Abstract
Patients with unresectable intrahepatic cholangiocarcinoma (ICC) have a poor prognosis and have historically benefited from very few treatment options. In the last 10 years, image-guided locoregional therapies have been developed to administer targeted treatment to the liver and are increasingly used in primary hepatic malignancies such as ICC. Locoregional therapies primarily consist of ablation and intra-arterial therapies, including conventional transarterial chemoembolization (cTACE), TACE with drug-eluting beads (DEB-TACE), and yttrium-90 radioembolization (Y90-RE). Current clinical evidence suggests that these treatments result in prolonged survival without significantly diminishing patient’s quality of life. This chapter aims to describe each locoregional therapy and the clinical evidence supporting its therapeutic application to unresectable ICC.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Intrahepatic cholangiocarcinoma (ICC)
- Locoregional therapy
- Intra-arterial therapy
- Transarterial chemoembolization
- cTACE
- DEB-TACE
- Y90-RE
- Radiation
- Ablation
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer after hepatocellular carcinoma, representing about 10% of all cholangiocarcinomas [1]. Incidence levels have been rising over the past 15 years across Europe, North America, and Asia [2, 3]. Though a majority of patients develop ICC de novo, risk factors such as infectious agents (viral hepatitis, liver flukes), biliary tract disease (primary sclerosing cholangitis, biliary cystic disease), toxic exposures, metabolic abnormalities, cirrhosis, and lifestyle factors (smoking, alcohol abuse) increase the likelihood of developing ICC [4]. Despite improvements in the treatment, the prognosis of patients with ICC remains poor, since patients commonly present at advanced disease stages when symptoms first arise [5]. Median survival is less than 27 months, and 5-year overall survival (OS) rates range from 15 to 45% [6].
Diagnosis of ICC requires combined clinical suspicion and confirmatory laboratory, endoscopic, and radiologic data. ICC is often detected incidentally on imaging obtained for other indications. Symptoms, if they exist, usually consist of upper right quadrant discomfort, cholestasis, and weight loss. Lab work-up includes assessment of tumor markers such as carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), and carbohydrate antigen 19-9 (CA19-9). CA19-9 values are the most useful for diagnosing ICC; CA19-9 levels >100 U/mL have a sensitivity and specificity of 53% and 75–90%, respectively [5, 7]. Combined increases in CA19-9 and AFP levels would suggest a mixed hepatocellular-cholangiocarcinoma, a distinction that is important to make since the two pathologies respond differently to treatment and have markedly different outcomes [8]. Cross-sectional imaging including contrast-enhanced helical computed tomography (CT), magnetic resonance imaging (MRI)/MR cholangiopancreatography (MRCP), and position emission tomography (PET) is used to support an ICC diagnosis [5]. Contrast CT is useful for detecting the degree of biliary obstruction, liver atrophy, and the location of tumor-adjacent vessels and organs. Triple-phase helical CT will detect ICC lesions greater than 1 cm but cannot determine resectability in a majority of patients [9, 10]. MRCP is used to assess the degree of biliary obstruction through 3-D images of the biliary tree and surrounding tissue [11]. ICC lesions have a median size between 4 and 8 cm [12]. Tumors are typically hypovascular in nature and display significant fibrosis on contrast-enhanced imaging, appearing hypoenhanced on the arterial phase [5, 13]. Substantial fibrosis reduces tumor uptake of chemotherapy [14, 15].
Cholangiocarcinoma lesions develop from epithelial cells of small intrahepatic ductules or large intrahepatic ducts proximal to the hepatic ducts and are first classified as intrahepatic or extrahepatic according to their anatomical location along the separation point of second-order bile ducts [16]. ICC is further subclassified according to macroscopic growth patterns such as intraductal infiltrative, mass forming, periductal, or a combination of mass forming and periductal [17].
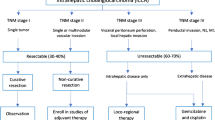
The advanced nature of ICC at the typical timepoint of diagnosis precludes a majority of patients from being eligible for surgical intervention, the only curative option. Patients with unresectable tumors go on to receive some combination of chemotherapy, radiation, and locoregional treatments. Locoregional therapy refers to targeted ablation of tumors or intra-arterial embolic therapies. Three of the most commonly utilized modalities of intra-arterial therapy include conventional transarterial chemoembolization (cTACE), TACE with drug-eluting beads (DEB-TACE), and yttrium-90 radioembolization (Y90-RE) (Fig. 9.1). These treatments work by exploiting the fact that tumoral tissue is primarily vascularized by the hepatic artery, while healthy parenchyma is mainly supplied by the portal vein. A catheter is advanced through the hepatic artery in order to deliver a combination of embolic particles, radiation, and chemotherapy drugs directly into tumors. This targeted approach reduces systemic chemotherapeutic side effects while maintaining a locally tumoricidal dose of drug. Evidence underscoring the importance of local tumor control in ICC continues to grow. In this chapter, locoregional treatments and current clinical evidence supporting their use in patients with unresectable ICC will be described.
Intra-arterial treatment visualization. This schematic demonstrates the differences between the three primary intra-arterial treatments for ICC: cTACE , DEB-TACE , and Y90-RE. cTACE involves the direct administration of a chemotherapy and Lipiodol suspension into the tumor region through the hepatic artery. DEB-TACE uses beads which release chemotherapy into the tumor vessels over time. Y90-RE utilizes the smallest microspheres which diffuse across the entire target lobe, enabling non-specific radioembolization of the tumor and surrounding area
Surgical Resection
Surgical resection is the only potentially curative intervention for patients with ICC, though up to 37% of patients with resectable tumors may not be offered the option of surgical resection [18]. While the goal of resection is to remove all disease while preserving liver volume, these procedures frequently require resection of the vena cava, extrahepatic biliary tree, or bowel, depending on the size and location of the tumor [5]. Lymphadenectomy is also necessary in a majority of cases [12].
Qualification for resection primarily relies on clinical judgment of whether the necessary resection is compatible with the level of functionality of the remaining liver tissue. Other factors considered include biochemical characteristics, the presence of metastatic lesions, and lymphatic involvement [19]. Tumors that are poorly differentiated are associated with unresectable disease, while other characteristics such as tumor size, histological origin, level of vascular invasion, and perineural invasion are not individually significant predictors of resectability [20].
A multi-institutional study reported resection outcomes for ICC patients and found that although clear intraoperative surgical margins occurred in 81.1% of patients, recurrence was observed in 53.5% of cases, with most recurrences occurring in the liver remnant [21, 22]. Positive margins, lymph node metastases, advanced cirrhosis with Child-Pugh scores beyond A, and portal hypertension are associated with poor outcomes for patients after resection [19]. Liver transplantation has poor reported outcomes and is not typically recommended for ICC [19].
Regional Liver-Directed Therapies
Regional therapies are the foundation of treatment for patients who are not eligible for surgical intervention, though ICC pathology presents unique technical challenges. Treatments targeting the hepatic artery may be less effective in ICC because tumors are relatively hypovascular. Fibrosis also reduces the penetrability of chemotherapy drugs [23]. As a result, locoregional therapies are both more technically challenging and less effective in ICC relative to other liver malignancies.
Of note, a meta-analysis across five major institutions in the United States demonstrated that median OS did not significantly differ among ICC patients receiving cTACE, DEB-TACE, and Y90-RE. Tumor response to treatment on follow-up imaging was the only predictor of improved survival [23]. Currently, selection of locoregional therapy is determined by clinical assessment of tumor characteristics, patient liver function and comorbidities, and treatment history. High-quality randomized studies of locoregional therapies are necessary in order to provide better evidence-driven guidelines for locoregional therapy selection.
Intra-arterial Therapies
Conventional Transarterial Chemoembolization
Background
The development of cTACE began in the 1970s as a treatment for hypervascular hepatocellular carcinoma. cTACE has since become the primary intra-arterial technique used to treat unresectable liver cancers, including ICC [24]. The therapy works through catheter-based administration of a suspension of chemotherapeutic drugs and an ethiodized contrast agent (Lipiodol) directly into tumor-supplying vasculature, typically a branch of one of the hepatic arteries. Then, an embolizing agent is administered in order to block the blood supply of the tumor, thereby inducing tumor necrosis. Embolic particles such as Gelfoam, polyvinyl alcohol (PVA), and trisacryl gelatin (TG) microspheres occlude more proximal blood vessels and further delay the washout of chemotherapy from the tumor [25]. The end result is a slow, sustained, and targeted delivery of chemotherapy with effective embolic blockade (Fig. 9.2).
Conventional TACE treatment in a patient diagnosed with mass-forming ICC. (a) Pre-treatment portal venous phase MR scan without contrast shows a tumor in the right lobe. (b) Digital angiography reveals diffuse blush in the right hepatic lobe. (c) CT scan 1 day after TACE shows Lipiodol deposition in tumor region. (d) Two-month follow-up MR scan shows necrosis in target lesion, indicating tumor response to treatment
In the United States and Europe, the chemotherapy combinations most frequently used for cTACE are gemcitabine and cisplatin or cisplatin, doxorubicin, and mitomycin-C [23]. Lipiodol is the primary contrast agent used and is advantageous in that it functions simultaneously as a drug transporter as well as an effective embolic agent that can penetrate tumor vasculature and reach capillaries [24]. Since Lipiodol is radiopaque on CT, it can be used to evaluate the technical success of the procedure. Lipiodol deposition on tumor has also been shown to correlate with tumor response [26].
Conventional TACE is generally well tolerated by patients. Adverse effects reported include fatigue, abdominal pain, nausea, and a transient increase in liver enzymes, often referred to as post-embolization syndrome [27,28,29]. Since its adoption, this technique has been applied to a wide spectrum of liver malignancies with successful results and is the mainstay therapy for patients with unresectable ICC [24, 30].
Evidence
Useful outcomes data of cTACE in ICC are limited because of a lack of standardized protocols. However, the role of cTACE in ICC as an adjuvant therapy to surgical resection and chemotherapy has been relatively well explored. One study of 125 patients compared various chemotherapy combinations with cTACE and demonstrated that patients treated with cTACE showed prolonged survival when compared to a control group who received chemotherapy alone (37.7% vs. 20.8% 5-year OS). Median OS was 5 months in patients who underwent surgical resection and 12 months in patients who received cTACE. Disease recurrence rates did not differ significantly between cTACE and resection groups [31]. Prospective trials are limited but have demonstrated that tumor downsizing is possible, resulting in resection eligibility after cTACE treatment in previously inoperable cases [30]. Another study identified a survival benefit for patients who had received systemic chemotherapy followed by cTACE compared to cTACE alone [32]. A third study of 42 patients showed good tumor response to cTACE treatment according to Response Evaluation Criteria in Solid Tumors (RECIST): 20 patients (48%) had stable disease (SD), 15 patients (36%) had progressive disease (PD), and 7 patients (17%) could not be evaluated. The median OS was 9.1 months. The choice of chemotherapy administered prior to cTACE is an important predictor of survival; gemcitabine combined with cisplatin resulted in a significant survival benefit when compared to gemcitabine alone (13.8 vs. 6.3 months) [33].
cTACE as a stand-alone treatment option for ICC has also been studied, though to a much lesser extent. As a stand-alone therapy, most studies suggest that if a tumor responds to the treatment, cTACE will produce a survival benefit. Additionally, current data shows that cTACE alone does not result in a survival benefit when compared to other intra-arterial therapies [23].
One study suggests that when compared to TACE, surgery does not result in increased survival for patients whose surgical procedure identifies positive lymph nodes or positive surgical margins. A retrospective study compared survival outcomes of 130 patients who underwent surgical resection, 32 patients who received cTACE, and 3 patients who received DEB-TACE. The median OS of surgical patients varied significantly if patients had positive lymph node status (9 months) or positive resection margin (11 months) when compared to patients with clear surgical margins (37 months). By contrast, the median OS of TACE patients (cTACE and DEB-TACE combined) was 11 months [34].
Drug-Eluting Beads Transarterial Chemoembolization
Background
TACE with drug-eluting beads (DEB-TACE) was developed in the last decade with the goal of addressing some limitations of conventional TACE , namely, challenges maintaining adequate drug dosing over time while continuing to minimize systemic toxicities. DEB microspheres aim to accomplish this by both embolizing and delivering chemotherapeutic agents in a manner similar to cTACE. The chemotherapeutic drugs are released more slowly when compared to cTACE, which could in theory make DEB-TACE a more controlled and targeted therapy [35]. The most common drug-eluting beads used in practice are DC or LC beads loaded with doxorubicin (DEBDOX). Beads are available in a range of diameters, typically 100–300 μm. Smaller beads such as the LC Bead M1 (diameter 70–150 μm) are currently being evaluated for efficacy. In theory, smaller beads can penetrate further into the tumor vessels, and initial studies have demonstrated they are more effective at delivering chemotherapy into tumors [36]. Irinotecan (DEBIRI) can also be used in place of doxorubicin [37]. Superabsorbent polymer (SAP) microspheres are another bead type that can be loaded with virtually any drug type, including irinotecan , cytotoxic antibiotics, and platinum-based agents [38] (Fig. 9.3).
TACE with drug-eluting beads (DEB-TACE) treatment in a patient with ICC. (a) Pretreatment arterial phase MR scan without contrast shows a large tumor in right hepatic lobe. (b) Digital angiography illustrates corresponding blush in the right lobe during treatment administration. (c) Post-embolization CT scan obtained 1 month after DEB-TACE treatment shows tumor reduction
Evidence
Though the safety of DEB-TACE has been validated, as with cTACE, the lack of standardized treatment protocols diminishes the utility of studies comparing outcomes of patients with varying forms of DEB-TACE treatment.
One study suggests that DEB-TACE in combination with systemic chemotherapy may be more effective than chemotherapy alone. This prospective study of seven patients with ICC found that DEB-TACE and systemic chemotherapy resulted in a higher median OS when compared to systemic chemotherapy alone (30 vs. 12.7 months), the largest reported improvement in survival rate. The patients receiving DEB-TACE received oxaliplatin-loaded beads in conjunction with systemic oxaliplatin and gemcitabine and were compared to a historical cohort of patients receiving only systemic oxaliplatin and gemcitabine [39].
DEB-TACE has been demonstrated to result in tumor downsizing to the extent where previously unresectable ICC can be surgically removed. A multi-institutional study enrolled 24 patients with unresectable ICC who were treated with DEB-TACE. 83.3% of patients had received prior chemotherapy. The DEB-TACE treatment used DC beads loaded with doxorubicin (150 mg) and irinotecan (75 mg), and in eight patients the treatment was combined with systemic chemotherapy. Three patients were eligible for surgical resection after DEB-TACE and systemic chemotherapy [40].
A third study demonstrated good tumor response after DEB-TACE was administered following chemotherapy or surgery. This prospective study treated 11 ICC patients with DEB-TACE following systemic chemotherapy or hepatic resection. The cohort received a median of three DEB-TACE sessions per patient and used DC Beads loaded with doxorubicin (75 mg/2 mL). The tumor response of the group was 100% according to RECIST, and the median OS was 13 months. One patient had a complete response, and nine patients had a partial response to the treatment [37].
Based on current evidence, DEB-TACE has not yet been demonstrated to lead to an improved survival benefit when compared to cTACE. While one study demonstrated prolonged median OS in patients treated with DEBIRI compared to cTACE and systemic chemotherapy, a larger meta-analysis found no differences in OS comparing DEB-TACE to cTACE and other intra-arterial therapies [22, 41]. Larger studies are needed to accurately evaluate the efficacy of DEB-TACE relative to cTACE and other treatment options for unresectable ICC.
Yttrium-90 Radioembolization
Background
Yttrium-90 radioembolization (Y90-RE) is a selective internal radiation therapy (SIRT) technique that uses microspheres to infuse radiolabeled particles through the hepatic artery, where the radioactive particles are trapped in the precapillary level and emit toxic ß-radiation. External beam radiation is used in a limited setting in liver malignancies because of the extreme sensitivity of liver tissue to radiation. The Y90-RE technique allows for higher levels of radiation to be used than what is permissible through external radiation, since exposure to surrounding parenchyma is limited. Target doses in Y90-RE are typically around 120 Gy [42]. Glass-based (TheraSphere®) or resin-based (SIR-Spheres®) microspheres are clinically used. Both produce similar outcomes, though glass microspheres are administered in higher doses [43, 44]. The small size of these microspheres allows them to penetrate tumors better than those used for DEB-TACE, but limits the embolic ability of the microsphere. Thus, Y90 microspheres are administered nonselectively across the entire lobes of the liver, resulting in a procedure that is less targeted than TACE but with more reproducible results [42, 45].
The nonselective administration of Y90-RE combined with the strong penetrative abilities of the particles often results in significant toxic side effects. After treatment, approximately half of patients will experience abdominal pain [46]. Up to 24% of patients may develop gastroduodenal ulcers, and this risk is significantly increased if Y90 spheres are administered close to a gastric artery, causing stasis in flow [47]. Angiographic imaging is vital in mitigating this risk; all patients are evaluated for arterial anatomical variants and arteriovenous shunting prior to Y90-RE treatment. If shunt vessels are identified, they may be sealed prior to treatment [48]. Since arteriovenous shunting to the lung is common in primary liver cancer, the risk of lung shunting is calculated prior to Y90 treatment and is used to modify the radiation dose [49] (Fig. 9.4).
Yttrium-90 radioembolization (Y90-RE) treatment in a patient with ICC. (a) Pre-treatment portal venous phase MR scan without contrast shows a tumor in right hepatic lobe. (b) Digital angiography illustrates diffuse blush corresponding to tumor location during treatment. (c) SPECT image shows diffuse area of radioembolization in green. (d) Post-treatment MR scan shows increased necrosis in tumor region, indicating treatment response
Despite these toxicities, Y90-RE can be administered in patients with portal involvement, since it does not induce ischemic effects [50]. Canada was the first country to approve Y90-RE for the treatment of liver malignancies, and the United States soon followed suit, although the procedure is only FDA approved for hepatocellular carcinoma. Hence, the use of Y90-RE in ICC currently requires IRB approval in the United States [51].
Evidence
As with other intra-arterial therapies, survival outcomes reported for Y90-RE are confounded by small patient cohorts with various prior treatment histories and heterogeneous dosing regimens. The safety of Y90-RE was evaluated in a study that used SIR-Spheres to treat 33 patients with cholangiocarcinoma. Patients had various previous treatments including chemotherapy and TACE. The study showed that patients had a median OS of 20 months, time to progression (TTP) of 9.8 months, and good ECOG performance status after treatment. Patients tolerated the procedure well and reported no significant toxicities [52].
A phase I trial was conducted to identify the maximum tolerable Y90-RE dose for ICC. In this study, 17 ICC patients were treated with Y90-RE using TheraSphere in combination with a radiosensitizing agent, capecitabine. The study evaluated progressively escalating doses of Y90 and found that Y90 > 170 Gy could be used with only two patients reporting dose-limiting toxicity of abdominal pain. The study concluded that radiosensitizing agents may enhance the technical success of Y90-RE and confirmed that high doses of Y90-RE can be tolerated by patients [53].
One prospective study suggests that patients naïve to systemic chemotherapy may benefit from Y90-RE more than patients with prior chemotherapy treatment. The study examined 24 patients with unresectable ICC who were treated with TheraSphere. Twenty-nine percent of patients had prior chemotherapy, and extrahepatic and bilobar diseases were present in 33% and 67% of patients, respectively. The study reported a median OS of 14.9 months, and 77% of patients observed significant tumor response. Patients who had not received prior systemic chemotherapy had a survival benefit compared to the treated group, although this may be due to the confounding factor of initial disease severity at the time of the treatment [51].
As with cTACE and DEB-TACE, Y90-RE can also downsize ICC tumors to become eligible for resection. One study reported that of 46 ICC patients treated with Y90-RE using glass-based microspheres, 5 tumors were converted to a resectable form [46].
Ablation Therapies
Background
Ablation therapy refers to a minimally invasive procedure used to directly destroy tumor tissue primarily using thermal energy. In the context of ICC, the most common ablative therapy is radiofrequency ablation (RFA ), though microwaves are also used. A radiofrequency generating electrode is inserted directly into the tumor under ultrasound image guidance [54]. When properly positioned, radiofrequency energy is delivered for a set amount of time, typically 10 min. Tissue temperature is monitored and maintained at an ideal temperature for tumor tissue destruction, typically around 105 °C. To achieve an optimal ablative margin of 0.5–1 cm, a single electrode is used for tumors less than 3 cm in diameter, and multiple or clustered electrodes are used for larger tumors [55]. Besides therapeutic efficacy, one of the primary advantages of image-guided thermal ablation is its cost-effectiveness [54].
Evidence
Modest literature is available on the use of ablative therapies for ICC, likely because ICC tumors are typically large in diameter and their central location near sensitive hilar structures limits heat application [56,57,58]. The reported technical success of RFA on eligible ICC lesions ranges from 80 to 100%. Tumor size is the primary factor in determining the success of RFA and its impact on survival; complete ablation in a single session is challenging for nodules larger than 4 cm [59, 60]. In patients with smaller tumors (<3 cm diameter), RFA or microwave ablation is nearly as effective as repeated hepatic resection, with significantly fewer complications [61]. The complication rate of ablative therapies is 3.9% on average, compared to a 46.9% complication rate in repeated resection [61]. In one review of 13 ICC patients treated with RFA, the progression-free survival (PFS) was 32.2 months. In this cohort, ten tumors measured less than 3 cm; five tumors were 3–5 cm. Two tumors were larger than 5 cm, and treatment failed in these tumors. The median OS was 38.5 months, and the 3- and 5-year survival rates were 51% and 15%, respectively [62]. A meta-analysis of 86 ICC patients treated with RFA found pooled 1-, 3-, and 5-year survival rates of 82, 47, and 24%, respectively. Complications occurred in five patients, with one death related to treatment complications [58].
Microwave ablation is only used in limited cases in ICC, and therefore data on its efficacy is extremely limited [55]. One study including 15 patients with a mean ICC tumor size of 3.2 cm treated with sonography-guided microwave ablation reported a 2-year survival rate of 60% [63]. Another study examined 18 patients who received either RFA or microwave ablation and reported a 3-year survival rate of 30.3%. A control group was not included [54].
The Role of Radiation Therapy
CT-guided high-dose brachytherapy (CT-HDRBT ) has been used since 2002 to treat liver malignancies. It is particularly well suited to tumors that are large or near critical blood vessels which are unsuitable for ablative treatment [64, 65]. The treatment works by inserting a coaxial needle to puncture the lesion. Next, an angiography guidewire is introduced and exchanged with the needle. The guidewire is then removed and replaced with a brachytherapy catheter, which sits inside the tumor. Fluoroscopy CT is used to aid in the positioning of the catheters. The tumor is then irradiated with a high dose of iridium-192 for a maximum of 90 min [66]. Though the technique has been determined to be safe, outcomes data supporting the use of brachytherapy in ICC is scarce. One retrospective study of 15 patients receiving 27 brachytherapy treatments reported a median OS of 14 months after treatment. The median dose administered was 20 Gy, and the mean targeted tumor volume was 131 mL [64, 67].
Stereotactic body radiation therapy (SBRT ) can also be used to treat small ICC tumors <5 cm in diameter. In this treatment, diagnostic imaging is first obtained to plan the procedure, including 4D imaging mapping of target lesion movement during patient respiratory cycles. Then, high doses of hypofractionated conformal external beam radiation are directed to the tumor, usually in less than five fractions. Usual doses of SBRT are 20–40 Gy and are delivered in 30–60 min sessions over the course of a week [68]. Study data of SBRT in ICC is also limited. One study followed 34 patients with intrahepatic and hilar cholangiocarcinoma receiving SBRT. The median SBRT dose was 30 Gy in three fractions. Median OS was 17 months, and PFS was 10 months. Four patients developed grade III toxicities [69]. Another retrospective dose-response study of 79 patients with large ICC tumors (7.9 cm median) treated with SBRT reported a median OS of 30 months and a 3-year OS rate of 44%. Patients in this study received an average dose of 58.05 Gy. Radiation dose was the most important prognostic factor that correlated with improved local control and OS [70].
Medical Therapy Options for Advanced Disease
Chemotherapy is the foundation of medical therapy for patients with advanced ICC and is used in patients regardless of resection eligibility. Systemic chemotherapy primarily includes fluorouracil, gemcitabine, or oxaliplatin. Gemcitabine is generally considered first-line therapy for any advanced biliary tract cancer. A recent phase III trial demonstrated that doublet therapy with gemcitabine and cisplatin resulted in improved ICC tumor response and prolonged PFS without additional toxicity when compared to gemcitabine alone. Overall survival was 11.7 months for the gemcitabine/cisplatin group compared to 8.1 months for gemcitabine alone [71].
Generally, systemic chemotherapy has demonstrated disappointing effectiveness, with a majority of regimens resulting in a median survival of 6–12 months [33]. One meta-analysis of 57 studies concluded that adjuvant chemotherapy combined with resection did not appear to increase OS or recurrence-free survival [12]. Currently, all forms of cholangiocarcinoma are treated with similar chemotherapeutic regimens. Emerging genomic sequencing data suggests that ICC contains a different genetic profile than extrahepatic bile duct and gallbladder tumors. This evidence suggests there may be room for future advances in more targeted medical therapy based on tumor genetic profile [72].
In cases when biliary obstruction is severe and the tumor is unresectable, stents can be placed through endoscopic retrograde cholangiopancreatography (ERCP ) or percutaneous transhepatic cholangiography (PTC ). Stents are typically plastic or metal, with plastic stents requiring replacement every 3 months [73]. Experimental therapies such as photodynamic therapy may also be considered for advanced ICC patients to restore biliary drainage. The therapy consists of intravenous administration of a photosensitizer followed by light illumination to relieve biliary blockade [74].
Conclusion
Intrahepatic cholangiocarcinoma is a relatively rare but serious cancer with poor prognosis. Surgical resection is the best curative option, but most patients are ineligible due to the advanced stage of the disease at the time of diagnosis. In this group of patients, locoregional therapies, including ablation as well as intra-arterial therapies such as cTACE, DEB-TACE, and Y90-RE, constitute the mainstay therapies. Radiation and systemic chemotherapy are used both as adjuvant and last resort therapies for advanced ICC. However, randomized trials are warranted to determine evidence-driven guidelines for the use of these therapies.
References
Buettner S, van Vugt JL, JN IJ, Groot Koerkamp B. Intrahepatic cholangiocarcinoma: current perspectives. Onco Targets Ther. 2017;10:1131–42.
Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10.
Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37(6):806–13.
Gupta A, Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(2):101–4.
Poultsides GA, Zhu AX, Choti MA, Pawlik TM. Intrahepatic cholangiocarcinoma. Surg Clin North Am. 2010;90(4):817–37.
Kim Y, Moris DP, Zhang XF, Bagante F, Spolverato G, Schmidt C, et al. Evaluation of the 8th edition American joint commission on cancer (AJCC) staging system for patients with intrahepatic cholangiocarcinoma: a surveillance, epidemiology, and end results (SEER) analysis. J Surg Oncol. 2017;116(6):643–50.
Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol. 2011;8(4):189–200.
Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366(9493):1303–14.
Valls C, Guma A, Puig I, Sanchez A, Andia E, Serrano T, et al. Intrahepatic peripheral cholangiocarcinoma: CT evaluation. Abdom Imaging. 2000;25(5):490–6.
Tillich M, Mischinger HJ, Preisegger KH, Rabl H, Szolar DH. Multiphasic helical CT in diagnosis and staging of hilar cholangiocarcinoma. AJR Am J Roentgenol. 1998;171(3):651–8.
Anderson CD, Pinson CW, Berlin J, Chari RS. Diagnosis and treatment of cholangiocarcinoma. Oncologist. 2004;9(1):43–57.
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565–74.
Savic LJ, Chapiro J, Geschwind JH. Intra-arterial embolotherapy for intrahepatic cholangiocarcinoma: update and future prospects. Hepatobiliary Surg Nutr. 2017;6(1):7–21.
Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217(4):736–50 e4.
Lee JI, Campbell JS. Role of desmoplasia in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol. 2014;61(2):432–4.
Wang K, Zhang H, Xia Y, Liu J, Shen F. Surgical options for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(2):79–90.
Vijgen S, Terris B, Rubbia-Brandt L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(1):22–34.
Tan JC, Coburn NG, Baxter NN, Kiss A, Law CH. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol. 2008;15(2):600–8.
Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–79.
Weber SM, Jarnagin WR, Klimstra D, DeMatteo RP, Fong Y, Blumgart LH. Intrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193(4):384–91.
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96.
Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153(6):811–8.
Hyder O, Marsh JW, Salem R, Petre EN, Kalva S, Liapi E, et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: a multi-institutional analysis. Ann Surg Oncol. 2013;20(12):3779–86.
Yamada R, Nakatsuka H, Nakamura K, Sato M, Itami M, Kobayashi N, et al. Hepatic artery embolization in 32 patients with unresectable hepatoma. Osaka City Med J. 1980;26(2):81–96.
Georgiades CS, Hong K, Geschwind JF. Radiofrequency ablation and chemoembolization for hepatocellular carcinoma. Cancer J. 2008;14(2):117–22.
Minami Y, Kudo M. Imaging modalities for assessment of treatment response to nonsurgical hepatocellular carcinoma therapy: contrast-enhanced US, CT, and MRI. Liver Cancer. 2015;4(2):106–14.
Cohen MJ, Levy I, Barak O, Bloom AI, Fernandez-Ruiz M, Di Maio M, et al. Trans-arterial chemo-embolization is safe and effective for elderly advanced hepatocellular carcinoma patients: results from an international database. Liver Int. 2014;34(7):1109–17.
Vogl TJ, Naguib NN, Nour-Eldin NE, Bechstein WO, Zeuzem S, Trojan J, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: results and prognostic factors governing treatment success. Int J Cancer. 2012;131(3):733–40.
Pomoni M, Malagari K, Moschouris H, Spyridopoulos TN, Dourakis S, Kornezos J, et al. Post embolization syndrome in doxorubicin eluting chemoembolization with DC bead. Hepato-Gastroenterology. 2012;59(115):820–5.
Burger I, Hong K, Schulick R, Georgiades C, Thuluvath P, Choti M, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: initial experience in a single institution. J Vasc Interv Radiol. 2005;16(3):353–61.
Shen WF, Zhong W, Liu Q, Sui CJ, Huang YQ, Yang JM. Adjuvant transcatheter arterial chemoembolization for intrahepatic cholangiocarcinoma after curative surgery: retrospective control study. World J Surg. 2011;35(9):2083–91.
Kiefer MV, Albert M, McNally M, Robertson M, Sun W, Fraker D, et al. Chemoembolization of intrahepatic cholangiocarcinoma with cisplatinum, doxorubicin, mitomycin C, ethiodol, and polyvinyl alcohol: a 2-center study. Cancer. 2011;117(7):1498–505.
Gusani NJ, Balaa FK, Steel JL, Geller DA, Marsh JW, Zajko AB, et al. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experience. J Gastrointest Surg. 2008;12(1):129–37.
Scheuermann U, Kaths JM, Heise M, Pitton MB, Weinmann A, Hoppe-Lotichius M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma--a single-center experience. Eur J Surg Oncol. 2013;39(6):593–600.
Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12(8):2563–7.
Lewis AL, Dreher MR, O'Byrne V, Grey D, Caine M, Dunn A, et al. DC BeadM1: towards an optimal transcatheter hepatic tumour therapy. J Mater Sci Mater Med. 2016;27(1):13.
Aliberti C, Benea G, Tilli M, Fiorentini G. Chemoembolization (TACE) of unresectable intrahepatic cholangiocarcinoma with slow-release doxorubicin-eluting beads: preliminary results. Cardiovasc Intervent Radiol. 2008;31(5):883–8.
Huppert P, Wenzel T, Wietholtz H. Transcatheter arterial chemoembolization (TACE) of colorectal cancer liver metastases by irinotecan-eluting microspheres in a salvage patient population. Cardiovasc Intervent Radiol. 2014;37(1):154–64.
Poggi G, Amatu A, Montagna B, Quaretti P, Minoia C, Sottani C, et al. OEM-TACE: a new therapeutic approach in unresectable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2009;32(6):1187–92.
Schiffman SC, Metzger T, Dubel G, Andrasina T, Kralj I, Tatum C, et al. Precision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survival. Ann Surg Oncol. 2011;18(2):431–8.
Kuhlmann JB, Euringer W, Spangenberg HC, Breidert M, Blum HE, Harder J, et al. Treatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur J Gastroenterol Hepatol. 2012;24(4):437–43.
Salem R, Thurston KG. Radioembolization with 90Yttrium microspheres: a state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: technical and methodologic considerations. J Vasc Interv Radiol. 2006;17(8):1251–78.
Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17(2):484–91.
Rafi S, Piduru SM, El-Rayes B, Kauh JS, Kooby DA, Sarmiento JM, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol. 2013;36(2):440–8.
Hoffmann RT, Paprottka PM, Schon A, Bamberg F, Haug A, Durr EM, et al. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35(1):105–16.
Mouli S, Memon K, Baker T, Benson AB 3rd, Mulcahy MF, Gupta R, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol. 2013;24(8):1227–34.
Konda A, Savin MA, Cappell MS, Duffy MC. Radiation microsphere-induced GI ulcers after selective internal radiation therapy for hepatic tumors: an underrecognized clinical entity. Gastrointest Endosc. 2009;70(3):561–7.
Rodriguez-Lago I, Carretero C, Herraiz M, Subtil JC, Betes M, Rodriguez-Fraile M, et al. Long-term follow-up study of gastroduodenal lesions after radioembolization of hepatic tumors. World J Gastroenterol. 2013;19(19):2935–40.
Mosconi C, Cappelli A, Ascanio S, Pettinari I, Modestino F, Renzulli M, et al. Yttrium-90 microsphere radioembolization in unresectable intrahepatic cholangiocarcinoma. Future Oncol. 2017;13(15):1301–10.
Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol. 2014;4:198.
Ibrahim SM, Mulcahy MF, Lewandowski RJ, Sato KT, Ryu RK, Masterson EJ, et al. Treatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot study. Cancer. 2008;113(8):2119–28.
Camacho JC, Kokabi N, Xing M, Prajapati HJ, El-Rayes B, Kim HS. Modified response evaluation criteria in solid tumors and European Association for the Study of the liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J Vasc Interv Radiol. 2014;25(2):256–65.
Hickey R, Mulcahy MF, Lewandowski RJ, Gates VL, Vouche M, Habib A, et al. Chemoradiation of hepatic malignancies: prospective, phase 1 study of full-dose capecitabine with escalating doses of yttrium-90 radioembolization. Int J Radiat Oncol Biol Phys. 2014;88(5):1025–31.
Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85(1016):1078–84.
Shindoh J. Ablative therapies for intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2017;6(1):2–6.
Slakey DP. Radiofrequency ablation of recurrent cholangiocarcinoma. Am Surg. 2002;68(4):395–7.
Simo KA, Halpin LE, McBrier NM, Hessey JA, Baker E, Ross S, et al. Multimodality treatment of intrahepatic cholangiocarcinoma: a review. J Surg Oncol. 2016;113(1):62–83.
Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol. 2015;26(7):943–8.
Giorgio A, Calisti G, DE Stefano G, Farella N, DI Sarno A, Amendola F, et al. Radiofrequency ablation for intrahepatic cholangiocarcinoma: retrospective analysis of a single Centre experience. Anticancer Res. 2011;31(12):4575–80.
Carrafiello G, Lagana D, Cotta E, Mangini M, Fontana F, Bandiera F, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010;33(4):835–9.
Zhang SJ, Hu P, Wang N, Shen Q, Sun AX, Kuang M, et al. Thermal ablation versus repeated hepatic resection for recurrent intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2013;20(11):3596–602.
Kim JH, Won HJ, Shin YM, Kim PN, Lee SG, Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. Eur J Radiol. 2011;80(3):e221–5.
Yu MA, Liang P, Yu XL, Cheng ZG, Han ZY, Liu FY, et al. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol. 2011;80(2):548–52.
Schnapauff D, Denecke T, Grieser C, Collettini F, Seehofer D, Sinn M, et al. Computed tomography-guided interstitial HDR brachytherapy (CT-HDRBT) of the liver in patients with irresectable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2012;35(3):581–7.
Ricke J, Wust P, Wieners G, Beck A, Cho CH, Seidensticker M, et al. Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J Vasc Interv Radiol. 2004;15(11):1279–86.
Ricke J, Wust P. Computed tomography-guided brachytherapy for liver cancer. Semin Radiat Oncol. 2011;21(4):287–93.
Koay EJ, Odisio BC, Javle M, Vauthey JN, Crane CH. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments? Hepatobiliary Surg Nutr. 2017;6(2):105–16.
Weiner AA, Olsen J, Ma D, Dyk P, DeWees T, Myerson RJ, et al. Stereotactic body radiotherapy for primary hepatic malignancies - report of a phase I/II institutional study. Radiother Oncol. 2016;121(1):79–85.
Mahadevan A, Dagoglu N, Mancias J, Raven K, Khwaja K, Tseng JF, et al. Stereotactic body radiotherapy (SBRT) for intrahepatic and hilar cholangiocarcinoma. J Cancer. 2015;6(11):1099–104.
Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. J Clin Oncol. 2016;34(3):219–26.
Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25(2):391–8.
Lee H, Ross JS. The potential role of comprehensive genomic profiling to guide targeted therapy for patients with biliary cancer. Therap Adv Gastroenterol. 2017;10(6):507–20.
Doherty B, Nambudiri VE, Palmer WC. Update on the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2017;19(1):2.
Zoepf T, Jakobs R, Arnold JC, Apel D, Riemann JF. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol. 2005;100(11):2426–30.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Murali, N., Savic, L.J., Nezami, N., Chapiro, J., Geschwind, JF. (2018). Regional Liver-Directed Therapies for Intrahepatic Cholangiocarcinoma. In: Cardona, K., Maithel, S. (eds) Primary and Metastatic Liver Tumors. Springer, Cham. https://doi.org/10.1007/978-3-319-91977-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-91977-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-91976-8
Online ISBN: 978-3-319-91977-5
eBook Packages: MedicineMedicine (R0)