Abstract
Microbial fuel cells (MFCs) are emerging wastewater treatment systems with a proven potential for denitrification. In this study, we have developed a high-rate denitrifying MFC. The anode consisted of cow manure and fruit waste and the cathode consisted of cow manure and soil. The initial chemical oxygen demand (COD)/nitrate nitrogen (NO3 −-N) was varied from 2 to 40 at the cathode while keeping the anode ratio fixed at 100. NO3 −-N removal rate of 7.1 ± 0.9 kg NO3 −-N/m3 net cathodic compartment (NCC)/day was achieved at cathode COD/NO3 −-N ratio 7.31 with the current density of 190 ± 9.1 mA/m2 and power density of 31.92 ± 4 mW/m2 of electrode surface area. We achieved an open-circuit voltage (OCV) of 410 ± 20 mV at initial cathodic NO3 −-N of 0.345 g/l. The cathode COD/NO3 −-N ratio had a significant influence on MFC’s OCV and nitrate removal rate. Lower OCV (<150 mV) and NO3 −-N removal rates were observed at COD/NO3 −-N ratio >12 and <7. Experiments done at different cathode pH values indicated that the optimum pH for denitrification was 7. Under optimized biochemical conditions, nitrate removal rate of 6.5 kg NO3 −-N/m3 net cathodic compartment (NCC)/day and power density of 210 mW/m2 were achieved in a low resistance MFC. The present study thus demonstrates the utility of MFCs for the treatment of high nitrate wastes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cells (MFCs) are emerging as potential wastewater treatment systems and are shown to remove and recover nutrients such as nitrogen and phosphorous (Kelly and He 2014). Nitrate indisputably is one of the most common contaminants in the environment and wastewater arising either from industrial operations or agricultural runoff. Nitrate is one of the nutrients causing eutrophication in lakes killing aquatic life forms. It causes blue baby syndrome in infants and is carcinogenic. Kelly and He (2014) had recently reviewed the literature on denitrification studies using MFC. The process appears promising in contrast to the conventional activated sludge process as the energy recovered from MFC can give an offset to the total energy requirements of the process.
The study on bioelectrochemical removal of nitrate was first done by Gregory et al. (2004) wherein the bioelectrodes poised at −500 mV (versus Ag/AgCl) effectively removed nitrate. MFC-mediated successful de-nitrification studies were then performed by Clauwaert et al. (2007) in a tubular reactor. The highest removal rate reported by this group was 0.146 kg NO3 −-N/m3 net cathodic compartment (NCC)/day. Several reports later emerged discussing simultaneous carbon and nitrogen removal using MFCs. Virdis et al. achieved a removal rate of 0.41 kg NO3 −-N/m3 (NCC)/day and Zhang et al. achieved 87.6 % of nitrogen removal in sediment-type photomicrobial fuel cell (Virdis et al. 2008; Zhang et al. 2011). Recent studies also demonstrated the utility of MFC for in situ nitrate removal. Tong and He (2013) found that applying an electric potential improved nitrate removal rate from ground water and reported a nitrate removal rate of 208.2 ± 13.3 g NO3 −-N/m3/day. In another study, Zhang and Angelidaki (2013) demonstrated nitrate removal from ground water using submerged microbial desalination-denitrification cell and achieved removal rate of 0.481 kg NO3 −-N/m3 total cathodic volume (TCV)/day. Recently, denitrification using membrane-less MFC (a low-cost alternative) was reported, but the reported NO3 −-N removal rate was limited to 0.186 g NO3 −-N/m3 liquid cathodic volume (LCV)/day (Zhu et al. 2013). Denitrifying MFCs offer low denitrification rates as compared to the conventional nitrification-denitrification process and anaerobic ammonium oxidation process (ANAMMOX) (Kelly and He 2014). Table 1 summarizes some of the studies performed on denitrifying MFCs. In view of increasing the process efficiency, high-rate denitrifying MFC needs to be developed. High-rate denitrification processes have been developed by previous researchers (Li et al. 2014), but denitrifying MFCs hold special interest as these are energy-generating devices. Thus, the dual purpose of waste treatment and energy generation can be accomplished using MFCs.
Certain wastes such as those arising from nuclear fuel cycle operations as well as certain industrial wastes are characterized by high nitrate content with initial nitrate concentration up to 10 g/l (Fredrickson et al. 2004). Therefore, high removal rates are required to process gallons of waste. Biological denitrification even in MFC is based on the denitrifying microbial populations. Moreover, the microbial consortium in MFC has to be bioelectrochemically active.
Most of the studies performed in denitrifying MFCs (cathodic denitrification) are based on autotrophic denitrification (Clauwaert et al. 2007; Ghafari et al. 2008; Puig et al. 2012; Kelly and He 2014). Anodic denitrification being heterotrophic offers slightly high nitrate removal rates and can carry out denitrification in high strength nitrate wastes (Zhang et al. 2013). Anodic denitrification, however, leads to a potential loss in the device’s columbic efficiency as nitrate competes with the anode as an electron acceptor (Sukkasem et al. 2008; Lee et al. 2012). In some studies, the synergistic effect of heterotrophic and autotrophic denitrification has been shown in biofilm electrode reactor leading to high nitrate removal efficiency and low nitrite and ammonia accumulation (Zhao et al. 2012). Recently, Xu et al.(2015) demonstrated that combined heterotrophic and autotrophic denitrification is highly effective for denitrification. In this study, they reported simultaneous removal of acetate, nitrate, and sulfide with an efficiency of 100, 80, and 100 %, respectively. Zhu et al.(2015) demonstrated simultaneous nitrification-denitrification in biocathode of microbial fuel cell fed with cyanobacteria solution. They achieved removal efficiencies of 0.064 ± 0.005 kg TN m3/day and 0.063 ± 0.005 kg NH4 +-N m3/day under the closed circuit condition which were 2.6 and 2.0 times greater than that under the open-circuit condition.
In the present study, high-rate denitrification system is developed using heterotrophic denitrification in MFC cathode, and device performance has been assessed. The redox potential difference between the anode and cathode was ensured by maintaining differences in net COD/NO3 −-N ratios of anode and cathode. Cow manure was chosen as a source of organic carbon, organic carbon-degrading bacteria, and denitrifying bacteria (Moral et al. 2005). Farm soil, a rich source of denitrifying bacteria, was added to enhance denitrifying bacterial diversity.
Materials and methods
MFC construction
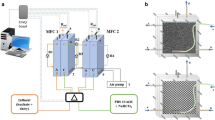
Two identical airtight cylindrical chambers were connected using a glass tube (length 7.5 cm and diameter 1 cm) containing salt bridge. Graphite rod (diameter 1.2 cm and length 5.5 cm) was placed in each chamber, namely, anode and cathode. The salt bridge consisted of 2 % agar-agar prepared in saturated KCl solution. Anode and cathode were connected using a copper wire (gauge 2 mm) and a 100 Ώ resistor was used for completing the circuit. The volume of total and net cathodic and anodic compartment was 0.150 and 0.143 l, respectively (Fig. 1).
Cow manure, soil, and fruit waste characterization
Soil samples were collected from an institute farmland (latitude and longitude 23.23 N and 73.06 E). The farm had been receiving nitrate fertilizers for over 6 years. The soil sample was collected from 9 to 13 cm below the ground surface. The soil sample was passed through a 2-mm sieve to remove rocks and large particles. The sieved soil was mixed with normal saline. Sewage water was collected from a sewage collection pipe. Cow manure was collected from a local dairy farm. Cow manure, soil, and fruit waste were characterized for pH, conductivity, chemical oxygen demand (COD), total nitrogen (NH4 +-N, NO3 −-N, and NO2 −-N), total phosphorus, and denitrifying colony-forming units (CFU/ml). In order to determine pH and conductivity, the sample was thoroughly mixed with double distilled water in 1:1 ratio and allowed to settle in two layers. Measurements were made on the top liquid layer.
COD, NO3 −-N, NO2 −-N, and total phosphorus were determined using Spectroquant kit (Merck) according to the manufacturer’s instructions. NH4 +-N was determined using calorimetric Nessler’s protocol (APHA 1992).
Total solids and total moisture were determined by the gravimetric method. Briefly, a pre-weighed sample (in a thin layer) was subjected to 103 °C for 1 h till a constant weight was achieved. Recorded weight was equivalent to the total solids and lost weight was equal to the total moisture content. COD/NO3 −-N ratio was calculated by dividing the total COD value (g/g) by total NO3 −-N (g/g).
Cultivable denitrifying bacteria in both cow manure and soil were determined using the protocol developed by Takaya et al. (2003). Briefly, the samples were serially diluted and plated in the denitrifier’s enrichment medium containing 0.1 % l-asparagine, 0.1 % KNO3, 0.1 % KH2PO4, 0.005 % FeCl2·6H2O, 0.02 % CaCl2·2H2O, 0.1 % MgSO4·7H2O, 1 ml of Bromothymol blue (BTB) l−1 (1 % in ethanol), and 2 % agar, and pH is 7.0 to 7.3. Plates with bacterial suspension were incubated for 3 days at 30 °C in completely anaerobic conditions (Anaerobic Jar; Himedia). This method is based on the change in the pH of a medium due to nitrate depletion by denitrifying bacteria. The blue colored colonies/halo indicated positive denitrification. The colonies were counted to determine denitrifying CFU/ml. Table 2 shows the physicochemical characteristics of cow manure, soil, and fruit pulp used in the study.
Preliminary screening for the possibility of cathodic denitrification using inoculums such as cow manure, sewage sludge, soil, and soil + cow manure was done in an MFC setup operated as described below. The anode in all these experiments was fixed. The COD/NO3 −-N ratio of anode and cathode in all these treatments was kept constant to make a comparison and identify the best source of denitrifying bacterial consortium usable in MFC conditions.
MFC operation
Pre-treatment of cow manure was done by preparing cow manure (20 % wet weight) slurry in 50 mM potassium dihydrogen phosphate (pH 4.5) followed by an incubation period of 6 h under aerobic conditions. This step was performed to suppress methanogens in cow manure. While this is true that aerobic sparging does not completely kill the methanogens but inhibit them, it has been observed that upon restoring anaerobic conditions, the methane emission rate is lowered (Keiner and Leisinger 1983; Fetzer et al. 1993). This is because the cultures exhibit a long lag phase recovering from the inhibitory effect of oxygen. Denitrifiers, on the other hand, are physiologically facultative anaerobes (Koike and Sorensen 1988); therefore, this step was expected not to kill denitrifying bacteria. Moreover, several classes of bacteria that catalyze hydrolysis, acidogenesis, and acetogenesis reactions can withstand a short duration of air exposure. In the subsequent step, the slurry was diluted using 50 mM dipotassium hydrogen phosphate to obtain final pH 7. The anode consisted of pre-treated cow manure (60 g/l) and homogenized fruit waste (62 g/l) as a co-substrate (COD/NO3 −-N ratio 100). The cathode consisted of a pre-treated cow manure slurry (6–60 g/l) and soil (62 g/l). Phosphate buffer (pH 7) concentration in both anode and cathode chambers was 50 mM. Potassium nitrate was added at the cathode in the concentration range of 0.5 to 7 g/l (or NO3 −-N 0.069 to 0.966 g/l) at a fixed cow manure concentration of 30 g/l. In a separate experiment, the cow manure concentration in the cathode was varied from 6 to 60 g/l while keeping NO3 −-N concentration fixed at 0.345 g/l, and the calculated cathode COD/ NO3 −-N ratios in all these experiments ranged from 2 to 40. In all the experiments, the COD/NO3 −-N ratio of anode remained ≥100. The anode and cathode compartment were thoroughly flushed with oxygen-free nitrogen for 30 min prior to incubation.
In order to study the effect of cathode pH on the device’s NO3 −-N removal efficiency, five different pH values were tested (2, 4, 6, 7, and 9). The phosphate buffer was used for all the pH values tested.
After a start-up period (10 days), stable open-circuit potential (OCV) value was achieved. The resistor (100 Ω) was connected to achieve denitrification. Samples were withdrawn every 10 min to measure nitrogen (nitrate, ammonia, and nitrite) and after every 6 h to measure COD. When the OCV dropped to very low values, the resistor was disconnected. When the NO3 −-N concentration was below 10 mg/l, additional nitrate was supplemented at the cathode. This led to a concomitant increase in the OCV.
Cathode head space nitrogen gas analysis was done using gas chromatography equipped with a thermal conductivity detector (TCD) (YL 6500; Young Lin Instruments Korea). Stainless steel packed column with an internal coating of zeolite was used for separation of gases. The oven and detector temperature was set at 35 and 120 °C, respectively. Argon was used as carrier gas with a flow rate of 15 ml/min. For cathodic head space nitrogen gas analysis, MFC chambers were thoroughly flushed with argon gas prior to operation.
Calculations
Voltage and current measurements were made at different intervals of time using a digital multimeter (METRAVI-451). The individual cathode and anode potentials were measured using Ag/AgCl reference electrode, and the values were noted to confirm the correctness of open-circuit cell voltage. Power (mW) and power density (mW/m2) were calculated using the formulas (V × I) and (V × I/electrode surface area), respectively, where V is the potential in volts (V) and I is the current in milliamperes (mA). The polarization curves were plotted by measuring potential and current at various resistances ranging from 40,000 to 1 Ω. The readings were recorded after a stable value of potential and current achieved. The columbic efficiency (ε) was calculated using the modified formula given by (Logan et al. 2006).
where M is the molecular weight of nitrate, F is Faraday’s constant, n is the number of electrons exchanged per mole of nitrate when reduced completely to molecular nitrogen, V an is the volume of anode liquid, and ΔCOD is the difference between initial and final COD value after time t.
Testing the optimized biochemical condition and feedstock concentration in a low resistance MFC
In a separate experiment, a dual chambered MFC with relatively low internal resistance was used wherein the distance between the anode and cathode was <2 cm (Cheng et al. 2006). The anode and cathode consisted of stainless steel mesh (5 × 5 cm) and activated carbon cloth (thickness 356 μm, 5 × 5 cm). The volume of anode and cathode chamber was 250 and 200 ml, respectively. This configuration was a sediment type MFC wherein the cathode chamber is on top of the anode chamber. Ultrafiltration membrane (Nylon-66 disc filter, pore size 0.2 μm, diameter 47 mm) was used as a separator. This setup was initiated with optimized biochemical conditions and cathode COD/NO3 −-N ratio 7.31.
Statistical analysis
All experiments were performed in triplicates. The results reported are the average values with the standard deviation ranging from 2 to 12 %.
Results
Preliminary screening of inoculum sources
Of all the sources of denitrifying bacteria tested for cathodic denitrification in an MFC setup, cow manure and soil combination showed highest denitrification potential with a lowest start-up period (10 days) and an OCV value of 257.8 ± 20 mV. Ninety-six percent NO3 −-N removal and 67 % COD removal was achieved at 100 Ω. Soil alone, however, gave the highest start-up time and a lowest OCV (66.5 ± 20 mV).
MFC inoculated with sewage water at cathode had a long start-up time and low denitrification. Cow manure alone also showed denitrification (80 %) after a start-up period of 10 days and OCV of 133.2 ± 25 mV as shown in Table 3.
Although the long start-up period could be attributed to low initial bacterial titers but keeping in view high OCV and denitrification potential, the combination of cow manure and soil was chosen for subsequent studies.
Effect of initial cow manure concentration on cell performance
At a fixed soil concentration (6 %) and NO3 −-N concentration (0.345 g/l), cow manure concentration at cathode was varied from 6 to 60 g/l. It was found that low cow manure concentration (6 g/l) lowered NO3 −-N removal rate and OCV. This could be attributed to unfavorable drop in COD/NO3 −-N ratio. With an increase in cow manure concentration up to 30 g/l, the nitrate removal rate increased from 3.5 ± 0.25 to 7.1 ± 0.9 kg/m3/day and OCV increased from 0.158 ± 0.01 to 0.410 ± 0.02 V. Further increase in the cow manure concentration led to a drop in cell OCV to 0.322 ± 0.02 V. However, the NO3 −-N removal rates were enhanced up to 8.2 ± 1.1 kg/m3/day, perhaps because of an abundance of organic matter for nitrate reduction (Table 4).
Effect of initial nitrate concentrations on cell performance
At a fixed total solids and COD at the cathode, initial NO3 −-N concentration was varied. It was found that with increasing initial NO3 −-N concentrations (from 0.069 to 0.345 g/l), a proportional increase in NO3 −-N removal rate from 2.5 ± 0.5 to 7.1 ± 0.9 kg/m3/day and OCV from 0.108 ± 0.01 to 0.410 ± 0.02 V was observed. Upon further increase in NO3 −-N concentration (from 0.345 to 0.966 g/l), a drop in OCV up to 0.114 ± 0.001 V and NO3 −-N removal rate up to 2.05 ± 0.2 kg/m3/day was observed. Initial NO3 −-N concentration of 0.345 g/l, COD/NO3 −-N ratio 7.31 ± 1.21 supported highest OCV of 0.410 ± 0.02 V and NO3 −-N removal rate (Table 4).
Effect of cathode COD/ NO3 −-N on cell performance
The effect of corresponding COD/NO3 −-N ratio was also evident from the above studies (Table 4). It was found that the cathode COD/NO3 −-N ratios in the range of 7–12 were optimal for the MFC operation. In this range, high denitrification rates ranging from 7.1 to 8.2 ± 1.1 kg/m3/day were achieved. Any value beyond this range did not support healthy MFC operation. At COD/NO3 −-N ratios <7 and >12, the voltage values dropped from 0.410 to 0.114 V and NO3 −-N removal rate from 8.2 ± 1.1 to 3.5 ± 0.25 kg/m3/day, columbic efficiency from 11.79 ± 0.7 to 3.11 ± 0.03 %, and also the total COD (cathodic + anodic) removal.
Nitrate nitrogen removal in open-circuit conditions
Nitrate removal was inevitable under open-circuit conditions because of surplus organic carbon and COD/NO3 −-N ratio value favoring the denitrification process. However, the rates of denitrification were two to three times lower under closed circuit conditions (Table 4, Fig. 2). As the concentration of nitrate increased in the cathode, in particular when the COD/NO3 −-N ratio was less than 7, the rates achieved in the closed circuit (2.05 ± 0.02 kg/m3/day) and open circuit(1.32 ± 0.02 kg/m3/day) were not significantly different from each other (Table 4).
Changes in NO3 −-N, NO2 −-N, and NH4 +-N with time under closed circuit conditions
Figure 3 shows the profile of NO3 −-N, NO2 —N, and NH4 +-N concentration with time at different initial NO3 −-N concentrations. The NO3 −-N removal rates increased with increasing initial nitrate concentrations. Nearly complete NO3 −-N removal was achievable in the period of 2 h (Fig. 3a, b).
The concentration of NO2 −-N within the analysis period remained below 6 mg/l. Thus, nitrite was not accumulating in the system. NO2 −-N concentration increased from 1 to 5.5 mg/l after 60 min of operation and then declined again to 1 mg/l in 90 min (Fig. 3c).
NH4 +-N remained below 15 mg/l during the analysis period but did not follow a specific trend. The final concentration of NH4 +-N was 5.2 mg/l (at initial NO3 −-N concentration of 345 mg/l) which was less than the initial concentration (Fig. 3d).
Effect of initial cathode pH on cell open-circuit potential and nitrate removal
pH 7 resulted in open-circuit voltage of 410 mV and high NO3 −-N removal at 100 Ω. pH 2, 4, 6, and 9, however, presented a low OCV of 150 mV and relatively lower NO3 −-N removal (0.25 kg/m3/day at initial NO3 −-N of 138 mg/l). The optimum pH was 7 (Fig. 4).
Performance of the MFC setup initiated with conditions optimized above
The setups initiated with cow manure (30 g/l) + soil (62.5 g/l) at cathode initially began with negative open-circuit potential. The potential increased steadily and reached a value of about 400 mV after a period of 10 days. The initial COD/NO3 −-N ratio at the anode was about 100 and it increased to about 150. The nitrate losses during the start-up period were nearly 20 % at the cathode and 25 % at the anode. The COD/NO3 −-N ratio of the cathode was maintained at 7.31 by intermittent nitrate and cow manure supplementation. The pH value at anode dropped gradually while at cathode a gradual rise in pH was observed during the start-up period. The values were maintained at near neutral by acid/base supplementation periodically. The denitrifying bacterial (CFU/ml) count at cathode under closed circuit conditions was 1.29 × 108 CFU/ml. The denitrifying bacterial numbers were 5.46 times higher than that under the open-circuit conditions. After 10 days, the OCV did not rise further. The cell was discharged at 100 Ω and NO3 −-N measurements were done to estimate removal rates.
Profile of NO3 −-N removal at 100 Ω, cell open-circuit potential, and current at 100 Ω during fed batch cycles
After optimizing cathode pH, cow manure concentration, initial NO3 −-N concentration, and optimal COD/NO3 −-N ratios, the nitrate removal from MFC cathode was performed at an external resistance value of 100 Ω. Ninety-six percent NO3 −-N removal was achieved within a period of 2 h. This was accompanied with a corresponding drop in cell voltage and current (Fig. 5a, b). When the cell voltage dropped below 50 mV, the resistors were disconnected. Nitrate supplementation at cathode along with COD adjustment resulted in the concomitant increase in the OCV. This reflected the role of nitrate ion in completing cell circuit and as a sole agent responsible for high cathode potential. About three cycles of NO3 −-N removal were achieved at MFC cathode. The total COD removal of the cathodic and anodic compartment was 69.39 and 67.62 %, respectively. The NO3 −-N removal rate was about 7.1 ± 0.9 kg NO3 −-N/m3 of net cathodic compartment (NCC)/day. This was higher than the theoretical maximum (electrochemically) as the heterotrophic carbon present at cathode presented a surplus source of electrons for NO3 −-N reduction.
An increase in head space nitrogen gas concentration was observed concomitant with the process of denitrification which also confirmed the biochemical/or electrochemical reduction of nitrate ions.
Effect of cathode COD/NO3 −-N ratio on columbic efficiency and total COD removal rate
At a fixed soil (62.5 g/l) and NO3 −-N (345 mg/l) concentration, when cow manure concentration was varied from 6 to 60 g/l, an increase in columbic efficiency was observed from 4.82 to 11.79 %. (Table 4).
The anode and cathode COD removal rates also increased with increasing COD/NO3 − N ratio. At low COD/NO3 −-N ratio <7, the anodic and cathodic COD removal rates were decreased to 53.21 ± 2.45 and 58.54 ± 6.5 %, respectively. With an increase in COD/NO3 −-N ratio >7, the anodic and cathodic COD removal rates were increased to 83.06 ± 5.24 and 84.52 ± 5.54 %, respectively (Table 4).
Polarization results
The polarization curve was plotted for the setup initiated with optimized conditions, viz. pH 7 and cathode COD/NO3 −-N ratio 7.31. The maximum power density (34.61 ± 4.2 mW/m2) value was obtained at an external resistance value of 400 Ώ. This corresponded to the current density value of 139.56 ± 6.2 mA/m2 and voltage of 248 ± 25 mV. Power density (31.92 ± 4 mW/m2) was obtained at 100 Ώ corresponding to the current density of 190 ± 9.1 mA/m2 and a cell voltage of 168 ± 15 mV (Fig. 6).
Effect of MFC configuration
The reproducibility of nitrate removal rates and proposed biochemical conditions were tested in a different MFC configuration (with low internal resistance). While the nitrate removal rate was nearly reproducible (6.5 kg NO3 −-N/m3/day), a significant enhancement in power density was obtained (220 mW/m2) which indicated the usefulness of the developed system for simultaneous wastewater treatment and power generation.
Discussion
In this study, we have successfully developed a high-rate denitrifying MFC wherein the synergistic effect of heterotrophic and bioelectrochemical denitrification is operating. We hypothesized that heterotrophic cathodic denitrification in MFC shall be better than autotrophic cathodic denitrification or heterotrophic anodic denitrification, and we were able to prove so. Nitrate, in the present experimental setup, was removed by both the processes namely heterotrophic denitrification and bioelectrochemical denitrification. In conventional high-rate heterotrophic denitrification systems, electron donor substrates such as acetate, methanol, and ethanol are used that make the process costly (Hayden and Gu 2008). Therefore, costlier substrates need to be replaced by cheap and easily available waste streams, and both cow manure and soil fit into that criteria. In our preliminary experiments, it was found that MFC’s cathode supplemented with soil and cow manure were indeed giving high denitrification rates without any compromise in OCV.
A dual chambered MFC was used in this study with two chambers connected to each other with a salt bridge. The anodic and cathodic solution involved rich medium containing both soluble and insoluble components, and it was believed that proton/ion exchange membrane will foul sooner. Therefore, agar agar salt bridge was used to separate the two chambers (Logan et al. 2006). Although agar agar salt bridge MFC offers high internal resistance (Min et al. 2005), our purpose was not to achieve high power output but high denitrification rate. Moreover, a salt bridge is a cheaper alternative and helps to keep the process of bioremediation overall economical.
Since MFC cathode supplemented with cow manure and soil gave good denitrification values, subsequent studies were performed with cathode containing both cow manure and soil. The process had to be optimized for nitrate removal, and therefore, to determine effective cow manure concentration, we varied cow manure from 6 to 60 g/l. The addition of organic carbon at the cathode is expected to lower the OCV; however, we did not observe any significant reduction in the MFC performance. We obtained a power density value of 31.92 ± 4 mW/m2 which is higher than values recently reported for heterotrophic cathodic denitrification (Zhu et al. 2015). Previous studies also demonstrated that the presence of organic matter in the cathodic chamber does not nullify the redox potential difference between anode and cathode (Vilar-Sanz et al. 2013).
It was found that cow manure and soil at cathode promotes denitrification and acts as a source of electron donor substrates for nitrate reduction. As the concentration of cow manure increased at the cathode, NO3 −-N removal rates increased in both closed and open-circuit conditions. This is attributable to the surplus electron donor substrate available for nitrate reduction. Although increased organics at the cathode did lower the OCV and current density, NO3 −-N removal rates were not affected. This indicated that surplus organics present at the cathode compensated well for the reduced bioelectrochemical performance in terms of denitrification (Table 4).
Experiments performed at various nitrate concentrations with fixed organic matter at cathode indicated the influence of COD/ NO3 −-N ratio. COD/NO3 −-N ratio turned out to be a critical criterion for MFC performance and denitrification. An optimal COD/NO3 −-N ratio for cathode ranged from 7 to 12. At a value of 7.31, an OCV of 410 ± 20 mV was achieved. Discharge of this potential through a 100-Ω resistance provided the NO3 −-N removal rate of 7.1 ± 0.9 kg NO3 −-N/m3 of net cathodic compartment (NCC)/day. This value is much higher than the value recently reported by Zhang et al. (2013) wherein heterotrophic anodic denitrification studies have been performed. In the given experimental condition, this study reports the highest denitrification rate achieved in the MFC system.
At low COD/NO3 −-N ratios (<7), increased NO3 − concentration in the system possibly leads to global inhibition causing reduced concentration of active denitrifying bacteria (Akunna et al. 1992) which in turn affects the utilization rate of electron donor substrate. We also observed a reduced COD reduction rate at cathode when COD/NO3 −-N ratio was less than 7. At high COD/NO3 −-N (>12), a large fraction of electron donor substrate get channeled towards the accumulation of organic intermediates or methane, which lower the OCV and reduce the electron motive force and the current passing through the cathode thereby reducing the contribution of bioelectrochemical denitrification. Thus, low overall nitrate reduction rates were observed. It was also determined that as long as the COD/NO3 −-N ratio is lower in the cathode (<12) and higher in the anode (≥100), the OCV in positive, denitrification is favored and high rates of NO3 −-N removal are achieved. Lower COD/NO3 −-N ratio tested in anaerobic digesters have been shown to promote denitrification activity (Akunna et al. 1992; Ruiz et al. 2006).
The presence of high nitrate concentration at cathode ensured high cathode potential and overall high OCV. Rise in water saturated soil (with fixed organic matter) redox potential from −300 to +200 mV upon nitrate addition had been observed in previous studies (Bailey and Beauchamp 1971). The potential again fell back to −300 mV as soon as the nitrate depleted from the soil (Bailey and Beauchamp 1971). A similar trend was observed in the present study. There was a concomitant fall in the OCV with the drop in cathode nitrate concentration while the potential values increased as the nitrate was supplemented in the cathodic compartment (Fig. 5a).
MFCs kept at open circuit exhibited denitrification that can be completely attributed to heterotrophic denitrification process. NO3 −-N removal rates ranged from 1 to 4 kg NO3 −-N/m3/day. These values are similar to the values reported in the majority of heterotrophic denitrification studies (Wang et al. 2009). In bioelectrochemical denitrification, denitrification rate is directly proportional to the current density (Clauwaert et al. 2007), and that sets a limit to achievable denitrification rate. With a current density of a 3.7 A/m3, the NO3 −-N removal rates are not expected to exceed 0.0092 kg NO3 −-N/m3/day as per the formula given by Clauwaert et al. (2007). This points to the fact that denitrification rate observed in this study is not a simple summation of bioelectrochemical and heterotrophic denitrification but a synergistic effect of the two mechanisms wherein the whole is greater than the sum of its parts. One explanation could be that the closed circuit conditions rather than contributing directly towards magnitude of nitrate removal contribute towards the effective microbial biofilm formation (Gregoire et al. 2014) or increased titers of denitrifying bacteria which are involved in heterotrophic denitrification, and thus it indirectly promotes denitrification. We also observed increased titers of denitrifying bacteria under closed under conditions. Similar observations were made by Zhu et al. (2015) where they observed two times more nitrogen removal under closed circuit than that under open circuit. Huang et al. (2014) also found that closed circuit conditions favored richer and more diverse microbial communities at the cathode. Also, biofilm processes exhibit high denitrification rates (Oh et al. 2001). Thus, closed circuit conditions indirectly augmented heterotrophic denitrification rates. This also explains that microbial activity prevailing in such a condition might be undergoing drastic changes. However, the exact mechanism needs to be elucidated. This would encompass complete metabolite, proteomic, and microbial analysis of the MFC cathode.
Also, in heterotrophic denitrification, organic matter can inhibit the activity of autotrophic bacteria, but the simultaneous existence of both autotrophic and heterotrophic processes cannot be completely ruled out in wastewater treatment systems (Zhao et al. 2012; Rocca et al. 2006; Qambrani et al. 2013). Biofilm stratification on the electrode surface is inevitable in nutrient-rich conditions. The microbial layer close to the electrode surface (where organic matter diffusion would be little) is expected to reduce nitrate with the use of electrons generating at the anode. The flow of current confirms the acceptance of electrons at the cathode.
The control setups without any soil and cow manure at cathode resulted in nil denitrification and power generation, which points to the fact that activation energy required to carry out electrochemical nitrate reduction is very high and a catalyst is required to mediate nitrate reduction. Microbial catalysts derived from cow manure and soil were thus quite effective in doing so. Also, there was no organic carbon for heterotrophic denitrification to occur in such a setup, which emphasizes on the contribution of heterotrophic denitrification operating at test MFC cathodic chamber.
The movement of nitrate ions from the cathodic to the anodic chamber is ruled out by two important observations; one, the anodic COD/NO3 −-N ratio increased gradually from 100 to about 150 during the course of operation, which indicates a decrease in nitrate concentration. Migration of nitrate would have resulted in decreased COD/NO3 −-N ratio. Second, upon disconnecting resistors during repeated batch denitrification (Fig. 5a, b), nitrate did not reappear in the catholyte. Reappearance was expected if it was simple absorption on the agar agar salt bridge. These observations indicated that nitrate ions are not getting absorbed and migrating from one chamber to another but getting converted biochemically.
The high COD removal achieved from the cathodic and anodic chamber suggested the device’s effectiveness in carbon removal. The columbic efficiency of the device also increased at an optimal COD/NO3 −-N ratio but declined at very low or high COD/NO3 −-N ratios at the cathode. The overall columbic efficiency in the present study was low, which could be attributed to undesirable metabolic products at the anode and the presence of high organic matter at the cathode. A similar phenomenon was explained by Virdis et al. (2009) wherein 40 % reduction in coulombic efficiency was attributed merely to anodic methane emissions.
NH4 +-N and NO2 −-N nitrogen concentrations at MFC cathode were low and followed a specific trend at different initial NO3 −-N concentrations tested. A slight increase in the NO2 −-N followed by a drop after some time was observed indicating the denitrification activity (Park et al. 2005).
The optimum cathode pH was in the neutral to an alkaline range, and this corresponds with an optimum pH for denitrification. At low pH values, there was a concomitant drop in OCV, the power density at 100 Ω and the denitrification rate. The pH values tend to increase at the cathode and decrease at the anode upon prolonged incubation periods. This warrants the continuous pH control for increasing the process longevity. A similar phenomenon was reported by Clauwaert et al. (2009) wherein the pH control in the system sustained high denitrification for a long time.
The usefulness of this system for nitrate removal with simultaneous power generation can be explained by the experiments performed in a low resistance MFC setup. We achieved a power density of 210 mW/m2 and a current density of 302.17 mA/m2.
In the present study, we employed cow manure and soil that gave us good results in terms of nitrate removal. However, the composition of soil microflora is subject to change with seasonal variations, prevailing climatic conditions, water activity, chemical composition, etc. (Wolsing and Prieme 2004). Therefore, the experiments involving direct use of soil is expected to give large variations in the data. It is also impossible to maintain identical cell loading/cell activities in duplicate experimental setups because of the prevailing heterogeneity in the samples used. Therefore, it is important to develop a microbial consortium originating from high performing MFC that are capable of sustaining high-rate denitrification. This will help solve the problem of the direct use of soil for MFC operation. But in general, the soil is a rich source of denitrifying bacteria (O ‘Leary et al. 2002), and this study emphasizes that cow manure amended soil offer high NO3 −-N removal rates and that this phenomenon can be exploited in MFC setup to treat nitrate contaminated wastewater with some energy recovery.
Conclusions
High-rate denitrification process has been developed using cow manure and soil at MFC cathode. Nitrate removal rate of 7.1 ± 0.9 kg NO3 −-N/m3 NCC/day was obtained at COD/NO3 −-N ratio of 7.31. Heterotrophic conditions at the cathode did not alter the energy generation from MFC. Closed circuit conditions supported high denitrifying bacterial titers at the cathode which in turn promoted high denitrification rates. Power density value of 210 mW/m2 with nearly reproducible nitrate removal rates in an MFC with low internal resistance emphasized the usefulness of such systems for wastes to energy conversion. The developed process is also useful for the wastes characterized by high nitrate concentrations.
References
Akunna JC, Bizeau C, Moletta R (1992) Denitrification in anaerobic digesters: possibilities and influence of wastewater COD/N-NOx ratio. Environ Sci Technol 13:825–836
APHA (1992) Standard methods for examination of water and waste-water, 18th edn. American Public Health Association, Washington, DC
Bailey LD, Beauchamp EG (1971) Nitrate reduction and redox potentials measured with permanently and temporarily placed platinum electrode in saturated soil. Can J Soil Sci 51:51–58
Cheng S, Liu H, Logan BE (2006) Increased power generation in a continuous flow MFC with advective flow through the porous anode and reduced electrode spacing. Environ Sci Technol 40:2426–2432
Clauwaert P, Rabaey K, Aelterman P, Schamphelaire LD, Pham TH, Boeckx P, Boon N, Verstraete W (2007) Biological denitrification in microbial fuel cells. Environ Sci Technol 41:3354–3360
Clauwaert P, Desloover J, Shea C, Nerenberg R, Boon N, Verstraete W (2009) Enhanced nitrogen removal in bio-electrochemical systems by pH control. Biotechnol Lett 31:1537–1543
Fetzer S, Bak F, Conrad R (1993) Sensitivity of methanogenic bacteria from paddy soil to oxygen and dessication. FEMS Microbiol Ecol 12:107–115
Fredrickson JK, Zachara JM, Balkwill DL, Kennedy D, Li SW, Kostandarithes HM, Daly MJ, Romine MF, Brockman FJ (2004) Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the Hanford Site Washington State. Appl Environ Microbiol 70:4230–4241
Ghafari S, Hasan M, Aroua MK (2008) Bio-electrochemical removal of nitrate from water and wastewater—a review. Bioresour Technol 99:3965–3974
Gregoire KP, Glaven SM, Hervey J, Lin B, Tenderb LM (2014) Enrichment of a high-current density denitrifying microbial biocathode. J Electrochem Soc 161:H3049–H3057
Gregory KB, Bond DR, Lovley DR (2004) Graphite electrodes as electron donors for anaerobic respiration. Environ Microbiol 6:596–604
Hayden AO, Gu AZ (2008) Comparisons of organic sources for denitrification: biodegradability, denitrification rates, kinetic constants and practical implication for their application in WWTPs. Proceedings of the Water Environment Federation. WEFTEC 253–273
Huang J, Wang Z, Zhu C, Ma J, Zhang X, Wu Z (2014) Identification of microbial communities in open and closed circuit bioelectrochemical MBRs by high-throughput 454 pyrosequencing. PLoS ONE 9(4):e93842
Keiner A, Leisinger T (1983) Oxygen sensitivity of methanogenic bacteria. Syst Appl Microbiol 4(3):305–312
Kelly PT, He Z (2014) Nutrients removal and recovery in bioelectrochemical systems: a review. Bioresour Technol 153:351–360
Koike I, Sorensen J (1988) Nitrate reduction and denitrification in marine sediments. In: Blackburn TH, Sorensen (ed). Wiley, New York 251–273
Lee CY, Ho KL, Lee DJ, Suc A, Chang JS (2012) Electricity harvest from nitrate/sulfide-containing wastewaters using microbial fuel cell with autotrophic denitrifier, Pseudomonas sp. C27. Int J Hydrog Energy 37:15827–15832
Li W, Zheng P, Guo J, Ji J, Zhang M, Zhang Z, Zhan E, Abbas G (2014) Characteristics of self-alkalization in high-rate denitrifying automatic circulation (DAC) reactor fed with methanol and sodium acetate. Bioresour Technol 154:44–50
Logan BE, Hamelers B, Rozendal R, Schroder U, Keller J, Freguia S et al (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Min B, Cheng S, Logan BE (2005) Electricity generation using membrane and salt bridge microbial fuel cells. Water Res 39:1675–1686
Moral R, Moreno-Caselles J, Perez-Murcia MD, Perez-Espinosa A, Rufete B, Paredes C (2005) Characterisation of the organic matter pool in manures. Bioresour Technol 96:153–158
O ‘Leary M, Rehm G, Schmitt M (2002) Understanding nitrogen in soils. University of Minnesota, Extension Service, Minneapolis, USA, p 5
Oh J, Yoon SM, Park JM (2001) Denitrification in submerged biofilters of concentrated nitrate wastewater. Water Sci Technol 43:217–223
Park H, Kim DK, Choi YJ, Pak D (2005) Nitrate reduction using an electrode as direct electron donor in a biofilm-electrode reactor. Process Biochem 40:3383–3388
Puig S, Coma M, Desloover J, Boon N, Colprim J, Balaguer MD (2012) Autotrophic denitrification in microbial fuel cells treating low ionic strength waters. Environ Sci Technol 46:2309–2315
Qambrani NA, Jung SH, Ok YS, Kim YS, Oh SE (2013) Nitrate-contaminated groundwater remediation by combined autotrophic and heterotrophic denitrification for sulphate and pH control: batch tests. Environ Sci Pollut Res 20:9084–9091
Rocca CD, Belgiorno V, Meric S (2006) An heterotrophic/autotrophic denitrification (HAD) approach for nitrate removal from drinking water. Process Biochem 41:1022–1028
Ruiz G, Jeison D, Chamy R (2006) Development of denitrifying and methanogenic activities in USB reactors for the treatment of wastewater: effect of COD/N ratio. Process Biochem 41:1338–1342
Sukkasem C, Xu S, Park S, Boonsawang P, Liu H (2008) Effect of nitrate on the performance of single chamber air cathode microbial fuel cells. Water Res 42:4743–4750
Takaya N, Antonina MB, Sakairi C, Sakaguchi Y, Kato I, Zhou Z, Shoun H (2003) Aerobic denitrifying bacteria that produce low levels of nitrous oxide. Appl Environ Microbiol 69:3152–3157
Tong Y, He Z (2013) Nitrate removal from groundwater driven by electricity generation and heterotrophic denitrification in a bioelectrochemical system. Hazard J Mater 262:614–619
Velvizhi G, Goud RK, Mohan SV (2014) Anoxic bio-electrochemical system for treatment of complex chemical wastewater with simultaneous bioelectricity generation. Bioresour Technol 151:214–220
Vilar-Sanz A, Puig S, Garcia-Lledo A, Trias R, Balaguer MD, Colprim J et al (2013) Denitrifying bacterial communities affect current production and nitrous oxide accumulation in a microbial fuel cell. PLoS One 8(5):e63460
Virdis B, Rabaey K, Yuan Z, Keller J (2008) Microbial fuel cells for simultaneous carbon and nitrogen removal. Water Res 42:3013–3024
Virdis B, Rabaey K, Yuan Z, Rozendal RA, Keller J (2009) Electron fluxes in a microbial fuel cell performing carbon and nitrogen removal. Environ Sci Technol 43:5144–5149
Wang LK, Pereira NC, Hung YT, Shammas NK (2009) Biological treatment processes, vol 8. Humana Press, New York, p 569
Wolsing M, Prieme A (2004) Observation of high seasonal variation in community structure of denitrifying bacteria in arable soil receiving artificial fertilizer and cattle manure by determining T-RFLP of nir gene fragments. FEMS Microbiol Ecol 48:261–271
Xu G, Peng J, Feng C, Fang F, Chen S, Xu Y, Wang X (2015) Evaluation of simultaneous autotrophic and heterotrophic denitrification processes and bacterial community structure analysis. Environ Biotechnol 99:6527–6536
Zhang Y, Angelidaki I (2013) A new method for in situ nitrate removal from groundwater using submerged microbial desalination–denitrification cell (SMDDC). Water Res 47:1827–1836
Zhang F, He Z (2012) Integrated organic and nitrogen removal with electricity generation in a tubular dual-cathode microbial fuel cell. Process Biochem 47:2146–2151
Zhang Y, Noori JS, Angelidaki I (2011) Simultaneous organic carbon, nutrients removal and energy production in a photomicrobial fuel cell (PFC). Energy Environ Sci 4:4340–4346
Zhang J, Zheng P, Zhang M, Chen H, Chen T, Xie Z, Jing C, Abbas G (2013) Kinetics of substrate degradation and electricity generation in anodic denitrification microbial fuel cell (AD-MFC). Bioresour Technol 149:44–50
Zhao Y, Zhang B, Feng C, Huang F, Zhang FP, Zhang Z et al (2012) Behavior of autotrophic denitrification and heterotrophic denitrification in an intensified biofilm-electrode reactor for nitrate-contaminated drinking water treatment. Bioresour Technol 107:159–165
Zhu G, Onodera T, Tandukar M, Pavlostathis SG (2013) Simultaneous carbon removal, denitrification and power generation in a membrane-less microbial fuel cell. Bioresour Technol 146:1–6
Zhu G, Chen G, Yu R, Li H, Wang C (2015) Enhanced simultaneous nitrification/denitrification in the biocathode of a microbial fuel cell fed with cyanobacteria solution. Process Biochem. doi:10.1016/j.procbio.2015.11.004
Acknowledgments
Author MC gratefully acknowledges Board of Research in Nuclear Sciences (BRNS), Department of Atomic energy (DAE), Government of India for financial support. Author AV thanks the Council of Scientific and Industrial Research (CSIR), India for her fellowship. Dr. Tessy Vincent from DAE is gratefully acknowledged for thoroughly reading the manuscript and Dr. SKS Sahoo from DAE is acknowledged for his timely support. All the authors thanks Mr. Bharat Pareek for his timely support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Vijay, A., Vaishnava, M. & Chhabra, M. Microbial fuel cell assisted nitrate nitrogen removal using cow manure and soil. Environ Sci Pollut Res 23, 7744–7756 (2016). https://doi.org/10.1007/s11356-015-5934-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5934-0