Abstract
Leishmaniasis has become a significant public health issue in several countries in the world. New products have been identified to treat against the disease; however, toxicity and/or high cost is a limitation. The present work evaluated the antileishmanial activity of a new naphthoquinone derivate, Flau-A [2-(2,3,4-tri-O-acetyl-6-deoxy-β-L-galactopyranosyloxy)-1,4-naphthoquinone], against promastigote and amastigote-like stages of Leishmania amazonensis and L. infantum. In addition, the cytotoxicity in murine macrophages and human red cells was also investigated. The mechanism of action of Flau-A was assessed in L. amazonensis as well as its efficacy in treating infected macrophages and inhibiting infection of pretreated parasites. Results showed that Flau-A was effective against promastigotes and amastigote-like forms of both parasite species, as well as showed low toxicity in mammalian cells. Results also highlighted the morphological and biochemical alterations induced by Flau-A in L. amazonensis, including loss of mitochondrial membrane potential, as well as increased reactive oxygen species production, cell shrinkage, and alteration of the plasma membrane integrity. The present study demonstrates for the first time the antileishmanial activity of Flau-A against two Leishmania species and suggests that the mitochondria of the parasites may be the main target organelle. Data shown here encourages the use of this molecule in new studies concerning treatment against Leishmania infection in mammalian hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis is a major public health problem in the world, where approximately 350 million people are at risk of contracting the infection, with an estimated 12 million people being clinically affected by the disease (World Health Organization 2016). The clinical manifestations of leishmaniasis have varied from self-healing cutaneous lesions present at the site of the phlebotomine vector bite to the visceral form of the disease, which can be fatal if untreated (Andrade et al. 2016).
Tegumentary leishmaniasis (TL) is usually found in Afghanistan, Saudi Arabia, Syria, Iran, Algeria, Iraq, Brazil, and Peru, the disease being mainly caused by Leishmania braziliensis, L. panamensis, L. guyanensis, and L. amazonensis species (Ullah et al. 2016). Visceral leishmaniasis (VL) leads to nearly 50,000 deaths annually and is caused by L. donovani complex parasites, such as L. donovani and L. infantum (Araújo et al. 2017).
The treatment against leishmaniasis is based on pentavalent antimonials. Other drugs, such as amphotericin B (AmpB), paramomycin, miltefosine, and pentamidine, have been used as second-line drugs (Copeland and Aronson 2015). However, these compounds present limitations, such as toxic side effects leading to hepatic, splenic, and renal toxicity; long duration of treatment; and/or parasite resistance. Due to few current alternatives available in the market, the identification of new compounds to be used to treat against leishmaniasis could be considered desirable (Chakravarty and Sundar 2010; Sundar and Chakravarty 2013; Chávez-Fumagalli et al. 2015).
As a consequence, the investigation of synthetic molecules presenting biological actions other than antileishmanials could allow applying these products against Leishmania parasites, being considered as a promising field of research for new pharmaceuticals (Cheuka et al. 2016). In this context, quinones consist of a variety of aromatic metabolites found in plants, fungi, algae, and bacteria. These molecules include anthraquinones, benzoquinones, and naphthoquinones and can easily be synthesized, allowing to obtain a high yield and lower cost of production to be tested as therapeutic candidates against diseases such as leishmaniasis (Pinto et al. 2014).
In this context, naphthoquinones have been used as promising anticancer, antiviral, trypanocidal, and/or antimicrobial compounds (Riffel et al. 2002; Su et al. 2010; Pinto et al. 2014). Among the 1,4-naphthoquinone derivatives, some molecules present also antimalarial activity (Rezende et al. 2013; Schuck et al. 2013), while others show activity against Mycobacterium tuberculosis (Ferreira et al. 2010), Plasmodium falciparum (Sharma et al. 2013), and Biomphalaria glabrata (Camara et al. 2008).
In the present study, aiming to identify new 1,4-naphthoquinone derivates with antileishmanial activity as well as with low toxicity to mammalian cells, the 2-(2,3,4-tri-O-acetyl-6-deoxy-β-L-galactopyranosyloxy)-1,4-naphthoquinone, namely Flau-A, was evaluated as an antiparasitic agent against L. infantum and L. amazonensis species. Also, aiming to investigate the action in Leishmania, the mechanism of action of Flau-A was evaluated in L. amazonensis.
Materials and methods
Synthesis of Flau-A
Lawsone 0.30 g (1.72 mmol) was dissolved in 5 mL of dichloromethane, and transferred to a 100-mL round bottom flask. A solution containing potassium carbonate (10% w/v, 5 mL) was added and the mixture was stirred at room temperature during 30 min. Next, 2,3,4-tri-O-acetyl-6-deoxy-β-L-galactopyranosyl bromide (2.0 g, 3.44 mmol) and n-Bu4NBr (0.12 g, 22% mol) were added, and the mixture was stirred at room temperature for 18 h. Then, hydrochloric acid 6 mol/L was added to obtain pH 3.0, and the mixture was transferred to a separatory funnel, aiming to separate the organic phase and extracting the aqueous phase with dichloromethane (3 × 50 mL). The organic phase was washed using distilled water (100 mL), dried over anhydrous sodium sulfate, and concentrated. The crude product was used in a column chromatography (hexane/ethyl acetate, 7:3) to obtain 0.26 g (34% yield) of the purified molecule.
Parasites and mice
L. infantum (MHOM/BR/1970/BH46) and L. amazonensis (IFLA/BR/1967/PH-8) were used. Parasites were grown at 24 °C in complete Schneider’s medium (Sigma-Aldrich, St. Louis, MO, USA), consisting of Schneider’s medium plus 20% heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich, USA) and 20 mM l-glutamine, pH 7.4. Stationary-phase promastigotes were cultured as described elsewhere (Coelho et al. 2003). To obtain the amastigote-like forms, a technical protocol was developed according to a previously described method, with a few modifications (Duarte et al. 2015). Briefly, 1 × 109 stationary-phase promastigotes were washed 3 times in sterile phosphate buffer saline (PBS) 1× and incubated for 48 or 72 h for L. amazonensis or L. infantum, respectively, in 3 mL FBS, at 37 °C. Then, parasites were washed 3 times in cold PBS 1×, and their morphology was evaluated after staining by the Giemsa method in an optical microscope. Murine peritoneal macrophages were collected from BALB/c mice (female, 8 weeks old), which were purchased from the Institute of Biological Sciences of the Federal University of Minas Gerais (UFMG). The study was developed in compliance with the National Guidelines of the Institutional Animal Care and approved by the Use Committee for the Ethical Handling of Research Animals (protocol number 085/2017). Also, this work was approved by the Human Research Ethics Committee of UFMG (protocol number CAAE–32343114.9.0000.5149).
Viability assay in Leishmania species
A previous titration curve was performed to determine the best time of inhibition of parasite growth. The inhibition of Leishmania growth was then evaluated by cultivating L. infantum or L. amazonensis stationary promastigotes or amastigote-like forms (1 × 106 parasites) in the presence of Flau-A (0.1 to 20.0 μg/mL) and in 96-well culture plates (Nunc, Nunclon, Roskilde, Denmark) for 48 h at 24 °C. AmpB (0.1 to 2.0 μg/mL, Sigma-Aldrich, USA) was used as a control. Cell viability was assessed by measuring the cleavage of 5 mg/mL of 3-(4.5-dimethylthiazol-2-yl)-2.5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich, USA). The optical density (OD) values were read in an ELISA microplate spectrophotometer (Molecular Devices, Spectra Max Plus, Canada) at 570 nm. The product concentration needed to inhibit 50% of the Leishmania viability (IC50) was determined by applying a sigmoidal regression of the dose-response curves. Data shown are representative of three independent experiments, performed in triplicate, which presented similar results.
Cytotoxicity in murine cells and selectivity index
To evaluate the toxicity against mammalian cells, Flau-A was incubated with murine macrophages. For this, a previous titration curve was performed to determine the best time of inhibition of macrophage viability. The inhibition of 50% of the macrophage viability (CC50) was calculated by cultivating them (5 × 105 cells, per well) with Flau-A (0.1 to 20.0 μg/mL) or AmpB (0.1 to 2.0 μg/mL), in 96-well plates (Nunc) for 48 h at 37 °C. Then, cell viability was evaluated by MTT. Selectivity index (SI) values were calculated by the ratio between the CC50 and IC50 results. Data shown are representative of three independent experiments, performed in triplicate, which presented similar results.
Cytotoxicity against human cells
To evaluate the toxicity against human cells, the hemolytic activity was investigated by incubating Flau-A (1.0 to 100.0 μg/mL) with a 5% red blood cell (human O type) suspension, which was obtained from three healthy donors, during 1 h at 37 °C. The suspension was centrifuged at 1000×g and 10 min, when the cell lysis percentage was determined spectrophotometrically at 570 nm. The absence (blank) or 100% of hemolysis was determined by replacing Flau-A for an equal volume of PBS 1× or distilled water, respectively. Results were calculated by the hemolysis percentage as compared to the negative (PBS 1×) and positive (distilled water) controls. Data shown are representative of three independent experiments, performed in triplicate, which presented similar results.
Treatment of infected macrophages
To evaluate the efficacy of Flau-A in treating infected macrophages, cells (5 × 105 per well) were seeded on round glass coverslips within 24-well plates in RPMI 1640 medium, which was supplemented with 20% FBS and 20 mM l-glutamine, pH 7.4, and incubated during 24 h at 37 °C in 5% CO2. Stationary-phase promastigotes (5 × 106) were added to the wells, and cultures were incubated for 48 h at 37 °C in 5% CO2. Free parasites were removed by extensive washing with RPMI 1640 medium, while infected macrophages were treated with Flau-A (0, 2.5, 5.0, and 10.0 μg/mL) or AmpB (0, 0.1, 0.5, and 1.0 μg/mL) for 48 h at 24 °C in 5% CO2. After fixation with 4% paraformaldehyde, macrophages were stained by the Giemsa method and the percentage of infected cells as well as the number of intramacrophage amastigotes was determined by counting 200 cells in an optical microscope. Data shown are representative of three independent experiments, performed in triplicate, which presented similar results.
Inhibition of macrophage infection
The inhibitory effect of Flau-A in Leishmania was evaluated by treating parasites with this molecule, and then used them to infect murine macrophages. For this, parasites (5 × 106) were incubated with Flau-A (0, 2.5, 5.0, and 10.0 μg/mL) or AmpB (0, 0.1, 0.5, and 1.0 μg/mL) for 1 h at 24 °C. Cells were washed 3 times in RPMI 1640 medium, quantified, and incubated with macrophages (5 × 105 cells) for 24 h at 37 °C in 5% CO2. Then, they were washed and stained by the Giemsa method to evaluate the percentage of infected macrophages as well as the number of intramacrophage amastigotes by counting 200 cells in triplicate and in an optical microscope. Data shown are representative of three independent experiments, performed in triplicate, which presented similar results.
Mechanism of action in L. amazonensis
Morphological studies in Flau-A-treated parasites
L. amazonensis promastigotes (1 × 107 cells) were untreated or treated with Flau-A (0.73 and 1.46 μg/mL, corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Next, parasites were fixed with 4% paraformaldehyde for 20 min, washed with PBS 1×, and placed on glass slides. After staining with Giemsa, slides were examined in an optical microscope and photographed. The volume of treated parasites was evaluated by a FACSCanto II flow cytometer (Becton Dickinson, Rutherford, NJ, USA), equipped with DIVA software (Joseph Trotter, Scripps Research Institute, La Jolla, CA, USA). The forward scatter (FSC) parameter was analyzed as presenting a correlation with the cell volume, and a total of 10,000 events were acquired, according to a method previously described (Ribeiro et al. 2013).
Measurement of the mitochondrial membrane potential (ΔΨ푚)
The evaluation of ΔΨ푚 was performed by using two fluorescent dyes: MitoTracker® Red CM-H2XROS (Life Technologies, USA) and Rh123 (Sigma-Aldrich, USA). For this, L. amazonensis promastigotes (1 × 107 cells) were untreated or treated with Flau-A (0.73 and 1.46 μg/mL, corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. The reduction of parasite viability was 29.42% when a concentration of 0.73 μg/mL was used and 37.53% when the concentration of 1.46 μg/mL was employed in the experiments, in comparison to untreated cells. Then, parasites were washed with PBS 1× and incubated with 50 nM MitoTracker® for 40 min at 24 °C (Coimbra et al. 2016). The fluorescence intensity was spectrofluorometrically measured (FLx800, BioTek Instruments, Inc., Winooski, VT, USA), at an excitation wavelength of 540 nm and an emission wavelength of 600 nm. Additional assays were performed using Rh123 as described elsewhere (Antinarelli et al. 2015). The data acquisition and interpretation were performed in a FACSCanto II flow cytometer, in a total of 10,000 events acquired using FITC channel. The variation index (VI), which allows quantifying changes in the fluorescence intensity of Rh123, was determined by the (MT − MC)/MC equation, with MT representing the median fluorescence of the treated parasites and the MC the median fluorescence of negative controls. Miltefosine (18.0 μg/mL, Sigma-Aldrich, USA) and carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP, 2.5 and 5.0 μg/mL) were used as controls.
Detection of the reactive oxygen species (ROS)
The ROS production was determined by using the fluorescent 2,7-dichlorodihydrofluorescein diacetate (H2DCFDA) probe, as described elsewhere (Coimbra et al. 2016). For this, L. amazonensis promastigotes (1 × 107 cells) were treated or untreated with Flau-A (0.73 and 1.46 μg/mL, corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Cells were washed with PBS 1× and incubated with H2DCFDA. The fluorescence intensity was measured in a spectrofluorometer (FLx800, BioTek Instruments, Inc., Winooski, VT, USA), with excitation and emission wavelengths of 485 and 528 nm, respectively. Miltefosine (18.0 μg/mL) was used as a control, and the experiments were performed in triplicate, presenting similar results.
Evaluation of the cell membrane integrity
L. amazonensis promastigotes (1 × 107 cells) were untreated or treated with Flau-A (0.73 and 1.46 μg/mL, corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Cells were washed with PBS 1× and incubated with propidium iodide (1.0 μg/mL) during 15 min in the dark at room temperature (Stroppa et al. 2017). The fluorescence intensity was measured using a spectrofluorometer (FLx800, BioTek Instruments, Inc., Winooski, VT, USA), with excitation and emission wavelengths of 540 and 600 nm, respectively. As a control, cells were heated at 65 °C for 10 min. Results were obtained from three independent experiments performed in triplicate, which presented similar results.
Evaluation of phosphatidylserine exposition on the cell membrane
The exposure of phosphatidylserine on the cell surface was evaluated by using Annexin V-FITC (Invitrogen, USA) and propidium iodide, as described elsewhere (Stroppa et al. 2017). For this, L. amazonensis promastigotes (1 × 107 cells) were untreated or treated with Flau-A (0.73 and 1.46 μg/mL, corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Then, parasites were washed with PBS 1× and resuspended in Annexin V binding buffer. For labeling, 10 μL propidium iodide (1.0 μg/mL) and 5 μL of Annexin-FITC were added for 15 min at room temperature and in the dark. Data acquisition and interpretation were performed in a FACSCanto II flow cytometer, and a total of 10,000 events were acquired. Miltefosine (18.0 μg/mL) used as a control.
Toxicology studies
A toxicological evaluation was performed in BALB/c mice, as previously described (Duarte et al. 2016; Mendonça et al. 2016). For this, mice (n = 8 per group) were inoculated subcutaneously once a day in their left hind footpad with saline, AmpB (1 mg/kg body weight), or Flau-A (5 mg/kg of body weight), during 15 days. Once a day, variations in the body weight and clinical signals were monitored. One day after the end of treatment, blood samples were collected for biochemical analysis, with the cardiac function being analyzed by the dosage of creatine kinase-MB and the hepatic function analyzed by the dosage of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Nephrotoxicity was evaluated by examining the blood urea nitrogen (BUN) and serum creatinine (CRTN) levels. Experiments were performed using commercial kits (Labtest Diagnostica®, Minas Gerais, Brazil), in an autoanalyzer apparatus (Thermo Plate TP analyzer, São Paulo, Brazil).
Statistical analysis
Results were evaluated in Microsoft Excel (version 10.0) and analyzed by the GraphPad PrismTM (version 6.0 for Windows). The IC50 and CC50 values were calculated from the mean percentage reduction of the promastigotes and amastigote-like forms (IC50) or murine macrophages (CC50), respectively, when compared to the untreated controls. The curves were determined by applying sigmoidal regression to logarithm concentration/response data. The one-way analysis of variance (ANOVA), followed by Dunnett’s test, was used for multiple comparisons between the groups. Results were expressed as mean ± standard deviation, and differences in relation to the untreated controls were considered significant when P < 0.05 (*), P < 0.01 (**), and P < 0.001 (***).
Results
Antileishmanial activity of Flau-A
The antileishmanial activity of Flau-A was evaluated against promastigotes and amastigote-like forms of L. infantum and L. amazonensis species. Results using L. infantum showed IC50 values of 0.73 and 0.07 μg/mL for Flau-A and AmpB against promastigotes, respectively, while against the amastigote-like forms, the values were 1.03 and 0.23 μg/mL, respectively. Results using L. amazonensis showed IC50 values of 0.73 and 0.10 μg/mL for Flau-A and AmpB against promastigotes, respectively, while against amastigote-like forms, the values were 1.57 and 0.38 μg/mL, respectively.
Cytotoxicity in mammalian cells
Regarding CC50 values, the results in murine macrophages presented values of 18.48 and 1.0 μg/mL for Flau-A and AmpB, respectively. With these data, SI values (CC50/IC50 ratio) were calculated and were 25.3 and 14.3 when Flau-A and AmpB were tested against L. infantum promastigotes, respectively, while against the amastigote-like forms, the values were 17.9 and 4.3, respectively. Results against L. amazonensis promastigotes presented values of 25.3 and 10.0, respectively, while against the amastigote-like forms, the values were 11.8 and 2.6, respectively. The hemolytic activity in human type O-type red cells was also investigated; however, no hemolysis was found when Flau-A was tested (data not shown).
Flau-A reduces parasite load in Leishmania-infected macrophages
The effect of Flau-A in treating infected macrophages was investigated by counting 200 cells in triplicate. In the results using L. amazonensis, infection reduction in treated and infected macrophages was 81.0 and 91.0%, respectively, when 2.5 and 10.0 μg/mL of this molecule was tested, with a reduction in the number of recovered amastigotes in the order of 92.0 and 98%, respectively (Table 1). Using AmpB (1.0 μg/mL), reductions in the infection degree and in the number of recovered amastigotes were 89.0 and 88.0%, respectively. When L. infantum was used, infection reduction in Flau-A-treated and infected macrophages (2.5 and 10.0 μg/mL) was 55.0 and 85.0%, respectively, while the reduction in the number of recovered amastigotes was 78.0 and 97.0%, respectively. Using AmpB (1.0 μg/mL), reductions in the infection degree and in the number of recovered amastigotes were 79.0 and 89.0%, respectively (Table 1).
Pretreatment with Flau-A inhibits macrophage infection
The infectivity of parasites that were first treated with Flau-A and later used to infect macrophages was also evaluated. In the results using L. amazonensis, infection reduction in treated and infected macrophages was 46.0 and 68.0%, respectively, when 2.5 and 10.0 μg/mL of Flau-A was used. In addition, the reduction in the number of recovered amastigotes was 90.0 and 97%, respectively (Table 2). Using AmpB (1.0 μg/mL), reductions in the infection degree and in the number of recovered amastigotes were 81.0 and 98.0%, respectively. When L. infantum was employed in the tests, infection reduction in Flau-A-treated and infected macrophages (2.5 and 10.0 μg/mL) was 70.0 and 83.0%, respectively, whereas the reduction in the number of recovered amastigotes was 86.0 and 96.0%, respectively. Using AmpB (1.0 μg/mL), reductions in the infection degree and in the number of recovered amastigotes were 89.0% in both cases (Table 2).
Morphological alterations induced by Flau-A in L. amazonensis
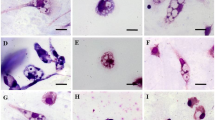
Aiming to evaluate the influence of Flau-A in the morphology of the parasites, we noticed the occurrence of morphological changes in L. amazonensis promastigotes that were treated with Flau-A. In the results, changes in the shape of the parasites were observed, with a rounded body and loss of cell volume, when compared to untreated controls, which showed an elongated cell body (Fig. 1). To confirm these results, the FSC parameter, which correlates with the cell volume, was also analyzed, and results showed changes in the cell population profile in relation to the parasite size, in a dose-dependent way. In addition, a gradual increase in this cellular population presenting reduced size was also observed (Fig. 2).
Morphology of L. amazonensis promastigotes after treatment using Flau-A. Parasites (1 × 107 cells) were untreated (a) or treated with 0.73 or 1.46 μg/mL of Flau-A (b and c, corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Cells were fixed with paraformaldehyde, stained with Giemsa, and visualized in an optical microscope (Olympus BX53). Scale, 10 μm
Representative histograms showing changes in the L. amazonensis volume after treatment using Flau-A. Parasites (1 × 107 cells) were treated with 0.73 or 1.46 μg/mL of Flau-A (corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Cells were analyzed by flow cytometry, and a total of 10,000 events were acquired. Experiments were performed 3 times and in triplicate. Miltefosine was used as a drug control. (**) and (***) indicate statistical differences in relation to the untreated control (P > 0.01 and P < 0.001, respectively)
Flau-A induces depolarization of ΔΨ푚, increases ROS production, and induces rupture of the L. amazonensis plasma membrane, but without phosphatidylserine exposure on the surface of the parasite membrane
Promastigotes treated with Flau-A and stained with MitoTracker® showed reduction in their ΔΨ푚, presenting similar results to those using FCCP, a classic protonophore uncoupler that was used as a positive control (Fig. 3a). The ΔΨ푚 was also evaluated through a flow cytometry assay using the Rh123 probe. The histogram of total Rh123 fluorescence (Fig. 3b) and results of the variation index (Fig. 3c) showed that the fluorescence was reduced. Promastigotes treated with 1 and 2 times the IC50 value showed a reduction in the fluorescence intensity in the order of 28.2 and 40.0%, respectively, when compared to the untreated controls. Cells treated with miltefosine showed a decrease in their ΔΨ푚 in the order of 62.7%, while FCCP reduced the ΔΨ푚 by 37.6 and 48.2%, when the concentrations of 2.5 and 5.0 μg/mL, respectively, were used. These results corroborated with the data obtained using MitoTracker®, indicating a depolarization of the ΔΨ푚 in Flau-A-treated parasites.
Alteration in the ΔΨ푚 of L. amazonensis after treatment using Flau-A. Parasites (1 × 107 cells) were treated with 0.73 or 1.46 μg/mL of Flau-A (corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Then, they were probed with MitoTracker® and analyzed spectrofluorometrically). FCCP (5.0 μg/mL) was used as controls (a). Representative histograms showing treated promastigotes stained with Rh123 by flow cytometry are shown. FCCP (5.0 μg/mL) and miltefosine (18.0 μg/mL) were used as controls (b). VI values were obtained by the (MT − MC)/MC equation, where MT corresponds to the median fluorescence of the treated parasites, and MC is the median fluorescence of the control parasites. FCCP (2.5 and 5.0 μg/mL) and miltefosine (18.0 μg/mL) were used as controls (c). Experiments were performed 3 times and in triplicate. (***) indicates statistical difference in relation to the untreated control (P < 0.001)
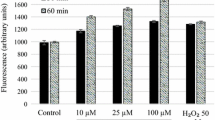
Oxidative stress is characterized by an increase in ROS production, and the mitochondria are one of the major intracellular sources of these reactive species (Chandrasekaran et al. 2013). Thus, we evaluated the ROS production in L. amazonensis promastigotes by using the fluorescent H2DCFDA probe. Results demonstrated that Flau-A-treated promastigotes by using 1 and 2 times the IC50 value expanded the ROS production in the order of 2.5 and 3.3 times, respectively, when compared to the untreated controls, while using miltefosine, the value was increased by 2 times (Fig. 4).
Production of ROS in Flau-A-treated L. amazonensis. Parasites (1 × 107 cells) were treated with 0.73 and 1.46 μg/mL of Flau-A (corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Cells were stained with H2DCFDA for 30 min at 24 °C, and the fluorescence was spectrofluorometrically measured. Miltefosine (18.0 μg/mL) was used as a drug control. Results were expressed as mean ± standard deviation. (*), (**), and (***) indicate statistical differences in relation to the untreated control (P > 0.05, P > 0.01, and P < 0.001, respectively)
The plasma membrane integrity was also evaluated by labeling it with propidium iodide, a fluorescent dye that is unable to cross the entire plasma membrane, but when it is ruptured, it is allowed to enter and bind to the DNA cell (Scariot et al. 2017). Results showed that Flau-A-treated promastigotes by using 1 and 2 times the IC50 value induced to an increase in their fluorescence intensity in the order of 3.0 and 4.1 times, when compared to the untreated controls, respectively (Fig. 5). Parasites heated at 65 °C for 10 min, which were used as positive controls, showed an increase in the fluorescence intensity in the order of 4.3 times.
Integrity of the Flau-A-treated L. amazonensis membrane. Parasites (1 × 107 cells) were treated with 0.73 and 1.46 μg/mL of Flau-A (corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Cells were stained with propidium iodide (PI) for 15 min at 24 °C, and the fluorescence intensity was spectrofluorometrically measured. Cells prewarmed at 65 °C for 10 min were used as positive control. Results were expressed as mean ± standard deviation. Three independent experiments were performed and they presented similar results. (***) indicates statistical difference in relation to the untreated control (P < 0.001)
Phosphatidylserine exposure is one characteristic of apoptotic cells (El-Hani et al. 2012). In the present study, treatment using Flau-A did not induce a statistical increase in the percentage of cells stained only with Annexin-FITC (4.99% in untreated control and 6.69% using 1.46 μg/mL of Flau-A; lower right quadrant). The dot plot analysis showed an increase in the percentage of propidium iodide-positive cells (5.13 and 13.05%, by using 0.73 and 1.46 μg/mL of Flau-A, respectively; upper left quadrant), while an increase in the double-stained Annexin+/PI+ cell percentage was also observed by using both concentrations, probably reflecting an initial rupture of the plasma membrane and subsequent entry of this reagent and Annexin probes into the cell. As a control, miltefosine-treated promastigotes exhibited percentages of 41.71 and 4.72% of Annexin- and propidium iodide-positive cells, respectively (Fig. 6).
Representative dot plots of phosphatidylserine exposure on the Flau-A-treated L. amazonensis membrane. Parasites (1 × 107 cells) were treated with 0.73 and 1.46 μg/mL of Flau-A (corresponding to 1 and 2 times the IC50 value obtained after 48 h of the in vitro cultures, respectively) for 2 h at 24 °C. Cells were stained with fluorescent probes containing Annexin V-FITC and propidium iodide (PI), and they were analyzed by flow cytometry. Miltefosine (18.0 μg/mL) was used as a drug control. The Annexin V-positive cell percentage is shown in the lower right quadrant, the PI+ cells are shown in the upper left quadrant, and the Annexin V+/PI+ cells are shown in the upper right quadrant. Dot plots are representative of three independent experiments, which were performed in triplicate, and presented similar results. (*), (**), and (***) indicate statistical differences in relation to the untreated control (P > 0.05, P > 0.01, and P < 0.001, respectively)
Flau-A is not toxic for mammalian models
A clinical and biochemical evaluation was performed in AmpB- or Flau-A-treated mice. In the results, no clinical signal or laboratorial abnormality was found in the treated animals, which showed also a positive variation in their weight in the order of 7.0%. On the other hand, AmpB-treated mice presented weakness and a negative variation in their weight in the order of 8.0%. These animals showed also higher levels of AST, ALT, BUN, and CRTN, indicating an organic toxicity caused by the administration of this drug.
Discussion
The treatment of leishmaniasis has been based on chemotherapy, although drugs are toxic and difficult to administer, and parasite resistance has increased (Chávez-Fumagalli et al. 2015). In this context, the search for less toxic, highly effective, and low-cost products is desirable. In the present study, a new naphthoquinone derivative showed an effective antileishmanial activity against Leishmania species able to cause tegumentary and visceral leishmaniasis in the world, and this molecule was also found to present a low toxicity in distinct mammalian cells.
The identification of new compounds presenting biological properties remains a challenge. Natural products have served as lead structures for the development of chemotherapeutics against diseases, due to their biological activity, bioavailability, high yield, and low cost of production (Winkler et al. 2007). In this context, naphthoquinones have been considered promising scaffolds against parasites, and some classes of molecules exhibit in vitro antileishmanial activity (Lezama-Dávila et al. 2012; Costa et al. 2014).
Leishmania parasites present two main morphological forms in their life cycle: promastigote and amastigote. Promastigotes are found in the gut of sand flies and live extracellularly, whereas amastigotes reside inside macrophages in infected mammalian hosts (Saraiva et al. 2005). Most of the studies aiming to identify new antileishmanial targets have tested molecules against the promastigote forms (Gao et al. 2012; Sazgarnia et al. 2012), although amastigotes should be considered also relevant, since they are responsible for the development of the active disease in the mammalian hosts (Muylder et al. 2011; Fernandes et al. 2012). As a consequence, promastigote assays could be used as prescreening, whereas intra-amastigote assays could be employed to follow the biological trials (Ullah et al. 2016).
In our study, Flau-A was effective against stationary promastigotes, amastigote-like forms, and intramacrophage amastigotes of both L. amazonensis and L. infantum species, besides presenting no significant toxicity in the two mammalian cells. In addition, a reduction in the percentage of infected macrophages was found when parasitized cells were treated with Flau-A, as well as a reduction in the infection of macrophages was visualized when this molecule was preincubated with parasites, then demonstrating an important biological activity against Leishmania, as well as efficacy in activating macrophages to kill parasites.
Since this study’s purpose was to identify new antileishmanial candidates to be used in the in vivo treatment against leishmaniasis, a toxicity study was performed in BALB/c mice by using Flau-A, which was administered in the animals during 15 days. As a control, AmpB was used. Higher levels of AST, ALT, BUN, and CRTN enzymes were found in the AmpB-treated animals, possibly indicating a hepatic and renal toxicity when this product was inoculated in mice. On the other hand, no toxicity was found when Flau-A was administered in the animals. Our experimental model used a subcutaneous route to administrate the molecules, as well as a predefined dose of Flau-A and AmpB. We understand that other routes and doses could eventually induce higher or lower toxicity in the animals; however, due to ethical limitations limiting the number of animals to be used to perform a dose-response curve, we have adopted routes and doses already described as effective in other studies (Duarte et al. 2016; Mendonça et al. 2016). In addition, similar organic alterations were also found in BALB/c mice, when the intravenous route was employed to administer AmpB, thus demonstrating the toxicity of this compound in the mammalian hosts (Deray 2002; Mishra et al. 2013; Ribeiro et al. 2014; Asad et al. 2015). As a consequence, we can infer that Flau-A is safe to administer in these hosts, which opens the possibility to test it in future studies for the treatment against leishmaniasis.
Works evaluating the mechanism of action of antileishmanial drugs could provide important information for the optimization of hit compounds, since the majority of the studies have shown an antileishmanial effect but without presenting the mechanism of action of the tested compounds (Fumarola et al. 2004; Tempone et al. 2011). In the present study, the mechanism of action of Flau-A was investigated in L. amazonensis. The modifications induced in Flau-A-treated promastigotes showed changes in parasite morphology, which were reflected by a marked decrease in their mobility and alterations in the flagellum size, possibly resulting from alterations in the cytoskeleton reorganization associated with modifications in their mitochondrial activity.
Undoubtedly, the mitochondria are relevant target organs in Leishmania, and our study showed also that Flau-A is acting in this organelle, as described by others studying different molecules and trypanosomatids (Menna-Barreto and Castro 2014; Lage et al. 2015). The mitochondria are organelles important in parasites, acting in the metabolism, cell differentiation, calcium homeostasis, cell death, and oxidative phosphorylation (Smith et al. 2012; Menna-Barreto and Castro 2014). Unlike eukaryotic organisms that present several, if not thousands, of mitochondria, trypanosomatids such as Leishmania have a single organelle in their cytoplasm (Souza et al. 2009), a fact that can favor the development of drugs acting on this Leishmania organelle (Menna-Barreto and Castro 2014; Antinarelli et al. 2015; Stroppa et al. 2017). The present study used two probes, namely Rh123 and MitoTracker®, which are considered to be ΔΨ푚-sensitive dyes, and results showed that treatment with Flau-A interferes in the electrochemical potential gradient of the parasites’ mitochondrial membrane, by reducing their ΔΨ푚. In addition, results revealed also an increase in ROS production, indicating that it may well be responsible for mitochondrial dysfunction. During mitochondrial metabolism, ROS is formed by the incomplete reduction of molecular oxygen (Forkink et al. 2010; Smith et al. 2012). Cells can protect this molecule in physiological conditions; however, oxidative stress can occur if ROS is produced in large amounts, then contributing to mitochondrial dysfunction and the parasite’s irreversible damage, culminating with cell death.
In addition to its effect on parasite mitochondria, treatment with Flau-A altered also the permeability of the plasma membrane of L. amazonensis promastigotes. Using propidium iodide, a probe able to cross the membrane in nonintact cells, it was observed that treatment with Flau-A affected the Leishmania membrane, allowing them to cause the rupture of the cell membrane. This fact was observed by both fluorimetry and flow cytometry techniques, with the rupture of the plasma membrane being usually considered as an event related to cell necrosis (Proto et al. 2013).
On the other hand, when Annexin V-FITC was used, we observed a low population of Annexin V-positive/propidium iodide-negative cells (~ 7.0%), revealing a loss of membrane asymmetry and exposure of phosphatidylserine residues at the outer plasma membrane of the parasites. These cells cannot necessarily indicate cell death due to treatment with Flau-A but suggest this action in a parasite subpopulation, as described by Santos et al. (2013), which showed that stationary-phase promastigotes of Leishmania, in contrast to the log-phase cells, present a subpopulation that exposes a high frequency of Annexin-V-positive cells. Other studies demonstrated also that Annexin-positive parasites can be result of a “apoptotic mimicry,” where Leishmania exposes phosphatidylserine with the purpose to infect macrophages, although this event seems to be usually found when the amastigote forms are evaluated (Wanderley and Barcinski 2010; El-Hani et al. 2012).
In summary, our data showed that Flau-A presents an effective antileishmanial activity against two important Leishmania species worldwide, besides a rapid in vitro effect manifesting changes in membrane permeability, mitochondrial functionality, and parasite morphology. In addition, the ability of this molecule in reducing the infection in murine macrophages, as well as its effectiveness in inhibiting the infection of these cells, when pretreated parasites are used, demonstrates that it could well be applied as a new candidate to treat against tegumentary and visceral leishmaniasis in mammalian hosts.
References
Andrade MA, Azevedo CD, Motta FN, Santos ML, Silva CL, Santana JM, Bastos IM (2016) Essential oils: in vitro activity against Leishmania amazonensis, cytotoxicity and chemical composition. BMC Complement Altern Med 16(1):444. https://doi.org/10.1186/s12906-016-1401-9
Antinarelli LM, Dias RM, Souza IO, Lima WP, Gameiro J, da Silva AD, Coimbra ES (2015) 4-Aminoquinoline derivatives as potential antileishmanial agents. Chem Biol Drug Des 86(4):704–714. https://doi.org/10.1111/cbdd.12540
Araújo MV, David CC, Neto JC, Oliveira LA, Silva KC, Santos JM, Silva JK, Brandão AVB, Silva TM, Camara CA, Alexandre-Moreira MS (2017) Evaluation on the leishmanicidal activity of 2-N,N’-dialkylamino-1,4-naphthoquinone derivatives. Exp Parasitol 176:46–51. https://doi.org/10.1016/j.exppara.2017.02.004
Asad M, Bhattacharya P, Banerjee A, Ali N (2015) Therapeutic and immunomodulatory activities of short-course treatment of murine visceral leishmaniasis with KALSOME™10, a new liposomal amphotericin B. BMC Infect Dis 15(1):188. https://doi.org/10.1186/s12879-015-0928-6
Camara CA, Silva TM, Silva TG, Martins RM, Barbosa TP, Pinto AC, Vargas MD (2008) Molluscicidal activity of 2-hydroxy-[1,4]naphthoquinone and derivatives. Na Acad Bras Cienc 80(2):329–334. https://doi.org/10.1590/S0001-37652008000200011
Chakravarty J, Sundar S (2010) Drug resistance in leishmaniasis. J Glob Infect Dis 167:176
Chandrasekaran S, Dayakar A, Veronica J, Sundar S, Maurya R (2013) An in vitro study of apoptotic like death in Leishmania donovani promastigotes by withanolides. Parasitol Intern 62(3):253–261. https://doi.org/10.1016/j.parint.2013.01.007
Chávez-Fumagalli MA, Ribeiro TG, Castilho RO, Fernandes SO, Cardoso VN, Coelho CS, Mendonça DV, Soto M, Tavares CA, Faraco AA, Coelho EA (2015) New delivery systems for amphotericin B applied to the improvement of leishmaniasis treatment. Rev Soc Bras Med Trop 48(3):235–242. https://doi.org/10.1590/0037-8682-0138-2015
Cheuka PM, Mayoka G, Mutai P, Chibale K (2016) The role of natural products in drug discovery and development against neglected tropical diseases. Molecules 22:E58
Coelho EAF, Tavares CAP, Carvalho FAA, Chaves KF, Teixeira KN, Rodrigues RC, Charest H, Matlashewski G, Gazzinelli RT, Fernandes AP (2003) Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect Immun 71(7):3988–3994. https://doi.org/10.1128/IAI.71.7.3988-3994.2003
Coimbra ES, Antinarelli LM, Silva NP, Souza IO, Meinel RS, Rocha MN, Soares RP, Silva AD (2016) Quinoline derivatives: synthesis, leishmanicidal activity and involvement of mitochondrial oxidative stress as mechanism of action. Chem Biol Interact 260:50–57. https://doi.org/10.1016/j.cbi.2016.10.017
Copeland NK, Aronson NE (2015) Leishmaniasis: treatment updates and clinical practice guidelines review. Curr Opin Infect Dis 28(5):426–437. https://doi.org/10.1097/QCO.0000000000000194
Costa L, Pinheiro RO, Dutra PM, Santos RF, Cunha-Júnior EF, Torres-Santos EC, Silva AJ, Costa PR, Silva AS (2014) Pterocarpanquinone LQB-118 induces apoptosis in Leishmania (Viannia) braziliensis and controls lesions in infected hamsters. PLoS One 9(10):e109672. https://doi.org/10.1371/journal.pone.0109672
Deray G (2002) Amphotericin B nephrotoxicity. J Antimicrob Chemother 49(suppl 1):37–41. https://doi.org/10.1093/jac/49.suppl_1.37
Duarte MC, Pimenta DC, Menezes-Souza D, Magalhães RD, Diniz JL, Costa LE, Chávez-Fumagalli MA, Lage PS, Bartholomeu DC, Alves MJ, Fernandes AP, Soto M, Tavares CA, Gonçalves DU, Rocha MO, Coelho EA (2015) Proteins selected in Leishmania (Viannia) braziliensis by an immunoproteomic approach with potential serodiagnosis applications for tegumentary leishmaniasis. Clin Vaccine Immunol 22(11):1187–1196. https://doi.org/10.1128/CVI.00465-15
Duarte MC, Lage LM, Lage DP, Martins VT, Carvalho AM, Roatt BM, Menezes-Souza D, Tavares CA, Alves RJ, Barichello JM, Coelho EA (2016) Treatment of murine visceral leishmaniasis using an 8-hydroxyquinoline-containing polymeric micelle system. Parasitol Int 65(6):728–736. https://doi.org/10.1016/j.parint.2016.07.005
El-Hani CN, Borges VM, Wanderley JL, Barcinski MA (2012) Apoptosis and apoptotic mimicry in Leishmania: an evolutionary perspective. Front Cell Infect Microbiol 2:96
Fernandes AP, Coelho EA, Machado-Coelho GL, Grimaldi G Jr, Gazzinelli RT (2012) Making an anti-amastigote vaccine for visceral leishmaniasis: rational, update and perspectives. Curr Opin Microbiol 15(4):476–485. https://doi.org/10.1016/j.mib.2012.05.002
Ferreira SB, Carvalho-da-Silva F, Bezerra FA, Lourenço MC, Kaiser CR, Pinto AC, Ferreira VF (2010) Synthesis of alpha- and beta-pyran naphthoquinones as a new class of antitubercular agents. Arch Pharm (Weinheim) 343(2):81–90. https://doi.org/10.1002/ardp.200900162
Forkink M, Smeitink JA, Brock R, Willems PH, Koopman WJ (2010) Detection and manipulation of mitochondrial reactive oxygen species in mammalian cells. Biochim Biophys Acta 97:1034–1044
Fumarola L, Spinelli R, Brandonisio O (2004) In vitro assays for evaluation of drug activity against Leishmania spp. Res Microbiol 155(4):224–230. https://doi.org/10.1016/j.resmic.2004.01.001
Gao J, Radwan MM, León F, Wang X, Jacob MR, Tekwani BL, Khan SI, Lupien S, Hill RA, Dugan FM, Cutler HG, Cutler SJ (2012) Antimicrobial and antiprotozoal activities of secondary metabolites from the fungus Eurotium repens. Med Chem Res 21(10):3080–3086. https://doi.org/10.1007/s00044-011-9798-7
Lage PS, Chávez-Fumagalli MA, Mesquita JT, Mata LM, Fernandes SO, Cardoso VN, Soto M, Tavares CA, Leite JP, Tempone AG, Coelho EA (2015) Antileishmanial activity and evaluation of the mechanism of action of strychnobiflavone flavonoid isolated from Strychnos pseudoquina against Leishmania infantum. Parasitol Res 114(12):4625–4635. https://doi.org/10.1007/s00436-015-4708-4
Lezama-Dávila CM, Isaac-Márquez AP, Kapadia G, Owens K, Oghumu S, Beverley S, Satoskar AR (2012) Leishmanicidal activity of two naphthoquinones against Leishmania donovani. Biol Pharm Bull 35(10):1761–1764. https://doi.org/10.1248/bpb.b12-00419
Mendonça DV, Lage LM, Lage DP, Chávez-Fumagalli MA, Ludolf F, Roatt BM, Menezes-Souza D, Faraco AA, Castilho RO, Tavares CA, Barichello JM, Duarte MC, Coelho EA (2016) Poloxamer 407 (Pluronic® F127)-based polymeric micelles for amphotericin B: in vitro biological activity, toxicity and in vivo therapeutic efficacy against murine tegumentary leishmaniasis. Exp Parasitol 169:34–42. https://doi.org/10.1016/j.exppara.2016.07.005
Menna-Barreto RF, Castro SL (2014) The double-edged sword in pathogenic trypanosomatids: the pivotal role of mitochondria in oxidative stress and bioenergetics. Biomed Res Int 2014:614014
Mishra J, Dey A, Singh N, Somvanshi R, Singh S (2013) Evaluation of toxicity & therapeutic efficacy of a new liposomal formulation of amphotericin B in a mouse model. Indian J Med Res 137(4):767–776
Muylder G, Ang KKH, Chen S, Arkin MR, Engel JC, Mc Kerrow JH (2011) A screen against Leishmania intracellular amastigotes: comparison to a promastigote screen and identification of a host cell-specific hit. PLoS Negl Trop Dis 5(7):e1253. https://doi.org/10.1371/journal.pntd.0001253
Pinto EG, Santos IO, Schmidt TJ, Borborema SE, Ferreira VF, Rocha DR, Tempone AG (2014) Potential of 2-hydroxy-3-phenylsulfanylmethyl-[1,4]-naphthoquinones against Leishmania (L.) infantum: biological activity and structure-activity relationships. PLoS One 9(8):e105127. https://doi.org/10.1371/journal.pone.0105127
Proto WR, Coombs GH, Mottram JC (2013) Cell death in parasitic protozoa: regulated or incidental? Nat Rev Microbiol 11(1):58–66. https://doi.org/10.1038/nrmicro2929
Rezende LC, Fumagalli F, Bortolin MS, Oliveira MG, Paula MH, Andrade-Neto VF, Emery FS (2013) In vivo antimalarial activity of novel 2-hydroxy-3-anilino-1,4-naphthoquinones obtained by epoxide ring-opening reaction. Bioorg Med Chem Lett 23(16):4583–4586. https://doi.org/10.1016/j.bmcl.2013.06.033
Ribeiro GA, Cunha-Júnior EF, Pinheiro RO, Da-Silva SA, Canto-Cavalheiro MM, Silva AJ, Costa PR, Netto CD, Melo RC, Almeida-Amaral EE, Torres-Santos EC (2013) LQB-118, an orally active pterocarpanquinone, induces selective oxidative stress and apoptosis in Leishmania amazonensis. J Antimicrob Chemoth 68(4):789–799. https://doi.org/10.1093/jac/dks498
Ribeiro TG, Chávez-Fumagall MA, Valadares DG, França JR, Rodrigues LB, Duarte MC, Lage PS, Andrade PH, Lage DP, Arruda LV, Abánades DR, Costa LE, Martins VT, Tavares CA, Castilho RO, Coelho EA, Faraco AA (2014) Novel targeting using nanoparticles: an approach to the development of an effective antileishmanial drug-delivery system. Int J Nanomedicine 9:877–890. https://doi.org/10.2147/IJN.S55678
Riffel A, Medina LF, Stefani V, Santos RC, Bizani D, Brandelli A (2002) In vitro antimicrobial activity of a new series of 1,4-naphthoquinones. Braz J Med Biol Res 35(7):811–818. https://doi.org/10.1590/S0100-879X2002000700008
Santos MG, Muxel SM, Zampieri RA, Pomorski TG, Floeter-Winter LM (2013) Transbilayer dynamics of phospholipids in the plasma membrane of the Leishmania genus. PLoS One 8:e55604. https://doi.org/10.1371/journal.pone.0055604
Saraiva EM, Pinto-da-Silva LH, Wanderley JL, Bonomo AC, Barcinski MA, Moreira ME (2005) Flow cytometric assessment of Leishmania spp metacyclic differentiation: validation by morphological features and specific markers. Exp Parasitol 110(1):39–47. https://doi.org/10.1016/j.exppara.2005.01.004
Sazgarnia A, Zabolinejad N, Layegh P, Rajabi O, Berenji F, Javidi Z, Salari R (2012) Antileishmanial activity of liposomal clarithromycin against Leishmania major promastigotes. Iran J Basic Med Sci 15(6):1210–1214
Scariot DB, Britta EA, Moreira AL, Falzirolli H, Silva CC, Ueda-Nakamura T, Dias-Filho BP, Nakamura CV (2017) Induction of early autophagic process on Leishmania amazonensis by synergistic effect of miltefosine and innovative semi-synthetic thiosemicarbazone. Front Microbiol 8:1–16
Schuck DC, Ferreira SB, Cruz LN, Rocha DR, Moraes M, Nakabashi M, Rosenthal PJ, Ferreira VF, Garcia CR (2013) Biological evaluation of hydroxynaphthoquinones as anti-malarials. Malar J 12(1):234. https://doi.org/10.1186/1475-2875-12-234
Sharma A, Santos IO, Gaur P, Ferreira VF, Garcia CR, Rocha DR (2013) Addition of thiols to o-quinone methide: new 2-hydroxy-3-phenylsulfanylmethyl[1,4]-naphthoquinones and their activity against the human malaria parasite Plasmodium falciparum (3D7). Eur J Med Chem 59:48–53. https://doi.org/10.1016/j.ejmech.2012.10.052
Smith RA, Hartley RC, Cochemé HM, Murphy MP (2012) Mitochondrial pharmacology. Trends Pharmacol Sci 33(6):341–352. https://doi.org/10.1016/j.tips.2012.03.010
Souza W, Attias M, Rodrigues JC (2009) Particularities of mitochondrial structure in parasitic protists (Apicomplexa and Kinetoplastida). Int J Biochem Cell Biol 41(10):2069–2080. https://doi.org/10.1016/j.biocel.2009.04.007
Stroppa PHF, Antinarelli LMR, Carmo AML, Gameiro J, Coimbra ES, Silva AD (2017) Effect of 1,2,3-triazole salts, non-classical bioisosteres of miltefosine, on Leishmania amazonensis. Bioorg Med Chem 25(12):3034–3045. https://doi.org/10.1016/j.bmc.2017.03.051
Su JC, Lin KL, Chien CM, Tseng CH, Chen YL, Chang LS, Lin SR (2010) Furano-1,2- naphthoquinone inhibits EGFR signaling associated with G2/M cell cycle arrest and apoptosis in A549 cells. Cell Biochem Funct 28:695–705
Sundar S, Chakravarty J (2013) Leishmaniasis: an update of current pharmacotherapy. Expert Opin Pharmacother 14(1):53–63. https://doi.org/10.1517/14656566.2013.755515
Tempone AG, Martins-de-Oliveira C, Berlinck RG (2011) Current approaches to discover marine antileishmanial natural products. Planta Med 77(06):572–585. https://doi.org/10.1055/s-0030-1250663
Ullah N, Nadhman A, Siddiq S, Mehwish S, Islam A, Jafri L, Hamayun M (2016) Plants as antileishmanial agents: current scenario. Phytother Res 30(12):1905–1925. https://doi.org/10.1002/ptr.5710
Wanderley JL, Barcinski MA (2010) Apoptosis and apoptotic mimicry: the Leishmania connection. Cell Mol Life Sci 67(10):1653–1659. https://doi.org/10.1007/s00018-010-0291-0
Winkler JD, Londregan AT, Hamann MT (2007) Synthetic modification of Manzamine A via Grubbs metathesis. Novel structures with enhanced antibacterial and antiprotozoal properties. Org Lett 9(22):4467–4469. https://doi.org/10.1021/ol701799c
World Health Organization (2010) Control of the leishmaniases. World Health Organ Tech Rep Ser 949:22–26
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mendonça, D.V.C., Lage, D.P., Calixto, S.L. et al. Antileishmanial activity of a naphthoquinone derivate against promastigote and amastigote stages of Leishmania infantum and Leishmania amazonensis and its mechanism of action against L. amazonensis species. Parasitol Res 117, 391–403 (2018). https://doi.org/10.1007/s00436-017-5713-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5713-6