Abstract
Coastal embayments have been and will continue to be constructed along the northwest shoreline of Lake Ontario to restore and create warmwater fish habitat. However, very little is known about the biological connections among embayments. Using otolith microchemistry on pumpkinseed, largemouth bass and yellow perch collected from three constructed embayments in 2006–2009, we confirm that these three species of fish each exist in a metapopulation. We find that juvenile pumpkinseed, largemouth bass and yellow perch occupy embayments different from their natal habitat after their first winter, and for at least pumpkinseed, continue to move among embayments after their second winter. We hypothesize that these fishes move among embayments after haphazardly dispersing from their overwintering habitat to the littoral zone each spring. Habitat restoration and remediation efforts in coastal Great Lakes habitats should take a system-based management approach that considers the spatial proximity of embayments, and attempts to create or preserve connected networks.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
The Laurentian Great Lakes primarily support a cold and coolwater fishery but maintain a warmwater fishery in wetlands and coastal embayments along sheltered areas of the shoreline. Wetlands and coastal embayments are productive areas; over 75% of the fish species in the Great Lakes use them for at least one stage of their life cycle (Stephenson 1990; Jude and Pappas 1992; Trebitz et al. 2005; Meixler et al. 2005; Mills et al. 2005), and they have higher total phosphorous, chlorophyll-a concentrations and zooplankton densities than unsheltered nearshore habitats (Hall et al. 2003). Unfortunately, destruction of coastal fish habitats in North America has occurred at an unprecedented rate throughout the last century (Whillans 1982; Quigley and Harper 2006a, b); approximately one seventh (20 million hectares) of wetlands in Canada have been lost (Rubec 1994) and almost 100% of the original coastal wetlands and embayments near urban centers are now gone (Whillans 1982).
Along the northwest shoreline of Lake Ontario (hereafter the Lake) small coastal embayments have been (and likely will continue to be) constructed to meet a Canadian legal requirement to replace warmwater fish habitats lost from urbanization. Especially for warmwater fish, these coastal embayments may allow the formation of fish metapopulations because thermal differences between the embayment and the adjacent lake may preclude migration between embayments within the summer (when large thermal gradients exist) but not during the fall through spring months when smaller thermal gradients exist (Murphy et al. 2011). The amount of exchange and the degree of subpopulation integrity would depend on the degree of phylopatry for the species: centrarchids especially are known to be highly phylopatric (Ridgway et al. 1991; McCairns and Fox 2004; Bartlett et al. 2010), so the possibility of extinctions of subpopulations could exist. In the remainder of the paper we refer to this population structure as a metapopulation, through some may feel that the population is merely a single population with substructure.
The biological linkages among coastal embayments are uncertain, perhaps because of the difficulty associated with tracking fishes in a large lake. However, a few recent studies suggest that fish move among embayments: Brazner et al. (2004) use otolith microchemistry (explained below) to suggest that adult yellow perch captured from a number of different wetlands in Lake Superior were hatched from a common source or that they represent a metapopulation originating from several sources; Murphy et al. (submitted) provide indirect evidence of a pumpkinseed metapopulation by showing that embayments that are predicted to be too cold to produce age-0 pumpkinseed are occupied by age ≥1 pumpkinseed. Both of these studies suggest that coastal embayment habitats are biologically linked but direct evidence is required to confirm embayment connectivity and, if possible, to identify source embayments that are especially valuable. If metapopulations exist among embayments an area-based habitat management plan that considers habitat linkages among embayments should be employed because metapopulations allow the regional fish population to persistent in the face of independent local extinctions of its subpopulations (Pulliam 1988; Hanski 1991; Dias 1996; Gonzalez et al. 1998).
The relatively recent development of methods to measure otolith microchemistry have made it possible to infer lifetime fish movements without extensive tagging or tracking studies. Otoliths create an excellent natural tag because they grow continuously, are never reabsorbed and incorporate ambient elemental conditions in the water, although not necessarily in direct proportion to environmental concentrations (Campana and Neilson 1985; Campana 1999; Hamer and Jenkins 2007). The trace elements that become incorporated into otoliths are collectively known as the “elemental fingerprint” of a fish. If elemental fingerprints are sufficiently distinct in different locations, then movement patterns among these locations can be inferred from changes in the composition of the layers of the otolith. For example, the elemental fingerprint can be used to indicate the age when anadromous fishes move into water of different salinity (Secor et al. 1995; Crook et al. 2006), or used to “match” an age ≥1 fish to its nursery habitat using the elemental fingerprint in the age-0 region of the otolith (Brazner et al 2004; Barbee and Swearer 2007; Schaffler and Winkelman 2008; Standish et al. 2008; Zeigler and Whitledge 2010, 2011).
In this work, we use microelemental analysis to investigate the connectivity among embayment populations of two warmwater fish species, pumpkinseed (Lepomis gibbosus) and largemouth bass (Micropterus salmoides), and a coolwater fish, yellow perch (Perca flavescens) in the Toronto region of Lake Ontario. We seek direct evidence of movements among embayments by these three species, and we attempt to determine the ages at which these movements occur.
Methods
Study sites
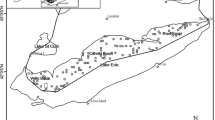
Along the northwest shoreline of Lake Ontario, in the city of Toronto, there are at least 25 river mouths and embayments that could be part of one or more metapopulations. The majority of embayments along the shoreline are located in the Toronto Harbour in Tommy Thompson Park and the Toronto Islands (Fig. 1). Tommy Thompson Park is a 5-km peninsula created by lake-infilling with uncontaminated construction refuse and riprap. Lake-infilling began in the 1950s and is ongoing. The Toronto Islands are a 230 ha natural land feature that has been beneficially modified by connecting isolated ponds to warmwater canals and improving aquatic vegetation. For this study, we selected two embayments within Tommy Thompson Park, Embayment C and Cell 2, and one within the Toronto Island, Trout Pond (Fig. 1). These three embayments were selected for this study because they were the only locations along the Toronto shoreline where electrofishing and seining had produced fish samples large enough to allow the necessary statistical comparisons.
Species selection and collections
Samples of three species of fish from two thermal guilds were sufficiently large for this study: the warmwater fishes pumpkinseed and largemouth bass, and the coolwater fish yellow perch. All three species are commonly found in the coastal embayments of the Great Lakes and are popular recreational fishing species.
We captured pumpkinseed, largemouth bass and yellow perch from Trout Pond, Embayment C and Cell 2 from 2006 to 2009 (Fig. 2). Fish were captured using a combination of seining and boat electrofishing. Seining was performed from the shore with a 22 m × 1 m bag seine (5 × 5 mm mesh). The embayments were seined approximately weekly between early-May and early-October in 2006 and early-May and late November in 2007 and in 2008. We used boat electrofishing in July 2009. Boat electrofishing transects ran parallel to the shore, in less than 3 m of water and lasted 1000 s. All captured fish were immediately bagged and preserved on ice until transported to a −20°C freezer. Thawed fish were weighed to the nearest 0.01 g and their total length measured to the nearest millimeter in the laboratory.
Otolith preparation
We prepared one sagittal otolith from each fish for microelemental analysis. Debris and connective tissues were removed from the otoliths, and they were then mounted on an acetate sheet with cyanoacrylate glue. The mounted otolith was ground with 30 μm lapping film (3M Company®), until the core was visible under a compound light microscope, and then polished with 3 μm lapping film. Once polishing was complete the excess acetate was removed, and the otolith on its acetate base was glued to a glass slide.
LA-ICP-MS analysis
The prepared otoliths were analyzed using an inductively coupled plasma mass-spectrometer (Thermo Elemental X7; Thermo Fisher Scientific Inc., Waltham, Mass.) coupled with a Continuum® Surelite® solid-state Nd:Yttrium Aluminum Garnet laser (wavelength = 266 nm, maximum power = 40 mJ, pulse rate = 20 Hz, primary beam width = 6 mm; Continuum Inc., Santa Clara, Calif.). Given the small size of the otoliths, the diameter of the laser ablation spot was set at 34 μm, the power set at 0.02 mJ/pulse, and the rate of travel at 5 μm/s. Element concentrations were read along a single linear transect, from the core of the otolith to the edge. We removed surface contamination from the otolith by first passing the laser at 20x the analytical speed (100 μm/s) over the intended element sampling path. In order to isolate the age-0 region from the age-1 or older regions of the otolith, the core and any annuli were visually identified before the transect began, and the time was recorded when the laser passed over these areas so that the composition of the different age bands of the otolith could be distinguished. To ensure that the site of collection of YOY fish corresponded to the region of the otolith we examined, we only used the outer 60 μm (12 s of ablation times the speed of the laser travel, 5 μm/s) of the otoliths to describe elemental fingerprints. To ensure fair comparisons with YOY fish, for fish age ≥1 only the 60 μm inside each annulus and inside the otolith edge are used to establish the elemental fingerprints.
We measured the concentrations of 16 trace elements - lithium (7Li), magnesium (25Mg), manganese (55Mn), iron (57Fe), nickel (60Ni), copper (65Cu), zinc (66Zn), rubidium (85Rb), strontium (86Sr), yttrium (89Y), cadmium (114Cd), tin (120Sn), barium (138Ba), cerium (140Ce), lead (208Pb) and bismuth (209Bi) - from our sample of sagittal otoliths. A glass reference standard (NIST 610) was analyzed twice before and twice after each sample of ≤20 otoliths, which allowed for quantification and correction of instrument drift and ablation yield. The argon carrier gas (i.e., background) was analyzed for 60 s before the elemental concentrations from each otolith were measured, allowing the limits of detection to be calculated for individual otoliths (Table 1). When a location on the otolith had element concentrations below the detection limits we replaced the missing values by multiplying the detection limits of the element by a random number uniformly distributed between 0.00 and 1.00 (Hand et al. 2008). If the concentration of an element was below detection limits for more than 1/3 of the 60 μm transect in more than 50% of the YOY otoliths, we removed that element from further analysis. Given that CaCO3 comprises nearly 100% of the otolith, we corrected for ablation-yield differences by normalizing element concentrations using calcium (measured as 43Ca) as an internal standard.
Data analysis
We used multivariate analysis of variance (MANOVA) to compare the elemental fingerprints of YOY fish captured in the same location but different years. We selected YOY pumpkinseed captured from Embayment C and from Trout Pond in 2007 and 2008 for this comparison because they were the only species available in sufficient quantities from the same location in different years to draw robust statistical conclusions. We also used MANOVA to assess differences between the elemental fingerprint of the YOY fish and the natal region of the otolith in the age-1 fish captured from that site in the subsequent year. We used Levene’s test to assess the equality of variances, and we graphically assessed multivariate normality with the squared mahalanobis distance in a quantile-quantile plot. Element concentrations were log10(x) transformed to normalize error distributions, when necessary (Table 1).
To visualize differences in the elemental fingerprints of fish, we used the elements that MANOVA indicated were significantly different (Table 2) in a linear discriminant analysis to reduce the number of dimensions. For each species of fish, the age groups and embayments were all identified as separate factors in the linear discriminant analysis. Since the number of factors in our discriminant function analysis never exceeded two (i.e. nursery sites, or age categories within one site) the elemental fingerprint can only be described as a single linear discriminant score along an axis that maximizes the difference between the two centroids in multivariate space. We plotted the mean discriminant scores and the 95% confidence intervals for the elemental fingerprint of YOY fish and the natal region of age ≥1 fish or the age-1 region of age-2 fish. All statistical analysis was done in R (R Development Core Team 2009)
Detecting significant differences in scores indicates that the multivariate centre of gravity of the sample of fish from one or more locations or times differs significantly in spatial position from the centers of gravity of scores from one or more other locations. Significant differences do not mean the two samples of fish all come from different locations, only that enough of the fish in one sample are from a different location to significantly displace the multivariate centre of gravity of the sample.
We used Monte Carlo analysis to determine if the standard deviation of the discriminant scores, in addition to the mean, can also be used to characterize movements of fish. Yellow Perch were selected for the Monte Carlo analysis because they were sufficient in number for statistical comparisons and the mean discriminant scores of the age-1 fish were similar to the YOY fish from one location but their variances appeared to differ substantially. For each site, the age-1 fish were randomly selected with replacement until we had a sample equal in size to the number of fish comprising the real sample from an embayment. Then we calculated the standard deviation of the randomly selected sample. This process of selection was repeated 1000 times. To determine the probability that the observed standard deviation of age-1 fish could have generated a standard deviation value as extreme as the observed sample of YOY fish, we counted the number of times the standard deviation from the real group of YOY fish was less than the standard deviation of the randomly selected groups of age-1 fish, divided that number by the number of iterations (1000) and then multiplied by two.
Comparisons of otolith regions
The otoliths we compare come from fish from different locations, years of capture and ages. When the fish were greater than one year old, we compared more than one age band of the otolith within the fish. To ensure our comparisions (Fig. 3) are communicated clearly, we introduce the following notation:
Hypothetical results for the locations of the mean discriminant scores of the elemental fingerprints of YOY (solid lines) and the natal region of age-1 fish (dashed lines) from two sites, and lines represent the 95% confidence interval of the multivariate centre of gravity from a sample of fish from one location or time. We communicate our comparisons with the following notation: Year of capture Age of fish at capture otolith age band, site of collection. The comparisons within each panel assume equal numbers of fish, so that the width of any confidence interval would not be affected by differences in sample size. Because scores on only one axis are being analyzed the confidence interval of the centre of gravity reduces to a horizontal line. a YOY fish in the two sites cannot be discriminated; b Juvenile fish return to their natal habitat; c YOY fish can be discriminated but mixing among juveniles is high and has broadened the fingerprints, homogenizing the populations across the embayments; d YOY from Site A are not represented among the juvenile cohort in the following year; e Juveniles move among embayments and are produced in a location for which we lack YOY elemental fingerprints
To assess the connectivity of fish subpopulations, we first had to confirm that embayments had elemental conditions that were sufficiently distinct so that fish subpopulations could be resolved. If YOY fish in different embayments from the same year cannot be discriminated (Fig. 3a), then the habitats older fish occupied in earlier years cannot be inferred. If the mean elemental fingerprints of the YOY fish were different, we proceeded to compare the mean natal elemental fingerprints of age ≥1 fish with those of the YOY fish.
We determined whether age ≥1 fish returned to their natal habitat after their first winter by comparing their mean elemental fingerprints from the age-0 region of the age ≥1 otolith with the mean elemental fingerprints of YOY fish captured from the same site, and in the same year as the older fish were hatched. If most fish return to their natal habitat after the winter, then the older fish will have similar mean elemental fingerprints as the YOY fish (Fig. 3b). If older fish mix as a metapopulation, then the mean elemental fingerprints of older fish in different sites will be similar mixtures of fingerprints from many embayments (Fig. 3c).
If the mean natal elemental fingerprints from older fishes were not represented in the YOY fingerprints, we assumed that the YOY from the unrepresented location (Site A) either did not survive their first winter or moved to another habitat (Fig. 3d). If older fish were produced in a site for which we lack YOY elemental fingerprints, then the mean natal elemental fingerprint of older fish will be distinct from the mean YOY elemental fingerprint for all locations (Fig. 3e).
We also determined if the post-juvenile age-2 pumpkinseed captured in 2009 occupied a different embayment in 2008, when they were age 1. To do this, we compared the mean elemental fingerprint in the age-1 region of the otolith (200921,A and 200921,B) with the mean elemental fingerprint from YOY pumpkinseed that grew up in 2008 (200800,A and 200800,B). We made similar assumptions and contrasts as described above about the movement of fish after their first winter.
Results
Pumpkinseed
Across years 2007 and 2008, MANOVA indicates the elemental fingerprint of YOY pumpkinseed differed within Embayment C (p<0.001) and within Trout Pond (p<0.001).
In 2007, the 23 YOY pumpkinseed from Embayment C and 16 YOY pumpkinseed from Trout Pond have significantly different mean elemental fingerprints (Fig. 4). The elements that are important for distinguishing the YOY subpopulations are Mg, Fe, Sr, log10(Sn) and log10(Ba) (Table 2). Since the mean elemental fingerprints of the YOY pumpkinseed hatched in Embayment C and Trout Pond are significantly different, we continued with our analysis to determine if older pumpkinseed moved among embayments.
Canonical variate plot based on linear discriminant analysis using the concentrations of Mg, Fe, Sr, log10(Sn) and log10(Ba) to distinguish YOY pumpkinseed in 2007 (black shapes) from the natal region of age-1 fish (uncoloured shapes) captured in 2008, and the natal region of age-2 fish captured in 2009 (uncoloured shapes with crosshairs). The vertical spread of the points is only to aid the visualization of the discriminant scores along the x-axis. Fish captured from Trout Pond are squares and those captured from Embayment C are circles. Overlap of the 95% confidence intervals of the mean discriminant scores in the grey band indicates nonsignificant differences between the mean canonical variate scores. Age-1 and age-2 fish are distinguished in the plot but are grouped together in calculating the confidence limits. Age-1 and 2 pumpkinseed captured in Embayment C appear to have been hatched in a location for which we lack an elemental fingerprint (Fig. 3e), or they have formed a hetergeneous population with pumpkinseed from Trout Pond, or from a location with a similar elemental fingerprint as Trout Pond (Fig. 3c). Any of these interpretations involves movement of individuals from their natal embayments as they age. The majority of juvenile pumpkinseed in Trout Pond appear to have hatched in Trout Pond (see Fig. 3b)
Most of the 33 age ≥1 pumpkinseed that we captured in Trout Pond appear to have hatched in Trout Pond, or another location with similar elemental conditions (Fig. 4). Most of the 36 age ≥1 pumpkinseed from Embayment C appear to have hatched from a location for which we lack an elemental fingerprint (Fig. 4) or the pumpkinseed hatched in Embayment C formed a heterogeneous group with the pumpkinseed from Trout Pond or from a location with similar elemental fingerprint as Trout Pond. The hypothetical comparisons that best describe the age ≥1 pumpkinseed from 2007/2008 after their first winter in Trout Pond is that illustrated in Fig. 3b; for Embayment C the most likely scenario is that of Fig. 3c or e.
In 2008, the 29 YOY pumpkinseed from Embayment C and 29 YOY pumpkinseed from Trout Pond have significantly different mean elemental fingerprints (Fig. 5). The elements that are important for distinguishing the YOY subpopulations are log10(Mg), Mn and Sr (Table 2). Since YOY embayment subpopulations can be distinguished, we continued with our analysis to determine if the age-2 pumpkinseed we captured in 2009 from Embayment C and Trout Pond moved from the locations they occupied in the previous year.
Canonical variate plot based on linear discriminant analysis using the concentrations of log10(Mg), log10(Mn) and log10(Sn) to distinguish YOY pumpkinseed in 2008 (black shapes) from the age-1 region of age-2 fish captured in 2009 (uncoloured shapes with crosshairs). The vertical spread of the points is to improve the visualization of the discriminant scores along the x-axis. Overlap of the 95% confidence intervals in the grey box indicates nonsignificant differences between the mean elemental fingerprints. Fish captured from Trout Pond are squares and those captured from Embayment C are circles. Age-1 pumpkinseed continue to move among embayments after their second winter (Fig. 3c)
Age-2 pumpkinseed were captured in locations different from the ones they occupied as age-1 fish. Some of the six age-2 pumpkinseed from Trout Pond appear to have moved from a location other than Trout Pond after their second winter. Their age-1 mean elemental fingerprint was significantly different from the mean elemental fingerprint of the YOY from Trout Pond. Some of the six age-2 pumpkinseed that were captured in Embayment C also appear to have occupied a location other than Embayment C as age-1 fish. Their mean age-1 elemental fingerprint was not significantly different from the mean elemental fingerprint of YOY in Trout Pond or Embayment C. The similar mean age-1 elemental fingerprints of age-2 pumpkinseed in Trout Pond and Embayment C suggest that age-1 pumpkinseed continue to move among embayments after their second winter. The hypothetical comparison that best describes the movement of age-2 pumpkinseed after their second winter in 2008\2009 is Fig. 3c.
Largemouth bass
In Cell 2, the mean elemental fingerprint of the 31 YOY largemouth bass captured in 2006 and the mean natal elemental fingerprint of the 8 age-1 largemouth bass captured in 2007 are significantly different (Fig. 6). The elements that distinguished the two groups of fish were log10(Mg), log10(Mn) and log10(Sn) (Table 2). YOY largemouth bass appear to move from their natal habitats after their first winter. The hypothetical comparision that best describes the contrasts between the YOY and age-1 largemouth bass in Cell 2 is Fig. 3e.
Canonical variate plot based on linear discriminant analysis using the concentrations of log10(Mg), log10(Mn) and log10(Sn) to distinguish YOY largemouth captured in Cell 2 in 2006 (black diamonds) from the age-0 region of age-1 bass captured in Cell 2 during 2007 (open diamonds). The vertical spread of the points is to improve the visualization of the discriminant scores along the x-axis. Overlap of the 95% confidence intervals in the grey box indicates nonsignificant differences between the mean elemental fingerprints.. Almost none of the age-1 largemouth bass in Cell 2 appear to been hatched in Cell 2 (Fig. 3e)
In 2008, the mean elemental fingerprint of the 28 YOY largemouth bass in Trout Pond and the 29 YOY largemouth bass from Embayment C are significantly different (Fig. 7). The elements that distinguished these subpopulations are log10(Mg), log10(Mn), and Ba (Table 2). Since the YOY populations could be distinguished, we continued with our analysis and determined if the age-1 largemouth bass hatched in 2008 moved to other locations after their first winter.
Canonical variate plot based on linear discriminant analysis from the concentrations of log10(Mg), log10(Mn), and Ba to distinguish YOY largemouth bass in 2008 (black shapes) from the natal region of age-1 fish (uncoloured shapes) captured in 2009. Fish captured from Trout Pond are squares and those captured from Embayment C are circles. The vertical spread of the points is to improve the visualization of the discriminant scores along the x-axis. Overlap of the 95% confidence intervals in the grey box indicate nonsignificant differences between the mean elemental fingerprint. Mixing among juvenile largemouth bass is high, the age-1 population appears to be a mixture of individuals from several locations (Fig. 3c)
Juvenile largemouth bass exist in a metapopulation. The mean natal elemental fingerprint of the 9 age-1 largemouth bass captured in Trout Pond is not significantly different from the mean elemental fingerprint of the YOY largemouth bass captured in either Trout Pond or Embayment C, which is similar to the hypothetical comparison Fig. 3c. Juvenile largemouth bass appear to move between embayments after their first winter.
Yellow perch
In 2008, the mean elemental fingerprint of the 29 YOY yellow perch from Embayment C and the 39 YOY from Trout Pond are significantly different (Fig. 8). The elements that distinguish the two subpopulations are log10(Mg), Mn, and log10(Sn) (Table 2). Since the YOY subpopulations could be distinguished, we continued with our analysis to determine if the age-1 yellow perch move to other locations after their first winter.
Canonical variate plot based on linear discriminant analysis from the concentrations of log10(Mg), Fe, and log10(Sn) of YOY yellow perch in 2008 (black shapes) and the natal region of age-1 yellow perch (uncoloured shapes) captured in 2009. Yellow perch captured from Trout Pond are squares, from Embayment C circles, and those from Cell 2 are diamonds. The vertical spread of the points is to improve the visualization of the discriminant scores along the x-axis. Overlap of the 95% confidence intervals in the grey box indicates nonsignificant differences between the mean elemental fingerprints. The age-1 yellow perch in Cell 2 and Trout Pond appear to be produced in Embayment C or in a location with similar elemental fingerprint to Embayment C (see Fig. 3d)
Age-1 yellow perch move from their natal habitats after their first summer. The mean natal elemental fingerprint of the 33 age-1 yellow perch captured in Trout Pond was significantly different from the mean elemental fingerprint of the YOY yellow perch in Trout Pond but not significantly different from the YOY in Embayment C (Fig. 8). The mean natal elemental fingerprints of the 13 age-1 yellow perch from Cell 2 and 33 from Trout Pond are not significantly different from each other but the variance among age-1 individuals in both locations is lower than the variance among YOY individuals from Embayment C (Table 3). The similar mean elemental fingerprints but lower variance suggests either the age-1 yellow perch were hatched in a location with similar elemental conditions as Embayment C but with less variability or the age-1 fish represent a subset of surviving YOY fish that experienced very similar elemental conditions and had higher overwinter survival rates. The hypothetical situation that best describes yellow perch movements after their first winter is Fig. 3e.
Discussion
Our findings suggest that age ≥1 yellow perch, largemouth bass and pumpkinseed exist in metapopulations. Given the movement of fishes among embayments, it was surprising that the elemental fingerprints among age-1 yellow perch in two embayments was less variable than for YOY yellow perch from a single embayment. Although the age-1 fish may have hatched in a less variable environment, higher rates of overwinter survival for some YOY fish may have made the age-1 population more homogenous. The surviving YOY fish may have a similar physiological condition or some microhabitat association that correlates with elemental composition, which may have increased overwinter survival by increasing growth rate, lipid reserves or modifying behavior.
A metapopulation has conservation implications among coastal embayments that would allow the regional fish population to persist despite extinctions of local subpopulations. For example, in some years cooler embayments are ecological traps that waste the reproductive effort of warmwater fish like pumpkinseed that spawn there. However, in warmer years cooler embayments produce YOY that can recruit to the adult population (Murphy et al. submitted). Without warm ‘source’ habitats the spawning pumpkinseed subpopulation in cool ‘sink’ embayments would be reduced, potentially to extinction, and would never be able to contribute YOY towards the regional adult population in warmer years. We assume there are similar advantages of a metapopulation for species other than pumpkinseed.
The metapopulation structure among embayments is likely created by the haphazard dispersal of winter fish aggregations in the spring. Yellow perch appear to remain active and continue to feed in the winter (Moffett and Hunt 1945), but tend to aggregate in deeper waters (Hasler 1945; Wang and Eckmann 1994), and centrarchid fishes in general seem to overwinter in deep, slow-moving water (Suski and Ridgeway 2009). Field observations of bluegill, a congener to pumpkinseed (Osenberg et al. 1988; Garvey et al. 2002), found they overwinter in loose aggregations and disperse in spring. Similarly, largemouth bass converge and overwinter in aggregations (Carlson 1992; Karchesky and Bennett 2004). All the embayments along the Toronto shoreline are within 40 km of one-another, and most within a 10 km strip. If there are a limited number of these overwintering locations, for instance near heated outflows (Cooke et al. 2004), fish subpopulations from different embayments might tend to congregate and mix at them. The breakup of these aggregations in the spring and the haphazard dispersal back to the littoral zone would result in the mixing of the regional fish subpopulation. Although we conclude that fish move among embayments after overwintering, we are unable to show from our data the distance fish travel between embayments. Obtaining an elemental fingerprint from more nursery locations so that the natal location of age ≥1 fishes can be classified or using acoustic telemetry to track fish movement might provide insight on the spatial scale of the metapopulation.
Although we show that fish disperse among embayments after winter, movements among embayments could also occur within the summer. Adult fishes may move among embayments if their home range size is greater than the distance that separates them. Minns (1995) estimated the home range of adult largemouth bass, yellow perch and pumpkinseed to be 34 403 m2, 9173 m2 and 9048 m2, respectively. Although it is possible that an individual fish may have a home range that includes more than one interconnected embayment (i.e. Embayment C and Cell 2), the distance between the contrasting embayments in this study (Embayment C and Trout Pond or Cell 2 and Trout Pond) is approximately 6.5 km, much greater than the size of their home range. YOY pumpkinseeds may move among embayments in their first summer if they swim offshore shortly after swim-up and return to the littoral zone several weeks later, as they do in smaller inland lakes (Rettig 1998; Garvey et al. 2002). Although it is believed that YOY largemouth bass are hatched and stay within the littoral zone for their first year of life (DeVries et al. 2009), their home range is sufficiently large to include other interconnected embayments (Hoffman and Bettoli 2005). Movement of yellow perch among embayments can take place at several stages. Newly hatched yellow perch are transported through advection to the open water (Wang and Eckmann 1994) and may select an alternative embayment when they return inshore in early summer. Yellow perch may also move among embayments during their first summer or after they travel to the limnetic zone and return to the littoral zone in late-summer (Whiteside et al. 1985; Stephenson 1990).
Fish habitat management for these metapopulations require a systems-based approach that considers the linkages among coastal embayments. Connections among embayments should be maintained because the linkages help to sustain the regional population abundance and lower the probability of local extinction of fish subpopulations. Concentrating fish habitat rehabilitation and construction in areas with multiple embayments, and preventing the destruction of nearby habitats are ways of maintaining and improving habitat connectedness.
References
Barbee NC, Swearer SE (2007) Characterizing natal source population signatures in the diadromous fish Galaxias maculatus, using embryonic otolith chemistry. Mar Ecol Prog Ser 343:273–282. doi:10.3354/meps06886
Bartlett JA, Ward MP, Landsman SJ, Epifanio JM (2010) Nest-site fidelity in parental male bluegill Lepomis macrochirus: spatial patterns and the influence of prior mating success. J Fish Biol 77(4):890–906. doi:10.1111/j.1095-8649.2010.02724.x
Brazner JC, Campana SE, Tanner DK, Schram ST (2004) Reconstructing habitat use and wetland nursery origin of yellow perch from Lake Superior using otolith elemental analysis. J Great Lakes Res 30(4):492–507. doi:10.1016/S0380-1330(04)70365-2
Campana SE (1999) Chemistry and composition of fish otoliths: pathways, mechanisms and applications. Mar Ecol Prog Ser 188:263–297. doi:10.3354/meps188263
Campana SE, Neilson JD (1985) Microstructure of fish otoliths. Can J Fish Aquat Sci 42(5):1014–1032. doi:10.1139/f85-127
Carlson D (1992) Importance of wintering refugia to the largemouth bass fishery in the Hudson River Estuary. J Freshw Ecol 7(2):173–180
Cooke SJ, Bunt CM, Schreer JF (2004) Understanding fish behavior, distribution, and survival in thermal effluents using fixed telemetry arrays: a case study of smallmouth bass in a discharge canal during winter. Environ Manag 33(1):140–150. doi:10.1007/s00267-003-0175-2
Crook DA, MacDonald JI, O’Connor JP, Barry B (2006) Use of otolith chemistry to examine patterns of diadromy in the threatened Australian grayling Prototroctes maraena. J Fish Biol 69(5):1330–1344. doi:10.1111/j.1095-8649.2006.01191.x
DeVries DR, Garvey JE, Wright RA (2009) In: Cooke SJ, Philipp DP (eds) Early life history and recruitment. Wiley-Blackwell, West Sussex, pp 105–133
Dias PC (1996) Sources and sinks in population biology. Trends Ecol Evol. doi:10.1016/0169-5347(96)10037-9
Garvey JE, Herra TP, Legget WC (2002) Protracted reproduction in sunfish: the temporal dimensions in fish recruitment revisited. Ecol Appl 12(1):194–205. doi:10.1890/1051-0761(2002)012[0194:PRISTT]2.0.CO;2
Gonzalez A, Lawton JH, Gilbert FS, Blackburn TM, Evans-Freke I (1998) Metapopulation dynamics, abundance, and distribution in a microecosystem. Science 281(5385):2045–2047. doi:10.1126/science.281.5385.2045
Hall SR, Pauliukonis NK, Mills EL, Rudstam LG, Schneider CP, Lary SJ, Arrhenius F (2003) A comparison of total phosphorus, chlorophyll a, and zooplankton in embayment, nearshore, and offshore habitats in Lake Ontario. J Great Lakes Res 29(1):54–69
Hamer PA, Jenkins GP (2007) Comparison of spatial variation in otolith chemistry of two fish species and relationships with water chemistry and otolith growth. J Fish Biol 71(4):1035–1055. doi:10.1111/j.1095-8649.2007.01570.x
Hand CP, Ludsin SA, Fryer BJ, Marsden JE (2008) Statolith microchemistry as a technique for discriminating among Great Lakes sea lamprey (Petromyzon marinus) spawning tributaries. Can J Fish Aquat Sci 65(6):1153–1164. doi:10.1139/F08-045
Hanski I (1991) Single-species metapopulation dynamics: concepts, models and observations. Biol J Linn Soc 41(1–2):17–38. doi:10.1111/j.1095-8312.1991.tb00548.x
Hasler AD (1945) Observations on the winter perch populations of Lake Mendota. Ecology 26(1):90–94, Available from http://www.jstor.org/stable/1931918 [accessed 4 of September 2005]
Hoffman KJ, Bettoli PW (2005) Growth, dispersal, mortality, and contribution of largemouth bass stocked into Chickamauga Lake, Tennessee. N Am J Fish Manage 25(4):1518–1527. doi:10.1577/M04-164.1
Jude DJ, Pappas J (1992) Fish utilization of Great Lakes coastal wetlands. J Great Lakes Res 18(4):651–672. doi:10.1016/S0380-1330(92)71328-8
Karchesky CM, Bennett DH (2004) Winter habitat use by adult largemouth bass in the Pend Oreille River, Idaho. N Am J Fish Manage 24(2):544–585. doi:10.1577/M02-175.1
McCairns SRJ, Fox MG (2004) Habitat and home range fidelity in a trophically dimorphic pumpkinseed sunfish (Lepomis gibooosus) population. Oecologia 140(2):271–279. doi:10.1007/s00442-004-1580-9
Meixler MS, Arend KK, Bain MB (2005) Fish community support in wetlands within protected embayments of Lake Ontario. J Great Lakes Res 31(suppl 1):188–196. doi:10.1016/S0380-1330(05)70298-7
Mills EL, Casselman JM, Dermott R, Fitzsimons JD, Gal G, Holeck KT, Hoyle JA, Johannsson OE, Lantry BF, Makarewicz JC, Millard ES, Munawar IF, Munawar M, O’Gorman R, Owens RW, Rudstam LG, Schaner T, Stewart TJ (2005) A synthesis of ecological and fish community changes in Lake Ontario, 1970–2000. Great Lakes Fish Comm Tech Rep 67
Minns CK (1995) Allometry of home range size in lake and river fishes. Can J Fish Aquat Sci 52(7):1499–1508
Moffett JW, Hunt BP (1945) Winter feeding habitat of bluegills, Lepomis Macrochirus Rafinesque, and yellow perch, Perca flavescens (Mitchill) in Ceader Lake, Washtenaw County. Michigan T Am Fish Soc 73(1):231–242. doi:10.1577/1548-8659(1943)73[231:WFHOBL]2.0.CO;2
Murphy SC, Collins NC, Doka SE (2011) Thermal habitat characteristics for warmwater fishes in coastal embayments of Lake Ontario. J Great Lakes Res 37(1):111–123. doi:10.1016/j.jglr.2010.12.005
Osenberg CW, Werner EE, Mittelback GG, Hall DJ (1988) Growth patterns in bluegill (Lepomis macrochirus) and pumpknseed (L gibbosus) sunfish: environmental variation and the importance of ontogenetic niche shifts. Can J Fish Aquat Sci 45(1):17–26
Pulliam RH (1988) Sources, sinks, and population regulation. Amer Nat 132(5):652–661, Available from http://www.jstor.org/stable/2461927 [accessed 15 March 2011]
Quigley JT, Harper DJ (2006a) Compliance with Canada’s Fisheries Act: A field audit of habitat compensation projects. Environ Manag 37(3):335–350. doi:10.1007/s00267-004-0114-x
Quigley JT, Harper DJ (2006b) Effectiveness of fish habitat compensation in Canada in achieving no net loss. Environ Manag 37(3):351–366. doi:10.1007/s00267-004-0263-y
R Development Core Team (2009) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Rettig JE (1998) Variation in species composition of the larval assemblage in four southwest Michigan lakes: Using allozyme analysis to identify larval sunfish. T Am Fish Soc 127(4):661–668. doi:10.1577/1548-8659(1998)127<0661:VISCOT>2.0.CO;2
Ridgway MS, MacLean JA, MacLeod JC (1991) Nest-site fidelity in a centrarchid fish, the smallmouth bass (Micropterus dolomieui). Can J Zool 69(12):3103–3105. doi:10.1139/z91-436
Rubec CDA (1994) Canada’s federal policy on wetland conservation: a global model. In: Mitsch WJ (ed) Global wetlands: old world and new. Elsevier, New York, pp 909–917
Schaffler JJ, Winkelman DL (2008) Temporal and spatial variability in otolith trace-element signatures of juvenile striped bass from spawning location in Lake Texoma, Oklahoma-Texas. T Am Fish Soc 137(3):818–829. doi:10.1577/T06-023.1
Secor DH, Henderson-Arzapalo A, Piccoli PM (1995) Can otolith microchemistry chart patterns of migration and habitat utilization in anadromous fishes. J Exp Mar Biol Ecol 192(1):15–33. doi:10.1016/0022-0981(95)00054-U
Standish JD, Sheehy M, Warner RR (2008) Use of otolith natal elemental signatures as natural tags to evaluate connectivity among open-coast fish populations. Mar Ecol Prog Ser 356:259–268. doi:10.3354/meps07244
Stephenson TD (1990) Fish reproductive utilization of coastal marshes of Lake Ontario near Toronto. J Great Lakes Res 16(1):71–81. doi:10.1016/S0380-1330(90)71399-8
Suski CD, Ridgeway MS (2009) Winter biology of centrarchid fishes. In: Cooke SJ, Philipp DP (eds) Centrarchid fishes. Wiley-Blackwell, West Sussex, pp 264–292
Trebitz AS, Morrice JA, Taylor DL, Anderson RL, West CW, Kelly JR (2005) Hydromorphic determinants of aquatic habitat variability in Lake Superior coastal wetlands. Wetlands 25(3):505–519. doi:10.1672/0277-5212(2005)025[0505:HDOAHV]2.0.CO;2
Wang N, Eckmann R (1994) Distribution of perch (Perca fluviatilis L.) during their first year of life in Lake Constance. Hydrobiologia 277(3):135–143. doi:10.1007/BF00007295
Whillans TH (1982) Changes in marsh area along the Canadian shore of Lake Ontario. J Great Lakes Res 8(3):570–577. doi:10.1016/S0380-1330(82)71994-X
Whiteside MC, Swindoll CM, Doolittle WL (1985) Factors affecting the early life history of yellow perch, Perca flavescens. Environ Biol Fishes 12(1):47–56. doi:10.1007/BF00007709
Zeigler JM, Whitledge GW (2010) Assessment of otolith chemistry for identifying source environment of fishes in the lower Illinois River, Illinois. Hydrobiologia 638(1):109–119. doi:10.1007/s10750-009-0033-1
Zeigler JM, Whitledge GW (2011) Otolith trace element and stable isotopic compositions differentiate fishes from the Middle Mississipi River, its tributaries, and floodplain lakes. Hydrobiologia 661(1):289–302. doi:10.1007/s10750-010-0538-7
Acknowledgements
The Toronto and Region Conservation Authority (TRCA) and the Department of Fisheries and Oceans through the Federal Great Lakes Action Plan provided funding for this research. Zhaoping Yang at the Great Lakes Institute for Environmental Research at the University of Windsor provided technical expertise with the LA-ICP-MS. G. MacPherson and R. Portiss of TRCA and the Department of Fisheries and Ocean’s Great Lakes Laboratory for Fisheries and Aquatic Sciences provided training, field facilities, equipment, logistical support, and support personnel. We also thank M. Correa for field expertise, R. Brown for map production, K. Rudmik and D. Bennett for field assistance, and T. Chaugule, S. Cheung, S. Khalid, A. Rooke, S. Stanescu and J. Torres for assistance in the laboratory. SCM was supported by the National Sciences and Engineering Research Council of Canada, an Ontario Graduate Scholarship and the Federal Great Lakes Action Plan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murphy, S., Collins, N.C., Doka, S.E. et al. Evidence of yellow perch, largemouth bass and pumpkinseed metapopulations in coastal embayments of Lake Ontario. Environ Biol Fish 95, 213–226 (2012). https://doi.org/10.1007/s10641-012-9978-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-012-9978-4