Abstract
Background
It is well known that carbon dioxide (CO2) is absorbed faster in the body than air and also that it is rapidly excreted through respiration. This study aimed to investigate the safety of CO2 insufflation used for esophageal and gastric endoscopic submucosal dissection (ESD) in patients under deep sedation.
Methods
Patients with either early gastric or esophageal cancers that could be resected by ESD were enrolled in this study from March 2007 to July 2008 and randomly assigned to undergo ESD procedures with CO2 insufflation (CO2 group) or air insufflation (air group). A TOSCA measurement system and TOSCA 500 monitor were used to measure and monitor both transcutaneous partial pressure of CO2 (PtcCO2) and oxygen saturation (SpO2).
Results
The study enrolled 89 patients and randomly assigned them to a CO2 group (45 patients) or an air group (44 patients). The mean CO2 group versus air group measurements were as follows: PtcCO2 (49.1 ± 5.0 vs. 50.1 ± 5.3 mmHg; nonsignificant difference [NS]), maximum PtcCO2 (55.1 ± 6.5 vs. 56.8 ± 7.0 mmHg; NS), PtcCO2 elevation (9.1 ± 5.4 vs. 11.4 ± 5.6 mmHg; p = 0.054), SpO2 (99.0 ± 0.7% vs. 99.0 ± 1.0%; NS), minimum SpO2 (96.5 ± 2.4% vs. 95.4 ± 3.3%; p = 0.085), and SpO2 depression (2.4 ± 2.3% vs. 3.3 ± 2.9%; NS). The PtcCO2 and SpO2 measurements were similar in the two groups, but the CO2 group was better than the air group in PtcCO2 elevation and minimum SpO2.
Conclusions
The findings demonstrated CO2 insufflation to be as safe as air insufflation for upper gastrointestinal tract ESDs performed for patients under deep sedation without evidencing any adverse effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several recent studies investigating colonoscopy and endoscopic retrograde cholangiopancreatography (ERCP) have reported that carbon dioxide (CO2) insufflation reduces abdominal pain and discomfort caused by bowel hyperextension and can be used as safely as air insufflation [1–6]. It is well known that CO2 is absorbed faster in the body than air and that it also is rapidly excreted through respiration unless some type of pulmonary dysfunction exists [1, 2]. To date, almost all endoscopic procedures have been performed using air insufflation, although it has led to some problems of abdominal pain and discomfort in routine examinations and perforation-related subcutaneous or mediastinal emphysema and pneumoperitoneum in endoscopic treatments [7, 8].
With the relatively recent development and increasingly widespread use of endoscopic submucosal dissection (ESD) as a minimally invasive treatment, performance of ESD for early gastrointestinal (GI) neoplasm in the esophagus, stomach, and colorectum has increased dramatically [9–16]. Quite naturally, the number of complications also has increased as a direct result, including perforations that occur during the technically difficult ESD procedure itself and the delayed bleeding experienced afterward [7, 8, 14, 17, 18]. In fact, the reported ESD perforation rate is 7% for cases involving the esophagus, 4% for cases involving the stomach, and 5% for cases involving the colorectum [10, 14, 15]. Perforation can cause peritonitis and mediastinitis, and possibly also thromboembolism due to blood flow congestion (compartment syndrome) when significant pneumatic leakage results in excess internal pressure [19–24]. It is anticipated that such associated problems will be minimized by further use of CO2 insufflation.
Colonoscopy with conscious sedation and the use of CO2 insufflation has become more generally accepted since the demonstration of the safety and effectiveness of CO2 insufflation in a previously published reported [5]. We previously conducted a case–control study that showed CO2 insufflation to be both safe and effective for colorectal ESD with conscious sedation [25]. However, the safety of CO2 insufflation has not been established for upper GI tract endoscopic treatment such as ESD with deep sedation in which CO2 retention and decreased oxygenation are more important factors than in colonoscopy performed with conscious sedation.
This study aimed to investigate the safety of CO2 insufflation for esophageal and gastric ESDs with deep sedation. Both operations are lengthy procedures.
Materials and methods
Patients
We prospectively assessed the safety of CO2 insufflation for upper GI tract ESDs performed with the patient under deep sedation compared with air insufflation from March 2007 to July 2008 at the National Cancer Center Hospital (NCCH) in Tokyo, Japan. The study enrolled 89 patients with either early gastric or esophageal cancer that could be resected by ESD and randomly assigned them to undergo ESD procedures with CO2 insufflation (CO2 group) or air insufflation (air group).
The study excluded patients with severe pulmonary disease including either chronic obstructive pulmonary disease (COPD) or disease resulting in less than 80% of vital capacity (%VC) or less than 70% of the forced expiratory volume in 1 s as a percentage of the forced vital capacity (FEV1%), patients with severe cardiovascular disease including NYHA III or IV heart failure or arrhythmia with any treatment history, patients with hepatic or renal dysfunction, and patients with a change in insufflation methods from CO2 to air or from air to CO2 for any reason during their ESDs.
Endoscopic procedures
All ESD procedures were performed with Olympus video endoscopes and a standard videoendoscope system (EVIS LUCERA; Olympus Optical Co., Ltd., Tokyo, Japan). For ESD procedures, an insulation-tipped diathermic knife (IT-knife; Olympus) was used from March to October 2007 and an improved IT-knife (IT-knife 2; Olympus) from November 2007 to July 2008 [11, 26, 27].
First, marking dots were made around the lesion using a needleknife (Olympus). This was followed by injection of diluted epinephrine with normal saline (1:200,000) to lift the submucosal layer and allow the tip of the IT-knife or IT-knife 2 to be inserted into the submucosal layer. A small initial incision then was made by a needleknife, and a complete circumferential mucosal incision around the periphery of the marking dots was performed with the IT-knife or IT-knife 2. After an additional submucosal injection, the submucosal layer beneath the lesion was directly dissected using the same IT-knife or IT-knife 2.
Although all ESDs were generally performed in this manner, we sometimes used not only other devices such as an argon plasma coagulation probe for the marking dots and a bipolar needleknife (B-knife; XEMEX Co., Tokyo, Japan) for the initial incision and submucosal dissection [15, 28], but also another injection solution, sodium hyaluronate (MucoUp; Johnson & Johnson Co., Ltd., Tokyo, Japan) diluted with normal saline (1:1), especially for esophageal ESDs [12, 29–31]. The final objective was to achieve successful en bloc resections for precise pathologic evaluations.
Patients received midazolam, propofol, or both for deep sedation, and oxygen (O2) was administered nasally (2 l/min) during ESD. Initially, 3–5 mg of midazolam was used for induction of venous anesthesia, with an additional 1–3 mg given repeatedly as necessary based on the judgment of the individual endoscopist. Propofol was administered initially at a dosage of 20 mg for induction, with another 0.1–0.5 mg/kg/h given continuously for maintenance depending on the condition of the patient.
CO2 insufflation and transcutaneous measurements
A CO2 regulator prototype (Olympus) connected to a CO2 bottle was used for CO2 insufflation until the Olympus UCR (Fig. 1) became commercially available in Japan in May 2008 [25]. During the procedure, CO2 insufflation was set at a constant rate of 1.2 l/min, which is a moderate level. In upper GI endoscopy, the UCR has three insufflation levels, which can be controlled by the use of three types of connecting tubes. These insufflation amounts are almost equivalent to the original three regulation levels of the EVIS LUCERA (Olympus).
Measurement of the arterial partial pressure of CO2 (partial pressure of carbon dioxide [PCO2] and arterial partial pressure of carbon dioxide [PaCO2]) is an invasive, intermittent, and unpleasant process widely used for various patients as the gold standard, but determining the variation of PaCO2 during ESD using CO2 insufflation has proved to be quite difficult.
In this study, a TOSCA measurement system and TOSCA 500 monitor (Linde Medical Sensors, Basel, Switzerland) (Fig. 2) was used to measure and monitor both transcutaneous partial pressure of CO2 (PtcCO2) and oxygen saturation (SpO2). This system, which takes measurements using a sensor attached by a low-pressure clip to the patient’s earlobe, is a noninvasive, continuous, trend-monitoring device for PtcCO2 reported in several studies to provide general agreement between PtcCO2 and PaCO2 measurements [32–37]. We used a default temperature setting of 42ºC for the earlobe sensor and recalibrated the TOSCA system to minimize the possibility of measurement error before each ESD. Procedure time was measured from endoscope insertion to its completed withdrawal after ESD, with PtcCO2 and SpO2 recorded every 3 s for both groups using the TOSCA system.
Statistical analysis
All variables in this study were described in terms of mean ± standard deviation as well as median and range. We used chi-square and t-tests to compare baseline characteristics and measurements between the two groups. All statistical analyses were performed using the SAS Statistical Package (SAS Institute, Tokyo, Japan), and a p value less than 0.05 was considered statistically significant.
Ethics
The ethics committee at NCCH approved the study protocol, and written informed consent was obtained from all patients before they were enrolled in the study.
Results
No significant differences in patient characteristics between the two groups were observed (Table 1). The CO2 group study consisted of 45 patients (39 men and 6 women) with 52 lesions. These 45 patients (involving 15 esophageal and 30 gastric ESD cases) had a mean age of 68.5 ± 8.8 years (range, 50–84 years). The air group consisted of 44 patients (38 men and 6 women) with 51 lesions. These 44 patients (involving 12 esophageal and 32 gastric ESD cases) had a mean age of 67.6 ± 8.0 years (range, 43–84 years).
The macroscopic types of tumors included 13 elevated lesions, 32 flat and depressed lesions, 6 combined lesions, and 1 residual lesion in the CO2 group and 11 elevated lesions, 34 flat and depressed lesions, 5 combined lesions, and 1 residual lesion in the air group (nonsignificant difference [NS]). In the CO2 group, the median size of the tumors, determined histopathologically, was 13 mm (range, 5–60 mm), and the 35 adenocarcinomas included 2 Barrett’s carcinomas, 15 squamous cell carcinomas (SCCs), and 2 adenomas. The median size of the tumors in the air group was 19 mm (range, 5–55 mm), and the 37 adenocarcinomas included 2 Barrett’s carcinomas, 13 SCCs, and 1 adenoma. The difference between the two groups was not significant. The median specimen size was 35 mm (range, 20–75 mm) in the CO2 group and 35 mm (range, 20–68 mm) in the air group (NS). The median procedure time was 115 min (range, 30–575 min) in the CO2 group and 96 min (range, 38–309) in the air group (NS). Midazolam was received by 30 patients at a median dosage of 12 mg (range, 5–20 mg) in the CO2 group and by 31 patients at a median dosage of 12 mg (range, 4–23 mg) in the air group (NS), and propofol was received by 15 patients at a median dosage of 640 mg (range, 130–2,460 mg) in the CO2 group and by 13 patients at a median dosage of 370 mg (range, 180–1,116) in the air group (NS).
All the tumors were resected en bloc by ESD except in one esophageal case in the air group, In this case, the patient’s main lesion was resected en bloc by ESD, whereas another smaller synchronous lesion was treated by using endoscopic mucosal resection (EMR) with a cap-fitted panendoscope, resulting in a piecemeal resection [38].
Measurements of PtcCO2 and SpO2
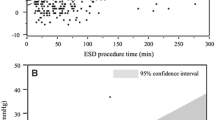
The mean CO2 group versus air group measurements were as follows: PtcCO2 (49.1 ± 5.0 vs. 50.1 ± 5.3 mmHg; NS), maximum PtcCO2 (55.1 ± 6.5 vs. 56.8 ± 7.0 mmHg; NS), PtcCO2 elevation (9.1 ± 5.4 vs. 11.4 ± 5.6 mmHg; p = 0.054), SpO2 (99.0 ± 0.7% vs. 99.0 ± 1.0%; NS), minimum SpO2 (96.5 ± 2.4% vs. 95.4 ± 3.3%; p = 0.085), and SpO2 depression (2.4 ± 2.3% vs. 3.3 ± 2.9%; NS) (Table 2; Fig. 3A–F). The PtcCO2 and SpO2 measurements were similar in the two groups, but in PtcCO2 elevation and minimum SpO2, the CO2 group was better than the air group.
The patient characteristics did not differ significantly between the two groups when esophageal and gastric ESD cases were considered separately, nor did the PtcCO2 and SpO2 measurements differ significantly between the two groups when only esophageal ESD cases were considered. The CO2 group versus air group measurements in gastric ESD cases were as follows: PtcCO2 elevation (8.0 ± 5.2 vs. 10.8 ± 5.7 mmHg; p = 0.049) and SpO2 depression (1.9 ± 1.8% vs. 2.8 ± 2.5%; p = 0.087). Although the PtcCO2 and SpO2 measurements again were similar for the two groups, when only gastric ESD cases were considered, the CO2 group was better than the air group in PtcCO2 elevation and SpO2 depression.
Five CO2 group patients and five air group patients experienced a maximum PtcCO2 exceeding 60 mmHg that continued for more than 5 min (NS). The median duration time was 12 min (range, 6–166 min) for the CO2 group and 35 min (range, 10–148 min) for the air group (NS). The maximum PtcCO2 was 72 mmHg in the CO2 group and 74 mmHg in the air group (NS) (Table 3). None of the cases in either group involved an SpO2 level lower than 90% that continued for more than 1 min, and no harmful oxygenation effects occurred. Temporary SpO2 depression lower than 90% for less than 1 min resulted from the aspiration of two patients in the air group, but the condition subsequently improved and did not impair treatment (Table 3).
No adverse effects were caused by CO2 insufflation in the CO2 group. Perforations involving CO2 insufflation occurred in three cases including two esophageal ESD cases and one gastric ESD case, but x-rays did not show any subcutaneous or mediastinal emphysema or pneumoperitoneum. As for the three patients in the CO2 group with perforations, histopathologic examinations of the one gastric ESD patient showed a well-differentiated intramucosal adenocarcinoma located in the cardia, and the two esophageal ESD patients had SCCs within the lamina propria mucosae located in either the middle or lower thoracic esophagus. Antibiotics were administrated for all three patients over 3 to 5 days. Oral diet intake was started on either postoperative day 2 or 4, and each patient was discharged on postoperative day 6 without any invasive intervention, as is the usual course for gastric and esophageal ESD patients at our hospital. All the CO2 group procedures were completed without delays, and none of the 45 CO2 insufflation patients required extended hospitalization.
Discussion
To the best of our knowledge, this is the first study to investigate the safety of CO2 insufflation in lengthy upper GI tract ESD procedures for patients under deep sedation. The results of our study indicate that CO2 insufflation can be used as safely as air insufflation without any adverse effects by continuous monitoring of PtcCO2 and SpO2 during both esophageal and gastric ESDs.
Bretthauer et al. [4, 6] reported no significant observed difference in PtcCO2 elevation between air and CO2 insufflation groups during ERCP with deep sedation, and no significant increase in end-tidal CO2 levels was demonstrated between the two groups in colonoscopy examinations without sedation, although patient abdominal discomfort was significantly less in the CO2 group. In our study, midazolam and propofol were used, so it was difficult to measure patient discomfort levels using a visual analog scale after ESD because of considerable differences in the rate of recovery between those two sedatives.
The PCO2 level basically depends on ventilation, so PCO2 elevation can be regarded generally as caused by depression of both the ventilation rate and the tidal volume. Nelson et al. [39] reported PtcCO2 elevation exceeding 40 mmHg and a maximum PtcCO2 greater than 100 mmHg in ERCP using air insufflation, although there were no evident adverse effects.
In our results, the maximum PtcCO2 per duration time, with PtcCO2 exceeding 60 mmHg, was 72 mmHg for 166 min in the CO2 group and 74 mmHg for 148 min in the air group, but with no adverse events in either group. No harmful oxygenation effects resulted from using CO2 insufflation during ESDs because all the patients received O2 nasally. These results suggest that PtcCO2 elevation, which registered a maximum value of 74 mmHg without SpO2 depression, did not represent a clinical problem, and no actual correlation was found between the two measurements in any of the cases. We believe that PtcCO2 elevation was not caused solely by CO2 insufflation but that other important factors were involved, including sedation levels and respiratory status, because the air group showed even higher PtcCO2 values than the CO2 group (Table 2; Fig. 3A–C) [5, 40].
Concerning the observation of differences between the two groups in PtcCO2 elevation and minimum SpO2 in all cases as well as PtcCO2 elevation and SpO2 depression in only the gastric ESD cases, we considered that ventilation rate and tidal volume were difficult to decrease because abdominal distension and diaphragm elevation were reduced to relieve bowel hyperextension. Accordingly, it also can be speculated that CO2 insufflation may stimulate the respiratory center, leading theoretically to hyperventilation. Except for patients with COPD, who were excluded from this study, PtcCO2 elevation may have been caused by hypoactivity of the respiratory center resulting from deep sedation rather than CO2 insufflation or oxygen administration.
In the upper GI tract, especially the esophagus, the most serious complications are arrhythmia, cardiac collapse, thromboembolism produced by blood flow congestion resulting from a perforation (compartment syndrome), and pneumothorax [19–24]. We also considered why no subcutaneous or mediastinal emphysema or pneumoperitoneum appeared, and we suspected that leaked CO2 in the three patients who experienced perforations probably was absorbed rapidly into the surrounding tissue [1, 2]. It can be expected that CO2 insufflation will reduce all such complications. Because CO2 insufflation was demonstrated to be safe in this study, it is recommended that to avoid any unexpected developments during treatment in the upper GI tract, particularly in the esophagus, ESD should be performed from the start using CO2 insufflation. In addition, CO2 insufflation is recommended for endoscopists with limited ESD experience, who likely will need more time to complete the procedure and may have a greater possibility of a perforation occurring because of their relative inexperience.
It generally is considered that a severe acidosis condition leads to arrhythmia, cardiac collapse, or hyperkalemia. If CO2 retention does occur, the CO2 can serve as a factor in decreasing the pH balance, although no clinical problem is involved if the pH balance is preserved within normal limits by other factors. Based on our findings, it appears that no adverse events may result if normal oxygenation is maintained even when a PtcCO2 exceeding 60 mmHg persists for some time. Although CO2 insufflation is not recommended for patients with severe pulmonary or cardiovascular disease, it is associated with no clinical disadvantage compared with air insufflation. We currently recommend, however, that PtcCO2 be measured for enhanced safety during upper GI ESDs.
Several studies have shown a close correlation between PtcCO2 and PaCO2, so PtcCO2 currently is regarded as a reliable and accurate measurement, although it is known that a discrepancy can exist between the two under certain body temperature and skin conditions [41]. No blood gas samples were taken in this study, so we have no data on actual patient pH levels and PaCO2 values during the ESD procedures.
We were able to perform continuous measurement of the PtcCO2 level and monitoring of its elevation during upper GI tract endoscopic treatments, neither of which had previously been completely certain. Although more than 2,000 upper GI tract ESDs have been performed for patients at NCCH [42], very few major respiratory-related problems with the use of air insufflation have occurred despite the lack of certainty about previous PtcCO2 levels. The advantage of having precise PtcCO2 data is avoidance of additional sedatives resulting in excessively deep sedation that may cause respiratory dysfunction because PCO2 elevation suggests depression of the ventilation rate and tidal volume. This also prevents tracheal intubation due to pulmonary arrest.
Use of a bispectral index (BIS) monitor that indicates a patient’s sedation level by monitoring brain waves has been reported recently, so it is conceivable that the combined use of CO2 insufflation with continuous PtcCO2 measurement and the BIS monitor could result in safer upper GI tract endoscopic treatment procedures in the future [43, 44].
Conclusions
This study demonstrated CO2 insufflation to be as safe as air insufflation for upper GI tract ESDs performed for patients under deep sedation without evidencing any adverse effects. We believe that CO2 insufflation may be particularly effective for esophageal cases in which severe subcutaneous or mediastinal emphysema can be caused by perforations that may occur during the ESD procedure.
References
Hussein AM, Bartram CI, Williams CB (1984) Carbon dioxide insufflation for more comfortable colonoscopy. Gastrointest Endosc 30:68–70
Stevenson GW, Wilson JA, Wilkinson J, Norman G, Goodacre RL (1992) Pain following colonoscopy: elimination with carbon dioxide. Gastrointest Endosc 38:564–567
Church J, Delaney C (2003) Randomized, controlled trial of carbon dioxide insufflation during colonoscopy. Dis Colon Rectum 46:322–326
Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E, Hoff G (2002) NORCCAP (Norwegian colorectal cancer prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut 50:604–607
Bretthauer M, Lynge AB, Thiis-Evensen E, Hoff G, Fausa O, Aabakken L (2005) Carbon dioxide insufflation in colonoscopy: safe and effective in sedated patients. Endoscopy 37:706–709
Bretthauer M, Seip B, Aasen S, Kordal M, Hoff G, Aabakken L (2007) Carbon dioxide insufflation for more comfortable endoscopic retrograde cholangiopancreatography: a randomized, controlled, double-blind trial. Endoscopy 39:58–64
Minami S, Gotoda T, Ono H, Oda I, Hamanaka H (2006) Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video). Gastrointest Endosc 63:596–601
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2006) Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy 38:1001–1006
Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y (2005) Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 3:67–70
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Shimizu Y, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2006) Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 4:688–694
Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S (2001) Endoscopic mucosal resection for treatment of early gastric cancer. Gut 48:225–229
Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K, Sugano K (2003) Successful en bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy 35:690–694
Yahagi N, Fujishiro M, Kakushima N, Kobayashi K, Hashimoto T, Oka M (2004) Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type). Dig Endosc 16:34–38
Oda I, Gotoda T, Hamanaka H (2005) Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time, and complications from a large consecutive series. Dig Endosc 17:54–58
Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D (2007) Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc 66:966–973
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K, Kawabe T, Ichinose M, Omata M (2007) Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol 5:678–683
Gotoda T (2007) Endoscopic resection of early gastric cancer. Gastric Cancer 10:1–11
Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H (2008) Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection: an analysis of risk factors. Endoscopy 40:179–183
Kimball EJ, Rollins MD, Mone MC, Hansen HJ, Baraghoshi GK, Johnston C, Day ES, Jackson PR, Payne M, Barton RG (2006) Survey of intensive care physicians on the recognition and management of intraabdominal hypertension and abdominal compartment syndrome. Crit Care Med 34:2340–2348
Williams C (1986) Who’s for CO2? Gastrointest Endosc 32:365–367
Hayakawa M, Gando S, Kameue T, Morimoto Y, Kemmotsu O (2002) Abdominal compartment syndrome and intrahepatic portal venous gas: a possible complication of endoscopy. Intensive Care Med 28:1680–1681
Peppriell JE, Bacon DR (2000) Acute abdominal compartment syndrome with pulseless electrical activity during colonoscopy with conscious sedation. J Clin Anesth 12:216–219
Rizzo AG, Sample GA (2003) Thoracic compartment syndrome secondary to a thoracic procedure: a case report. Chest 124:1164–1168
van Mook WN, Huslewe-Evers RP, Ramsay G (2002) Abdominal compartment syndrome. Lancet 360:1502
Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D (2007) A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc 65:537–542
Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T (1999) A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 50:560–563
Ono H, Hasuike N, Inui T, Takizawa K, Ikehara H, Yamaguchi Y, Otake Y, Matsubayashi H (2008) Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 11:47–52
Saito Y, Takisawa H, Suzuki H, Takizawa K, Yokoi C, Nonaka S, Matsuda T, Nakanishi Y, Kato K (2008) Endoscopic submucosal dissection of recurrent or residual superficial esophageal cancer after chemoradiotherapy. Gastrointest Endosc 67:355–359
Yamamoto H, Yahagi N, Oyama T, Gotoda T, Doi T, Hirasaki S, Shimoda T, Sugano K, Tajiri H, Takekoshi T, Saito D (2008) Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid “cushion” in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc 67:830–839
Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Enomoto S, Kakushima N, Kobayashi K, Hashimoto T, Iguchi M, Shimizu Y, Ichinose M, Omata M (2004) Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy 36:579–583
Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Matsuura T, Enomoto S, Kakushima N, Imagawa A, Kobayashi K, Hashimoto T, Iguchi M, Shimizu Y, Ichinose M, Omata M (2004) Different mixtures of sodium hyaluronate and their ability to create submucosal fluid cushions for endoscopic mucosal resection. Endoscopy 36:584–589
Parker SM, Gibson GJ (2007) Evaluation of a transcutaneous carbon dioxide monitor (“TOSCA”) in adult patients in routine respiratory practice. Respir Med 101:261–264
Maniscalco M, Zedda A, Faraone S, Carratu P, Sofia M (2008) Evaluation of a transcutaneous carbon dioxide monitor in severe obesity. Intensive Care Med 34:1340–1344
Carter BG, Wiwczaruk D, Hochmann M, Osborne A, Henning R (2001) Performance of transcutaneous PCO2 and pulse oximetry monitors in newborns and infants after cardiac surgery. Anaesth Intensive Care 29:260–265
Eberhard P, Gisiger PA, Gardaz JP, Spahn DR (2002) Combining transcutaneous blood gas measurement and pulse oximetry. Anesth Analg 94:76–80
Rohling R, Biro P (1999) Clinical investigation of a new combined pulse oximetry and carbon dioxide tension sensor in adult anaesthesia. J Clin Monit Comput 15:23–27
Sumanac K, Zealley I, Fox BM, Rawlinson J, Salena B, Marshall JK, Stevenson GW, Hunt RH (2002) Minimizing postcolonoscopy abdominal pain by using CO2 insufflation: a prospective, randomized, double blind, controlled trial evaluating a new commercially available CO2 delivery system. Gastrointest Endosc 56:190–194
Inoue H, Endo M, Takeshita K, Yoshino K, Muraoka Y, Yoneshima H (1992) A new simplified technique of endoscopic esophageal mucosal resection using a cap-fitted panendoscope (EMRC). Surg Endosc 6:264–265
Nelson DB, Freeman ML, Silvis SE, Cass OW, Yakshe PN, Vennes J, Stahnke LL, Herman M, Hodges J (2000) A randomized, controlled trial of transcutaneous carbon dioxide monitoring during ERCP. Gastrointest Endosc 51:288–295
Bell GD (2000) Premedication, preparation, and surveillance. Endoscopy 32:92–100
Bolliger D, Steiner LA, Kasper J, Aziz OA, Filipovic M, Seeberger MD (2007) The accuracy of noninvasive carbon dioxide monitoring: a clinical evaluation of two transcutaneous systems. Anaesthesia 62:394–399
Oda I, Gotoda T, Sasako M, Sano T, Katai H, Fukagawa T, Shimoda T, Emura F, Saito D (2008) Treatment strategy after noncurative endoscopic resection of early gastric cancer. Br J Surg 95:1495–1500
Imagawa A, Fujiki S, Kawahara Y, Matsushita H, Ota S, Tomoda T, Morito Y, Sakakihara I, Fujimoto T, Taira A, Tsugeno H, Kawano S, Yagi S, Takenaka R (2008) Satisfaction with bispectral index monitoring of propofol-mediated sedation during endoscopic submucosal dissection: a prospective, randomized study. Endoscopy 40:905–909
Lazzaroni M, Porro Bianchi (2005) Preparation, premedication, and surveillance. Endoscopy 37:101–109
Acknowledgments
We thank Mr. Christopher Dix for editing the manuscript. This study was partly supported by the Japanese Foundation for Research and Promotion of Endoscopy. Although the CO2 regulator prototype was provided by the Olympus Optical Co., Ltd., Tokyo, Japan, this was not a collaborative study.
Disclosures
Satoru Nonaka, Yutaka Saito, Hajime Takisawa, Yongmin Kim, Tsuyoshi Kikuchi, and Ichiro Oda have no conflict of interests or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nonaka, S., Saito, Y., Takisawa, H. et al. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc 24, 1638–1645 (2010). https://doi.org/10.1007/s00464-009-0824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-009-0824-5