain
The results of the in vitro antagonistic study showed that the B. coagulans induced an inhibition zone against A. hydrophila and V. harveyi of 4.1 and 6.5 mm, respectively. The results indicated that the probiotic strain have potential antagonistic activity against pathogenic bacteria. There were no signs of disease in probiotic-injected prawn as neither mortalities nor morbidities were observed during 10 days of probiotic-challenge examination. This confirms the non-pathogenicity of B. coagulans in freshwater prawn.
Water quality
The effect of probiotic, B. coagulans, on water quality parameters in freshwater prawn tanks are presented in Table 2. The average temperature, pH and DO in treated tanks ranged from 27.9 ± 2.8 to 28.6 ± 3.1 °C, 7.7 ± 2.9 to 7.8 ± 2.5 and 7.4 ± 2.1 to 7.6 ± 3.9 mg l−1, respectively, while that of control were 28.6 ± 2.5 °C, 7.8 ± 2.8 and 7.3 ± 2.4 mg l−1, respectively. There was no significant difference among control and probiotic-treated tanks (P > 0.05) during the experimental period in terms of temperature, pH and DO. Free carbon dioxide (FCO2) remained nil throughout the experimental period in all treatments including control. Moreover, no significant difference were observed (P > 0.05) throughout the experimental period in NH4-N concentration although the higher level was determined in control compared with T1, T2 and T3. As for the NO2-N concentration, analysis showed no difference (P > 0.05) in group treated with B. coagulans, as compared with the control although NO2-N concentration in T3 had a relatively lower content (Table 2).
Survival, growth and feed utilization
The growth performance and associated nutritional indices are presented in Table 3. Prawn fed with B. coagulans showed significantly higher (P < 0.05) survival rate over the control (80.0 ± 11.5 %) after 60 days of culture. After 60 days of feeding, the growth performance and feed utilization of the prawn groups fed probiotic incorporated diet was significantly improved compared to those of prawn fed control diet. Inclusion of probiotic in diet significantly (P < 0.05) enhanced final body weight, specific growth rate, protein efficiency ratio as compared to control. Prawn fed C (control) diet showed lowest significant final body weight, specific growth rate and protein efficiency ratio (Table 3). Improved non-significant differences were found in feed conversion ratio of prawn fed probiotic-supplemented diets; however, inferior significant value was recorded in control group.
Digestive enzyme activity
Specific protease activity in prawn was significantly different between probiotic treatments and control (Table 4). The highest protease activity was observed in T3 followed by T1 and T2. There was no significant difference (P < 0.05) between T2 and control treatments in protease activity. Amylase activity in T3 was significantly higher (P < 0.05) than T2 and T1. As for the lipase activity of prawn, assay showed significant difference (P < 0.05) in treatments with different levels of B. coagulans over the control. However, a concentration-dependant response of probiotic strains in T3 and T1 was not observed in the present study. Also, there was no significant difference (P < 0.05) in lipase activity between the T1 and T2.
Immune response
The innate immune response parameters are presented in Table 5. There was no significant difference among probiotic fed dietary treatments in lysozyme activity (P > 0.05). Prawn group fed diet supplemented with B. coagulans exhibited the highest lysozyme activity than control. The respiratory burst activities (RBA) in the haemolymph showed significant differences among the experimental groups. Prawn fed probiotic-supplemented diets showed significant higher RBA than control (C) fed basal diet only. However, the differences among the probiotic fed prawn were insignificant (P > 0.05).
Resistance against infection
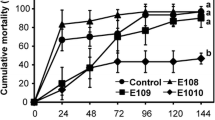
The challenge test revealed that long-term dietary administration of B. coagulans-supplemented diet enhanced the resistance of M. rosenbergii to infection (Fig. 1). Prawn mortality after injection of V. harveyi increased significantly (P < 0.05) with time and observed after 24 h of post injection and onwards. Significant higher post-challenge survival (P < 0.05) was observed in the prawn fed diet containing B. coagulans. The prawn fed the control diet exhibited the lowest survival (33.33 %) followed by T1 (46.67 %); however, highest was observed in positive/unchallenged control (93.33 %) (Fig. 1).
Survival rate of freshwater prawn M. rosenbergii fed diet containing different levels of B. coagulans as probiotic after challenge with V. harveyi. Control (C): prawn fed with basal diet. T1, T2 and T3: prawn fed with basal diet supplemented with different levels of B. coagulans @ 105, 107 and 109 cfu g−1 feed, respectively. PC: positive/unchallenged control. Data are presented as mean ± SE. Different superscripts indicate statistically significant differences (P < 0.05) between treatments
Discussion
Bacillus species are gaining more and more importance and are widely used in aquaculture due to their longer stability, easy preparedness, antagonistic effects on pathogens and enhancement of immunity (Hong et al. 2005; Gupta et al. 2014). The results of the in vitro antibacterial activity of the probiotic strain used against the pathogenic A. hydrophila and V. harveyi revealed that B. coagulans has probiotic potential. Previous works have established that Bacillus like B. coagulans and B. licheniformis and Paenibacillus like P. polymyxa and P. thiaminolyticus produce antibacterial compounds, such as polymyxin, octopytin and baciphelacin (Slepecky and Hemphill 1992).
It is necessary to provide freshwater prawn with a healthy environment, and probiotics has a great deal of potential (Zhou et al. 2009). Zhou et al. (2009) found that the treatment of P. monodon with different levels of B. coagulans did not significantly affected water quality compared with the control. However, Wang et al. (2005) investigated the effect of commercial probiotic on water quality in P. vannamei ponds, and the results showed that probiotics could significantly reduce the concentrations of nitrogen and phosphorous in pond water. In this study, the use of B. coagulans in freshwater prawn as feed additive had shown inconsistent results. In contrast, there was no obvious effect of probiotic on the water quality during the study. This result may be explained by the good water quality and culture conditions in this study in contrast to theirs. In fact, the pH and DO values and concentrations of ammonium and nitrite determined in this study were stable and within acceptable ranges, as required for the culture of freshwater prawn (Gupta et al. 2007; Gupta and Dhawan 2013).
The supplementation of B. coagulans in the diet resulted in improved growth performance of freshwater prawn. These results are supported by feeding of Bacillus spp. in the Indian white shrimp (Fenneropenaeus indicus) (Ziaei-Nejad et al. 2006); pacific white shrimp (Litopenaeus vannamei) (Wang 2007; Far et al. 2009; Wang and Gu 2010); M. rosenbergii (Keysami et al. 2007; Gupta and Dhawan 2012, 2013); and Penaeus monodon (Rahiman et al. 2010). Kumar et al. (2012) observed no significant differences in the survival, whereas significant increase in growth parameters and decrease in FCR were observed in M. rosenbergii fed diets supplemented with different concentrations of B. licheniformis. Rahiman et al. (2010) reported an increase in the weight gain and SGR in M. rosenbergii fed diet supplemented with Bacillus species without any significant increase in the survival. In contradiction with our results, Shariff et al. (2001) found that the treatment of P. monodon with a commercial Bacillus probiotic did not significantly increase survival. However, it is difficult to directly assess different studies using probiotics, because the efficacy of a probiotic application depended on many factors (Lara-Flores 2011; Wang et al. 2012) such as species composition and viability, administration level, mode and frequency of application, diet composition, prawn age and environmental stress factors.
Prawn fed diet supplemented with B. coagulans showed remarkable increase in digestive enzyme activities. Lovett and Felder (1990) and Kamarudin et al. (1994) investigated that the shrimp digestive system was activated during larval and post-larval stages, where the probiotics would have the utmost effect. Moreover, a wide variety of exoenzymes are secreted by bacteria, predominantly by members of the genus Bacillus (Moriarty 1996, 1998). Furthermore, the exogeneous enzymes secreted by the probiotic bacteria would contribute a little to the total enzyme activity of the gut (Ding et al. 2004; Ziaei-Nejad et al. 2006). Hence, we could not distinguish between activity due to exoenzymes synthesized by probiotic bacteria and activity due to endoenzymes synthesized by prawn. In the present study, different concentrations of probiotic had different consequences on enzyme activity. The protease, amylase and lipase activity increased significantly with increase in concentration of probiotic. The higher level of enzyme activity obtained with diets containing B. coagulans might have improved the digestion of protein, starch, fat and cellulose, which in turn led to enhanced digestion and proper absorption of food that serves as the reason for better growth observed with probiotic-supplemented diets.
Lysozyme in serum prevents adherence and colonization of microorganisms, thus helps in circumvent the disease (Alexander and Ingram 1992). In the present study, tremendous increase of lysozyme activity were demonstrated in all treatments fed Bacillus spp. compared to control. Similar results were obtained by Gullian et al. (2004) using Bacillus, as a probiotic, that showed better immunostimulatory features in L. vannamei. Our study showed that prawn groups fed with different levels of probiotic had significantly (P < 0.05) higher respiratory bursts than the control group, confirming that innate immune response was enhanced in prawn fed diets containing B. coagulans. We assume that B. coagulans inhabits for long term in probiotic-treated prawn guts and hence provides a long-term immune stimulation for freshwater prawn. Similar trend in stimulation of respiratory burst activity after dietary probiotic supplementation has been reported in M. rosenbergii fed Bacillus spp. (Rahiman et al. 2010) and L. vannamei fed Arthrobacter species (Li et al. 2008).
Probiotics help in achieving natural resistance and high survivability of fish (Abraham et al. 2007). The dietary supplementation of B. coagulans in the present study showed good antibacterial activity against V. harveyi and significantly increased survival rate of freshwater prawn after challenged with pathogenic bacteria. In a previous study, Bacillus administration has also been shown to increase survival by enhancing resistance to pathogens by acting both cellular and humoral immune defence in shrimp and prawn (Rengpipat et al. 2000; Gupta and Dhawan 2013). Bacillus surface antigens or their metabolites were also reported to act as immunogens for shrimp by stimulating the phagocytic activity of granulocytes (Itami et al. 1998). Protection was achieved in eel (Anguilla anguilla) and Indian carp (Catla catla) against A. hydrophila infection after the fish were fed with diet supplemented with B. amyloliquefaciens (Cao et al. 2011; Das et al. 2013). Such enhancement in prawn disease resistance can be partially due to the facilitated innate immune responses of fed prawn where significantly higher lysozyme and RBA (P < 0.05) level were obtained. The results indicate the involvement of an activated and prolonged immune status of freshwater prawn to resist V. harveyi infection.
Conclusion
The results of present study demonstrated that the use of B. coagulans at approximately 109 cfu g−1 as a dietary probiotic could significantly improve survival rate, growth performance, feed utilization and digestive enzyme activity of freshwater prawn M. rosenbergii. The negative environment effects on freshwater prawn could thus be mitigated through the use of B. coagulans, which is known to enhance immune activities and disease resistance against V. harveyi as seen in the present investigation.
References
Abraham TJ, Babu CHS, Mondal S, Banerjee T (2007) Effects of dietary supplementation of commercial human probiotic and antibiotic on the growth rate and content of intestinal microflora in ornamental fishes. Bangladesh J Fish Res 11:57–63
Alexander JB, Ingram GA (1992) Noncellular nonspecific defence mechanisms of fish. Ann Rev Fish Dis 2:249–279
Aly SM, Abdel-Galil Ahmed Y, Abdel-Aziz Ghareeb A, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25:128–136
Anderson DP, Siwicki AK (1995) Basic haematology and serology for fish health programs. In: Shariff M, Authur JR, Subasinghe RP (eds) Diseases in asian aquaculture II. Phillipines Fish Health Section, Asian Fisheries Society, Manila, pp 185–202
Anson ML (1938) The estimation of pepsin, trypsin, papain and cathepsin with hemoglobin. J Gen Physiol 22:79–89
APHA (1989) Standard methods for the examination of water and waste water, 19th edn. American Public Health Association, Washington
Balcazar JL, Decamp O, Vendrell D, De Blas I, Ruiz-Zarzuela I (2006) Health and nutritional properties of probiotics in fish and shellfish. Microb Ecol Health Dis 18:65–70
Balcazar JL, Rojas-Luna T, Cunningham DP (2007) Effect of the addition of four potential probiotic strains on the survival of Pacific white shrimp (Litopenaeus vanamei) following immersion challenge with Vibrio parahaemolyticus. J Invertebr Pathol 96:147–150
Borlongan IG (1990) Studies on the digestive lipases of milkfish, Chanos chanos. Aquaculture 89:315–325
Cao H, He S, Wei R, Diong M, Lu L (2011) Bacillus amyloliquefaciens G1: a potential antagonistic bacterium against eel-pathogenic Aeromonas hydrophila. Evid Based Complement Altern Med 7 Article ID 824104. doi:10.1155/2011/824104
Das KM, Tripathi SD (1991) Studies on the digestive enzymes of grass carp, Ctenopharyngodon idella (V). Aquaculture 92:21–32
Das A, Nakhro K, Chowdhury S, Kamilya D (2013) Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish Shellfish Immunol 35:1547–1553
Ding X, Li ZJ, Chen YQ, Lin HZ, Yang YY, Yang K (2004) Effects of probiotics on growth and activities of digestive enzymes of Pennaus vannamei. J Fish Sci China 11:580–584
Esiobu N, Armenta L, Ike J (2002) Antibiotic resistance in soil and water environments. Int J Environ Heal Res 12:133–144
Far HZ, Saad CRB, Daud HM, Harmin SA, Shakibazadeh S (2009) Effect of Bacillus subtilis on the growth and survival rate of shrimp (Litopenaeus vannamei). Afr J Biotechnol 14:3369–3376
Fuller R (1989) Probiotics in man and animals. J Appl Bacteriol 66:365–378
Gatesoupe FJ (1999) The use of probiotics in aquaculture. Aquaculture 180:147–165
Gullian M, Thompson F, Rodrıguez J (2004) Selection of probiotic bacteria and study of their immunostimulatory effect in Penaeus vannamei. Aquaculture 233:1–14
Gupta A, Dhawan A (2011) Effect of supplementing probiotics (Prosol) on the performance of giant freshwater prawn (Macrobrachium rosenbergii) juveniles. Indian J Anim Nutr 28:457–463
Gupta A, Dhawan A (2012) Effect of dietary probiotic Improval (Lactobacillus sporogenes and Saccharomyces cerevisiae) on growth and feed utilization of Macrobrachium rosenbergii post larvae. Anim Nutr Feed Tech 12:209–217
Gupta A, Dhawan A (2013) Probiotic based diets for freshwater prawn Macrobrachium rosenbergii (de Man). Indian J Fish 60:103–109
Gupta A, Sehgal HS, Sehgal GK (2007) Growth and caracass composition of giant freshwater prawn, Macrobrachium rosenbergii (De Man), fed different isonitrogenous and isocaloric diets. Aquac Res 38:1355–1363
Gupta A, Gupta P, Dhawan A (2014) Dietary supplementation of probiotics affects growth, immune response and disease resistance of Cyprinus carpio fry. Fish Shellfish Immunol 41:113–119
He S, Liu W, Zhou Z, Mao W, Ren P, Marubashi T (2011) Evaluation of probiotic strain Bacillus subtilis C-3102 as feed supplement for koi carp (Cyprinus carpio). J Aquac Res Dev S 1:005. doi:10.4172/2155-9546.S1-005
Hong HA, le Duc H, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Microbiol Rev 29:813–835
Itami T, Asano M, Tokushige K, Kubono K, Nakagawa A, Takeno N, Nishimura H, Maeda M, Kondo M, Takahashi Y (1998) Enhancement of disease resistance of kuruma shrimp, Penaeus japonicus after oral administration of peptidoglycan derived from Bifidobacterium thermophilum. Aquaculture 164:277–288
Jiang CK (1982) Activity measuring for implemental enzyme. Science and Technology Press, Shanghai
Jin ZL (1995) Evaluation principle and method of functional food. Beijing Publishers, Beijing
Kamarudin MS, Jones DA, Vay L, Abidin AZ (1994) Ontogenetic changes in digestive enzyme activity during larval development of Macrobrachium rosenbergii. Aquaculture 123:323–333
Kawai S, Ikeda S (1972) Studies on digestive enzymes of fishes. Effect of dietary change on the activities of digestive enzymes in carp intestine. Bull Jpn Soc Sci Fish 38:265–270
Kesarcodi-Watson A, Kaspar H, Lategan MJ, Gibson L (2008) Probiotics in aquaculture: the need, principles and mechanisms of action and screening processes. Aquaculture 274:1–14
Keysami MA, Saad CR, Sijam K, Daud HM, Alimon AR (2007) Effect of Bacillus subtilis on growth development and survival of postlarvae Macrobrachium rosenbergii (de Man). Aquacult Nutr 13:131–136. doi:10.1111/j.1365-2095.2007.00463.x
Kim DH, Austin B (2006) Innate immune responses in rainbow trout (Oncorhynchus mykiss, Walbaum) induced by probiotics. Fish Shellfish Immunol 21:513–524
Kumar R, Mukherjee SC, Prasad KK, Pal AK (2006) Evaluation of Bacillus subtilis as a probiotic to Indian Major carp Labeo rohita (Ham.). J Aquacult Res 37:1215–1221
Kumar R, Mukherjee SC, Ranjan R, Nayak SK (2008) Enhanced innate immune parameters in Labeo rohita (Ham.) following oral administration of Bacillus subtilis. Fish Shellfish Immunol 24:168–172
Kumar NR, Raman RP, Jadhao SB, Brahmchari RK, Kumar K, Dash G (2012) Effect of dietary supplementation of Bacillus licheniformis on gut microbiota, growth and immune response in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879). Aquacult Int. doi:10.1007/s10499-012-9567-8
Lara-Flores M (2011) The use of probiotic in aquaculture: an overview. Int Res J Microbiol 2:471–478
Li JQ, Tan BP, Mai KS, Ai QS, Zhang WB, Liufu ZG, Xu W (2008) Immune response and resistance against Vibrio parahaemolyticus induced by probiotic bacterium Arthrobacter XE-7 in pacific white shrimp, Litopenaeus vannamei. J World Aquac soc 39:477–489
Liu CH, Chen JC (2004) Effect of ammonia on the immune response of white shrimp Litopenaeus vannamei and its susceptibility to Vibrio alginolyticus. Fish Shellfish Immunol 16:321–334
Lovett DL, Felder DL (1990) Ontogenetic change in digestive enzyme activity of larval and postlarval white shrimp Penaeus setiferus (Crustacea, Decapoda, Penaeidae). Biol Bull 178:144–159
Lowry OH, Rosenbrough WJ, Fair AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Moriarty DJW (1996) Microbiology biotechnology: a key ingredient for sustainable aquaculture. Info Fish Int 4:29–33
Moriarty DJW (1998) Control of luminous Vibrio species in penaeid aquaculture ponds. Aquaculture 164:351–358
New MB (2005) Freshwater prawn farming: global status, recent research and a glance at the future. Aquac Res 36:210–230
Panigrahi A, Kiron V, Puangkaew J, Kobayashi T, Satoh S, Sugita H (2005) The viability of probiotic bacteria as a factor influencing the immune response in rainbow trout Oncorhynchus mykiss. Aquaculture 243:241–254
Rahiman KMM, Jesmi Y, Thomas AP, Hatha AAM (2010) Probiotic effect of Bacillus NL110 and Vibrio NE17 on the survival, growth performance and immune response of Macrobrachium rosenbergii (de Man). Aquatic Res 41:120–134
Ranjeet K, Kurup BM (2002) Management strategies associating batch-graded and size-graded post larvae can reduce heterogeneous individual growth in Macrobrachium rosenbergii (de Man). Aquac Res 33:1221–1231
Rengpipat S, Runkpratanporn S, Piyatiratitivorakul S, Menasaveta P (2000) Immunity enhancement on black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture 191:271–288
Sankaran K, Gurnani S (1972) On the variation in the catalytic activity of lysozyme in fishes. Ind J Biochem Biophys 9:162–165
Shariff M, Yusoff FM, Devaraja TN, Rao SPS (2001) The effectiveness of a commercial microbial product in poorly prepared tiger shrimp, Penaeus monodon (Fabricius), ponds. Aquac Res 32:181–187
Slepecky RA, Hemphill HE (1992) The genus Bacillus-nonmedical. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer KH (eds) The Prokaryotes, 2nd edn. Springer, New York, pp 1663–1696
Verma G, Gupta A (2015) Probiotics application in aquaculture: improving nutrition and health. J Anim Feed Sci Tech 3:53–64
Wang YB (2007) Effect of probiotics on growth performance and digestive enzyme activity of the shrimp Penaeus vannamei. Aquaculture 269:259–264
Wang YB, Gu QQ (2010) Effect of probiotics on white shrimp (Penaeus vannamei) growth performance and immune response. Mar Biol Res 6:327–332
Wang YB, Xu ZR, Xia MS (2005) The effectiveness of commercial probiotics in Northern White Shrimp (Penaeus vanname iL.) ponds. Fish Sci 71:1034–1039
Wang YB, Li JR, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquaculture 281:1–4
Wang YB, Fu LL, Lin J (2012) Probiotic (Bacillus coagulans) cells in the diet benefit the white shrimp Litopenaeus vannamei. J Shellfish Res 31:855–860
Worthington V (1993) Worthington enzyme manual. Enzymes and related biochemicals. Worthington Chemical, New Jersey
Zhou X, WangY Li W (2009) Effect of probiotic on larvae shrimp (Penaeus vannamei) based on water quality, survival rate and digestive enzyme activities. Aquaculture 287:349–353
Zhou X, Tian Z, Wang Y, Li W (2010) Effect of treatment with probiotics as water additives on tilapia (Oreochromis niloticus) growth performance and immune response. Fish Physiol Biochem 36:501–509
Ziaei-Nejad S, Rezaei MH, Takami GA, Lovett DL, Mirvaghefi AR, Shakouri M (2006) The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture 252:516–524
Acknowledgments
This research work is financially supported by the University Grants Commission, New Delhi, in the form of Major Research Project (F. No. 41-87/2012-SR). The authors wish to thank the Dean, College of Fisheries, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, A., Verma, G. & Gupta, P. Growth performance, feed utilization, digestive enzyme activity, innate immunity and protection against Vibrio harveyi of freshwater prawn, Macrobrachium rosenbergii fed diets supplemented with Bacillus coagulans . Aquacult Int 24, 1379–1392 (2016). https://doi.org/10.1007/s10499-016-9996-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-9996-x