Abstract
Subterranean fauna can be divided into two broad groups – stygofauna are aquatic and occur in groundwater, while troglofauna are air-breathing and occur in the unsaturated zone from depths of a metre or so below the ground surface down to the water table. Defining exactly which species are covered by the term subterranean fauna is quite complex, because of the different life histories of many vertebrate and invertebrate species, and the habitat differences between caves and the much more extensive, but less studied underground matrix outside caves. However, a useful starting point for understanding the general characteristics of subterranean species is provided by various schemes that categorise species according to their dependence on the underground environment. These schemes are discussed in detail by Sket (2008), but, in summary, species occurring only in the aphotic zone of caves or deep underground are classified as troglobites or stygobites (Table 1). The terms troglophiles and stygophiles are applied to species found in parts of caves where there is some penetration of light or to species that use surface habitats for one (usually short) part of their life history. Occasionally, troglophilic or stygophilic species may have some surface populations and some wholly subterranean populations. The third category, trogloxenes and stygoxenes, is applied to primarily surface species that regularly make use of caves or underground habitats, often as a refugium during periods of adverse conditions (such as drought) in their usual surface habitat.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Introduction
Subterranean fauna can be divided into two broad groups – stygofauna are aquatic and occur in groundwater, while troglofauna are air-breathing and occur in the unsaturated zone from depths of a metre or so below the ground surface down to the water table. Defining exactly which species are covered by the term subterranean fauna is quite complex, because of the different life histories of many vertebrate and invertebrate species, and the habitat differences between caves and the much more extensive, but less studied underground matrix outside caves. However, a useful starting point for understanding the general characteristics of subterranean species is provided by various schemes that categorise species according to their dependence on the underground environment. These schemes are discussed in detail by Sket (2008), but, in summary, species occurring only in the aphotic zone of caves or deep underground are classified as troglobites or stygobites (Table 1). The terms troglophiles and stygophiles are applied to species found in parts of caves where there is some penetration of light or to species that use surface habitats for one (usually short) part of their life history. Occasionally, troglophilic or stygophilic species may have some surface populations and some wholly subterranean populations. The third category, trogloxenes and stygoxenes, is applied to primarily surface species that regularly make use of caves or underground habitats, often as a refugium during periods of adverse conditions (such as drought) in their usual surface habitat.
In this chapter, the terms subterranean fauna, troglofauna and stygofauna refer principally to troglobites, stygobites, troglophiles and stygophiles (Table 1). Species in these categories are clearly dependent on caves and other subterranean habitats for their survival. Trogloxenes and stygoxenes are mostly excluded from coverage, although there are occasions and situations in which subterranean habitat is important for these species. Bats and cave-nesting birds are excluded from consideration, largely because this chapter deals with what is happening in the broad landscape outside caves. Aquatic invertebrates found in the bed of streams are also not treated as subterranean for the purpose of this chapter, although, globally, much study of stygofauna is conducted in stream beds (Hancock et al. 2005; Dole-Olivier et al. 2009). Similarly, there is little consideration of fish and reptiles, because nearly all subterranean fauna species in Australia are invertebrates, although some stygofauna fish and troglofaunal reptiles do occur (e.g. Aplin 1998; Larson et al. 2013).
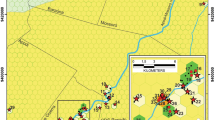
The major difference between this chapter and most accounts of subterranean fauna is the focus on underground habitats other than caves. Globally, most subterranean fauna studies deal with caves. However, the arid zone of Australia has very few large caves; in fact, there are arguably only two large cave systems in the arid zone. These are on the Nullarbor Plain (Webb and James 2006) and around Camooweal, north-west of Mount Isa (Grimes 1988; Eberhard 2003) (Fig. 1). Despite their large size, both systems have relatively depauperate subterranean faunas (Richards 1971; Eberhard 2003) which is perhaps unsurprising given the lack of wider occurrence of caves. Other cave systems on the edge of the arid zone, such as the Judbarra/Gregory karst area in the Northern Territory, are also depauperate (Moulds and Bannick 2012) (Fig. 1). Largely as a result of the paucity of cave fauna and the traditional emphasis of subterranean fauna studies on caves, it is only during the last 20 years that the richness of the Australian arid zone subterranean fauna outside caves has begun to be appreciated (Guzik et al. 2010).
This chapter provides a summary of current information on subterranean fauna in the Australian arid zone, particularly in the western half of the arid zone where most richness appears to occur. Some of the main issues in contemporary subterranean fauna research are discussed, together with issues that are relevant to the conservation of that subterranean fauna. A recent account with greater focus on the evolution of subterranean fauna in Australia is provided by Humphreys (2016).
Subterranean Fauna Habitats
While many stygofauna species occur in groundwater below stream beds and in cave streams and ponds, most of this chapter deals with stygofauna in deeper regional aquifers that extend more widely across the landscape. The habitats of troglofauna outside caves are less well documented, but in general terms they comprise the habitat between the ‘dry’ ground surface and the water table. This habitat is often referred to as the vadose zone (e.g. Halse and Pearson 2014), although the term ‘unsaturated zone’ is also apt. Juberthie (1983) was the first to pay much attention to the unsaturated zone, although his focus was on the upper part of the zone, and he applied the term milieu souterrain superficial (MSS) to shallow detrital habitats across the landscape that support troglofauna (see also Mammola et al. 2016). Culver and Pipan (2008) applied the term shallow superficial habitats (SSH) to a wider variety of situations (including near-surface stygofauna habitat) but in doing so implied that caves comprised the major deep subterranean habitat. This is not the case in the Pilbara and Yilgarn regions of Western Australia, where stygofauna and troglofauna may be found tens of metres below the surface in various types of very small spaces that are widespread across the landscape. These small spaces are mostly what Howarth (1983) termed microcaverns (<5 mm in width) or, less commonly, mesocaverns (5–500 mm) (Fig. 2). A variety of other terms are used to describe the spaces, with the names often providing some information about their genesis [e.g. interstitial spaces in alluvium, vugs (or small spaces) in weathered rock, fissures in fractured rock].
Habitats in which subterranean fauna species occur. (a) Iron ore range in the Pilbara, with deep gullies and mesocaverns in hardcap; (b) drill core through saturated calcrete; (c) schematic illustration of subterranean habitat where calcrete is present; (d) Yilgarn palaeochannel containing calcrete below ground (red sand dune in foreground); (e) drill core through mineralised iron ore formation; and (f) schematic illustration of subterranean habitat in iron ore range
The factors determining the occurrence of subterranean fauna are still in the early stages of investigation. While the size, pattern and quantity of spaces in a habitat is important for the occurrence of fauna, poor connectivity with the surface may constrain the number of species present (Halse et al. 2014), because most energy (as carbon) comes from the surface. Studies elsewhere suggest that the chemistry of the host substrate is not particularly important in most cases beyond its influence on habitat structure (Hahn and Fuchs 2009). The importance of spaces to the occurrence of subterranean fauna is most clearly documented for stygofauna, where the likelihood of species occurring is related in a positive way to the transmissivity of the aquifer (Maurice and Bloomfield 2012). At the same time, studies by Bradford et al. (2013) and others have shown there is currently poor understanding of how the finer details of habitat structure affect subterranean fauna occurrence. One example of fine-scale variation in occurrence, however, is provided by Hose et al. (2017) who showed differences in the abundance of stygofauna species in different alluvial substrates over vertical distances of less than 1 m. Such fine-scale control of distributions probably occurs in many species.
While predictions about the suitability of habitats for subterranean fauna at a fine scale tend to be unreliable, existing work enables some general observations to be made. Despite subterranean fauna occurring in a wide range of geologies in the arid zone, they tend to occur most abundantly in three broad geological units: karst (especially calcrete), alluvium and colluvium and mineralised or weathered iron formations, especially banded iron formation and channel iron deposit. They are sometimes abundant in weathered volcanics and ultramafics but mostly occur in low numbers in other geologies.
The relationship between large numbers of stygofauna and calcrete bodies in palaeochannels in the Yilgarn region of Western Australia is well documented (Humphreys 2001, 2008; Guzik et al. 2010), with up to 75 species recorded from a single calcrete body (EPA 2016a). Another well-studied relationship is the occurrence of large numbers of troglofauna species in mineralised banded iron formations and channel iron deposits of the Pilbara, with more than 120 species being recorded from sections of banded iron formation in the Hamersley Range and up to 25 species being recorded from individual small mesas in the Robe Valley (Harvey et al. 2008; G. Humphreys and M. Curran, unpublished data). It should also be noted that it is not uncommon for a site to yield high numbers of stygofauna and low numbers of troglofauna or vice versa. This is sometimes the result of different geologies in the unsaturated zone and in the underlying groundwater aquifer; in other situations the flow of water may have kept spaces open in the aquifer, whereas they have been filled by fine sediment in the unsaturated zone.
Environmental Parameters
While physical habitat structure, connectivity and the extent of surface connection are determinants of subterranean fauna occurrence across the broad landscape matrix, some of the factors critical to subterranean fauna occurrence in caves are equally important across the landscape. These include the absence of light, stable temperatures and, in the non-saturated zone, high relative humidity (Culver and Pipan 2008). Differences in relative humidity are probably a key factor distinguishing between the habitats of soil fauna and subterranean fauna in the arid zone, with subterranean habitats probably usually being very close to saturated (Fig. 3), while surface habitats in the Pilbara and Yilgarn are often quite dry as a consequence of annual pan evaporation being 3000–5000 mm.
Groundwater in much of the arid zone is saline, and salinity is another factor potentially controlling the occurrence of species in groundwater, in the same way as it does in surface systems (Pinder et al. 2005). While it has been widely thought that stygofauna in the Pilbara and Yilgarn are unlikely to occur at salinities much above seawater concentration (35,000 mg L−1 or 50,000 μS cm−1) (e.g. Watts and Humphreys 2006), there are recent records of stygofauna in the northern Yilgarn at salinities of approximately 100,000 mg L−1 (Outback Ecology 2012). Despite these records suggesting some stygofauna species are strongly salt-adapted, most species are restricted to salinities of less than 10,000 mg L−1 in the Pilbara (Halse et al. 2014) and 25,000 mg L−1 in the Yilgarn (Humphreys et al. 2009) (Fig. 4). The broader environmental tolerances of stygofauna in Australia have been reviewed by Korbel and Hose (2011).
Subterranean Fauna of the Western Shield
Sampling of stygofauna outside caves in the arid zone began only in the late 1990s, when Bill Humphreys and colleagues extended their coastal sampling at Cape Range into the Pilbara and Yilgarn regions of Western Australia (Pesce et al. 1996; Humphreys 1999, 2001). Troglofauna sampling began about 10 years later, with the collection of schizomids and other troglofauna in the Robe Valley in the Pilbara (Harvey et al. 2008). Examples of the fauna collected are shown in Fig. 5.
Species of subterranean fauna. (a) Billibathynella sp. (syncarid), (b) Lagynochthonius sp. (pseudoscorpion), (c) Haifameira pori (copepod), (d) Lathrobiina sp. (beetle), (e) Gomphodella yandii (ostracod), (f) Stenoniscidae gen. nov. sp. (isopod), (g) Mangkurtu kutjarra (spelaeogriphacid), (h) Limbodessus sp. (beetle), (i) Nocticola sp. (cockroach), (j) Linyphiidae sp. (spider), (k) Draculoides sp. (schizomid), (l) Hydrobiidae sp. (snail), and (m) Japygidae sp. (dipluran)
As the amount of sampling of both stygofauna and troglofauna has increased, the richness of these two groups in arid parts of Western Australia has become ever more apparent. For example, Humphreys (2008) reported the occurrence of 560 stygofauna species on the Western Shield (principally the Pilbara and Yilgarn regions) (Fig. 1). This was followed by Eberhard et al. (2009) estimating, based on a regional sampling program, that 500–550 stygofauna species occur in the Pilbara alone, after which Guzik et al. (2010) estimated that 4140 subterranean fauna species, comprising 2680 stygofauna and 1460 troglofauna species, occur in the western half of Australia, mostly in the arid zone. More recent work suggests that even Guzik et al.’s (2010) large figure underestimates the richness of the fauna.
Halse (2015) proposed, based on a combination of sampling results and extrapolation based on the pattern of increasing richness seen with additional sampling, that nearly 3000 species of subterranean fauna occur in the Pilbara (Table 2). While they do not represent systematic sampling, the results of surveys for environmental impact assessments suggest that the Yilgarn has similar stygofauna richness to that in the Pilbara but fewer troglofauna species. Therefore, it is considered likely that more than 4500 stygofauna and troglofauna species occur in the Pilbara and Yilgarn and that approximately 5500 subterranean species occur in the western half of Australia. By way of context, the known vascular plant richness in the Pilbara is less than 1800 species, with approximately 15% of these being endemic to the region (Erickson and Merritt 2016). Nearly all stygofauna and troglofauna species in the Pilbara (and Yilgarn and elsewhere) are endemic to the region in which they occur (Humphreys et al. 2009; Halse et al. 2014).
New comparisons of the richness of subterranean fauna in the Western Shield with other parts of the world are not made here, because there are substantial differences in the habitats that have been sampled in different countries, what species are treated as subterranean and the way in which numbers of species have been determined (Culver et al. 2013). However, both Halse et al. (2014) and Guzik et al. (2010) provide some comparisons between the richness of stygofauna in the Pilbara and western half of the arid zone and the richness of assemblages in other parts of the world. Halse et al. (2014) suggested the Pilbara has higher known density of stygofauna species than any other region in the world, except the Dinaric karst in south-eastern Europe, while Guzik et al. (2010) suggested arid Australia is uniquely rich in stygofauna compared with other areas of the world. Few easy comparisons of troglofauna richness can be made, because regional inventories are rarely compiled, but, with an estimated 1511 species, the Pilbara is likely to be one of the richest regions in the world for troglofauna. Only 995 troglofauna species have been recorded in the well-studied Dinaric karst (Sket et al. 2004), which is widely regarded as an area of global importance for troglofauna.
Stygofauna
A detailed account of the stygofauna of the Pilbara is given by Halse et al. (2014). Information on the Yilgarn is less consolidated, and the first moderately comprehensive overview is provided here, although general information is also available in Humphreys (2001) and Humphreys et al. (2009).
There is relatively little overlap in composition of stygofauna assemblages below the stream bed and in deeper groundwater aquifers of the Pilbara (Halse et al. 2002, 2014). This is probably because the groundwater associated with the alluvium of the ephemeral rivers and creeks of the Western Shield is poorly connected to regional groundwater (Dogramaci et al. 2012). Thus, while some species typical of the streambed fauna are found in regional aquifers, such as darwinulid ostracods, the candonid ostracod Candonocypris tenuis, many cyclopoid copepods and possibly phreatoicid isopods (Knott and Halse 1999; Schön et al. 2010; Pinder et al. 2010), the reverse rarely occurs.
The stygofauna assemblages of the Pilbara and Yilgarn show similar patterns at higher taxonomic levels, despite some differences in the proportions of the major groups (Fig. 6). Further comments on six of the groups are made here. First, based on the number of animals collected, copepods dominate stygofaunal communities of both the Pilbara and Yilgarn, comprising approximately 60% of the fauna in the Yilgarn and 40% in the Pilbara. However, copepod species are often represented by large numbers of animals, and, based on species richness, copepods comprise 44% of the fauna in the Yilgarn and 20% in the Pilbara (Halse et al. 2014; S. Halse, unpublished data). Complementing this overall picture, there appears to have been explosive speciation of harpacticoid copepods in some calcretes of the Yilgarn (Karanovic and Cooper 2011, 2012), where copepod species may represent almost half of the species at a site. As already noted, global comparisons are difficult to make, because of differences in habitats sampled and analytical methods, but copepods may be regarded as comprising 17% of the species in a typical stygofauna community elsewhere (Eberhard et al. 2005) or 20–40% of the species in European communities (Galassi et al. 2009). The diversity of copepods in the Pilbara conforms to global patterns, while the diversity in the Yilgarn is relatively high.
Ostracods represent 24% and 13%, respectively, of the stygofaunal animals in the Pilbara and Yilgarn (Fig. 6) and 30% and 10% of the species in these regions. The greater contribution of ostracod species in the Pilbara reflects the enormous radiation of candonid ostracods in this region, consisting of 11 described endemic genera and more than 108 collected species (Reeves et al. 2007; Karanovic 2007; S.A. Halse, unpublished data). By global standards, where ostracods typically constitute about 3% of species in groundwater communities (Eberhard et al. 2005), both the Pilbara and Yilgarn are rich in ostracods.
Another outstanding characteristic of the stygofauna of the Yilgarn is the occurrence of more than 90 described species of dytiscid beetle in it and nearby regions (Watts and Humphreys 2009; Eberhard et al. 2016). In contrast, there is only one described beetle species in the Pilbara (Watts and McRae 2013), and this disparity, together with the difference in abundance of candonid ostracods, forms the clearest indications of the biogeographic differences between the Pilbara and Yilgarn. Despite their diversity, beetles are not a dominant component of the Yilgarn fauna in terms of either animal abundance or species richness. Beetles represent 0.01% and 2.2%, respectively, of the animals in the Pilbara and Yilgarn and 0.01% and 4.5% of the species in these regions. (Far more sites, including some outside the Yilgarn, have been sampled for beetles than for other stygofaunal groups which is why a larger number of species have been described than their proportional representation in the Yilgarn suggests.)
Amphipods are common in most stygofauna communities, typically representing about 19% of species globally. They represent 16% and 7%, respectively, of the animals in the Pilbara and Yilgarn (Fig. 6) and 17% and 20% of the species in these regions (Halse et al. 2014; S.A. Halse, unpublished data). Much of the focus of Yilgarn and Pilbara stygofauna research has been on amphipods (Finston et al. 2004, 2007; Cooper et al. 2007; Bradford et al. 2010, 2013; King et al. 2012), and comparisons of the results of this research with that from the northern hemisphere provide some insights into the different factors structuring communities in Australia and Europe.
Syncarids comprise 3.1% and 4.1%, respectively, of the stygofaunal animals in the Pilbara and Yilgarn and 11% of the stygofaunal species in both regions. This is a substantially larger proportion of the stygofauna community than is typically represented elsewhere by syncarids (Eberhard et al. 2005). Despite the relatively high number of species present and some recent morphological (e.g. Cho 2005; Cho and Humphreys 2010) and genetic work (e.g. Guzik et al. 2008), there is relatively poor understanding of the diversity and distributions of syncarids in the Pilbara and Yilgarn, especially for the family Bathynellidae (Perina et al. 2018).
Oligochaetes represent 9% and 8%, respectively, of the stygofaunal animals in the Pilbara and Yilgarn (Fig. 5) and 11% and 8% of the species, compared with a global average of 2% of the species in stygofauna communities (Eberhard et al. 2005). Many oligochaetes are quite widespread, and the proportion of stygobitic species in groundwater communities is often quite low (Creuze des Chatelliers et al. 2009), which may have affected global calculations. However, the greater number of stygal species in the Pilbara and Yilgarn is mostly attributable to the collection of relatively large numbers of enchytraeid species, despite phreodrilids and other oligochaete groups also occurring (Pinder 2008; Brown et al. 2015). Only 11% of the stygofaunal oligochaete species listed by Creuze des Chatelliers et al. (2009) are enchytraeids, which are considered to be a predominantly terrestrial family with unstable taxonomy and uncertain ecological attributes, whereas they comprise 31% of Pilbara and 50% of Yilgarn oligochaete species.
Troglofauna
Information on the occurrence of troglofauna outside caves comes almost entirely from environmental impact assessment surveys associated with mining proposals and is strongly biased towards the sampling of hard rock geologies. Areas of calcrete have usually been sampled at low intensity, if at all, because of the difficulty maintaining open holes without casings of soft substrata. Halse and Pearson (2014) have provided an analysis of the taxonomic composition of troglofauna in the Pilbara, but the first account of the overall composition of Yilgarn troglofaunal assemblages is presented here.
One of the peculiarities of the information on troglofauna in the Pilbara and Yilgarn is that there has been no attempt to assess the occurrence of troglofaunal mites and collembolans. Both groups occur as subterranean fauna in other parts of the world (Ortuño et al. 2013; Kováč et al. 2016) and have been observed in Pilbara and Yilgarn samples (Greenslade 2002). Leaving aside mites and collembolans, there are substantial differences between the troglofaunal assemblages of the Pilbara and Yilgarn, with isopods dominating the Yilgarn fauna and several of the groups that are prominent in the Pilbara (cockroaches, schizomids, dipterans) being absent, or nearly so, from the Yilgarn (Fig. 7). The occurrence of 10 groups is considered in detail.
Based on the number of animals collected, isopods comprise 6% and 43% of the troglofauna abundance in the Pilbara and Yilgarn, respectively. The difference between the regions is reduced somewhat when species richness is examined, with isopods comprising 12% of troglofauna species in the Pilbara and 30% of those in the Yilgarn. Comparisons of these proportions with typical troglofaunal communities elsewhere in the world are difficult to make, because of differences in the habitats sampled (cave or broader unsaturated zone), the completeness of lists and whether lists include all troglofauna species or only troglobites. Nevertheless, the Yilgarn appears to have an unusually high proportion of isopods compared with other parts of the world, such as the Balkan Peninsula and various high-yielding sites where, in both cases, 12% of species are isopods (Culver and Sket 2000; Sket et al. 2004). On the other hand, in the small fauna of Portugal, isopods occur in proportions similar to the Yilgarn (26% of species; Reboleira et al. 2013). More than 72 troglofauna isopod species have been recorded in the Yilgarn, including 20 troglomorphic species listed by Javidkar et al. (2016) that are not included in Fig. 7. This is nearly three times as many isopod species as recorded from Brazilian caves (Campos-Filho et al. 2014), albeit that very few Yilgarn isopods are described (Taiti 2014).
Hemipterans, mostly belonging to the family Meenoplidae, are very abundant in the Pilbara and to a lesser extent the Yilgarn, representing 23% and 10%, respectively, of all animals, but only 3.4% of the troglofauna species in each region (Fig. 7). Some troglophilic meenoplid species are widespread in the Pilbara and Yilgarn. Other potentially troglobitic species appear to have small ranges, but further taxonomic and ecological work is required to confirm their status as troglobitic. Culver and Pipan (2008) considered troglobitic hemipterans to be more common in shallow subterranean habitats than caves, although meenoplid species considered to be troglobitic are found in caves of northern Australia (Hoch 1993; Moulds and Banninck 2012).
Cockroaches, mostly belonging to the family Nocticolidae, are also very abundant in the Pilbara, where they represent 19% of animals but only 4% of all troglofauna species. However, cockroaches are one of the many groups in which use of genetic species concepts is likely to substantially increase the number of species recognised (Trotter et al. 2017). No cockroaches have been collected from the Yilgarn. Elsewhere, cockroaches often occur in low numbers (Roth 1991; Moulds and Banninck 2012), and their diversity in the unsaturated zone of the Pilbara appears to be comparatively high.
Millipedes are relatively abundant in both the Pilbara and Yilgarn, representing 9% and 6% of all troglofaunal animals, respectively, in these regions (Fig. 7). However, this is largely the result of the widespread occurrence of the circum-tropical, troglophilic Lophoturus madecassus (see Car et al. 2013), and millipedes comprise only 3.5% and 1.7% of the species present, respectively, in the two regions. In comparison, millipedes comprise 10% of the fauna in the Balkan Peninsula (Sket et al. 2004) and 8% of the fauna at selected sites around the world that are rich in troglofauna (Culver and Sket 2000).
Schizomids occur moderately often in caves and the unsaturated zone across Northern Australia (e.g. Harvey 2001) as well as in the tropics more generally (Monjaraz-Ruedas 2013). However, in iron ore ranges of the Pilbara, they are found with a diversity that appears to be exceptionally high for the group, and they comprise 7% of all animals and 9% of all species in Pilbara troglofaunal assemblages (Fig. 7). Some of the diversity of schizomids in the Robe Valley of the Pilbara was documented in detail by Harvey et al. (2008), while Harms et al. (2016) have discussed issues around species delineation in the Hamersley Range. At least in Australia, the occurrence of schizomids is indicative of a taxonomically rich troglofauna community. As with cockroaches, schizomids are absent from the Yilgarn.
Diplurans usually comprise a small to moderate proportion of troglofaunal assemblages (1.1% in the Balkan peninsula, Sket et al. 2004; 1.4–6% in superficial subterranean habitats, Culver and Pipan 2008; 7% in Portugal, Reboleira et al. 2013). An unpublished report by Markus Koch in 2009 highlights the richness of diplurans in Western Australia, especially in the Pilbara, Yilgarn and Kimberley regions. However, troglofaunal dipluran species tend to occur at low abundance and comprise only 2.9% and 3.0% of animals in the Pilbara and Yilgarn, respectively, despite accounting for 13% and 7% of species. In fact, diplurans are the most species-rich troglofaunal group in the Pilbara, although some species are certainly troglophiles and the exact proportion of troglobitic species is unclear. The estimated median linear range of 4.5 km for Pilbara species (Table 3) suggests the proportion of troglobites may be quite high.
Pauropods, symphylans and, perhaps to a lesser extent, palpigrads provide even greater difficulty distinguishing troglofauna from surface species, because they occur in soil and lack eyes and pigment. While troglobitic palpigrads and symphylans are regularly recorded elsewhere in the world (e.g. Sket et al. 2004), there are few records of troglobitic pauropods anywhere (Vandel 1965). However, Halse and Pearson (2014) suggested that at least some of the pauropod species collected from the unsaturated zone in the Pilbara are likely troglobites, because of their small ranges and, more particularly, the hostile surface soil conditions in the hot, arid Pilbara. More documentation of the surface soil faunas of pauropods, symphylans and palpigrads would help determine the status of species collected in subterranean sampling (see Trajano and Bichette 2010). Based on current interpretations of surface and subterranean status, it is considered that all three groups are relatively species-rich in subterranean habitats of the Yilgarn and Pilbara. Pauropods, symphylans and palpigrads are thought to comprise 2.8%, 2.3% and 1.5% of all troglofaunal animals in the Pilbara and 9%, 9% and 2.0% of Yilgarn animals, respectively (Fig. 7). As a result of most species being collected at low abundance, these animals represent 4.9%, 5.6% and 2.6% and 6.3%, 13.6% and 2.3% of all troglofaunal species, respectively.
Compared with other parts of the world, the low proportions of beetles in the troglofauna assemblages of the Pilbara and Yilgarn are quite startling. It is also a contrast to the relative richness of stygofaunal beetles in the Yilgarn. Only 6% and 3% of troglofaunal animals and 10% and 9% of species are beetles in the Pilbara and Yilgarn, respectively. Typically, beetles comprise about one-third of the species in troglofaunal communities (30% at selected species-rich sites around the world, Culver and Sket 2000; 39% in the Balkan Peninsula, Sket et al. 2004; approximately 40% in Tennessee, Niemiller and Zigler 2013). While further taxonomic investigations are likely to substantially increase the number of beetles known from the Pilbara and Yilgarn (e.g. Baehr and Main 2016; Table 2), beetles are likely to continue to be poorly represented compared with other parts of the world.
Species Delimitation
In addition to the capacity for study of subterranean fauna in the Pilbara and Yilgarn to provide new insights into evolutionary and biogeographic processes (see Humphreys 2016), the potential for mining and groundwater abstraction to threaten the persistence of subterranean species is an important driver of stygofauna and troglofauna research. The process of identifying species as units for conservation (whether or not formally described), and, more particularly, the ranges of those species, is a fundamental step in assessing the likely conservation impacts of mining and groundwater developments.
There are challenges for both morphologists and geneticists when it comes to delimiting species in subterranean habitats. Identifying species through use of traditional morphological characters can be difficult, because of the occurrence of both convergent evolution on some characters (Ornelas-Garcia et al. 2008) and relaxed selection pressure on others that results in phenotypic plasticity, especially in relation to segmentation and setae on left and right sides of animals (Karanovic et al. 2013). In addition, the mostly low numbers in which species are collected, and high proportions of immature animals, means that the number of suitable specimens for morphology is usually very small. Consequently, species descriptions are occasionally based on single immature or damaged animals, making it difficult to achieve certainty when trying to align subsequently collected specimens with the described species.
Genetic recognition of species can also be challenging, because the likelihood of very limited dispersal capacity below ground means genetic structuring within a species is to be expected. Consequently, there may be almost as much intraspecific as interspecific variation in genes used for species discrimination (e.g. CO1; Bradford et al. 2010), which can cause problems in determining the number of species present (Ferguson 2002; Ross et al. 2008). The extent of sequence variation appears to differ among taxonomic groups, with insects being relatively conservative (Leys et al. 2003; Guzik et al. 2009), while arachnids and, particularly, crustaceans show greater variation (Harvey et al. 2008; Finston et al. 2007, 2011). Some variation in crustaceans in calcrete bodies is likely to be the result of successive isolation events for animal populations in refugia within the calcrete, followed by expansion events as higher water levels expand the area of saturated calcrete and permit wider gene flow. More generally, high intraspecific variability in subterranean fauna is often accompanied by low nucleotide diversity (Guzik et al. 2009; Bradford et al. 2013), and it is likely that accurate genetic delimitation of species will often require the use of multiple genes (Bazin et al. 2006; Asmyhr and Cooper 2012; Bradford et al. 2013) and collection of a large number of samples across the species’ range (Bergstein et al. 2012). However, adequate sampling is usually difficult to achieve when access to the species’ habitat is via pre-existing drill holes that probably cover only part of the species’ range and, in fact, may often not intersect the species’ preferred microhabitat (Fig. 2).
The way in which sampling and identification effort may affect the recognition of troglofauna species was illustrated by Harms et al. (2016), who showed that limited sampling led to eight species being recognised in the schizomid genus Draculoides from a small part of the Hamersley Range. Applying the results of barcoding with the CO1 gene and a small amount of additional sampling led to 15 species being recognised. However, further sampling in the same area and phylogenetic analysis using CO1 results reduced this to 12 species. This changing number of species highlights the complexity of delimiting species units in the subterranean environment, especially when the broad biological characteristics of the environment are still being investigated and there is a poor ecological and life-history framework in which to interpret results. Genetic data may provide no clearer guidance about species boundaries than morphological information, and, in such situations, use of a combination of genetics and morphology likely provides better-informed taxonomic decisions (De Queiroz 2007; Javidkar et al. 2016).
Species Distributions
As a group, subterranean fauna species are characterised by small ranges. This is especially so for troglofauna species (Halse and Pearson 2014), which in the Pilbara appear to have ranges that are mostly at least an order of magnitude smaller than those of stygofauna species (Eberhard et al. 2009; Halse et al. 2014). Linear ranges of 1–2 km appear to be moderately common among arid-zone troglofauna species in Australia (Table 3), while existing data suggest only about 5% of Pilbara stygofauna species are likely to have linear ranges of <30 km (Halse et al. 2014). There is probably less difference, however, between ranges of stygofauna and troglofauna species in the Yilgarn, particularly in calcretes.
Perhaps the best-known generalisation about the pattern of occurrence of subterranean fauna species is the ‘calcrete island hypothesis’ of Steve Cooper and others to explain the restricted distributions of many stygofauna species in calcrete bodies of the Yilgarn (Cooper et al. 2002, 2007). It also seems to apply to troglofauna species in Yilgarn calcretes (Javidkar et al. 2016). Under this hypothesis, most species in calcretes of the Yilgarn region are expected to be restricted to individual calcrete bodies that may have linear ranges of only tens of kilometres at most. The area between calcrete bodies, which includes intervening sections of the palaeochannel valleys hosting the calcretes, is considered to be unsuitable for stygofauna and troglofauna, because of high salinity (Humphreys et al. 2009), lack of suitable voids and spaces or otherwise inhospitable habitat for a variety of reasons. A series of papers by Tomislav Karanovic on the copepods of the Yeelirrie calcrete illustrate the extreme levels of geographic replacement and local endemism that may occur, with some species appearing to have linear ranges of <5 km (Karanovic and Cooper 2011, 2012; Karanovic et al. 2015).
Another apparent generalisation is that mineralised and weathered iron ore formations provide rich troglofaunal habitat. The occurrence of rich troglofauna communities in iron ore ranges in Australia is analogous to the occurrence of troglofauna in iron ore mining areas of Brazil (Silva et al. 2011), although in Brazil the animals have mostly been collected from caves, rather than from microcaverns across the iron ore deposit (Fig. 2). While iron ore formations are used extensively by troglofauna, the factors affecting the extent to which different types of deposits are used are still being studied and, for example, the reasons why banded iron formations in the Pilbara support greater numbers of troglofauna species than banded iron formations in the Yilgarn are not understood. Pilbara communities are more complex in structure and, as already mentioned, support groups such as schizomids and cockroaches that are absent from the Yilgarn.
In terms of species ranges, the microcaverns comprising most of the spaces in vuggy banded iron and other rock formations are unlikely to provide many pathways for long-distance lateral dispersal (Fig. 2). Therefore, the troglobitic species found in rock habitats would be expected to have smaller ranges than species inhabiting various types of detritals (scree and alluvium/colluvium) where the potential for dispersal through the matrix is likely greater. Despite this, based on current understanding of the habitats occupied by species, the ranges of troglofauna species in the Pilbara are not strongly determined by the type of geology in which the species occur (Table 3). Probably the most important factor is whether species are troglophilic and have a surface dispersal phase (rather than the lateral below-ground dispersal of troglobites), but intrinsic biological differences among groups may also affect ranges.
The finding that stygofaunal syncarids in alluvial aquifers in New South Wales display some genetic structuring over distances of 50 m in an apparently homogeneous alluvial aquifer (Asmhyr et al. 2013) highlights that lateral movement through the substrate by subterranean species is limited in many habitats. Furthermore, it may be vertically constrained by the physical structure of the substrate or its chemistry (Hose et al. 2017). With the limited information about subterranean habitats that is available from the surface (albeit often augmented with data from drill holes), it is usually difficult to determine whether species ranges are constrained by subtle habitat variation acting as barriers to reduce movement (Guzik et al. 2009; Trontelj et al. 2009) or innate life-history characteristics. This latter phenomenon is widely recognised in trapdoor spiders (Bond et al. 2001; Cooper et al. 2011) and, given the considerable variation in species’ ranges between different taxonomic groups, may also be important in subterranean fauna.
Conservation Challenges
There are three issues that make it challenging to put together an appropriate program for the conservation of subterranean fauna in the Australian arid zone. These issues apply in other regions as well. The first issue is that the small ranges of subterranean fauna species make them particularly vulnerable to even single development projects where subterranean habitat is removed. The second issue is the limited information about the ecology and distribution of subterranean species across the arid zone as a whole. There has been only one regional survey to identify the local areas of high species richness (Halse et al. 2014), as well as to identify the extent to which different geologies are used by subterranean species. The third issue is perhaps the biggest challenge. It is the low level of awareness of subterranean fauna among policymakers and the public at large. There is no impetus to protect species that people know little about and which have poorly documented ecological roles.
Species Ranges in Relation to Project Impacts
Large mining operations may have open mine pits that extend 20 km or more, with annual dewatering requirements of up to 150 gigalitres and substantially larger areas of drawdown than the mine pits themselves (e.g. EPA 2015). In some cases, the pits may be hundreds of metres deep (e.g. EPA 2002). There may also be requirements for mine processing water or, where dewatering produces more water than can be used, the excess may be reinjected below ground. Irrigation projects in the arid zone are another potential user of large amounts of groundwater. All of these developments may pose a conservation threat to subterranean species. Given that many troglofauna and stygofauna species, respectively, have linear ranges of 1–2 km and < 30 km (and sometimes <5 km), such developments have the potential to threaten significant numbers of species through direct loss of habitat. There is also potential for reduction in stygofauna populations, and perhaps even extinction of species under some circumstances, as a result of changes in water chemistry associated with mining and irrigation. Potential changes include increased salinity, reduced carbon and nutrient inputs as a result of mining and increased nutrient loads as a result of irrigated agriculture (Hancock et al. 2005; Humphreys 2009; Nevill et al. 2010).
Documenting Distributions and Managing Threats
While assessments of the potential impacts of development on subterranean fauna usually focus on threats to species, another important conservation value to consider is the overall richness and biological uniqueness of the subterranean fauna assemblage in a development area. Regional surveys provide a framework of information that enables the relative value of assemblages to be assessed, as well as enabling prediction of the likely values of an area in advance of survey.
Regional surveys also help provide information on the distributional characteristics of species of different taxonomic groups, sometimes enabling a species’ range to be predicted from that of related species. This type of information will be refined over time as more ecological and life-history studies of species are undertaken. The other important aspect of distributions, particularly from the viewpoint of predicting and managing impact, is the vertical occurrence of species. This may largely be controlled by the geological preferences of the species or, in the case of stygofauna, their salinity tolerance. Current sampling methods provide relatively unreliable information about the depths at which animals occur, although use of packers and other new techniques would improve the quality of information obtained when sampling (Sorensen et al. 2013).
In many situations, the threat to species is likely to be partial, whereby a considerable amount (but not all) of habitat is lost or the quality of the habitat is affected by development. If the biology of the species is well understood, then its likely impacts can be reduced or mitigated through management actions. One scenario where better information may allow mining to proceed without threat to species is where the vertical distribution of suitable habitat for a subterranean species can be shown to extend deeper than the mine pit or the extent of groundwater drawdown. However, sometimes the process of mining, groundwater abstraction or reinjection will alter conditions in the deeper ‘refuge’, rendering it unsuitable for the species. Sound ecological understanding is critical to decision-making in such situations.
Awareness of Subterranean Fauna
Of the government agencies in Australia, the Western Australian Environmental Protection Authority has responded most strongly to the threats to subterranean fauna, with stygofauna featuring in assessments in the arid zone as early as 1997 (EPA 1997, 1998) and troglofauna in 2006 (EPA 2006, 2007). This is largely a reflection of the richness of subterranean fauna on the Western Shield and the large amount of mining that occurs in Western Australia.
A series of guidelines on subterranean fauna assessment has been released in Western Australia, with the most recent describing the principles of assessment (EPA 2016b). Elsewhere in Australia, the Queensland Government released a subterranean fauna assessment guideline in 2014, while South Australia released a discussion document in 2015 (Goonan et al. 2015). Subterranean fauna have been identified as an issue requiring assessment in many development projects in New South Wales (e.g. Eco Logical 2015), but the framework for assessment is less formal than in Western Australia and Queensland.
Maintaining or increasing agencies’ interest in the conservation of subterranean fauna would be assisted by clearer demonstration of the ecological services provided by these animals. There has been greater effort in this regard for stygofauna (e.g. Danielopol et al. 2003; Steube et al. 2009; Griebler and Avramov 2015) than for troglofauna, where the focus has been more taxonomic and biogeographic. However, troglofauna are likely to provide similar ecological services to those provided by soil fauna (e.g. Lavelle et al. 2006), albeit at greater depth. Plant roots, especially of trees, extend much deeper than the zones occupied by soil fauna and probably often rely on subterranean fauna to maintain a suitable environment.
Concluding Remarks
In summary, the arid zone of Western Australia contains very significant stygofaunal and troglofaunal biodiversity at the global scale. Largely because the animals are mostly tiny, and live underground, they have usually been overlooked in conservation planning, and their scientific importance has frequently been underestimated compared with surface plants and animals. While the large, iconic Karijini National Park in the Pilbara is probably uniquely rich in troglofauna, there is no information whatsoever about the species within it.
The historical lack of interest in subterranean fauna is currently being redressed to some extent by research funding agencies, with many interesting research results emerging. In terms of management, both stygofauna and troglofauna provide taxonomic challenges when determining what comprises a species. Legislation for protecting fauna uses species as the operational unit, which means taxonomy will continue to be an important component of subterranean fauna research. New genetic techniques, such as environmental DNA, may assist in documenting the distributions of rare or ‘difficult to collect’ species, while more intensive studies of individual species and how particular microhabitats are used by subterranean fauna will provide the basis for informed management of subterranean fauna and for mitigation of threats to them. It is also important that research and reviews focus on providing more information about the environmental services provided by subterranean fauna and the sensitivity of these services to the loss of different types of species.
References
Aplin KP (1998) Three new blind snakes (Squamata, Typhlopidae) from north western Australia. Rec West Mus 19:1–12
Asmyhr MG, Cooper SJB (2012) Difficulties barcoding in the dark; the case of crustacean stygofauna from Eastern Australia. Invertebr Syst 26:583–591
Asmyhr MG, Hose G, Graham P, Stow AJ (2013) Fine-scale genetics of subterranean syncarids. Freshw Biol 59:1–11
Baehr M, Main D (2016) New genera and species of subterranean anilline Bembidiini from the Pilbara, north-western Australia (Insecta: Coleoptera: Carabidae: Bembidiini: Anillina). Rec West Aust Mus 31:59–89
Bazin E, Glemin S, Galtier N (2006) Population size does not influence mitochondrial genetic diversity in animals. Science 312:570–572
Bergsten J, Bilton DT, Fujisawa T, Elliott M, Monaghan MT, Balke M, Hendrich L, Geijer J, Herrmann J, Foster GN, Ribera I, Nilsson AN, Barraclough TG, Vogler AP (2012) The effect of geographical scale of sampling on DNA barcoding. Syst Biol 61:851–869
Bond JE, Hedin MC, Ramirez MG, Opell BD (2001) Deep molecular divergence in the absence of morphological and ecological change in the Californian coastal dune endemic trapdoor spider Aptostichus simus. Mol Ecol 10:899–910
Bradford T, Adams M, Humphreys WF, Austin AD, Cooper SJB (2010) DNA barcoding of stygofauna uncovers cryptic amphipod diversity in a calcrete aquifer in Western Australia’s arid zone. Mol Ecol Resour 10:41–50
Bradford TM, Adams M, Guzik MT, Humphreys WF, Austin AD, Cooper SJ (2013) Patterns of population genetic variation in sympatric chiltoniid amphipods within a calcrete aquifer reveal a dynamic subterranean environment. Heredity 111:77–85
Brown L, Finston T, Humphreys G, Eberhard S, Pinder A (2015) Groundwater oligochaetes show complex genetic patterns of distribution in the Pilbara region of Western Australia. Invertebr Syst 29:405–420
Campos-Filho IS, Araujo PB, Bichuette ME, Trajano E, Taiti S (2014) Terrestrial isopods (Crustacea: Isopoda: Oniscidea) from Brazilian caves. Zool J Linnean Soc 172:360–425
Car CA, Short M, Huynh C, Harvey MS (2013) The millipedes of Barrow Island, Western Australia (Diplopoda). Rec West Aust Mus Suppl 83:209–219
Cho J-L (2005) A primitive representative of the Parabathynellidae (Bathynellacea, Syncarida) from the Yilgarn Craton of Western Australia. J Nat Hist 39:3422–3433
Cho J-L, Humphreys WF (2010) Ten new species of the genus Brevisomabathynella Cho, Park and Ranga Reddy, 2006 (Malacostraca, Bathynellacea, Parabathynellidae) from Western Australia. J Nat Hist 44:993–1079
Cooper SJB, Hinze S, Leys R, Watts CHS, Humphreys WF (2002) Islands under the desert: molecular systematics and evolutionary origins of stygobitic water beetles (Coleoptera: Dytiscidae) from Central Western Australia. Invertebr Syst 16:589–598
Cooper SJB, Bradbury JH, Saint KM, Leys R, Austin AD, Humphreys WF (2007) Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from Central Western Australia. Mol Ecol 16:1533–1544
Cooper SJB, Harvey MS, Saint KM, Main BY (2011) Deep phylogeographic structuring of populations of the trapdoor spider Moggridgea tingle (Migidae) from southwestern Australia: evidence for long-term refugia within refugia. Mol Ecol 20:3219–3236
Creuzé d, Châtelliers M, Juget J, Lafont M, Martin P (2009) Subterranean aquatic Oligochaeta. Freshw Biol 54:678–690
Culver DC, ad Pipan T (2008) Superficial subterranean habitats – gateway to the subterranean realm? Cave Karst Sci 5:5–12
Culver DC, Sket B (2000) Hotspots of subterranean biodiversity in caves and wells. J Cave Karst Stud 6:11–17
Culver DC, Trontelj P, Zagmajster M, Pipan T (2013) Paving the way for standardized and comparable biodiversity studies. Subterranean Biol 10:43–50
Danielopol DL, Griebler C, Gunatilaka A, Notenboom J (2003) Present state and future prospects for groundwater ecosystems. Environ Conserv 30:104–130
De Queiroz K (2007) Species concepts and species delimitation. Syst Biol 56:879–886
Dogramaci S, Skrzypek G, Dodson W, Grierson PF (2012) Stable isotope and hydrochemical evolution of groundwater in the semi-arid Hamersley Basin of subtropical northwest Australia. J Hydrol 475:281–293
Dole-Olivier M-J, Malard F, Martin D, Lefébure T, Gibert J (2009) Relationships between environmental variables and groundwater biodiversity at the regional scale. Freshw Biol 54:797–813
Eberhard S (2003) Nowranie Caves and the Camooweal Karst Area, Queensland: hydrology, geomorphology and speleogenesis, with notes on aquatic biota. Helicitite 38:27–38
Eberhard SM, Halse SA, Humphreys WF (2005) Stygofauna in the Pilbara region, north-west Western Australia: a review. J R Soc West Aust 88:167–176
Eberhard SM, Halse SA, Williams MR, Scanlon MD, Cocking JS, Barron HJ (2009) Exploring the relationship between sampling efficiency and short range endemism for groundwater fauna in the Pilbara region, Western Australia. Freshw Biol 54:885–901
Eberhard SM, Watts CHS, Callan SK, Leijs R (2016) Three new subterranean diving beetles (Coleoptera: Dytiscidae) from the Yeelirrie groundwater calcretes, Western Australia, and their distribution between several calcrete deposits including a potential mine site. Rec West Aust Mus 31:27–40
Eco Logical (2015) Bylong coal project environmental impact statement: stygofauna impact assessment. Prepared for Hansen Bailey. Ecological Australia, Sutherland, NSW
EPA (1997) Extensions to exmouth water supply borefield. Bulletin 843. Environmental Protection Authority, Perth
EPA (1998) Newman satellite development; mining of Orebody 23 below the water table. Bulletin 888. Environmental Protection Authority, Perth
EPA (2002) Iron ore mine, downstream processing (direct-reduced and hot-briquetted iron) and port, Cape Preston, WA. Bulletin 1056. Environmental Protection Authority, Perth
EPA (2006) Gorgon gas development, Barrow Island Nature Reserve. Bulletin 1221. Environmental Protection Authority, Perth
EPA (2007) Mesa A/Warramboo iron ore project. Bulletin 1251. Environmental Protection Authority, Perth
EPA (2015) Increase in abstraction and reinjection at Cloubreak mine. Report 1547. Environmental Protection Authority, Perth
EPA (2016a) Yeelirrie uranium project. Report 1574. Environmental Protection Authority, Perth
EPA (2016b) Environmental factor guideline: subterranean fauna. Environmental Protection Authority, Perth
Erickson TE, Merritt DJ (2016) Introduction to plant diversity of the Pilbara. In: Erickson TE, Barrett RL, Merritt DJ, Dixon KW (eds) Pilbara seed atlas and field guide: plant restoration in Australia’s arid northwest. CSIRO, Melbourne, pp 1–5
Ferguson JWH (2002) On the use of genetic divergence for identifying species. Biol J Linn Soc 75:509–516
Finston TL, Bradbury JH, Johnson MS, Knott B (2004) When morphology and molecular markers conflict: a case history of subterranean amphipods from the Pilbara, Western Australia. Anim Biodivers Conserv 27:83–94
Finston TL, Johnson MS, Humphreys WF, Eberhard SM, Halse SA (2007) Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape. Mol Ecol 16:355–365
Finston TL, Johnson MS, Eberhard SE, Cocking JS, McRae JM, Halse SA, Knott B (2011) New genus and species of groundwater paramelitid amphipods from the Pilbara, Western Australia: a combined molecular and morphological approach. Rec West Aust Mus 26:154–178
Galassi DMP, Huys R, Reid JW (2009) Diversity, ecology and evolution of groundwater copepods. Freshw Biol 54:691–708
Goonan P, Jenkins C, Hill R, Klainig T (2015) Subsurface groundwater ecosystems: a briefing report on the current knowledge, monitoring considerations and future plans for South Australia. Environment Protection Authority, Adelaide
Greenslade P (2002) Systematic composition and distribution of Australian cave collembolan faunas with notes on exotic taxa. Helicite 38:11–15
Griebler C, Avramov M (2015) Groundwater ecosystem services: a review. Freshw Sci 34:355–367
Grimes KG (1988) The Barkly karst region, north-west Queensland. In: Pearson L (ed) 17th biennial conference of the Australian Speleological Federation, TROPICON, Lake Tinaroo, Cairns. Australian Speleological Federation, pp 16–24
Guzik MT, Abrams KM, Cooper SJB, Humphreys WF, Cho J-L, Austin AD (2008) Phylogeography of the ancient Parabathynellidae (Crustacea: Bathynellacea) from the Yilgarn region of Western Australia. Invertebr Syst 22:205–216
Guzik MT, Cooper SJB, Humphreys WF, Austin AD (2009) Fine-scale comparative phylogeography of a sympatric sister species triplet of subterranean diving beetles from a single calcrete aquifer in Western Australia. Mol Ecol 18:3683–3698
Guzik MT, Austin AD, Cooper SJB, Harvey MS, Humphreys WF, Bradford T, Eberhard SM, King RA, Leys R, Muirhead KA, Tomlinson M (2010) Is the Australian subterranean fauna uniquely diverse? Invertebr Syst 24:407–418
Hahn HJ, Fuchs A (2009) Distribution patterns of groundwater communities across aquifer types in south-western Germany. Freshw Biol 54:848–860
Halse SA (2015) Subterranean biodiversity in the Pilbara provides difficulties for environmental assessment. In: Abstracts, invertebrate biodiversity and conservation conference 2015, Fremantle. Society of Australian Systematic Biologists and Western Australian Museum, p 12
Halse SA, Pearson GB (2014) Troglofauna in the vadose zone: comparison of scraping and trapping results and sampling adequacy. Subterranean Biol 13:17–34
Halse SA, Scanlon MD, Cocking JS (2002) Do springs provide a window to the groundwater fauna of the Australian arid zone? In: Yinfoo D (ed) Balancing the groundwater budget: proceedings of an international groundwater conference, Darwin 2002. International Association of Hydrogeologists, pp 1–12
Halse SA, Scanlon MD, Cocking JS, Barron HJ, Richardson JB, Eberhard SM (2014) Pilbara stygofauna: deep groundwater of an arid landscape contains globally significant radiation of biodiversity. Rec West Aust Mus Suppl 78:443–483
Hancock PJ, Boulton AJ, Humphreys WF (2005) Aquifers and hyporheic zones: towards an ecological understanding of groundwater. Hydrogeol J 13:98–111
Harms D, Halse S, McRae J, Scanlon M, Curran M (2016) Biodiversity in complex subterranean systems: a tale of arachnids in arid Western Australia. In: Abstracts, international conference on subterranean biology 2016, Fayetteville, Arkansas. International Society for Subterranean Biology, p 36
Harvey MS (2001) New cave-dwelling schizomids (Schizomida: Hubbardiidae) from Australia. Rec West Aust Mus Suppl 64:171–185
Harvey MS, Berry O, Edward KL, Humphreys G (2008) Molecular and morphological systematics of hypogean schizomids (Schizomida: Hubbardiidae) in semiarid Australia. Invertebr Syst 22:167–194
Hoch H (1993) A new troglobitic plant hopper species (Hemiptera: Fulgoroidea: Meenoplidae) from Western Australia. Rec West Aust Mus 16:393–398
Hose GC, Fryirs KA, Bailey J, Ashby N, White T, Stumpp C (2017) Different depths, different fauna: habitat influences on 1 the distribution of groundwater invertebrates. Hydrobiologia 791:145–157
Howarth FG (1983) Ecology of cave arthropods. Annu Rev Entomol 28:365–389
Humphreys WF (1999) Relict stygofaunas living in sea salt, karst and calcrete habitats in arid northwestern Australia contain many ancient lineages. In: Ponder W, Lunney D (eds) The other 99%: the conservation and biodiversity on invertebrates. Royal Zoological Society of New South Wales, Sydney, pp 219–227
Humphreys WF (2001) Groundwater calcrete aquifers in the Australian arid zone: the context of an unfolding plethora of stygal biodiversity. Rec West Aust Mus Suppl 64:63–83
Humphreys WF (2008) Rising from down under: developments in subterranean biodiversity in Australia from a groundwater fauna perspective. Invertebr Syst 22:85–101
Humphreys WF (2009) Hydrogeology and groundwater ecology: does each inform the other? Hydrogeol J 17:5–21
Humphreys WF (2016) Australasian subterranean biogeography. In: Ebach MC (ed) Handbook of Australasian biogeography. CRC Press, Baca Raton, pp 269–293
Humphreys WF, Watts CHS, Cooper SJB, Leijs R (2009) Groundwater estuaries of salt lakes: buried pools of endemic biodiversity on the western plateau, Australia. Hydrobiologia 626:79–95
Javidkar M, Cooper SJB, King RA, Humphreys WF, Bertozzi T, Stevens MI, Austin AD (2016) Molecular systematics and biodiversity of oniscidean isopods in the groundwater calcretes of central Western Australia. Mol Phylogenet Evol 104:83–98
Juberthie C (1983) Le milieu souterrain: étendu et compositon. Mémoires de Biospéologie 10:17–65
Karanovic I (2007) Candoninae (Ostracoda) from the Pilbara region in Western Australia. Crustaceana Monogr 7:1–432
Karanovic T, Cooper SJB (2011) Molecular and morphological evidence for short range endemism in the Kinnecaris solitaria complex (Copepoda: Parastenocarididae), with descriptions of seven new species. Zootaxa 3026:1–64
Karanovic T, Cooper SJB (2012) Explosive radiation of the genus Schizopera on a small subterranean island in Western Australia (Copepoda : Harpacticoida): unravelling the cases of cryptic speciation, size differentiation and multiple invasions. Invertebr Syst 26:115–192
Karanovic T, Eberhard SM, Perina G, Callan S (2013) Two new subterranean ameirids (Crustacea : Copepoda : Harpacticoida) expose weaknesses in the conservation of short-range endemics threatened by mining developments in Western Australia. Invertebr Syst 27:540–566
Karanovic T, Djurakic M, Eberhard SM (2015) Cryptic species or inadequate taxonomy? Implementation of 2D geometric morphometrics based on integumental organs as landmarks for delimitation and description of copepod taxa. Syst Biol 65:304–327
King RA, Bradford T, Austin AD, Humphreys WF, Cooper SJB (2012) Divergent molecular lineages and not-so-cryptic species: the first descriptions of stygobitic chiltoniid amphipods (Talitroidea: Chiltoniidae) from Western Australia. J Crustac Biol 32:465–488
Knott B, Halse SA (1999) Pilbarophreatoicus platyarthricus n.gen., n.sp. (Isopoda: Phreatoicidae: Amphisopodidae) from the Pilbara region of Western Australia. Rec Aust Mus 51:33–42
Korbel KL, Hose GC (2011) A tiered framework for assessing groundwater ecosystem health. Hydrobiologia 661:329–349
Kováč Ľ, Parimuchová A, Miklisová D (2016) Distributional patterns of cave Collembola (Hexapoda) in association with habitat conditions, geography and subterranean refugia in the Western Carpathians. Biol J Linn Soc 119:571–592
Larson HK, Foster R, Humphreys WF, Stevens MI (2013) A new species of the blind cave gudgeon Milyeringa (Pisces: Gobioidei, Eleotridae) from Barrow Island, Western Australia, with a redescription of M. veritas Whitley. Zootaxa 3616:135–150
Lavelle P, Decaëns T, Aubert M, Barot S, Blouin M, Bureau F, Margerie P, Mora P, Rossi JP (2006) Soil invertebrates and ecosystem services. Eur J Soil Biol 42(Suppl 1):S3–S15
Leys R, Watts CHS, Cooper SJB, Humphreys WF (2003) Evolution of subterranean diving beetles (Coleoptera: Dytiscidae, Hydroporini, Bidessini) in the arid zone of Australia. Evolution 57:2819–2834
Mammola S, Giachino PM, Piano E, Jones A, Barberis M, Badino G, Isaia M (2016) Ecology and sampling techniques of an understudied subterranean habitat: the milieu souterrain superficiel (MSS). Sci Nat 103:1–24
Maurice L, Bloomfield J (2012) Stygobitic invertebrates in groundwater – a review from a hydrogeological perspective. Fr Rev 5:51–71
Monjaraz-Ruedas R (2013) A new species of Protoschizomus (Schizomida: Protoschizomidae) from a cave in Guerrero, Mexico. J Arachnol 41:420–424
Moulds T, Bannink P (2012) Preliminary notes on the cavernicolous arthropod fauna of Judbarra/Gregory karst area, northern Australia. Helictite 41:75–85
Nevill JC, Hancock PJ, Murray BR, Ponder WF, Humphreys WF, Phillips ML, Groom PK (2010) Groundwater-dependent ecosystems and the dangers of groundwater overdraft: a review and an Australian perspective. Pac Conserv Biol 16:187–208
Niemiller ML, Zigler KS (2013) Patterns of cave biodiversity and endemism in the Appalachians and interior plateau of Tennessee, USA. PLoS One 8:e64177
Ornelas-Garcia CP, Dominguez-Dominguez O, Doadrio I (2008) Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evol Biol 8:340. https://doi.org/10.1186/1471-2148-8-340
Ortuño VM, Gilgado JD, Jiménez-Valverde A, Sendra A, Pérez-Suárez G, Herrero-Borgoñón JJ (2013) The “alluvial mesovoid shallow substratum”, a new subterranean habitat. PLoS One 8:e76311
Outback Ecology (2012) Lake Maitland uranium project: stygofauna assessment. Outback Ecology Services, Jolimont
Perina G, Camacho AI, Huey J, Horwitz P, Koenders A (2018) Understanding subterranean variability: the first genus of Bathynellidae (Bathynellacea, Crustacea) from Western Australia described through a morphological and multigene approach. Invertebr Syst 32:423–447
Pesce GL, de Laurentiis P, Humphreys WF (1996) Copepods from ground waters of Western Australia. I. The genera Metacyclops, Mesocyclops, Microcyclops and Apocyclops (Crustacea: Copepoda: Cyclopidae). Rec West Aust Mus 18:67–76
Pinder AM (2008) Phreodrilidae (Clitellata: Annelida) in north-western Australia with descriptions of two new species. Rec West Aust Mus 24:459–468
Pinder AM, Halse SA, McRae JM, Shiel RJ (2005) Occurrence of aquatic invertebrates of the wheatbelt region of Western Australia in relation to salinity. Hydrobiologia 543:1–24
Pinder AM, Halse SA, Shiel RJ, McRae JM (2010) An arid zone awash with diversity: patterns in the distribution of aquatic invertebrates in the Pilbara region of Western Australia. Rec West Aust Mus Suppl 78:205–246
Reboleira ASPS, Goncalves F, Oromi P (2013) Literature survey, bibliographic analysis and a taxonomic catalogue of subterranean fauna from Portugal. Subterranean Biol 10:51–60
Reeves JM, De Deckker P, Halse SA (2007) Groundwater ostracods from the arid Pilbara region of northwestern Australia: distribution and water chemistry. Hydrobiologia 585:99–118
Richards AM (1971) An ecological study of the cavernicolous fauna of the Nullarbor Plain Southern Australia. J Zool 164:1–60
Ross HA, Murugan S, Li WLS (2008) Testing the reliability of genetic methods of species identification via simulation. Syst Biol 57:216–230
Roth LM (1991) A new cave-dwelling cockroach from Western Australia (Blattaria: Nocticolidae). Rec West Aust Mus 15:17–21
Schön I, Martens K, Halse S (2010) Genetic diversity in Australian ancient asexual Vestalenula (Ostracoda, Darwinulidae) – little variability down-under. Hydrobiologia 641:59–70
Silva M, Martins R, Ferreira R (2011) Cave lithology determining the structure of the invertebrate communities in the Brazilian Atlantic rain forest. Biodivers Conserv 20:1713–1729
Sket B (2008) Can we agree on an ecological classification of subterranean animals? J Nat Hist 42:1549–1563
Sket B, Paragamian K, Tontelj P (2004) A census of the obligate subterranean fauna of the Balkan Peninsula. In: Griffith HI (ed) Balkan biodiversity. Kluwer Academic, Dordrecht, pp 309–322
Sorensen JPR, Maurice L, Edwards FK, Lapworth DJ, Read DS, Allen D, Butcher AS, Newbold LK, Townsend BR, Williams PJ (2013) Using boreholes as windows into groundwater ecosystems. PLoS ONE 8:e70264
Steube C, Richter S, Griebler C (2009) First attempts towards an integrative concept for the ecological assessment of groundwater ecosystems. Hydrogeol J 17:23–35
Taiti S (2014) New subterranean Armadillidae (Crustacea, Isopoda, Oniscidea) from Western Australia. Trop Zool 27:153–164
Trajano E, Bichuette ME (2010) Diversity of Brazilian subterranean invertebrates, with a list of troglomorphic taxa. Subterranean Biol 7:1–16
Trontelj P, Douady CJ, Fišer C, Gibert J, Gorički Š, Lefébure T, Sket B, Zakšek V (2009) A molecular test for cryptic diversity in ground water: how large are the ranges of macro-stygobionts? Freshw Biol 54:727–744
Trotter AJ, McRae JM, Main DC, Finston TL (2017) Speciation in fractured rock landforms: towards understanding the diversity of subterranean cockroaches (Dictyoptera: Nocticolidae: Nocticola) in Western Australia. Zootaxa 4232:361–375
Vandel A (1965) Biospeleology: the biology of cavernicolous animals. Pergamon Press, London
Watts CHS, Humphreys WF (2006) Twenty-six new Dytiscidae (Coleoptera) of the genera Limbodessus Guignot and Nirripirti Watts and Humphreys, from underground waters in Australia. Trans R Soc Aust 130:123–185
Watts CHS, Humphreys WF (2009) Fourteen new Dytiscidae (Coleoptera) of the genera Limbodessus Guignot, Paroster Sharp, and Exocelina Broun from underground waters in Australia. Trans R Soc S Aust 133:62–107
Watts CHS, McRae JM (2013) Limbodessus bennetti sp. nov.: first stygobitic Dytiscidae from the Pilbara region of Western Australia. Rec West Aust Mus 28:141–143
Webb JA, James JM (2006) Karst evolution of the Nullarbor plain, Australia. In: Harmon RS, Wicks C (eds) Perspectives on karst geomorphology, hydrology, and geochemistry. Geological society of America special paper, pp 65–78
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Halse, S.A. (2018). Subterranean Fauna of the Arid Zone. In: Lambers, H. (eds) On the Ecology of Australia’s Arid Zone. Springer, Cham. https://doi.org/10.1007/978-3-319-93943-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-93943-8_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-93942-1
Online ISBN: 978-3-319-93943-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)