Abstract
Purpose of Review
To discuss endoscopic resection techniques of early gastrointestinal malignancy. The review will focus on the indications and outcomes of endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD).
Recent Findings
EMR is indicated for upper GI lesions less than 20 mm provided they can be easily lifted and have a low risk of submucosal invasion (SMI). ESD should be considered for esophageal and gastric lesions that are bulky, show intramucosal carcinoma, or have a risk of superficial submucosal invasion. With regard to colonic polyps, EMR is acceptable for the removal of large colonic polyps using a piecemeal technique. ESD can be reserved for rectal neuroendocrine tumors, fibrotic polyps, or polyps harboring early malignancy.
Summary
In selected cases, particularly in lesions less than 2 cm in size, EMR can be safe and effective. For larger lesions or lesions with submucosal invasion, ESD is effective and curative. Choosing the best approach can be tailored for each patient depending on lesion size, pathology, and availability of local expertise.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoscopic resection is a technique which allows the removal of large benign lesions and early precancerous or cancerous tumors from the lumen of the gastrointestinal tract for either diagnostic or therapeutic purposes [1••]. Endoscopic resection includes endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD). In EMR, a snare is used to remove the lesion en bloc or in a piecemeal fashion; usually the lesion size is less than 2 cm for en bloc, margin-negative resection [1••]. In ESD, various endoscopic tools are used to resect the lesion at the submucosa plane. ESD can be used for large size lesions; however, ESD is technically more difficult than EMR [1••]. In this review, we will discuss techniques for performing the procedure, in addition to its indications, efficacy, and possible adverse events.
Techniques
EMR Techniques
Prior to the procedure, the lesion selected for EMR should be evaluated visually using normal white light endoscopy, magnified endoscopy, or chromoendoscopy to assess the resectability of the lesion. Marking the lesion’s boundaries is helpful to ensure complete resection. Marking can be achieved with the tip of the snare or argon plasma coagulation (APC) [2]. There are many techniques for performing EMR. We will discuss the four most commonly used techniques.
Injection-Assisted EMR
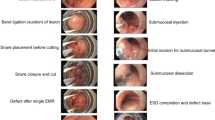
Injection-assisted EMR is performed by injecting a solution into the submucosa under the lesion to separate the lesion from the muscular layer and create a cushion that lifts the lesion. The created cushion provides a zone that protects the underlying structures from mechanical and electrocautery damage, which can occur while the lesion is being removed by the snare [2, 3]. The lesion is removed en bloc or in piecemeal fashion (Fig. 1).
Injected solutions include normal saline, a fibrinogen mixture, sodium hyaluronate, hydroxypropyl methylcellulose (HPMC), hydroxyethyl starch 6%, or 10% glycerol [4,5,6,7,8]. Currently, the preferred solution is HPMC due to its wide availability, effectiveness, and longer lift prior to absorption [9,10,11]. Injections are performed using a needle in a stepwise manner and can be assisted by a pressure pump for uniform lifting [12]. Staining dyes are frequently added to the injected fluid to facilitate identification of lateral and deep margins of the lesions [13]. Diluted epinephrine can be added to the injected solution for the theoretical benefit of decreased bleeding and delayed absorption of injected material by decreasing vascular flow [14]. However, it carries a small risk of tissue ischemia and may also rarely cause myocardial infarction [15] [16].
Cap-Assisted EMR
For cap-assisted EMR, like injection-assisted EMR, the steps mentioned above are performed to lift the lesion, and an EMR kit is then used to remove the lesion. The endoscopic EMR kit includes a clear plastic cap (soft or hard) with a straight or oblique tip [17]. The capped endoscope is placed over the lesion, followed by suction to engulf the lesion inside the cap, and a snare is then used to remove the lesion [3, 18]. Some EMR kits utilize a band deployed over the suction tissue. A snare can be used to dissect the lesion above or below the band.
Underwater EMR
In this technique, luminal air is suctioned, and water is introduced to fill the gastrointestinal (GI) lumen in order to immerse the lesion. The technique creates a “floatation” force on the lesion without the submucosal injection described above [19]. Underwater EMR has the theoretical advantage of avoiding possible displacement of neoplastic cells deeper into the GI tract during injections and can be used in cases where submucosal injection is difficult, for example, where fibrosis may be present from previous EMR attempts [20,21,22].
Cold Snare EMR
The technique was developed to avoid the risk of electrocautery-induced damage in select cases [23]. Similar steps of injection-assisted EMR are performed. However, a smaller and stiffer snare is used to complete the resection without application of cautery energy [24]. Electrocautery has increased risk of delayed bleeding, as it may burn the wall of an arterial branch of a vessel that can later slough off and bleed [24].
Specimen Handling
Due to the relatively larger size of EMR specimens, they should be oriented and mounted on a wax block before submerging in a fixative.
ESD Techniques
ESD is a more complex procedure accomplished in a stepwise manner using an assortment of tools and devices. ESD evolved over the last two decades and is currently used beyond its original intent of endoscopic resection of GI lesions.
Technique
-
i.
Marking the lesion boundary as mentioned above after careful inspection.
-
ii.
Injection of a lifting solution around the perimeter of the lesion.
-
iii.
Incision of the mucosa followed by a circumferential or semi-circumferential cut around the lesion using an electrosurgical knife.
-
iv.
Dissection of the lesion by pressurized water jet or a series of injections and cutting tools through the submucosa plane to separate the lesion from the underlying muscle layer. During the dissection process, various tools are used including an electrosurgical knife, traction, and retrieval devices.
-
v.
Addressing intraprocedural events like bleeding and perforation using clips, coagulation, or sutures.
-
vi.
In certain locations such as the duodenum or right side of the colon, closure of the exposed submucosa by clips or endoscopic suturing devices to decrease post-procedure abdominal pain or bleeding is suggested [25,26,27].
Devices for ESD
Besides the standard tools for endoscopy, ESD utilizes an array of specialized tools to resect the lesions at the submucosa plane. An example of modified tools is the addition of a ceramic tip ball to the surgical knife to prevent inadvertent deep dissection of the muscularis propria and perforation [28]. In the USA, availability of newer ESD tools is limited, in part due to regulatory reasons and approval by the US Food and Drug Administration. Table 1 discusses commonly used ESD knives:
-
1)
Dissection and Hemostasis Devices:
-
a.
ITKnife and ITKnife2: Are used for circumferential incision and dissection of gastric lesions. ITKnife2 also has a triangular electrode beneath the ceramic ball that facilitates cutting.
-
b.
ITKnife nano: Used for circumferential incision and dissection of esophageal and colorectal lesions. It features a recessed electrode for dissection.
-
c.
Hook knife: The tip of the knife is bent at a right angle to create an L-shaped edge. The shape of the knife allows hooking and retraction of the lesion; it is especially useful in fibrotic lesions.
-
d.
Triangle tip knife: Has a non-insulated triangular electrode, mainly used for peroral endoscopic myotomy procedures.
-
e.
Dual knife: Has a very small non-insulated dome-shaped electrode at the tip of the cutting knife (Fig. 1)
-
f.
Flex knife: Comprises a braided cutting knife with a loop-shaped tip at the distal aspect that may be extended to variable lengths from the catheter tip.
-
g.
Hybrid knife: Has a central capillary within the cutting knife that can serve as an ultrafine water jet. The pressurized water jet can help lift the lesion from the mucosa without needle punctures for injections. The tip has multiple configurations: I-type, T-type (used mainly for peroral endoscopic myotomy), and O-type. The knife tip can be extended to variable lengths.
-
h.
Hemostatic forceps: Monopolar and bipolar hemostatic forceps are used to address bleeding. Gastric forceps are coarser and thicker than colonic forceps in order to accommodate different wall thickness.
-
2)
Traction devices:
Traction of the lesion occurs during the dissection phase of ESD. It helps shorten procedure time and allows a tangential view of the lesion. Traction utilizes various tools, for example:
-
a.
Clip-with-line method: A 3–0 silk line is tied to the arm part of a clip and inserted through the accessory channel of the endoscope. Once it is hooked to the lesion, a pulling force is applied to pull the lesion toward the oral side [29, 30].
-
b.
External forceps method: External grasping forceps are inserted with the help of second grasping forceps and anchored at the distal margin of the lesion; it is then pulled toward the oral direction [31].
-
c.
Clip-and-snare method: Uses a hemoclip and snare. Traction occurs by pulling and pushing on the lesion using a hemoclip grasped with the snare [32].
-
d.
Internal traction method: Dissection is achieved by pulling a rubber band or medical ring attached to anchors on the opposite side of the lesion under the mucosa [33, 34].
Double-scope method: A small-caliber endoscope is inserted along the main scope, allowing a two-hand model [35].
Double-balloon lumen assisting device with dynamic traction: This method utilizes a suture attached to the proximal balloon to be anchored with the clip to the tip of the lesion to create traction (Fig. 2).
-
3)
Ancillary tools for improved visualization and dissection:
-
a.
Cap: Using a cap applied to the tip of the endoscope is essential to prevent the tissue from obscuring the lens during resection. Newer ESD caps are equipped with irrigation ports, cutting wires, or snares to assist ESD.
-
b.
Dyes: Coloring agents are useful in ESD. For example, spray chromoendoscopy aids in marking the lesion surface and identifying borders [36]. Adding the dye to the injectable solution helps in plane identification during dissection.
-
c.
Endoscopes: Multi-bending endoscopes with high-definition imaging can help in approaching anatomically difficult lesions. Endoscopes with two channels allow two devices to be used concurrently such as an ESD knife and forceps.
-
d.
Electrosurgical units (ESU): An ESU with advanced features aids in achieving a successful ESD. For example, units with impedance sensing can respond accordingly to maintain constant voltage. Units with adjustable duty cycles and peak voltage can be adjusted according to the wall thickness of the lesion and the anatomical site.
-
e.
Gas insufflation: Luminal insufflation with CO2 has some advantages over other agents, including a faster resorption time, which could decrease abdominal discomfort during and after the procedure [37]. In case of perforation, CO2 is less likely to cause pneumomediastinum or pneumoperitoneum [38].
Hybrid EMR and ESD Techniques
Hybrid techniques include a combined use of EMR and ESD to shorten the procedure time while achieving the same results. Two examples of combined techniques are:
-
1-
Precut EMR: An ESD knife is used for incision before snaring the lesion in order to achieve en bloc resection. The precut can be partially or fully circumferential [39]. In precutting EMR, there is no dissection. Precutting EMR is not suitable for lesions larger than 30 mm due to the size limit of the snare.
-
2-
Hybrid EMR/ESD: Is a modified ESD where two-thirds of the lesion is dissected by a conventional ESD method as mentioned above, and the last third is removed using an EMR snare [40]. Hybrid EMR/ESD was evaluated in a recent retrospective study of 220 patients. Ninety-three patients underwent hybrid ESD of colon lesions and achieved similar results to the group of conventional ESD in less time [41]; a shorter procedure time is associated with fewer complications as discussed below.
Specimen Handling
Specimens should be affixed to a flat surface using pins or needles to facilitate the pathology review of margin and depth [42].
Complications
ER carries risks that are inversely proportional to the experience of the endoscopist [43]. Multiple adverse events include perforation, bleeding, and stricture formation. Due to the nature of ESD, its steep learning curve, and longer procedural time, it has higher risks and more complications than the simpler EMR [44]. Using data from the Diagnosis Procedure Combination (DPC) database in Japan, a study for esophageal ESD that included 12,899 esophageal ESD found a total complication rate of 3.3% [45]. In a different study using the same database, 32,943 gastric ESD had a total complication rate of 3.5% [46]. A study evaluating colorectal ESD in Japan found a complication rate of 4.4% in 7567 colorectal ESD [47]. In this review, we will focus on a few of the most commonly encountered adverse events during endoscopic resection (Table 2).
Bleeding
Bleeding is the most common complication of ER. Bleeding is classified as immediate or delayed, with immediate bleeding defined as more than 2 g of Hb loss within 24 h after the procedure [48]. Post-procedure bleeding after gastric ESD was 5.1% in a meta-analysis that included 62 prospective and retrospective trials of gastric ESD. The most common risk factors for bleeding after ESD were larger specimen (greater than 2 cm), longer duration of the procedure, ulcerated lesions, and lesions present on the lesser curvature [49]. In a prospective study of 1172 patients who underwent colonic EMR, 133 patients had intraprocedural bleeding that was managed during the procedure. Seventy-three patients (6.2%) had post-endoscopy bleeding managed by conservative measures or by repeat endoscopy or surgery [50]. Prophylactic clipping did not decrease the risk of post-procedural bleeding in a meta-analysis of 1526 cases of colonic EMR conducted in 2017 [51]. However, a recent multicenter prospective randomized trial by Pohl et al., which included 919 patients who underwent colonic EMR with and without clipping, found that selective endoclipping after removal of large colon polyps reduced the risk of bleeding from 7.1 to 3.5% [52]. In regard to intraprocedural bleeding during ESD, bleeding can be minimized using a high-definition view, adequate submucosal injection, preventive coagulation of visible blood vessels before dissection (Fig. 3), wise choice of traction tools, and a slower pace of dissection [53]. Suturing, discussed below, was reported by Kantsevoy to be a superior technique for addressing bleeding and perforation as it decreased the need for surgery and post-procedural hospitalization [54,55,56].
Delayed bleeding is defined as more than 2 g Hb loss after being stable for the initial 24 h. The patient may need an intervention to control the bleeding [57,58,59]. Risk factors for delayed bleeding include using antiplatelet agents, anticoagulants, steroids, and nonsteroidal anti-inflammatory drugs [58, 60]. It is important to identify serious bleeding and request arterial embolization by interventional radiology when endoscopy fails to control bleeding. According to multiple studies, the most significant risk of bleeding is tumor location [57, 61]. In a retrospective analysis of 478 gastric ESD cases, bleeding occurred at sites where the submucosal plane had rich blood vessels [57]. For colonic and gastric resection, the proximal colon and the lesser curvature are associated with a higher risk of bleeding.
Perforation
The odds of perforation are higher in ESD than EMR. Perforation can be immediate or delayed and is usually diagnosed visually. If severe, it may lead to increased intraabdominal pressure and increased peak pressures on the ventilator [62]. Perforation risk can be minimized by employing the same measures used to prevent bleeding. Additionally, appropriate sedation to prevent gag reflex or body movements helps lower the risk of perforation [63]. Risk factors for perforation include tumor size (greater than 2 cm) and longer procedure time (more than 2 h) [64, 65]. In a prospective study of 143 patients who underwent esophageal ESD, perforation occurred in 4% of the cases, causing pneumoperitoneum and pneumothorax. All cases were treated during the procedure with clips and resolved without surgical treatment [66]. Perforation after gastric ER occurs more often with ESD than EMR. A meta-analysis comparing EMR versus ESD in the management of early gastric cancer found that gastric perforation occurred in 1% of EMR cases compared to 4.5% of gastric ESD cases [67]. Regarding colonic ESD (C-ESD), perforations were reported in up to 10% of cases. A prospective study of 1111 colorectal ESD reported a perforation rate of 5%. In the majority of cases, perforation was addressed during endoscopy, with 0.5% requiring surgical intervention [68, 69•, 70,70,71,72,73,74]. A large study of over 1000 lesions reported that the risk factors for perforation during colonic ESD included large lesions and minimal provider experience [75]. Duodenal ESD is associated with a very high risk of intraprocedural perforation or delayed perforation. In a meta-analysis of 14 studies comparing duodenal ESD to duodenal EMR, surgical repair of delayed perforation was performed in up to 33% of duodenal ESD cases in some of the included studies [76] (Fig. 4). Newer methods are under investigation to lower the risk of delayed perforation following duodenal ESD, including the use of polyglycolic acid sheets and fibrin glue to shield ESD ulcer [77].
If perforation occurs, it can be addressed by endoclipping or suturing. In 2016, a retrospective study by Kantsevoy et al. reported that endoscopic suturing was superior to clipping in managing ESD defects and perforation. Suturing led to a decreased need for surgical intervention when compared to clipping of post-resection defects [78]. Antibiotics should be administered if perforation occurs, and decompression of pneumoperitoneum should be attempted to prevent tension penumoperitoneum [64]. Delayed perforation is a rare event and is considered a surgical emergency [79]. Due to the rarity of the event, the risk factors are inadequately understood; however, in theory, excessive thermal damage of the muscular layer may lead to delayed perforation. Therefore, excessive coagulation of visible vessels should be avoided, particulary in the thin colon wall. It is worth noting that micro-perforation during ESD is an adverse event that can be easily treated during endoscopy without post-procedural adverse event.
Pain
Post-procedure pain has been described after ESD, especially in the colon and rectum, as post endoscopic submucosal dissection electrocoagulation syndrome (PEECS). PEECS can manifest as pain, fever, leukocytosis, and rebound tenderness without perforation. Forty percent of patients reported PEECS in a study of 89 patients treated with ESD [80]. PEECS is managed conservatively with intravenous antibiotics and pain medications.
Bacteremia
Transient bacteremia following cap-assisted EMR has been reported; however, repeat cultures after 4 h were negative in a study that recommended against routine prophylactic antibiotics [81].
Aspiration Pneumonia
Post-procedure pneumonia occurred in up to 6% of patients undergoing ESD [82, 83]. Risk factors are old age (above 75 years) and long procedure time (more than 2 h) [83]. Diagnosis can be made based on clinical symptoms and imaging modalities. To prevent aspiration pneumonia, removal of oral secretions by suction is advised prior to endoscopy and avoidance of excessive air insufflation [82]. Treatment for pneumonia in this setting follows general standard of care guidelines, including short course of antibiotics treatment.
Venous Thromboembolism (VTE)
In a prospective cohort study that involved 60 patients who underwent gastric ESD, 10% developed venous thromboses. Risk factors were prolonged immobility during and after the procedure [84]. Elastic stocking and sequential compression devices (SCDs) are usually used before and after the procedure to lower the risk of thrombosis; however, a recent study of 31,824 patients found that SCDs were not associated with lower VTE incidence during the hospital stay [85]. Usually a short course of anticoagulation is sufficient for the treatment of VTE in this setting.
Stricture
Esophageal stricture is common after ER greater than three-fourths of the esophageal circumference [86]. A steroid injection, such as triamcinolone, may decrease stricture formation [87]. Esophageal stricture can be easily treated by balloon dilatation [88]. Rectal stricture after ESD is rare but can happen after circumferential colonic resection. In a retrospective study of 370 rectal ESD, 26 patients underwent rectal ESD for a lesion greater than three quarters of the circumference; only one out of 26 patients developed rectal stenosis [89].
Evidence Supporting Endoscopic Resection
In the eastern hemisphere, ER is the gold standard treatment for early GI cancers. Publications since the 1980s have validated the effectiveness, safety, and superiority of ER. In the western hemisphere, ESD is still a novel intervention with limited availability and continued debate. Although EMR is simpler and faster, it fails to address larger lesions and has a higher rate of lesion recurrence compared to ESD, along with other disadvantages. Although ESD is a complex intervention with a challenging learning curve and potentially a higher complication rate, it offers better outcome, lower morbidity, and possibly lower cost [44]. The optimal choice would be based on the organ involved, type and size of the lesion, patient goals, and availability of expertise. The evidence for performing ESD for early gastric cancer is well documented in eastern literature. Given the low prevalence of early gastric cancer in western countries, we will focus on the evidence of endoscopic resection for esophageal and colonic lesions in this section.
Esophageal Lesions
With an increasing detection rate due to technological advancements, ER has become a superior choice for superficial tumors of the esophagus [44]. Barrett’s esophagus (BE) is the main condition treated by ER in western countries.
BE is a precursor of esophageal adenocarcinoma [90]. Compared to surgery, ER is cost-effective, with fewer complications and superior outcomes [44]. The choice between EMR and ESD for nodular or dysplastic esophageal lesions depends on the size. EMR can be safely performed for lesions less than 2 cm that can be removed en bloc and allow adequate histology review for depth of invasion and grade of differentiation [91]. EMR also offers a curative outcome for low risk lesions [92]. The recurrence rate of dysplasia after EMR can be as high as 10% [93]. Recurrence rates for EMR are worse when piecemeal resection is performed, without ablation for large lesions [94]. The recurrence rate is lower if ablation therapy is performed on the target area after EMR [95]. The leading disadvantage of EMR is the fragmented specimen, which compromises the pathology review, making it impossible to assume curative resection [96]. According to a recent review published in 2019 [44], ER is considered curative if the peripheral and deep margins are free from macroscopic or microscopic tumor (R0 resection), depth of invasion is less than 500 μm below the muscularis mucosa, poorly differentiated or mucinous histology is absent, and there is no lymphovascular or perineural involvement. EMR for esophageal lesions is associated with fewer adverse events compared to surgery. Bleeding and perforation are rare; these events can be managed by endoscopy [97].
The main advantage of ESD over EMR is en bloc resection of any lesion irrespective of its size, allowing precise pathology review of margin and depth [98, 99]. In a recent meta-analysis of 11 studies including a total of 501 patients who underwent ESD for early esophageal adenocarcinoma, the mean lesion size was 27 mm, and procedure time was under 2 h, with en bloc and curative resection of 75% and 65%, respectively. The recurrence rate after curative resection was 0.17% at a mean follow-up period of 23 months [100•]. Strictures occurred in 11.6% of all cases, and in all cases, it was resolved by dilation in a repeat endoscopy. A recent meta-analysis of eight studies compared EMR to ESD. The study included 1081 patients and found ESD superior to EMR, with a 92% curative rate for ESD compared to 53% for EMR. ESD achieved a lower recurrence rate of 0.3% versus 12% for EMR [101]. The same study concluded that ESD took longer and had more perforation events; however, bleeding and stricture formation were similar in EMR and ESD. Another meta-analysis of 16 studies comparing multiband EMR (MB-EMR) to ESD found a 2.6% recurrence rate for MB-EMR and a 0.7% recurrence rate for ESD (P value = 0.06) [102]. In a prospective randomized controlled study of 40 patients with superficial esophageal neoplasia, en bloc resection with ESD was 100% compared to 15% for EMR, and curative resection was 53% for ESD and 12% for EMR [103].
Based on the above evidence, EMR may be suitable for lesions less than 20 mm, which are easily lifted with less risk of submucosal invasion (SMI) [42]. ESD should be considered for all other lesions, such as large bulky lesions with a higher risk for SMI [44]. Lesions showing intramucosal carcinoma should be treated with ESD due to frequent upstaging of the tumor after resection [104, 105]. Lesions showing a positive margin or previous incomplete resection should be treated by ESD [44]. Although EMR might seem more cost-effective, repeated procedures without curative outcome can reach higher cumulative cost.
Colonic Lesions
Early detection and removal of colon polyps significantly reduce the death rate due to cancer [106]. ER has been proven to have a clear advantage over surgery for removal of colorectal lesions; the superiority of ER over surgery encompass all aspects, including cost and outcome [107, 108]. Endoscopic resection is associated with low mortality compared to surgical resection. In a study of 1050 patients with advanced colonic polyps who were treated with EMR, the actual 30-day mortality after EMR was 0% in the subset of patients with multiple comorbidities and predicated surgical mortality of 5% or higher [109]. ER offers an advantage for the resection of recurrent lesions after prior surgical resection. In a small retrospective study of 11 patients with post-surgical colorectal lesions at the anastomotic site, successful en bloc resection was achieved without perforation in 88.9%, despite the fact that ESD at anastomotic sites is difficult due to severe fibrosis. ESD in this limited study prevented the need for repeat surgical resection [110].
With regard to lesion selection for EMR versus ESD, there is no clear evidence showing the superiority of one technique over the other in the management of colonic polyps. Removal of colorectal lesions in piecemeal fashion is effective but may lead to recurrence requiring endoscopic reintervention. The recurrence rate with piecemeal EMR could be as high as 40% [111,112,113]. The use of soft tip coagulation or argon plasma coagulation may decrease the recurrence rate following piecemeal EMR [114]. ESD is superior for lesions with possible superficial submucosal invasion. The Japan Gastroenterological Endoscopy suggested that ESD can cure SMI with the following criteria [115]:
-
i.
Resection with margin negative (R0)
-
ii.
Depth of the invasion less than 1000 um below muscularis mucosa
-
iii.
Absence of poorly differentiated or mucinous pathology
-
iv.
Absence of lymphovascular invasions and tumor budding
It is advisable to remove early colonic neoplasia with suspected SMI with ESD. Approaching SMI lesions with the piecemeal EMR technique will require follow-up surgery [116].
Colonic ESD reported en bloc and curative resection rates of 88% and 89%, respectively, in a prospective study of Saito et al., which included 1111 colonic lesions ranging from tubular adenoma to superficial submucosal cancer [69•]. A direct comparison of EMR to ESD was conducted by meta-analysis of eight studies and reported the outcomes of ER of 2229 colorectal neoplastic lesions. In this meta-analysis, the en bloc and curative resection was superior in ESD compared to the EMR group, and the rate of recurrence was lower in the ESD group. However, ESD was associated with more complications such as perforation and the potential need for surgery [98]. A similar meta-analysis of 66 studies involving 18,000 lesions showed the en bloc resection rate by ESD in 91% of lesions compared to 63% in lesions resected by EMR [117]. Both meta-analyses confirmed a lower perforation rate for EMR, as previously discussed.
In regard to patient selection for colonic ESD, the Japan Gastroenterological Endoscopy Society [98], the European Society of Gastrointestinal Endoscopy [42], and recent proposal guidelines from the USA [44, 118, 119] recommend ESD for the following colonic lesions: large flat lateral spreading non granular polyps, recurrent colonic lesions, lesions with submucosal fibrosis, and lesions with suspicion of submucosal invasion.
Conclusion
Endoscopic resection techniques are increasingly becoming the first-line treatment for superficial lesions in the GI tract. EMR in selected cases can be safe and effective. For larger lesions and lesions with submucosal invasion, ESD could be effective and curative. ESD has a steep learning curve, longer procedural time, and a higher rate of adverse events. The choice for the best approach can be tailored for each patient depending on lesion size, pathology, care goals, and availability of local expertise.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
•• Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute clinical practice update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol. 2019;17(1):16–25.e1 This reference summarizes ESD indications for the commonly performed procedures in the US.
Hwang JH, et al. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82(2):215–26.
Jung YS, Park DI. Submucosal injection solutions for endoscopic 581 mucosal resection and endoscopic submucosal dissection of gastrointestinal neoplasms. Gastrointestinal Intervention. 2013;2(2):73–7
Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Matsuura T, et al. Different mixtures of sodium hyaluronate and their ability to create submucosal fluid cushions for endoscopic mucosal resection. Endoscopy. 2004;36(7):584–9.
Fujishiro M, Yahagi N, Kashimura K, Mizushima Y, Oka M, Enomoto S, et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36(7):579–83.
Lee SH, Park JH, Park DH, Chung IK, Kim HS, Park SH, et al. Clinical efficacy of EMR with submucosal injection of a fibrinogen mixture: a prospective randomized trial. Gastrointest Endosc. 2006;64(5):691–6.
Yamamoto H, Yahagi N, Oyama T, Gotoda T, Doi T, Hirasaki S, et al. Usefulness and safety of 0.4% sodium hyaluronate solution as a submucosal fluid "cushion" in endoscopic resection for gastric neoplasms: a prospective multicenter trial. Gastrointest Endosc. 2008;67(6):830–9.
Fasoulas K, Lazaraki G, Chatzimavroudis G, Paroutoglou G, Katsinelos T, Dimou E, et al. Endoscopic mucosal resection of giant laterally spreading tumors with submucosal injection of hydroxyethyl starch: comparative study with normal saline solution. Surg Laparosc Endosc Percutan Tech. 2012;22(3):272–8.
Feitoza AB, Gostout CJ, Burgart LJ, Burkert A, Herman LJ, Rajan E. Hydroxypropyl methylcellulose: a better submucosal fluid cushion for endoscopic mucosal resection. Gastrointest Endosc. 2003;57(1):41–7.
Polymeros D, Kotsalidis G, Triantafyllou K, Karamanolis G, Panagiotides JG, Ladas SD. Comparative performance of novel solutions for submucosal injection in porcine stomachs: an ex vivo study. Dig Liver Dis. 2010;42(3):226–9.
Conio M, Rajan E, Sorbi D, Norton I, Herman L, Filiberti R, et al. Comparative performance in the porcine esophagus of different solutions used for submucosal injection. Gastrointest Endosc. 2002;56(4):513–6.
Kahler GF, et al. Selective tissue elevation by pressure injection (STEP) facilitates endoscopic mucosal resection (EMR). Surg Technol Int. 2007;16:107–12.
Hirao M, et al. Endoscopic resection with local injection of hypertonic saline epinephrine for the treatment of early gastric cancer. Gan To Kagaku Ryoho. 1988;15(4 Pt 2–3):1466–72.
Tanaka M, Ono H, Hasuike N, Takizawa K. Endoscopic submucosal dissection of early gastric cancer. Digestion. 2008;77(Suppl 1):23–8.
Probst A, Maerkl B, Bittinger M, Messmann H. Gastric ischemia following endoscopic submucosal dissection of early gastric cancer. Gastric Cancer. 2010;13(1):58–61.
Kim HH, et al. Myocardial infarction thought to be provoked by local epinephrine injection during endoscopic submucosal dissection. J Clin Med Res. 2011;3(3):143–6.
Poppers DM, Haber GB. Endoscopic mucosal resection of colonic lesions: current applications and future prospects. Med Clin North Am. 2008;92(3):687–705 x.
Fleischer DE, Wang GQ, Dawsey S, Tio TL, Newsome J, Kidwell J, et al. Tissue band ligation followed by snare resection (band and snare): a new technique for tissue acquisition in the esophagus. Gastrointest Endosc. 1996;44(1):68–72.
Binmoeller, K.F., et al., "Underwater" EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc. 2012;75(5):1086–91.
Arebi N, et al. Endoscopic mucosal resection of 161 cases of large sessile or flat colorectal polyps. 2007;42(7):859–66.
Friedland S, et al. Endoscopic management of nonlifting colon polyps. 2013;2013.
Luigiano, C., et al., Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy. 2009;41(10):829–35.
Thoguluva Chandrasekar V, et al. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled-analysis. Gastrointest Endosc. 2019;89(5):929–936.e3.
Thoguluva Chandrasekar V, et al. Cold snare endoscopic resection of nonpedunculated colorectal polyps larger than 10 mm: a systematic review and pooled-analysis. Gastrointest Endosc. 2019;89(5):929–936.e3.
Burgess NG, Bourke MJ, Byth K. Prophylactic clip closure. Gastrointest Endosc. 2013;78(2):386–7.
Shioji K, Suzuki Y, Kobayashi M, Nakamura A, Azumaya M, Takeuchi M, et al. Prophylactic clip application does not decrease delayed bleeding after colonoscopic polypectomy. Gastrointest Endosc. 2003;57(6):691–4.
Zhang QS, Han B, Xu JH, Gao P, Shen YC. Clip closure of defect after endoscopic resection in patients with larger colorectal tumors decreased the adverse events. Gastrointest Endosc. 2015;82(5):904–9.
Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50(4):560–3.
Jeon WJ, You IY, Chae HB, Park SM, Youn SJ. A new technique for gastric endoscopic submucosal dissection: peroral traction-assisted endoscopic submucosal dissection. Gastrointest Endosc. 2009;69(1):29–33.
Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45(4):375–8.
Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, et al. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38(10):1007–10.
Baldaque-Silva F, Vilas-Boas F, Velosa M, Macedo G. Endoscopic submucosal dissection of gastric lesions using the "yo-yo technique". Endoscopy. 2013;45(3):218–21.
Matsumoto K, et al. A new traction device for facilitating endoscopic submucosal dissection (ESD) for early gastric cancer: the "medical ring". Endoscopy. 2011;43(Suppl 2 UCTN):E67–8.
Parra-Blanco A, Nicolas D, Arnau MR, Gimeno-Garcia AZ, Rodrigo L, Quintero E. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video). Gastrointest Endosc. 2011;74(5):1137–41.
Fujii L, Onkendi EO, Bingener-Casey J, Levy MJ, Gostout CJ. Dual-scope endoscopic deep dissection of proximal gastric tumors (with video). Gastrointest Endosc. 2013;78(2):365–9.
Monkemuller K, Wilcox CM. Interventional chromoendoscopy. Gastrointest Endosc. 2013;78(2):346–50.
Wu J, Hu B. The role of carbon dioxide insufflation in colonoscopy: a systematic review and meta-analysis. Endoscopy. 2012;44(2):128–36.
Maeda Y, et al. A pilot study to assess mediastinal emphysema after esophageal endoscopic submucosal dissection with carbon dioxide insufflation. Endoscopy. 2012;44(6):565–71.
Yoshida N, Inoue K, Dohi O, Itoh Y. Precutting EMR with full or partial circumferential incision with a snare tip for the en-bloc resection of difficult colorectal lesions. VideoGIE. 2018;3(12):378–80.
Toyonaga T, Man-I M, Morita Y, Azuma T. Endoscopic submucosal dissection (ESD) versus simplified/hybrid ESD. Gastrointest Endosc Clin N Am. 2014;24(2):191–9.
Wang, X.Y., et al., Efficacy and safety of hybrid endoscopic submucosal dissection compared with endoscopic submucosal dissection for rectal neuroendocrine tumors and risk factors associated with incomplete endoscopic resection. Ann Transl Med. 2020;8(6):368.
Pimentel-Nunes P, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47(9):829–54.
Saito I, Tsuji Y, Sakaguchi Y, Niimi K, Ono S, Kodashima S, et al. Complications related to gastric endoscopic submucosal dissection and their managements. Clinical endoscopy. 2014;47(5):398–403.
Yang D, Othman M, Draganov PV. Endoscopic Mucosal Resection vs Endoscopic Submucosal Dissection For Barrett’s Esophagus and Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2019;17(6):1019–28.
Odagiri H, Yasunaga H, Matsui H, Matsui S, Fushimi K, Kaise M. Hospital volume and adverse events following esophageal endoscopic submucosal dissection in Japan. Endoscopy. 2017;49(4):321–6.
Murata A, Okamoto K, Muramatsu K, Matsuda S. Time trend of medical economic outcomes of endoscopic submucosal dissection for gastric cancer in Japan: a national database analysis. Gastric Cancer. 2014;17(2):294–301.
Odagiri H, Yasunaga H, Matsui H, Fushimi K, Iizuka T, Kaise M. Hospital volume and the occurrence of bleeding and perforation after colorectal endoscopic submucosal dissection: analysis of a national administrative database in Japan. Dis Colon Rectum. 2015;58(6):597–603.
Oda I. et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. 2005;17(1):54–8.
Libanio D, et al. Risk factors for bleeding after gastric endoscopic submucosal dissection: a systematic review and meta-analysis. Gastrointest Endosc. 2016;84(4):572–86.
Burgess NG, et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol. 2014;12(4):651–61.e1–3.
Nishizawa T, Suzuki H, Goto O, Ogata H, Kanai T, Yahagi N. Effect of prophylactic clipping in colorectal endoscopic resection: a meta-analysis of randomized controlled studies. United Eur Gastroenterol J. 2017;5(6):859–67.
Pohl H, et al. Clip Closure Prevents Bleeding After Endoscopic Resection of Large Colon Polyps in a Randomized Trial. Gastroenterology. 2019;157(4):977–984.e3.
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Tateishi A, Omata M. Management of bleeding concerning endoscopic submucosal dissection with the flex knife for stomach neoplasm. Dig Endosc. 2006;18:S119–S122.
Aihara H, Kumar N, Ryou M, Abidi W, Ryan MB, Thompson CC. Facilitating endoscopic submucosal dissection: the suture-pulley method significantly improves procedure time and minimizes technical difficulty compared with conventional technique: an ex vivo study (with video). Gastrointest Endosc. 2014;80(3):495–502.
Kantsevoy SV, Wagner A, Mitrakov AA, Thuluvath AJ, Berr F. Rectal reconstruction after endoscopic submucosal dissection for removal of a giant rectal lesion. VideoGIE. 2019;4(4):179–81.
Kantsevoy SV, Bitner M, Hajiyeva G, Mirovski PM, Cox ME, Swope T, et al. Endoscopic management of colonic perforations: clips versus suturing closure (with videos). Gastrointest Endosc. 2016;84(3):487–93.
Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45(1):30–6.
Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, et al. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16(23):2913–7.
Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD study group multicenter study. Gastrointest Endosc. 2009;69(7):1228–35.
Kataoka Y, Tsuji Y, Sakaguchi Y, Minatsuki C, Asada-Hirayama I, Niimi K, et al. Bleeding after endoscopic submucosal dissection: risk factors and preventive methods. World J Gastroenterol. 2016;22(26):5927–35.
Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Digestive Endoscopy. 2006;18:S123–7. [Internet]. Wiley Online Library. Available from: https://doi.org/10.1111/j.1443-1661.2006.00645.x.
Veekash G, Wei LX, Su M. Carbon dioxide pneumoperitoneum, physiologic changes and anesthetic concerns. Ambul Surg. 2010;16:41–6.
Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63(2):243–9.
Nonaka, S., et al., Endoscopic submucosal dissection for early gastric cancer in the remnant stomach after gastrectomy. Gastrointest Endosc. 2013;78(1):63–72.
Watari J, Tomita T, Toyoshima F, Sakurai J, Kondo T, Asano H, et al. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: a prospective pilot study. World J Gastrointest Endosc. 2013;5(6):281–7.
Li QL, Yao LQ, Zhou PH, Xu MD, Chen SY, Zhong YS, et al. Submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a large study of endoscopic submucosal dissection (with video). Gastrointest Endosc. 2012;75(6):1153–8.
Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25(8):2666–77.
Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, et al. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42(9):723–9.
• Saito Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72(6):1217–25 One of the largest prospective studies discussing colonic ESD..
Hayashi N, Tanaka S, Nishiyama S, Terasaki M, Nakadoi K, Oka S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc. 2014;79(3):427–35.
Kiriyama S, Saito Y, Yamamoto S, Soetikno R, Matsuda T, Nakajima T, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy. 2012;44(11):1024–30.
Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, et al. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39(5):418–22.
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, et al. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006;38(10):1001–6.
Yoshida N, Wakabayashi N, Kanemasa K, Sumida Y, Hasegawa D, Inoue K, et al. Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy. 2009;41(9):758–61.
Lee EJ, Lee JB, Lee SH, Kim DS, Lee DH, Lee DS, et al. Endoscopic submucosal dissection for colorectal tumors--1,000 colorectal ESD cases: one specialized institute's experiences. Surg Endosc. 2013;27(1):31–9.
Perez-Cuadrado-Robles E, et al. ESD versus EMR in non-ampullary superficial duodenal tumors: a systematic review and meta-analysis. Endosc Int Open. 2018;6(8):E998–E1007.
Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26(Suppl 2):46–9.
Kantsevoy SV, Bitner M, Mitrakov AA, Thuluvath PJ. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos). Gastrointest Endosc. 2014;79(3):503–7.
Ikezawa K, Michida T, Iwahashi K, Maeda K, Naito M, Ito T, et al. Delayed perforation occurring after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer. 2012;15(1):111–4.
Jung D, Youn YH, Jahng J, Kim JH, Park H. Risk of electrocoagulation syndrome after endoscopic submucosal dissection in the colon and rectum. Endoscopy. 2013;45(9):714–7.
Lee TH, Hsueh PR, Yeh WC, Wang HP, Wang TH, Lin JT. Low frequency of bacteremia after endoscopic mucosal resection. Gastrointest Endosc. 2000;52(2):223–5.
Watari J, Tomita T, Toyoshima F, Sakurai J, Kondo T, Asano H, et al. The incidence of "silent" free air and aspiration pneumonia detected by CT after gastric endoscopic submucosal dissection. Gastrointest Endosc. 2012;76(6):1116–23.
Park CH, Kim H, Kang YA, Cho IR, Kim B, Heo SJ, et al. Risk factors and prognosis of pulmonary complications after endoscopic submucosal dissection for gastric neoplasia. Dig Dis Sci. 2013;58(2):540–6.
Kusunoki, M., et al., The incidence of deep vein thrombosis in Japanese patients undergoing endoscopic submucosal dissection. Gastrointest Endosc, 2011;74(4):798–804.
Dhakal P, Wang L, Gardiner J, Shrotriya S, Sharma M, Rayamajhi S. Effectiveness of sequential compression devices in prevention of venous thromboembolism in medically ill hospitalized patients: a retrospective cohort study. Turk J Haematol. 2019;36(3):193–8.
Yoshida M, Hanashi T, Momma K, Yamada Y, Sakaki N, Koike M, et al. Endoscopic mucosal resection for radical treatment of esophageal cancer. Gan To Kagaku Ryoho. 1995;22(7):847–54.
Hashimoto S, Kobayashi M, Takeuchi M, Sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc. 2011;74(6):1389–93.
Sato H, Inoue H, Kobayashi Y, Maselli R, Santi EGR, Hayee B'H, et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc. 2013;78(2):250–7.
Abe S, Sakamoto T, Takamaru H, Yamada M, Nakajima T, Matsuda T, et al. Stenosis rates after endoscopic submucosal dissection of large rectal tumors involving greater than three quarters of the luminal circumference. Surg Endosc. 2016;30(12):5459–64.
Singh S, et al. Incidence of esophageal adenocarcinoma in Barrett's esophagus with low-grade dysplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2014;79(6):897–909.e4.
Moss A, Bourke MJ, Hourigan LF, Gupta S, Williams SJ, Tran K, et al. Endoscopic resection for Barrett's high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105(6):1276–83.
Ell C, May A, Pech O, Gossner L, Guenter E, Behrens A, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer). Gastrointest Endosc. 2007;65(1):3–10.
Small AJ, et al. Comparative risk of recurrence of dysplasia and carcinoma after endoluminal eradication therapy of high-grade dysplasia versus intramucosal carcinoma in Barrett's esophagus. Gastrointest Endosc. 2015;81(5):1158–66.e1–4.
Pech O, Behrens A, May A, Nachbar L, Gossner L, Rabenstein T, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett’s oesophagus. Gut. 2008;57(9):1200–6.
Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol. 2016;111(1):30–50 quiz 51.
Martelli MG, Duckworth LV, Draganov PV. Endoscopic submucosal dissection is superior to endoscopic mucosal resection for histologic evaluation of Barrett's esophagus and Barrett's-related neoplasia. Am J Gastroenterol. 2016;111(6):902–3.
Desai M, et al. Efficacy and safety outcomes of multimodal endoscopic eradication therapy in Barrett’s esophagus-related neoplasia: a systematic review and pooled analysis. Gastrointest Endosc. 2017;85(3):482–495.e4.
Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, et al. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81(3):583–95.
Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4(6):688–94.
• Yang D, et al. Endoscopic submucosal dissection for early Barrett's neoplasia: a meta-analysis. Gastrointest Endosc. 2018;87(6):1383–93 This is the largest meta-analysis discussing the outcomes of ESD for esophageal adenocarcinoma.
Guo HM, Zhang XQ, Chen M, Huang SL, Zou XP. Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J Gastroenterol. 2014;20(18):5540–7.
Komeda Y, Bruno M, Koch A. EMR is not inferior to ESD for early Barrett's and EGJ neoplasia: an extensive review on outcome, recurrence and complication rates. Endosc Int Open. 2014;2(2):E58–64.
Terheggen G, Horn EM, Vieth M, Gabbert H, Enderle M, Neugebauer A, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017;66(5):783–93.
Yang D, Coman RM, Kahaleh M, Waxman I, Wang AY, Sethi A, et al. Endoscopic submucosal dissection for Barrett’s early neoplasia: a multicenter study in the United States. Gastrointest Endosc. 2017;86(4):600–7.
Coman RM, Gotoda T, Forsmark CE, Draganov PV. Prospective evaluation of the clinical utility of endoscopic submucosal dissection (ESD) in patients with Barrett's esophagus: a western center experience. Endosc Int Open. 2016;4(6):E715–21.
Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–96.
Jayanna M, et al. Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions. Clin Gastroenterol Hepatol. 2016;14(2):271–8.e1–2.
Ahlenstiel G, Hourigan LF, Brown G, Zanati S, Williams SJ, Singh R, et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014;80(4):668–76.
Ahlenstiel G, Hourigan LF, Brown G, Zanati S, Williams SJ, Singh R, et al. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014;80(4):668–76.
Maehata, T., Kato, M., Ochiai, Y. et al. Feasibility of endoscopic submucosal dissection for colorectal neoplasia at anastomotic sites: a retrospective study. Surg Endosc. 2020.
Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, et al. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian colonic EMR (ACE) study. Gut. 2015;64(1):57–65.
Oka S, Tanaka S, Saito Y, Iishi H, Kudo SE, Ikematsu H, et al. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015;110(5):697–707.
Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109(2):183–9.
Holmes I, Kim HG, Yang DH, Friedland S. Avulsion is superior to argon plasma coagulation for treatment of visible residual neoplasia during EMR of colorectal polyps (with videos). Gastrointest Endosc. 2016;84(5):822–9.
Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27(4):417–34.
Benson AB 3rd, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2017;15(3):370–98.
De Ceglie A, et al. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: a systematic review. Crit Rev Oncol Hematol. 2016;104:138–55.
Burgess NG, et al. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology. 2017;153(3):732–742.e1.
Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140(7):1909–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Mohamed Othman MD, is a consultant for Olympus, Boston Scientific, Conmed, Abbvie, and Lumendi.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Large Intestine
Rights and permissions
About this article
Cite this article
Ahmed, Y., Othman, M. EMR/ESD: Techniques, Complications, and Evidence. Curr Gastroenterol Rep 22, 39 (2020). https://doi.org/10.1007/s11894-020-00777-z
Published:
DOI: https://doi.org/10.1007/s11894-020-00777-z