Abstract
Diseases caused by trypanosomatids include leishmaniasis (Leishmania spp.), Chagas disease (Trypanosoma cruzi), and sleeping sickness (Trypanosoma brucei) that affect millions of people, especially low-income populations, being classified as neglected tropical diseases. Limitations in the clinical treatment, associated with the huge number of cases, make these infections a health and socioeconomic problem worldwide. To complete their life cycle, trypanosomatids survive to environmental changes in different hosts, including oxidative stress. A paradoxal role of reactive oxygen species (ROS) has been proposed, such as signaling as a proliferation regulator or even presenting cytotoxic activity, depending on the concentration. Mitochondrial electron transport chain, especially complex III, is figured as one of the most important ROS resources in trypanosomatids. In relation to antioxidant defenses, trypanothione pathway plays a crucial role, being a peculiar thiol-redox system responsible for the maintenance of protozoa functions mediated by thiol-dependent processes. In this chapter, we discuss the biological aspects of oxidative stress in trypanosomatids and its implications for the success of the infection. The possible ROS resources in these protozoa and their consequent antioxidant machinery involved in detoxification were also focused in this review, including alternative strategies for the development of new drugs for these diseases based on oxidative stress modulation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The World Health Organization (WHO) defined neglected tropical diseases (NTDs) as a group of illnesses that affects low-income populations in tropical and subtropical areas, without the adequate sanitary conditions, reaching over than a billion people worldwide. These conditions which are far from the ideal, together with severe limitations in the current chemotherapy, led to high mortality and morbidity rates of these diseases in developing countries [1]. Trypanosomatid parasites are responsible for some of the most important NTDs. Leishmania species, Trypanosoma cruzi, and Trypanosoma brucei cause leishmaniasis, Chagas disease, and sleeping sickness, respectively [2], and the implication of oxidative metabolism in the success of these infections is the focus of this review.
1.1 Leishmania spp. and Leishmaniasis
Leishmaniasis is a complex of diseases caused by over 20 species of parasites of the genus Leishmania [3]. This disease is endemic in 98 countries, with more than 12 million people infected and 350 million in risk areas [4]. The parasite life cycle is digenetic, with two hosts: an invertebrate host (sandfly), being also the transmitter of the disease, and the vertebrate host (mammalian), including humans and animals such as rodents, canines, marsupials, primates, and others [5,6,7,8]. Leishmania is responsible for two major clinical manifestations, tegumentary and visceral leishmaniasis. The tegumentary form can be divided into cutaneous, diffuse, and mucocutaneous; however, it is the visceral form which can lead to death by affecting internal organs, such as the liver and spleen [9]. Leishmaniasis chemotherapy has been based over seven decades on pentavalent antimonials; however, even being the first choice of treatment in several countries, its side effects and longstanding therapy added to all the resistance reports led to alternative drug choices, such as amphotericin B, pentamidine, paramomycin, and miltefosine [10, 11]. Amphotericin B and its lipid formulation, which reduces side effects but has a higher cost, is the first-line therapy in some countries and an alternative in cases of antimonial failure. Nevertheless, along with pentamidine and paramomycin, there is a vast report of failure cases, with side effects, longstanding treatment, and resistance reports [9]. Miltefosine, the first oral drug for leishmaniasis, has been emerging in leishmaniasis therapeutic scenario, but the resistance case reports and teratogenic effects limit its use [12].
Leishmania biological cycle presents two major hosts and two forms. When the hematophagous sandfly host bites the infected mammal, it ingests a mixture of blood and phagocytic cells infected with amastigote forms, which are released upon cellular rupture. Inside the insect gut (midgut or hindgut, depending on the Leishmania subgenus), amastigotes differentiate as procyclic promastigotes and develop in the sandfly. Promastigotes migrate to the proboscis and differentiate into metacyclic promastigotes, the mammalian infective form. When the infected sandfly bites a mammalian host, it regurgitates promastigotes, which are phagocytized by mononuclear phagocytic cells. Once, inside these cells, promastigotes transform into amastigotes, that proliferates up to host cell rupture, being phagocytized by other phagocytic cells, disseminating the infection [13].
1.2 Trypanosoma cruzi and Chagas Disease
Chagas disease, which is caused by the hemoflagellate protozoa T. cruzi, is typically a Latin American endemic illness. With the reduction in the transmission by triatominae vector, other transmission routes emerge, such as contaminated food or liquid ingestion in Brazil and transfusional transmission in Europe and North America, due to an increase in the migratory flux of infected people [14, 15]. In relation to clinical manifestations, Chagas disease presents an acute phase defined by high parasitemia in the patient bloodstream and a chronic phase where severe cardiac and/or digestive alterations are observed in 30–40% of the infected individuals decades after the acute infection [16, 17]. Up to now, benznidazole and nifurtimox are the clinical options for Chagas disease chemotherapy, being strongly effective on acute cases. On the other hand, both nitroderivatives show severe side effects as well as important activity limitations in the symptomatic chronic patients, particularly. Differences in the susceptibility of the parasite stocks isolated from distinct areas to these drugs also emphasize the necessity of the development of alternative compounds [18, 19].
The complexity of T. cruzi biological cycle, with different parasite evolutive stages and two hosts, also contributes for the delay in the development of novel active trypanocidal compounds. During the bloodmeal, triatomines ingest trypomastigotes with blood of the infected host. Reaching the midgut, bloodstream forms differentiate into proliferative epimastigotes that colonizes the insect digestive tract. The migration of epimastigotes to the low nutrient and acid environment of the triatomine posterior rectum triggers a new differentiation process in the parasite, and nonreplicative metacyclic trypomastigotes present in the insect feces will infect the vertebrate, invading host cells. In the intracellular environment, metacyclic forms differentiate into amastigotes that proliferates quickly. A new intracellular differentiation occurs to trypomastigotes that disrupt host cell and disseminate the infection by bloodstream [20, 21].
1.3 Trypanosoma brucei and Sleeping Sickness
Sleeping sickness, which is caused by the trypanosomatid T. brucei rhodesiense or T. brucei gambiense, is a disease restricted to sub-Saharan Africa, where the vector tsetse flies (Glossina spp.) is present. The transmission occurs by the insect bite, being T. b. gambiense the most abundant infection (98% of all cases), which is especially distributed in the Democratic Republic of Congo [22]. An estimation of WHO pointed to 70 million people under risk of infection and about 20,000 new cases per year [23,24,25]. Clinical manifestations of sleeping sickness mainly involve cognitive impairment, including mental confusion, personality alterations, and seizures, among others, deriving from the direct injury caused by the parasite presence in central nervous system of the host, being present for many years or months in T. b. gambiense or T. b. rhodesiense infections, respectively [26]. Unfortunately, this disease progression can lead to the patient death, if untreated. Due to the variety of illness stages and parasite subspecies, adaptations in chemotherapy approach are critical. Suramin and pentamine are the first-line drugs for early phase of T. b. rhodesiense and T. b. gambiense infections, respectively. On the other hand, the treatment of later stages is more complicated, once the compounds need to cross the blood-brain barrier. Melarsoprol and eflornithine (only for T. b. gambiense) are the current drugs for this stage, sometimes in combination with nifurtimox; however, their high toxicity associated with difficulties in the administration encourages the search for alternative therapies [27,28,29].
The evasion of the host immune system by the parasite represents a crucial challenge for the efficacy of novel drugs. Variant surface glycoproteins (VSGs) of T. brucei mammalian stages are produced constantly, varying the composition of the protozoa surface coat and hampering their recognition by host phagocytic cells [30]. The biological cycle of T. brucei starts during the tsetse fly foraging, when metacyclic trypomastigotes are inoculated after the insect bite, reaching the mammalian bloodstream. The first differentiation step takes place, and long slender forms proliferate, maintaining the infection. Central nervous system, as well as different other tissues, are infected when this stage crosses the endothelia. In order to guarantee the parasite survival in the tsetse environment, long slender forms differentiate into short stumpy forms that will be ingested by the insect. In the midgut, a new differentiation process occurs, and procyclic trypomastigotes will colonize the digestive tract of the flies. The migration of procyclic forms to the salivary glands takes place, tissue where the last parasite differentiation step will occur. Infective metacyclic trypomastigotes presented in the insect saliva will reach vertebrate host during tsetse bloodmeal [31].
2 Oxidative Stress
The harmful consequences of free radicals production for biological systems and its implications in aging and diseases were proposed only half century after the first description of these species by Moses Gomberg [32]. Curiously, many years later, it was demonstrated that free radicals can also present a beneficial role for the cells and tissues, and it was postulated their involvement in the killing of pathogens, and help in the immune system [33, 34]. So, the participation of these molecules has been demonstrated in a great variety of biochemical pathways, acting as regulators [35].
Even today, the terms “reactive species” and “free radicals” are employed as synonyms, generating a little confusion. Free radicals are instable and represent reactive molecules with an unpaired electron in their orbital [36, 37]. Some reactive species does not present an unpaired electron, being not considered a free radical [38]. Reactive nitrogen species (RNS) comprehend nitric oxide (NO), nitrogen dioxide (NO2), peroxynitrite (ONOO–), and nitroxyl (HNO) that participate in many crucial cellular processes [39], while also reactive oxygen species (ROS) include superoxide anion (O2•–), hydrogen peroxide (H2O2), and hydroxyl radical (HO–). Here, we only focused on the mechanisms of oxidative stress and their detoxification in pathogenic trypanosomatids and also its implications for the infection success.
In oxidative conditions, antioxidant machinery is highly expressed in order to downregulate the reactive species to physiological levels. Indeed, when such balance is broken, the concentration of ROS and/or RNS becomes higher, defining the oxidative stress condition that presents different biological implications, depending on molecular targets oxidized and the efficacy of antioxidant defenses, among other factors [40, 41]. The increase in ROS generation usually promotes this disbalance; however, the decrease in levels of antioxidant enzymes, due to reduction in expression or inactivation, also contributed for the oxidative regulation [42]. Regardless of the causes, the availability of reactive species scavengers strongly modulates almost all of biochemical pathways [43].
In the oxidative context, among all organelles and cellular structures, the mitochondrion stands out. Nobel Prize winner, Peter Mitchel, in 1961, described the co-dependency of cellular respiration and oxidative phosphorylation, being the oxygen metabolism and the ATP production causes of each other [44,45,46]. In the mitochondrial cristae, oxidative phosphorylation takes place, being the proton electrochemical gradient generated by electron transport through the complexes, leading to ATP synthesis [47, 48]. In aerobic conditions, the great majority of the oxygen directly reduced to water by complex IV (cytochrome c oxidase) in an electron transport chain (ETC) [49]. The partial reduction of oxygen by ETC electrons leakage represents one of the main ROS sources in eukaryotic cells, usually occurring in complexes I and III, as well as in coenzyme Q [50]. Alternatively to ETC, other enzymatic reactions, such as those catalyzed by oxidases, also can represent important ROS resource. In ETC, coenzyme Q shows an oxidized (UQ) or a reduced state (partially reduced UQ– or fully reduced UQH2), becoming one of the largest ROS generators in the mitochondrion. It is directly associated with its role in oxidative phosphorylation, where coenzyme Q reduction by complexes I and II allows the electrons targeting to complex III, in a step dependent of the full reduction of ubiquinone. This process includes oxidized ubiquinone that is partially reduced to semiquinone (transference of only one electron). During the return to stable state, coenzyme Q needs to be reduced again, and such reduction occurs with the semiquinone formation. This reaction is not so quick; many molecules including oxygen could be also reduced by semiquinone, producing mitochondrial ROS [51,52,53]. Chronologically, O2•– is the first ROS generated during ETC electron leakage, derived from oxygen reduction by only one electron. Due to its unstability, superoxide anion is detected in low concentrations in cells under physiological conditions, situation where superoxide dismutase (SOD) and other specific antioxidant enzymes are expressed in order to detoxify these free radicals [41, 52].
After superoxide anion production, the next ROS generated is H2O2 by the O2•– reduction with two protons accepted, concomitantly. H2O2 does not present an unpaired electron, being more stable and less reactive than O2•–. Once such molecule is still more reactive than molecular oxygen, it is called ROS but it is not considered a free radical. Peroxidases and catalases are specific antioxidant enzymes involved in H2O2 removal [41]. In a new reduction step, HO– is produced from H2O2, a highly reactive free radical that interacts with more protons and electrons, generating water as a product from Fenton reaction [54]. For sure, HO– is the most damaging ROS, impairing a great variety of biological processes; however, due to its high instability, this molecule presents low half-life in cells compared to other ROS [41, 55, 56]. In summary, cellular physiology is directly affected by the consequences of these species production, damaging macromolecules, organelles, and structures, which can lead to phospholipids peroxidation, including in the plasma membrane, and causing subsequent cell rupture [57] (Fig. 8.1).
During catabolic pathways, reduced equivalents are produced and enter in the respiratory chain at complexes I and II and at type-II NADH dehydrogenases (DHII), reducing coenzyme Q (UQH2) pool in the inner membrane. Electrons from UQH2 are successively transported to complex III, to cytochrome C (C), and to complex IV, where they reduce molecular O2 to H2O. Electron transference through complexes III and IV is coupled to H+ translocation from the mitochondrial matrix to intermembrane space (IMS). This process induces the formation of an electrochemical gradient between both sides of the inner mitochondrial membrane, which is used to produce ATP from ADP and inorganic phosphate (Pi) by the ATP synthase. In T. brucei, the respiratory chain possesses an alternative oxidase (AOX) that catalyzes the reduction of O2 to H2O by UQH2. The electron transference between coenzyme Q and complex III or AOX is a process dependent to the full reduction of ubiquinone (UQH2). The partial reduction of this molecule generates a semiquinone (UQ−), an unstable molecule that can reduce O2, forming O2•−. Naturally (dashed arrows) or by the action of superoxide dismutase (SOD), O2•− is transformed into H2O2, which can be reduced by the action of peroxidases or can react with metal ions, such as Fe2+ in a Fenton reaction, producing HO−
2.1 Oxidative Metabolism in Pathogenic Trypanosomatids
The mitochondrial metabolic processes that occur in the majority of the organisms are also present in trypanosomatids, despite some specific peculiarities of this organelle [58] (Fig. 8.1). ETC exhibits unique features when comparing their enzymatic complexes to the canonical system. In higher eukaryotes, the complex I (NADH: ubiquinone oxidoreductase) contains up to 30 accessory subunits whose function remains largely unknown [59,60,61]. It catalyzes the transfer of electrons from NADH to ubiquinone, restoring the NAD+, with the concomitant translocation of four protons across the mitochondrial inner membrane [62]. In trypanosomatids, this complex consists in 19 subunits which were determined by proteomic analysis or deduced from genome sequence searches [63]. However, the functionality of complex I in these protozoa has been debated. Among the described subunits, all molecules known to participate in electron transfer are present, but four membrane subunits supposedly involved in proton translocation are missing. Indeed, NADH-dependent substrates are not able to stimulate ATP production in isolated mitochondrion [64]. Natural T. cruzi mutants which showed deletions in ND4, ND5, and ND7 genes coding for complex I subunits presented no significant differences in oxygen consumption, respiratory control ratio, and mitochondrial membrane potential in the presence of NADH-dependent substrates or FADH2-generating succinate. In mammals, the complex I is also a site of ROS production; however, H2O2 formation induced by different substrates was not associated to complex I subunit deletions, demonstrating that these mutations are not important for the control of oxidative burst in trypanosomatids [65]. In T. brucei, NADH-induced respiration is sensitive to the complex I inhibitor rotenone, at a higher concentration than that dose required for inhibiting this complex in other models [66, 67], which is suggestive of the inhibition of other electron carriers [68] or even a characteristic of an atypical complex I. Besides that, previous findings pointed to an increased mRNA levels of several kDNA encoded complex I subunits in bloodstream compared to T. brucei procyclic forms [69, 70], suggesting a more important role for complex I in this parasite stage. Nevertheless, RNAi knockdown of three subunits indicates that complex I is not required for normal culture growth of T. brucei procyclic forms [71], the same phenotype was observed in T. cruzi natural knockouts [65].
Trypanosomatids, as many other organisms, have alternative pathways to transfer electrons without concomitant proton translocation, such as type-II NAD(P)H dehydrogenases and alternative oxidases [57]. The contribution of the alternative NADH oxidizing enzymes to the entry point of electrons into the respiratory chain is not completely established. Type-II NAD(P)H dehydrogenases are single polypeptides that catalyze the transfer of two electrons from NAD(P)H to ubiquinone without coupled proton pumping [72, 73]. These enzymes are able to oxidize both NADH and NADPH produced either in cytosol or in the mitochondrial matrix, depending on the orientation of NADH binding site. In trypanosomatids, the alternative NADH dehydrogenase was first described in T. brucei, where a rotenone-insensitive NADH oxidation activity and superoxide production in procyclic forms were reported [74, 75]. Contrasting to the lack of phenotype observed for complex I mutants, the knockdown of type-II NAD(P)H dehydrogenases affects parasite growth and decreases mitochondrial membrane potential [71, 76, 77].
In most trypanosomatids, succinate is the major end product of glucose metabolism. This substrate of complex II (succinate:ubiquinone oxidoreductase) is produced in the glycosome and/or in the mitochondrial matrix by NADH-dependent fumarate reductases. These enzymes oxidize NADH generating succinate from fumarate [78,79,80,81]. The excretion of succinate probably indicates that the respiratory chain is not able to deal with the input of reducing equivalents, supporting the exclusive occurrence of oxidative phosphorylation from complex II to complex IV in trypanosomatids. Some data suggest that malonate, an inhibitor of complex II, impaired oxygen consumption when complex I and II substrates were added [64, 65, 82]. Complex II of T. cruzi epimastigotes and L. donovani promastigotes produce ROS after the treatment with thenoyltrifluoroacetone (TTFA), another inhibitor of complex II [83, 84].
The ubiquinol:cytochrome c oxidoreductase, or complex III, works similarly in trypanosomatids and other eukaryotes. This complex carries the electrons flow to cytochrome c, which reduces the complex IV. As well as in other organisms, trypanosomatid complex III is usually the major source of mitochondrial ROS, being responsible for O2•– formation in L. donovani promastigotes and T. cruzi epimastigotes induced by antimycin A, a classical inhibitor of this enzyme [83]. The only exception was T. brucei, in which this complex is not considered a potential ROS producer site [75]. Trypanosomatids possess two terminal oxidases: a classic complex IV KCN-sensitive and a KCN-insensitive alternative oxidase (AOX) [85,86,87]. The classic complex IV is similar to mammalian cytochrome c oxidase, transferring electrons from cytochrome c to the final acceptor, an oxygen molecule. This complex is important to mitochondrial functions, causing a decrease in this organelle membrane potential, reduced ATP production via oxidative phosphorylation, and redirected oxygen consumption to AOX when their indispensable subunits were repressed in T. brucei procyclic stage [88]. The impairment of cytochrome c oxidase also resulted in other severe mitochondrial phenotypes. Without active complex IV, the electrons flow through complex III is not able to completely reduce the oxygen. Therefore, the electrons flow from cytochrome-mediated pathway can be deviated to AOX. Gnipová et al. [88] also showed that knockdown of T. brucei complex IV subunits affects the complex III activity, suggesting that these subunits are responsible for the signaling mechanism that allows communication between these two sequential respiratory complexes. It can contribute to ROS production detected when cytochrome c oxidase was repressed in these parasites.
AOX, the second terminal oxidase in trypanosomatids, is restricted to the inner mitochondrial membrane of both T. brucei bloodstream and procyclic forms, not exhibiting proton translocation capacity and subsequent contribution to proton gradient that drives ATP formation. In bloodstream forms, the complex IV can be completely replaced by AOX, while in the insect stage, the enzyme coexists with this complex. Interestingly, KCN, a complex IV inhibitor, does not completely abolish T. cruzi and L. donovani respiratory rates, suggesting the existence of an AOX as an alternative to cytochrome c oxidase. However, the activity of salicylhydroxamic acid, an AOX inhibitor, was observed only in T. cruzi, suggesting a role of this enzyme in this parasite energetic metabolism [89, 90]. In L. donovani, the respiration KCN-insensitive still remains unclear, needing further investigation [91]. Fang and Beattie [72] showed that the inhibition of AOX by salicylhydroxamic acid stimulates ROS formation in T. brucei, resulting in an increase in oxidation of cellular proteins. Besides that, AOX activity increased when parasites were incubated in the presence of H2O2 and antimycin A, which leads to high ROS levels. These data suggested that the excess of reducing equivalents was removed by AOX in T. brucei transferring these equivalents to oxygen, preventing ROS production.
Kinetoplastid parasites have a complex life cycle in which they transit between invertebrate and vertebrate hosts. During the cycle, the protozoa change their morphology and metabolic profile, adaptating to diverse environmental conditions [92, 93]. In T. cruzi, the comparison between the energetic and oxidative metabolisms of bloodstream trypomastigotes and epimastigotes showed more active complex II–III and a restriction in electron flux to complex IV, reducing oxygen consumption and resulting in increased H2O2 generation in bloodstream forms [94]. These findings can be explained by the access to glucose at constant concentration that trypomastigotes have in vertebrate bloodstream. Similar results were found in T. brucei bloodstream stage that is essentially glycolytic, also living in an environment that presents high glucose levels. Besides that, these protozoa also lack many tricarboxylic acid cycle enzymes and cytochromes, affecting energy production [95,96,97]. In this regard, the invertebrate environment is glucose poor but is rich in amino acids released from intense digestion of blood proteins [92, 98, 99], resulting in high hemolymphatic levels of histidine [100, 101]. Several groups have discussed the possibility of oxidative environment as a stimulus to trypanosomatid growth. In T. cruzi epimastigotes, an increase in parasite proliferation was observed in response to H2O2 incubation, mediated by calmodulin kinase II activation. The exposure of epimastigotes to different redox-state molecules including heme, a pro-oxidant molecule derived from the insect blood digestion, increased mitochondrial ROS production and parasite replication, whereas mitochondrion-targeted antioxidant reduces ROS generation, impairing protozoa proliferation and increasing metacyclogenesis [102,103,104].
2.2 Oxidative Metabolism in the Hosts
Both invertebrate and vertebrate hosts of trypanosomatids share a common machinery of oxidative metabolism, which occurs in the mitochondrion. After decades of research in this field, critical proteins and molecules involved in the oxidative metabolism were described. The sequenced mitochondrial genome and uncoupling proteins (UCP) assays were extremely important for the overall comprehension of this process [105,106,107]. As described above for trypanosomatids, ETC is presented in the mitochondrial inner membrane, being the complexes I, II, III, IV, and ATP synthase functional [46]. The electrons entry in ETC occurs in complexes I or II, passing to complex III through coenzyme Q. Having received the electrons from complexes I or II, complex III uses cytochrome c to pass the electrons to complex IV, responsible for the reduction reaction that generates H2O from O2 [108]. As in trypanosomatids, hosts mitochondria are the main source of cellular ROS. To date, 11 sites of superoxide and/or hydrogen peroxide in mammalian mitochondria have been described, depending on the substrate metabolism, electron transport, and oxidative phosphorylation. The majority of these site-specific mitochondrial ROS production has been studied, measuring the maximum capacities of these sites under optimal conditions [52, 109]. As described above, complexes II and IV are not important ROS sources in mammals. Here, these two complexes are the main ROS-generating enzymes.
Differently of trypanosomatids, in mammals, complex I is the only entry point of electrons from NADH into the respiratory chain. This enzyme presents two domains: a hydrophilic portion located into mitochondrial matrix and a hydrophobic one, embedded in the inner mitochondrial membrane. All the known redox centers of complex I, the flavin mononucleotide cofactor (FMN), and eight FeS clusters are located in the hydrophilic domain [110]. The complex I has been recognized for a long time as one of the main sources of ROS production by the mammalian mitochondrial respiratory chain. This process was previously demonstrated, where the reduction of coenzyme Q pool and the generation of a large ΔΨm by succinate led to H2O2 production [111]. Subsequently, other authors showed that isolated complex I, in the presence of NADH, produces O2•– and that this production is enhanced by rotenone [112]. The mechanism of O2•– generation by isolated complex I is well understood, by the reaction of O2 with the fully reduced FMN (set by NADH/NAD+ ratio), which explains the enzyme inhibition by rotenone [113, 114]. During stress conditions, as ETC inhibition by damage, loss of cytochrome c, or low ATP demand and consequent low cellular respiration, the ratio of NADH/NAD+ increases, leading to O2•– production [52]. The overexpression of a yeast NADH dehydrogenase in mammalian mitochondria reduces O2•– generation through the NADH/NAD+ decrease [115]. As described previously to trypanosomatids, in mammals, the ROS production by complex III is dependent of coenzyme Q-cycle and semiquinone formation [116].
The oxidation of energetic substrates generates reducing cofactors, as NADH and FADH2, which donate electrons to ETC. During the electron flow between the mitochondrial complexes, the dissipated energy is used by complexes I, III, and IV to translocate protons to intermembrane space, generating an ΔΨm across the inner membrane. Protons return to the mitochondrial matrix through ATP synthase, decrease the electrochemical gradient, and promote ATP synthesis [44]. However, the oxidative phosphorylation is partially coupled since protons can return to the mitochondrial matrix independently of ATP synthase and thereby without ATP synthesis [44, 117]. The energy-dissipating process (proton translocation to intermembrane space followed by the proton re-entry in mitochondrial matrix) apparently is present in all eukaryotic cells in a high proportion of cellular metabolic rates (up to 25% of the basal metabolic rate in the rats) and could prevent the oversupply of electrons to ETC, minimizing the probability of electron leak and O2•– production [118, 119]. Some authors showed the close relationship between the proton leakage and ROS production: uncoupler molecules and ADP, which increase respiration rate, stimulate ATP synthesis, and decrease ΔΨm, are known for impaired ROS production in isolated mitochondria [120, 121].
There are at least two types of proton leakage: a basal and an inducible. The basal proton leak is unregulated and depends only on the presence but not on the activity of carrier proteins. In this case, the proton return to mitochondrial matrix occurs through the lipid bilayer and has low impact. The inducible proton leakage is a protein-mediated process that is regulated and could be reversibly activated and/or inhibited. Among the inner mitochondrial membrane carriers, the UCPs, proteins belonging to mitochondrial anion carrier protein (MACP) family, are the mitochondrial carriers whose participation in ROS production is better understood [122, 123]. In the late 1970s, the first UCP was described in mammalian brown adipose tissue and was designated UCP1; afterwards, UCP1 homologues were found in mammalian tissues (UCP1-5) [124,125,126,127]. While proton leakage mediated by UCP1 is crucial for adaptative thermogenesis in the cold [124, 128], the function of these protein homologues is not yet fully elucidated. One of the differences between UCP1 and their homologues is the abundance in individual cells and nonthermogenic tissues, influencing the oxidative phosphorylation and ROS production [129]. The role of UCP in H2O2 generation was first demonstrated in 1997, showing that the inhibition of UCP2 by GDP results in higher ΔΨm and ROS production [130]. Studies with UCP2 knockout mice demonstrated an oxidative burst in macrophages and liver and also improved resistance to Toxoplasma gondii. In contrast, UCP2 overexpression decreased ROS generation. In addition, O2•– and lipid peroxidation products (4-hydroxy-2-nonenal (HNE)) have been described to activate UCPs [131, 132]. Chemical uncouplers, such as 2,4-dinitrophenol (DNP) and FCCP (carbonyl cyanide p-tri-fluoromethoxyphenylhydrazone), also had a protector effect, where they contributed to a decrease in oxidative stress in the skeletal muscle, heart, and brain [133,134,135].
3 Antioxidant Machinery
As discussed above, mitochondrion is one of the major ROS sources. It is well-known that the reactions involving ATP synthesis are able to release toxic products such as ROS and RNS. ROS accumulation led to severe biological consequences, and a machinery to regulate oxidative stress is essential to minimize its deleterious effects [136]; therefore, eukaryotic cells, among them, trypanosomatids and their hosts, possess enzymatic and nonenzymatic antioxidant defenses. Antioxidants are molecules responsible for the prevention of substrates oxidation (or delay), decreasing the intensity of the characteristic oxidative phenotype that includes genotoxicity and injury in crucial molecules such as proteins and lipids, among others [137]. Eukaryotic cells use two distinct strategies against the oxidative stress described up to now: the blockage of radical formation by antioxidant molecules (enzymes or not) or even the increase in the expression of specific enzymes that remove oxidized biomolecules [138, 139].
Considering the molecular mass, antioxidants are classified into (i) high molecular mass antioxidants (≥10 KDa), which include antioxidant enzymes, such as transferrin, albumin, and ferritin, which strongly bind to metal ions, among other pro-oxidant molecules with high oxidizing potential [43], and (ii) low molecular mass antioxidants (≤1 KDa), such as tocopherol (vitamin E), ascorbic acid (vitamin C), anthocyanins, carotenoids, uric acid, and polyphenols, which are obtained during the alimentation of almost all organisms [43, 138].
3.1 Antioxidant Defenses in Pathogenic Trypanosomatids
3.1.1 Trypanothione/Trypanothione Reductase
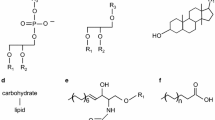
The antioxidant system of trypanosomatids is based on trypanothione/trypanothione reductase, an alternative system to glutathione/glutathione reductase, absent in these protozoa [140] (Fig. 8.2). The trypanothione [N1, N8-bis (glutathionyl) spermidine] is well-characterized, being formed by the binding of two glutathiones (GSH) and one spermidine (SPD) molecule in the cytosol [141,142,143,144,145]. Trypanothione biosynthesis is divided into three different steps, including GSH and SPD synthesis, similar to the pathway in other organisms.
The trypanothione is formed by the binding of two glutathione (GSH) molecules and one spermidine molecule in a reaction catalyzed by trypanothione synthetase (TryS). In this system, the maintenance of reduced trypanothione [dihydrotrypanothione – T(SH)2] is dependent on trypanothione reductase (TryR) action by NADPH consumption. T(SH)2 directly reduces tryparedoxin (TXN), dehydroascorbate (dhASC) to ascorbate (ASC), and glutathione disulfide (GSSG) to GSH. Trypanothione disulfide (TS2); 2-Cys peroxiredoxin (2-Cys PRX); non-selenium glutathione peroxidase-like enzymes (nsGPx); ascorbate peroxidase (APx); hydroperoxides (ROOH)
GSH synthesis begins with the formation of γ-glutamylcysteine from the binding of L-glutamate and L-cysteine, catalyzed by γ-glutamylcysteine synthase (GCS). Such step is a limiter of the reaction in mammals and trypanosomatids. Previous studies showed that low GCS levels in L. infantum reduce the resistance to oxidative stress and consequently parasite survival in activated macrophages [146,147,148,149]. In T. brucei, the reduction in GCS levels resulted in a decrease in GSH and trypanothione concentrations; however, GCS knockdown led to an increase in GSH uptake, reversing trypanothione levels in this parasite [145, 150]. Once human blood has low GSH levels, the GSH uptake is not the primary source of these molecules in T. brucei bloodstream forms [151]. Curiously, GSH transporters were not described in Leishmania spp. and T. cruzi [145]. For GSH synthesis, it is necessary the presence of cysteine, an amino acid with a thiol group, that confers its redox capacity. In trypanosomatids, cysteine can be taken and/or biosynthesized, although, despite Leishmania spp. and T. cruzi express cysteine carriers, the uptake levels are much lower than in T. brucei, which expressed highly efficient transporters [152,153,154,155]. Alternatively, Leishmania spp. and T. cruzi have two biosynthetic pathways, also present in other eukaryotes, de novo or cysteine assimilatory pathway (CAP) and trans-sulfuration pathway (TSP). Increased levels of protein expression and activity of cysteine synthase and cystathionine β-synthase in L. braziliensis, key enzymes of these pathways, led to elevated thiol concentrations in response to oxidative and nitrosative stresses, confirming the association between cysteine biosynthesis and stress response. Promastigotes and amastigotes expressed differently these pathways, with TSP pathway increased in insect form and higher de novo synthesis in mammalian form [156]. T. brucei possesses gene sequences only of TSP pathway; nevertheless, this parasite does not synthesize cysteine [145, 152,153,154,155]. The stage/species-specific regulation of cysteine biosynthetic pathways may be due to complex life cycle and exogenous nutrients, which differ considerably between invertebrate and mammalian hosts. The de novo pathway occurs predominantly in the mammalian intracellular form, while the TSP is present in insect form. These observations are consistent with the fact that T. brucei and Trypanosoma rangeli, parasites without an intracellular stage, possess only TSP pathway [155,156,157,158,159].
Polyamines are simple aliphatic compounds that are found in all mammalian tissues and also in microorganisms. These molecules are essential for cell growth and differentiation and have several biological roles. In trypanosomatids, SPD is one of the most important polyamines, being involved in crucial cellular processes including synthesis of trypanothione. Polyamines could be obtained by de novo synthesis from ornithine and in some cases from arginine, or by the uptake from the extracellular medium [160]. T. brucei synthesize SPD through the ornithine decarboxylation mediated by ornithine decarboxylase, which generates putrescine, a substrate for spermidine synthase, while Leishmania spp., beyond de novo synthesis, can also uptake this molecule [161, 162]. In T. cruzi, enzymes of putrescine biosynthetic pathway are absent, being this parasite auxotrophic for polyamines [163, 164]. An increase in T. cruzi polyamine transport improves the resistance to oxidative stress in this protozoa, which can be generated by incubation with H2O2 or trypanocidal drugs, such nifurtimox and benznidazole [165].
The last step in trypanothione biosynthesis is the binding of GSH and SPD. This process is exclusive of few organisms, including trypanosomatids [145]. Trypanothione biosynthesis consists in an ATP-dependent addition of two GSH molecules to SPD amino groups by different pathways, depending on the protozoa. In pathogenic trypanosomatids, trypanothione is synthesized by trypanothione synthetase (TryS) in two steps: first this enzyme catalyzes the binding of one GSH molecule and SPD, forming glutathionylspermidine (intermediate product). After this, the same enzyme added a second GSH, forming the trypanothione molecule. In addition, TryS presents amidase function, hydrolyzing trypanothione and glutathionylspermidine to form GSH and SPD, suggesting an involvement in polyamine levels [145, 166,167,168,169].
In trypanosomatids, trypanothione can be found in reduced form dihydrotrypanothione (T(SH)2) and/or in the oxidized form trypanothione disulfide (TS2). T(SH)2 is more reactive than GSH, being a dithiol, which favors the reduction of disulfides (different pKa values: 7.4 and 8.4 to trypanothione and GSH, respectively) [170,171,172]. Depending on the reactive species, T(SH)2 can suffer one- or two-electron oxidation, forming thiyl radicals (RS–) or sulfenic acid (RSOH), respectively. The thiyl radicals are formed in the reactions with peroxyl and hydroxyl radicals, nitrogen dioxide, and others, whereas the sulfenic acid is formed during the reactions with H2O2 and peroxynitrite [145]. Moreover, T(SH)2 participate in many antioxidant pathways, reducing intermediate molecules which transfer electrons to peroxidases [173].
The T(SH)2 levels are maintained by NADPH-dependent flavoenzyme trypanothione reductase (TryR). TryR has 40% identity with mammalian glutathione reductase and shares several physical and chemical characteristics with host enzyme, being their specificity of disulfides the main difference. The enzyme reduces positively charged oxidized forms of glutathionyl-polyamine conjugates, such as trypanothione, glutathionylspermidine, and others [174], whereas glutathione reductase only accepts negatively charged oxidized glutathione as a substrate. This characteristic is determined by the presence of five amino acid residues in the catalytic site, make this part wider, more hydrophobic and negatively charged when compared with glutathione reductase [175]. TryR distribution is still discussed, but some previous data suggested cytosolic and mitochondrial locations, while other reports proposed a glycosome localization [176,177,178]. T. cruzi and T. brucei have a carboxy-terminal extension with a tripeptide segment that would direct the enzyme to glycosome [179,180,181]. TryR is a potential drug target due to its importance for the survival of trypanosomatids. L. donovani mutants that presents only 15% of the wild-type TryR activity presented a normal growth in culture, but also showed an increased susceptibility to oxidative stress and a reduced viability inside macrophages. Similarly, the depletion of this enzyme in T. brucei led to an increase in sensitivity to H2O2 of the parasite, which then cannot successfully infect mice [182,183,184].
3.1.2 Tryparedoxin, a Trypanothione-Dependent Peroxidase System
Catalase and glutathione peroxidase (GPx) are absent in pathogenic trypanosomatids, making these parasites more susceptible to high concentrations of hydroperoxides [185, 186]. For many years, the elimination of low H2O2 concentrations was attributed to trypanothione [187, 188]. Recent studies showed the presence of three different classes of peroxidases; however, trypanothione remains essential for hydroperoxide removal (Fig. 8.2). Among these peroxidases, tryparedoxin (TXN) is a member of thioredoxin superfamily (Trx) that transfers reducing equivalents to different thiol proteins, which are found exclusively on Kinetoplastida [189, 190]. In contrast to classical thioredoxins, TXN is not directly reduced by a NADPH-dependent flavoprotein; however, these molecules are reduced by T(SH)2/TryR system at the expenses of NADPH in the parasite [191,192,193]. Experimental analyses pointed cytosol as the localization of TXN in different trypanosomatids, but in silico approaches also suggested the mitochondrion and endoplasmic reticulum as possible target organelles to the enzyme in Leishmania spp. and T. cruzi [173, 177, 191,192,193,194,195]. TXN depletion in L. infantum and T. brucei promoted the impairment of the antioxidant metabolism, compromising the parasite survival [195, 196]. Studies with L. infantum and L. donovani showed that TXN is essential for promastigotes and amastigotes stages, particularly during the establishment of infection [195, 197, 198].
Tryparedoxin peroxidase (TXNPx) is a class of enzymes that use TXN as electron donor. This process occurs in two steps: (1) T(SH)2 reduces TXN, being considered a regulatory reaction in the pathway; and (2) TXNPx is reduced by TXN [199]. The TXNPx includes two types of peroxidases, the peroxiredoxins (PRXs) and non-selenium glutathione-like enzymes (nsGPx). PRXs are a family of antioxidant enzymes that are present in several organisms, detoxifying hydroperoxides and peroxynitrite through cysteine (Cys) residues. These enzymes are divided in two categories, depending on the quantity of Cys residues involved in the reaction. The enzyme of trypanosomatids is a typical 2-Cys peroxiredoxin (2-Cys PRX) distributed in cytosol and mitochondrion, although, in Leishmania spp., one of the genes that encode this protein has a glycosomal signal sequence [190, 200]. The 2-Cys PRX has two identical reactions centers, being the substrate reduced by one of the Cys residues present in these centers, forming a cysteine sulfenic acid (Cys-SOH). Then, this residue is attacked by a Cys residue, forming a stable disulfide bond that is removed by TXN oxidoreductase [201]. 2-Cys PRX overexpression in Leishmania spp. and T. cruzi increased the resistance to hydroperoxides and peroxynitrite; however, the excess of substrate can reduce the enzyme activity in T. cruzi [202,203,204,205].
The second class of TNXPx, the nsGPxs, is structurally similar to glutathione peroxidase, but the selenocysteine residue in the active site of this enzyme was replaced for Cys residue [206]. These enzymes are classified as nsGPx-AI-III and nsGPx-B with different localizations. In T. cruzi, nsGPx-A1 is found in glycosome and cytosol, while nsGPX-AII-III are restricted to the cytoplasm. Interestingly, nsGPx-B is exclusively located in the endoplasmic reticulum in this parasite [177, 207]. In T. brucei and Leishmania spp., nsGPX-AI-II exhibit cytosolic localization, but on the other hand, nsGPx-AIII shows glycosomal and mitochondrial signal peptides [181, 208]. The nsGPx mechanism of action is similar to 2-Cys PRX, with the two Cys residues in the active site being responsible for substrate oxidation. The affinity of nsGPxs to substrates is a peculiarity of these enzymes. The depletion and/or mutation of amino acid residues in the catalytic site is responsible for a decrease in glutathione-binding capacity [207]. In this way, nsGPx-A uses TXN during substrate reduction, while nsGPx-B shows low affinity to both molecules [177, 209, 210].
3.1.3 Ascorbate Peroxidase
Despite the first description by Clark et al. [211], the biological relevance of ascorbic acid (vitamin C) in trypanosomatids was demonstrated almost a decade later [212]. This molecule acts as a cofactor for a wide range of enzymes involved in distinct metabolic processes, among them the antioxidant system [213]. The ascorbate peroxidase (APx), an antioxidant enzyme that uses ascorbic acid as a reductor agent, is a heme-containing peroxidase that catalyzes H2O2 reduction [209]. This enzyme has an endoplasmic reticulum localization in T. cruzi and Leishmania spp., whereas in T. brucei, there are no reports about this existence [185, 214, 215]. APx acts by cleaving O-O bound in H2O2 through the reaction between heme and reactive species, producing water. This process generates an intermediate that is reduced by ascorbate in two sequential electron transfer, restoring APx. Dehydroascorbate, the oxidized molecule, returns to the reduced form in two nonenzymatic reactions that may include T(SH)2 and TXN [212, 216, 217]. Taylor et al. [218] showed that APx overexpression in T. cruzi confers protection against H2O2 exposure, whereas its depletion results in enhanced sensitivity. The enzyme activity is not required to parasite fundamental processes, such as replication and virulence.
3.1.4 Fe-Superoxide Dismutase
The SOD is an antioxidant enzyme described in most eukaryotes, being responsible for O2•– dismutation in H2O2 and O2 [219]. This enzyme action is dependent on a metallic cofactor, such as Cu/ZnSOD and MnSOD, which are found in mammalian cytosol and mitochondria, respectively. In trypanosomatids, in silico analyses showed four SOD genes that use iron as cofactor. These isoforms are expressed in different organelles; FeSOD-B is distributed between cytoplasm and glycosomes of T. cruzi and T. brucei, whereas FeSOD-A and FeSOD-C are found in T. brucei mitochondrial matrix and intermembrane space, while only the first isoform was described in T. cruzi [220,221,222,223]. FeSOD-D was described in the three pathogenic trypanosomatids, being its activity related with apoptotic-like death in T. cruzi and L. donovani, while in T. brucei, it is considered nonessential [57, 224, 225]. The downregulation of FeSOD-A in Leishmania spp. increased the parasite sensitivity to menadione, a O2•– producer, whereas this enzyme overexpression protects the parasite from the oxidative stress. Furthermore, the SOD expression is related to differentiation and replication processes in Leishmania spp. Promastigotes in stationary phase showed increased resistance to oxidative stress and higher SOD activity during amastigote differentiation. In contrast, low SOD activity and ROS accumulation were found during promastigote logarithmic phase [226, 227]. In T. cruzi, the enzyme also has an important role in vertebrate host adaptation, with the enzymatic levels increasing during metacyclogenesis [92].
3.2 Antioxidant Defenses in the Hosts
3.2.1 Glutathione/Glutathione Reductase System
GSH (L-glutamyl-L-cysteine-L-glycine) is the most abundant low molecular mass thiol in eukaryotic cells [228, 229]. Its reduced form is also the active form (GSH) that is oxidized to glutathione disulfide (GSSG) in the oxidative stress. GSH is synthesized in cytosol and is transported into mitochondrion by dicarboxylate carrier protein and 2-oxoglutarate carrier protein [230]. Glutathione reductase (GR) is the enzyme responsible for maintain glutathione in its reduced form since the abundance of its oxidized form leads to a decrease in GSH/GSSG ratio, which serves as a warning of oxidative stress [229]. GR has been found in all organisms analyzed thus far, being a highly conserved enzyme in highly divergent organisms, such as Homo sapiens and E. coli [231]. GR has two cysteines in the catalytic domain and two other domains that bind to FAD and NADPH. Glutathione is found in cytoplasm, nucleus, mitochondria, and endoplasmic reticulum, as well as it seems to be present in lysosomes [232]. The formation of disulfides between GSH and protein cysteine residues constitutes a protective mechanism for thiols, which prevents their further oxidation, protecting cells from oxidative stress [233]. However, the formation of protein disulfides can alter the function of thiol-based proteins, such as receptors, protein kinases, and transcription factors, impairing cell signaling. In this context, GSSG is able to play a role in a nonspecific cell signalization [230]. GSH is considered the most important redox molecule present in organisms, above all, mammals. This molecule is able to play several roles, such as a redox buffer, acting as cofactor scavenger for antioxidant enzymes such as GPx. This enzyme is able to detoxify H2O2 and lipid peroxidation products by the reaction of selenocysteines (presents in its active site) with peroxide group, forming GSSG and H2O [228, 230, 233, 234].
GSH is not only found in cytosol but also in endoplasmic reticulum, nucleus, and intermembrane space. The transport of this molecule to nucleus is thought to bcl-2 facilitating passive diffusion via nuclear pores [235], and nuclear pool of GSH is responsible for regulating the redox state of protein sulfhydryls, in order to prevent DNA oxidative damage [229, 230]. The regulation of cytosolic GSH transport to the nucleus appears to follow cell cycle progression, balancing the GSH cytosolic/GSH nuclear according to cellular proliferation [236]. Reaching the endoplasmic reticulum, GSH is responsible for maintaining the homeostasis and the thiol levels in catalytic sites for PDI (protein disulfide isomerase) protein folding. Besides endoplasmic reticulum capacity to produce ROS, other oxidative inducers can lead to an unbalanced environment, impairing natural protein folding [237, 238]. Inside mitochondria, GSH is able to control mitochondria ROS generation by ETC and preserve mitochondrial proteins and lipids integrity [239, 240]. GSH also prevents toxic effects of free intracellular metals, such as iron, preventing its reaction with O2 and Fenton reaction [228].
3.2.2 Other Thiol-Dependent Enzymes
Trx is part of a major system called TRX system, which includes, besides the Trx, the thioredoxin reductase (TrxR) and NADPH. Trx is a ubiquitous protein with a redox-active dithiol/disulfide site [241]. As GSH/GSSG, Trx appears in a reduced form [Trx(SH)2], with a dithiol group, and in an oxidized form (TrxS2), with a disulfide bound. Trx contains a conserved site Cys-Gly-Pro-Cys found in all organisms. In mammalian cells, Trx is described by having two isoforms, known as Trx-1 and Trx-2. Trx-1 is localized in cytosol, being transported to the nucleus during oxidative stress [229], while Trx-2 is the exclusive mitochondrial Trx isoform, regulating mitochondrial homeostasis. As in GSH/GSSG system, the maintenance of reduced and oxidized Trx ratio is extremely important in TRX system. TrxR is an oxidoreductase, responsible for regulating Trx(SH)2/TrxS2 [241, 242].
PRXs are ubiquitous thiol-dependent enzymes, known as an ancient family of proteins, being evolutionarily conserved and present in all kinds of organisms [243]. This peroxidase was first observed in Saccharomyces cerevisiae, demonstrating an antioxidant activity [244]. Unlike GSH and Trx, PRXs are selenium and heme-free molecules, with a peroxidatic cysteine (Cp) conserved in N-terminal domain. PRX mechanism of action consists in Cp attack to O–O bonds present in peroxide (reaction 3), which is oxidized into cysteine sulfenic acid [245]. PRX uses Trx as a hydrogen donor, creating an electron flow. Besides the H2O2, PRXs are able to act as a scavenger for peroxynitrite and lipid peroxidation products. The ability to scavenge peroxides protects prokaryotic and eukaryotic cells from DNA, lipids, and proteins oxidative damages, caused by ROS and RNS [243, 245]. Some groups have been demonstrating the relation of H2O2 signaling with PRX inactivation, describing new roles for this protein. Low concentrations of H2O2 appear to generate PRX inactivation by hyperoxidation, a reversible reaction promoted by sulfiredoxin [246, 247]. This reaction appears to follow a circadian rhythm, whereas the H2O2 signaling is required for different cell functions [248]. In structural terms, PRXs are divided in two subcategories: 1-Cys and 2-Cys peroxiredoxins, depending on the quantity of Cys residues involved in the reaction. There are six PRX isoforms present in mammalian: PRX I, II, III, and IV (2-Cys PRX), PRX V (atypical 2-Cys PRX), and PRX VI (1-Cys PRX) [242, 243, 245, 249]. PRX I and II are found in cytosol and nucleus. PRX III has an affinity to mitochondria, while PRX IV is found at endoplasmic reticulum. PRX V has been already detected at cytosol, mitochondria, and peroxisomes [250].
Glutaredoxin (GRX) is a small dithiol protein, also known as thioltransferase, required at the redox system. GSH is able to reduce GRX, and it has been described the potential role of its sensing changes in GSH/GSSG ratio [251]. GRX has the Trx family motif Cys-X-X-Cys, and it is able to form a disulfide bond within GSH. GRXs are also under two forms, an oxidized and a reduced form, and its reduction reaction is done by GSH, GR, and NADPH [249]. GRX is also found inside the intermembrane space, endoplasmic reticulum, and cytosol. It has been described that GRX can also display a sensing role at GSH-dependent glucose deprivation, as its interactions result in mediated cell death [251]. GRX-GSH is extremely important when TRX system decreases its activity under circumstances such as lack of selenium or Trx/TrxR inhibition, acting as a backup redox system [252, 253]. It has been described that mitochondrial GRX serves as TrxR substrate, maintaining mitochondrial redox homeostasis.
3.2.3 Superoxide Dismutase and Catalase
In the enzymatic redox system, SOD and catalase appear as the main enzymes in cellular detoxification. SOD is the first defense against superoxide, converting O2•– in H2O2 and O2. This enzyme is dependent on metal as a cofactor, and in mammals, there are three different isoforms: SOD1, a Cu/ZnSOD found in cytoplasm, nucleus, and plasma membrane; SOD2, a MnSOD found in mitochondrial matrix; and SOD3, a Cu/ZnSOD found in extracellular compartments, scavenging O2•– released in inflammatory cascades [254, 255]. High H2O2 concentrations are very dangerous for the cells, being necessary its elimination. Catalase is an enzyme that works directly connected with SOD, in order to complete O2•– detoxification, converting H2O2 into H2O and O2 [256]. The catalase types include Fe (heme)/Mn dependent [257]. These enzymes are largely found in all mammalian tissues, especially in red blood cells, and have been described as a cardiac and neural aging protectors [255, 258, 259].
4 Role of Oxidative Metabolism in Hosts/Trypanosomatid Infection
These parasites must thrive endogenous toxic metabolites produced by its aerobic metabolism and deal with the oxidative burst derived from the host immune system, which include ROS production. Once some antioxidant machinery in trypanosomatids such as catalase and classical GSH/GPx system is lacking, many authors suggested that ROS production by the hosts is a defense against parasite infection. However, it is well described the ability of trypanosomatids to overcome this situation, using ROS and RNS as important signaling molecules for their survival [260,261,262].
Both Leishmania spp. and T. cruzi have an intracellular stage inside the vertebrates. Macrophages and neutrophils are phagocytic cells, responsible for recognizing, internalizing, and destroying pathogens, being the first contact of infective metacyclic forms, performing a key role in infection control [263, 264]. For a successful infection, T. cruzi metacyclic trypomastigotes must invade macrophages and survive to oxidative burst found inside the phagosome. Previous data showed that cruzipain, an immunogenic glycoprotein, induces an increase in ROS production in murine cells during parasite invasion [265]. O2•– is produced by an associated-membrane NADPH oxidase, contributing to the formation of an oxidative environment during phagocytosis [266]. Besides that, the increase in H2O2 formation is also related with NADPH oxidase activation, once that spontaneous reactions (enzymatic or not) can convert O2•– in this molecule [267]. When the parasite is phagocytosed, a signaling cascade is triggered, culminating in the oxidative burst. The complex NADPH oxidase, known as NOX family, is a transmembrane multimeric protein able to transfer electrons to O2 by NADPH oxidation [268, 269]. Cytochemical data demonstrated O2•– production when the parasite is attached to the macrophage surface, due to NADPH oxidase activation [270]. These enzymes need calcium or cytosolic proteins to be activated and thus lead to ROS production. In early stages of infection, NADPH oxidase is activated and its subunits are directed to phagosome membrane. Seven NADPH oxidase homologues have been identified in several cells types, being the NADPH oxidase 2 (NOX 2) the homologue present in phagocytic cells [269, 271].
Other important reactive species found during macrophages infection is NO. This RNS is a highly reactive free-radical produced by the oxidation of L-arginine by nitric oxide synthase (NOS) [272], participating in trypanosomatids killing, both directly or through the interaction with other free radicals, such as O2•–, forming ONOO– [273, 274]. NO is not a strong reactive species by itself, being unlikely to account for a direct damage to the parasite. The NO can inhibit the respiratory chain in mammalian via interactions with cytochrome c oxidase, which is accompanied by a steady-state level of reduced respiratory complexes, which favors intramitochondrial O2•– formation [275,276,277]. Proinflammatory cytokine production by macrophages, such as IL-12, INF-γ, and TNF-α, activates the inducible nitric oxide synthase (iNOS), generating high amounts of NO, which is maintained by 24 h during infection [264, 278]. The upregulation of iNOS expression in BALB/c mice is protected from infection by Leishmania major. Furthermore, T. cruzi infection-resistant C57BL/6 mice produced larger amounts of NO, which is correlated with a better control of acute-phase parasitemia [279, 280]. In addition to NADPH oxidases and iNOS, myeloperoxidase (MPO) is another enzyme present in phagocytic cells, mainly neutrophils, which is pointed out as responsible for oxidative burst. MPO is able to catalyze the reaction of hypochlorite production, one of the major neutrophil antimicrobial responses [269]. This enzyme is a lysosomal hemoprotein, member of cyclooxygenase superfamily, stored in neutrophils azurophilic granules [281, 282]. When neutrophils are activated, the MPO is released in cytoplasm during degranulation, which also releases H2O2. The MPO-H2O2 system is able to convert halide ions such as Cl– , Br–, and I–, producing their oxidized forms of hypohalous acids (HOX), potent antimicrobial molecules [283, 284].
The levels of parasite antioxidant defenses during macrophage invasion may improve pathogen survival [285]. Chronic chagasic cardiomyopathy is characterized by the presence of pseudo cysts of amastigotes nests in the cardiac fiber. During T. cruzi invasion in cardiomyocytes, there is an increase in the production of inflammatory mediators, such as TNF-α and IL-1β, and the induction of iNOS, with subsequent NO generation [286, 287]. The biochemical and genetic heterogeneity among T. cruzi strains is, in part, responsible for the diverse clinical manifestations of the disease, controlling several pathogenesis aspects [288]. Taking into account the establishment of a nitroxidative stress during the acute and chronic stages of Chagas disease, T. cruzi antioxidant enzymes are important virulence factors and become decisive for the infection success. In this context, several proteomic analyses showed an overexpression of T. cruzi antioxidant machinery in the infective metacyclic trypomastigotes compared with noninfective epimastigotes [92]. Such increase, found during metacyclogenesis of different T. cruzi strains, may act as a general preadaptation process to allow the parasite survival in the nitroxidative environment found in the vertebrate host. Piacenza et al. [289] showed a positive association between parasite virulence and levels of antioxidant enzymes in vivo.
In Leishmania spp. infection, some strategies to escape the oxidative burst present in phagocytic cells were demonstrated. Lipophosphoglycan (LPG), a molecule widely distributed in promastigotes surface, plays an important role in intracellular survival of these parasites. The protective effect of LPG is restricted to the establishment of infection during differentiation of promastigotes into amastigotes. In vitro experiments showed that LPG decreased oxidative burst in activated monocytes through inhibition of p67phox and p47phox recruitment to NADPH oxidase complex in phagosomes [290, 291]. LPG is also able to reduce NO• production, regulating the iNOS expression in macrophages [292]. Additionally, O2•– generation after the infection by promastigotes and amastigotes is substantially different [272]. The hypothesis for this difference is the deficiency of NADPH oxidase activity after amastigote infection. Monocytes of patients with visceral leishmaniasis produce lower levels of O2•– and H2O2 and have a decreased NADPH activity when compared with healthy controls [293, 294]. To accomplish the successful NADPH oxidase complex assembly, the maturation of gp91phox is necessary, which is dependent on heme availability. During the infection, Leishmania pifanoi amastigotes induces the heme oxygenase-1 expression, an enzyme responsible for heme degradation, thereby blocking gp91phox maturation and preventing NADPH activity [295, 296]. Amastigotes also induced lower levels of p47phox phosphorylation mediated by protein kinase C, decreasing the phagosomal recruitment of p67phox and p47phox to NADPH oxidase complex. Interestingly, unlike promastigotes, such effect is not attributed to LPG in amastigotes and remains unclear [291, 297, 298].
Although ROS are expected to be responsible for pathogen elimination during oxidative burst as described above, evidences suggest that ROS production could also have a beneficial role to T. cruzi macrophage infection. Oxidative stress mobilizes iron from host intracellular storages, which is an essential for amastigote replication. Such process occurs by the regulation of elF2α kinase. In the absence of heme, elF2α kinase is active and promotes cell growth arrest, leading to the differentiation of proliferative amastigotes into nonproliferative trypomastigotes [299]. ROS also participate in Leishmania spp. differentiation in a process dependent on iron availability. In this case, changes in intracellular iron levels activate a ROS-dependent signaling pathway that induces promastigotes differentiation into infective amastigotes [300]. Trypanosomatids also have contact with ROS and RNS inside the invertebrate host, in which the blood digestion, derived from the hematophagic behavior, causes an increase of heme concentration and an oxidative burst [260, 301]. Heme-induced and mitochondrial ROS stimulates epimastigote proliferation, being the contribution of mitochondrial ROS in epimastigote growth confirmed by the use of mitochondrial-targeted antioxidant that impairs parasite proliferation [103, 104]. The contribution of mitochondrial ROS to epimastigote growth was confirmed using the mitochondrion-targeted antioxidant MitoTEMPO that decreased ROS and ATP production induced by heme, strongly impairing the cell proliferation.
5 Oxidative Mechanisms of Action of Anti-trypanosomatid Drugs
Considering all mechanistic studies of preclinical compounds performed in trypanosomatids, mitochondrion stands out among the most recurrent targets in these parasites, and mitochondrial damage has been described as part of the mode of action of distinct drugs classes [83, 84, 149, 302,303,304,305,306,307,308,309,310,311]. Surprisingly, the components and molecular events involved in the mitochondrial susceptibility to drugs, usually detected by phenotypes such as swelling and/or depolarization of the organelle, are completely undescribed [312]. Such scarcity of molecular information about the mechanisms of action of the great majority of the compounds makes hard to prove the mitochondrial direct effect. The specificity of this organelle as a target is very controversial, representing its injury a secondary target, derived from primary effects on another biochemical processes in different cellular structures. Independently of the origin or intensity, the mitochondrial damage caused by drugs regularly promotes a calcium homeostasis and/or ROS production [313].
Additionally, the activity of mitochondrial-specific inhibitors has been evaluated in trypanosomatid parasites, essentially targeting ETC complexes. Due to the fact that the biological activity of complex I had not been demonstrated in trypanosomatids up to now, the effect of the classical inhibitor of NADH dehydrogenase, rotenone, is very debatable [68, 77, 83, 314]. On the other hand, ROS generation was stimulated in parasites treated with complexes II and III inhibitors (noyltrifluoroacetone and antimycin A, respectively), being correlated to the mitochondrial depolarization and an apoptotic-like events in some cases [66, 75, 83, 94, 315]. Interestingly, the inhibition of complex II promoted a potentialization of leishmanicidal effect of the clinical drug pentamidine in vitro [83]. In L. donovani, 4,4′-bis((tri-n-pentylphosphonium)methyl)benzophenone dibromide and sitamaquine, complex II inhibitors, led to a ROS production and deep mitochondrial alterations, including reduction in oxygen consumption and ATP levels as well as the remarkable swelling of the organelle and consequent cell cycle arrestment [316, 317]. Similar alterations could also be observed after treatment of L. donovani with tafenoquine and miltefosine, which inhibited complexes III and IV, respectively [318, 319]. Furthermore, in almost all pathogenic trypanosomatids, complex IV activity was, at least partially, inhibited by KCN, a classical cytochrome c oxidase inhibitor in higher eukaryotes [84, 91, 94, 320,321,322]. The absence of AOX in humans makes this oxidase another interesting mitochondrial drug target [323]. In T. brucei, it was demonstrated that ascofuranone affects ubiquinol oxidase activity in vitro, producing an apoptotic-like phenotype [323,324,325,326]. The alkyl lysophospholipid analogue edelfosine interferes in ATP synthase activity in Leishmania, being suggested a correlation between this biological effect to the leishmanicidal mechanism of action [327].
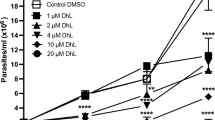
It is well-established that some chemical characteristics confer high redox potential to some compounds, leading to ROS generation [328, 329]. In this context, structural differences presented in the quinoidal nucleus directly influenced the oxidative capacity of quinones [330]. The effect of naturally occurring quinones and derivatives has been investigated in different Leishmania spp., T. cruzi, and T. brucei, and a promising activity was observed [331,332,333,334,335,336,337,338,339]. The first description of the oxidative effect of a quinone in trypanosomatids was performed in the late 1970s, demonstrating that beta-lapachone induced ROS generation in T. cruzi epimastigotes [340, 341]. More recently, other naphthoquinones also showed similar mode of action, producing considerable amounts of reactive species in this parasite [321, 342,343,344]. Almost a decade ago, a mechanistic proposal was raised by our research group, in order to explain the anti-T. cruzi effect of naphthofuranquinones. In 2009, our data pointed to the mitochondrial depolarization, derived from electron flow impairment, probably due to the electrons deviation from ubiquinone to the compounds. It compromises electron flux, producing ROS, leading to the impairment of the mitochondrial function, reflected by the reduction in respiratory rates, complexes I–III activity, and the dilation of the organelle [321]. Natural products also promoted mitochondrial damage/ROS production on different species of Leishmania. Quercetin, apigenin, and epigallocatequin-3-gallate are flavonoids working as potent ROS inductors in both Leishmania forms, causing mitochondrial dysfunction such as a decrease in ATP levels by altering mitochondrial membrane potential [345,346,347,348,349]. Thiosemicarbazones, 1,3,4-thiadiazole, and triazoles derivatives, or even other classes of drugs such as LQB-118, Flau-A, clioquinol, and pyrazyl/pyridylhydrazones derivatives, also induced morphological injury in the mitochondrion and ROS production, suggesting the trigger of parasite cell death [350,351,352,353,354,355,356]. Interestingly, a trypanothione reductase inhibitor, cyclobenzaprine also increased ROS levels, which may be the mechanism of the antileishmanial effect caused by this compound [357].
After half century of the development of nifurtimox and benznidazole, the available clinical options for Chagas disease, their mode of action is still debatable. The first mechanistic hypothesis for the trypanocidal activity of both compounds was proposed in early 1980s, indicating O2•– and H2O2 generation induced by nifurtimox; however, such production was not observed after the treatment with benznidazole [358,359,360,361]. In this way, the effect of nifurtimox depends on the type-II nitroreductases activity that transforms nitroanion radical, producing ROS and their subsequent biological consequences such as lipids peroxidation [359, 362, 363]. Unfortunately, Boiani et al. [364] showed no correlation between anti-T. cruzi effect of nifurtimox and ROS production, which was demonstrated by the absence of redox cycling at trypanocidal concentrations together with low molecular weight thiol reduction. Recently, the trypanocidal activities of nifurtimox and also benznidazole were associated with type-I nitroreductase in oxygen-independent way, and nitroso and hydroxylamine intermediates would generate amine, using NADH as a cofactor [365,366,367]. Based on the nitroderivative, different reactions of these intermediates would take place. For example, furane ring would be cleaved, producing a highly reactive unsaturated open chain nitrile in case of nifurtimox [367, 368]. For benznidazole, such cleavage led to glyoxal and other metabolites production, which directly interacts with DNA [367].
Moreover, despite the current clinical drugs for sleeping sickness or leishmaniasis have not been associated with oxidative stress, a great variety of compounds leads to the mitochondrial functional impairment, increasing ROS production during the treatment. The molecular targets of anti-trypanosomatid drugs involved in these protozoa oxidative stress events reported experimentally were described in Fig. 8.3.
6 Conclusions
During the macrophage/parasite interaction, the host cell triggers a signaling cascade, recruiting immune cells to combat the infection. Macrophages induce NO production via iNOS and O2., which is also required in initial phagocytic steps; therefore, an oxidative burst against the parasites is developed [262, 369, 370]. Both superoxide and NO can also generate peroxynitrite, a toxic-free radical for pathogens [371]. Neutrophils, the first-line defense at pathogen infection, are also able to induce NO production in order to kill parasites, but in a smaller scale than macrophages [371, 372]. However, both leishmania and trypanosome parasites developed several evasion systems, being able to fool host oxidative burst mechanisms.
Antioxidant machinery of trypanosomatids, especially trypanothione/trypanothione reductase pathway, is considered an interesting drug target, and many efforts have been made to the design of novel-specific inhibitors that do not interfere with mammalian systems [184]. Trypanothione is the most characterized antioxidant system in these parasites, once glutathione/glutathione peroxidase pathway and classical enzymes such as catalase are absent [185, 373]. Unfortunately, up to now, no promising inhibitors of any antioxidant enzyme of pathogenic trypanosomatids were found, despite all the efforts employed [374]. The high susceptibility of these protozoa to ROS in relation to their hosts is an old-fashioned concept, due to the presence of efficient scavengers in trypanosomatids [51, 57, 375].
Several preclinical studies associated oxidative stress to the mode of action of anti-trypanosomatid compounds, being the parasites mitochondrion, the main ROS source [98]. Curiously, the participation of this organelle in pathogenic trypanosomatids treated with different drugs has been extensively demonstrated, but the molecular mechanisms involved are still unknown in a large number of cases. The mitochondrial injury could be derived from a randomic outcome of an indirect effect (probably in the great majority of the cases) or even resulted from ETC-specific inhibition that generates a redox imbalance [312]. Previous data published showed high host toxicity of anti-trypanosomatid compounds with redox mode of action, suggestive of low drug specificity to these protozoa.
As it was mentioned, the significative role of ROS in cell signaling cannot be neglected. The pro-oxidant molecule heme triggers an oxidative stress, leading to calmodulin kinase II activation and consequent proliferation of T. cruzi epimastigotes [103]. On the other hand, ROS production also represents a crucial step for the success of these parasites in different hosts, and mitochondrial plasticity (morphological and molecular) has been postulated as an important adaptation, being ETC impairment directly associated to oxidative stress and loss of the redox balance [50]. The clinical correlation between the efficiency of mitochondrial antioxidant machinery (especially trypanothione synthetase and peroxiredoxins) of trypanosomatids and these parasites virulence was proposed, favoring the progression of the disease [277]. Studies about the molecular regulation of oxidative stress processes could base promising strategies for the development of new drugs.
References
World Health Organization (2016) Neglected tropical diseases. http://www.who.int/trypanosomiasis_african/en/index.html. Accessed 10 July 2018
World Health Organization (2008) The global burden of disease: 2004 update. http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/. Accessed 10 July 2018
World Health Organization (2018) What is leishmaniasis? http://www.who.int/leishmaniasis/disease/en/. Accessed 10 July 2018
Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, Team WLC (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7(5):e35671. https://doi.org/10.1371/journal.pone.0035671
Handman E (2001) Leishmaniasis: current status of vaccine development. Clin Microbiol Rev 14(2):229–243. https://doi.org/10.1128/cmr.14.2.229-243.2001
Anversa L, Tiburcio MGS, Richini-Pereira VB, Ramirez LE (2018) Human leishmaniasis in Brazil: a general review. Rev Assoc Med Bras (1992) 64(3):281–289. https://doi.org/10.1590/1806-9282.64.03.281
Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, López-Vélez R, García-Hernández R, Pountain AW, Mwenechanya R, Papadopoulou B (2017) Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Negl Trop Dis 11(12):e0006052. https://doi.org/10.1371/journal.pntd.0006052
Croft SL, Olliaro P (2011) Leishmaniasis chemotherapy – challenges and opportunities. Clin Microbiol Infect 17(10):1478–1483. https://doi.org/10.1111/j.1469-0691.2011.03630.x
Sundar S, Singh B (2018) Emerging therapeutic targets for treatment of leishmaniasis. Expert Opin Ther Targets 22(6):467–486. https://doi.org/10.1080/14728222.2018.1472241
Tiuman TS, Santos AO, Ueda-Nakamura T, Filho BP, Nakamura CV (2011) Recent advances in leishmaniasis treatment. Int J Infect Dis 15(8):e525–e532. https://doi.org/10.1016/j.ijid.2011.03.021
Ndjonka D, Rapado LN, Silber AM, Liebau E, Wrenger C (2013) Natural products as a source for treating neglected parasitic diseases. Int J Mol Sci 14(2):3395–3439. https://doi.org/10.3390/ijms14023395
Khalil NM, de Mattos AC, Carraro TC, Ludwig DB, Mainardes RM (2013) Nanotechnological strategies for the treatment of neglected diseases. Curr Pharm Des 19(41):7316–7329
Centers for Disease Control and Prevention (2018) Parasites – Leishmaniasis. https://www.cdc.gov/parasites/leishmaniasis/biology.html. Accessed 10 July 2018
Schmunis GA, Yadon ZE (2010) Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115(1–2):14–21. https://doi.org/10.1016/j.actatropica.2009.11.003
Schofield CJ, Jannin J, Salvatella R (2006) The future of Chagas disease control. Trends Parasitol 22(12):583–588. https://doi.org/10.1016/j.pt.2006.09.011
Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375(9723):1388–1402. https://doi.org/10.1016/s0140-6736(10)60061-x
Rassi A, Marcondes de Rezende J (2012) American trypanosomiasis (Chagas disease). Infect Dis Clin North Am 26(2):275–291. https://doi.org/10.1016/j.idc.2012.03.002
Urbina JA (2010) Specific chemotherapy of Chagas disease: relevance, current limitations and new approaches. Acta Trop 115(1–2):55–68. https://doi.org/10.1016/j.actatropica.2009.10.023
Soeiro MN, de Castro SL (2011) Screening of potential anti-Trypanosoma cruzi candidates: in vitro and in vivo studies. Open Med Chem J 5:21–30. https://doi.org/10.2174/1874104501105010021
Tyler KM, Engman DM (2001) The life cycle of Trypanosoma cruzi revisited. Int J Parasitol 31(5–6):472–481
Clayton J (2010) Chagas disease: pushing through the pipeline. Nature 465(7301):S12–S15. https://doi.org/10.1038/nature09224
Welburn SC, Molyneux DH, Maudlin I (2016) Beyond tsetse-implications for research and control of human african Trypanosomiasis epidemics. Trends Parasitol 32(3):230–241. https://doi.org/10.1016/j.pt.2015.11.008
Lumsden WH (1970) Trypanosomiasis. Trop Dis Bull 67(5):465–481
Malvy D, Chappuis F (2011) Sleeping sickness. Clin Microbiol Infect 17(7):986–995. https://doi.org/10.1111/j.1469-0691.2011.03536.x
World Health Organization (2016) Human African trypanosomiasis. http://www.who.int/trypanosomiasis_african/en/. Accessed 10 July 2018
Kennedy PG (2013) Clinical features, diagnosis, and treatment of human African trypanosomiasis (sleeping sickness). Lancet Neurol 12(2):186–194. https://doi.org/10.1016/s1474-4422(12)70296-x
Steverding D (2010) The development of drugs for treatment of sleeping sickness: a historical review. Parasit Vectors 3(1):15. https://doi.org/10.1186/1756-3305-3-15
Brun R, Don R, Jacobs RT, Wang MZ, Barrett MP (2011) Development of novel drugs for human African trypanosomiasis. Fut Microbiol 6(6):677–691. https://doi.org/10.2217/fmb.11.44
Simarro PP, Franco J, Diarra A, Postigo JA, Jannin J (2012) Update on field use of the available drugs for the chemotherapy of human African trypanosomiasis. Parasitology 139(7):842–846. https://doi.org/10.1017/s0031182012000169
Horn D (2014) Antigenic variation in African trypanosomes. Mol Biochem Parasitol 195(2):123–129. https://doi.org/10.1016/j.molbiopara.2014.05.001
Langousis G, Hill KL (2014) Motility and more: the flagellum of Trypanosoma brucei. Nat Rev Microbiol 12(7):505–518. https://doi.org/10.1038/nrmicro3274
Harman D (2009) Origin and evolution of the free radical theory of aging: a brief personal history, 1954–2009. Biogerontology 10(6):773–781. https://doi.org/10.1007/s10522-009-9234-2
Babior BM, Kipnes RS, Curnutte JT (1973) Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest 52(3):741–744. https://doi.org/10.1172/jci107236
Rossi F, Della Bianca V, de Togni P (1985) Mechanisms and functions of the oxygen radicals producing respiration of phagocytes. Comp Immunol Microbiol Infect Dis 8(2):187–204
Shaikhali J, Heiber I, Seidel T, Ströher E, Hiltscher H, Birkmann S, Dietz KJ, Baier M (2008) The redox-sensitive transcription factor Rap2.4a controls nuclear expression of 2-Cys peroxiredoxin A and other chloroplast antioxidant enzymes. BMC Plant Biol 8:48. https://doi.org/10.1186/1471-2229-8-48
Riley PA (1994) Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 65(1):27–33
Poljsak B, Šuput D, Milisav I (2013) Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013:956792. https://doi.org/10.1155/2013/956792
Sies H (2015) Oxidative stress: a concept in redox biology and medicine. Redox Biol 4:180–183. https://doi.org/10.1016/j.redox.2015.01.002
Chen X, Wang F, Hyun JY, Wei T, Qiang J, Ren X, Shin I, Yoon J (2016) Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem Soc Rev 45(10):2976–3016. https://doi.org/10.1039/c6cs00192k
Sies H (1991) Role of reactive oxygen species in biological processes. Klin Wochenschr 69(21–23):965–968
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 97:55–74. https://doi.org/10.1016/j.ejmech.2015.04.040
Xing J, Wang G, Zhang Q, Liu X, Gu Z, Zhang H, Chen YQ, Chen W (2015) Determining antioxidant activities of lactobacilli cell-free supernatants by cellular antioxidant assay: a comparison with traditional methods. PLoS One 10(3):e0119058. https://doi.org/10.1371/journal.pone.0119058
Lushchak VI (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 224:164–175. https://doi.org/10.1016/j.cbi.2014.10.016
Mitchell P (1961) Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191:144–148
Vercesi AE, Oliveira HCF (2018) Contribution to mitochondrial research in Brazil: 10th anniversary of the mitomeeting. Cell Biol Int 42(6):626–629. https://doi.org/10.1002/cbin.10898
Guo R, Zong S, Wu M, Gu J, Yang M (2017) Architecture of Human Mitochondrial Respiratory Megacomplex I. Cell 170(6):1247–1257.e1212. https://doi.org/10.1016/j.cell.2017.07.050
Chance B, Williams GR (1956) The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem 17:65–134
Schägger H (2001) Respiratory chain supercomplexes. IUBMB Life 52(3–5):119–128. https://doi.org/10.1080/15216540152845911
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12(5):913–922. https://doi.org/10.1007/s10495-007-0756-2
Venditti P, Di Stefano L, Di Meo S (2013) Mitochondrial metabolism of reactive oxygen species. Mitochondrion 13(2):71–82. https://doi.org/10.1016/j.mito.2013.01.008
Boveris A, Stoppani AO (1977) Hydrogen peroxide generation in Trypanosoma cruzi. Experientia 33(10):1306–1308
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417(1):1–13. https://doi.org/10.1042/bj20081386
Wang Y, Hekimi S (2016) Understanding Ubiquinone. Trends Cell Biol 26(5):367–378. https://doi.org/10.1016/j.tcb.2015.12.007
Gutteridge JM (1994) Biological origin of free radicals, and mechanisms of antioxidant protection. Chem Biol Interact 91(2–3):133–140
Gutteridge JM, Halliwell B (1992) Comments on review of free radicals in biology and medicine, second edition, by Barry Halliwell and John M. C. Gutteridge. Free Radic Biol Med 12(1):93–95
Kohen R, Nyska A (2002) Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30(6):620–650. https://doi.org/10.1080/01926230290166724
Tomás AM, Castro H (2013) Redox metabolism in mitochondria of trypanosomatids. Antioxid Redox Signal 19(7):696–707. https://doi.org/10.1089/ars.2012.4948
Tielens AG, van Hellemond JJ (2009) Surprising variety in energy metabolism within Trypanosomatidae. Trends Parasitol 25(10):482–490. https://doi.org/10.1016/j.pt.2009.07.007
Marques I, Duarte M, Assunção J, Ushakova AV, Videira A (2005) Composition of complex I from Neurospora crassa and disruption of two “accessory” subunits. Biochim Biophys Acta 1707(2-3):211–220. https://doi.org/10.1016/j.bbabio.2004.12.003
Carroll J, Fearnley IM, Walker JE (2006) Definition of the mitochondrial proteome by measurement of molecular masses of membrane proteins. Proc Natl Acad Sci U S A 103(44):16170–16175. https://doi.org/10.1073/pnas.0607719103
Galkin A, Moncada S (2017) Modulation of the conformational state of mitochondrial complex I as a target for therapeutic intervention. Interface Focus 7(2):20160104. https://doi.org/10.1098/rsfs.2016.0104
Hatefi Y (1985) The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem 54:1015–1069. https://doi.org/10.1146/annurev.bi.54.070185.005055
Opperdoes FR, Michels PA (2008) Complex I of Trypanosomatidae: does it exist? Trends Parasitol 24(7):310–317. https://doi.org/10.1016/j.pt.2008.03.013
Turrens JF (1989) The role of succinate in the respiratory chain of Trypanosoma brucei procyclic trypomastigotes. Biochem J 259(2):363–368
Carranza JC, Kowaltowski AJ, Mendonça MA, de Oliveira TC, Gadelha FR, Zingales B (2009) Mitochondrial bioenergetics and redox state are unaltered in Trypanosoma cruzi isolates with compromised mitochondrial complex I subunit genes. J Bioenerg Biomembr 41(3):299–308. https://doi.org/10.1007/s10863-009-9228-4
Beattie DS, Obungu VH, Kiaira JK (1994) Oxidation of NADH by a rotenone and antimycin-sensitive pathway in the mitochondrion of procyclic Trypanosoma brucei brucei. Mol Biochem Parasitol 64(1):87–94
Beattie DS, Howton MM (1996) The presence of rotenone-sensitive NADH dehydrogenase in the long slender bloodstream and the procyclic forms of Trypanosoma brucei brucei. Eur J Biochem 241(3):888–894
Hernandez FR, Turrens JF (1998) Rotenone at high concentrations inhibits NADH-fumarate reductase and the mitochondrial respiratory chain of Trypanosoma brucei and T. cruzi. Mol Biochem Parasitol 93(1):135–137
Jasmer DP, Feagin JE, Stuart K (1985) Diverse patterns of expression of the cytochrome c oxidase subunit I gene and unassigned reading frames 4 and 5 during the life cycle of Trypanosoma brucei. Mol Cell Biol 5(11):3041–3047
Souza AE, Myler PJ, Stuart K (1992) Maxicircle CR1 transcripts of Trypanosoma brucei are edited and developmentally regulated and encode a putative iron-sulfur protein homologous to an NADH dehydrogenase subunit. Mol Cell Biol 12(5):2100–2107
Verner Z, Cermáková P, Skodová I, Kriegová E, Horváth A, Lukes J (2011) Complex I (NADH:ubiquinone oxidoreductase) is active in but non-essential for procyclic Trypanosoma brucei. Mol Biochem Parasitol 175(2):196–200. https://doi.org/10.1016/j.molbiopara.2010.11.003
Fang J, Beattie DS (2003) Alternative oxidase present in procyclic Trypanosoma brucei may act to lower the mitochondrial production of superoxide. Arch Biochem Biophys 414(2):294–302
Fisher N, Bray PG, Ward SA, Biagini GA (2007) The malaria parasite type II NADH:quinone oxidoreductase: an alternative enzyme for an alternative lifestyle. Trends Parasitol 23(7):305–310. https://doi.org/10.1016/j.pt.2007.04.014
Fang J, Beattie DS (2003) Identification of a gene encoding a 54 kDa alternative NADH dehydrogenase in Trypanosoma brucei. Mol Biochem Parasitol 127(1):73–77
Fang J, Beattie DS (2002) Rotenone-insensitive NADH dehydrogenase is a potential source of superoxide in procyclic Trypanosoma brucei mitochondria. Mol Biochem Parasitol 123(2):135–142
Verner Z, Skodová I, Poláková S, Durišová-Benkovičová V, Horváth A, Lukeš J (2013) Alternative NADH dehydrogenase (NDH2): intermembrane-space-facing counterpart of mitochondrial complex I in the procyclic Trypanosoma brucei. Parasitology 140(3):328–337. https://doi.org/10.1017/s003118201200162x
Surve S, Heestand M, Panicucci B, Schnaufer A, Parsons M (2012) Enigmatic presence of mitochondrial complex I in Trypanosoma brucei bloodstream forms. Eukaryot Cell 11(2):183–193. https://doi.org/10.1128/ec.05282-11
Boveris A, Hertig CM, Turrens JF (1986) Fumarate reductase and other mitochondrial activities in Trypanosoma cruzi. Mol Biochem Parasitol 19(2):163–169
Besteiro S, Biran M, Biteau N, Coustou V, Baltz T, Canioni P, Bringaud F (2002) Succinate secreted by Trypanosoma brucei is produced by a novel and unique glycosomal enzyme, NADH-dependent fumarate reductase. J Biol Chem 277(41):38001–38012. https://doi.org/10.1074/jbc.M201759200
Coustou V, Biran M, Besteiro S, Rivière L, Baltz T, Franconi JM, Bringaud F (2006) Fumarate is an essential intermediary metabolite produced by the procyclic Trypanosoma brucei. J Biol Chem 281(37):26832–26846. https://doi.org/10.1074/jbc.M601377200
Coustou V, Besteiro S, Rivière L, Biran M, Biteau N, Franconi JM, Boshart M, Baltz T, Bringaud F (2005) A mitochondrial NADH-dependent fumarate reductase involved in the production of succinate excreted by procyclic Trypanosoma brucei. J Biol Chem 280(17):16559–16570. https://doi.org/10.1074/jbc.M500343200
Coustou V, Biran M, Breton M, Guegan F, Rivière L, Plazolles N, Nolan D, Barrett MP, Franconi JM, Bringaud F (2008) Glucose-induced remodeling of intermediary and energy metabolism in procyclic Trypanosoma brucei. J Biol Chem 283(24):16342–16354. https://doi.org/10.1074/jbc.M709592200
Mehta A, Shaha C (2004) Apoptotic death in Leishmania donovani promastigotes in response to respiratory chain inhibition: complex II inhibition results in increased pentamidine cytotoxicity. J Biol Chem 279(12):11798–11813. https://doi.org/10.1074/jbc.M309341200
Silva TM, Peloso EF, Vitor SC, Ribeiro LH, Gadelha FR (2011) O2 consumption rates along the growth curve: new insights into Trypanosoma cruzi mitochondrial respiratory chain. J Bioenerg Biomembr 43(4):409–417. https://doi.org/10.1007/s10863-011-9369-0
Evans DA, Brown RC (1973) The inhibitory effects of aromatic hydroxamic acids on the cyanide-insensitive terminal oxidase of Trypanosoma brucei. Trans R Soc Trop Med Hyg 67(2):258
Edwards C, Chance B (1982) Evidence for the presence of two terminal oxidases in the trypanosomatid Crithidia oncopelti. J Gen Microbiol 128(7):1409–1414. https://doi.org/10.1099/00221287-128-7-1409
Opperdoes FR, Butenko A, Flegontov P, Yurchenko V, Lukeš J (2016) Comparative metabolism of free-living Bodo saltans and parasitic trypanosomatids. J Eukaryot Microbiol 63(5):657–678. https://doi.org/10.1111/jeu.12315
Gnipová A, Panicucci B, Paris Z, Verner Z, Horváth A, Lukeš J, Zíková A (2012) Disparate phenotypic effects from the knockdown of various Trypanosoma brucei cytochrome c oxidase subunits. Mol Biochem Parasitol 184(2):90–98. https://doi.org/10.1016/j.molbiopara.2012.04.013
Chaudhuri M, Ott RD, Hill GC (2006) Trypanosome alternative oxidase: from molecule to function. Trends Parasitol 22(10):484–491. https://doi.org/10.1016/j.pt.2006.08.007
Horváth A, Horáková E, Dunajcíková P, Verner Z, Pravdová E, Slapetová I, Cuninková L, Lukes J (2005) Downregulation of the nuclear-encoded subunits of the complexes III and IV disrupts their respective complexes but not complex I in procyclic Trypanosoma brucei. Mol Microbiol 58(1):116–130. https://doi.org/10.1111/j.1365-2958.2005.04813.x
Santhamma KR, Bhaduri A (1995) Characterization of the respiratory chain of Leishmania donovani promastigotes. Mol Biochem Parasitol 75(1):43–53
Atwood JA, Weatherly DB, Minning TA, Bundy B, Cavola C, Opperdoes FR, Orlando R, Tarleton RL (2005) The Trypanosoma cruzi proteome. Science 309(5733):473–476. https://doi.org/10.1126/science.1110289
Shah-Simpson S, Pereira CF, Dumoulin PC, Caradonna KL, Burleigh BA (2016) Bioenergetic profiling of Trypanosoma cruzi life stages using Seahorse extracellular flux technology. Mol Biochem Parasitol 208(2):91–95. https://doi.org/10.1016/j.molbiopara.2016.07.001
Gonçalves RL, Barreto RF, Polycarpo CR, Gadelha FR, Castro SL, Oliveira MF (2011) A comparative assessment of mitochondrial function in epimastigotes and bloodstream trypomastigotes of Trypanosoma cruzi. J Bioenerg Biomembr 43(6):651–661. https://doi.org/10.1007/s10863-011-9398-8
Tielens AG, Van Hellemond JJ (1998) Differences in energy metabolism between trypanosomatidae. Parasitol Today 14(7):265–272
Clayton CE, Michels P (1996) Metabolic compartmentation in African trypanosomes. Parasitol Today 12(12):465–471
Priest JW, Hajduk SL (1994) Developmental regulation of Trypanosoma brucei cytochrome c reductase during bloodstream to procyclic differentiation. Mol Biochem Parasitol 65(2):291–304
Bringaud F, Rivière L, Coustou V (2006) Energy metabolism of trypanosomatids: adaptation to available carbon sources. Mol Biochem Parasitol 149(1):1–9. https://doi.org/10.1016/j.molbiopara.2006.03.017
Silber AM, Tonelli RR, Lopes CG, Cunha-e-Silva N, Torrecilhas AC, Schumacher RI, Colli W, Alves MJ (2009) Glucose uptake in the mammalian stages of Trypanosoma cruzi. Mol Biochem Parasitol 168(1):102–108. https://doi.org/10.1016/j.molbiopara.2009.07.006
Harington JS (1956) Histamine and histidine in excreta of the blood-sucking bug Rhodnius prolixus. Nature 178(4527):268
Harington JS (1961) Studies of the amino acids of Rhodnius prolixus I. Analysis of the haemolymph. Parasitology 51:309–318
Finzi JK, Chiavegatto CW, Corat KF, Lopez JA, Cabrera OG, Mielniczki-Pereira AA, Colli W, Alves MJ, Gadelha FR (2004) Trypanosoma cruzi response to the oxidative stress generated by hydrogen peroxide. Mol Biochem Parasitol 133(1):37–43
Nogueira NP, de Souza CF, Saraiva FM, Sultano PE, Dalmau SR, Bruno RE, Gonçalves RL, Laranja GA, Leal LH, Coelho MG, Masuda CA, Oliveira MF, Paes MC (2011) Heme-induced ROS in Trypanosoma cruzi activates CaMKII-like that triggers epimastigote proliferation. One helpful effect of ROS. PLoS One 6(10):e25935. https://doi.org/10.1371/journal.pone.0025935
Nogueira NP, Saraiva FMS, Oliveira MP, Mendonça APM, Inacio JDF, Almeida-Amaral EE, Menna-Barreto RF, Laranja GAT, Torres EJL, Oliveira MF, Paes MC (2017) Heme modulates Trypanosoma cruzi bioenergetics inducing mitochondrial ROS production. Free Radic Biol Med 108:183–191. https://doi.org/10.1016/j.freeradbiomed.2017.03.027
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG (1981) Sequence and organization of the human mitochondrial genome. Nature 290(5806):457–465
Vercesi AE, Martins LS, MAP S, HMF L, Cuccovia IM, Chaimovich H (1995) PUMPing plants. Nature 375:24. https://doi.org/10.1038/375024a0
Vercesi AE, Borecký J, Maia IG, Arruda P, Cuccovia IM, Chaimovich H (2006) Plant uncoupling mitochondrial proteins. Annu Rev Plant Biol 57:383–404. https://doi.org/10.1146/annurev.arplant.57.032905.105335
Hassanpour SH, Dehghani MA, Karami SZ (2018) Study of respiratory chain dysfunction in heart disease. J Cardiovasc Thorac Res 10 (1):1–13. https://doi.org/10.15171/jcvtr.2018.01
Wong HS, Dighe PA, Mezera V, Monternier PA, Brand MD (2017) Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. J Biol Chem 292(41):16804–16809. https://doi.org/10.1074/jbc.R117.789271
Sazanov LA, Hinchliffe P (2006) Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311(5766):1430–1436. https://doi.org/10.1126/science.1123809
Hinkle PC, Butow RA, Racker E, Chance B (1967) Partial resolution of the enzymes catalyzing oxidative phosphorylation. XV. Reverse electron transfer in the flavin-cytochrome beta region of the respiratory chain of beef heart submitochondrial particles. J Biol Chem 242(22):5169–5173
Cadenas E, Boveris A, Ragan CI, Stoppani AO (1977) Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch Biochem Biophys 180(2):248–257
Kussmaul L, Hirst J (2006) The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A 103(20):7607–7612. https://doi.org/10.1073/pnas.0510977103
Hirst J, King MS, Pryde KR (2008) The production of reactive oxygen species by complex I. Biochem Soc Trans 36(Pt 5):976–980. https://doi.org/10.1042/bst0360976
Seo BB, Marella M, Yagi T, Matsuno-Yagi A (2006) The single subunit NADH dehydrogenase reduces generation of reactive oxygen species from complex I. FEBS Lett 580(26):6105–6108. https://doi.org/10.1016/j.febslet.2006.10.008
Bleier L, Dröse S (2013) Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim Biophys Acta 1827(11–12):1320–1331. https://doi.org/10.1016/j.bbabio.2012.12.002
Cadenas S (2018) Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta. https://doi.org/10.1016/j.bbabio.2018.05.019
Rolfe DF, Brand MD (1996) Contribution of mitochondrial proton leak to skeletal muscle respiration and to standard metabolic rate. Am J Physiol 271(4 Pt 1):C1380–C1389. https://doi.org/10.1152/ajpcell.1996.271.4.C1380
Stuart JA, Cadenas S, Jekabsons MB, Roussel D, Brand MD (2001) Mitochondrial proton leak and the uncoupling protein 1 homologues. Biochim Biophys Acta 1504(1):144–158
Boveris A, Chance B (1973) The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J 134(3):707–716
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416(1):15–18
Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD (2010) Mitochondrial proton and electron leaks. Essays Biochem 47:53–67. https://doi.org/10.1042/bse0470053
Woyda-Ploszczyca AM, Jarmuszkiewicz W (2017) The conserved regulation of mitochondrial uncoupling proteins: From unicellular eukaryotes to mammals. Biochim Biophys Acta 1858(1):21–33. https://doi.org/10.1016/j.bbabio.2016.10.003
Nicholls DG, Bernson VS, Heaton GM (1978) The identification of the component in the inner membrane of brown adipose tissue mitochondria responsible for regulating energy dissipation. Experientia Suppl 32:89–93
Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH (1997) Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 15(3):269–272. https://doi.org/10.1038/ng0397-269
Boss O, Samec S, Paoloni-Giacobino A, Rossier C, Dulloo A, Seydoux J, Muzzin P, Giacobino JP (1997) Uncoupling protein-3: a new member of the mitochondrial carrier family with tissue-specific expression. FEBS Lett 408(1):39–42
Mao W, Yu XX, Zhong A, Li W, Brush J, Sherwood SW, Adams SH, Pan G (1999) UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett 443(3):326–330
Klingenberg M (1990) Mechanism and evolution of the uncoupling protein of brown adipose tissue. Trends Biochem Sci 15(3):108–112
Sluse FE (2012) Uncoupling proteins: molecular, functional, regulatory, physiological and pathological aspects. Adv Exp Med Biol 942:137–156. https://doi.org/10.1007/978-94-007-2869-1_6
Nègre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Pénicaud L, Casteilla L (1997) A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J 11(10):809–815
Azzu V, Jastroch M, Divakaruni AS, Brand MD (2010) The regulation and turnover of mitochondrial uncoupling proteins. Biochim Biophys Acta 1797(6-7):785–791. https://doi.org/10.1016/j.bbabio.2010.02.035
Mailloux RJ, Harper ME (2011) Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic Biol Med 51(6):1106–1115. https://doi.org/10.1016/j.freeradbiomed.2011.06.022
MacLellan JD, Gerrits MF, Gowing A, Smith PJ, Wheeler MB, Harper ME (2005) Physiological increases in uncoupling protein 3 augment fatty acid oxidation and decrease reactive oxygen species production without uncoupling respiration in muscle cells. Diabetes 54(8):2343–2350
Tahara EB, Navarete FD, Kowaltowski AJ (2009) Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46(9):1283–1297. https://doi.org/10.1016/j.freeradbiomed.2009.02.008
Toime LJ, Brand MD (2010) Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic Biol Med 49(4):606–611. https://doi.org/10.1016/j.freeradbiomed.2010.05.010
Brigelius-Flohé R, Flohé L (2011) Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal 15(8):2335–2381. https://doi.org/10.1089/ars.2010.3534
Scandalios JG (2005) Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res 38 (7):995–1014. doi:S0100-879x2005000700003
Babior BM, Curnutte JT, Kipnes RS (1975) Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med 85(2):235–244
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106(1):207–212
Fairlamb AH, Blackburn P, Ulrich P, Chait BT, Cerami A (1985) Trypanothione: a novel bis(glutathionyl)spermidine cofactor for glutathione reductase in trypanosomatids. Science 227(4693):1485–1487
Shames SL, Fairlamb AH, Cerami A, Walsh CT (1986) Purification and characterization of trypanothione reductase from Crithidia fasciculata, a newly discovered member of the family of disulfide-containing flavoprotein reductases. Biochemistry 25(12):3519–3526
Krauth-Siegel RL, Enders B, Henderson GB, Fairlamb AH, Schirmer RH (1987) Trypanothione reductase from Trypanosoma cruzi. Purification and characterization of the crystalline enzyme. Eur J Biochem 164(1):123–128
Sullivan FX, Walsh CT (1991) Cloning, sequencing, overproduction and purification of trypanothione reductase from Trypanosoma cruzi. Mol Biochem Parasitol 44(1):145–147
Aboagye-Kwarteng T, Smith K, Fairlamb AH (1992) Molecular characterization of the trypanothione reductase gene from Crithidia fasciculata and Trypanosoma brucei: comparison with other flavoprotein disulphide oxidoreductases with respect to substrate specificity and catalytic mechanism. Mol Microbiol 6(21):3089–3099
Manta B, Comini M, Medeiros A, Hugo M, Trujillo M, Radi R (2013) Trypanothione: a unique bis-glutathionyl derivative in trypanosomatids. Biochim Biophys Acta 1830(5):3199–3216. https://doi.org/10.1016/j.bbagen.2013.01.013
Lueder DV, Phillips MA (1996) Characterization of Trypanosoma brucei gamma-glutamylcysteine synthetase, an essential enzyme in the biosynthesis of trypanothione (diglutathionylspermidine). J Biol Chem 271(29):17485–17490
Grondin K, Haimeur A, Mukhopadhyay R, Rosen BP, Ouellette M (1997) Co-amplification of the gamma-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J 16(11):3057–3065. https://doi.org/10.1093/emboj/16.11.3057
Olin-Sandoval V, González-Chávez Z, Berzunza-Cruz M, Martínez I, Jasso-Chávez R, Becker I, Espinoza B, Moreno-Sánchez R, Saavedra E (2012) Drug target validation of the trypanothione pathway enzymes through metabolic modelling. FEBS J 279(10):1811–1833. https://doi.org/10.1111/j.1742-4658.2012.08557.x
Mukherjee P, Majee SB, Ghosh S, Hazra B (2009) Apoptosis-like death in Leishmania donovani promastigotes induced by diospyrin and its ethanolamine derivative. Int J Antimicrob Agents 34(6):596–601. https://doi.org/10.1016/j.ijantimicag.2009.08.007
Huynh TT, Huynh VT, Harmon MA, Phillips MA (2003) Gene knockdown of gamma-glutamylcysteine synthetase by RNAi in the parasitic protozoa Trypanosoma brucei demonstrates that it is an essential enzyme. J Biol Chem 278(41):39794–39800. https://doi.org/10.1074/jbc.M306306200
Jones DP, Carlson JL, Samiec PS, Sternberg P, Mody VC, Reed RL, Brown LA (1998) Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta 275(2):175–184
Duszenko M, Ferguson MA, Lamont GS, Rifkin MR, Cross GA (1985) Cysteine eliminates the feeder cell requirement for cultivation of Trypanosoma brucei bloodstream forms in vitro. J Exp Med 162(4):1256–1263
Duszenko M, Mühlstädt K, Broder A (1992) Cysteine is an essential growth factor for Trypanosoma brucei bloodstream forms. Mol Biochem Parasitol 50(2):269–273
Hesse F, Selzer PM, Mühlstädt K, Duszenko M (1995) A novel cultivation technique for long-term maintenance of bloodstream form trypanosomes in vitro. Mol Biochem Parasitol 70(1-2):157–166
Williams RA, Westrop GD, Coombs GH (2009) Two pathways for cysteine biosynthesis in Leishmania major. Biochem J 420(3):451–462. https://doi.org/10.1042/bj20082441
Romero I, Téllez J, Romanha AJ, Steindel M, Grisard EC (2015) Upregulation of Cysteine Synthase and Cystathionine β-Synthase Contributes to Leishmania braziliensis Survival under Oxidative Stress. Antimicrob Agents Chemother 59(8):4770–4781. https://doi.org/10.1128/aac.04880-14
Romero I, Téllez J, Yamanaka LE, Steindel M, Romanha AJ, Grisard EC (2014) Transsulfuration is an active pathway for cysteine biosynthesis in Trypanosoma rangeli. Parasit Vectors 7:197. https://doi.org/10.1186/1756-3305-7-197
Marciano D, Santana M, Nowicki C (2012) Functional characterization of enzymes involved in cysteine biosynthesis and H(2)S production in Trypanosoma cruzi. Mol Biochem Parasitol 185(2):114–120. https://doi.org/10.1016/j.molbiopara.2012.07.009
Okalang U, Nanteza A, Matovu E, Lubega GW (2013) Identification of coding sequences from a freshly prepared Trypanosoma brucei brucei expression library by polymerase chain reaction. Int J Biochem Mol Biol 4(2):73–82
Colotti G, Ilari A (2011) Polyamine metabolism in Leishmania: from arginine to trypanothione. Amino Acids 40(2):269–285. https://doi.org/10.1007/s00726-010-0630-3
Hasne MP, Ullman B (2005) Identification and characterization of a polyamine permease from the protozoan parasite Leishmania major. J Biol Chem 280(15):15188–15194. https://doi.org/10.1074/jbc.M411331200
Hasne MP, Ullman B (2011) Genetic and biochemical analysis of protozoal polyamine transporters. Methods Mol Biol 720:309–326. https://doi.org/10.1007/978-1-61779-034-8_19
Carrillo C, Cejas S, González NS, Algranati ID (1999) Trypanosoma cruzi epimastigotes lack ornithine decarboxylase but can express a foreign gene encoding this enzyme. FEBS Lett 454(3):192–196
Carrillo C, Cejas S, Huber A, González NS, Algranati ID (2003) Lack of arginine decarboxylase in Trypanosoma cruzi epimastigotes. J Eukaryot Microbiol 50(5):312–316
Reigada C, Sayé M, Vera EV, Balcazar D, Fraccaroli L, Carrillo C, Miranda MR, Pereira CA (2016) Trypanosoma cruzi Polyamine Transporter: Its Role on Parasite Growth and Survival Under Stress Conditions. J Membr Biol 249(4):475–481. https://doi.org/10.1007/s00232-016-9888-z
Smith K, Nadeau K, Bradley M, Walsh C, Fairlamb AH (1992) Purification of glutathionylspermidine and trypanothione synthetases from Crithidia fasciculata. Protein Sci 1(7):874–883. https://doi.org/10.1002/pro.5560010705
Oza SL, Tetaud E, Ariyanayagam MR, Warnon SS, Fairlamb AH (2002) A single enzyme catalyses formation of Trypanothione from glutathione and spermidine in Trypanosoma cruzi. J Biol Chem 277(39):35853–35861. https://doi.org/10.1074/jbc.M204403200
Oza SL, Ariyanayagam MR, Aitcheson N, Fairlamb AH (2003) Properties of trypanothione synthetase from Trypanosoma brucei. Mol Biochem Parasitol 131(1):25–33
Comini M, Menge U, Wissing J, Flohé L (2005) Trypanothione synthesis in crithidia revisited. J Biol Chem 280(8):6850–6860. https://doi.org/10.1074/jbc.M404486200
Gilbert HF (1990) Molecular and cellular aspects of thiol-disulfide exchange. Adv Enzymol Relat Areas Mol Biol 63:69–172
Moutiez M, Aumercier M, Schöneck R, Meziane-Cherif D, Lucas V, Aumercier P, Ouaissi A, Sergheraert C, Tartar A (1995) Purification and characterization of a trypanothione-glutathione thioltransferase from Trypanosoma cruzi. Biochem J 310(Pt 2):433–437
Fraser-L’Hostis C, Defrise-Quertain F, Coral D, Deshusses J (1997) Regulation of the intracellular pH in the protozoan parasite Trypanosoma brucei brucei. Biol Chem 378(9):1039–1046
Irigoín F, Cibils L, Comini MA, Wilkinson SR, Flohé L, Radi R (2008) Insights into the redox biology of Trypanosoma cruzi: Trypanothione metabolism and oxidant detoxification. Free Radic Biol Med 45(6):733–742. https://doi.org/10.1016/j.freeradbiomed.2008.05.028
Ariyanayagam MR, Oza SL, Mehlert A, Fairlamb AH (2003) Bis(glutathionyl)spermine and other novel trypanothione analogues in Trypanosoma cruzi. J Biol Chem 278(30):27612–27619. https://doi.org/10.1074/jbc.M302750200
Stoll VS, Simpson SJ, Krauth-Siegel RL, Walsh CT, Pai EF (1997) Glutathione reductase turned into trypanothione reductase: structural analysis of an engineered change in substrate specificity. Biochemistry 36(21):6437–6447. https://doi.org/10.1021/bi963074p
Meziane-Cherif D, Aumercier M, Kora I, Sergheraert C, Tartar A, Dubremetz JF, Ouaissi MA (1994) Trypanosoma cruzi: immunolocalization of trypanothione reductase. Exp Parasitol 79(4):536–541
Wilkinson SR, Meyer DJ, Taylor MC, Bromley EV, Miles MA, Kelly JM (2002) The Trypanosoma cruzi enzyme TcGPXI is a glycosomal peroxidase and can be linked to trypanothione reduction by glutathione or tryparedoxin. J Biol Chem 277(19):17062–17071. https://doi.org/10.1074/jbc.M111126200
Wilkinson SR, Prathalingam SR, Taylor MC, Ahmed A, Horn D, Kelly JM (2006) Functional characterisation of the iron superoxide dismutase gene repertoire in Trypanosoma brucei. Free Radic Biol Med 40(2):198–209. https://doi.org/10.1016/j.freeradbiomed.2005.06.022
Smith K, Opperdoes FR, Fairlamb AH (1991) Subcellular distribution of trypanothione reductase in bloodstream and procyclic forms of Trypanosoma brucei. Mol Biochem Parasitol 48(1):109–112
Sommer JM, Wang CC (1994) Targeting proteins to the glycosomes of African trypanosomes. Annu Rev Microbiol 48:105–138. https://doi.org/10.1146/annurev.mi.48.100194.000541
Schlecker T, Schmidt A, Dirdjaja N, Voncken F, Clayton C, Krauth-Siegel RL (2005) Substrate specificity, localization, and essential role of the glutathione peroxidase-type tryparedoxin peroxidases in Trypanosoma brucei. J Biol Chem 280(15):14385–14394. https://doi.org/10.1074/jbc.M413338200
Tovar J, Cunningham ML, Smith AC, Croft SL, Fairlamb AH (1998) Down-regulation of Leishmania donovani trypanothione reductase by heterologous expression of a trans-dominant mutant homologue: effect on parasite intracellular survival. Proc Natl Acad Sci U S A 95(9):5311–5316
Krieger S, Schwarz W, Ariyanayagam MR, Fairlamb AH, Krauth-Siegel RL, Clayton C (2000) Trypanosomes lacking trypanothione reductase are avirulent and show increased sensitivity to oxidative stress. Mol Microbiol 35(3):542–552
Leroux AE, Krauth-Siegel RL (2016) Thiol redox biology of trypanosomatids and potential targets for chemotherapy. Mol Biochem Parasitol 206(1–2):67–74. https://doi.org/10.1016/j.molbiopara.2015.11.003
Boveris A, Sies H, Martino EE, Docampo R, Turrens JF, Stoppani AO (1980) Deficient metabolic utilization of hydrogen peroxide in Trypanosoma cruzi. Biochem J 188(3):643–648
Kraeva N, Horáková E, Kostygov AY, Kořený L, Butenko A, Yurchenko V, Lukeš J (2017) Catalase in Leishmaniinae: With me or against me? Infect Genet Evol 50:121–127. https://doi.org/10.1016/j.meegid.2016.06.054
Penketh PG, Kennedy WP, Patton CL, Sartorelli AC (1987) Trypanosomatid hydrogen peroxide [corrected] metabolism. FEBS Lett 221(2):427–431
Carnieri EG, Moreno SN, Docampo R (1993) Trypanothione-dependent peroxide metabolism in Trypanosoma cruzi different stages. Mol Biochem Parasitol 61(1):79–86
Nogoceke E, Gommel DU, Kiess M, Kalisz HM, Flohé L (1997) A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol Chem 378(8):827–836
Castro H, Tomás AM (2008) Peroxidases of trypanosomatids. Antioxid Redox Signal 10(9):1593–1606. https://doi.org/10.1089/ars.2008.2050
Castro H, Sousa C, Novais M, Santos M, Budde H, Cordeiro-da-Silva A, Flohé L, Tomás AM (2004) Two linked genes of Leishmania infantum encode tryparedoxins localised to cytosol and mitochondrion. Mol Biochem Parasitol 136(2):137–147
Castro H, Romao S, Gadelha FR, Tomás AM (2008) Leishmania infantum: provision of reducing equivalents to the mitochondrial tryparedoxin/tryparedoxin peroxidase system. Exp Parasitol 120(4):421–423. https://doi.org/10.1016/j.exppara.2008.09.002
Castro H, Romao S, Carvalho S, Teixeira F, Sousa C, Tomás AM (2010) Mitochondrial redox metabolism in trypanosomatids is independent of tryparedoxin activity. PLoS One 5(9):e12607. https://doi.org/10.1371/journal.pone.0012607
El-Sayed NM, Myler PJ, Blandin G, Berriman M, Crabtree J, Aggarwal G, Caler E, Renauld H, Worthey EA, Hertz-Fowler C, Ghedin E, Peacock C, Bartholomeu DC, Haas BJ, Tran AN, Wortman JR, Alsmark UC, Angiuoli S, Anupama A, Badger J, Bringaud F, Cadag E, Carlton JM, Cerqueira GC, Creasy T, Delcher AL, Djikeng A, Embley TM, Hauser C, Ivens AC, Kummerfeld SK, Pereira-Leal JB, Nilsson D, Peterson J, Salzberg SL, Shallom J, Silva JC, Sundaram J, Westenberger S, White O, Melville SE, Donelson JE, Andersson B, Stuart KD, Hall N (2005) Comparative genomics of trypanosomatid parasitic protozoa. Science 309(5733):404–409. https://doi.org/10.1126/science.1112181
Romao S, Castro H, Sousa C, Carvalho S, Tomás AM (2009) The cytosolic tryparedoxin of Leishmania infantum is essential for parasite survival. Int J Parasitol 39(6):703–711. https://doi.org/10.1016/j.ijpara.2008.11.009
Comini MA, Krauth-Siegel RL, Flohé L (2007) Depletion of the thioredoxin homologue tryparedoxin impairs antioxidative defence in African trypanosomes. Biochem J 402(1):43–49. https://doi.org/10.1042/bj20061341
Suman SS, Equbal A, Zaidi A, Ansari MY, Singh KP, Singh K, Purkait B, Sahoo GC, Bimal S, Das P, Ali V (2016) Up-regulation of cytosolic tryparedoxin in Amp B resistant isolates of Leishmania donovani and its interaction with cytosolic tryparedoxin peroxidase. Biochimie 121:312–325. https://doi.org/10.1016/j.biochi.2015.12.017
Suman SS, Amit A, Singh KP, Gupta P, Equbal A, Kumari A, Topno RK, Ravidas V, Pandey K, Bimal S, Das P, Ali V (2018) Cytosolic tryparedoxin of Leishmania donovani modulates host immune response in visceral leishmaniasis. Cytokine 108:1–8. https://doi.org/10.1016/j.cyto.2018.03.010
Gommel DU, Nogoceke E, Morr M, Kiess M, Kalisz HM, Flohé L (1997) Catalytic characteristics of tryparedoxin. Eur J Biochem 248(3):913–918
Barr SD, Gedamu L (2001) Cloning and characterization of three differentially expressed peroxidoxin genes from Leishmania chagasi. Evidence for an enzymatic detoxification of hydroxyl radicals. J Biol Chem 276(36):34279–34287. https://doi.org/10.1074/jbc.M104406200
Poole LB (2007) The catalytic mechanism of peroxiredoxins. Subcell Biochem 44:61–81
Levick MP, Tetaud E, Fairlamb AH, Blackwell JM (1998) Identification and characterisation of a functional peroxidoxin from Leishmania major. Mol Biochem Parasitol 96(1-2):125–137
Castro H, Budde H, Flohé L, Hofmann B, Lünsdorf H, Wissing J, Tomás AM (2002) Specificity and kinetics of a mitochondrial peroxiredoxin of Leishmania infantum. Free Radic Biol Med 33(11):1563–1574
Flohé L, Budde H, Bruns K, Castro H, Clos J, Hofmann B, Kansal-Kalavar S, Krumme D, Menge U, Plank-Schumacher K, Sztajer H, Wissing J, Wylegalla C, Hecht HJ (2002) Tryparedoxin peroxidase of Leishmania donovani: molecular cloning, heterologous expression, specificity, and catalytic mechanism. Arch Biochem Biophys 397(2):324–335. https://doi.org/10.1006/abbi.2001.2688
Budde H, Flohé L, Hecht HJ, Hofmann B, Stehr M, Wissing J, Lünsdorf H (2003) Kinetics and redox-sensitive oligomerisation reveal negative subunit cooperativity in tryparedoxin peroxidase of Trypanosoma brucei brucei. Biol Chem 384(4):619–633. https://doi.org/10.1515/bc.2003.069
Wilkinson SR, Meyer DJ, Kelly JM (2000) Biochemical characterization of a trypanosome enzyme with glutathione-dependent peroxidase activity. Biochem J 352(Pt 3):755–761
Wilkinson SR, Taylor MC, Touitha S, Mauricio IL, Meyer DJ, Kelly JM (2002) TcGPXII, a glutathione-dependent Trypanosoma cruzi peroxidase with substrate specificity restricted to fatty acid and phospholipid hydroperoxides, is localized to the endoplasmic reticulum. Biochem J 364(Pt 3):787–794. https://doi.org/10.1042/bj20020038
Colasante C, Ellis M, Ruppert T, Voncken F (2006) Comparative proteomics of glycosomes from bloodstream form and procyclic culture form Trypanosoma brucei brucei. Proteomics 6(11):3275–3293. https://doi.org/10.1002/pmic.200500668
Herbette S, Roeckel-Drevet P, Drevet JR (2007) Seleno-independent glutathione peroxidases. More than simple antioxidant scavengers. FEBS J 274(9):2163–2180. https://doi.org/10.1111/j.1742-4658.2007.05774.x
Hillebrand H, Schmidt A, Krauth-Siegel RL (2003) A second class of peroxidases linked to the trypanothione metabolism. J Biol Chem 278(9):6809–6815. https://doi.org/10.1074/jbc.M210392200
Clark D, Albrecht M, Arévalo J (1994) Ascorbate variations and dehydroascorbate reductase activity in Trypanosoma cruzi epimastigotes and trypomastigotes. Mol Biochem Parasitol 66(1):143–145
Wilkinson SR, Obado SO, Mauricio IL, Kelly JM (2002) Trypanosoma cruzi expresses a plant-like ascorbate-dependent hemoperoxidase localized to the endoplasmic reticulum. Proc Natl Acad Sci U S A 99(21):13453–13458. https://doi.org/10.1073/pnas.202422899
Halliwell B (2001) Vitamin C and genomic stability. Mutat Res 475(1-2):29–35
Docampo R, de Boiso JF, Boveris A, Stoppani AO (1976) Localization of peroxidase activity in Trypanosoma cruzi microbodies. Experientia 32(8):972–975
Adak S, Datta AK (2005) Leishmania major encodes an unusual peroxidase that is a close homologue of plant ascorbate peroxidase: a novel role of the transmembrane domain. Biochem J 390(Pt 2):465–474. https://doi.org/10.1042/bj20050311
Krauth-Siegel RL, Lüdemann H (1996) Reduction of dehydroascorbate by trypanothione. Mol Biochem Parasitol 80(2):203–208
Reckenfelderbäumer N, Krauth-Siegel RL (2002) Catalytic properties, thiol pK value, and redox potential of Trypanosoma brucei tryparedoxin. J Biol Chem 277(20):17548–17555. https://doi.org/10.1074/jbc.M112115200
Taylor MC, Lewis MD, Fortes Francisco A, Wilkinson SR, Kelly JM (2015) The Trypanosoma cruzi vitamin C dependent peroxidase confers protection against oxidative stress but is not a determinant of virulence. PLoS Negl Trop Dis 9(4):e0003707. https://doi.org/10.1371/journal.pntd.0003707
McCord JM, Fridovich I (1969) The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem 244(22):6056–6063
Dufernez F, Yernaux C, Gerbod D, Noël C, Chauvenet M, Wintjens R, Edgcomb VP, Capron M, Opperdoes FR, Viscogliosi E (2006) The presence of four iron-containing superoxide dismutase isozymes in trypanosomatidae: characterization, subcellular localization, and phylogenetic origin in Trypanosoma brucei. Free Radic Biol Med 40(2):210–225. https://doi.org/10.1016/j.freeradbiomed.2005.06.021
Ismail SO, Paramchuk W, Skeiky YA, Reed SG, Bhatia A, Gedamu L (1997) Molecular cloning and characterization of two iron superoxide dismutase cDNAs from Trypanosoma cruzi. Mol Biochem Parasitol 86(2):187–197
Temperton NJ, Wilkinson SR, Meyer DJ, Kelly JM (1998) Overexpression of superoxide dismutase in Trypanosoma cruzi results in increased sensitivity to the trypanocidal agents gentian violet and benznidazole. Mol Biochem Parasitol 96(1–2):167–176
Taylor MC, Kelly JM (2006) pTcINDEX: a stable tetracycline-regulated expression vector for Trypanosoma cruzi. BMC Biotechnol 6:32. https://doi.org/10.1186/1472-6750-6-32
Piacenza L, Irigoín F, Alvarez MN, Peluffo G, Taylor MC, Kelly JM, Wilkinson SR, Radi R (2007) Mitochondrial superoxide radicals mediate programmed cell death in Trypanosoma cruzi: cytoprotective action of mitochondrial iron superoxide dismutase overexpression. Biochem J 403(2):323–334. https://doi.org/10.1042/bj20061281
Getachew F, Gedamu L (2012) Leishmania donovani mitochondrial iron superoxide dismutase A is released into the cytosol during miltefosine induced programmed cell death. Mol Biochem Parasitol 183(1):42–51. https://doi.org/10.1016/j.molbiopara.2012.01.005
Alzate JF, Arias AA, Moreno-Mateos D, Alvarez-Barrientos A, Jiménez-Ruiz A (2007) Mitochondrial superoxide mediates heat-induced apoptotic-like death in Leishmania infantum. Mol Biochem Parasitol 152(2):192–202. https://doi.org/10.1016/j.molbiopara.2007.01.006
Mittra B, Laranjeira-Silva MF, Miguel DC, Perrone Bezerra de Menezes J, Andrews NW (2017) The iron-dependent mitochondrial superoxide dismutase SODA promotes. J Biol Chem 292(29):12324–12338. https://doi.org/10.1074/jbc.M116.772624
Filomeni G, Rotilio G, Ciriolo MR (2002) Cell signalling and the glutathione redox system. Biochem Pharmacol 64(5-6):1057–1064
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48(6):749–762. https://doi.org/10.1016/j.freeradbiomed.2009.12.022
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84. https://doi.org/10.1016/j.biocel.2006.07.001
Couto N, Wood J, Barber J (2016) The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med 95:27–42. https://doi.org/10.1016/j.freeradbiomed.2016.02.028
Chiang HS, Maric M (2011) Lysosomal thiol reductase negatively regulates autophagy by altering glutathione synthesis and oxidation. Free Radic Biol Med 51(3):688–699. https://doi.org/10.1016/j.freeradbiomed.2011.05.015
Bindoli A, Fukuto JM, Forman HJ (2008) Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal 10(9):1549–1564. https://doi.org/10.1089/ars.2008.2063
Flohé L (2010) Changing paradigms in thiology from antioxidant defense toward redox regulation. Methods Enzymol 473:1–39. https://doi.org/10.1016/s0076-6879(10)73001-9
Voehringer DW, McConkey DJ, McDonnell TJ, Brisbay S, Meyn RE (1998) Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc Natl Acad Sci U S A 95(6):2956–2960
Markovic J, Borrás C, Ortega A, Sastre J, Viña J, Pallardó FV (2007) Glutathione is recruited into the nucleus in early phases of cell proliferation. J Biol Chem 282(28):20416–20424. https://doi.org/10.1074/jbc.M609582200
Chakravarthi S, Jessop CE, Bulleid NJ (2006) The role of glutathione in disulphide bond formation and endoplasmic-reticulum-generated oxidative stress. EMBO Rep 7(3):271–275. https://doi.org/10.1038/sj.embor.7400645
Jessop CE, Bulleid NJ (2004) Glutathione directly reduces an oxidoreductase in the endoplasmic reticulum of mammalian cells. J Biol Chem 279(53):55341–55347. https://doi.org/10.1074/jbc.M411409200
Jocelyn PC, Kamminga A (1974) The non-protein thiol of rat liver mitochondria. Biochim Biophys Acta 343(2):356–362
Schnellmann RG (1991) Renal mitochondrial glutathione transport. Life Sci 49(5):393–398
Holmgren A, Björnstedt M (1995) Thioredoxin and thioredoxin reductase. Methods Enzymol 252:199–208
Lee S, Kim SM, Lee RT (2013) Thioredoxin and thioredoxin target proteins: from molecular mechanisms to functional significance. Antioxid Redox Signal 18(10):1165–1207. https://doi.org/10.1089/ars.2011.4322
Perkins A, Nelson KJ, Parsonage D, Poole LB, Karplus PA (2015) Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem Sci 40(8):435–445. https://doi.org/10.1016/j.tibs.2015.05.001
Kim K, Kim IH, Lee KY, Rhee SG, Stadtman ER (1988) The isolation and purification of a specific "protector" protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixed-function oxidation system. J Biol Chem 263(10):4704–4711
Knoops B, Argyropoulou V, Becker S, Ferté L, Kuznetsova O (2016) Multiple roles of peroxiredoxins in inflammation. Mol Cells 39(1):60–64. https://doi.org/10.14348/molcells.2016.2341
Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG (2010) Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell 140(4):517–528. https://doi.org/10.1016/j.cell.2010.01.009
Rhee SG, Woo HA (2011) Multiple functions of peroxiredoxins: peroxidases, sensors and regulators of the intracellular messenger H2O2, and protein chaperones. Antioxid Redox Signal 15(3):781–794. https://doi.org/10.1089/ars.2010.3393
Edgar RS, Green EW, Zhao Y, van Ooijen G, Olmedo M, Qin X, Xu Y, Pan M, Valekunja UK, Feeney KA, Maywood ES, Hastings MH, Baliga NS, Merrow M, Millar AJ, Johnson CH, Kyriacou CP, O’Neill JS, Reddy AB (2012) Peroxiredoxins are conserved markers of circadian rhythms. Nature 485(7399):459–464. https://doi.org/10.1038/nature11088
Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH (2013) Thioredoxins, glutaredoxins, and peroxiredoxins-molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal 19(13):1539–1605. https://doi.org/10.1089/ars.2012.4599
Knoops B, Clippe A, Bogard C, Arsalane K, Wattiez R, Hermans C, Duconseille E, Falmagne P, Bernard A (1999) Cloning and characterization of AOEB166, a novel mammalian antioxidant enzyme of the peroxiredoxin family. J Biol Chem 274(43):30451–30458
Song JJ, Rhee JG, Suntharalingam M, Walsh SA, Spitz DR, Lee YJ (2002) Role of glutaredoxin in metabolic oxidative stress. Glutaredoxin as a sensor of oxidative stress mediated by H2O2. J Biol Chem 277(48):46566–46575. https://doi.org/10.1074/jbc.M206826200
Lu J, Holmgren A (2012) Thioredoxin system in cell death progression. Antioxid Redox Signal 17(12):1738–1747. https://doi.org/10.1089/ars.2012.4650
Lu J, Holmgren A (2014) The thioredoxin antioxidant system. Free Radic Biol Med 66:75–87. https://doi.org/10.1016/j.freeradbiomed.2013.07.036
Nightingale H, Kemp K, Gray E, Hares K, Mallam E, Scolding N, Wilkins A (2012) Changes in expression of the antioxidant enzyme SOD3 occur upon differentiation of human bone marrow-derived mesenchymal stem cells in vitro. Stem Cells Dev 21(11):2026–2035. https://doi.org/10.1089/scd.2011.0516
Ye ZW, Zhang J, Townsend DM, Tew KD (2015) Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim Biophys Acta 1850(8):1607–1621. https://doi.org/10.1016/j.bbagen.2014.11.010
Putnam CD, Arvai AS, Bourne Y, Tainer JA (2000) Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism. J Mol Biol 296(1):295–309. https://doi.org/10.1006/jmbi.1999.3458
Nicholls P (2012) Classical catalase: ancient and modern. Arch Biochem Biophys 525(2):95–101. https://doi.org/10.1016/j.abb.2012.01.015
Liao AC, Craver BM, Tseng BP, Tran KK, Parihar VK, Acharya MM, Limoli CL (2013) Mitochondrial-targeted human catalase affords neuroprotection from proton irradiation. Radiat Res 180(1):1–6. https://doi.org/10.1667/rr3339.1
Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS (2009) Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation 119(21):2789–2797. https://doi.org/10.1161/circulationaha.108.822403
Nogueira NP, Saraiva FM, Sultano PE, Cunha PR, Laranja GA, Justo GA, Sabino KC, Coelho MG, Rossini A, Atella GC, Paes MC (2015) Proliferation and differentiation of Trypanosoma cruzi inside its vector have a new trigger: redox status. PLoS One 10(2):e0116712. https://doi.org/10.1371/journal.pone.0116712
Sterkel M, Oliveira JHM, Bottino-Rojas V, Paiva-Silva GO, Oliveira PL (2017) The dose makes the poison: nutritional overload determines the life traits of blood-feeding arthropods. Trends Parasitol 33(8):633–644. https://doi.org/10.1016/j.pt.2017.04.008
Carneiro PP, Conceição J, Macedo M, Magalhães V, Carvalho EM, Bacellar O (2016) The role of nitric oxide and reactive oxygen species in the killing of Leishmania braziliensis by monocytes from patients with Cutaneous Leishmaniasis. PLoS One 11(2):e0148084. https://doi.org/10.1371/journal.pone.0148084
Kierszenbaum F, Knecht E, Budzko DB, Pizzimenti MC (1974) Phagocytosis: a defense mechanism against infection with Trypanosoma cruzi. J Immunol 112(5):1839–1844
Alvarez MN, Peluffo G, Piacenza L, Radi R (2011) Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J Biol Chem 286(8):6627–6640. https://doi.org/10.1074/jbc.M110.167247
Guiñazú N, Carrera-Silva EA, Becerra MC, Pellegrini A, Albesa I, Gea S (2010) Induction of NADPH oxidase activity and reactive oxygen species production by a single Trypanosoma cruzi antigen. Int J Parasitol 40(13):1531–1538. https://doi.org/10.1016/j.ijpara.2010.05.012
Alvarez MN, Piacenza L, Irigoín F, Peluffo G, Radi R (2004) Macrophage-derived peroxynitrite diffusion and toxicity to Trypanosoma cruzi. Arch Biochem Biophys 432(2):222–232. https://doi.org/10.1016/j.abb.2004.09.015
Fridovich I (1997) Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem 272(30):18515–18517
Babior BM (1984) The respiratory burst of phagocytes. J Clin Invest 73(3):599–601. https://doi.org/10.1172/jci111249
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87(1):245–313. https://doi.org/10.1152/physrev.00044.2005
de Carvalho TU, de Souza W (1987) Cytochemical localization of NADH and NADPH oxidases during interaction of Trypanosoma cruzi with activated macrophages. Parasitol Res 73(3):213–217
Brandes RP, Weissmann N, Schröder K (2014) Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic Biol Med 76:208–226. https://doi.org/10.1016/j.freeradbiomed.2014.07.046
Van Assche T, Deschacht M, da Luz RA, Maes L, Cos P (2011) Leishmania-macrophage interactions: insights into the redox biology. Free Radic Biol Med 51(2):337–351. https://doi.org/10.1016/j.freeradbiomed.2011.05.011
Peluffo G, Piacenza L, Irigoín F, Alvarez MN, Radi R (2004) L-arginine metabolism during interaction of Trypanosoma cruzi with host cells. Trends Parasitol 20(8):363–369. https://doi.org/10.1016/j.pt.2004.05.010
Radi R (2013) Peroxynitrite, a stealthy biological oxidant. J Biol Chem 288(37):26464–26472. https://doi.org/10.1074/jbc.R113.472936
Cassina A, Radi R (1996) Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch Biochem Biophys 328(2):309–316. https://doi.org/10.1006/abbi.1996.0178
Ferrer-Sueta G, Radi R (2009) Chemical biology of peroxynitrite: kinetics, diffusion, and radicals. ACS Chem Biol 4(3):161–177. https://doi.org/10.1021/cb800279q
Piacenza L, Peluffo G, Alvarez MN, Martínez A, Radi R (2013) Trypanosoma cruzi antioxidant enzymes as virulence factors in Chagas disease. Antioxid Redox Signal 19(7):723–734. https://doi.org/10.1089/ars.2012.4618
Piacenza L, Alvarez MN, Peluffo G, Radi R (2009) Fighting the oxidative assault: the Trypanosoma cruzi journey to infection. Curr Opin Microbiol 12(4):415–421. https://doi.org/10.1016/j.mib.2009.06.011
Mattner J, Wandersee-Steinhäuser A, Pahl A, Röllinghoff M, Majeau GR, Hochman PS, Bogdan C (2004) Protection against progressive leishmaniasis by IFN-beta. J Immunol 172(12):7574–7582
Costa VM, Torres KC, Mendonça RZ, Gresser I, Gollob KJ, Abrahamsohn IA (2006) Type I IFNs stimulate nitric oxide production and resistance to Trypanosoma cruzi infection. J Immunol 177(5):3193–3200
Kinkade JM, Pember SO, Barnes KC, Shapira R, Spitznagel JK, Martin LE (1983) Differential distribution of distinct forms of myeloperoxidase in different azurophilic granule subpopulations from human neutrophils. Biochem Biophys Res Commun 114(1):296–303
Anand U, Anand CV (2012) Myeloperoxidase: a new twist to an old tale. Indian J Clin Biochem 27(2):107–109. https://doi.org/10.1007/s12291-012-0220-0
Lazarević-Pasti T, Leskovac A, Vasić V (2015) Myeloperoxidase Inhibitors as Potential Drugs. Curr Drug Metab 16(3):168–190
Segal AW (2005) How neutrophils kill microbes. Annu Rev Immunol 23:197–223. https://doi.org/10.1146/annurev.immunol.23.021704.115653
Piacenza L, Peluffo G, Alvarez MN, Kelly JM, Wilkinson SR, Radi R (2008) Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage- and endogenously-derived peroxynitrite. Biochem J 410(2):359–368. https://doi.org/10.1042/bj20071138
Chandrasekar B, Melby PC, Troyer DA, Colston JT, Freeman GL (1998) Temporal expression of pro-inflammatory cytokines and inducible nitric oxide synthase in experimental acute Chagasic cardiomyopathy. Am J Pathol 152(4):925–934
Machado FS, Martins GA, Aliberti JC, Mestriner FL, Cunha FQ, Silva JS (2000) Trypanosoma cruzi-infected cardiomyocytes produce chemokines and cytokines that trigger potent nitric oxide-dependent trypanocidal activity. Circulation 102(24):3003–3008
Luquetti AO, Miles MA, Rassi A, de Rezende JM, de Souza AA, Póvoa MM, Rodrigues I (1986) Trypanosoma cruzi: zymodemes associated with acute and chronic Chagas’ disease in central Brazil. Trans R Soc Trop Med Hyg 80(3):462–470
Piacenza L, Zago MP, Peluffo G, Alvarez MN, Basombrio MA, Radi R (2009) Enzymes of the antioxidant network as novel determiners of Trypanosoma cruzi virulence. Int J Parasitol 39(13):1455–1464. https://doi.org/10.1016/j.ijpara.2009.05.010
McNeely TB, Turco SJ (1990) Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J Immunol 144(7):2745–2750
Lodge R, Diallo TO, Descoteaux A (2006) Leishmania donovani lipophosphoglycan blocks NADPH oxidase assembly at the phagosome membrane. Cell Microbiol 8(12):1922–1931. https://doi.org/10.1111/j.1462-5822.2006.00758.x
Proudfoot L, Nikolaev AV, Feng GJ, Wei WQ, Ferguson MA, Brimacombe JS, Liew FY (1996) Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc Natl Acad Sci U S A 93(20):10984–10989
Kumar R, Pai K, Sundar S (2001) Reactive oxygen intermediates, nitrite and IFN-gamma in Indian visceral leishmaniasis. Clin Exp Immunol 124(2):262–265
Kumar P, Pai K, Pandey HP, Sundar S (2002) NADH-oxidase, NADPH-oxidase and myeloperoxidase activity of visceral leishmaniasis patients. J Med Microbiol 51(10):832–836. https://doi.org/10.1099/0022-1317-51-10-832
DeLeo FR, Burritt JB, Yu L, Jesaitis AJ, Dinauer MC, Nauseef WM (2000) Processing and maturation of flavocytochrome b558 include incorporation of heme as a prerequisite for heterodimer assembly. J Biol Chem 275(18):13986–13993
Pham NK, Mouriz J, Kima PE (2005) Leishmania pifanoi amastigotes avoid macrophage production of superoxide by inducing heme degradation. Infect Immun 73(12):8322–8333. https://doi.org/10.1128/iai.73.12.8322-8333.2005
McNeely TB, Rosen G, Londner MV, Turco SJ (1989) Inhibitory effects on protein kinase C activity by lipophosphoglycan fragments and glycosylphosphatidylinositol antigens of the protozoan parasite Leishmania. Biochem J 259(2):601–604
Watson F, Robinson J, Edwards SW (1991) Protein kinase C-dependent and -independent activation of the NADPH oxidase of human neutrophils. J Biol Chem 266(12):7432–7439
da Silva AL, Moretti NS, Ramos TC, de Jesus TC, Zhang M, Castilho BA, Schenkman S (2015) A membrane-bound eIF2 alpha kinase located in endosomes is regulated by heme and controls differentiation and ROS levels in Trypanosoma cruzi. PLoS Pathog 11(2):e1004618. https://doi.org/10.1371/journal.ppat.1004618
Mittra B, Cortez M, Haydock A, Ramasamy G, Myler PJ, Andrews NW (2013) Iron uptake controls the generation of Leishmania infective forms through regulation of ROS levels. J Exp Med 210(2):401–416. https://doi.org/10.1084/jem.20121368
Graça-Souza AV, Maya-Monteiro C, Paiva-Silva GO, Braz GR, Paes MC, Sorgine MH, Oliveira MF, Oliveira PL (2006) Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol 36(4):322–335. https://doi.org/10.1016/j.ibmb.2006.01.009
Rodrigues JC, Bernardes CF, Visbal G, Urbina JA, Vercesi AE, de Souza W (2007) Sterol methenyl transferase inhibitors alter the ultrastructure and function of the Leishmania amazonensis mitochondrion leading to potent growth inhibition. Protist 158(4):447–456. https://doi.org/10.1016/j.protis.2007.05.004
Rodrigues JC, de Souza W (2008) Ultrastructural alterations in organelles of parasitic protozoa induced by different classes of metabolic inhibitors. Curr Pharm Des 14(9):925–938
Sen N, Majumder HK (2008) Mitochondrion of protozoan parasite emerges as potent therapeutic target: exciting drugs are on the horizon. Curr Pharm Des 14(9):839–846
Cavalli A, Bolognesi ML (2009) Neglected tropical diseases: multi-target-directed ligands in the search for novel lead candidates against Trypanosoma and Leishmania. J Med Chem 52(23):7339–7359. https://doi.org/10.1021/jm9004835
Menna-Barreto RF, Corrêa JR, Cascabulho CM, Fernandes MC, Pinto AV, Soares MJ, De Castro SL (2009) Naphthoimidazoles promote different death phenotypes in Trypanosoma cruzi. Parasitology 136(5):499–510. https://doi.org/10.1017/s0031182009005745
de Souza W, Attias M, Rodrigues JC (2009) Particularities of mitochondrial structure in parasitic protists (Apicomplexa and Kinetoplastida). Int J Biochem Cell Biol 41(10):2069–2080. https://doi.org/10.1016/j.biocel.2009.04.007
Vannier-Santos MA, De Castro SL (2009) Electron microscopy in antiparasitic chemotherapy: a (close) view to a kill. Curr Drug Targets 10(3):246–260
Fidalgo LM, Gille L (2011) Mitochondria and trypanosomatids: targets and drugs. Pharm Res 28(11):2758–2770. https://doi.org/10.1007/s11095-011-0586-3
Buckner FS, Urbina JA (2012) Recent developments in sterol 14-demethylase Inhibitors for Chagas disease. Int J Parasitol Drugs Drug Resist 2:236–242. https://doi.org/10.1016/j.ijpddr.2011.12.002
Sangenito LS, Menna-Barreto RFS, Oliveira ACS, d’Avila-Levy CM, Branquinha MH, ALS S (2018) Primary evidences of the mechanisms of action of HIV aspartyl peptidase inhibitors on Trypanosoma cruzi trypomastigote forms. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2018.03.021
Menna-Barreto RF, de Castro SL (2014) The double-edged sword in pathogenic trypanosomatids: the pivotal role of mitochondria in oxidative stress and bioenergetics. Biomed Res Int 2014:614014. https://doi.org/10.1155/2014/614014
Docampo R, Gadelha FR, Moreno SN, Benaim G, Hoffmann ME, Vercesi AE (1993) Disruption of Ca2+ homeostasis in Trypanosoma cruzi by crystal violet. J Eukaryot Microbiol 40(3):311–316
Affranchino JL, De Tarlovsky MN, Stoppani AO (1985) Respiratory control in mitochondria from Trypanosoma cruzi. Mol Biochem Parasitol 16(3):289–298
Peloso EF, Vitor SC, Ribeiro LH, Piñeyro MD, Robello C, Gadelha FR (2011) Role of Trypanosoma cruzi peroxiredoxins in mitochondrial bioenergetics. J Bioenerg Biomembr 43(4):419–424. https://doi.org/10.1007/s10863-011-9365-4
Luque-Ortega JR, Reuther P, Rivas L, Dardonville C (2010) New benzophenone-derived bisphosphonium salts as leishmanicidal leads targeting mitochondria through inhibition of respiratory complex II. J Med Chem 53(4):1788–1798. https://doi.org/10.1021/jm901677h
Carvalho L, Luque-Ortega JR, López-Martín C, Castanys S, Rivas L, Gamarro F (2011) The 8-aminoquinoline analogue sitamaquine causes oxidative stress in Leishmania donovani promastigotes by targeting succinate dehydrogenase. Antimicrob Agents Chemother 55(9):4204–4210. https://doi.org/10.1128/aac.00520-11
Luque-Ortega JR, Rivas L (2007) Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob Agents Chemother 51(4):1327–1332. https://doi.org/10.1128/aac.01415-06
Carvalho L, Luque-Ortega JR, Manzano JI, Castanys S, Rivas L, Gamarro F (2010) Tafenoquine, an antiplasmodial 8-aminoquinoline, targets leishmania respiratory complex III and induces apoptosis. Antimicrob Agents Chemother 54(12):5344–5351. https://doi.org/10.1128/aac.00790-10
Njogu RM, Whittaker CJ, Hill GC (1980) Evidence for a branched electron transport chain in Trypanosoma brucei. Mol Biochem Parasitol 1(1):13–29
Menna-Barreto RF, Goncalves RL, Costa EM, Silva RS, Pinto AV, Oliveira MF, de Castro SL (2009) The effects on Trypanosoma cruzi of novel synthetic naphthoquinones are mediated by mitochondrial dysfunction. Free Radic Biol Med 47(5):644–653. https://doi.org/10.1016/j.freeradbiomed.2009.06.004
Moore AL, Shiba T, Young L, Harada S, Kita K, Ito K (2013) Unraveling the heater: new insights into the structure of the alternative oxidase. Annu Rev Plant Biol 64:637–663. https://doi.org/10.1146/annurev-arplant-042811-105432
Nihei C, Fukai Y, Kita K (2002) Trypanosome alternative oxidase as a target of chemotherapy. Biochim Biophys Acta 1587(2–3):234–239
Minagawa N, Yabu Y, Kita K, Nagai K, Ohta N, Meguro K, Sakajo S, Yoshimoto A (1996) An antibiotic, ascofuranone, specifically inhibits respiration and in vitro growth of long slender bloodstream forms of Trypanosoma brucei brucei. Mol Biochem Parasitol 81(2):127–136
Tsuda A, Witola WH, Ohashi K, Onuma M (2005) Expression of alternative oxidase inhibits programmed cell death-like phenomenon in bloodstream form of Trypanosoma brucei rhodesiense. Parasitol Int 54(4):243–251. https://doi.org/10.1016/j.parint.2005.06.007
Yabu Y, Suzuki T, Nihei C, Minagawa N, Hosokawa T, Nagai K, Kita K, Ohta N (2006) Chemotherapeutic efficacy of ascofuranone in Trypanosoma vivax-infected mice without glycerol. Parasitol Int 55(1):39–43. https://doi.org/10.1016/j.parint.2005.09.003
Villa-Pulgarín JA, Gajate C, Botet J, Jimenez A, Justies N, Varela-M RE, Cuesta-Marbán Á, Müller I, Modolell M, Revuelta JL, Mollinedo F (2017) Mitochondria and lipid raft-located FOF1-ATP synthase as major therapeutic targets in the antileishmanial and anticancer activities of ether lipid edelfosine. PLoS Negl Trop Dis 11(8):e0005805. https://doi.org/10.1371/journal.pntd.0005805
Powis G (1987) Metabolism and reactions of quinoid anticancer agents. Pharmacol Ther 35(1–2):57–162
O’Brien PJ (1991) Molecular mechanisms of quinone cytotoxicity. Chem Biol Interact 80(1):1–41
Monks TJ, Jones DC (2002) The metabolism and toxicity of quinones, quinonimines, quinone methides, and quinone-thioethers. Curr Drug Metab 3(4):425–438
Lima NM, Correia CS, Leon LL, Machado GM, Madeira MeF, Santana AE, Goulart MO (2004) Antileishmanial activity of lapachol analogues. Mem Inst Oswaldo Cruz 99(7):757–761. doi:/S0074-02762004000700017
da Silva Júnior EN, de Melo IM, Diogo EB, Costa VA, de Souza Filho JD, Valença WO, Camara CA, de Oliveira RN, de Araujo AS, Emery FS, dos Santos MR, de Simone CA, Menna-Barreto RF, de Castro SL (2012) On the search for potential anti-Trypanosoma cruzi drugs: synthesis and biological evaluation of 2-hydroxy-3-methylamino and 1,2,3-triazolic naphthoquinoidal compounds obtained by click chemistry reactions. Eur J Med Chem 52:304–312. https://doi.org/10.1016/j.ejmech.2012.03.039
da Silva Júnior EN, Jardim GAM, Menna-Barreto RFS, de Castro SL (2014) Anti-Trypanosoma cruzi compounds: our contribution for the evaluation and insights on the mode of action of naphthoquinones and derivatives. J Braz Chem Soc 25(10):1780–1798. https://doi.org/10.5935/0103-5053.20140180
Ramírez-Macías I, Marín C, Es-Samti H, Fernández A, Guardia JJ, Zentar H, Agil A, Chahboun R, Alvarez-Manzaneda E, Sánchez-Moreno M (2012) Taiwaniaquinoid and abietane quinone derivatives with trypanocidal activity against T. cruzi and Leishmania spp. Parasitol Int 61(3):405–413. https://doi.org/10.1016/j.parint.2012.02.001
Diogo EB, Dias GG, Rodrigues BL, Guimarães TT, Valença WO, Camara CA, de Oliveira RN, da Silva MG, Ferreira VF, de Paiva YG, Goulart MO, Menna-Barreto RF, de Castro SL, da Silva Júnior EN (2013) Synthesis and anti-Trypanosoma cruzi activity of naphthoquinone-containing triazoles: electrochemical studies on the effects of the quinoidal moiety. Bioorg Med Chem 21(21):6337–6348. https://doi.org/10.1016/j.bmc.2013.08.055
Lam CF, Pearce AN, Tan SH, Kaiser M, Copp BR (2013) Discovery and evaluation of thiazinoquinones as anti-protozoal agents. Mar Drugs 11(9):3472–3499. https://doi.org/10.3390/md11093472
Pieretti S, Haanstra JR, Mazet M, Perozzo R, Bergamini C, Prati F, Fato R, Lenaz G, Capranico G, Brun R, Bakker BM, Michels PA, Scapozza L, Bolognesi ML, Cavalli A (2013) Naphthoquinone derivatives exert their antitrypanosomal activity via a multi-target mechanism. PLoS Negl Trop Dis 7(1):e2012. https://doi.org/10.1371/journal.pntd.0002012
Hata Y, Ebrahimi SN, De Mieri M, Zimmermann S, Mokoka T, Naidoo D, Fouche G, Maharaj V, Kaiser M, Brun R, Potterat O, Hamburger M (2014) Antitrypanosomal isoflavan quinones from Abrus precatorius. Fitoterapia 93:81–87. https://doi.org/10.1016/j.fitote.2013.12.015
Ellendorff T, Brun R, Kaiser M, Sendker J, Schmidt TJ (2015) PLS-prediction and confirmation of hydrojuglone glucoside as the antitrypanosomal constituent of Juglans Spp. Molecules 20(6):10082–10094. https://doi.org/10.3390/molecules200610082
Boveris A, Docampo R, Turrens JF, Stoppani AO (1977) Effect of beta and alpha-lapachone on the production of H2O2 and on the growth of Trypanosoma cruzi. Rev Asoc Argent Microbiol 9(2):54–61
Cruz FS, Docampo R, de Souza W (1978) Effect of beta-lapachone on hydrogen peroxide production in Trypanosoma cruzi. Acta Trop 35(1):35–40
Molina Portela MP, Fernandez Villamil SH, Perissinotti LJ, Stoppani AO (1996) Redox cycling of o-naphthoquinones in trypanosomatids. Superoxide and hydrogen peroxide production. Biochem Pharmacol 52(12):1875–1882
Salomão K, De Santana NA, Molina MT, De Castro SL, Menna-Barreto RF (2013) Trypanosoma cruzi mitochondrial swelling and membrane potential collapse as primary evidence of the mode of action of naphthoquinone analogues. BMC Microbiol 13:196. https://doi.org/10.1186/1471-2180-13-196
Lara LS, Moreira CS, Calvet CM, Lechuga GC, Souza RS, Bourguignon SC, Ferreira VF, Rocha D, Pereira MCS (2018) Efficacy of 2-hydroxy-3-phenylsulfanylmethyl-[1,4]-naphthoquinone derivatives against different Trypanosoma cruzi discrete type units: Identification of a promising hit compound. Eur J Med Chem 144:572–581. https://doi.org/10.1016/j.ejmech.2017.12.052
Fonseca-Silva F, Inacio JD, Canto-Cavalheiro MM, Almeida-Amaral EE (2011) Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS One 6(2):e14666. https://doi.org/10.1371/journal.pone.0014666
Fonseca-Silva F, Canto-Cavalheiro MM, Menna-Barreto RF, Almeida-Amaral EE (2015) Effect of Apigenin on Leishmania amazonensis is associated with reactive oxygen species production followed by mitochondrial dysfunction. J Nat Prod 78(4):880–884. https://doi.org/10.1021/acs.jnatprod.5b00011
Fonseca-Silva F, Inacio JD, Canto-Cavalheiro MM, Menna-Barreto RF, Almeida-Amaral EE (2016) Oral efficacy of apigenin against cutaneous leishmaniasis: involvement of reactive oxygen species and autophagy as a mechanism of action. PLoS Negl Trop Dis 10(2):e0004442. https://doi.org/10.1371/journal.pntd.0004442
Inacio JD, Canto-Cavalheiro MM, Almeida-Amaral EE (2013) In vitro and in vivo effects of (-)-epigallocatechin 3-O-gallate on Leishmania amazonensis. J Nat Prod 76(10):1993–1996. https://doi.org/10.1021/np400624d
Inacio JD, Gervazoni L, Canto-Cavalheiro MM, Almeida-Amaral EE (2014) The effect of (-)-epigallocatechin 3-O--gallate in vitro and in vivo in Leishmania braziliensis: involvement of reactive oxygen species as a mechanism of action. PLoS Negl Trop Dis 8(8):e3093. https://doi.org/10.1371/journal.pntd.0003093
Martins SC, Lazarin-Bidóia D, Desoti VC, Falzirolli H, da Silva CC, Ueda-Nakamura T, Silva SO, Nakamura CV (2016) 1,3,4-Thiadiazole derivatives of R-(+)-limonene benzaldehyde-thiosemicarbazones cause death in Trypanosoma cruzi through oxidative stress. Microbes Infect 18(12):787–797. https://doi.org/10.1016/j.micinf.2016.07.007
Soares RO, Echevarria A, Bellieny MS, Pinho RT, de Leo RM, Seguins WS, Machado GM, Canto-Cavalheiro MM, Leon LL (2011) Evaluation of thiosemicarbazones and semicarbazones as potential agents anti-Trypanosoma cruzi. Exp Parasitol 129(4):381–387. https://doi.org/10.1016/j.exppara.2011.08.019
Britta EA, Scariot DB, Falzirolli H, Ueda-Nakamura T, Silva CC, Filho BP, Borsali R, Nakamura CV (2014) Cell death and ultrastructural alterations in Leishmania amazonensis caused by new compound 4-Nitrobenzaldehyde thiosemicarbazone derived from S-limonene. BMC Microbiol 14:236. https://doi.org/10.1186/s12866-014-0236-0
Lapier M, Zuniga-Lopez MC, Aguilera-Venegas B, Adam R, Abarca B, Ballesteros R, Lopez-Munoz R, Maya JD, Olea-Azar C (2017) Evaluation of the Novel Antichagasic Activity of [1,2,3]Triazolo[1,5-a]pyridine Derivatives. Curr Top Med Chem 17(4):399–411
Ribeiro GA, Cunha-Júnior EF, Pinheiro RO, da Silva SA, Canto-Cavalheiro MM, da Silva AJ, Costa PR, Netto CD, Melo RC, Almeida-Amaral EE, Torres-Santos EC (2013) LQB-118, an orally active pterocarpanquinone, induces selective oxidative stress and apoptosis in Leishmania amazonensis. J Antimicrob Chemother 68(4):789–799. https://doi.org/10.1093/jac/dks498
Mendonça DVC, Lage DP, Calixto SL, Ottoni FM, Tavares GSV, Ludolf F, Chávez-Fumagalli MA, Schneider MS, Duarte MC, Tavares CAP, Alves RJ, Coimbra ES, Coelho EAF (2018) Antileishmanial activity of a naphthoquinone derivate against promastigote and amastigote stages of Leishmania infantum and Leishmania amazonensis and its mechanism of action against L. amazonensis species. Parasitol Res 117(2):391–403. https://doi.org/10.1007/s00436-017-5713-6
Tavares GSV, Mendonça DVC, Lage DP, Granato JDT, Ottoni FM, Ludolf F, Chávez-Fumagalli MA, Duarte MC, Tavares CAP, Alves RJ, Coimbra ES, Coelho EAF (2018) Antileishmanial activity, cytotoxicity and mechanism of action of clioquinol against Leishmania infantum and Leishmania amazonensis species. Basic Clin Pharmacol Toxicol. https://doi.org/10.1111/bcpt.12990
Cunha-Júnior EF, Andrade-Neto VV, Lima ML, da Costa-Silva TA, Galisteo Junior AJ, Abengózar MA, Barbas C, Rivas L, Almeida-Amaral EE, Tempone AG, Torres-Santos EC (2017) Cyclobenzaprine Raises ROS levels in Leishmania infantum and reduces parasite burden in infected mice. PLoS Negl Trop Dis 11(1):e0005281. https://doi.org/10.1371/journal.pntd.0005281
Polak A, Richle R (1978) Mode of action of the 2-nitroimidazole derivative benznidazole. Ann Trop Med Parasitol 72(1):45–54
Docampo R, Moreno SN (1984) Free radical metabolites in the mode of action of chemotherapeutic agents and phagocytic cells on Trypanosoma cruzi. Rev Infect Dis 6(2):223–238
Moreno SN, Docampo R, Mason RP, Leon W, Stoppani AO (1982) Different behaviors of benznidazole as free radical generator with mammalian and Trypanosoma cruzi microsomal preparations. Arch Biochem Biophys 218(2):585–591
Díaz de Toranzo EG, Castro JA, Franke de Cazzulo BM, Cazzulo JJ (1988) Interaction of benznidazole reactive metabolites with nuclear and kinetoplastic DNA, proteins and lipids from Trypanosoma cruzi. Experientia 44(10):880–881
Docampo R, Stoppani AO (1979) Generation of superoxide anion and hydrogen peroxide induced by nifurtimox in Trypanosoma cruzi. Arch Biochem Biophys 197(1):317–321
Moreno SN, Mason RP, Docampo R (1984) Reduction of nifurtimox and nitrofurantoin to free radical metabolites by rat liver mitochondria. Evidence of an outer membrane-located nitroreductase. J Biol Chem 259(10):6298–6305
Boiani M, Piacenza L, Hernández P, Boiani L, Cerecetto H, González M, Denicola A (2010) Mode of action of nifurtimox and N-oxide-containing heterocycles against Trypanosoma cruzi: is oxidative stress involved? Biochem Pharmacol 79(12):1736–1745. https://doi.org/10.1016/j.bcp.2010.02.009
Wilkinson SR, Taylor MC, Horn D, Kelly JM, Cheeseman I (2008) A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc Natl Acad Sci U S A 105(13):5022–5027. https://doi.org/10.1073/pnas.0711014105
Wilkinson SR, Kelly JM (2009) Trypanocidal drugs: mechanisms, resistance and new targets. Expert Rev Mol Med 11:e31. https://doi.org/10.1017/s1462399409001252
Hall BS, Wilkinson SR (2012) Activation of benznidazole by trypanosomal type I nitroreductases results in glyoxal formation. Antimicrob Agents Chemother 56(1):115–123. https://doi.org/10.1128/aac.05135-11
Hall BS, Bot C, Wilkinson SR (2011) Nifurtimox activation by trypanosomal type I nitroreductases generates cytotoxic nitrile metabolites. J Biol Chem 286(15):13088–13095. https://doi.org/10.1074/jbc.M111.230847
Channon JY, Roberts MB, Blackwell JM (1984) A study of the differential respiratory burst activity elicited by promastigotes and amastigotes of Leishmania donovani in murine resident peritoneal macrophages. Immunology 53(2):345–355
Cardoni RL, Antunez MI, Morales C, Nantes IR (1997) Release of reactive oxygen species by phagocytic cells in response to live parasites in mice infected with Trypanosoma cruzi. Am J Trop Med Hyg 56(3):329–334
Horta MF, Mendes BP, Roma EH, Noronha FS, Macêdo JP, Oliveira LS, Duarte MM, Vieira LQ (2012) Reactive oxygen species and nitric oxide in cutaneous leishmaniasis. J Parasitol Res 2012:203818. https://doi.org/10.1155/2012/203818
Charmoy M, Megnekou R, Allenbach C, Zweifel C, Perez C, Monnat K, Breton M, Ronet C, Launois P, Tacchini-Cottier F (2007) Leishmania major induces distinct neutrophil phenotypes in mice that are resistant or susceptible to infection. J Leukoc Biol 82(2):288–299. https://doi.org/10.1189/jlb.0706440
Turrens JF (2004) Oxidative stress and antioxidant defenses: a target for the treatment of diseases caused by parasitic protozoa. Mol Aspects Med 25(1-2):211–220. https://doi.org/10.1016/j.mam.2004.02.021
Machado-Silva A, Cerqueira PG, Grazielle-Silva V, Gadelha FR, Peloso EF, Teixeira SM, Machado CR (2016) How Trypanosoma cruzi deals with oxidative stress: Antioxidant defence and DNA repair pathways. Mutat Res Rev Mutat Res 767:8–22. https://doi.org/10.1016/j.mrrev.2015.12.003
Brookes PS, Levonen AL, Shiva S, Sarti P, Darley-Usmar VM (2002) Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med 33(6):755–764
Acknowledgments
The authors thank Dra. Claudia M. d´Ávila-Levy for her critical reading and English grammar corrections. The present study was supported by grants from Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Papes/Fundação Oswaldo Cruz (Fiocruz).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Bombaça, A.C.S., de Oliveira, L.G.F., Almeida-Amaral, E.E., Menna-Barreto, R.F.S. (2019). The Biological Impact of Oxidative Metabolism in Trypanosomatid Parasites: What Is the Perfect Balance Between Reactive Species Production and Antioxidant Defenses?. In: Chakraborti, S., Chakraborti, T., Chattopadhyay, D., Shaha, C. (eds) Oxidative Stress in Microbial Diseases. Springer, Singapore. https://doi.org/10.1007/978-981-13-8763-0_8
Download citation
DOI: https://doi.org/10.1007/978-981-13-8763-0_8
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8762-3
Online ISBN: 978-981-13-8763-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)