Abstract
Endoscopic resection emerged as a less invasive alternative for treatment of esophageal neoplasm associated with Barrett’s esophagus (BE). The Western guidelines recommend endoscopic resection for all visible early esophageal neoplasms for staging as well as treatment. Endoscopic mucosal resection (EMR) is currently the most commonly available treatment of BE associated neoplasm particularly in the West, because of technically easy and simple procedure. Endoscopic submucosal dissection (ESD) can provide en bloc resection and thus a more complete assessment of histology regardless of the size and location. R0 resection of ESD is higher than that of EMR; however, this has not translated into clinically meaningful benefits to date. Thus, ESD for BE-related neoplasia is commonly performed in some Asian countries; however, it is still not yet ready for widespread clinical use. Further prospective studies are warranted to clarify the effectiveness of ESD for esophageal neoplasm associated with BE.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Barrett’s adenocarcinoma
- Esophageal adenocarcinoma
- High-grade dysplasia
- Endoscopic resection
- Endoscopic mucosal resection
- Endoscopic submucosal dissection
1 Introduction
Historically, radical esophagectomy was the standard of care for the management of esophageal cancer including high-grade dysplasia (HGD) and early esophageal adenocarcinoma (EAC) associated with Barrett’s esophagus. However, esophageal surgery is associated with major morbidity and high mortality rates [1,2,3,4]. Endotherapy is minimally invasive treatment option for early gastrointestinal cancer including Barrett’s adenocarcinoma, which allows for curative resection for the lesion without risk of lymph node metastasis while preserving organ function. Endoscopic resection emerged as a less invasive alternative for treatment of superficial esophageal cancer and is currently the gold standard. Endotherapy for Barrett’s adenocarcinoma is generally divided into endoscopic resection and ablation by the use of thermal therapy or cryogens. Particularly, the former allows for removal of visible lesions, which serves to provide accurate histologic staging (distinguishing dysplasia and mucosal adenocarcinoma from submucosal adenocarcinoma) and determine subsequent management of the patient. This chapter presents an overview of indication, technique, and treatment outcomes of endoscopic resection for esophageal dysplasia and adenocarcinoma associated with Barrett’s esophagus.
2 Criteria for Curative Endoscopic Resection
Curability of endoscopic resection is generally determined by completeness of the primary tumor removal and nil possibility of lymph node metastasis. The endoscopically resected specimen allows for optimal histological staging including the depth of invasion, which provides further strategy for patients.
A recent systematic review by Dunber et al. revealed that no metastases were found in 524 patients with HGD, whereas 26 of the 1350 patients with intramucosal carcinoma had positive lymph nodes (1.93%, 95% CI 1.19–2.66%) [5]. Manner et al. retrospectively analyzed 72 patients who had a proven maximum invasion depth of SM1 (<500 μm) [6]. The rate of lymph node metastasis was 2% (1/49) in the low-risk group (well- or moderately differentiated tumor grade and absence of tumor invasion into lymphatics or blood vessels) and 9% (2/23) in the high-risk group (other than low-risk group). Although the treatment strategy for patients with a T1b is controversial, endoscopic resection might be considered for the low-risk group because risk of lymph node metastasis could be lower than the mortality rate of esophagectomy.
Given the evidence, American College of Gastroenterology (ACG) clinical guidelines stated as follows: (1) Endoscopic therapy is the preferred therapeutic approach, being both effective and well tolerated in patients with T1a esophageal adenocarcinoma. (2) Endoscopic therapy may be an alternative strategy to esophagectomy, especially in those with SM1 with low risk of metastasis although consultation with multidisciplinary surgical oncology team should occur before embarking on endoscopic therapy [7]. Similarly, ESGE position statement determined that the optimal treatment strategy in patients with T1b EAC depended on histopathological characteristics of the endoscopic resection specimen, and endoscopic resection might be a valid alternative to surgery and was recommended in patients who were borderline fit for surgery, if the endoscopic resection specimen met all of aforementioned criteria [8]. However, experience in Europe is limited above all in relation to the not yet completely appropriate preparation of endoscopist and pathologists who deal with this topic. Recently, a multicenter retrospective study from Japan demonstrated that no metastasis was detected in patients who had lesions without lymphovascular involvement, a poorly differentiated component with invasion into the deep muscularis mucosa (0/88) and superficial submucosa (≤500 μm) 30 mm or less in size (0/32) [9].
In summary, additional surgery can be avoided if the resected specimen showed HGD, T1a EAC, or selected T1b EAC (well- or moderately differentiated EAC, SM1 (<500 μm) in depth without lymph node metastasis or positive deep margin, strictly ≤30 mm in size). Additional surgery should be considered given the risk of lymph node metastasis if the histology doesn’t meet the criteria.
3 Preoperative Diagnosis for Endoscopic Resection
In general, endoscopic resection is local treatment and thus indicated for the gastrointestinal cancer which has negligible risk of lymph node metastasis. In addition, minimal risk of lymph node metastasis for endoscopic resection is acceptable if the mortality of surgery exceeds its risk. Thus, careful patient selection by accurate staging is essential to embark on curative endoscopic therapy. Preoperative endoscopic staging for gastrointestinal neoplasms is commonly performed based on careful inspection of the target lesion, histological diagnosis with forceps biopsy, and the depth diagnosis using conventional endoscopy and endoscopic ultrasound (EUS).
In terms of endoscopic appearance, Oda et al. found that mucosal esophagogastric junction adenocarcinoma was significantly smaller than submucosal invasive lesions. Non-polypoid type without mixed type (0-IIa, 0-IIb, or 0-IIc) lesions had a significantly lower risk for SM invasion compared to polypoid type (0-I) and mixed type (0-IIa + IIc or 0-IIc + IIa) lesions. In the polypoid type lesions, the risk for SM invasion was significantly lower for the pedunculated subtype (0-Ip) than for the sessile subtype (0-Is) lesions. Although this study included non-Barrett adenocarcinoma and didn’t subclassify SM1 and SM2 in depth, this simple determination of endoscopic macroscopic type may be useful in depth diagnosis [10].
Preoperative both random/targeted biopsy and endoscopic ultrasound (EUS) are performed in addition to endoscopic assessment of the target lesion. In the West, a recently published meta-analysis reported that EUS detected advanced disease in only a minority of patients with HGD or early EAC and therefore was considered of limited utility [11]. In addition, endoscopic forceps biopsy correlated with EMR findings in only 50% of patients [12]. Thus, the Western guidelines recommend that irrespective of the endoscopic forceps biopsy results, all visible lesions associated with Barrett’s esophagus should be removed by means of endoscopic resection techniques, generally EMR in order to obtain optimal histopathological staging [7, 8, 12].
4 Endoscopic Mucosal Resection (EMR)
EMR is currently the most commonly available treatment of BE associated neoplasia particularly in the West, because of technically easy and simple procedure. The principle of technique is mainly based on the creation of a “cushion” by submucosal injection of a saline solution or other materials which allow the detachment of neoplastic lesion from muscularis propria. Following technique is snare application for the flat target in the BE with use of some devices.
EMR is performed in order to remove visible early neoplastic lesion in Barrett’s Esophagus as possible alternative to surgery with a low related procedure morbidity (0–14% risk of bleeding and 1.8% perforation risk with no death) [12,13,15]. In addition, EMR plays an important role of precise histological assessment, because it can change diagnosis in approximately 30% of BE in comparison with pre EMR biopsy [16].
Two main technique for carrying out EMR are present in literature reports: cap assisted mucosectomy (EMR-C) and multiband mucosectomy (MBM) (Fig. 15.1). A randomized controlled trial comparing between EMR-C and MBM demonstrated that the cap technique with submucosa injection and the ligation technique without submucosa injection were similar with respect to efficacy and safety for endoscopic resection of early stage esophageal adenocarcinoma [14].
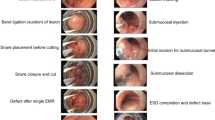
A band-assisted EMR. (a) White light endoscopy demonstrated a flat elevated lesion on the left side of long-segment Barrett’s esophagus. (b) Chromoendoscopy with indigo carmine spraying visualized the margin of the lesion. (c) A band was ligated around the lesion following suction. (d) The resected specimen histologically revealed high-grade dysplasia, 5 mm in size
It is considered safe and effective with complete remission in 98% of patients after 40 months of follow-up [17]. However, EMR can only achieve en bloc resection of lesions smaller than 15–20 mm due to the limited size of snare, and one of the risk factors most frequently associated with recurrence is piecemeal resection [18]. However, ablation therapy is generally scheduled for the background BE in order to treat the residual tumor and intestinal metaplasia. Actually it seems EMR for any visible lesions plus eradication of residual metaplastic mucosa is safe and efficacious so the need for esophagectomy has been eliminated for high-grade dysplasia and greatly reduced for mucosal cancer [19].
5 Endoscopic Submucosal Dissection (ESD)
Endoscopic submucosal dissection can provide en bloc resection and thus a more complete understanding of the lateral margins of a lesion regardless of the size and location of gastrointestinal cancer. This technique was firstly introduced in early gastric cancer and then applied to colorectal cancer and esophageal squamous cell carcinoma [19,20,22]. Esophageal ESD is technically challenging due to the following reasons: (1) The narrow lumen of the esophagus makes gravity counter traction less effective. (2) The resected specimen retracts distally making it difficult to maintain good traction and orientation. (3) The thin wall of the esophagus increases the risk of perforation. Some items mentioned below should be used for safe procedure.
5.1 Items for Safe and Effective Esophageal ESD
First, distal endocap is essential to stabilize the operation field against respiratory movements; it helps us to access the submucosal plane facilitating the submucosal dissection.
Second, high viscous injection solution is strongly recommended to safe and efficient ESD because esophageal wall is very thin compared with that of stomach. In Asia, sodium hyaluronate 0.4% (MucoUp; Boston scientific, Tokyo, Japan) is widely used, with the disadvantage of being expensive [23]. Glycerol (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) has also been used in Japan, with the advantages of being inexpensive and producing a long-lasting lift [24]. In the West, hydroxyethyl starch (Voluven, Fresenius/Hospira, Germany) and 0.4% hydroxypropyl methylcellulose has been typically used [25, 26]. Recently, a polymer- and methylene blue-containing solution (Eleview™, Cosmo Technologies Ltd., Dublin, Ireland) was approved by the Food and Drug Administration (FDA) for submucosal lift of lesions in the upper and lower gastrointestinal tract. A blinded randomized controlled trial in an ex-vivo porcine model comparing different submucosal injection solutions demonstrated the superior long-lasting lifting effect of Eleview and Volven to the submucosal injection fluids available in the West [27].
Third, CO2 insufflation can be rapidly absorbed allowing for the reduction of patient’s abdominal fullness and pain in addition to minimal air leak in case of perforation [28]. Moreover, monitored anesthesia care and deep sedation is preferred for esophageal ESD [29]. General anesthesia can be considered for less-experienced endoscopists, because of the long procedure times, and the risk of aspiration of secretions or blood. In addition, positive pressure of mediastinum in general anesthesia can help minimize air leak in case of perforation.
5.2 Technical Tips and Tricks of ESD of Visible Lesions Associated with Barrett’s Esophagus (Fig. 15.2)
The following technical tips and tricks are recommended to perform the advanced procedure safely.
-
(a)
Marking
Appropriate identification, mapping, and demarcation of the lesion is mandatory before starting ESD. Circumferential marking should be carefully performed. The tip of a needle-type or argon plasma coagulation can be used to sharply and clearly mark at 3–5 mm from the edge of the lesion. Soft coagulation mode (effect 5, 80 W in VIO 300D (ERBE Tuebingen, Germany) or 50 W in ESG100 (Olympus)) or forced APC mode (effect 3, 30 W) is recommended to avoid perforation of the thin wall of the esophagus (Fig. 15.2a, b).
-
(b)
Submucosal Injection
As mentioned, high viscous injection solution allows for safe and efficient ESD. These lifting solutions can be easily injected to muscle layer when injected deeply, it is essential to make sure good submucosal elevation by normal saline prior to the high viscous solutions.
-
(c)
Mucosal incision
In esophageal ESD, partial circumferential incision is preferred to prevent the escape of fluid from the submucosal layer. Additionally, it is very important to firstly incise muscularis mucosa to expose the lucent submucosal plane following enough submucosal lifting (Fig. 15.2c). Suction of the air makes submucosal cushion thicker and helps perform safe and effective mucosal incision.
The oral and anal incisions are made first. A retrograde approach is often required for part of the resection when the lesion is located on or near the EGJ (Fig. 15.2e). Mucosal incision along the left lateral border mucosal lesion is then performed allowing the lesion to retract away from the water pool on the gravity dependent side. Circumferential incision of the right lateral wall is then completed when approximately three-fourths of the lesion had been dissected.
-
(d)
Submucosal dissection
After enough exposure of the submucosal layer of the proximal side, the lesion is then lifted with injection of a lifting solution. The submucosa can be dissected with a needle-type device or IT knife nano (KD-612 L/U; Olympus) by hooking and cutting the submucosa. It is important to enter the submucosa with the use of the tip of endocap for direct visualization of submucosa, allowing for safe submucosal dissection avoiding perforation. Similarly to mucosal incision, submucosal dissection should be started from the left side allowing the lesion to retract away from the water pool on the gravity dependent side when performed in left lateral position (Fig. 15.2d).
A standard ESD of Barrett’s adenocarcinoma. (a) Flat elevated lesion was seen on the right side of esophagogastric junction. (b) Marking. (c) Partial mucosal incision of the left side. (d) Identification of submucosal plane to dissect with the use of endocap. (e) Submucosal dissection by retroflexed approach. (f) Mucosal defect after ESD. (g) The resected specimen histologically revealed well-differentiated tubular adenocarcinoma, 16 mm, T1a-LPM, ly(−), v(−), pHM0, pVM0
5.3 Current Technical Innovation of ESD
In esophageal ESD, it is generally difficult to keep good tissue traction during the procedure, particularly for the distal side in a large lesion. Recently clip line traction method is commonly used for submucosal dissection in ESD of esophageal squamous cell carcinoma [30]. A catheter was inserted through an accessory channel of the endoscope, with an endoclip attached to the catheter. The loaded clip was left half-open. A length of commercial line was tied directly to the arm of the endoclip. Subsequently, the endoclip with line was placed in the accessory channel to enable reinsertion of the endoscope into the stomach, followed by re-exposure of the endoclip and anchoring to backside of the proximal side of the mucosal flap for per-oral traction (Fig. 15.3g). It allows for improved exposure of submucosa allowing easier identification of the edges of exposed submucosa to direct dissection (Fig. 15.3h). One prospective study showed clip line traction contributed to significantly shorten the procedure time in ESD of esophageal squamous cell carcinoma [31].
An extensive ESD with the use of innovative techniques. (a) Large flat elevated lesion involving almost complete luminal circumference. (b) Marking of the proximal side. (c) Marking of the distal side. (d) Circumferential mucosal incision of the proximal side. (e) Circumferential mucosal incision of the distal side. (f) Tunneling dissection of the left side. (g) Clip line traction technique. (h) Well-visualized submucosa with tissue retraction by clip-line traction. (i) Mucosal defect after complete Barrett excision. (j) A syringe shaped specimen. (k) The opened specimen (l) The resected specimen histologically revealed moderately to well-differentiated tubular adenocarcinoma, 52 mm, T1a-MM, ly(−), v(−), pHM0, pVM0
In addition, submucosal tunneling method is proposed to keep nice visualization of submucosal layer and submucosal fluid cushion. This technique allows for safe ESD procedure shortening time [32]. It can be performed with use of IT knife nano device even for large esophageal Barrett’s adenocarcinoma involving complete luminal circumference (Fig. 15.3e). Although these techniques were originally developed in ESD of esophageal squamous cell carcinoma, both are applicable for ESD of Barrett’s adenocarcinoma.
5.4 Short- and Long-Term Outcomes of ESD
Although most of the paper regarding ESD in Barrett’s adenocarcinoma and HGD consisted of small case series reporting single center experience, a recent meta-analysis evaluated the safety and efficacy of ESD in the treatment of early BE neoplasia [33]. It included 11 studies, of which 10 were cohort studies and 1 was a randomized controlled trial. Seven studies were from Europe, three from Asia, and one from the United States. Mean lesion size was 27 mm (20.9–33.1) and average procedure time was 107.5 min (86.4–128.5). The pooled en bloc resection rate was 92.9% (95%CI, 90.3–95.2%), while the R0 and curative resection rates were 74.5% (95%CI, 66.3–81.9%) and 64.9% (95%CI, 55.7–73.6%), respectively. This meta-analysis reported highly favorable outcomes and safety profiles, comparable to those in gastric and colorectal ESD. Interestingly, this study found significant heterogeneity in R0 and curative resection rates [33]. Variation has been attributed to whether both HGD and Barrett’s adenocarcinoma were included, differences including lesion location and length of Barrett’s esophagus between the East and West, or infiltrated lateral margins that were not evident before endoscopic resection.
Two recent multicenter analyses demonstrated the efficacy and safety of ESD in the West for resection of BE associated HGD and EAC. The multicenter retrospective analysis from five academic tertiary referral centers in the United States reported en bloc and curative resection rates of 96% and 70%, respectively. Post-ESD bleeding was noted in 6% of the patients, perforation in 2.1%, and esophageal strictures in 15% [34]. The European multicenter study which included large (≥2 cm), nodular or fibrotic lesions similarly revealed the en bloc resection rate of 90.8% and curative resection rate of 65.8%. The learning curve portraying en bloc resection revealed that it plateaued after 30 procedures. Post-ESD was 1.4%, perforation 0%, and stricture 2.1% [35]. These findings highlight the potential role of ESD for the assessment and management of neoplastic lesions associated with Barrett’s esophagus, and provide reassurance on the safety of ESD when performed by experts in high-volume centers.
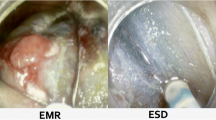
6 Comparison Between EMR and ESD
In recent years also thanks to the use of ESD, some works have appeared in the literature that demonstrate the superiority of this technique compared to the traditional EMR in terms of en bloc resection and reducing the risk of local recurrence [36]. Indeed, a randomized controlled trial by Terheggen et al. demonstrated that R0 resection was achieved more frequently with ESD (59% vs 12%) [37]. However, this study didn’t show any clinical advantage of ESD over EMR in terms of need for surgery, neoplasia remission or recurrence. Thus, although a compelling argument can be made regarding the theoretical advantages of en bloc resection made possible by ESD, this has not translated into clinically meaningful benefits to date. However, this RCT had short follow-up period and it is still unclear if the higher R0 resection rates achieved by ESD might translate into lower rates of neoplasia recurrence over longer periods of time. Therefore, further prospective studies with longer follow-up periods are warranted to conclude the clinical questions.
Conclusions
EMR is currently the most commonly available treatment of BE associated neoplasia because of technically easy and simple procedure. Although en bloc resection rate and R0 resection rate were inferior to that of ESD, the following ablation therapy is generally scheduled for the remaining Barrett’s esophagus in order to achieve complete eradication of intestinal metaplasia. This multimodality strategy is applied in the Western country where the neoplasia is mainly seen in the long-segment Barrett esophagus.
On the other hand, ESD allows for higher en bloc resection rate compared with that of EMR and it is widely spread in the East. Although there are still some clinical issues such as technical difficulty, long-time procedure, and financial reimbursement, it has been gradually accepted in the West. If patients have short-segment, non-circumferential areas of BE-related neoplasia, ESD will provide better clinical outcomes.
It is essential to understand the differences in endoscopic treatment strategies in the East and the West, and the method of endoscopic resection should be determined considering the length of Barrett esophagus, lesion location, availability of subsequent ablation therapy, and the skill and experience of ESD.
References
Seder CW, Raymond DP, Wright CD, Gaissert HA, Chang AC, Clinton S, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database 2017 Update on Outcomes and Quality. Ann Thorac Surg. 2017;103(5):1378–83.
Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Ann Surg. 2007;246(3):363–72; discussion 72–4.
McCulloch P, Ward J, Tekkis PP, ASCOT group of surgeons, British Oesophago-Gastric Cancer Group. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ. 2003;327(7425):1192–7.
Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27.
Dunbar KB, Spechler SJ. The risk of lymph-node metastases in patients with high-grade dysplasia or intramucosal carcinoma in Barrett’s esophagus: a systematic review. Am J Gastroenterol. 2012;107(6):850–62; quiz 63.
Manner H, Pech O, Heldmann Y, May A, Pohl J, Behrens A, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11(6):630–5; quiz e45
Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG Clinical Guideline: diagnosis and management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111(1):30–50; quiz 1.
Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49(2):191–8.
Ishihara R, Oyama T, Abe S, Takahashi H, Ono H, Fujisaki J, et al. Risk of metastasis in adenocarcinoma of the esophagus: a multicenter retrospective study in a Japanese population. J Gastroenterol. 2016;52(7):800–8.
Oda I, Abe S, Kusano C, Suzuki H, Nonaka S, Yoshinaga S, et al. Correlation between endoscopic macroscopic type and invasion depth for early esophagogastric junction adenocarcinomas. Gastric Cancer. 2011;14(1):22–7.
Qumseya BJ, Brown J, Abraham M, White D, Wolfsen H, Gupta N, et al. Diagnostic performance of EUS in predicting advanced cancer among patients with Barrett's esophagus and high-grade dysplasia/early adenocarcinoma: systematic review and meta-analysis. Gastrointest Endosc. 2015;81(4):865–74. e2
Thota PN, Sada A, Sanaka MR, Jang S, Lopez R, Goldblum JR, et al. Correlation between endoscopic forceps biopsies and endoscopic mucosal resection with endoscopic ultrasound in patients with Barrett’s esophagus with high-grade dysplasia and early cancer. Surg Endosc. 2017;31(3):1336–41.
Ell C, May A, Gossner L, Pech O, Gunter E, Mayer G, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett’s esophagus. Gastroenterology. 2000;118(4):670–7.
May A, Gossner L, Behrens A, Kohnen R, Vieth M, Stolte M, et al. A prospective randomized trial of two different endoscopic resection techniques for early stage cancer of the esophagus. Gastrointest Endosc. 2003;58(2):167–75.
Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett's esophagus. Gastrointest Endosc. 2000;52(3):328–32.
Wani S, Abrams J, Edmundowicz SA, Gaddam S, Hovis CE, Green D, et al. Endoscopic mucosal resection results in change of histologic diagnosis in Barrett’s esophagus patients with visible and flat neoplasia: a multicenter cohort study. Dig Dis Sci. 2013;58(6):1703–9.
Bahin FF, Jayanna M, Hourigan LF, Lord RV, Whiteman D, Williams SJ, et al. Long-term outcomes of a primary complete endoscopic resection strategy for short-segment Barrett’s esophagus with high-grade dysplasia and/or early esophageal adenocarcinoma. Gastrointest Endosc. 2016;83(1):68–77.
Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57(9):1200–6.
Lada MJ, Watson TJ, Shakoor A, Nieman DR, Han M, Tschoner A, et al. Eliminating a need for esophagectomy: endoscopic treatment of Barrett esophagus with early esophageal neoplasia. Semin Thorac Cardiovasc Surg. 2014;26(4):274–84.
Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1):1–11.
Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72(6):1217–25.
Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;7 Suppl 1:S67–70.
Yamamoto H, Yahagi N, Oyama T, Gotoda T, Doi T, Hirasaki S, et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid “cushion” in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc. 2008;67(6):830–9.
Uraoka T, Saito Y, Yamamoto K, Fujii T. Submucosal injection solution for gastrointestinal tract endoscopic mucosal resection and endoscopic submucosal dissection. Drug Des Devel Ther. 2009;2:131–8.
Draganov PV, Gotoda T, Chavalitdhamrong D, Wallace MB. Techniques of endoscopic submucosal dissection: application for the Western endoscopist? Gastrointest Endosc. 2013;78(5):677–88.
Arantes V, Albuquerque W, Benfica E, Duarte DL, Lima D, Vilela S, et al. Submucosal injection of 0.4% hydroxypropyl methylcellulose facilitates endoscopic mucosal resection of early gastrointestinal tumors. J Clin Gastroenterol. 2010;44(9):615–9.
Mehta N, Strong AT, Franco M, Stevens T, Chahal P, Jang S, et al. Optimal injection solution for endoscopic submucosal dissection: a randomized controlled trial of Western solutions in a porcine model. Dig Endosc. 2017;30(3):347–53.
Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24(7):1638–45.
Nonaka S, Kawaguchi Y, Oda I, Nakamura J, Sato C, Kinjo Y, et al. Safety and effectiveness of propofol-based monitored anesthesia care without intubation during endoscopic submucosal dissection for early gastric and esophageal cancers. Dig Endosc. 2015;27(6):665–73.
Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45(4):375–8.
Koike Y, Hirasawa D, Fujita N, Maeda Y, Ohira T, Harada Y, et al. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc. 2015;27(3):303–9.
Huang R, Cai H, Zhao X, Lu X, Liu M, Lv W, et al. Efficacy and safety of endoscopic submucosal tunnel dissection for superficial esophageal squamous cell carcinoma: a propensity score matching analysis. Gastrointest Endosc. 2017;86(5):831–8.
Yang D, Zou F, Xiong S, Forde JJ, Wang Y, Draganov PV. Endoscopic submucosal dissection for early Barrett’s neoplasia: a meta-analysis. Gastrointest Endosc. 2017;87(6):1383–93.
Yang D, Coman RM, Kahaleh M, Waxman I, Wang AY, Sethi A, et al. Endoscopic submucosal dissection for Barrett’s early neoplasia: a multicenter study in the United States. Gastrointest Endosc. 2017;86(4):600–7.
Subramaniam S, Chedgy F, Longcroft-Wheaton G, Kandiah K, Maselli R, Seewald S, et al. Complex early Barrett’s neoplasia at 3 Western centers: European Barrett’s Endoscopic Submucosal Dissection Trial (E-BEST). Gastrointest Endosc. 2017;86(4):608–18.
Guo HM, Zhang XQ, Chen M, Huang SL, Zou XP. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. 2014;20(18):5540–7.
Terheggen G, Horn EM, Vieth M, Gabbert H, Enderle M, Neugebauer A, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017;66(5):783–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Abe, S., Catalano, F., Saito, Y. (2019). Endoscopic Resections: EMR and ESD. In: Galloro, G. (eds) Revisiting Barrett's Esophagus. Springer, Cham. https://doi.org/10.1007/978-3-319-92093-1_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-92093-1_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92092-4
Online ISBN: 978-3-319-92093-1
eBook Packages: MedicineMedicine (R0)