Abstract
Reduction and recycling of wastes are very serious problems all over the world for the limiting final disposal sites and decreasing environmental loads. The purpose of this study is to investigate a performance of soil microbial fuel cell (SMFC) to generate electricity in accordance with the metabolism of microorganisms in composting of organic wastes. The microbial fuel cell is a device that utilizes the organic matter decomposition of microbes and directly converts chemical energy to electrical energy. In the present study, useful microorganism, namely, photosynthetic bacteria culture, is used for progressing anaerobic fermentation of organic waste, namely, the cutting grass. Rice bran and leaf mould are used for increasing various microorganisms. From the observed results, it is found that the electric power generation of more than a month has increased by using activated bamboo charcoal with iron wire as anode.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
Recently, the amount of waste materials has been decreased due to recycling. However a large amount of organic wastes has been disposed at final landfill site by incineration process . To resolve resource and environmental issues such as global warming and depletion of fossil fuels , suppressing as much as possible the dependence on fossil fuels is important. The annual organic waste generated from the food industries and kitchen garbage in Japan is about 20 million tons per year (Koike et al. 2009). Most of these wastes are directly incinerated with other combustible waste, and the residual ash is disposed of in landfills. However, incineration of this water-containing waste is energy consuming. Microbial fuel cell (MFC) functions to primarily harness bioelectricity through microbial redox reactions and is gaining prominence due to its sustainable applications in multiple domains (Swathi et al. 2018).
Microbial fuel cells (MFCs) are devices that exploit microorganisms to generate electricity from a variety of reduced materials, including organic matter (Logan et al. 2006). MFCs have the prime function of harnessing the bioelectricity through microbial redox reactions and exhibit the multifaceted applications with environmental sustainability. There are several researches on electricity generation of MFCs from organic wastes or wastewaters that are conducted all over the world in the era of green energy generation for sustainable environment and future generation (Bennetto 1990; Miyahara et al. 2016). Researchers have also used MFCs to recover electricity from marine sediments (Reimers et al. 2001) and rice paddy fields (Kaku et al. 2008). MFC in hybrid composting method by reusing the kitchen garbage as a raw material is also proposed (Moqsud et al. 2010, 2013).
In this study, a soil microbial fuel cell (SMFC) that generates electricity by the biodegradation of organic matter is developed. Influences of mixing materials and conditions of electrodes in the SMFC are investigated. A performance of the soil microbial fuel cell by composting under anaerobic condition is discussed based on the experimental results.
7.2 Test Materials and Methods
Generally microbial fuel cell is used under conditions of aerobic cathode with air and anaerobic anode in wastewater. Proton exchange membrane is also used as a separator between the cathode and anode in the microbial fuel cell. In this study, a new type of MFC with compost of organic wastes is developed. Cutting grass (organic waste), leaf mould and rice bran were mixed together with water and photosynthetic bacteria for promoting the fermentation process.
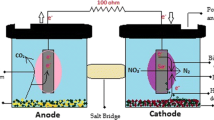
Activated bamboo carbon was used for anode and cathode. The schematic diagram of the experimental device of the SMFC is shown in Fig. 7.1. A plastic container (100 × 70 × 50 mm) is used as a cell. Then cutting grass, leaf mould and rice bran together with water and photosynthetic bacteria are blended properly and filled in the container and shown in Fig. 7.2. Test conditions of SMFC in different mixing ratio of the samples are shown in Table 7.1. For a purpose of increasing a performance of SMFC, anode with iron wire is used. SMFC test in a different electrode distance is also performed under the condition (Case E-1, E-2 and E-6). The anode is inserted into the sample, and the cathode is placed on a surface of the sample. Both the anode and cathode are related to a data logger. A filter paper is used to separate the anode and cathode. SMFC test is performed by wrapping the container up in plastic film. Aerobic or anaerobic condition was applied in the test. In aerobic condition, small holes on the surface of plastic film were opened using pin. In anaerobic condition, the container was sealed up without hole. The data logger is set to measure the voltage in every 10 min’ interval. The laboratory test is conducted in a constant room temperature of 30 °C.
Electrode output is measured in volts (V) against time. The current I in amperes (A) is calculated using Ohm’s law, I = V/R, where V is the measured voltage in volts (V) and R is the known value of the external load resistor in ohms. From this it is possible to calculate the electric power output P in watts (W) of the SMFC by taking the product of the voltage and current, i.e. P = I × V. For obtaining a maximum power of SMFC, values of voltage are measured using three different resistances (10, 100 and 1 kΩ).
7.3 Results and Discussion
7.3.1 Influence of Leaf Mould
In the mixture blend of organic waste , rice bran, photosynthetic bacteria and leaf mould, each constituent plays a vital role towards the generation of electricity as a consequence of biochemical process. With the cutting grass as organic waste, the mixed rice bran functions as a fermenting and nutritious material towards the multiplication of microorganism. The photosynthetic bacteria make its participation to promote the fermentation process under anaerobic condition. Leaf mould is a product of slow decomposition of deciduous shrub and tree leaves. It is also a form of compost produced primarily by fungal breakdown and is retentive of water and fertiliser. In order to investigate the influence of leaf mould from our experimental cases (A-1 to A-6), the amount of leaf mould was varied from 20 g to 120 g by keeping the amount of the other materials constant with the addition of adequate volume of water .
The relationship between voltage and elapsed time on the SMFCs at different mixed amounts of leaf mould during the time period of 48 h is represented in Fig. 7.3. The graphs illustrated that the SMFCs in cases A-1, A-2 and A-6 keep relatively high voltage. On the other hand, the voltage of other SMFCs in cases A-3, A-4 and A-5 was rather unstable and discrete. The recorded voltage of more than 0.4 V in 48 h for A-6 was the highest among the other cases (Fig. 7.4) with 120 g of leaf mould. However, the influence of leaf mould in voltage generation remains unclear .
7.3.2 Influence of Photosynthetic Bacteria
Photosynthetic bacteria have been used for the treatment of various wastewater and biodegradable solids (Choi et al. 2002). These bacteria have a relatively simple nutritional requirement and can grow actively, regardless of the oxygen diffusion rate under aerobic/anaerobic conditions in the light or aerobic conditions in the dark .
In order to investigate the influence of photosynthetic bacteria, samples without photosynthetic bacteria in different mixing proportions of leaf mould were prepared as shown in cases B-1, B-2 and B-6. Figure 7.5 depicts the relationship between voltage and elapsed time for the SMFCs without photosynthetic bacteria for 45 h. In the case of B-2 and B-6, the maximum voltage generation in the range of 0.2–0.3 V could be studied up to 12 h of time which later decreased at a faster rate of 0.0116–0.0204 Vh−1 and declines at the end of 37 h and 23 h, respectively. Unlike the above cases, B-1 could be recorded with 0.14 V as the maximum initially and gradually falls to 0.1 V at the end of 20 h and then remained consistent. The significance of photosynthetic bacteria in the improved performance of SMFCs could be envisaged from the present observations.
7.3.3 Influences of Rice Bran
Many types of raw materials are effectively used as organic fertiliser (Sethuraman and Naidu 2008), and rice bran is among them. As a by-product of the rice milling industry, rice bran constitutes about 10% of the rough rice by its weight. It has the primary composition of aleurone, pericarp and subaleurone layer and germ and is a rich source of vitamins, minerals, essential fatty acids, dietary fibre and other sterols. Based on the above, it has been considered as a good fermentation material under aerobic and anaerobic conditions . Due to the sufficiently contained nutrients in rice bran, it enables the microorganisms for the electricity generation both in pure and mineral water as studied by Takahashi et al. (2016).
The influential characteristics of rice bran could be studied on comparing the SMFC conditions in the absence and presence of rice bran as denoted in cases C-1, C-2 and C-6. It could be well illustrated from Fig. 7.6 that the SMFC system in absentia of rice bran was measured with the potential raised to 0.18 V, 0.07 V and 0.12 V for C-1, C-2 and C-6, respectively, during the initial hours. Later the cell voltage began to fall very quickly to zero at the end of 8 h for C-2 and C-6, but a comparatively lesser declining rate in C-1 was observed which became zero after 20 h with a residual amount of leaf mould. It is quite explicable that the output potential of SMFCs in the presence of rice bran was higher and consistent throughout the time period of 48 h as compared to the insubstantiality of voltage rate and the prolonged output during its absence. Hence the significance of rice bran towards the influence of potential in SMFCs becomes evident on the basis of the recorded results .
7.3.4 Influences of Aerobic Condition
The aerobic and anaerobic conditional environments are quite significant to drive the performance of SMFCs. It is rather decisive to maintain the aerobic and anaerobic maintenance of cathode and anode, respectively. Many types of membranes such as cation-exchange membranes, anion-exchange membranes, polymer/composite membranes and porous membranes have usually been applied in MFC systems which further extended to membraneless technology (Leong et al. 2013). In the present MFC setup, the separation of electrodes has been attempted with filter paper as it is deemed to be cost-effective. But at the same time, the appropriate ambience of the electrodes was failed from its adoption. The probable factor was that the anaerobically conditioned anode becomes oxidized feasibly on separating the aerobically set cathode with the filter paper.
On investigating the preponderance of aerobic condition , MFCs resembling the cases D-1, D-2 and D-6 were constructed with cathode set at defined leaf mould proportions.
Although the voltage–time graph (Fig. 7.7) for cases D1, D2 and D6 illustrated with curve dips and peaks initially, the potential was consistent with 0.07 V and 0.05 V for the prolonged time of 44 h for D-2 and D-3 cases, respectively. However case D-1 was observed with very gradual decrease in potential down to 0.02 V during the time period of 44 h. Evidently, the potential generated under anaerobic condition was several times higher than the potential generated under aerobic condition. The anaerobically conditioned cases such as A-1, A-2 and A-6 were greater in the consistent potential by 16, 3.4 and 8.4 times than the aerobically conditioned D-1, D-2 and D-6 cases during the time period of 44 h. It could be considered that the aerobic condition of cathode is suitable for this type of SMFCs with the priority of wrapping the container up using a plastic film to facilitate a perfect anaerobic (anode) and aerobic (cathode) conditions in SMFCs.
7.3.5 Influence Due to the Distance Between the Electrodes
The distance between anode and cathode, it plays a significant role in deciding the performance of SMFC as it affects the diffusion of protons from anode to cathode.
The anode/cathode distance is known to influence MFC performance, since it affects proton diffusion from anode to cathode (Cheng et al. 2006). Accordingly, cases E-1, E-2 and E-6 with reference to Table 7.1 were executed by mimicking the conditions of case A-2 with the distance between the electrodes as a variable. The voltage–time relationship is represented in Fig. 7.8a where the measurement of open-circuit voltage (equivalent to electromotive force) was carried out under no external load condition against time for the SMFC performance for 48 h. In the case of open-circuit MFCs , the potential of an anode becomes more negative, but on reconnecting the circuit, it tends to become less negative which ultimately results in greater power output and lower energy capture by bacterial organisms (Logan 2009).
The voltage–time graphs were observed to be identical in patterns by exhibiting a voltage drop from a maximum to a minimum followed by an increase which later attains constancy. The attainment of voltage constancy of E-1, E-2 and E-6 was measured with 0.12 V, 0.13 V and 0.17 V, respectively, and hence the cases were in the order: E-6 > E-2 > E-1. These observations revealed that the voltage was directly proportional to the distance between the electrodes and associated probably with the minimum oxygen diffusion towards the anode. Due to the diffusion of oxygen towards the anode, the possible reduction of oxygen molecule into oxide takes place (Eq. 7.1). Oxygen tends to undergo reduction by accepting electrons generated from the anode as its standard electrode potential is positive (Eq. 7.2). As the depth of anode is progressing higher, a highly anaerobic and oxygen-restricted condition prevails which facilitates the flow of electrons towards increasing the power rather than their consumption due to reduction by oxygen species (Fig. 7.9):
Figure 7.8b shows the polarization profiles plotted between the voltage and current obtained for SMFCs E-1, E-2 and E-6 with 24 h of elapsed time. The curves exhibited a linearity in all the three cases where the slope and the intercept represent the internal resistance and electromotive force, respectively. The electromotive force was found to ascend from 122 mV to 191 mV proportionally to the increasing distance between the electrodes. On the other hand, the internal resistance was almost the same and found to be independent to the distance as shown in Table 7.2. It can be inferred that the extension of anodic distance multiplied the maximum electric power per anodic area of about 0.0045 m2. It could also be conceivable with the fact that the depth variations of 20 mm and 40 mm from the cathode decrease the rate of oxygen diffusion towards anode and hence resulted in an increased voltage and maximum electric power per anodic area.
7.3.6 Influence of Anode Modified with Iron Winding
The role of anodic component is noteworthy as it is the primary source for electrons in MFCs. Recently, researchers intend to undertake experiments on MFCs with modified anodes with certain organic and inorganic compounds. The results established the enhanced performance of MFCs and proved that the modifications could improve the potential with economically feasible route (Hindatu et al. 2017; Sonawane et al. 2017).
The voltage–time profiles in Fig. 7.10a and b depict the prolonged voltage consistency between cases A and F. These two assembled SMFC cases are one and the same but with modified anodic parts, i.e. with (F cases) and without iron winding (A cases). Unlike the linear profiles of A (1, 2 and 6) cases, F (1, 2 and 6) cases appeared with initial voltage fluctuations. In cases F-1 and F-6, the consistency of output voltage was recorded after 12 h whereas in F-2 it was attained early after 5 h. The SMFC obtained voltage of F-1, F-2 and F-6 was 1.81, 1.84 and 1.88 times higher than the corresponding A-1, A-2 and A-6 cases. The remarkable raise in the voltage of SMFCs as a cause of synergistic effect in F cases could be substantiated with the modification by iron wire winding in the anode. The contribution of Fe oxidation also played a prominent role in the generation of voltage from the cell. The standard reduction potential (E0) for iron is 0.44 V (Eq. 7.3):
The oxidation of Fe to Fe (II) could be feasible as the standard potential value is more negative to drive the process. During the process, the loss of electron contributes for the potential increase along with the release of Fe (II) which facilitates the growth of microorganisms as a nutrient as shown in Fig. 7.10c.
The polarization profiles for F-1, F-2 and F-3 cases were linear for the time of 24 h as shown in Fig. 7.11. Among the three curves, F-1 and F-2 appear with a meagre difference of 12.82 Ω in internal resistance, but F-6 recorded with a lower value of 108.23 Ω.
Even though the electromotive force was higher for F-1 (800 mV), the role of internal resistance decreased the maximum power to 146.7 mW.m−2. On the other hand, in F-6, the lower electromotive force of 671 mV was able to generate the power of 231.1 mW.m−2 due to lesser internal resistance of 108.23 Ω. In F-2, the paradoxical influence due to the lower electromotive force and higher internal resistance, could generate the lowest power of 79.7 mW.m−2. Hence the impact of multiplied addition (six times of F-1 and three times of F-2) of leaf mould ought to be the reason behind the decreased resistance to drive more output power from the SMFC system as given in Table 7.3.
7.3.7 Power Generation
The low-power output of SMFCs seems to be one of the major issues towards upscaling and practicable applications. Although the electric power of the SMFC is small, it is expected that the power generation will continue for a long period. Also it would be possible to increase the power by connecting several SMFCs in series and parallel.
Figure 7.11a illustrated the voltage variation every day for F cases. The voltage was measured under no external load condition (open-circuit), equivalent to an electromotive force. Remarkably, high voltage in these cases of SMFC continued for a long term more than a month of time period.
Case F-2 with the leaf mould quantity of 40 g was measured with higher voltage in the range of 560–670 mV from the 12th day of the total time period (31 days). On the other hand, in cases F-1 and F-6, the voltage was dropped down from the 14th day around 350 mV. The maximum recorded voltages for F-2 and F-6 cases are about 800 mV (3–7 days) and 700 mV (1–5 days), respectively. When a resistance is connected in the circuit as an external load, the electric power is calculated as a product of voltage and current. Assuming the values of internal resistance in Table 7.3 to be constant, the maximum power generation of respective SMFCs was calculated. Electric energy of the SMFCs was estimated by the integration of the power generation and elapsed time. The maximum electrical energy of the SMFCs generated in cases F-1, F-2 and F-6 during 31 days is represented in Fig. 7.11b. The electric power generated from SMFC of F-6 case was higher, and the value of 412.6 mWh equals the electric capacity of a small-sized dry battery. The electric power generated is of the following order: F-6 > F-3 > F-1. As the electric power of SMFC continued to generate more than a month, the ultimate solid residue can be used as a compost once the power generation is exhausted .
7.4 Conclusions
Soil microbial fuel cell that generates electricity through organic biodegradation was developed. The mixture of cutting grass, leaf mould and rice bran together is added with photosynthetic bacteria for promoting fermentation. Main conclusions drawn from the experimental cases are as follows:
-
1.
The electromotive force of SMFCs assembled using anode made from activated bamboo charcoal was almost doubled due to modification of anode winding by iron wire. The maximum electric power density was recorded with 231.1 mW.m−2 for the modified anode.
-
2.
The performance of SMFC was proportional to the distance between the electrodes. It was ascertained that the restricted oxygen diffusion at greater anodic depth was favourable for electronic ejection rather from anode than reduction of oxygen.
-
3.
The SMFC with Fe wire-wound anode continued to generate the output power of 412.6 mWh during 31 days of time period that was equivalent to that of an electric capacity of a small-sized dry battery.
-
4.
Apart from the bioenergy output , the residual solid remaining in the exhausted SMFC can be utilized as a compost in an agricultural field . Future studies would be aimed at the maximization of power by adopting SMFC modifications with respect to anode, different organic waste and proportions and series and parallel SMFC connections.
References
Bennetto HP (1990) Electricity generation by micro-organisms. Biotechnol Educ 1(4):163–168
Cheng S, Liu H, Logan BE (2006) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40:2426–2432
Choi H-P, Kang H-J, Seo H-C, Sung H-C (2002) Isolation and identification of photosynthetic bacterium useful for wastewater treatment. J Microbiol Biotechnol 12(4):643–648
Hindatu Y, Annuar MSM, Gumel AM (2017) Mini-review: anode modification for improved performance of microbial fuel cell. Renew Sust Energ Rev 73:236–248
Koike Y, An M, Tang Y, Syo T, Osaka N, Morimura S, Kida K (2009) Production of fuel ethanol and methane from garbage by high-efficiency two-stage fermentation process. J Biosci Bioeng 108:508–512
Kaku N, Yonezawa N, Kodama Y, Watanabe K (2008). Plant/microbe cooperation for electricity generation in a rice paddy field. Appl Microbiol Biotechnol 79:43–49. https://doi.org/10.1007/s00253-008-1410-9.
Leong JX, Dauda WRW, Ghasemi M, Liew KB, Ismail M (2013) Ion exchange membranes as separators in microbial fuel cells for bioenergy conversion: a comprehensive review. Renew Sust Energ Rev 28:575–587
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Logan BE (2009) Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7:375–381
Moqsud MA., Omine K, Hayashi S (2010) Microbial fuel cells by using kitchen garbage in hybrid composting method. Proceedings of the 5th civil engineering conference in the Asian region and Australasian structural engineering conference, Sydney
Moqsud MA, Omine K, Yasufuku N, Hyodo M, Nakata Y (2013) Microbial fuel cell (MFC) for bioelectricity generation from organic wastes. Waste Manag 33:2465–2469
Miyahara STM, Kouzuma A, Watanabe K (2016) Electricity generation from rice bran in microbial fuel cells. Bioresour Bioprocess 3:50. https://doi.org/10.1186/s40643-016-0129-1
Reimers CE, Tender LM, Fertig S, Wang W (2001) Harvesting energy from the marine sediment-water interface. Environ Sci Technol 35:192–195
Sethuraman G, Naidu S (2008) Soils and composts. In: International encyclopaedia of agricultural science and technology, vol 4. Mittal Publications, New Delhi
Takahashi S, Miyahara M, Kouzuma A, Watanabe K (2016) Electricity generation from rice bran in microbial fuel cells. Bioresour Bioprocess 3: 1–5
Sonawane JM, Yadav A, Ghosh PC, Adeloju SB (2017) Recent advances in the development and utilization of modern anode materials for high performance microbial fuel cells. Biosens Bioelectron 90:558–576
Swathi K, Sarkar O, Butt SK, Velvizhi G, Venkat Mohan S (2018) Stacking of microbial fuel cells with continuous mode operation for higher bioelectrogenic activity. Bioresour Technol 257:210–216
Acknowledgement
The authors thank Nagasaki University, Nagasaki, Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Omine, K., Sivasankar, V., Chicas, S.D. (2018). Bioelectricity Generation in Soil Microbial Fuel Cells Using Organic Waste. In: Sivasankar, V., Mylsamy, P., Omine, K. (eds) Microbial Fuel Cell Technology for Bioelectricity. Springer, Cham. https://doi.org/10.1007/978-3-319-92904-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-92904-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92903-3
Online ISBN: 978-3-319-92904-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)