Abstract
The yellow perch Perca flavescens and the walleye Sander vitreus are native North American percid fishes, which have considerable fishery and ecological importance across their wide geographic ranges. Over the past century, they were stocked into new habitats, often with relative disregard for conserving local genetic adaptations. This chapter focuses on their comparative population structure and genetic diversity in relationship to historical patterns, habitat connectivity, dispersal ability, distributional abundances, and reproductive behavior. Both species possess considerable genetic structure across their native ranges, exhibiting similar patterning of discontinuities among geographic regions. The two species significantly differ in levels of genetic diversity, with walleye populations possessing overall higher genetic variability than yellow perch. Genetic divergence patterns follow the opposite trend, with more pronounced differences occurring among closely spaced spawning aggregations of yellow perch than walleye. Results reveal broad-scale correspondence to isolation by geographic distance, however, their fine-scale population structures show less relationship, often with pronounced genetic differences among some nearby reproductive groups. Genetic composition of spawning groups is stable from year to year in walleye, according to two decades of data, and is less consistent in yellow perch. These patterns appear to reflect fundamental behavioral differences between the two species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction to Yellow Perch and Walleye Population Genetics
The yellow perch Perca flavescens and walleye Sander vitreus are North American percid fishes of very significant fishery value and ecological importance as top piscivores. Both genera, Perca and Sander, are exclusively native to North America and Eurasia, with their respective species significantly diverging between the two continents. In North America, Perca contains just a single species – the yellow perch, whereas Sander has two species – the walleye and the sauger S. canadensis. There are two native Eurasian species of Perca: the European perch P. fluviatilis and the Balkash perch P. schrenkii, whereas Sander has three: the pikeperch S. lucioperca, the Volga pikeperch S. volgensis, and the sea pikeperch S. marinus. Their respective phylogenies are discussed by Stepien and Haponski in Chap. 1 of this book.
Yellow perch and walleye share wide native geographic distributions across much of the northeast and north central regions of North America, with both having a few isolated relict populations in the southeast. They inhabit a wide diversity of lacustrine and fluvial habitats, ranging from large to small in geographic sizes, with their most extensive habitats and greatest abundances occurring in the Laurentian Great Lakes – especially in Lake Erie (Scott and Crossman 1973; Hubbs and Lagler 2004). Dating to the mid-1800s, walleye and yellow perch were stocked (artificially introduced to new areas), throughout much of North America to provide fishing opportunity (USFWS/GLFC 2010). These introductions often mixed nonindigenous hatchery broodstock with local genotypes, and may have influenced the overall adaptedness of some indigenous populations. Today’s fishery managers increasingly recognize the importance of preserving local population variability, and it is advisable to perform any supplementation solely with native genotypes specific to that particular locale. The most prudent action is to protect the habitats of locally adapted populations and avoid negative effects of overexploitation, thereby circumventing any need to stock.
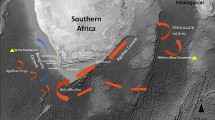
In the northern regions of the ranges of yellow perch and walleye, the habitats and basins of the Great Lakes region were formed and reshaped by the Laurentian Ice Age glaciations, leading to their present configuration about 4000–12,000 years ago (ya). During the glaciations, yellow perch, walleye, and other aquatic species migrated southward of the ice sheets, where their populations were concentrated in restricted glacial refugia areas. Three primary North American glacial refugia are recognized (marked on Fig. 25.1): the Mississippian Refugium in the central U.S., the Missourian Refugium to the west, and the Atlantic Refugium to the east (Bailey and Smith 1981; Crossman and McAllister 1986; Mandrak and Crossman 1992). Following the glacial meltwaters, aquatic taxa migrated along tributary pathways leading from the three refugia into the reformed waterbodies of the Great Lakes and other northerly habitats (see Fig. 25.1). Today’s northern populations of yellow perch, walleye, and other fishes appear to retain the signatures of their genetic origins from the respective glacial refugia (summarized by Sepulveda-Villet and Stepien (2012) for yellow perch and Stepien et al. (2009) for walleye). Yellow perch and walleye, although they now are adapted to the large inland “seas” that comprise the Great Lakes, have their ecological and evolutionary origins in fluvial systems rather than large, lacustrine basins.
Maps showing sampled spawning populations for (a) yellow perch (triangles) and walleye (circles) across North America, and genetic barriers for (b) yellow perch and (c) walleye. (a) Box shows the HEC (Huron-Erie Corridor), thick dashed line indicates maximum extent of the Wisconsinan glaciations, arrows denote likely routes of post-glacial population colonizations (adapted from Mandrak and Crossman 1992). (b, c) Grey lines (solid = microsatellite data, dashed = mtDNA control region sequences) define major barriers to gene flow calculated based on geographic coordinates (latitude and longitude) and genetic divergence (F ST) from BARRIER v2.2 analysis (Manni et al. 2004; http://ecoanthropologie.mnhn.fr/software/barrier.html). These are ranked I–V for the microsatellite and 1–5 for the mtDNA control region data sets, in order of their decreasing magnitude (Barriers are modified from results of Stepien et al. (2009), Sepulveda-Villet and Stepien (2012), and Haponski and Stepien (2014a))
The genetic composition and structure of yellow perch, walleye, and other aquatic species have been shaped from the past through the present by their relative (1) dispersal abilities and migration behavior, (2) abundances and distributions across waterways, (3) habitat requirements, quality, and connectivity, and (4) reproductive behavior and spawning site fidelity. Large connected spans of suitable habitats, as found in the Great Lakes basins and tributaries, offer a variety of environmental resources for diverse populations, reflecting an interplay between migration opportunity and localized adaptation (see Lindsay et al. 2008; Vandewoestijne et al. 2008; Kunin et al. 2009). Aquatic habitats frequently are connected by narrow and relatively ephemeral connections that link populations during migration and dispersal, but whose habitats may pose distinct biological challenges. For example, small connecting channels extensively vary in size and habitat complexity, and differ in available food and shelter. These factors then influence population variability and local adaptations. In contrast, isolated relict populations with little connectivity may possess lower overall genetic diversity due to the influences of genetic drift, bottlenecks, and selection (Moran and Hopper 1983; Petit et al. 2003; Coulon et al. 2012). Such relict and “rear edge” populations (those found in fringe latitudinal portions of the native range, as in the southeastern U.S. for yellow perch and walleye) may serve as critical repositories of genetic diversity and provide possible sources for future range expansion in the face of climate change (see Hampe and Petit 2005; Diekmann and Serrão 2012). These hypotheses are explored and discussed in this chapter for yellow perch and walleye, in order to provide an understanding of their shared and different population patterns.
2 Reproduction of Yellow Perch and Walleye in Relation to Their Population Genetic Structure
Cued by gradual changes in water temperature and photoperiod, walleye aggregate to reproduce in early spring in slow-moving tributaries or on shallow reef complexes in lacustrine systems (Craig 1987; Kreiger et al. 1983; Colby et al. 1994). Yellow perch reproduce in similar habitats, about a month later at warmer temperatures (Scott and Crossman 1973; Carlander 1997; Jansen et al. 2009). Spawning of the two species occurs much earlier in the southern U.S., and much later in the Canadian north (Carlander 1997; Craig 2000).
Walleye often migrate long distances to propagate (Kreiger et al. 1983; Colby et al. 1994; Craig 2000), homing to their natal sites, as indicated by tagging studies (Ferguson and Derkson 1971; Wolfert and Van Meter 1978; Jennings et al. 1996) and genetic divergence data among their spawning groups (Stepien and Faber 1998; Strange and Stepien 2007; Stepien et al. 2009, 2010, 2012). Notably, mark-and-recapture studies have recovered most Lake Erie and Lake St. Clair walleye near their original spawning sites during the subsequent spring reproductive season(s) (Wang et al. 2007). A study by Jennings et al. (1996) tracked the spawning returns of laboratory-reared walleye after release in the field, indicating that natal homing is a genetically based response to environmental cues.
Spring spawning migrations of yellow perch are shorter and it is presumed that they also return to specific natal sites in shallow waters (Aalto and Newsome 1990; Carlander 1997; Craig 2000). Yellow perch that were captured and tagged during the reproductive season and released many km distant in the eastern basin of Lake Erie, then returned to their tagging locations (MacGregor and Witzel 1987) – implicating homing. Separate studies by Clady (1977), Rawson (1980), and the Ontario Ministry of Natural Resources (OMNR 2011) likewise found that most yellow perch tagged during spawning were recovered at or very close to their initial reproductive locations in subsequent years. For example, yellow perch spawning groups located just a few km apart (17 km) in central Lake Erie were found to diverge from one another in genetic and morphological composition (Kocovsky et al. 2013). This suggests that spawning populations likely comprise natal groups at specific locations (Kocovsky et al. 2013; Sullivan and Stepien 2015). Aalto and Newsome (1990) removed yellow perch egg masses from given spawning sites, which led to fewer fish returning to that location in subsequent years than in control sites, suggesting that they returned to the same spawning areas year after year. It is hypothesized that imprinting occurs during the early life history of walleye and yellow perch, with their highly developed olfactory systems used to detect natal sites and/or the pheromones of neighbors (see Horrall 1981; Gerlach et al. 2001).
Stepien et al. (2012) analyzed the genetic structure of walleye reproducing at three well-known spawning locations in Lake Erie, encompassing 6 years of spawning runs from 1995 to 2008. The genetic composition of each population group, based on nine nuclear microsatellite loci (see Tables 25.1 and 25.2), remained similar from year to year, among age cohorts, between the sexes, and from generation to generation (Stepien et al. 2012). In contrast, although the genetic composition of yellow perch spawning aggregations differed significantly from location to location across broad and fine geographic scales (Sepulveda-Villet and Stepien 2011), there were some significant differences at the same location from year to year (Sullivan and Stepien 2015). This suggests that although yellow perch may reproduce together with a specific population group (believed to be their natal group), specific spawning locales may vary from year to year. Thus, the site fidelity to specific reproductive locations may differ between yellow perch and walleye, which remains to be experimentally investigated.
During their respective spring reproductive seasons, males move into the spawning areas first, arriving before females by a few weeks and lingering longer at the sites (Scott and Crossman 1973; Craig 2000; Simon and Wallus 2006). Single females spawn with several males. The female yellow perch lays a long gelatinous egg strand (to 2.1 m long), which contains 10,000–40,000 eggs, over submerged vegetation or other structures at night or in early morning. As the egg mass is released by the female, it is externally fertilized by a cluster of 2–25 male yellow perch, who closely follow her and often are in close proximity to other spawning clusters (Scott and Crossman 1973; Mangan 2004; Simon and Wallus 2006). In contrast, each female walleye releases thousands of separate eggs at night, which are externally fertilized by a swarm of one to six males (Kerr et al. 1997). The walleye eggs settle into crevices, where they adhere to rocks or gravel with their sticky outer coating of muco-polysaccharides (Craig 2000; Barton and Barry 2011). Female walleye tend to spawn over several days, and males of both species fertilize the eggs of multiple females over several days or weeks (Craig 2000; Simon and Wallus 2006).
Neither species provides any parental care (Trautman 1981; Zhao et al. 2009). After spawning the adults move to nearby bays and other littoral areas, and then travel offshore to summer feeding grounds (Kerr et al. 1997; Craig 2000). Eggs of both species hatch over a period ranging from 10 days to three weeks, depending on water temperature (Simon and Wallus 2006). More rapid development and better survival occurs at warmer temperatures (Scott and Crossman 1973; Kerr et al. 1997; Roseman et al. 2005).
3 Movements and Behavior of Larval and Juvenile Yellow Perch and Walleye, in Relation to Their Population Genetics
Larvae of yellow perch (4–7 mm) and walleye (6–9 mm) hatch with small yolk sacs (Scott and Crossman 1973; Bozek et al. 2011a). Yellow perch larvae swim in a coordinated manner at hatching (Fulford et al. 2006). In contrast, walleye are reported to swim to the surface aided by a large oil globule in the yolk, then sink back to the bottom, and by the second day are free swimming (Simon and Wallus 2006). Larvae of both species drift with currents to nearshore nursery areas in shallow vegetated areas (Craig 2000; Jones et al. 2003; Roseman et al. 2005). They begin to feed after their yolk sacs are absorbed, consuming phytoplankton and small zooplankton, and grouping with conspecifics of similar size in schools (Collette et al. 1977; Kerr et al. 1997; Craig 2000; Simon and Wallus 2006). It is hypothesized, but untested, that they may school with their natal site cohorts.
Successful location and capture of prey items of appropriate size and nutrition are critical for survival of the larvae, as well as avoiding predation (see Fulford et al. 2006; Beletsky et al. 2007). These two factors – feeding and avoiding predation – impose high selective pressures, and favor those with better-developed sensory organs and greater swimming ability (Li and Mathias 1982; Craig 2000; Simon and Wallus 2006). Water turbidity is believed to augment survival, facilitating predator avoidance (Roseman et al. 2005; Manning et al. 2013). The larval pelagic stage lasts for about 30–40 days, with yellow perch transitioning to become increasingly benthic, but remaining in schools throughout life (summarized by Fulford et al. 2006; Simon and Wallus 2006). In contrast, most walleye remain more pelagic and become solitary, whereas other individuals are reported to school (Craig 2000; Simon and Wallus 2006).
The young-of-year move into deeper water as juveniles in late spring (Craig 2000). Larger juvenile walleye become piscivorous, eating a variety of fish species (including conspecifics), as well as zooplankton – especially when forage fish are low in abundance (Collette et al. 1977; Craig 2000; Bozek et al. 2011a). In the Great Lakes, the invasive Eurasian round goby Neogobius melanostomus increasingly has become important in the diets of both yellow perch and walleye (Truemper and Lauer 2005; Kornis et al. 2012).
Parker et al. (2009) found that age-1 juvenile yellow perch differed in morphology and genetically at 12 nuclear DNA microsatellite loci between populations in Lake Huron and Lake Michigan, with those from Lake Huron having deeper, longer bodies and larger dorsal fins. The researchers also discerned morphological and genetic differences between juveniles living in nearshore versus wetland habitats in Lake Michigan. Juveniles inhabiting nearshore areas from both lakes had deeper, longer bodies and larger dorsal fins than did those occupying wetlands, which might reflect an adaptive response to predators and open-water cruising. Differences between habitats across the lakes were hypothesized to reflect plasticity between phenotypic and genetic divergence (Parker et al. 2009).
4 Non-reproductive Movements and Behavior of Adult Yellow Perch and Walleye in Relation to Population Genetics
After the reproductive season, movements of adult yellow perch and walleye largely are determined by habitat complexity, food availability, and foraging capacity (Roseman et al. 2005; Radabaugh et al. 2010). Juvenile and adult yellow perch typically are found in schools, which likely facilitate foraging and predator avoidance (Helfman 1984; Craig 2000). In comparison, walleye are either solitary or found in smaller schools as adults (Craig 2000; Simon and Wallus 2006). Whether the schools of yellow perch are structured based on kinship has not yet been evaluated (see Sullivan and Stepien 2015). However, schools of the closely related European perch have been shown to contain large numbers of related individuals, which recognize one another via chemical and physical cues (Gerlach et al. 2001; Behrmann-Godel et al. 2006).
Walleye typically range widely to feed at non-reproductive times of the year, travelling distances from 50 to 300 km (see Colby et al. 1979; Wang et al. 2007; Bozek et al. 2011b). In contrast, a study of yellow perch tag returns by Haas et al. (1985) determined that post-spawning movements are moderate; individuals tagged at Lake Erie spawning sites did not move upstream through the Huron-Erie Corridor (HEC; see Fig. 25.1), which connects Lake Huron to Lake Erie via the St. Clair River, Lake St. Clair, and the Detroit River. Some yellow perch that were tagged in Lake St. Clair migrated to nearby tributaries (Haas et al. 1985). Likewise, Dumont (1996) found little movement of tagged yellow perch along the St. Lawrence River. There thus appears to be much greater tendency for mixing among spawning groups of walleye than of yellow perch during non-reproductive times of the year. Note that although individuals may move among water bodies to feed, their reproductive groups determine their overall population genetic structures.
Evidence of yellow perch metapopulations inhabiting Lake Ontario embayments was discerned using otolith microchemistry (Murphy et al. 2012). Results described discrete assemblages in connected bays and impoundments (Murphy et al. 2012). This type of metapopulation structure characterized yellow perch reproductive groups along Lake Erie coastal sites (Sepulveda-Villet and Stepien (2011); these populations likely display some seasonal mixing, as described by Parker et al. (2009). In the Baltic Sea, European perch and pikeperch showed no appreciable genetic distinction among sites during the mid-summer, despite being separated by coastal features and salinity regimes (Sruoga et al. 2008). Post-spawning populations of Perca and Sander often intermingle, obscuring genetic population identities other than during reproduction.
Maturity is reported to be reached between ages 2 and 3 in yellow perch and at about age 3 in walleye (Collette et al. 1977; Barton and Barry 2011). However, fall survey results in Lake Erie showed that over half of age-1 male yellow perch and walleye are sexually mature, whereas less than half of the age-3 females had reached maturity (Dr. Patrick Kocovsky, USGS Great Lakes Science Center, personal communication, 2014). Female yellow perch and walleye mature later, grow more rapidly throughout their lives, and reach larger sizes than do the males; the females also live longer (summarized by Carlander 1997). Yellow perch attain ages of 6–21 (Craig 2000), whereas walleye reach 6–19 years (Carey and Judge 2000; Craig 2000) and live to a maximum of ~30 years (Bozek et al. 2011a). In northern habitats, both species grow more slowly and have longer life expectancies (Carlander 1997; Craig 2000). With climate warming, we predict that they likely will grow faster and may die earlier, which will influence their population structures.
5 Genetic Origins of Northern Yellow Perch and Walleye Populations Tracing to Glacial Refugia
Contemporary haplotypes of yellow perch appear to trace to ~6.0 million years ago (Mya; Fig. 25.2a; Sepulveda-Villet and Stepien 2012), whereas walleye date to ~10.6 Mya during the late Miocene Epoch (Fig. 25.2b). The northern haplotypes of yellow perch share a common ancestry estimated as ~0.6–4.2 Mya (Fig. 25.2a; Sepulveda-Villet and Stepien 2012), and those for walleye similarly date to ~1–2.5 Mya (Fig. 25.2b).
Bayesian phylogenetic trees of mtDNA control region sequence haplotypes for (a) yellow perch and (b) walleye, calculated using MrBAYES v3.2.1 (Ronquist and Huelsenbeck 2003; http://mrbayes.sourceforge.net/). Values above nodes = Bayesian posterior probability/percentage support from 2000 bootstrap pseudo-replications in ML with PHYML v3.0 (Guindon et al. 2010; http://www.atgc-montpellier.fr/phyml/); those with ≥0.50 pp and ≥50 % bootstrap support are reported. jMODELTEST v2 (Darriba et al. 2012; https://code.google.com/p/jmodeltest2/) selected the most likely model of nucleotide substitution for construction of the phylogenetic trees and divergence time estimates. Values below nodes in italics = estimated divergence times (given as millions of years) as determined in BEAST v1.71 (Drummond et al. 2012; http://beast2.org). Divergence times were calibrated using three fossil and four molecular calibration points following Haponski and Stepien (2013). Letters in parentheses denote sampling sites in which haplotypes were recovered (see Fig. 25.1 map). Vertical bars denote general geographical regions
Northerly populations of yellow perch and walleye were subjected to drastic climate change and habitat losses during the Pleistocene glaciations 10,000 ya–2.6 Mya. As the ice sheets formed, the fish populations gradually migrated to survive in southerly areas – the Missourian, Mississippian, and Atlantic glacial refugia (Fig. 25.1; Petit et al. 2003; Hewitt 2004; Provan and Bennett 2008). Following the glacial meltwaters, migrants from these refugia surged northward, recolonizing drastically changed habitats, including the new basins of the Laurentian Great Lakes. Notably, an estimated 90 fish species migrated northward from the Mississippian glacial refugium to found modern Great Lakes populations, another 14 expanded up from the Atlantic coastal refugium, and some from each met and mixed (see Mandrak and Crossman 1992). To the west, colonists from the Missourian refugium founded yellow perch and walleye populations in the Northwest Plains, including Lake Winnipeg and the upper Mississippi River region, as well as contributing to western Lake Superior (Billington 1996; Stepien et al. 2009, 2010; Backhouse-James and Docker 2012). Patterns of genetic divergences among northern fish populations today reflect these differential contributions originating from refugia, which were subsequently modified by drainage connections and basin isolation (Bailey and Smith 1981; Mandrak and Crossman 1992; Stepien et al. 2009, 2010).
Yellow perch and walleye in the Northwest Lake Plains region (Fig. 25.1: Lake Winnipeg and the upper Mississippi River) trace their descent to Missourian refugium colonists (Stepien et al. 2009, 2010; Sepulveda-Villet and Stepien 2012). Today, the overall differences in these populations from other regions is apparent in Figs. 25.1, 25.2, 25.3, and 25.4 (the latter depicts Bayesian STRUCTURE analyses of populations, based on nuclear DNA microsatellite loci from Tables 25.1 and 25.2), showing that the Lake Winnipeg and Upper Mississippi River populations are different from those in most of the Great Lakes. Yellow perch from western Lake Superior also are very distinctive in the nuclear microsatellite data (Fig. 25.4a; Sepulveda-Villet and Stepien 2012). Walleye reproducing in Lake Superior show a mixture of descent from the Missourian and Mississippian refugia (Fig. 25.4b; Stepien et al. 2009, 2010). Populations of lake sturgeon Acipenser fulvescens in the Hudson Bay drainage (Ferguson and Duckworth 1997) and brown bullhead Ameiurus nebulosus in western Lake Superior (Murdoch and Hebert 1997) likewise have been attributed to Missourian refugium ancestry.
Statistical parsimony network constructed using TCS v1.21 (Clement et al. 2000, http://darwin.uvigo.es/software/tcs.html) among mtDNA control region haplotypes of (a) yellow perch (Modified from Sepulveda-Villet et al. (2009) and Sepulveda-Villet and Stepien (2012)) and (b) walleye (Modified from Haponski and Stepien (2014a)). Circles are sized according to total observed frequencies of the haplotypes. Lines indicate a single mutational step between the haplotypes. Small, unlabelled circles represent hypothesized unsampled haplotypes. Dashed lines enclosing haplotype groups denote major regional delineations. Circle colours also reflect haplotype identities as portrayed in Fig. 25.4a. Note that there is no correspondence between the colors and haplotypes of yellow perch and walleye (a and b are entirely independent)
Estimated comparative population structure from Bayesian STRUCTURE v2.3.3 analyses (Pritchard et al. 2000; Pritchard and Wen 2004; http://pritchardlab.stanford.edu/structure.html) for (a) 17 yellow perch groups using 15 nuclear DNA microsatellite loci (Modified from Sepulveda-Villet and Stepien 2012), and (b) 9 walleye groups using 9 loci (Modified from Stepien et al. 2009, 2010). Analyses were run with 100,000 burn-in and 500,000 replicates. Optimal K values were determined by posterior probabilities (Pritchard et al. 2000) and the ΔK method of Evanno et al. (2005). Thin vertical lines represent individuals and thicker bars separate spawning groups at given locations; these are partitioned into K colored segments that represent estimated population group membership. Note that there is no correspondence between the colors of yellow perch and walleye (a and b are entirely independent)
Glacial Lake Agassiz initially occupied much of the Hudson Bay watershed (including Lake Winnipeg), which probably had some southern drainage to Lake Superior (Mandrak and Crossman 1992; Rempel and Smith 1998), facilitating fish movements 8500–13,000 ya. Ice later blocked this passage (Saarnisto 1974; Teller and Mahnic 1988), isolating the yellow perch and walleye populations in the Northwest Lake Plains sites, as is shown by their high divergences from other areas (denoted by distinct colors on Figs. 25.4 and 25.5; Stepien et al. 2009, 2010; Sepulveda-Villet and Stepien 2012; Haponski and Stepien 2014a). These northwestern fish populations always have been small in size (Hoagstrom and Berry 2010). The Lake Superior region was long covered in ice, except for glacial Lake Duluth in the west until ~8500–9000 ya, thus isolating its walleye and yellow perch gene pools.
Estimated population structure from mtDNA control region frequencies for (a) 26 yellow perch haplotypes (Modified from Sepulveda-Villet and Stepien 2012) and (b) 28 walleye haplotypes (Adapted from Haponski and Stepien 2014a) using GENEPOP v4.0 (Rousset 2008; http://kimura.univ-montp2.fr/%7Erousset/Genepop.htm), and Microsoft Excel 2008 (Redmond, VA). Vertical black lines separate different spawning groups (lettered). Major geographic regions are indicated in the bottom rule for each chart. Note that there is no correspondence between the colors and haplotypes of yellow perch and walleye (a and b are entirely independent)
Most of the Great Lakes fauna – especially in Lakes Huron, Michigan, St. Clair, and western Lake Erie (Underhill 1986; Mandrak and Crossman 1992; Todd and Hatcher 1993) – trace their origins to the Mississippian refugium, as indicated for yellow perch (Sepulveda-Villet et al. 2009; Sepulveda-Villet and Stepien 2012), walleye (Stepien and Faber 1998; Stepien et al. 2009, 2010), smallmouth bass Micropterus dolomieu (Stepien et al. 2007), rainbow darter Etheostoma caeruleum (Haponski et al. 2009), and lake sturgeon (Ferguson and Duckworth 1997). There were apparent genetic contributions from both the Atlantic and the Mississippian refugium into the lower Great Lakes for walleye (Stepien and Faber 1998; Strange and Stepien 2007; Stepien et al. 2009, 2010), smallmouth bass (Stepien et al. 2007), brown bullhead (Murdoch and Hebert 1997), lake trout Salvelinus namaycush (Wilson and Hebert 1996), and ninespine stickleback Pungitius pungitius (Aldenhoven et al. 2010).
The closer genetic relationship between walleye reproducing in Lake Michigan and Lake Huron proper (including Saginaw Bay) likely reflects their former connection as glacial Lake Algonquin ~2000–10,600 ya, which drained west to the Mississippi River system (Bailey and Smith 1981). Lake Huron walleye diverged ~11,500 ya when Georgian Bay (the former glacial Lake Hough) was isolated from the main basin population (the former glacial Lake Stanley) (Lewis et al. 1994).
Lake Erie’s formation dates to glacial Lake Maumee (~14,000 ya), which then drained west via the Ohio River to the Mississippi, changing outlets during several lake stages, to its current outlet east into Lake Ontario (~10,000 ya) (Underhill 1986; Larson and Schaetzl 2001). Lake Erie yellow perch and walleye populations today appear geographically isolated and genetically differentiated from most other Great Lakes populations. Lake Erie physically is separated from Lake Ontario by Niagara Falls and from the upper Great Lakes by the narrow and short Detroit River, which drains Lake St. Clair. Yellow perch from Lake St. Clair are separated from those spawning in Lake Erie (note the color difference between the purple-colored population from Lake St. Clair versus the mixed colors in Lake Erie in Fig. 25.4a); these appear on opposite sides of a genetic barrier IV shown in Fig. 25.1b. However, there is greater genetic exchange between walleye reproducing in Lakes St. Clair and Erie, as illustrated by their similar color on Fig. 25.4b (see Haponski and Stepien 2014b).
Yellow perch mtDNA control region haplotype 1 (Figs. 25.2a and 25.3a) likely already was widespread pre-glacially and then represented in the Mississippian and Atlantic refugia populations, but was more common in the west. Today, yellow perch haplotype 1 remains more abundant in the west (see Fig. 25.5a). Walleye possesses a similar pattern, with its most common haplotype 1 also being more common to the west, and increasing proportions of haplotypes 2, 3, and 4 to the east (Fig. 25.5b). This west-east pattern occurs in both species and presumably reflects retention of original colonization proportions from the Mississippian and Atlantic refugium to the present day.
The Atlantic coastal refugium (Fig. 25.1) formed a warm enclave of diverse habitats in coastal plains and estuaries east of the Appalachian Mountains (Schmidt 1986; Bernatchez 1997); yellow perch and walleye from that refugium migrated north to colonize the northeastern and northcentral regions after the glaciations (Russell et al. 2009). The northeastern migrating populations split to found the yellow perch populations in Maine (colored blue, sites X and Y, on Fig. 25.4a) and the Hudson River (colored light blue, site Z); both are very divergent today (also see mtDNA haplotypes on Fig. 25.5a).
Lake Champlain (site W) drains into the St. Lawrence River and its yellow perch appear to trace to joint origins from the Atlantic and Mississippian refugia, but today have a very divergent genetic composition from other locations (see unique haplotype 20 on Figs. 25.2a and 25.3a and distinct colors for Lake Champlain yellow perch on Figs. 25.4a and 25.5a, denoting different genetic composition). Lake Champlain received meltwaters from glacial Lake St. Lawrence (~11,600 ya), and then Lake Agassiz (~8000–10,900 ya) and glacial Lake Barlow-Ojibway (~8000–9,500 ya). This produced an extensive freshwater habitat that replaced the former saline Champlain Sea, which was a temporary inlet of the Atlantic Ocean formed by the retreating glaciers (Rodrigues and Vilks 1994). Regional flooding presumably led to colonization of Lake Champlain by aquatic taxa from the Atlantic refugium, as suggested by genetic evidence from lake cisco Coregonus artedi (Turgeon and Bernatchez 2001); the Lake Champlain yellow perch population appears to reflect joint contribution from the Atlantic and Mississippian refugia (see Fig. 25.5a).
The overall similarities among northern populations of yellow perch, walleye, and other aquatic taxa reveal a general pattern that originated with recolonization from multiple glacial refugia, which then became modified by changes in connections and drainages, and has been maintained by reproductive site philopatry from generation through generation. Refugium origins have been shown to translate to differences in walleye growth patterns (Zhao et al. 2008); thus the influences of evolutionary history appear to persist in their physiological, life history, and genetic adaptations today.
6 Atlantic and Gulf Coastal Populations of Yellow Perch and Walleye
The southern genotypes of yellow perch and walleye clearly are differentiated from the northern ones, with the southern ones being older (see Fig. 25.2; Stepien and Faber 1998; Stepien et al. 2009; Sepulveda-Villet and Stepien 2012; Haponski and Stepien 2014a). The most divergent walleye identified are from the New and Ohio Rivers (Fig. 25.2b). Divergence of southern walleye haplotypes in the New/Ohio River and Gulf coastal systems was estimated at ~7.2–10.6 Mya (this chapter), whereas dates for southerly yellow perch appear to be later at ~2.5–3.6 Mya (Fig. 25.2a; Sepulveda-Villet and Stepien 2012).
The Atlantic coastal area supports high species richness and endemism today (Griffiths 2010), as discerned for its yellow perch and walleye populations (Stepien et al. 2009, 2010; Sepulveda-Villet and Stepien 2012; Haponski and Stepien 2014a). These populations have relatively high genetic diversity, possessing unique alleles (see Table 25.2). Results support the hypothesis that greater genetic diversity in southerly, unglaciated populations may be due to their long undisturbed history for evolution and local adaptation (Petit et al. 2003). The South Atlantic coastal yellow perch populations are adapted to mesohaline conditions, and likely can readily migrate from fresh to brackish waters (Grzybowski et al. 2010). In response to ongoing climate change, the unique genetic diversity of these euryhaline populations of yellow perch might provide an important genetic reservoir in the event that some inland waters become more saline.
The South Atlantic and Gulf coastal haplotypes of yellow perch are more closely related to each other than to those from the North Atlantic region (Sepulveda-Villet and Stepien 2012).
In comparison, the southern Gulf relict populations of yellow perch and walleye have relatively lower heterozygosity (Table 25.2), characteristic of their small population sizes, bottlenecks, and genetic drift. Those populations also possess high numbers and proportions of private alleles, indicative of their long-term isolation and distinctiveness (Tables 25.2 and 25.3; Figs. 25.1, 25.2, 25.3, and 25.4). The Gulf coastal walleye population is small but persistent, and is believed to represent a long-isolated unique historic strain (Boschung and Mayden 2004; Stepien et al. 2009). This also appears to be the case for yellow perch, except that there is closer relationship of its relict Gulf coastal population to the southeast Atlantic seaboard (Sepulveda-Villet et al. 2009; Sepulveda-Villet and Stepien 2012). The distinctiveness of the Gulf Coast walleye was described by Hackney and Holbrook (1978) based on life history characters, faster growth, and spawning at higher temperatures. This differentiation also was indicated by population genetic data using allozymes (Murphy 1990; Billington and Maceina 1997), mtDNA restriction haplotypes (Billington et al. 1992; Billington and Strange 1995; Billington and Maceina 1997), nuclear microsatellite loci (Stepien et al. 2009), and mtDNA sequence data (Haponski and Stepien 2014a). Boschung and Mayden (2004) noted that this unique walleye strain is in danger of potential introgression with introduced northern strains of walleye entering from the Tennessee River drainage via the Tennessee-Tombigbee Waterway.
7 Comparative Genetic Diversity of Yellow Perch and Walleye Populations
The overall genetic diversity of yellow perch is much lower than walleye; this is clearly evident in Tables 25.1 and 25.2, and is true for both nuclear DNA (mean heterozygosity is 0.53 for yellow perch and 0.73 for walleye) and mtDNA sequence variability (mean haplotypic diversity is 0.31 for yellow perch and 0.77 for walleye). The relatively low diversity for yellow perch likewise has been revealed by other genetic data sets, including allozymes (Leary and Booke 1982; Todd and Hatcher 1993; Moyer and Billington 2004), mtDNA restriction fragment length polymorphisms (RFLPs) (Billington 1993; Moyer and Billington 2004), mtDNA sequences (Sepulveda-Villet et al. 2009; Sepulveda-Villet and Stepien 2011, 2012), as well as nuclear microsatellites (Miller 2003; Sepulveda-Villet and Stepien 2011, 2012). Values for yellow perch here (Table 25.2) correspond to the overall microsatellite heterozygosity average of 0.54 for 13 other freshwater fishes described by DeWoody and Avise (2000), whereas those for walleye are high.
Overall mtDNA genetic diversity of yellow perch roughly matches that of the European perch (Refseth et al. 1998; Nesbø et al. 1998, 1999), which also exhibits relatively low allozymic genetic diversity (Gyllensten et al. 1985; Bodaly et al. 1989). Relatively low genetic diversity in both mtDNA and nuclear DNA thus appears characteristic of the genus Perca. Moreover, mtDNA control region sequences revealed low variation in the ruffe Gymnocephalus cernua across Eurasia (Stepien et al. 1998, 2005), which is closely related to Perca (see phylogenies of Faber and Stepien 1997; Song et al. 1998; Sloss et al. 2004, and Chap. 1 of this book). This finding appears to suggest that modest genetic diversity may be characteristic of the Perca-Gymnocephalus lineage. Such low diversity may be a product of the life history of Perca species, with the yellow perch displaying considerable genetic divergence among closely-spaced reproductive groups within an aquatic system, such as large lakes (Sepulveda-Villet and Stepien 2011; Kocovsky et al. 2013; Sullivan and Stepien 2014, 2015).
Some individual yellow perch spawning groups possess a relatively high degree of kin relationship, which may result in lower diversity within samples (Sullivan and Stepien 2015). Genetically similar individuals of European perch have been found to aggregate with one another (Gerlach et al. 2001), recognizing their relatives via olfactory cues at the fry life stage and beyond (Behrmann-Godel et al. 2006). Kin recognition and olfactory cues have not yet been studied in yellow perch or walleye, but might yield important insights on their fine-scale population structure and the distribution of their respective diversities.
Across most of their North American ranges, yellow perch and walleye populations exhibit relatively consistent levels of genetic variability for the nuclear microsatellite data (Table 25.2 and Fig. 25.4). Some exceptions are that populations of both species are less variable in some of the northwest populations and the Gulf coastal region. Genetic diversities for both species are high across the Great Lakes. The southeastern populations of both species, which were never glaciated, also have high diversity.
Levels of mitochondrial DNA diversity, measured as haplotypic diversity (Table 25.2) are more subject to bottlenecks as the effective population size is one fourth that of nuclear DNA (see Avise 2004). Values of haplotypic diversity of walleye are similar to those obtained from the nuclear microsatellite data, however, those for yellow perch are much lower. This may reflect a history of bottlenecks for yellow perch. Most of the Great Lakes, as well as the northwestern populations, are dominated by a single yellow perch haplotype (haplotype 1 of Fig. 25.5). Likewise, the southern populations possess few haplotypes. In contrast, the eastern and Atlantic coastal yellow perch populations have many more haplotypes. By comparison, the diversity of walleye haplotypes is much greater across the range and is more consistent (Table 25.2 and Fig. 25.5).
Private alleles are those that are found only in a specific population or set of populations. In the Great Lakes region overall, 14 % of nuclear microsatellite alleles were private for yellow perch and 22 % for walleye (Table 25.2). For the mitochondrial DNA sequences, just 7 % of the Great Lakes’ haplotypes were private in yellow perch, whereas 59 % were private in walleye. In Lake Erie, 9 % of the microsatellite alleles and 3 % of the mitochondrial haplotypes were private in yellow perch; in contrast, only 1 % of the microsatellite alleles and 50 % of the haplotypes were private for walleye. There thus appears to be a fundamental difference in the isolation and differentiation of the Lake Erie populations between the two species. In the Gulf Costal populations, 7 % of yellow perch microsatellite alleles were private, and 11 % of the walleye. Those proportions of mitochondrial haplotypes were 13 % for yellow perch and 50 % for walleye; thus, the trend was similar (Table 25.2 and Fig. 25.5).
8 Broadscale Genetic Divergence Patterns of Yellow Perch and Walleye Populations Across Their Native Ranges
Yellow perch and walleye congruently show greatest divergences between their populations from the upper Mississippi River and the Gulf Coast, reaching F ST = 0.361 for yellow perch and 0.300 for walleye for the microsatellite data (Table 25.3a). The greatest difference for the mitochondrial DNA data likewise occurs between the same population pairs: F ST = 0.949 for yellow perch and 0.793 for walleye (Table 25.3b). The largest divergences overall thus reflect long-term geographic separation. Most population group comparisons significantly differ, except between some adjacent systems (Table 25.3). Some of these apparent exceptions, however, actually differ at finer scales because here we grouped together different spawning groups within systems, for the purpose of comparing across large areas.
Pronounced genetic demarcations that delineate the most unique yellow perch populations identify six major geographic regions: Northwest Lake Plains, Great Lakes watershed, Lake Champlain, North Atlantic coastal, South Atlantic coastal, and Gulf coastal (Fig. 25.1b). A similar pattern is apparent for the genetic structure of walleye populations: Northwest Lake Plains (Lake Winnipeg, McKim Lake in Ontario, and the upper Mississippi River), the Great Lakes watershed (divided into six groups: Lake Superior, Lakes Michigan/Huron, Lake Huron’s Georgian Bay, Lake St. Clair, Lake Erie, and Lake Ontario), North Atlantic coastal, South Atlantic coastal, and Gulf coastal groups (Stepien et al. 2009, 2010; see Fig. 25.1).
The most divergent population groups overall for yellow perch are from: the Gulf coast (mean F ST = 0.275 among 11 pairwise comparisons), the upper Mississippi River (0.257), the southeast (0.224), and then Lake Winnipeg (0.203). Three of these also are the most different with mtDNA, in order of: the Gulf Coast (mean F ST = 0.786), the southeast (0.506), Lake Winnipeg (0.424), and then Lake Erie (0.255). According to the walleye microsatellite data, the most divergent groups likewise occur on the peripheries of the range, including: the Gulf Coast (mean F ST = 0.275), the northeast (0.153), the upper Mississippi River (0.148), and Lake Winnipeg (0.131). Two of these match those most divergent discerned by walleye mtDNA, comprising: the Gulf Coast (mean F ST = 0.484), the southeast (0.470), Lake Ontario (0.346), and the upper Mississippi River (0.322).
In the northwest, yellow perch from the Lake Winnipeg region comparatively are more different from the populations in the upper Mississippi River system and Lake Superior than are walleye; this pattern is congruent between the nuclear and mitochondrial DNA data sets (Table 25.3). However, walleye diverge more between the upper Mississippi River and Lake Superior systems than do yellow perch populations. Within the Great Lakes, the Lake Superior population samples are the most differentiated from others for both species. The greatest overall differences among the Great Lakes population groups are some of the most geographically distant ones, notably between Lakes Superior and Ontario (F ST = 0.213 for yellow perch and 0.038 for walleye microsatellites), as is predicted by the hypothesis of genetic isolation with geographic distance. This relationship between genetic and geographic distances (measured by nearest waterway) is illustrated on Fig. 25.6. Another very distant relationship occurs between Lake Superior and St. Clair yellow perch (F ST = 0.226). Walleye from Lake Superior also are very divergent from those in Lakes Michigan, Huron, St. Clair, and Erie (F ST = 0.035–0.037), and those in Lake Michigan are markedly distinct from the Lakes St. Clair and Erie populations (0.037).
Mantel (1967) pairwise tests for the relationship between genetic distance (θ ST/1- θ ST) and the natural logarithm of geographic distance (km) across the native North American range of (a) yellow perch and (b) walleye. Results determined using GENEPOP v4.0 (Rousset 2008; http://kimura.univ-montp2.fr/%7Erousset/Genepop.htm), with 10,000 permutations. Equations for: (a) yellow perch populations. p < 0.001, R 2 = 0.39, y = 0.14x – 0.57 (Modified from Sepulveda-Villet et al. 2012), and (b) walleye populations. p = 0.005, R 2 = 0.23, y = 0.06x – 0.29 (Adapted from Stepien et al. 2009; Haponski and Stepien 2014a)
A positive regression relationship of genetic versus geographic distance is supported for both species, as shown in Fig. 25.6 from the microsatellite data. Yellow perch have greater divergence per geographic distance than do walleye. Genetic BARRIER analyses, depicted in Fig. 25.1b (yellow perch) and 25.1c (walleye), denote divisions among populations that are distinguished by much greater than expected genetic differences. Several of these are congruent between the species, including those isolating the Gulf coastal region, the southeast, the Lake Winnipeg region, the upper Mississippi River, Lake Superior, and the northeastern populations. Significant demarcation of the Lake St. Clair population of yellow perch is not found in walleye, which shows more genetic exchange with nearby spawning groups (Fig. 25.1). The Bayesian STRUCTURE analyses support a greater number of distinctive population groups of yellow perch (K = 17, Fig. 25.4a) than found for walleye (K = 9, Fig. 25.4b). Yellow perch and walleye share many congruent population areas that are denoted by marked distinctiveness, including Lake Winnipeg, the upper Mississippi River, Lake Superior, the northeastern populations, the southeastern populations, and the Gulf Coast. Additional unique groups of yellow perch occur in Lake St. Clair (congruent with the BARRIER analysis results from Fig. 25.1b), Lake Ontario, and the Hudson River (Fig. 25.4a). Findings thus demonstrate considerable genetic divergences among most population regions for both species (Table 25.3), reflecting both broad- and fine-scale patterns of differentiation.
9 Fine Scale Genetic Divergence Patterns of Yellow Perch and Walleye
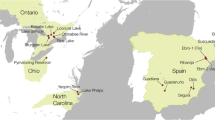
Although relationships among yellow perch and walleye populations typically follow a broad-scale pattern of genetic isolation by geographic distance (Fig. 25.6), those among reproductive groups within individual lakes do not reflect geographic distance (Fig. 25.7). In both species, some closely situated spawning populations are markedly different, whereas others are more closely related. Fine-scale relationships among yellow perch and walleye reproductive groups within Lake Erie appear to be driven by spawning aggregations, natal homing behavior, and localized adaptations, rather than due to simple geographic connectivity (see Stepien et al. 2009, 2012; Sepulveda-Villet et al. 2011, 2012) (Fig. 25.8).
Mantel (1967) pairwise tests in GENEPOP v4.0 (Rousset 2008; http://kimura.univ-montp2.fr/%7Erousset/Genepop.htm), with 10,000 permutations, for the relationship between genetic distance (θ ST/1- θ ST) and natural logarithm of geographical distance (km) across Lake Erie spawning groups of (a) yellow perch (p = 0.212, R 2 = 0.024, y = 0.016x – 0.038; Sepulveda-Villet and Stepien 2011), and (b) walleye (p = 0.827, R 2 = 0.015, y = −0.003x – 0.051; Strange and Stepien 2007)
Estimated population structure from Bayesian STRUCTURE v2.3.3 analyses (Pritchard et al. 2000; Pritchard and Wen 2004; http://pritchardlab.stanford.edu/structure.html) for Lake Erie spawning groups of yellow perch (a) K = 10 and (b) K = 4 four (Adapted from Sepulveda-Villet and Stepien 2011) and walleye (c) K = 3 (Adapted from Strange and Stepien 2007); in reference to outlying populations from Lake St. Clair and L. Ontario (the latter for yellow perch only). Analyses were run with 100,000 burn-in and 500,000 replicates. Optimal K values were determined by posterior probabilities (Pritchard et al. 2000) and the ΔK method of Evanno et al. (2005). Thin vertical lines, partitioned into colored segments, represent individual fish. Black lines separate spawning groups from different locations. Note that there is no correspondence between the colors and haplotypes of yellow perch and walleye (a + b versus c are entirely independent)
Sepulveda-Villet and Stepien (2011) found significant differences at 15 microsatellite loci among Lake Erie yellow perch reproductive populations (shown in Fig. 25.9), discerning no relationship between genetic distance and geographic distance between sampling locations. Kocovsky and Knight (2012) reported similar trends using morphometric data from yellow perch sampled from many of the same spawning locations used by Sepulveda-Villet and Stepien (2011). Yellow perch reproductive populations in the central basin of Lake Erie that are separated by 17–94 km were distinguished by significant genetic divergences of F ST = 0.016–0.056 using the same 15 loci, and also displayed significant morphological differences (Kocovsky et al. 2013). Similar fine-scale differentiation also was evident among yellow perch reproductive groups in the St. Lawrence River system, which comprised four distinct genetic clusters along a 310 km-long corridor (LeClerc et al. 2008). Grzybowski et al. (2010) described fine-scale genetic structure between yellow perch spawning in Lake Michigan open water versus those in Green Bay, also using microsatellite data (F ST = 0.126).
Fine-scale map of Lake Erie showing locations of spawning groups evaluated for yellow perch (triangles, with capital letters) and walleye (circles, with lowercase letters). Yellow perch sampling sites are: I. Monroe, MI; J. Cedar Pt., OH; K. S. Bass Isl., OH; L. Sturgeon Ck., ON; M. Erieau, ON; N. Cleveland, OH; O. Fairport, OH; P. Perry, OH; Q. Ashtabula, OH; R. Erie, PA; S. Long Pt. Bay, ON; T. Pt. Colborne, ON; U. Dunkirk, NY (Modified from Sepulveda-Villet and Stepien 2011, 2012). Sites for walleye include: n. Belle Isl.; o. Fighting Isl.; p. Grosse Ile; q. Huron R.; r. Hen Isl.; s. Western Reefs; t. Chickenolee Reef; u. Maumee R.; v. Sandusky R.; w. Grand R. OH; x. Grand R. ON; y. Van Buren Bay; z. Cattaraugus Ck.; aa. Smoke’s Ck (Modified from Strange and Stepien 2007; Stepien et al. 2009, 2010, 2012; Haponski and Stepien 2014a)
Relatively large genetic separations likewise delineated some walleye reproductive populations located in close proximity, including between the Moon and Musquash Rivers (site l) in Georgian Bay of Lake Huron (F ST = 0.034), between the Thames (m) and the Detroit Rivers (n) in Lake St. Clair (F ST = 0.012) and among spawning locations in eastern Lake Erie (sites x–aa; mean F ST = 0.036, range = 0.034–0.058; Stepien et al. 2009, 2010). Conversely, walleye spawning aggregations along the southern shore in western and central Lake Erie (q–w), which are the largest in population numbers, were linked by more connectivity and gene flow (discussed in detail by Strange and Stepien 2007; Stepien et al. 2012). This connectivity also was described by other researchers using a variety of genetic techniques (Merker and Woodruff 1996; Stepien and Faber 1998; Strange and Stepien 2007). A study by McParland et al. (1999) using mtDNA RFLPs and allozymes found no differentiation between walleye reproducing at Chickenolee Reef (site t on Fig. 25.9) and the Huron River (site q) in western Lake Erie, but this comparison differed significantly when higher-resolution microsatellites were used (Stepien et al. 2009, 2010). The latter data revealed more site-specific differentiation among walleye spawning populations, which was greater in eastern Lake Erie.
Such differentiation among reproducing populations groups within a continuous system appears to result from spawning site philopatry to specific natal locations, maintained from generation to generation. European perch form long-term population groups of full and half siblings, according to microsatellite data (Bergek and Björklund 2007; Behrmann-Godel and Gerlach 2008). Reproductive success was significantly lower in non-kin groups, with reduced pre-zygotic and post-zygotic fitness manifested by lower fertilization rates and less hatching success (Behrmann-Godel and Gerlach 2008). One of the likely barriers to gene flow for European perch thus is reproductive isolation, either via kin recognition using olfactory cues (Gerlach et al. 2001) or due to reduced hybrid fitness between sympatric but divergent cohorts (Behrmann-Godel and Gerlach 2008). Likewise, it is possible that yellow perch and walleye returning to their natal locations are guided by olfactory information imprinted during early stages of their life history. If so, it may be the primary mechanism for maintaining divergence among spawning aggregations, but this hypothesis remains to be tested.
There is no effect of gender in the establishment of these fine-scale genetic structure trends, as both male and female yellow perch and walleye have analogous genetic patterns, and thus appear to have similar site fidelity (Stepien and Faber 1998; Stepien et al. 2009, 2012; Sepulveda-Villet et al. 2011, 2012). Eight yellow perch populations from Lake Erie locations (sites on Fig. 25.9) were all genetically distinguishable from one other (mean F ST = 0.068 ± 0.008, range = 0.002–0.168), but some also varied in allelic composition between two sampling time periods (2001–2004 versus 2009), at ~1/4 the magnitude of the difference among locations. Sullivan and Stepien (2015) found significant differences among yellow perch spawning groups and between sampling years at some of these sites. An example of annual variation within the yellow perch reproductive group sampled at Van Buren Bay (site y on Fig. 25.9) in eastern Lake Erie is given in Table 25.4a. A study by Demandt (2010) likewise found significant variations in microsatellite allelic frequencies of European perch among sampling years for a population in Sweden. This suggests a similar trend for annual variability at spawning sites for both European and yellow perch.
Yellow perch spawning groups varied among individual sampling years and age cohorts, with the 2003 cohort being the most distinctive of those sampled (Sepulveda-Villet and Stepien 2011, 2012; Sullivan and Stepien 2015). This 2003 cohort was an especially large and successful group for both yellow perch and walleye recruitment in Lake Erie (WTG 2014, YPTG 2014). Reproductive groups of yellow perch contained high numbers of full siblings (mean = 18.5 %, ranging to 75 % for the 2001 age cohort spawning at Van Buren Bay in eastern Lake Erie; Sullivan and Stepien 2015). Temporal genetic divergence at reproductive locations was not explained by genetic isolation over time, but appeared due to yellow perch spawning in kin-related groups that varied slightly from year to year. Spatial patterns were attributed to limited migration and natal homing, whereas temporal patterns may reflect kin group structuring and differential reproductive success.
In contrast to yellow perch, walleye from three of the largest spawning populations in Lake Erie (Maumee River; site u on Fig. 25.9, Sandusky River, site v, and Van Buren Bay reefs, site y), exhibited both temporal and spatial consistency across 14 years (collected in 1995, 1998, 2003, 2007, and 2008) using nine nuclear DNA microsatellite loci (Stepien et al. 2012). There was overall year-to-year genetic consistency within walleye reproductive groups; no significant differences were found among collection dates within an annual run, between the sexes, or among age-cohorts. An example demonstrating consistency among walleye runs in the Maumee River is presented in Table 25.4b (Stepien et al. 2012). Overall, walleye spawning at the Van Buren Bay reefs were genetically divergent from those reproducing in the Maumee and Sandusky rivers, reflecting geographic distance; the latter two groups were genetically closer, with slight differences that suggested more recent divergence, higher gene flow, or both. The Van Buren Bay population group in 1995 was the most divergent sample and had the greatest genetic self-assignment (100 %); this might indicate some slight changes in its distinctive genetics over time, as it was the oldest sample examined (Stepien et al. 2012). Results illustrated the importance of sampling over several years of spawning runs to understand overall patterns of population structure, which showed remarkable genetic consistency across an open-lake system for walleye.
10 Color Variants and Genetics: The Extinct “Blue Pike” and Turquoise Mucus Walleye and Yellow Perch
Dark steel grey-blue colored walleye reportedly once were common in the deeper cooler eastern basin of Lake Erie and reported from western Lake Ontario. These were called “blue pike” and regarded as either a species (Hubbs 1926) or as a subspecies S. vitreus “glaucus” (Trautman 1981). Trautman (1981) documented a preponderance of morphological intergrades between the “blue pike” and common “yellow” walleye.
The “blue pike” was reported to vary from “normal” yellow-colored walleye by having a steel-grey blue color, larger eyes located higher on the head, a smaller interorbital distance, and a greater angle between the preopercle bone and branchiostegal rays (Bailey and Smith 1981; Trautman 1981; Hubbs and Lagler 2004). However, all of these characters – including color – overlapped extensively with those of the abundant and widespread “yellow” walleye, which was sympatric throughout Lakes Erie and Ontario (Scott and Crossman 1973; Trautman 1981).
The “blue pike” was reported to mostly inhabit deeper cooler waters of eastern Lake Erie, but also was caught in western Lake Erie (Trautman 1981). Spawning of the “blue pike” was reported to occur somewhat later and in deeper areas than other walleye (Stone 1948). The “blue pike” and “yellow” walleye shared a popular commercial fishery, with the “blue pike” collapsing in 1959 – attributed to exploitation, pollution, and/or habitat alteration – with walleye numbers declining concurrently (Trautman 1981). The “blue pike” had disappeared by the early 1960s (Hubbs and Lagler 2004), and officially was declared extinct in 1983 by the US Fish and Wildlife Service (Noecker 1998).
The “blue pike’s” popular saga has been confused by the facts that (1) it is not a member of the pike family (i.e., is not in the family Esocidae, but is in the Percidae), it apparently had no distinctive characters, including color (Trautman 1981; Haponski and Stepien 2014a), and (3) walleye in northern waters (the Canadian Shield Lakes) frequently are bright turquoise blue in color from a protein in the mucus, termed sandercyanin (Yu et al. 2008). The turquoise-mucus colored-walleye do not match the deep steel-grey blue color or other morphological attributes of the “blue pike” (Stone 1948; Scott and Crossman 1973; Trautman 1981; Campbell 1987). The turquoise mucus walleye are reported to have a combination of sandercyanin pigment and lack of yellow xanthophores (Yu et al. 2008).
Both turquoise-mucus and yellow-colored walleye occur sympatrically in the same water bodies in Canada, with both secreting the blue mucus (Paradis and Magnan 2005; Yu et al. 2008). Some of the turquoise blue color typically “rubs off” when the fish is collected (Yu et al. 2008, CAS, personal observation). The turquoise mucus additionally has been reported on some yellow perch in those northern waters (Trautman 1981; Haponski and Stepien 2014a). It has been hypothesized that the production of the turquoise mucus and sandercyanin in the Canadian Shield lakes is a response to ultraviolet light levels (Yu et al. 2008).
A recent study by Haponski and Stepien (2014a) collected new morphological and genetic data from the original “blue pike” specimens named and described by Hubbs (1926). They examined all morphological and meristic characters that had been alleged to be diagnostic. They also sequenced the mtDNA control region and analyzed variation at nuclear DNA microsatellite loci from the “blue pike” specimens, common “yellow” walleye from the same period, and contemporary walleye across North America from 23 spawning sites. The findings showed that the “blue pike” was indistinguishable from common yellow-colored walleye collected from that same period in Lake Erie. It is possible that the “blue pike” comprised one or more reproductive groups in eastern Lake Erie, but there is no evidence to support any greater distinctiveness than found in other walleye spawning groups.
Haponski and Stepien (2014a) also examined the genetic characters of typical “yellow” walleye and turquoise-colored mucus walleye that were collected together in McKim Lake, Ontario Canada using mtDNA control region sequence data and nine nuclear microsatellite loci. They found no genetic differences between fish of the two colors. Moreover, the McKim Lake samples showed no population genetic relationship to the extinct “blue pike” paratypes from Lake Erie (the same individuals that Carl Hubbs had named as “blue pike” Hubbs 1926). The turquoise mucus and “yellow” walleye from McKim Lake also did not show a population genetic origin shared with walleye from Lakes Erie or Ontario (see Figs. 25.4b and 25.5b). Stepien and Faber (1998) likewise analyzed several assorted turquoise mucus walleye from a variety of Canadian Shield lakes using entire mtDNA control region sequences and found no genetic distinction from the normal variation range of walleye.
Paradis and Magnan (2005) morphologically compared sympatric yellow and turquoise mucus walleye in five Canadian Shield lakes near Quebec, reporting shorter head lengths and smaller interorbital distances in the latter. Laporte et al. (2011) alleged slight genetic difference between turquoise mucus and yellow walleye populations sampled within a lake using AFLP (amplified fragment length polymorphism) markers and assignment tests, but had no diagnostic alleles and their genetic distance analyses lacked significant bootstrap support. They reported that their assignments indicated that they could respectively genetically diagnose either the yellow or turquoise mucus type within a lake, but could not assign them as a whole when more than one lake was considered. Laporte et al. (2011) also stated that turquoise mucus walleye did not warrant taxonomic recognition as a subspecies. It may be that there are some differentiated walleye variants with color differences within some lakes across the range of the Canadian Shield; many distinctions at the population level are found among walleye spawning groups within Lake Erie and other Great Lakes basins (Strange and Stepien 2007; Stepien et al. 2009, 2010, 2012; Haponski and Stepien 2014a, b).
Occasional steel-grey/blue colored walleye and yellow perch have been reported from Lake Erie (Trautman 1981; Hubbs and Lagler 2004). A recent example is that a Lake Erie commercial fisherman (Jeff Herr, personal communication, 2013) caught a dark steel-grey/blue yellow perch in summer 2013 at Fairport OH and steel-grey/blue walleye recently have been caught in the central and western basins of Lake Erie (AEH personal observation, 2013). A steel-grey/blue walleye individual sampled in the western basin near Sandusky OH had mtDNA haplotype 1 (Haponski and Stepien 2014a), which is the most common walleye haplotype (see Figs. 25.3b and 25.5b). A skin scraping showed no evidence of the turquoise mucus. A recent study by Wayne Schaeffer (personal communication, University of Wisconsin, 2013) found no turquoise mucus or sandercyanin in Lake Erie walleye. Overall, no diagnosable genetic or morphological characters have been found that distinguish historic “blue pike” from walleye, rendering its subspecies status invalid (Haponski and Stepien 2014a). In contrast, many walleye spawning groups genetically differ from one another (Stepien and Faber 1998; Stepien et al. 2009, 2010, 2012; Haponski and Stepien 2014a).
11 The Value of Genetic Data for Evaluating Restoration
As molecular-based population dynamics and structure analyses increasingly provide a way to better assess past and present levels of diversity in fish populations, a need for greater use of these techniques has been proposed in concert with traditional management approaches. When demographic data on exploited fisheries are collected on a larger scale than population subunits, valuable data may be lacking for management decisions to conserve local genetic and morphological diversity and adaptedness, as appears to be the case for Lake Erie yellow perch (Kocovsky et al. 2013). Likewise, a landscape genetics study by LeClerc et al. (2008) described four distinct populations of yellow perch along a 310 km stretch of the St. Lawrence River, from ten microsatellite loci. These results were in contrast with recognized fisheries management units in Quebec. Here we examine use of a combined approach linking fisheries management, conservation genetics, and historical assessment of Great Lakes’ walleye populations.
Fish habitats in the Great Lakes and connecting tributaries were subject to extensive and deleterious changes in the twentieth and twenty-first centuries, with loss of wetlands, channelization of major streams, construction of dams, oxygen depletion, shoreline modification, siltation of spawning areas, nutrient enrichment, water-quality deterioration, sand and gravel extraction, and invasive species introductions (Trautman 1981; Bolsenga and Herdendorf 1993; Fielder 2002; Hoff 2002; Ryan et al. 2003). For example, Lake Huron’s Saginaw Bay once housed the second-largest walleye fishery in the Great Lakes (Schneider and Leach 1977; Fielder 2002), which collapsed in the 1940s from spawning-habitat degradation and overfishing (Jude and Leach 1999). Similarly, Lake Michigan yellow perch populations underwent extensive declines, particularly in the 1980s and persisting to this day, manifested in erratic recruitment patterns and the dependence on a single year class (recently 2005) for most reproductive effort at spawning grounds in the southern lake (Redman et al. 2013). Lake Erie has the largest walleye populations, which declined throughout the 1960s and the early 1970s due to reduced habitat and water quality, coupled with high exploitation (Regier and Hartman 1973).
International management regulations reduced exploitation and improved environmental conditions, leading to the recovery of Lake Erie walleye during the 1980s and increases to historical abundance levels during the 1990s (Knight 1997). Commercial walleye fishing was banned in Lake Huron’s Saginaw Bay in 1969, and a sport fishery supported by stocking came into prominence in the 1980s (Fielder 2002; USFWS/GLFC 2010). Walleye numbers in the St. Louis River of western Lake Superior also increased following river cleanup, which today comprises the largest spawning group in that lake (MacCallum and Selgeby 1987; Hoff 2002; Schram et al. 2010).
A recent study by Haponski and Stepien (2014a) found that genetic diversity of Lake Erie walleye in historic samples from the 1920s to 1940s was much less than in contemporary samples (mtDNA H D = 0.05 ± 0.01 vs. 0.79 ± 0.00; μsat H O = 0.47 ± 0.06 vs. 0.72 ± 0.01; compare to Table 25.2), suggesting population recovery after intense pollution and exploitation. The genetic composition, as shown by microsatellite alleles, underwent significant changes (F ST = 0.336). This was attributed to Lake Erie’s ecological recovery from pollution and increasing fishery regulations, with walleye abundances increasing to ~80 million fish during the 1980s and then declining to ~23 million in 2014 (see WTG 2014). Similarly, Guinand et al. (2003) found that genetic diversity of lake trout populations from Lakes Superior, Michigan, and Huron, based on five microsatellite loci, was less in 1940–1959 (H O = 0.47) than in 1995–1999 (0.51). Likewise, Stott et al. (2013) found that lake whitefish Coregonus clupeaformis from Lakes Huron and Erie had lower diversity at seven microsatellite loci in 1927 (H O = 0.60) than in 1997–2005 (0.65). These studies indicate that many fish populations recovered across the Great Lakes during the past decades.
In the past decade, Lake Erie walleye numbers have declined by about 60 % from the 1990s (Locke et al. 2005; WTG 2014). Yellow perch also have declined (YPTG 2014). Understanding and maintaining yellow perch and walleye population structure are critically important fisheries-management goals designated by the Great Lakes Fishery Commission (Ryan et al. 2003; GLFC 2011). Genomics investigations by Bélanger-Deschênes et al. (2013) and Bougas et al. (2013) examined functional polymorphisms of yellow perch from sites having an 85 year history of cadmium (Cd) and nickel (Ni) metal contamination versus clean lakes. Bélanger-Deschênes et al. (2013) discerned two non-synonymous point substitutions involving dissimilar amino acids. The authors suggest that potentially adaptive evolution selected for alleles that may increase perch fitness in polluted environments. Bougas et al. (2013) found that 475 genes had significantly different transcription levels across temperature variations, and 287 and 176 genes were differentially transcribed at different concentrations of Ni and Cd, respectively. Those metals influenced the transcription levels of genes involving iron metabolism, vitamin metabolism, blood coagulation, and calcium transport. These studies foretell the insights to be gained by investigating functional genomic adaptations of populations.
The effect of contaminants such as heavy metals have an additional, and perhaps unexpected effect on percid biology; Azizishirazi et al. (2013) discerned that yellow perch living in metal contaminated lakes had reduced olfactory acuity as compared to those living in clean lakes. Such reduction in detecting olfactory cues might negatively affect kin recognition ability described by Gerlach et al. (2001). Later improvement in water quality and the removal of metallic contaminants resulted in rapid recovery of olfactory capacities in previously impaired yellow perch populations (Azizishirazi et al. 2013), underscoring the benefits of habitat restoration beyond physical or water quality improvements.
Somatic and genetic markers were employed to evaluate the reproductive health of yellow perch populations for which fisheries monitoring revealed reduced recruitment, in urbanized and developed streams of the Chesapeake Bay watershed (Blazer et al. 2013). Results showed gonadal anomalies and changes in DNA integrity in those yellow perch population samples. These findings suggest that pollution can significantly impact reproduction and recruitment, the effects of which can be detected with molecular markers.
Genetic findings to date, as illustrated in this chapter, reveal that most yellow perch and walleye populations have appreciable genetic diversity and significantly differ from other populations, both nearby and distant, despite anthropogenic influences. These diversity and divergence patterns translate to localized adaptations, which merit preservation. Accordingly, we recommend conserving their genetic composition and differentiation patterns by maintaining and restoring spawning habitats, and continued careful management of fisheries.
12 Use of Genetic Data to Resolve Questions About Stocking
A literature search recovered no studies of the genetic effects of yellow perch stocking. For the most part, walleye were just occasionally stocked in the Great Lakes, showing little prevalent influence on the genetic structure and diversity of native populations. For example, Gatt et al. (2002) found that mtDNA restriction fragment length polymorphism (RFLP) diversity of walleye reproducing in Lake Huron’s Georgian Bay declined across three decades, from H D = 0.50 in the 1960s to 0.15 in the 1990s, which they attributed to exploitation and stocking. Haponski and Stepien (2014a) also recovered similarly low mtDNA control region sequence diversity (H D = 0.15) for walleye spawning in the Moon/Musquash Rivers of Georgian Bay, showing that mtDNA diversity remains low there today. However, that bottleneck effect was restricted to mtDNA, since average levels of nuclear DNA variability were described for the same samples (Stepien et al. 2009, 2010; Haponski and Stepien 2014a).
Studies have revealed introgression among hatchery stocks and wild populations of walleye in inland lakes from Ontario, Canada (Cena et al. 2006; Walter et al. 2012). Despite a long history of stocking activity (>60 years) for a number of these inland lakes, the overall historical genetic signatures of native populations remain well preserved (Walter et al. 2012). The Grand River, Ontario in northeastern Lake Erie was stocked in the 1980–1990s with tagged adult walleye from the Thames River in Lake St. Clair, in effort to increase population abundance (MacDougall et al. 2007). The endeavor was regarded as unsuccessful as most adults did not remain at the site to spawn and were recaptured to the west (Timmerman 1995), which was attributed to natal homing (see Olson and Scidmore 1962; MacDougall et al. 2007). The stocking effort appeared to have little effect on the genetic composition of that native reproductive population (MacDougall et al. 2007).
Similarly, Garner et al. (2013) found that the native genetic structure of walleye reproducing in Lake Superior’s Black Bay was maintained despite large releases of foreign fingerlings (from the St. Marys River) in 2004 and 2005. Although the stocked fingerlings composed 45–71 % of the individuals in their respective age classes, they appeared to have lower reproductive success and utilized different habitats from the native Black Bay walleye. The genetic composition of that spawning population, based on nine nuclear μsat loci (six were the same as used here), was not altered towards the stocked individuals (Garner et al. 2013). In contrast, walleye stocking in Escanaba Lake, Wisconsin substantially changed the genetic composition of the native population between 1952 and 2002, despite retaining consistent diversity levels (mean H O = 0.76) (Franckowiak et al. 2009).
The New York State Department of Environmental Conservation (NYSDEC) stocked Cattaraugus Creek NY in eastern Lake Erie with 2.2 million fry and 44,000 fingerlings from western Lake Erie’s Maumee River in 1995–2000. However, a historic group of walleye returns to spawn each spring in territorial waters of the Seneca Nation in Cattaraugus Creek. Walleye are no longer being stocked there, and the stockings were considered unsuccessful (D. Einhouse, New York Department of Environmental Conservation, personal communication 2014). Genetic analyses indicated that the stocking did not appear to genetically affect the native genotypes or diversity of walleye spawning in Cattaraugus Creek (Stepien et al. 2004; Haponski et al. 2014). There was no significant difference between samples pre- versus post-stocking, according to results from nine nuclear microsatellite loci (F ST = 0.003) and mtDNA control region sequence data (F ST < 0.001); the spawners also significantly differed from the Maumee River broodstock that was introduced (F ST = 0.090). These studies indicate that it is important to retain the genetic signature of the historic reproductive groups when stocking percids and other fishes.
13 Genetic Patterns in the Face of Climate Change
Temperatures in temperate regimes are predicted to increase over the next 50 years, with those in the Great Lakes region predicted to increase by 5–5.5 °C to become more like today’s Gulf Coast (Hayhoe et al. 2010). Warmer temperatures likely will alter growth rates and change maximum sizes and ages of yellow perch and walleye populations (see Carlander 1997). Climate change may disproportionally increase or decrease genetic variability across a taxon’s range due to shifts in physical conditions or biological resources (Hewitt 1999; Petit et al. 2003; Hampe and Jump 2011), as occurred during Pleistocene glaciations (Oberdorff et al. 1997; Davis and Shaw 2001; Soltis et al. 2006) and is ongoing today (Araújo and Rahbek 2006; Harris and Taylor 2010).
Cena et al. (2006) found a positive relationship between walleye population heterozygosity and early growth rate, which was correlated with fitness and additionally may be influenced by temperature. Dupont et al. (2007) and Zhao et al. (2008) discerned that population evolutionary origin was a significant differentiating factor in biological characteristics among walleye populations. Bergek et al. (2010) suggested that environmental factors other than geographic distance distinguished among European perch spawning groups, which might have led to differences in their genetic compositions, with the most likely factor being water temperature differences among habitats during the spring reproductive season. Temperature differences may have led to genetic isolation of various spawning populations, with those in shallow waters reproducing earlier (Bergek et al. 2010). Similarly, both walleye and yellow perch were significantly affected by water level fluctuations of glacial lakes in North Dakota, with their highest recorded abundances and body weights occurring during high water periods (Dembkowski et al. 2013), underscoring potential deleterious effects of increased evaporation and water losses linked to climate change.
These findings highlight the importance of spawning habitat and localized variations among their associated reproductive groups. It appears likely that the genetic structure among spawning localities will continue to develop as a product of the interplay between ancestral lineages and environmental variation among reproductive areas, rather than isolation by distance. If this concept holds true, then we should expect effects on genetic diversity and composition from the increasing pace of climate change and higher surface water temperatures with shifting population distributions.
Evaluating diversity and divergence patterns resulting from post-glacial dispersal and adaptation in new environments, and the genetic reservoirs comprising isolated relict populations, may help us to predict the challenges faced by taxa during this era of rapid climate and habitat alterations. Populations along the lower latitudinal fringes of a species’ native range likely house valuable genetic adaptations to warmer climates (Hampe and Petit 2005). In effect, global warming patterns rapidly are extending the northward post-glacial expansion trajectory of many taxa; meanwhile their southerly rear-edge groups may experience greater isolation, habitat reduction, and bottlenecks. Moreover, these southern genotypes may move northward, given connection or transport opportunity. For example, the diverse South Atlantic coastal yellow perch populations may prove especially well-adapted to tolerating salinity fluctuations and increasing water temperatures. This may facilitate their northward coastal migration, if sea levels rise to eventually connect low-lying estuaries that are currently isolated by barrier island and sandbar systems. Similarly, the large river walleye populations present in tributaries and main stem of the Mississippi River system may provide unique adaptability and resilience to warmer temperatures, and may undergo a northerly shift. These distributional changes in populations are significant in the context that they may interbreed with long-term native populations in the north. It is possible that native population adaptedness may be either positively or negatively influenced by these changes.
Warming temperatures and increases in storm events may influence fish population structure and overall productivity via biological and climate-related effects, as outlined by Newbrey and Ashworth (2004). For example, Hill and Magnuson (1990) suggested that changes in bioenergetics accompanying climate change might modify growth and prey consumption, thereby affecting food-web dynamics. Shuter and Post (1990) suggested that an increase of 4 °C may shift the distributional limit of yellow perch northward and, depending on lake morphometry and productivity, might also greatly affect survival, relative year-class strength, and ecosystem carrying capacity. To date, populations of yellow perch and walleye possess relatively consistent levels of genetic diversity and high local distinctiveness. These appear to have been maintained despite anthropogenic habitat loss, degradation, fragmentation, and exploitation, likely offset by their large population sizes and the relative abundance of habitats throughout the Great Lakes and other systems.
Genetic structure of today’s yellow perch and walleye populations reflects interplay among climatic events, ephemeral waterway connections, population sizes, and likely spawning group philopatry. Delineation of the genomic adaptations that underlie the patterns of genetic diversity and diversity described here will aid predictions of likely response to changing environments, new habitat areas, and exploitation pressures (see Allendorf et al. 2010; Avise 2010). A combined fisheries management and genetics/genomic approach provides a bridge for understanding the unique challenges faced by aquatic taxa due to their constrained dispersal and gene flow via habitat connectivity. Understanding the historical and present day factors that shaped today’s populations may aid their continued conservation in the face of future challenges.
References
Aalto SK, Newsome (1990) Additional evidence supporting demic behavior of a yellow perch (Perca flavescens) population. Can J Fish Aquat Sci 47:1959–1962
Aldenhoven JT, Miller MA, Corneli PS, Shapiro MD (2010) Phylogeography of ninespine sticklebacks. Ecology 19:4061–4076
Allendorf FW, Hohenlohe PA, Luikart G (2010) Genomics and the future of conservation genetics. Nat Rev Genet 11:697–710
Araújo MB, Rahbek C (2006) How does climate change affect biodiversity? Science 313:1396–1397
Avise JC (2004) Molecular markers, natural history, and evolution, 2nd edn. Sinauer Associates, Sunderland
Avise J (2010) Conservation genetics enters the genomics era. Conserv Genet 11:665–669
Azizishirazi A, Dew WA, Forsyth HL, Pyle GG (2013) Olfactory recovery of wild yellow perch from metal contaminated lakes. Ecotoxicol Environ Saf 88:42–47
Backhouse-James SM, Docker MF (2012) Microsatellite and mitochondrial DNA markers show no evidence of population structure in walleye (Sander vitreus) in Lake Winnipeg. J Great Lakes Res 38:47–57
Bailey RM, Smith GR (1981) Origin and geography of the fish fauna of the Laurentian Great Lakes basin. Can J Fish Aquat Sci 38:1539–1561
Barton BA, Barry TP (2011) Reproduction and environmental biology. In: Barton BA (ed) Biology, management, and culture of walleye and sauger. American Fisheries Society, Bethesda, pp 199–231
Behrmann–Gödel J, Gerlach G (2008) First evidence for postzygotic reproductive isolation between two populations of Eurasian perch (Perca fluviatilis L.) within Lake Constance. Front Zool 5:1–7
Behrmann-Godel J, Gerlach G, Eckmann R (2006) Kin and population recognition in sympatric Lake Constance perch (Perca fluviatilis L.): can assortative shoaling drive population divergence? Behav Ecol Sociobiol 59:461–468
Bélanger-Deschênes S, Couture P, Campbell PG, Bernatchez L (2013) Evolutionary change driven by metal exposure as revealed by coding SNP genome scan in wild yellow perch (Perca flavescens). Ecotoxicology 22:938–957
Beletsky D, Mason DM, Schwab DJ, Rutherford ES, Janssen J, Clapp DF, Dettmers JM (2007) Biophysical model of larval yellow perch advection and settlement in Lake Michigan. J Great Lakes Res 33:842–866
Bergek S, Björklund M (2007) Cryptic barriers to dispersal within a lake allow genetic differentiation of Eurasian perch. Evolution 61:2035–2041
Bergek S, Sundblad G, Björklund M (2010) Population differentiation in perch Perca fluviatilis: environmental effects on gene flow? J Fish Biol 76:1159–1172
Bernatchez L (1997) Mitochondrial DNA analysis confirms the existence of two glacial races of rainbow smelt Osmerus mordax and their reproductive isolation in the St Lawrence River estuary (Quebec, Canada). Mol Ecol 6:73–83
Billington N (1993) Genetic variation in Lake Erie yellow perch (Perca flavescens) demonstrated by mitochondrial DNA analysis. J Fish Biol 43:941–943
Billington N (1996) Geographical distribution of mitochondrial DNA (mtDNA) variation in walleye, sauger, and yellow perch. Ann Zool Fenn 33:699–706
Billington N, Maceina MJ (1997) Genetic and population characteristics of walleyes in the Mobile drainage of Alabama. Trans Am Fish Soc 126:804–814
Billington N, Strange RM (1995) Mitochondrial DNA analysis confirms the existence of a genetically divergent walleye population in northeastern Mississippi. Trans Am Fish Soc 124:770–776
Billington N, Barrette RJ, Hebert PDN (1992) Management implications of mitochondrial DNA variation in walleye stocks. N Am J Fish Manag 12:276–284
Blazer VS, Pinkney AE, Jenkins JA, Iwanowicz LR, Minkkinen S, Draugelis-Dale RO, Uphoff JH (2013) Reproductive health of yellow perch Perca flavescens in selected tributaries of the Chesapeake Bay. Sci Total Environ 447:198–209
Bodaly RA, Ward RD, Mills CA (1989) A genetic stock study of perch, Perca fluviatilis L., in Windermere. J Fish Biol 34:965–967
Bolsenga SJ, Herdendorf CE (1993) Lake Erie and Lake St. Clair handbook. Wayne State University Press, Detroit
Borer S, Miller LM, Kapuscinski AR (1999) Microsatellites in walleye Stizostedion vitreum. Mol Ecol 8:336–338
Boschung HT, Mayden RL (2004) Fishes of Alabama. Smithsonian Institution, Washington, DC
Bougas B, Normandeau E, Pierron F, Campbell PGC, Bernatchez L, Couture P (2013) How does exposure to nickel and cadmium affect the transcriptome of yellow perch (Perca flavescens) – results from a 1000 candidate-gene microarray. Aquat Toxicol 142–143C:355–364
Bozek MA, Baccante DA, Lester NP (2011a) Walleye and sauger life history. In: Barton BA (ed) Biology, management, and culture of walleye and sauger. American Fisheries Society, Bethesda, pp 233–301
Bozek MA, Haxton TJ, Raabe JK (2011b) Walleye and sauger habitat. In: Barton BA (ed) Biology, management, and culture of walleye and sauger. American Fisheries Society, Bethesda, pp 133–198
Campbell RR (1987) Status of the blue walleye, Stizostedion vitreum glaucum, in Canada. Can Field Nat 101:245–252
Carey JR, Judge DS (2000) Longevity records: life spans of mammals, birds, amphibians, reptiles, and fish. Odense University Press, Odense
Carlander KD (1997) Handbook of freshwater fishery biology, vol. 3: life history data on ichthyopercid and percid fishes of the United States and Canada. Iowa State University Press, Ames
Cena CJ, Morgan GE, Malette MD, Heath DD (2006) Inbreeding, outbreeding and environmental genetic diversity in 46 walleye (Sander vitreus) populations. Mol Ecol 15:303–320
Clady MD (1977) Distribution and relative exploitation of yellow perch tagged on spawning grounds in Oneida Lake. NY Fish Game J 24:46–52
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1660. Available at http://darwin.uvigo.es/software/tcs.html
Colby PJ, McNicol RE, Ryder RA (1979) Synopsis of biological data on the walleye Stizostedion v. vitreum, vol 119, FAO fisheries synopsis. Food and Agriculture Organization of the United Nations, Rome
Colby PJ, Lewis CA, Eschenroder RL, Haas RC, Hushak LJ (1994) Walleye- rehabilitation guidelines for the Great Lakes area. Great Lakes Fishery Commission, technical report, Ann Arbor
Collette BB, Ali MA, Hokanson KEF, Nagiec M, Smirnov SA, Thorpe JE, Weatherly AH, Willemsen J (1977) Biology of the percids. J Fish Res Board Can 34:1891–1899
Coulon A, Fitzpatrick JW, Bowman R, Lovette IJ (2012) Mind the gap: genetic distance increases with habitat gap size in Florida scrub jays. Biol Lett 8:582–585
Craig JF (1987) The biology of perch and related fish. Croom Helm, London
Craig JF (2000) Percid fishes systematics, ecology, and exploitation. Blackwell Science, Oxford
Crossman EJ, McAllister DE (1986) Zoogeography of freshwater fishes of the Hudson Bay Drainage, Ungava Bay and the Arctic Archipelago. In: Hocutt CH, Wiley EO (eds) The zoogeography of North American freshwater fishes. Wiley, New York, pp 53–104
Darriba D, Taboada GL, Doallom R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. Available at http://code.google.com/p/jmodeltest2/
Davis MB, Shaw RG (2001) Range shifts and adaptive responses to quaternary climate change. Science 292:673–679
Demandt MH (2010) Temporal changes in genetic diversity of isolated populations of perch and roach. Conserv Genet 11:249–255
Dembkowski DJ, Chipps SR, Blackwell BG (2013) Response of walleye and yellow perch to water-level fluctuations in glacial lakes. Fish Manag Ecol. doi:10.1111/fme.12047
DeWoody JA, Avise JC (2000) Microsatellite variation in marine, freshwater and anadromous fishes compared with other animals. J Fish Biol 56:461–473
Diekmann OE, Serrão EA (2012) Range-edge genetic diversity: locally poor extant southern patches maintain a regionally diverse hotspot in the seagrass Zostera marina. Mol Ecol 7:1647–1657
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol 29:1969–1973. Available at http://beast2.org
Dumont P (1996) Comparaison de la dynamique des populations de perchaudes (Perca flavescens) soumises a des niveaux differents de stress anthropique. Ministere de l’Environmement et de la Faune, Service de l’amenagemetn et de l’exploitation de la faune. Report technique 06–46, Montreal
Dupont PP, Bourret V, Bernatchez L (2007) Interplay between ecological, behavioural and historical factors in shaping the genetic structure of sympatric walleye populations (Sander vitreus). Mol Ecol 26:937–951
Eldridge WH, Bacigalupi MD, Adelman IR, Miller LM, Kapuscinski AR (2002) Determination of relative survival of two stocked walleye populations and resident natural-origin fish by microsatellite DNA parentage assignment. Can J Fish Aquat Sci 59:282–290
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Excoffier L, Lischer HE (2010) ARLEQUIN suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567. Available at http://cmpg.unibe.ch/software/arlequin35/
Faber JE, Stepien CA (1997) The utility of mitochondrial DNA control region sequences for analyzing phylogenetic relationships among populations, species, and genera of the Percidae. In: Kocher TD, Stepien CA (eds) Molecular systematics of fishes. Academic, London, pp 129–144
Ferguson RG, Derkson AJ (1971) Migrations of adult and juvenile walleyes (Stizostedion vitreum vitreum) in southern Lake Huron, Lake St. Clair, Lake Erie, and connecting waters. J Fish Res Board Can 8:1133–1142
Ferguson MM, Duckworth GA (1997) The status and distribution of lake sturgeon, Ascipenser fulvescens, in the Canadian provinces of Manitoba, Ontario and Quebec: a genetic perspective. Environ Biol Fishes 48:299–309
Fielder DG (2002) Sources of walleye recruitment in Saginaw Bay, Lake Huron. N Am J Fish Manag 22:1032–1040
Franckowiak RP, Sloss BL, Bozek MA, Newman SP (2009) Temporal effective size estimates of a managed walleye Sander vitreus population and implications for genetic-based management. J Fish Biol 74:1086–1103
Fulford RS, Rice JA, Miller TJ, Binkowski FP, Dettmers JM, Belonger B (2006) Foraging selectivity by larval yellow perch (Perca flavescens): implications for understanding recruitment in small and large lakes. Can J Fish Aquat Sci 63:28–42
Garner SR, Bobrowicz SM, Wilson CC (2013) Genetic and ecological assessment of population rehabilitation: walleye in Lake Superior. Ecol Appl 23:594–605
Gatt MH, Fraser DJ, Liskauskas AP, Ferguson MM (2002) Mitochondrial DNA variation and stock structure of walleye from eastern Lake Huron: an analysis of contemporary and historical samples. Trans Am Fish Soc 131:99–108
Gerlach G, Schardt U, Eckmann R, Meyer A (2001) Kin–structured subpopulations in Eurasian perch (Perca fluviatilis L.). Heredity 86:213–221
Glaubitz JC (2004) CONVERT: a user–friendly program to reformat diploid genotypic data for commonly used population genetic software packages. Mol Ecol Notes 4:309–310. http://www.agriculture.purdue.edu/fnr/html/faculty/rhodes/students%20and%20staff/glaubitz/software.htm
GLFC (Great Lakes Fishery Commission) (2011) Strategic vision of the Great Lakes Fishery Commission 2011–2020. Great Lakes Fishery Commission, special publication, Ann Arbor. Available at http://www.glfc.org/pubs/SpecialPubs/StrategicVision2012.pdf
Goudet J (1995) Fstat version 1.2: a computer program to calculate Fstatistics. J Hered 86:485–486
Goudet J (2002) Fstat version 2.9.3.2. Available at http://www2.unil.ch/popgen/softwares/fstat.htm
Griffiths D (2010) Pattern and process in the distribution of North American freshwater fish. Biol J Linn Soc 100:46–61
Grzybowski M, Sepulveda-Villet OJ, Stepien CA, Rosauer D, Binkowski F, Klaper R, Shepherd BS, Goetz F (2010) Genetic variation of 17 wild yellow perch populations from the Midwest and east coast analyzed via microsatellites. Trans Am Fish Soc 139:270–287
Guinand B, Scribner KT, Page KS, Burnham-Curtis MK (2003) Genetic variation over space and time: analyses of extinct and remnant lake trout populations in the Upper Great Lakes. Proc R Soc Lond B 270:425–433
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New Algorithms and methods to estimate maximum–likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. Available at http://www.atgc-montpellier.fr/phyml/
Gyllensten ULF, Ryman N, Ståhl G (1985) Monomorphism of allozymes in perch (Perca fluviatilis L.). Hereditas 102:57–61
Haas RC, Bryant WC, Smith KD, Nuhfer AJ (1985) Movement and harvest of fish in Lake St. Clair, St. Clair River, and Detroit River. Final Report Winter Navigation Study U.S. Army Corps of Engineers
Hackney PA, Holbrook JA (1978) Sauger, walleye, and yellow perch in the southeastern United States. Am Fish Soc Spec Pub 11:74–81
Hampe A, Jump AS (2011) Climate relicts: past, present, future. Ann Rev Ecol Evol Syst 42:313–333
Hampe A, Petit R (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467
Haponski AE, Stepien CA (2013) Phylogenetic and biogeographic relationships of the Sander pikeperches (Perciformes: Percidae): patterns across North America and Eurasia. Biol J Linn Soc 110:156–179
Haponski AE, Stepien CA (2014a) A population genetic window in the past and future of the walleye Sander vitreus: relation to historic walleye and the extinct blue pike S. v. “glaucus”. BMC Evol Biol 14:133
Haponski AE, Stepien CA (2014b) Genetic connectivity and diversity of walleye (Sander vitreus) spawning groups in the Huron–Erie corridor. J Great Lakes Res 40:89–100
Haponski AE, Bollin TL, Jedlicka MA, Stepien CA (2009) Landscape genetic patterns of the rainbow darter Etheostoma caeruleum: a catchment analysis of mitochondrial DNA sequences and nuclear microsatellites. J Fish Biol 75:2244–2268
Haponski AE, Dean H, Blake BE, Stepien CA (2014) Genetic history of walleye (Sander vitreus) spawning in Lake Erie’s Cattaraugus Creek: a comparison of pre- and post-stocking. Trans Am Fish Soc. 143:1295–1307
Harris LN, Taylor EB (2010) Pleistocene glaciations and contemporary genetic diversity in a Beringian fish, the broad whitefish, Coregonus nasus (Pallas): inferences from microsatellite DNA variation. J Evol Biol 23:72–86
Hayhoe K, VanDorn J, Croley T, Schlegal N, Wuebbles D (2010) Regional climate change projections for Chicago and the US Great Lakes. J Great Lakes Res 36:7–21
Helfman G (1984) School fidelity in fishes: the yellow perch pattern. Anim Behav 32:663–672
Hewitt GM (1999) Post-glacial re-colonization of European biota. Biol J Linn Soc 68:87–112
Hewitt GM (2004) Genetic consequences of climatic oscillations in the Quaternary. Phil Trans R Soc B 359:183–195
Hill DK, Magnuson JJ (1990) Potential effects of global climate warming on the growth and prey consumption of Great Lakes fish. Trans Am Fish Soc 119:265–275
Hoagstrom CW, Berry CR (2010) The native range of walleyes in the Missouri River drainage. N Am J Fish Manag 30:642–654
Hoff MH (2002) A rehabilitation plan for walleye populations and habitats in Lake Superior, Great Lakes Fishery Commission miscellaneous publication 2003-01. Great Lakes Fishery Commission, Ann Arbor
Horrall RM (1981) Behavioral stock-isolating mechanisms in Great Lakes fishes with special reference to homing and site imprinting. Can J Fish Aquat Sci 38:1481–1496
Hubbs CL (1926) A check–list of the fishes of the Great Lakes and tributary waters, with nomenclatorial notes and analytical keys, vol 15, University of Michigan Museum of Zoology miscellaneous publication. University of Michigan Museum of Zoology, Ann Arbor
Hubbs CL, Lagler KF (2004) Fishes of the Great Lakes Region. (Smith GR, revised). University of Michigan, Ann Arbor
Jansen AC, Graeb BDS, Willis DW (2009) Effect of a simulated cold-front on hatching success of yellow perch eggs. J Freshw Ecol 24:651–655
Jennings MJ, Claussen JE, Philipp DP (1996) Evidence for heritable preferences for spawning habitat between two walleye populations. Trans Am Fish Soc 125:978–986
Jones ML, Netto JK, Stockwell JD, Mion JB (2003) Does the value of newly accessible spawning habitat for walleye (Stizostedion vitreum) depend on its location relative to nursery habitats? Can J Fish Aquat Sci 60:1527–1538
Jude DJ, Leach J (1999) Great Lakes fisheries. In: Kohler CC, Hubert WA (eds) Inland fisheries management in North America, 2nd edn. American Fisheries Society, Bethesda, pp 623–656
Kerr SJ, Corbett BW, Hutchinson NJ, Kinsman D, Leach JH, Puddister D, Stanfield L, Ward N (1997) Walleye habitat: a synthesis of current knowledge with guidelines for conservation. Percid Community Synthesis, Walleye Habitat Working Group, Ontario Ministry of Natural Resources, Peterborough
Knight RL (1997) Successful interagency rehabilitation of Lake Erie walleye. Fisheries 22:16–17
Kocovsky PM, Knight CT (2012) Morphological evidence for discrete stocks of yellow perch in Lake Erie. J Great Lakes Res 38:534–539
Kocovsky PM, Sullivan TJ, Knight CT, Stepien CA (2013) Genetic and morphometric differences demonstrate fine–scale population substructure of the yellow perch Perca flavescens: need for redefined management units. J Fish Biol 82:2015–2030
Kornis MS, Mercado-Silva N, Vander Zanden MJ (2012) Twenty years of invasion: a review of Neogobius melanostomus biology, spread, and ecological implications. J Fish Biol 80:235–285
Kreiger DA, Terrell JW, Nelson PC (1983) Habitat suitability information: yellow perch. U.S. Fish and Wildlife Service FWS/OBS-83/10.55. Washington, DC
Kunin WE, Vergeer P, Kenta T, Davey MP, Burke T, Woodward FI, Quick P, Mannarelli M-E, Watson-Haigh NS, Butlin R (2009) Variation at range margins across multiple spatial scales: environmental temperature, population genetics and metabolomic phenotype. Proc R Soc Lond B 276:1495–1506
Laporte M, Magnan P, Angers B (2011) Genetic differentiation between the blue and the yellow phenotypes of walleye (Sander vitreus): an example of parallel evolution. Ecoscience 18:124–129
Larson G, Schaetzl R (2001) Origin and evolution of the Great Lakes. J Great Lakes Res 27:518–546
Leary R, Booke HE (1982) Genetic stock analysis of yellow perch from Green Bay and Lake Michigan. Trans Am Fish Soc 111:52–57
LeClerc E, Mailhot Y, Mingelbier M, Bernatchez L (2008) The landscape genetics of yellow perch (Perca flavescens) in a large fluvial ecosystem. Mol Ecol 17:1702–1717
Lewis CFM, Moore TC, Rea DK, Dettman DL, Smith AM, Mayer LA (1994) Lakes of the Huron basin: their record of runoff from the Laurentide Ice Sheet. Quat Sci Rev 13:891–922
Li S, Mathias JA (1982) Causes of high mortality among cultured larval walleyes. Trans Am Fish Soc 111:710–721
Li L, Wang HP, Givens C, Czesny S, Brown B (2007) Isolation and characterization of microsatellites in yellow perch (Perca flavescens). Mol Ecol Notes 7:600–603
Lindsay DL, Barr KR, Lance RF, Tweddale SA, Hayden TJ, Leberg PL (2008) Habitat fragmentation and genetic diversity of an endangered, migratory songbird, the golden-cheeked warbler (Dendroica chrysoparia). Mol Ecol 17:2122–2133
Locke B, Belore M, Cook A, Einhouse D, Kenyon R, Knight R, Newman K, Ryan P, Wright E (2005) Walleye management plan. Lake Erie Committee Great Lakes Fishery Commission. Available at http://www.glfc.org/lakecom/lec/WTG_docs/other_reports_and_docs/wmp20051207.pdf
MacCallum WR, Selgeby JH (1987) Lake Superior revisited 1984. Can J Fish Aquat Sci 44:23–36
MacDougall TM, Wilson CC, Richardson LM, Lavender M, Ryan PA (2007) Walleye in the Grand River, Ontario: an overview of rehabilitation efforts, their effectiveness, and implications for eastern Lake Erie fisheries. J Great Lakes Res 33:103–117
MacGregor RB, Witzel LD (1987). A twelve year study of the fish community in the Nanticoke Region of Long Point Bay, Lake Erie. Lake Erie Fisheries Assessment Unit report 1987-3. Ontario Ministry of Natural Resources, Port Dover
Mandrak NE, Crossman EJ (1992) Postglacial dispersal of freshwater fishes into Ontario. Can J Zool 70:2247–2259
Mangan MT (2004) Yellow perch production and harvest strategies for semi- permanent wetlands in Eastern South Dakota. MSc thesis, Wildlife and Fisheries Sciences, South Dakota State University
Manni F, Guérard E, Heyer E (2004) Geographic patterns of (genetic, morphologic, linguistic) variation: how barriers can be detected by using Monmonier’s algorithm. Hum Biol 76:173–190. Available at http://ecoanthropologie.mnhn.fr/software/barrier.html
Manning NF, Mayer CM, Bossenbroek JM, Tyson JT (2013) Effects of water clarity on the length and abundance of age-0 yellow perch in the Western Basin of Lake Erie. J Great Lakes Res 39:295–302
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
McParland TL, Ferguson MM, Liskauskas AP (1999) Genetic population structure and mixed-stock analysis of walleyes in the Lake Erie-Lake Huron Corridor using allozymes and mitochondrial DNA markers. Trans Am Fish Soc 128:1055–1067
Merker RJ, Woodruff RC (1996) Molecular evidence for divergent breeding groups of walleye (Stizostedion vitreum) in tributaries to western Lake Erie. J Great Lakes Res 22:280–288
Miller LM (2003) Microsatellite DNA loci reveal genetic structure of yellow perch in Lake Michigan. Trans Am Fish Soc 132:503–513
Moran GF, Hopper SD (1983) Genetic diversity and the insular population structure of the rare granite rock species, Eucalyptus caesia Benth. Aust J Bot 31:161–172
Moyer GR, Billington N (2004) Stock structure among yellow perch populations throughout North America determined from allozyme and mitochondrial DNA analysis. In: Barry TP, Malison JA (eds) Proceedings of Percis III: the third international percid fish symposium, University of Wisconsin Sea Grant Institute, Madison, pp 96–97
Murdoch MH, Hebert PD (1997) Mitochondrial DNA evidence of distinct glacial refugia brown bullhead (Ameiurus nebulosus). Can J Fish Aquat Sci 54:1450–1460
Murphy BR (1990) Evidence for a genetically unique walleye population in the upper Tombigbee River system of northeastern Mississippi. Proc SE Fish Counc 22:14–16
Murphy S, Collins N, Doka S, Fryer B (2012) Evidence of yellow perch, largemouth bass and pumpkinseed metapopulations in coastal embayments of Lake Ontario. Environ Biol Fish 95:213–226
Nesbø CL, Magnhagen C, Jakobsen KS (1998) Genetic differentiation among stationary and anadromous perch (Perca fluviatilis) in the Baltic Sea. Hereditas 129:241–249
Nesbø CL, Fossheim T, Vollestad LA, Jakobsen KS (1999) Genetic divergence and phylogeographic relationships among European perch (Perca fluviatilis) populations reflect glacial refugia and postglacial colonization. Mol Ecol 8:1387–1404
Newbrey MG, Ashworth AC (2004) A fossil record of colonization and response of lacustrine fish populations to climate change. Can J Fish Aquat Sci 61:1807–1816
Noecker RJ (1998) Endangered species list revisions: a summary of delisting and downlisting. CRS report for congress 98-32 ENR
Oberdorff T, Hugueny B, Guégan J-F (1997) Is there an influence of historical events on contemporary fish species richness in rivers? Comparisons between western Europe and North America. J Biogeogr 24:461–467
Olson DE, Scidmore WJ (1962) Homing behavior of spawning walleyes. Trans Am Fish Soc 91:355–361
OMNR (Ontario Ministry of Natural Resources) (2011) 2006–2009 annual report. Lake Erie MU
Paradis Y, Magnan P (2005) Phenotypic variation of walleye, Sander vitreus, in Canadian Shield lakes: new insights on percid polymorphism. Environ Biol Fish 73:357–366
Parker AD, Stepien CA, Sepulveda-Villet OJ, Ruehl CB, Uzarski DG (2009) The interplay of morphology, habitat, resource use, and genetic relationships in young yellow perch. Trans Am Fish Soc 138:899–914
Petit RJ, Aguinaglade I, de Beaulieu J-L, Bittkau C, Brewer S, Cheddadi R, Ennos R, Fineschi S, Grivet D, Lascoux M, Mohanty A, Müller-Starck G, Demesure-Musch B, Palmé A, Martín JP, Rendell S, Vendramin GG (2003) Glacial refugia: hotspots but not melting pots of genetic diversity. Science 300:1563–1565
Pritchard JK, Wen W (2004) Documentation for STRUCTURE software: ver. 2.3.3. Stanford University. Available at http://pritchardlab.stanford.edu/software.html
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Provan J, Bennett KD (2008) Phylogeographic insights into cryptic glacial refugia. Trends Ecol Evol 23:564–571
Radabaugh NB, Bauer WF, Brown ML (2010) A comparison of seasonal movement patterns of yellow perch in simple and complex lake basins. N Am J Fish Manag 30:179–190
Rawson MR (1980) Yellow perch movements. Ohio Department of Natural Resources Job Program Report, Dingell–Johnson project number F-35-R-18, study number 4, 1 Nov 1979–30 Jun 1980
Redman RA, Czesny SJ, Dettmers JM (2013) Yellow perch population assessment in Southwestern Lake Michigan. INHS technical report 2013 (25), Division of Fisheries, Illinois Department of Natural Resources, Champaign
Refseth UH, Nesbø CL, Stacy JE, Vøllestad LA, Fjeld E, Jakobsen KS (1998) Genetic evidence for different migration routes of freshwater fish into Norway revealed by analysis of current perch (Perca fluviatilis) populations in Scandinavia. Mol Ecol 7:1015–1027
Regier HA, Hartman WL (1973) Lake Erie’s fish community: 150 years of cultural stresses. Science 180:1248–1255
Rempel LL, Smith DG (1998) Postglacial fish dispersal from the Mississippi refuge to the Mackenzie River basin. Can J Fish Aquat Sci 55:893–899
Rice RM (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rodrigues CG, Vilks G (1994) The impact of glacial lake runoff on the Goldthwait and Champlain Seas: the relationship between glacial Lake Agassiz runoff and the younger dryas. Quat Sci Rev 13:923–944
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. http://mrbayes.sourceforge.net/. (v3.1.2 2005)
Roseman EF, Taylor WW, Hayes DB, Tyson JT, Haas RC (2005) Spatial patterns emphasize the importance of coastal zones as nursery areas for larval walleye in western Lake Erie. J Great Lakes Res 31:28–44
Rousset F (2008) Genepop’008: a complete re-implementation of the Genepop software for Windows and Linux. Mol Ecol Resour 8:103–106. Available at http://kimura.univ-montp2.fr/%7Erousset/Genepop.htm
Russell DA, Rich FJ, Schneider V, Lynch-Stieglitz J (2009) A warm thermal enclave in the Late Pleistocene of the south-eastern United States. Biol Rev 84:173–202
Ryan PA, Knight R, MacGregor R, Towns G, Hoopes R, Culligan W (2003) Fish-community goals and objectives for Lake Erie, Great Lakes Fishery Commission special publication 03-02. Great Lakes Fishery Commission, Ann Arbor
Saarnisto M (1974) The deglaciation history of the Lake Superior region and its climatic implications. Quatern Res 4:316–339
Schmidt RE (1986) Zoogeography of the northern Appalachians. In: Hocutt CH, Wiley EO (eds) The zoogeography of North American freshwater fishes. Wiley, New York, pp 137–159
Schneider JC, Leach JH (1977) Walleye (Stizostedion vitreum vitreum) fluctuations in the Great Lakes and possible causes, 1800–1975. J Fish Res Board Can 34:1878–1889
Schram ST, Seider MJ, Furlong PD, Friday MJ (2010) Status of walleye in Lake Superior. In: Roseman E, Kocovsky P, Vandergoot C (eds) Status of walleye in the Great Lakes: proceedings of the 2006 symposium. Great Lakes Fishery Commission technical report 69, Ann Arbor, pp 1–13
Scott WB, Crossman EJ (1973) Freshwater fishes of Canada. J Fish Res Board Can 184:1–196
Sepulveda-Villet OJ, Stepien CA (2011) Fine-scale population genetic structure of the yellow perch Perca flavescens in Lake Erie. Can J Fish Aquat Sci 68:1435–1453
Sepulveda–Villet OJ, Stepien CA (2012) Waterscape genetics of the yellow perch (Perca flavescens): patterns across large connected ecosystems and isolated relict populations. Mol Ecol 21:5795–5826
Sepulveda-Villet OJ, Ford AM, Williams JD, Stepien CA (2009) Population genetic diversity and phylogeographic divergence patterns of the yellow perch (Perca flavescens). J Great Lakes Res 35:107–119
Shuter BJ, Post JR (1990) Climate, population viability, and the zoogeography of temperate fishes. Trans Am Fish Soc 119:314–336
Simon TP, Wallus R (2006) Reproductive biology and early life history of fishes in the Ohio River drainage vol. 4: Percidae – perch, pikeperch, and darters. CRC Taylor and Francis, Boca Raton
Sloss BL, Billington N, Burr BM (2004) A molecular phylogeny of the Percidae (Teleostei, Perciformes) based on mitochondrial DNA sequence. Mol Phylogenet Evol 32:545–562
Soltis DE, Morris AB, McLachlan JS, Manos PS, Soltis PS (2006) Comparative phylogeography of unglaciated eastern North America. Mol Ecol 15:4261–4293
Song CB, Near TJ, Page LM (1998) Phylogenetic relations among percid fishes as inferred from mitochondrial cytochrome b DNA sequence data. Mol Phylogenet Evol 10:343–353
Sruoga A, Butkauskas D, Rashal I (2008) Evaluation of genetic diversity of perch (Perca fluviatilis) and pikeperch (Sander lucioperca) populations from Curonian lagoon and inshore waters of the Baltic Sea. Acta Biol Univ Daugavpils 8:81–88
Stepien CA, Faber JE (1998) Population genetic structure, phylogeography, and spawning philopatry in Walleye (Stizostedion vitreum) from mtDNA control region sequences. Mol Ecol 7:1757–1769
Stepien CA, Dillon AK, Chandler MD (1998) Genetic identity, phylogeography, and systematics of ruffe Gymnocephalus in the North American Great Lakes and Eurasia. J Great Lakes Res 24:361–378
Stepien CA, Taylor CD, Einhouse DW (2004) An analysis of genetic risk to a native spawning stock of walleye Sander vitreus (Stizostedion vitreum) due to stocking in Cattaraugus Creek. In: Barry TP, Malison JA (eds) Proceedings of Percis III: the third international percid fish symposium, University of Wisconsin Sea Grant Institute, Madison, pp 93–94
Stepien CA, Brown JE, Neilson ME, Tumeo MA (2005) Genetic diversity of invasive species in the Great Lakes versus their Eurasian source populations: insights for risk analysis. Risk Anal 25:1043–1060
Stepien CA, Murphy DJ, Strange RM (2007) Broad- to fine-scale population genetic patterning in the smallmouth bass Micropterus dolomieu across the Laurentian Great Lakes and beyond: an interplay of behaviour and geography. Mol Ecol 16:1605–1624
Stepien CA, Murphy DJ, Lohner RN, Sepulveda–Villet OJ, Haponski AE (2009) Signatures of vicariance, postglacial dispersal, and spawning philopatry: population genetics and biogeography of the walleye Sander vitreus. Mol Ecol 18:3411–3428
Stepien CA, Murphy DJ, Lohner RN, Haponski AE, Sepulveda–Villet OJ (2010) Status and delineation of walleye (Sander vitreus) genetic stock structure across the Great Lakes. In: Roseman E, Kocovsky P, Vandergoot C (eds) Status of walleye in the Great Lakes: proceedings of the 2006 symposium. Great Lakes Fishery Commission technical report 69, Ann Arbor, pp 189–223
Stepien CA, Banda JA, Murphy DJ, Haponski AE (2012) Temporal and spatial genetic consistency of walleye (Sander vitreus) spawning groups. Trans Am Fish Soc 141:660–672
Stone FL (1948) A study of the taxonomy of the blue and yellow pikeperches (Stizostedion) of Lake Erie and Lake Ontario. Unpublished PhD dissertation, University of Rochester, Rochester
Stott W, Ebener MP, Mohr L, Hartman T, Johnson J, Roseman EF (2013) Spatial and temporal genetic diversity of lake whitefish (Coregonus clupeaformis (Mitchill)) from Lake Huron and Lake Erie. Adv Limnol 64:205–222
Strange RM, Stepien CA (2007) Genetic divergence and connectivity among river and reef spawning groups of walleye (Sander vitreus) in Lake Erie. Can J Fish Aquat Sci 64:437–448
Sullivan TJ, Stepien CA (2014) Genetic diversity and divergence of yellow perch spawning populations across the Huron–Erie Corridor, from Lake Huron through western Lake Erie. J Great Lakes Res 40:101–109
Sullivan TJ, Stepien CA (2015) Temporal population genetic structure of yellow perch spawning groups in the lower Great Lakes. Trans Am Fish Soc 144:211–226
Teller JT, Mahnic P (1988) History of sedimentation in the northwestern Lake Superior basin and its relation to Lake Agassiz overflow. Can J Earth Sci 25:1660–1673
Timmerman AJ (1995) Walleye assessment and enhancement projects in the middle Grand River watershed 1987–1995. Ontario Ministry of Natural Resources, Cambridge District, Guelph
Todd TN, Hatcher CO (1993) Genetic variability and glacial origins of yellow perch (Perca flavescens) in North America. Can J Fish Aquat Sci 50:1828–1834
Trautman MB (1981) The fishes of Ohio. Ohio State University Press, Columbus
Truemper HA, Lauer TE (2005) Gape limitation and piscine prey size-selection by yellow perch in the extreme southern area of Lake Michigan, with emphasis on two exotic prey items. J Fish Biol 66:135–149
Turgeon J, Bernatchez L (2001) Mitochondrial DNA phylogeography of lake cisco (Coregonus artedi): evidence supporting extensive secondary contacts between two glacial races. Mol Ecol 10:987–1001
Underhill JC (1986) The fish fauna of the Laurentian Great Lakes, the St. Lawrence lowlands, Newfoundland, and Labrador. In: Hocutt CH, Wiley EO (eds) The zoogeography of North American freshwater fishes. Wiley, New York, pp 105–136
USFWS/GLFC (United States Fish and Wildlife Service/Great Lakes Fishery Commission) (2010) Great Lakes Fish Stocking database. U.S. Fish and Wildlife Service, Region 3 Fisheries Program, and Great Lakes Fishery Commission. Available at http://www.glfc.org/fishstocking/
Vandewoestijne S, Schtickzelle N, Baguette M (2008) Positive correlation between genetic diversity and fitness in a large, well-connected metapopulation. BMC Biol 6:46
Walter RP, Cena CJ, Morgan GE, Heath DD (2012) Historical and anthropogenic factors affecting the population genetic structure of Ontario’s inland lake populations of walleye (Sander vitreus). J Hered 103:831–841
Wang HY, Rutherford ES, Cook HA, Einhouse DW, Haas RC, Johnson TB, Kenyon R, Locke B, Turner MW (2007) Movement of walleye in Lakes Erie and St. Clair inferred from tag return and fisheries data. Trans Am Fish Soc 136:539–551
Weir BS, Cockerham CC (1984) Estimating F–statistics for the analysis of population structure. Evolution 38:1358–1370
Wilson CC, Hebert PD (1996) Phylogeographic origins of lake trout (Salvelinus namaycush) in eastern North America. Can J Fish Aquat Sci 53:2764–2775
Wirth T, Saint-Laurent R, Bernatchez L (1999) Isolation and characterization of microsatellite loci in the walleye (Stizostedion vitreum), and cross–species amplification within the family Percidae. Mol Ecol 8:1960–1962
Wolfert DR, Van Meter HD (1978) Movements of walleyes tagged in eastern Lake Erie. N Y Fish Game J 25:16–22
WTG (Walleye Task Group of the Lake Erie Committee, Great Lakes Fishery Commission) (2014) Report for 2013 by the Lake Erie walleye Task Group. Great Lakes Fishery Commission Ann Arbor. Available at http://www.glfc.org/lakecom/lec/WTG_docs/annual_reports/WTG_report_2014.pdf
YPTG (Yellow Perch Task Group of the Lake Erie Committee, Great Lakes Fishery Commission) (2014) Report of the Lake Erie yellow perch task group. Great Lakes Fishery Commission Ann Arbor. Available at http://www.glfc.org/lakecom/lec/YPTG_docs/annual_reports/YPTG_report_2014.pdf
Yu C, Ferraro D, Ramaswamy S, Schmitz MH, Schaefer WF, Gibson DT (2008) Purification and properties of sandercyanin, a blue protein secreted in the mucus of blue forms of walleye, Sander vitreus. Environ Biol Fish 82:51–58
Zhao Y, Shuter BJ, Jackson DA (2008) Life history variation parallels phylogeographical patterns in North American walleye (Sander vitreus) populations. Can J Fish Aquat Sci 65:198–211
Zhao Y, Jones ML, Shuter BJ, Roseman EF (2009) A biophysical model of Lake Erie walleye (Sander vitreus) explains interannual variations in recruitment. Can J Fish Aquat Sci 66:114–125
Acknowledgements
This is publication #2015-07 from the University of Toledo’s Lake Erie Research Center. We thank Timothy Sullivan, who completed his master’s degree in 2013 from our Great Lakes Genetics Laboratory under CAS for supplying his latest results. Support for our research reported here came from the National Science Foundation NSF GK-12 DGE#0742395, the USEPA #CR-83281401-0, NOAA Ohio Sea Grant R/LR-013, and USDA ARS 3655-31000-020-00D funded to CAS. We greatly appreciate samples supplied by the USGS (Bruce Manny, Patrick Kocovsky, Wendylee Stott, Edward Roseman, Jeffrey Williamson), Ohio DNR (Kevin Kayle, Cary Knight, Roger Knight, Matthew Turner, Jeffrey Tyson, Christopher Vandergoot), Michigan DNR (David Clapp, Robert Haas, Michael Thomas), New York Department of Environmental Conservation (Brian Beckwith, Donald Einhouse), Pennsylvania Fish and Boat Commission (Roger Kenyon), Ontario Ministry of Natural Resources (Christopher Wilson, Timothy Johnson), and from various colleagues including Louis Bernatchez, Neil Billington, Eric Hallerman, Wolfgang Jansen, Brandon Kulik, Christine Mayer, Ellen Marsden, Douglas Nelson, Alex Parker, Webb Pearsall, Lars Rudstam, Wayne Schaefer, Roy Stein, and Matthew White. The manuscript benefitted from careful review by Patrick Kocovsky and Edward Roseman.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Stepien, C.A., Sepulveda-Villet, O.J., Haponski, A.E. (2015). Comparative Genetic Diversity, Population Structure, and Adaptations of Walleye and Yellow Perch Across North America. In: Kestemont, P., Dabrowski, K., Summerfelt, R. (eds) Biology and Culture of Percid Fishes. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7227-3_25
Download citation
DOI: https://doi.org/10.1007/978-94-017-7227-3_25
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7226-6
Online ISBN: 978-94-017-7227-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)