Abstract
Soils are rich in organics, particularly those that support growth of plants. These organics are possible sources of sustainable energy, and a microbial fuel cell (MFC) system can potentially be used for this purpose. Here, we report the application of an MFC system to electricity generation in a rice paddy field. In our system, graphite felt electrodes were used; an anode was set in the rice rhizosphere, and a cathode was in the flooded water above the rhizosphere. It was observed that electricity generation (as high as 6 mW/m2, normalized to the anode projection area) was sunlight dependent and exhibited circadian oscillation. Artificial shading of rice plants in the daytime inhibited the electricity generation. In the rhizosphere, rice roots penetrated the anode graphite felt where specific bacterial populations occurred. Supplementation to the anode region with acetate (one of the major root-exhausted organic compounds) enhanced the electricity generation in the dark. These results suggest that the paddy-field electricity-generation system was an ecological solar cell in which the plant photosynthesis was coupled to the microbial conversion of organics to electricity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial fuel cells (MFCs) are devices that exploit microorganisms to generate electric power from organic matter and are potentially applicable to the treatment of organic wastes (Logan et al. 2006). Attempts have also been made to apply MFC systems to recover electric power from marine and river beds (termed sediment MFC [SMFC] or benthic MFC; Reimers et al. 2001; Tender et al. 2002; Reimers et al. 2006). These systems utilize the natural potential gradient between the sediment and upper oxic water, and electrons released by the microbial oxidation of organic matter flow from the anode (in sediment) to the cathode (in water) through an external circuit. It was initially reported that marine SMFC systems generated stable outputs of 10 to 20 mW/m2 (per anode projection areas; Reimers et al. 2001; Tender et al. 2002). Recently, since the low output was due in part to a low organic content in the sediment, a laboratory experiment was conducted to demonstrate that power generation could be improved by supplementing the sediment with organic substrates (Rezaei et al. 2007). Although the power output from SMFCs is moderate, such levels of output are considered to be sufficient to serve as a remote power source in aquatic environments.
A paddy field is a flooded parcel of arable land used for growing rice and other semiaquatic crops. In Japan, rice paddy fields cover 2.5 million ha and occupy more than 50% of the total arable land areas in this country (Ministry of Agriculture, Forestry and Fisheries 2006). When a paddy field is flooded, the soil immediately below the surface becomes anaerobic (Takai 1969), and a community of anaerobic microbiota (comprised mainly of sulfate-reducing bacteria, iron-reducing bacteria, fermenting bacteria and methanogenic archaea) is established (Grosskopf et al. 1998; Chin et al. 1999). Since a potential gradient is known to be formed between the soil and the flooded water, it was anticipated that an SMFC system could operate in a paddy field.
In the present study, we installed an electricity-generating system similar to an SMFC in a rice paddy field during the rice-cropping season (from May to September) and examined how much electricity was generated. In addition, in order to understand the mechanisms for electricity generation, we analyzed plant-root exhausts and bacterial populations occurring on the electrodes. Based on the results obtained, we suggest that the power-generating system in the rice paddy field is an ecological solar cell in which plant photosynthesis was coupled to the microbial conversion of organics into electricity.
Materials and methods
Paddy field
An area (approximately 1 m × 5 m) of the experimental rice paddy field in the university farm of Yamagata University (Tsuruoka, Japan) was used for electricity-generation experiments during the rice-cropping season (from May to September) in 2007. The soil of the university farm was classified as sandy loam. The average total carbon and nitrogen contents of the soil were 1.5% and 0.2% (w/w), respectively. The field was annually supplemented with rice straws (4 t ha−1) and barnyard manure (20 t ha−1) as organic matter over the past 3 years. Rice straws were spread on the soil surface when rice grains were harvested in the previous autumn and plowed into the soil at the time of plowing in April as described elsewhere (Kaku et al. 2000). Barnyard manure was applied in late March. A basal application was carried out with 39–39–39 kg ha−1 of N–P2O5–K2O at the beginning of irrigation, and a topdressing was carried out with 14–2–17 kg ha−1 of N–P2O5–K2O at the panicle formation stage. The plowing and beginning of irrigation were performed on April 19th, and transplanting (22.2 hills m−2 of planting density and 5 rice [Oryza sativa L. cv. Sasanishiki] seedlings hill−1) was performed on May 15th. A midseason drainage was carried out from July 9th to July 20th. After the midseason drainage, an intermittent irrigation was performed until the drainage of residual water on September 10th. The maximum-tiller-number stage and heading stage were July 20th and August 7–10th, respectively. Rice was harvested on October 13th. The temperature ranged from 10°C to 35°C during the experimental period. Amount of global solar radiation was ∼30 MJ/m2/day1 in sunny days, while it was ∼20 MJ/m2/day1 in cloudy and rainy days. The following herbicides were applied to the rice paddy fields on June 4th: Mogeton granules (containing 9% [w/w] 2-amino-3-chloro-1,4-naphthoquinone, a photosynthetic inhibitor for algae and duckweed [Agro Kanesho, Tokyo, Japan]) and Apirotop granules (containing 1.8% Pyriftalid and 1.8% Pretilachlor as the main components, protein synthetic inhibitors for suppressing soil-surface weeds [Syngenta, Tokyo, Japan]). Oxidation/reduction potential (ORP) and pH were measured using a TOA HM-11P pH Meter (TOA Electronics, Nagoya, Japan). Methane emission was measured by the closed chamber method (Yagi and Minami 1990) using plastic chambers that covered four rice-plant hills. A ferrous-ion concentration in rhizosphere soil was determined by the ferrozine method (Stookey et al. 1970) after extraction with 0.5 N HCl as described previously (Lovley et al. 1987; Kaku et al. 2005). Plant growth was evaluated by measuring the number of stalks, the height of leaves, and crop yield.
Electricity-generating system
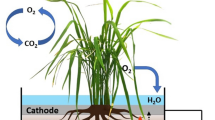
An anode (a projection area of 1,350 cm2) and a cathode (900 cm2) were made of graphite felt (3 mm in thickness, GF-80-3F; Sohgoh Carbon [Yokohama, Japan]). These electrodes covered an area of four rice-plant hills (see panels a to c in Fig. 1). In order to stably plant rice seedings, four holes were cut in the electrodes. The anode was set approximately 5 cm below the surface of soil, while the cathode was put immediately above the soil surface. These electrodes were connected via epoxy-encapsulated wires, and the circuit was completed using an external resister of 100 Ω. The voltage across the resister was monitored every 5 min using a data logger (Tachologger DQ5000; Graphtec, Tokyo, Japan). Graphite felts that were not connected via an external circuit were also set as described above and served as a control system. The power-generating system and control system were set in triplicate. Polarization curves and power density–current curves were made by using different resisters, and internal resistances and power densities were calculated as described elsewhere (Logan et al. 2006).
The paddy-field power-generating system. a Schematic illustration showing positions of the anode and cathode relative to rice plants. b The power-generating system immediately after staring the operation (May 16th). c The power-generating system in operation (June 23rd). d The backside of an anode graphite felt showing that rice roots penetrated the anode (August 7th)
To examine the effects of the sunlight, rice plants and the cathode surface were shaded using hard papers or black polymer sheets which almost completely blocked the sunlight. An acetate solution (500 ml of 1 mM sodium acetate, pH 7.0) was evenly injected into several different locations of the anode region using a syringe.
Analysis of root exudates
Hills of rice plants (147 ± 10 g [mean ± SE], n = 3) that had been shaded for 1 day were carefully pulled from the paddy field, and their roots were washed with water to remove the soils. The rice plants were put in a flask, and their roots were soaked in 500 ml of distilled water supplemented with antibiotics (tetracycline, carbenicillin, chloramphenicol, and kanamycin; each 20 mg/ml). They were incubated in the sunlight (n = 3), and water samples were taken 2 h after initiating the incubation. The rice plants were also incubated in the dark (n = 3) to reveal the effects of sunlight. The total organic carbon (TOC) of a water sample was measured using a TOC analyzer (TOC5000; Shimadzu, Kyoto, Japan) according to the manufacturer’s instructions. Organic acids (pyruvate, malate, succinate, lactate, fumarate, acetate, propionate, and butyrate) were measured by liquid chromatography using a LC-6A HPLC system equipped with an electroconductivity detector (CDD-10AVP; Shimadzu), a Shim-pack SCR-102H column (8 mm i.d. × 300 mm; Shimadzu), and a guard column (SCR-102H, 6 mm i.d. × 50 mm; Shimadzu) according to the manufacturer’s instructions. Organic acids were eluted with 5 mM p-toluenesulfonic aqueous solution as solvent at a flow rate of 0.8 ml per min at 45°C. Before being introduced to the detector, acidic eluents were reacted with 20 mM bis(2-hydroxyethyl)-iminotris(hydroxymetyl) methane aqueous solution containing 5 mM p-toluenesulfonic acid and l00 μM ethylenediaminetetraacetic acid. Eluents were identified and quantified based on retention times and peak areas, respectively, according to those of pure chemicals. Sugars (glucose, galactose, maltose, fructose, lactose, and sucrose) were measured by enzyme assays using the F-kit (J.K. International, Tokyo, Japan) according to the manufacturer’s instructions.
Analysis of bacterial populations
Small pieces of graphite felt (approximately 1 cm × 1 cm) were cut off in triplicate from central parts of the electrodes, put into sterile tubes, and stored at −20°C before use. Soil samples were taken in triplicate from the rice rhizospere where no electrode was set. Total DNA was extracted from the graphite felt and soil (approximately 0.5 g) using the FAST DNA spin kit for soil (Q-BIO gene, Irvine, CA, USA) according to the manufacturer’s instructions and finally dissolved in 50 μl of the DES solution supplied in the kit. Polymerase chain reaction (PCR) amplification of 16S rRNA gene fragments was performed using the extracted DNA solution (1 μl) and primers 341f-GC and 534r as described elsewhere (Watanabe et al. 2001). After amplified fragments were checked by electrophoresis through 1.5% (w/v) agarose gel (LO3, Takara) in a TBE buffer (Sambrook et al. 1989), they were analyzed by denaturing gradient gel electrophoresis (DGGE) using a DCode instrument (Bio-Rad laboratories; Richmond, CA, USA) as described previously (Watanabe et al. 1998). Ten percent (w/v) polyacrylamide gels with a 40–60% denaturant gradient (Watanabe et al. 1998) were used, and electrophoresis was performed for 3.5 h at 200 V at 58°C. Nucleotide sequences of DGGE bands were determined as described previously (Watanabe et al. 2001). Sequence homology searches were conducted using the GenBank nucleotide sequence library and the BLAST program (Atschul et al. 1990) through the National Center for Biotechnology Information (NCBI) internet site (http://www.ncbi.nlm.nih.gov/BLAST). The nucleotide sequences reported in this paper have been deposited in the DDBJ, EMBL, and NCBI nucleotide sequence databases under accession numbers AB363639 to AB363641.
Results
Trends in electric outputs
The pH in the rhizosphere soil was moderately acidic (e.g., 5.48 ± 0.40 [mean ± SE, n = 3] in August 7th). After flooding, the soil immediately became anaerobic, and ORP values were around −200 mV (e.g., −201 ± 6.3 [mean ± SE, n = 5] in August 7th). In contrast, ORP values in the flooded water were always higher than 0 mV (e.g., 123 ± 6.3 mV). After the power-generation system was set in the rice paddy field, an electric output of approximately 0.05 V was immediately detected, which then increased to more than 0.2 V within 7 days (Fig. 2a). Figure 1 shows the appearance of the electricity-generating system immediately after setting (panel b) and under operation (panels c and d). We found that rice roots penetrated the anode graphite felt and occasionally entwined with the graphite fibers (panel d). On June 4th, the herbicides were applied to the paddy field, resulting in the immediate drop of the electric output down to almost 0 V (Figs. 2a and 3), after which it took 1 week to recover the output to the original level. The output was decreased in July (a rainy season in Japan), while high outputs (as high as 0.3 V) were observed in August when hot sunny days continued. Dependency of the output on weather was observed throughout the monitoring period. We also found that the electric output increased in the daytime and decreased at night (Fig. 2b). These observations implied that the electric output was dependent on the sunlight. Such circadian oscillation was observed throughout the experiment.
Electric outputs from the paddy-field electricity-generating system. a Daily records of voltage at 11:00 in the morning. A The data logger failed, B rainy days continued during this period, C hot sunny days continued during this period. The arrow indicates the day when the herbicides were applied (June 4th). b Hourly records of voltage during a week showing a representative circadian oscillation in the electric output
We also analyzed the effects of electricity generation on the growth of rice plants (the number of stalks, the height of leaves, and crop yields), methane emission, and ferrous-ion concentration in soil, and no significant effects (as assessed by the t test, P = 0.05) were observed on these points when compared to the controls throughout the experimental period (data not shown).
Polarization and power-density curves
We obtained polarization data in the daytime by varying the circuit external resistance on June 23 and August 7, and power-density curves were drawn based on the polarization data (Fig. 4). Performance indices, i.e., the maximum power density (mW/m2, normalized to the anode projection area), internal resistance (Ω), and open-circuit voltage (V), were estimated to be 5.75, 156, and 0.701, respectively, for the June system, while those for the August system were 5.85, 58.2, and 0.428, respectively.
Polarization (voltage–current) curves (diamond) and power density–current curves (square) of the paddy-field electricity-generating system on June 23rd (white) and August 7th (black). First-order and second-order approximation curves were presented for the voltage–current and power density–current relationships, respectively
Effects of sunlight
In order to examine the effects of sunlight on the electric output, we shaded either rice plants or the surface of the cathode and then monitored the electric output. Figure 3 shows the relative levels of the output after the output became stable (∼2 h). This figure indicates that the shading of rice plants largely reduced the output, while there was no significant effect when only the cathode surface was shaded. This result suggests that plant photosynthesis was necessary for electricity generation.
Rice-root exudates
It has been reported that plants exhaust photosynthesized organic compounds from their roots (Jones 1998; Walker et al. 2003), and organic acids and sugars are the main components (Jones 1998). In order to examine if rice plants in the paddy field also exhausted organic compounds, we pulled rice plants from the paddy field and incubated them under the light and dark conditions by soaking their roots in water supplemented with the antibiotics. TOC, organic acids, and sugars in the root-soaked water were analyzed 2 h after commencing the incubation and compared with values at hour 0 (Fig. 5). We found that sunlight largely affected the amounts of organics exhausted from the roots. When the rice plants were incubated in the dark, the TOC value was slightly decreased from a value at hour 0 (28.6 ± 1.3 mg l−1 [mean ± SE, n = 3]); this may have been ascribable to microbial activities that were not completely inhibited by the antibiotics. Among the organic compounds detected, acetate was the most abundant, followed by glucose, and their production was also found to be sunlight dependent.
Since acetate was found to be the major organic exhausted from the rice roots, we investigated as to whether or not acetate artificially added to the rhizosphere/anode region enhanced the electricity generation in the dark (under the condition where rice plants did not produce organics). As presented in Fig. 3, the acetate addition resulted in the electric output rebounding to approximately 50% of the original level (the electric output before shading the rice plants), indicating that acetate was utilized by microbes for electricity generation.
Bacterial populations occurring on the electrodes
In MFCs, bacteria are considered to be responsible for electricity generation (Logan and Regan 2006; Lovley 2006). We analyzed the bacterial populations attaching onto the anode and cathode of the rice paddy-field power system by DGGE of PCR-amplified 16S rRNA gene fragments. The DGGE profiles were compared with those of bacteria associated with the graphite felts in the control system (electricity not generated) and also those in soil, allowing us to identify bacteria that specifically occurred in response to electricity generation. In addition, all samples were analyzed in triplicate (small pieces of graphite felt were cut out from different locations in the electrodes), allowing us to identify whether or not they occurred widely on the electrodes. As shown in Fig. 6, we detected several bands that specifically occurred on the anode or cathode of the power system (bands A1, A2, and C3), and these bands were excised for sequencing. Database searches revealed that band A1 was closely related to Natronocella acetinitrilica (accession number EF103127, 96% identical in nucleotide sequence), A2 was closely related to uncultured Beijerinckiaceae bacterium clone GASP-38KB-490-B06 (EU044137, 97%), and Rhizobiales bacterium A48 (AB081581, 96%), while C3 was closely related to Rhodobacter gluconicum (DQ363135, 99%). These bacteria were considered to be involved in the electricity generation observed in the rice paddy field.
DGGE profiles showing bacterial populations attaching onto graphite felts in the power and control systems and those in soil. Lanes 1 to 3 the anode felt in the power system, 4 to 6 the felt in the rhizosphere of the control system, 7 to 9 the cathode felt in the power system, 10 to 12 the felt in flooded water in the control system, 13 to 15, soil. Bands A1, A2, and C3 were excised and sequenced
Discussion
In the present study, we examined electricity generation in a rice paddy field using an SMFC-type power system by placing the anode in the rhizosphere and the cathode in the flooded water. Based on the results of our experiments, we concluded that the electricity-generating system in the rice paddy field was an ecological solar cell in which plant photosynthesis was linked to the microbial conversion of organics into electricity. The following observations supported this conclusion. First, the electric output was sunlight dependent; the shading of rice plants inhibited the electric output during the daytimes (but not the surface of the cathode, Fig. 4). Second, rice plants exhausted organic compounds from their roots in response to sunlight (Fig. 5). Third, supplementation of the anode region with acetate (one of the major root-exhausted organic compounds) enhanced electricity generation in the dark (Fig. 4). Fourth, herbicide addition, which had minimal effects on the rice plants, almost completely inhibited electricity generation, indicating that organisms other than the rice plants were necessary for electricity generation. Fifth, several bacterial populations specifically occurred on the anode during the electricity generation (Fig. 6). Since the organic compounds exhausted from the rice roots (including acetate and glucose [Fig. 5]) should have been easily metabolizable substrates for bacteria, this cooperation of the plants and bacteria could have been established relatively rapidly (∼1 week). In contrast, soil organic matter (such as humics) should have not been good substrates for microbes, resulting in the low output at night.
The maximum power density of the paddy-field power system was estimated to be approximately 6 mW/m2 (Fig. 3); this was lower than values reported for marine power systems (∼20 mW/m2 [Reimers et al. 2001]). Comparison of the June and August systems revealed that internal resistance was decreased during this period, while OCP was also decreased, resulting in the maximum power density being almost unchanged (Fig. 3). It was likely that the enrichment of electricity-generating bacteria resulted in lowering the internal resistance, while there were other rate-limiting steps that suppressed an increase in the power density. Several reasons could be considered for the low output. First, marine systems are considered to generate more electricity than freshwater systems in terms of high electrolyte (e.g., NaCl) concentrations [Reimers et al. 2001]. Second, we did not use platinum catalysts that could accelerate the cathode reaction (conversion of oxygen, proton plus electron to water; Oh et al. 2004) since there were concerns about its effects on rice production. Furthermore, since the anode had contact with small parts of rice roots, it could be deduced that root exhausts were only partially utilized for electricity generation. We suggest that anode design should be important for developing a more efficient electricity-generation system for paddy fields.
We have previously analyzed a laboratory-scale MFC reactor in which rice paddy field soil was used as an inoculum, and cellulose was used as the major organic substrate. Molecular phylogenetic analyses revealed that bacteria related to Rhizobiales bacteria (including strain A48) were abundantly detected in the anode biofilm communities that had been enriched for electricity generation (Ishii et al. 2008). A homologous sequence (band A2 in Fig. 6) was also detected in the present study, suggesting that this group of bacteria is important for electricity generation in MFC systems. Strain A48 was isolated from rice roots and is known to respire with ferric ions (Satoh et al. 2002). It is likely that this group of bacteria is capable of utilizing root exudates and respiring with an electrode in an MFC system. The cathode organisms represented by band C3 may have affected the cathode activity (the reduction of oxygen) since a recent manuscript have described that open-air biocathode enabled effective electricity generation in a microbial fuel cell (Clauwaert et al. 2007).
In conclusion, we observed for the first time plant/microbe cooperation in the sunlight-dependent biological generation of electricity. We consider that such plant/microbe-involving solar cells are widely applicable for electricity generation in plant-inhabiting wetlands. Our results presented herein will be useful for developing more efficient power systems in future engineering studies.
References
Atschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Chin KJ, Hahn D, Hengstmann U, Liesack W, Janssen PH (1999) Characterization and identification of numerically abundant culturable bacteria from the anoxic bulk soil of rice paddy microcosms. Appl Environ Microbiol 65:5042–5049
Clauwaert P, Van der Ha D, Boon N, Verbeken K, Verhaege M, Rabaey K, Verstraete W (2007) Open air biocathode enables effective electricity generation with microbial fuel cells. Environ Sci Technol 41:7564–7569
Grosskopf R, Janssen PH, Liesack W (1998) Diversity and structure of the methanogenic community in anoxic rice paddy soil microcosms as examined by cultivation and direct 16S rRNA gene sequence retrieval. Appl Environ Microbiol 64:960–969
Ishii S, Hotta Y, Watanabe K (2008) Methanogenesis versus electrogenesis: morphological and phylogenetic comparisons of microbial communities. Biosci Biotechnol Biochem 72:286–294
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant soil 205:25–44
Kaku N, Ueki A, Fujii H, Ueki K (2000) Methanogenic activities on rice roots and plant residue and their contributions to methanogenesis in wetland rice field soil. Soil Biol Biochem 32:2001–2010
Kaku N, Ueki A, Ueki K, Watanabe K (2005) Methanogenesis as an important terminal electron accepting process in estuarine sediment at the mouth of Orikasa River. Microb Environ 20:41–52
Logan BE, Regan JM (2006) Electricity-producing bacterial communities in microbial fuel cells. Trend Microbiol 14:512–518
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Lovley RD (2006) Microbial fuel cells: novel microbial physiologies and engineering approaches. Curr Opin Biotechnol 17:327–332
Lovley DR, Phillips EJP (1987) Rapid assay for microbially reducible ferric iron in aquatic sediments. Appl Environ Microbiol 52:751–757
Ministry of Agriculture, Forestry and Fisheries (2006) Statistics of agriculture, forestry and fisheries. Ministry of Agriculture, Forestry and Fisheries, Japan
Oh S, Min B, Logan BE (2004) Cathode performance as a factor in electricity generation in microbial fuel cells. Environ Sci Technol 38:4900–4904
Reimers CE, Tender LM, Fertig S, Wang W (2001) Harvesting energy from the marine sediment-water interface. Environ Sci Technol 35:192–195
Reimers CE, Girguis P, Stecher HA III, Tender M, Ryckelynck N, Whaling P (2006) Microbial fuel cell energy from an ocean cold seep. Geobiol 4:123–136
Rezaei F, Richard TL, Brennan RA, Logan BE (2007) Substrate-enhanced microbial fuel cells for improved remote power generation from sediment-based systems. Environ Sci Technol 41:4053–4058
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA
Satoh A, Watanabe M, Ueki A, Ueki K (2002) Physiological properties and phylogenetic affiliations of anaerobic bacteria isolated from roots of rice plants cultivated on a paddy field. Anaerobe 8:233–246
Stookey LL (1970) Ferrozine; a new spectrophometric reagent for iron. Anal Chem 42:779–781
Takai Y (1969) The mechanism of reduction in paddy soil. Jpn Agri Res 4:20–23
Tender LM, Reimers CE, Stecher HA III, Holmes DE, Bond DR, Lowy DA, Pilobello K, Fertig SJ, Lovley DR (2002) Harnessing microbially generated power on the seafloor. Nat Biotechnol 20:821–825
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132:44–51
Watanabe K, Kodama Y, Harayama S (2001) Design and evaluation of PCR primers to amplify bacterial 16S ribosomal DNA fragments used for community fingerprinting. J Microbiol Methods 44:253–262
Watanabe K, Teramoto M, Futamata H, Harayama S (1998) Molecular detection, isolation, and physiological characterization of functionally dominant phenol-degrading bacteria in activated sludge. Appl Environ Microbiol 64:4396–4402
Yagi K, Minami K (1990) Effect of organic matter application on methane emission from some Japanese paddy fields. Soil Sci Plant Nutr 36:599–610
Acknowledgments
We thank Katsuji Ueki and Atsuko Ueki for valuable discussions, Yoichi Kikuchi and Yuka Sasaki for paddy-field management, Midori Sato for technical assistance, and Greg Newton for critical reading of this manuscript. This work was supported by Japan Society for Promotion of Science (JSPS). K.W. was also supported by Japan Science and Technology Agency (JST).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaku, N., Yonezawa, N., Kodama, Y. et al. Plant/microbe cooperation for electricity generation in a rice paddy field. Appl Microbiol Biotechnol 79, 43–49 (2008). https://doi.org/10.1007/s00253-008-1410-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1410-9