Abstract
At regional and catchment scales, geology and hydrogeology strongly influence the distribution of groundwater invertebrates (stygofauna), but the fine scale distribution of stygofauna in sedimentary aquifers remains poorly studied. In this study, we examine the small-scale distribution of stygofauna in sediments of a perched aquifer in an upland swamp in south eastern Australia. We installed a series of piezometers which accessed either the full sediment profile or one of four discrete sedimentary layers in the swamp. Piezometers were sampled for stygofauna and 2H and 18O isotopes in the groundwater. The swamp contained a taxonomically diverse and abundant stygofauna, which was distributed throughout the swamp and similar in composition to that of other aquifers in the region. There were strong temporal changes in the faunal assemblages but the stimuli for these changes remain unknown. Isotope analysis indicated that the swamp water was well mixed despite localised inputs of groundwater from springs. Accordingly, we could not explore the relative influence of groundwater inputs on fauna; however, we have shown clearly that stygofauna were strongly influenced by sediment properties, with the abundance of stygofauna in the dense, fine sandy sediments being significantly lower than in the coarser sedimentary layers above and below.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aquifers are a vital source of water to over 1.5–2.8 billion people globally (Morris et al., 2003), and provide habitat to a diverse suite of organisms, particularly invertebrates, that are adapted to the dark, low-energy groundwater environment (Humphreys, 2008). Invertebrates adapted to conditions in aquifers (collectively referred to as stygofauna) are a significant component of regional biodiversities (Eberhard et al., 2005; Boulton et al., 2008; Gibert et al., 2009; Robertson et al., 2009; Glanville et al., 2016) and provide important ecosystem services (Griebler & Avramov, 2015).
Despite increasing recognition of the importance of groundwater ecosystems, and the frequent occurrence of stygofauna in the landscape, knowledge of groundwater ecology remains limited in many areas (Larned, 2012; Maurice & Bloomfield, 2012; Sorensen et al., 2013). At regional scales (100–1000 km), the distribution of stygofauna is most influenced by factors such as geological history, climate and geomorphic evolution (Maurice & Bloomfield, 2012; Zagmajster et al., 2014). At catchment scales (10–100 s of km), aquifer type (hydrogeology) is a key determinant of fauna type and distribution (Maurice & Bloomfield, 2012; Stein et al., 2012). At local scales, sediment structure (Korbel et al., 2013b; Korbel & Hose, 2015) and groundwater/surface water exchange (Mencio et al., 2014; Schmidt et al., 2007) are relatively strong correlates of faunal distributions. However, there have been few studies of the stygofauna distributions at finer (<1 km) spatial scales.
Upland swamps are a relatively common landscape feature on sandstone plateaus of south eastern Australia, where they occur in shallow depressions near the headwaters of low-gradient water courses. Sediments accumulated in the depressions create shallow perched aquifers that are fed by precipitation and regional groundwater from seeps or springs. Many of the swamps support stygofauna assemblages (Hose, 2008, 2009), which share compositional similarities with fauna of other groundwater environments (Hose et al., 2015).
The sediments in upland swamps are typically 2–4 m deep and fully saturated (Fryirs et al., 2014a). Sediments comprise discrete stratigraphic layers, each with very different structure and properties, which are stacked in a relatively consistent sequence across different swamps (Fryirs et al., 2014a; Cowley et al., 2016). Surface layers, which contain high concentrations of organic matter, overlie sand- and clay-dominated layers that sit above deeper sand and gravel layers, which in turn overlie saprolite (Fryirs et al., 2014a; Freidman & Fryirs, 2015; Cowley et al., 2016). The shallow depth, consistent profile structure and diverse sediment types provide an ideal environment to examine the small-scale spatial distribution of groundwater biota in relation to sediment structure and groundwater inputs.
In this study, we describe the stygofauna assemblages of an upland swamp and examine how the distribution of stygofauna varies spatially within the swamp, and over time. We expect that because of the distinctly different sedimentary layers and localised groundwater inputs within the swamp, there will be strong spatial differences in stygofauna distribution. We also expect that the sediment layers will provide different habitat conditions and thus support different invertebrate assemblages.
Materials and methods
Study area
The Budderoo Plateau is located in the Southern Highlands, south-west of Sydney, New South Wales, Australia (Fig. 1). The Budderoo Plateau has a temperate climate with an average annual rainfall of between 1500 and 1800 mm/year. Average daily temperatures range from 2 to 13°C in winter to 12 to 26°C in summer (Bureau of Meteorology, 2016).
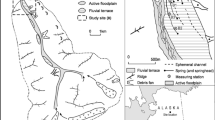
Location of the study swamp at Budderoo National Park. NSW, Australia. From Fryirs et al. (2014b)
The study swamp is one of over 3000 such swamps mapped in the region and is part of the Temperate Highland Peat Swamps on Sandstone ecological community that is listed as threatened under the Australian Environment Protection and Biodiversity Conservation Act (1999) Act. The study swamp is located in the headwaters of the Kangaroo River (34°37′S, 150°40′E) and wholly within the Budderoo National Park (Fig. 1). The plateau is approximately 600 m above sea level and is primarily composed of Triassic Hawkesbury Sandstone with small remnants of the Triassic Wianamatta Group and Tertiary basalts. Soils derived from the Hawkesbury sandstone dominate the plateau. This site was chosen for study because of its undisturbed condition and similarity to other swamps in the region in terms of size, geomorphic setting and vegetation. Detailed descriptions of the hydrology, vegetation and geomorphology of the site are given in Hose et al. (2014) and Fryirs et al. (2014b). The swamp sits at an altitude of 597–604 m asl and is approximately 150 m wide and 600 m long.
Swamp sedimentology
The sedimentology of the study swamp is described in detail in Fryirs et al. (2014a, b). The swamp is elongate in shape and has a lobe-like longitudinal morphology (Fig. 2). The sediments that make up the swamp sit in a basin and are a maximum of 3.5 m deep (Fig. 2). The swamp began forming in this valley about 14,000 years before present (Fryirs et al., 2014a). The swamp is composed of a sequence of five, stacked sedimentary layers (see Fryirs et al., 2014a, b; Freidman & Fryirs, 2015; Cowley et al., 2016, Fig. 2). The swamp sediments remain saturated throughout the year by precipitation and groundwater from two springs (Fryirs et al., 2014b; Fig. 3). By comparing with other swamp systems, this swamp (and others in this region) consists of mineral-rich sediments, derived from the surrounding sandstone geology. While these sediments do have peat-forming potential, the peat is not well developed in comparison to swamp systems formed in other locations and under different environmental conditions (Fryirs et al., 2014a). The basal layer in the swamp consists of coarse medium, poorly sorted, quartz sands and gravels. This basal sands and gravels (BSG) layer is continuous in extent and relatively uniform in depth throughout the study swamp (Fig. 2). Organic content is low at between 1 and 5.3%. Saturated hydraulic conductivity (K sat) values are moderate and bulk density is the highest of all layers within the study site (1.32 g cm−3; Table 1).
Longitudinal cross section (east–west) and representative valley wide cross section showing the sediment profile and piezometer transect locations in Budderoo swamp. Modified from Fryirs et al. (2014b)
Geomorphic zones, and transects and piezometer locations in Budderoo swamp. Modified from Fryirs et al. (2014b)
A thin, fine cohesive sand (FCS) layer occurs in the upper portion of the swamp and lies above the BSG layer (Fig. 2). This layer has a fine sandy loam texture with low–moderate organic content, a moderate bulk density and some of the lowest K sat values measured (to 2 × 10−8 ms−1; Table 1).
Above the FCS layer, organic-rich accumulations dominate the sediment profile. Two key ‘swamp-forming’ layers occur: the alternating organic sands (AOS) and the surface organic fines (SOF) (see Fryirs et al., 2014a; Cowley et al., 2016; Fig. 2). The AOS layer makes up the majority of the swamp and consists of primarily silt and clay with only a minor (<10%) sand fraction. This layer has low concentrations of coarse organic matter but a high organic content (average between 27 and 45%), a moderate bulk density and relatively low K sat values (Table 1). The SOF layer is characterised by a high proportion of live and dead coarse organic matter and therefore has the highest organic content at around 45–50%. This unit occurs uniformly across the swamp to a depth of about −50 cm. Bulk density is low and some of the highest K sat values were measured for this unit (up to 1.6 × 10−4 ms−1) (Table 1).
Stygofauna sampling
To examine patterns in the groundwater fauna, six transects were established approximately 50 m apart, each running perpendicular to the longitudinal axis of the swamp, and positioned in order to capture any variation in hydrology, chemistry or sediment layers within the swamp (Fig. 3). Between two and four, piezometers were installed along each transect and positioned 20–40 m apart with the aim of measuring the cross-sectional and longitudinal variability in the groundwater environment. In total, 18 piezometers were installed in September 2009. Each piezometer was constructed with 50-mm-diameter PVC pipe, slotted (1 mm width) along its entire length with a basal cap and removable top cap. The piezometers extended through the alluvial sediment to saprolite or bedrock and are therefore of varying depth. These piezometers are referred to hereafter as ‘fully slotted’ piezometers. Groundwater fauna were collected from these fully slotted piezometers on three occasions: 7 December 2009 (Time 1), 6 August 2010 (Time 2) and 18 August 2011 (Time 3).
To address the question of differences in stygofauna assemblages between sedimentary layers, we installed a further 21 piezometers on 18 August 2011. These piezometers (hereafter ‘partially slotted piezometers’) were installed to access particular sedimentary layers. Each piezometer was constructed with 50-mm-diameter PVC pipe, with a 200-mm-long slotted (1 mm width) section at the base, with a basal cap. The piezometers were installed adjacent to existing fully slotted piezometers where detailed depth profiles of the sediment were available (see Fryirs et al., 2014b). The partially slotted piezometers were installed at locations where the target sediment layer was >400 mm thick. The piezometers were installed so that the slotted section was wholly within a specific sediment layer, with at least 100 mm between the top or bottom of the slotted section and the boundary of that layer. Invertebrates were collected in each of the partially slotted piezometers on 15 November 2011 (spring) and 26 March 2012 (autumn).
Data on the depth, organic matter content, particle size, hydraulic conductivity and bulk density of the sediment layers (Table 1) were taken from Fryirs et al. (2014a, b). Data for saturated hydraulic conductivity (K sat) were supplemented with additional values measured using a desktop KSAT soil hydraulic conductivity instrument using the falling head method (UMS, Munich Germany).
All stygofauna samples were collected by removing four litres of groundwater from each piezometer using a bailer. This volume was chosen because it could be extracted consistently at all sites. The water was sieved at 63 µm. The contents of the sieve were preserved in 100% ethanol and later sorted using a dissecting microscope (60× magnification) and identified to the lowest practical taxonomic level using available keys (e.g. De Deckker, 2002; Serov, 2002; Wells, 2007; Camacho & Hancock, 2010). In the absence of suitable taxonomic keys, we sequenced the Cytochrome Oxidase 1 gene for a number of copepod specimens (see Hose et al., 2016). Taxa were further classified as either stygofauna (animals having an affinity with groundwater) based on the based on the presence of stygomorphic characters (absence of pigmentation and eyes (Sket, 2008) or as surface water fauna. Limited taxonomic and autecological knowledge of groundwater fauna of this region prohibited more detailed classification of the fauna. As such, our category of stygofauna includes taxa that are likely to be stygophiles (occasional groundwater inhabitants) and stygobionts (obligate groundwater inhabitants) (Sket, 2008).
The body size of a representative selection of copepods and syncarids was measured from photographs using an OIympus ZX16 microscope and Motic Images Plus software (Version 2.0; Motic China Group Co Ltd). Length was determined as head to end of the last abdominal/anal segment (excluding the telson or rami) and width was measured as the largest cross-sectional diameter in the thorax.
Swamp water quality
Water in the swamp typically has low electrical conductivity (<100 μS/cm), low hardness (8–37 mg/l as CaCO3), low pH (4.6–5.0), low dissolved oxygen (<50% saturation) and high concentrations of total organic carbon (21–35 mg C/l) (Hose et al., 2016). Samples of water were taken from the partially slotted piezometers on 26 March 2012 and were analysed for pH in the field using a portable metre (Hanna 9125, Hanna Inst., USA) and later for concentrations of major ions (Br−, Ca2+, Cl−, K+, Mg2+, Li+, Na+, NH4 +, NO2 −, NO3 −, HPO4 2−, SO4 2−) using ion chromatography (IC-1100, Dionex).
Swamp water isotope analysis
Water samples were collected from the partially slotted piezometers on 15 November 2011 and 26 March 2012. Samples were analysed for stable isotopes of water (δ 2H and δ 18O) by laser-based spectroscopy (Picarro, L2120-i) with a precision of 1.0 and 0.15‰ for δ 2H and δ 18O. Results are reported as δ-values relative to the Vienna Standard Mean Ocean Water. Long-term isotope averages were taken from Lucas Heights (Hughes & Crawford, 2013), which is approximately 68 km to the north but is the closest station with available records of isotopes in precipitation. These data were used to estimate a local meteoric water line.
Data analysis
Spatial and temporal patterns in invertebrate assemblages were analysed using non-metric multidimensional scaling (nMDS). Data were square root transformed and analysed using the Bray–Curtis similarity coefficient (Clarke & Green, 1988), with a dummy variable of 1 added to all samples to facilitate inclusion of otherwise empty (0 abundance) samples. Similarity Percentages (SIMPER) analysis (Clarke, 1993) was used to identify the taxa which contributed most to the differences in assemblages. Analysis of similarity (ANOSIM) was used to compare invertebrate assemblages over time. All multivariate analyses were done using PRIMER (version 7.0.9, Primer-E Ltd, UK).
The abundance of invertebrate taxa and isotope concentrations in groundwater was compared between sedimentary layers and times using 2-way ANOVA, with time and depth as fixed factors. Water quality variables were compared between sedimentary layers using 1-way ANOVA. Data were log(x + 1) transformed where necessary to meet assumptions of homogeneity of variance. The significance level (α) used for all inferential tests was 0.05.
Results
Fully slotted piezometers: stygofauna assemblages in the swamp
Over 19,000 invertebrates were collected over the three time periods. Samples were collected from all piezometers on all occasions except for piezometers 14 and 15 which were dry when sampled on Time 1 (Summer 2009). Details of taxa collected are given in Table S1. The stygofauna assemblages were dominated by Cyclopoida copepods (Cyclopidae), with Harpacticoida (Ameiridae) copepods also common (Fig. 4) (Genbank accession Nos KX244830-38). Fauna collected also included Bathynellidae (Bathynella sp.) and Parabathynellidae (Atopobathynella sp.) syncarids, Ostracoda (Candonidae), Amphipoda (Neoniphargidae), Isopoda, Nematoda, Oligochaeta and Acarina (Fig. 4). Maximum and minimum sizes of the copepod and syncarida specimens are given in Table 2.
Mean abundance of common taxa collected in 4-l groundwater samples from fully slotted piezometers in Budderoo swamp over time. Error bars reflect standard error associated with the mean total number of invertebrates per sample. Numbers in parentheses indicate sample size. Time 1 = Summer 2009, Time 2 = Winter 2010, Time 3 = Winter 2011. Sample volume = 4 l
The crustaceans all showed traits (blindness, lack of body pigments) that are typical of groundwater-adapted invertebrates and were included as stygofauna in all analyses. Bathynellidae and Parabathynellidae syncarids were rare and pooled at the order level for analysis. Acarina that were not pigmented were also included but those that were pigmented were excluded. A number of surface water insects, e.g. Chironomidae (Diptera), Scirtidae larvae (Coleoptera) and Collembola, were collected but excluded from analysis.
There was a significant difference in the stygofauna assemblage structure over time (ANOSIM, P = 0.001), which is evident from the distinct clustering by sampling occasion in Fig. 5. Temporal differences in assemblages were driven mostly by cyclopoid, harpacticoid and ostracod abundances (SIMPER), rather than changes in assemblage composition. Copepod nauplii were absent at Time 1, rare at Time 2 (total 2 individuals collected) but abundant at Time 3, suggesting a recent reproductive event for that population (Fig. 4). Interestingly, bathynellid and parabathynellid syncarids did not co-occur in any samples. Bathynellid syncarids were limited to piezometers in the upstream sections (transects 1 and 2, Figs. 2, 3) of the swamp, and parabathynellids were limited to middle transects 3 and 4 (Figs. 2, 3). Isopoda were rare and only recorded in three samples over the study period.
Partially slotted piezometers: water sources, water quality and stygofauna variability relative to sediment layers
Isotope analysis indicated that water in the swamp is well mixed throughout. There was no significant difference in the mean isotope concentrations between sediment layers or over time (P > 0.05; Fig. 6), nor was the time x sediment layer interaction significant (P > 0.05). This is supported by similar averages and small standard deviations for both sampling campaigns (Nov.: δ 18O = −4.58 ± 0.26‰; Mar.: δ 18O = −4.67 ± 0.23‰). Both δ 18O and δ 2H plot close to the Meteoric Water Line from Lucas Heights (Hughes & Crawford, 2013, Fig. 6).
18-O and 2-H concentrations in water samples with different sediment layers in the swamp over time. Open symbols November 2011, closed symbols March 2012. Open triangle, filled triangle surface organic fines layer, open square, filled square alternating organic sands layer, open diamond, filled diamond fine cohesive sands layer and open circle, filled circle basal gravels and sands layer. Dotted line indicates the meteoric water line for Lucas Heights, NSW (from Hughes & Crawford, 2013)
Consistent with the isotope analyses, there were no significant differences in pH, cation or anion concentrations between sediment layers (data not shown). Notably, concentrations of NH4 +, NO2 −, NO3 − and HPO4 2− were below or very close to detection limits in all samples.
The invertebrate assemblages in the nested piezometers were, like the fully slotted piezometers, highly variable. The composition of fauna in the fine cohesive sand (FCS) layers was different to all others largely due to the overall lower abundance of animals in the FCS samples compared to those in other layers (Fig. 7). Notably, more than half (5/8) of the samples from FCS layer had no animals. Those few FCS samples that did contain stygofauna had fewer than four individuals. A small number of surface fauna were found in the FCS layer, namely Tanypodinae and Ceratopogonidae larvae and an Anisoptera (Odonata) nymph.
Abundance of invertebrate taxa in samples from partially slotted piezometers targeting specific sediment layers. A all stygofauna, B all surface water taxa, C Cyclopoid copepods, D Harpacticoid copepods, E Syncarids, F Ostracoda collected from each sediment layer over time. SOF Surface organic fines, AOS alternating organic sands, FCS fine cohesive sands, BSG basal sands and gravel. Dark bars indicate samples collected at time 1 (summer 2011), and light bars indicate samples collected on time 2 (autumn 2012). Error bars indicate standard error. P values indicate significance of two-factor ANOVA (where run)
There was a significant difference (P < 0.05) in the abundance of stygofauna and harpacticoids between sediment layers (Fig. 7). However, there was no significant difference in the abundance of either group over time, nor were the time × sediment layers interactions significant (P > 0.05). Cyclopoids were most abundant in the AOS and SOF layers (Fig. 7) and rare in the FCS layer, although the differences in abundance among sediment layers were not significant (P = 0.289). Bathynellid syncarids were only found in the SOF layer. The abundance of ostracods differed significantly over time (P = 0.116) but not between sediment layers (P = 0.386). Amphipods and isopods, although found in the fully slotted piezometers, were not recorded in the partially slotted piezometers.
Discussion
Stygofauna were widely distributed throughout the swamp and the assemblage composition was similar to that found in the regional aquifers and swamps (Hose, 2008, 2009), and in aquifers more broadly (Humphreys, 2006; Hose et al., 2015). The stygofauna in the study swamp were numerically dominated by Copepoda, but with other common stygobitic crustacean taxa, such as Syncarida, Amphipoda, Isopoda and Ostracoda, also present.
The vertical distribution of stygofauna observed here reflects the physical properties and stacking of the sedimentary layers that make up the swamp and their suitability as stygofauna habitat. The bulk density of sediments in the swamp increases with depth, and hence the porosity decreases. The presence or absence of stygofauna in aquifers is, however, not directly correlated with total available pore space but rather the size of pores, which, in unconsolidated matrices, is indirectly related to the particle size (Arya & Paris, 1981). Consequently, fauna are typically most abundant in coarse sediments and less abundant in clay and other finer-grained sediments (Korbel & Hose, 2015).
In this swamp, stygofauna were abundant in the uppermost organic SOF, AOS layers and the BSG layer, but were uncommon in the intermediate FCS layer. This pattern of distribution indicates that other factors beyond simply a depth gradient associated with decreasing oxygen and nutrient concentrations are influencing the distribution and abundance of fauna, as is seen commonly in other aquifer types (e.g. Datry et al., 2005; Hancock & Boulton, 2008). The low abundance of fauna in the FCS layer is a likely consequence of its small mean particle size, high bulk density and low saturated hydraulic conductivity relative to the other sediment layers. The FCS layer has a high bulk density, meaning that the small particles (mean 177–250 µm) are densely packed, providing only small voids for invertebrates to occupy. The density of fine particles also accounts for the low hydraulic conductivities observed for these sediments (Table 1). Sandy sediments of this size and density may have void spaces with diameters in the order of 70–90 μm (Stakman, 1966), which may explain the low abundance of copepods, which were typically in the size range of 60–450 μm maximum width. The absence of the longer, thinner Syncarida (max width range 62–166 μm) further suggests an absence of large, connected pores in the FCS layer. Despite the likely low suitability of this layer as stygofauna habitat, it has not presented a barrier to dispersal; fauna still occur in the deeper BSG layer. The FCS layer is absent in some downstream areas of the swamp (Fryirs et al., 2014b; Fig. 2), allowing the BSG layer to interface directly with the upper SOF and AOS layers and thus likely permitting dispersal between those layers.
The BSG layer, which underlies the FCS layer, had bulk densities similar to those of the FCS layer, but had considerably larger-sized sediment particles (500–707 µm). These larger particles likely provide sufficient pore spaces (in the order of 150–200 µm, Stakman, 1966) for invertebrates to occupy, which explains the greater abundance of animals in this layer compared to the FCS layer. However, it appears that the BSG layer only contains some stygofauna taxa. While the BSG layer supports an abundant stygofauna, the fauna collected were limited to small-sized taxa such as the copepods and ostracods. Amphipods, which were the largest of the stygofauna taxa collected in the swamp, were only recorded in the fully slotted piezometers 2 and 7, and not in the adjacent partially slotted piezometers that were accessing the BSG layers. This suggests that the large amphipods may not be able to use the BSG habitat of this swamp. The dispersal of the copepods, even to this deeper layer, is consistent with the vertical dispersal of copepods from this swamp observed in laboratory studies (Stumpp & Hose, 2013).
The SOF and AOS layers have different properties from the BSG and FCS layers. They are highly organic with low bulk density, have small mean particle size and relatively high saturated hydraulic conductivity. Based on particle size alone, it would seem unlikely that stygofauna would be found in these layers (c.f. Korbel & Hose, 2015; Korbel unpublished data), but the low density of the sediments indicates a large void volume making these layers suitable habitat in these systems. Organic-rich swamp sediments that contain large amounts of fibric and sapric organic material and plant roots commonly contain macro pores and pipes which contribute significantly to water flow through various sedimentary layers of these swamps (Holden & Burt, 2003; Holden, 2009; Fryirs et al., 2014a, b). These properties are also likely to make it relatively easy for stygofauna to move through and between these layers. Interestingly, the low-density SOF layer allowed larger bodied stygofauna such as Syncarida to occur. Syncarida were not found in the other, coarser layers, suggesting that particle size in the more fibric-rich SOF layer was not a limiting factor for the fauna.
The relatively infrequent collection of syncarids overall makes it difficult to draw strong conclusions about the apparent spatial separation of the Bathynellidae and Parabathynellidae syncarids. This spatial separation may simply reflect low sampling intensity, although our collection of over 40 samples from >10 piezometers is consistent with recommended strategies for stygofauna sampling (e.g. WA EPA, 2007). Previous studies of alluvial aquifers in eastern Australia have reported co-occurrence of syncarids from these families (Tomlinson, 2008; Korbel et al., 2013a, b), but little is known of biotic interactions among these taxa and among stygofauna in general (Strayer, 1994; Hahn, 2006; Humphreys, 2008). On the basis of their co-occurrence in previous studies, it seems unlikely that there would be competitive exclusion occurring between the syncarid taxa, but further sampling is needed to resolve this question. Interestingly, Psammaspides (Syncarida; Anaspidacea) were not recorded in this swamp despite being recorded in other swamps of the region (SMEC, 2006; Hose, 2009).
Stygofauna assemblages often vary with groundwater–surface water exchange and input from the unsaturated zone (e.g. Schmidt et al., 2007; Schmidt & Hahn, 2012). However, in this site, the groundwater and surface water were well mixed, as evidenced by consistent isotope ratios within the swamp water samples and lack of differences in water quality variables. This is despite the direct input of groundwater from springs in some parts of the swamp.
The isotope concentrations in the swamp groundwaters were similar to those from other swamps in the region (PB, 2006; Cowley et al., subm.), and plotted close to the meteoric water line suggesting no isotopic enrichment due to evaporation. Isotope fractionation due to evaporation is expected for the surface water in these areas (Jacobsen et al., 1991). The low range of variability in isotope values also indicates that water in the swamp is well mixed with small fractions of recently infiltrated precipitation. Some of the groundwater in our study swamp was probably not recharged locally, and includes the inputs of at least two springs in the swamp (Fryirs et al., 2014b). Interestingly, there was little variation in the isotopic signatures and other analytes with depth, suggesting good mixing of the groundwater throughout the sediment profile. This homogenous water quality may facilitate the broad distribution of the stygofauna across the swamp; thus, water flow itself is not a limiting factor for fauna migration.
The temporal variability observed in the assemblages from both the fully and partially slotted piezometers is consistent with the general pattern of heterogeneity considered characteristic of subterranean fauna (Gibert et al., 1994; Maurice & Bloomfield, 2012). The limited extent of our temporal sampling limits our ability to identify factors that potentially influence the assemblages, particularly the factors leading to the large increase in the abundance of copepod nauplii in the fully slotted piezometers at Time 2. We could find no literature on the triggers for stygofauna recruitment. Past studies have linked stygofauna abundance to increases in carbon concentrations in groundwater (e.g. Mauclaire et al., 2000; Datry et al., 2005) such as occurs following rainfall (e.g. Korbel et al., 2013a, b). However, with the swamp being at the very headwaters of the catchment, there is relatively little scope for runoff and allochthonous nutrient input by this means. Furthermore, the swamp waters are typically carbon rich (from organic-rich surface sediments; Table 1); thus, it seems unlikely that a small influx of carbon from the catchment would be a strong trigger for recruitment. It thus remains unclear what may be the stimulus for reproduction of these fauna in this swamp system.
The sample volume that we collected from each piezometer was relatively small compared to that typically extracted from groundwater piezometers (e.g. Hancock & Boulton, 2009; Korbel et al., 2017) and is more akin to volumes used for sampling hyporheic assemblages (e.g. Boulton et al., 2003, 2007). Undoubtedly, a greater sample volume will increase (to a point) the richness and abundance of fauna collected (see Hancock & Boulton, 2009) but sampling in these shallow, organic-rich groundwater systems often yields samples containing a large amount of fine organic material, creating a trade-off between sample volume and sample processing efficiency and accuracy.
Our findings supported our expectations of spatial and temporal variation in stygofauna assemblages within this upland swamp system. Fauna were distributed across the swamp and showed a high degree of temporal variation in assemblage structure. There were consistent differences in the abundance of fauna associated with different sedimentary layers, most notably with fauna abundance being significantly lower in the fine particle-dominated FCS layer compared to the more porous upper organic and lower sand-dominated layers.
References
Arya, L. M. & J. F. Paris, 1981. A physicoempirical model to predict the soil moisture characteristic from particle size distribution and bulk density data. Soil Science Society of America Journal 45: 1023–1030.
Boulton, A. J., M.-J. Dole-Olivier & P. Marmonier, 2003. Optimizing a sampling strategy for assessing hyporheic invertebrate biodiversity using the Bou-Rouch method: within-site replication and sample volume. Archiv für Hydrobiologie 156: 431–456.
Boulton, A. J., P. Marmonier & P. E. X. Sarriquet, 2007. Hyporheic invertebrate community composition in streams of varying salinity in south-western Australia: diversity peaks at intermediate thresholds. River Research and Applications 23: 579–594.
Boulton, A. J., G. Fenwick, P. Hancock & M. Harvey, 2008. Biodiversity, functional roles and ecosystem services of groundwater invertebrates. Invertebrate Systematics 22: 103–116.
Bureau of Meteorology (BOM) 2016. Climate data online. Australian Bureau of Meteorology [available on internet at http://www.bom.gov.au/climate/data/index.shtml, accessed November 2016].
Camacho, A. I. & P. Hancock, 2010. A new genus of Parabathynellidae (Crustacea: Bathynellacea) in New South Wales, Australia. Journal of Natural History 44: 1081–1094.
Clarke, K. R., 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143.
Clarke, K. R. & R. H. Green, 1988. Statistical design and analysis for a ‘biological effects’ study. Marine Ecology Progress Series 46: 213–226.
Cowley, K., K. Fryirs & G. C. Hose, 2016. Identifying key sedimentary indicators of geomorphic structure and function of upland swamps in the Blue Mountains for use in condition assessment and monitoring. Catena. 147: 564–577.
Cowley K, K. Fryirs, R. Chisari & G. C. Hose, subm. Water sources and storage in Upland Swamps of Eastern NSW: implications for groundwater management and climate change. Journal of Hydrology. In review.
Datry, T., F. Malard & J. Gibert, 2005. Response of invertebrate assemblages to increased groundwater recharge rates in a phreatic aquifer. Journal of the North American Benthological Society 24: 461–477.
De Deckker, P., 2002. Australian Non-Marine Ostracoda (Crustacea) Murray Darling Freshwater Research Centre Taxonomy Workshop Presentation 1 February 2002. Murray Darling Freshwater Research Centre, Wodonga.
Eberhard, S. M., S. A. Halse & W. F. Humphreys, 2005. Stygofauna in the Pilbara region, north-west Western Australia: a review. Journal of the Royal Society of Western Australia 88: 167–176.
Freidman, B. L. & K. Fryirs, 2015. Rehabilitating upland swamps using environmental histories: a case study of the Blue Mountains Peat Swamps, Eastern Australia. Geografiska Annaler, Series A: Physical Geography 97: 337–353.
Fryirs, K., J. Gough & G. C. Hose, 2014a. The geomorphic character and hydrological function of an upland swamp, Budderoo Plateau, Southern Highlands, NSW, Australia. Physical Geography 35: 313–334.
Fryirs, K., B. Freidman, R. Williams & G. Jacobsen, 2014b. Peatlands in Eastern Australia? Sedimentology and age structure of Temperate Highland Peat Swamps on Sandstone (THPSS) in the Southern Highlands and Blue Mountains of NSW, Australia. The Holocene. 24(11): 1527–1538.
Gibert, J., J. A. Stanford, M. J. Dole-Oliver & J. Ward, 1994. Basic Attributes of Groundwater Ecosystems and Prospects for Research. In Gibert, J., D. Danielopol & J. Stanford (eds), Groundwater Ecology. Academic Press, California: 7–40.
Gibert, J., D. Culver, M. Dole-Olivier, F. Malard, M. Christman & L. Deharveng, 2009. Assessing and conserving groundwater biodiversity: synthesis and perspectives. Freshwater Biology 54: 930–941.
Glanville, K., C. Schulz, M. Tomlinson & D. Butler, 2016. Biodiversity and biogeography of groundwater invertebrates in Queensland, Australia. Subterranean Biology 17: 55–76.
Griebler, C. & M. Avramov, 2015. Groundwater ecosystem services: A review. Freshwater Sciences 34: 355–367.
Hahn, H. J., 2006. The GW-Fauna-Index: a first approach to a quantitative ecological assessment of groundwater habitats. Limnologica 36: 119–137.
Hancock, P. J. & A. J. Boulton, 2008. Stygofauna biodiversity and endemism in four alluvial aquifers in eastern Australia. Invertebrate Systematics 22: 117–126.
Hancock, P. J. & A. J. Boulton, 2009. Sampling groundwater fauna: efficiency of rapid assessment methods tested in bores in eastern Australia. Freshwater Biology 54: 902–917.
Holden, J., 2009. Flow through macropores of different size classes in blanket peat. Journal of Hydrology 364: 342–348.
Holden, J. & T. P. Burt, 2003. Hydrological studies on blanket peat: the significance of the acrotelm-catotelm model. Journal of Ecology 91: 6–102.
Hose, G. C. 2008. Stygofauna baseline assessment for Kangaloon Borefield Investigations- Southern Highlands, NSW. Report to Sydney Catchment Authority, Access Macquarie Ltd, North Ryde
Hose, G. C. 2009. Stygofauna baseline assessment for Kangaloon Borefield Investigations- Southern Highlands, NSW. Supplementary Report – Stygofauna molecular studies. Report to Sydney Catchment Authority, Access Macquarie Ltd, North Ryde.
Hose, G. C., J. Bailey, C. Stumpp & K. Fryirs, 2014. Groundwater depth and topography correlate with vegetation structure of an upland peat swamp, Budderoo Plateau, NSW, Australia. Ecohydrology 7: 1392–1402.
Hose, G. C., M. G. Asmyhr, S. J. B. Cooper & W. F. Humphreys, 2015. Down Under Down Under: Austral Groundwater Life. In Stow, A., N. Maclean & G. I. Holwell (eds), Austral Ark. Cambridge University Press, Cambridge: 512–536.
Hose, G. C., K. Symington, M. J. Lott & M. J. Lategan, 2016. The toxicity of arsenic (III), chromium (VI) and zinc to groundwater copepods. Environmental Science and Pollution Research 23: 18704–18713.
Hughes, C. E. & J. Crawford, 2013. Spatial and temporal variation in precipitation isotopes in the Sydney Basin, Australia. Journal of Hydrology 489: 42–55.
Humphreys, W. F., 2006. Aquifers: the ultimate groundwater dependent ecosystem. Australian Journal Botany 54: 115–132.
Humphreys, W. F., 2008. Rising from down under; developments in subterranean biodiversity in Australia from groundwater fauna perspective. Invertebrate Systematics 22: 85–101.
Jacobsen, G., J. Jankowski & R. S. Abell, 1991. Groundwater and surface water interaction at Lake George, New South Wales. BMR Journal of Australian Geology and Geophysics. 12: 161–190.
Korbel, K. & G. C. Hose, 2015. Water quality, habitat, site or climate? Identifying environmental correlates of the distribution of groundwater biota. Freshwater Sciences 34: 329–343.
Korbel, K., P. J. Hancock, P. Serov, R. P. Lim & G. C. Hose, 2013a. Groundwater ecosystems change with landuse across a mixed agricultural landscape. Journal of Environmental Quality 42: 380–390.
Korbel, K., R. P. Lim & G. C. Hose, 2013b. An inter-catchment comparison of groundwater biota in the cotton growing region of NW NSW. Crop and Pasture Science 64: 1195–1208.
Korbel, K., A. Chariton, P. Greenfield, S. Stephenson & G. C. Hose, 2017. Wells provide a distorted view of life in the aquifer: implications for sampling, monitoring and assessment of groundwater ecosystems. Scientific Reports. 7: 40702.
Larned, S. T., 2012. Phreatic groundwater ecosystems: research frontiers for freshwater ecology. Freshwater Biology. 57: 885–906.
Mauclaire, L., J. Gibert & C. Claret, 2000. Do bacteria and nutrients control faunal assemblages in alluvial aquifers? Archiv für Hydrobiologie 148: 85–98.
Maurice, L. & J. Bloomfield, 2012. Stygobitic Invertebrates in Groundwater – A Review from a Hydrogeological Perspective. Freshwater Reviews 5: 51–71.
Mencio, A., K. L. Korbel & G. C. Hose, 2014. River-aquifer interactions and their relationship to stygofauna assemblages: a case study of the Gwydir River alluvial aquifer (New South Wales, Australia). Science of the Total Environment 479(480): 292–305.
Morris, B. L., A. R. L. Lawrence, P. J. C. Chilton, B. Adams, R. C. Calow & B. A. Klinck, 2003. Groundwater and Its Susceptibility to Degradation: A Global Assessment of the Problem and Options for Management. Early Warning and Assessment Report Series, RS. 03-3. United Nations Environment Programme, Nairobi, Kenya.
PB, 2006. Hydrochemical and Environmental Isotope Sampling Program – Upper Nepean Groundwater Investigation Sites. Parsons Brinckerhoff, Sydney
Robertson, A. L., J. W. N. Smith, T. Johns & G. S. Proudlove, 2009. The distribution and diversity of stygobites in Great Britain: an analysis to inform groundwater management. Quarterly Journal of Engineering Geology and Hydrogeology 42: 359–368.
Schmidt, S. I. & H. J. Hahn, 2012. What is groundwater and what does this mean to fauna? – An opinion. Limnologica - Ecology and Management of Inland Waters 42: 1–6.
Schmidt, S. I., H. J. Hahn, T. J. Hatton & W. F. Humphreys, 2007. Do faunal assemblages reflect the exchange intensity in groundwater zones? Hydrobiologia 583: 1–19.
Serov, P.A., 2002. A preliminary identification of Australian Syncarida (Crustacea). MDFRC Identification Guide No.44, CRC Freshwater Ecology, Albury, NSW, Australia.
Sket, B., 2008. Can we agree on an ecological classification of subterranean animals? Journal of Natural History 42: 1549–1563.
SMEC 2006. Baseline Groundwater Dependent Ecosystem Evaluation Study –Upper Nepean Groundwater Pilot Studies – Final Report. Report to Sydney Catchment Authority. SMEC Australia Pty Ltd, Sydney.
Sorensen, J. P. R., L. Maurice, F. K. Edwards, D. J. Lapworth, D. S. Read, D. Allen, A. S. Butcher, L. K. Newbold, B. R. Townsend & P. J. Williams, 2013. Using boreholes as windows into groundwater ecosystems. PLoS ONE 8: e70264.
Stakman, W. P., 1966. The relation between particle size, pore size and hydraulic conductivity of sand separates. In Proceedings of the Wageningen Symposium. Water in the unsaturated zone, pages. International Association of Scientific Hydrology, Wageningen, The Netherlands: 373–384.
Stein, H., C. Griebler, S. E. Berkhoff, D. Matzke, A. Fuchs & H. J. Hahn, 2012. Stygoregions - a promising approach to a bioregional classification of groundwater systems. Nature Scientific Reports 2: 673.
Strayer, D.L., 1994. Limits to biological distributions in groundwater. In Groundwater Ecology. Academic Press, New York: 287–310.
Stumpp, C. & G. C. Hose, 2013. Impact of water table drawdown and drying on subterranean aquatic fauna in in vitro experiments. PLoS ONE 8(11): e78502.
Tomlinson, M. 2008. A Framework for Determining Environmental Water Requirements for Alluvial Aquifer Ecosystems. Unpublished PhD thesis, University of New England, Armidale, Australia.
WA EPA, 2007. Sampling methods and survey considerations for subterranean fauna in Western Australia, Technical Appendix to Guidance Statement No. 54, Western Australia Environmental Protection Authority, Perth.
Wells, J. B. J., 2007. An annotated checklist and keys to the species of Copepoda Harpacticoida. Zootaxa 1568: 1–872.
Zagmajster, M., D. Eme, C. Fiser, D. Galassi, P. Marmonier, F. Stoch, J. Cornu & F. Malard, 2014. Geographic variation in range size and beta diversity of groundwater crustaceans: insights from habitats with low thermal seasonality. Global Ecology and Biogeography 23: 1135–1145.
Acknowledgements
This research was funded by a Macquarie University Research and Development Grant, a grant awarded under the Department of Sustainability, Environment, Water, Population and Communities (DSEWPaC), and Australian National University (ANU) Research Program on Temperate Highland Peat Swamps on Sandstone (THPSS) and an Australian Research Council (ARC) Linkage Grant (LP130100120), all awarded to GH and KF at Macquarie University. This work was conducted under a NSW NPWS Scientific License. We are grateful for the comments of Associate Editor Stuart Halse and two anonymous reviewers which improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Stuart Anthony Halse
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hose, G.C., Fryirs, K.A., Bailey, J. et al. Different depths, different fauna: habitat influences on the distribution of groundwater invertebrates. Hydrobiologia 797, 145–157 (2017). https://doi.org/10.1007/s10750-017-3166-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3166-7