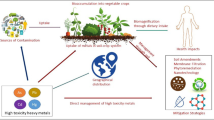
Abstract
The production of quality vegetables is a crucial issue worldwide due to consistently deteriorating soil health. Plants including vegetables absorb a number of metals from soil, some of which have no biological function, but some are toxic at low concentrations, while others are required at low concentration but are toxic at higher concentrations. As vegetables constitute a major source of nutrition and are an important dietary constituent, the heavy metal uptake and bioaccumulation in vegetables is important since it disrupts production and quality of vegetables and consequently affects human health via food chain. Considering the serious threat of metals to vegetables, an attempt in this chapter is made to highlight the effects of certain metals on vegetables grown in different agroclimatic regions of the world. Also, the bioremediation strategies adopted to clean up the metal-contaminated soil is discussed. The results of different studies conducted across the globe on metal toxicity and bioremediation strategies presented in this chapter are likely to help vegetable growers to produce fresh and contaminant-free vegetables.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Vegetables play an important role in humans’ diet by providing and assisting the body with a variety of important constituents such as minerals, vitamins, complex carbohydrate, high dietary fibre, low levels of fat and high amount of water. Due to these, the consumption of vegetables is encouraged in human dietary systems. Consequently, there is an increasing demand of fresh and healthy vegetables which, however, may be contaminated by pathogens, heavy metals and/or toxins (Mello 2003). Mostly, growers engaged in vegetable production in horticulture practices worldwide often use poor-quality irrigation water due to unavailability of good-quality water (Drechsel and Keraita 2014). Apart from poor-quality waters, soil, human handling, organic fertilizers and wastewater are the major factors in contaminating the fresh vegetables. Among these factors, organic fertilizers and wastewater are considered the major source of vegetable contamination (Grant 2011). Depending on the source of contamination, the industrial wastewater contributes significant amounts of metals, metalloids and volatile or semi-volatile compounds, while domestic wastewater is most harmful due to its pathogenic load (Fiona et al. 2003). International Water Management Institute (IWMI 2006) has reported that at least 3.5 million ha is irrigated globally with untreated, partly treated, diluted or treated wastewater. Such poor-quality water containing toxic materials after uptake by plants may cause severe toxicity to vegetables. The consumption of such contaminated vegetables in turn affects the human health. And, hence, due to increasing concern of food safety, proper practices and methods of production have to be developed, and the hazards and the risks associated with toxic elements like heavy metals have to be fully understood before they pose any serious and consequential threat to consumer health. The heavy metals cannot be degraded by any biological, physical or chemical processes (Naz et al. 2015) and, hence, persist in the environment. However, numerous traditional physicochemical processes are available for remediations of polluted sites which are expensive and quite often inefficient as they do not permanently eradicate the pollutants. Also, the byproducts generated in the process become hazardous to human health (Singh et al. 2011). On the other hand, biological methods are more acceptable as they do not pose such problems, are easy to operate and do not produce secondary pollution. The biological approach generally called as bioremediation is considered a safe and inexpensive technique since they are based on the use of living organisms, microorganisms and plants (Karigar and Rao 2011) For instance, microorganisms adopt several mechanisms such as biotransformation (Xiong et al. 2010) and have varied ability of interacting with heavy metals. Another heavy metal removal strategy involves the use of plants, called as phytoremediation, wherein plants partially or completely remediate selected contaminants from soil, sludge, sediments, wastewater and groundwater. It is a cost-effective, efficient and eco-friendly in situ remediation technology driven by solar energy. There are however, certain drawbacks associated with this technology such as the pollutants or their metabolites accumulate within plant tissues, which in turn shorten plant life and releases contaminants into the atmosphere via volatilization.
8.2 Heavy Metals: A Brief Account
A heavy metal is defined as a member of a loosely defined subset of elements that exhibits metallic properties and mainly includes the transition metals, some metalloids, lanthanides and actinides. However, based on density, atomic number or atomic weight and chemical properties or toxicity, heavy metals have been defined variously (John 2002). For instance, any metallic chemical element that has a relatively higher density and is toxic or poisonous even at low concentration is defined as heavy metal (Alloway 1990). On the other hand, the elements such as cadmium, copper, nickel, mercury and lead which are commonly associated with pollution and exhibiting significant toxicity are considered heavy metals by Fiona et al. (2003). However, based on their importance as a nutrient, metals have been classified as (1) essential (e.g. Zn, Cu, Fe, Mn and Se), (2) probably essential (e.g. V and Co) and (3) potentially toxic (As, Cd, Pb, Hg and Ni) (Ebdon 2001). Besides this, all metals, in general, have toxic effects when there is excessive exposure (Woimant and Trocello 2014).
Heavy metals are a serious concern throughout the globe due to their toxic, mutagenic and teratogenic effects even at very low concentrations (Oluwole et al. 2013). While growing in metal-polluted soils, the plant can absorb metals through roots, or they can be deposited on foliar surfaces (Jassir et al. 2005). Heavy metal enters the human body mainly through inhalation of dust, direct ingestion of soil, consumption of food plants grown in metal-contaminated soil and drinking contaminated water. Due to non-destructive nature, heavy metals consequently accumulate in human vital organs and cause varying degrees of illnesses (Lenntech 2006). Elimination of heavy metals deposited on the surface, however, can often be accomplished simply by washing prior to consumption, whereas bio-accumulated metals are difficult to remove and are, therefore, of major concern (Michio 2005).
8.3 Sources of Vegetable Contamination by Heavy Metals
Because of soil contamination, heavy metal stress is becoming a major challenge to crop plants, particularly to vegetable crops. The heavy metals are derived from city/industrial effluent (Cai et al. 2012; Wang et al. 2013), mining and smelting (Zhao et al. 2012), fertilizers and pesticides (Nacke et al. 2013; Yu et al. 2013), electronic waste recycling/dismantling activities (Liu et al. 2013) and auto mobile depositions (Turer and Maynard 2003). Additionally, wastewater/sewage water can be another major source of heavy metals in areas where raw sewage water is used for irrigation (Li et al. 2013; Wang et al. 2013). Vegetable growing areas which are often situated in or near sources of atmospheric deposits have an elevated risk of potential contamination. Ingestion of vegetables that have been produced with contaminated water poses a serious risk to human health including various chronic diseases, particularly after prolonged dietary intakes (Sharma et al. 2009). Different vegetable species, however, tend to accumulate different metals based on environmental conditions, metal species and plant available forms of heavy metals (Lokeshwari and Chandrappa 2006). Uptake through roots depends on many factors such as soil pH, plant growth stages, the soluble content of heavy metals in soil, as well as type of crops, fertilizers and soil (Sharma et al. 2006). Most common heavy metals often found in vegetables include Cd, Cu, As, Cr, Pb, Zn, Co and Ni. When present in trace quantities, some of them act as micronutrients. Comparing the accumulated concentrations of metals with permissible limits of the Indian Standard (Awashthi 1999) and safe limits given by WHO/FAO (WHO/FAO 2007), several studies have found that metal concentrations were higher in vegetables grown in metal-polluted soil as compared to the safe limits given by commission regulation (EU 2006) (Table 8.1).
Other than safety concerns, excessive heavy metals significantly deteriorate the fertility of soil and consequently affect the growth and quality of crops (Muchuweti et al. 2006). Several studies have indicated that vegetables, particularly leafy vegetables grown in heavy metal-contaminated soils, have higher concentrations of heavy metals as compared to those grown in non-polluted soil. The symptoms of phytotoxicity of heavy metals, however, vary from metal to metal (Table 8.2). Routine monitoring of heavy metal concentrations in soils and also in crops is, therefore, essential to know the pollution levels and to devise strategies to minimize contamination, in order to reduce the risks to human health.
8.4 Bioaccumulation of Heavy Metals: A Serious Concern
Contamination and subsequent accumulation of heavy metals in leafy (Table 8.3) and non-leafy (Table 8.4) vegetables from different sources have been widely reported. However, the concentration of heavy metals in vegetables varies from below the detection limit to above the safe limit depending upon the source of contamination. Among heavy metals, Cd, a relatively rare element (WHO 1992), is used in electroplating and galvanization processes, in batteries, in the production of pigments, as chemical reagent and in miscellaneous industrial processes such as smelting (ATSDR 1989). Cadmium compounds have varying degrees of solubility ranging from highly soluble to nearly insoluble which affects their absorption and toxicity (ATSDR 1989). Cadmium among metals is the most toxic heavy metal because it bioaccumulates, has a long half-life (about 30 years) and may cause health disorders even at low doses (Lenntech 2006). The increase in Cd uptake by plant tissues occurs due to the use of contaminated water for irrigation, fertilizers, sewage and composts. The absorption of Cd by plants, however, depends on genotypes and physical and chemical properties of plants (Jing and Logan 1992). Several workers have reported that the concentration of Cd was high and not suitable for human consumption in vegetables such as lettuce, spinach, radish, etc. (Prabu 2009), brinjal (Jamali et al. 2007), carrot and potato (Ding et al. 2014) and cucumber, tomato, green pepper, parsley, onion, bean, eggplant, pepper mint, pumpkin and okra (Demirezen and Aksoy 2006). In a study, Jassir et al. (2005) reported that the levels of Cd in the garden rocket vegetable species were high in both washed and unwashed samples which could possibly be due to the relatively easy uptake of Cd by food crops, especially by leafy vegetables. Also it may be due to the foliar absorption of atmospheric deposits on plant leaves (Midio and Satake 2003). In a similar investigation, significant variation in Cd concentration within different tomato genotypes was found (Hussain et al. 2015). The heavy metals that accumulated within tissues of various metal-tolerant tomato genotypes followed the order, shoot > fruit > leaf > root, while in susceptible genotypes, the order was fruit, shoot, leaf and root. The genotypic variation of a crop species makes it possible to select either Cd-accumulating cultivars to remediate contaminated soils or Cd-excluding cultivars to avoid Cd excessive uptake.
Chromium is yet another important metal which may enter through air, drinking water or eating food containing Cr or even through skin contact (Dinis and Fiúza 2011). However, for human and animals, it is considered as an essential metal for carbohydrate and lipid metabolism, and the recommended dietary intake of Cr is 50–200 μg/day for adults. However, exceeding normal concentrations (50–200 ug/day) lead to accumulation and toxicity that can result in hepatitis, gastritis, ulcers and lung cancer (Garcia et al. 2001). Several studies have demonstrated that some vegetables like cabbage and lettuce accumulate higher levels of Cr and could contribute to dietary problems (Biego et al. 1998; Castro 1998). Chromium has a low mobility and moves very slowly from roots to above-ground parts of plants (Skeffington et al. 1996). And, hence, the concentration of Cr is low in the upper organs of plants. Several studies have shown that the Cr concentration was higher than the maximum permitted metal concentration in lettuce, cabbage (Itanna 2002), spinach (Banerjee et al. 2011), luffa, brinjal, ladyfinger, cucumber and gourd (Kumar et al. 2016). In contrast, the Cr contents in different vegetables grown in the lands irrigated with wastewater were found within the safe limits (Sharma et al. 2006).
Mankind is exposed to the highest levels of lead (Pb) that occurs naturally as a sulphide compound and is a soft bluish-white, silvery grey metal that melts at 327.5° C (Budavari 2001). There are different sources of environmental pollution with Pb as Pb alkyl additives in petrol and manufacturing processes. This can bring Pb into the human food chain through uptake of food (about 65%), water (up to 20%) and from air and dust (about 15%) (IPCS 1992). Like other heavy metals, Pb can bioaccumulate over time and remain in the body for long periods. It therefore becomes important to detect such metals even at very low concentrations. In Sudan, for example, Dafelseed (2007) determined the level of Pb in selected fresh vegetables and reported 0.35, 0.86, 0.60 and 0.48 mg/kg in carrot, sweet pepper, garden rock and tomato, respectively. On the contrary, the FAO/WHO (2001) has reported that the maximum permissible level of Pb in vegetables is 0.3 mg/kg.
Human carcinogen, arsenic, is a well-known toxic element, widely distributed in the environment in both inorganic and organic forms (Hughes et al. 2011). It is well-recognized that inorganic arsenic is probably the most dangerous form of arsenic in food, being As (III) more toxic than As (V) (Pizarro et al. 2016). There are many routes by which As can enter the human body (1) via food chain (ingestion by water and food sources) and (2) occupational exposure (Rahman et al. 2009). There have been several reports of arsenic speciation in vegetables growing in natural or contaminated soils (Pell et al. 2013). Broccoli, lettuce, potato, carrots, etc., for example, can accumulate arsenic when such crops are grown in soil irrigated with As (V) containing water. In most of the vegetables, arsenic is taken up by plant roots via macronutrient transporters (Zhao et al. 2010; Wu et al. 2011). In a study, it was observed that the calculated accessible doses of As expressed as microgram arsenic per year are about 470 for carrots, 550 for beets and 180 for quinoa considering the maximum intake of 2.5 kg per year of quinoa and of 6 kg per year of carrots and beets. Therefore, quinoa seems to be the vegetable with the lower toxicological risk (Pizarro et al. 2016). When taken up by plants, significant changes have been observed in the growth, yield and accumulation characteristics of okra spiked with 20 and 50 mg/kg of As(III), As(V) and dimethylarsinic acid (DMA) (Chandra et al. 2016). The arsenic concentration in the aerial part followed the order As(V) > As(III) > DMA while it was As(III) > As(V) > DMA in the roots. Thus, the plant has the capacity to tolerate As stress and can be considered as a resistive variety. Similarly, arsenic accumulation has also been reported in different vegetables beyond the permissible limit. For instance, Santra et al. 2013 found that tuberous vegetables accumulated higher amount of arsenic than leafy vegetables which was followed by fruity vegetable. This is supported by the fact that the accumulation of As in plants occurs primarily through the root system and, hence, the highest As concentrations have been reported in plant roots and tubers (Marin et al. 1993). In this study, the highest arsenic accumulation was observed in potato, brinjal, arum, amaranth, radish, lady’s finger and cauliflower, whereas lower level of arsenic accumulation was observed in beans, green chilli, tomato, bitter guard, lemon and turmeric. Rehman et al. (2016) in contrast reported that the As concentration in edible portions of vegetable ranged from 0.03 to 1.38 mg/kg. Similarly, the trend of As bioaccumulation in vegetables irrigated with arsenic contaminated water was spinach > tomato > radish > carrot, and this distribution of As in vegetable tissues was species dependent; As was mainly found in the roots of tomato and spinach, but accumulated in the leaves and skin of root crops as reported by Bhatti et al. (2013).
8.4.1 Vegetable Toxicity by Multiple Metals
The interactions between different metals in soil may lead to increased uptake of one or the other metal by plants which in effect may cause toxicity to animals and humans via food chain. In a study carried out in Slovakia, Musilova et al. (2016) reported the accumulation of Cd, Pb and Zn in potato tubers in a concentration-dependent manner. The correlation between heavy metal content in soil and its content in potato tubers followed the order cv. Laura-Spissky Stvrtok (Cd), cv. Red Anna-Odorin (Pb) and Marabel and Red Anna-Odorin, cv. Marabel-Belusa and cv. Volumia-Imel (Zn). Also, heavy metals have been found several folds higher than the safe limit in other vegetables like Colocasia, Amaranthus, cauliflower, etc. (Saha et al. 2015). Of the various metals detected, the concentrations of Pb, Cd and Ni were above the permissible limit in all vegetables, while Colocasia and Amaranthus accumulated highest metal contents. The highest mean transfer coefficients (TCs) values recorded for Zn, Cu, Pb, Cd and Ni were 0.59 (cauliflower), 0.67 (Colocasia), 0.93 (Amaranthus), 1.02 (Colocasia) and 1.09 (Amaranthus), respectively. The results further revealed that the maximum single element pollution index (SEPI) value was found for Cd which ranged from 2.93 to 6.03 with a mean of 5.32. In yet another investigation, Tiwari et al.(2011) assessed the edible parts of five vegetables such as spinach, radish, tomato, chilli and cabbage growing in field receiving mixed industrial effluent and reported a high level of toxic metals (As, Cd, Cr, Pb and Ni). It was concluded from this study that the cultivation of such vegetables is not safe under heavy metal-stressed soil. Similarly, parsley, followed by spinach, contained the highest concentration of heavy metals besides onion that contained high levels of toxic heavy metals. The content of Cu in parsley and spinach and Pb in onion exceeded the Codex limits (Osaili et al. 2016). In the western region of Saudi Arabia, the human health problems were found associated with the consumption of metal-contaminated okra (Balkhair and Ashraf 2016). The levels of Ni, Pb, Cd and Cr in the edible parts were 90, 28, 83 and 63%, respectively, above the safe limit. The uptake and accumulation of heavy metals by the edible portions followed the order Cr > Zn > Ni > Cd > Mn > Pb > Cu > Fe. Moreover, the health risk index (HRI) was >1 which indicated a significant health risk, and hence, okra among vegetables was not safe for human consumption.
Antonious et al. (2012) reported that regardless of soil amendments, the overall bioaccumulation factor (BAF) of seven heavy metals in cabbage leaves and broccoli heads were poor. For leafy vegetables collected in 15 ha of squatted land belonging to the international airport of Cotonou, total concentrations of metal (loids) measured in consumed parts of Lactuca sativa L. and Brassica oleracea were 52.6–78.9, 0.02–0.3, 0.08–0.22, 12.7–20.3, 1.8–7.9 and 44.1–107.8 mg/kg for Pb, Cd, As, Sb, Cu and Zn, respectively (Uzu et al. 2014). Ferri et al. (2015) reported that 60 and 10% of spinach samples exceeded maximum Pb and Cd European standards and recommended that washing before consuming vegetables can reduce toxicity risk to humans. Moreover, crude or untreated wastewater, the treated wastewater and groundwater used for irrigating vegetables also contribute significantly to bioaccumulation of heavy metals. As an example, Ghosh et al. (2012) in a study found that radish, turnip and spinach, irrigated with sewage water, were grouped as hyperaccumulator of heavy metals, whereas brinjal and cauliflower accumulated less heavy metal though the metal concentrations did not exceed the permissible limit and, hence, were considered safe for consumption.
Industrial activities also add heavy metals to the environment that pose risk to both human and plant health. Also, the atmospheric deposition through the particulate matter released from transport creates heavy metal pollution among vegetables grown along roadside or during marketing. The health risk assessment methods of United States Environmental Protection Agency (USEPA) are used to establish the potential health hazards of heavy metals in soils growing vegetables in different regions of the world. In a study conducted in three economically developed areas of Zhejiang Province, China suffering from increasing heavy metal damages from various pollution sources including agriculture, traffic, mining and Chinese typical local private family-sized industry, 268 vegetable samples which included celery, cabbages, carrots, asparagus lettuces, cowpeas, tomatoes and cayenne pepper and their corresponding soils were collected. Metal concentrations were measured in soil, settled atmospheric particulate matter (PM) and vegetables at two different sites near a waste incinerator and a highway. A risk assessment was performed using both total- and bio-accessible metal concentrations in vegetables. At both sites, total Cr, Cd and Pb concentrations in vegetables were found above or just under the maximum limit levels for foodstuffs according to Chinese and European Commission regulations. High metal bio-accessibility in the vegetables (60–79%, with maximum value for Cd) was observed (Xiong et al. 2016). In another study, leaves of mature cabbage and spinach were exposed to manufactured mono-metallic oxide particles (CdO, Sb2O3 and ZnO) or to complex process. Particulate matter was mainly enriched with lead, and it was found that high quantities of Cd, Sb, Zn and Pb were taken up by the plant leaves. The levels of metals depend on both the plant species and nature of the PM. A maximum of 2% of the leaf surfaces were covered up to 12% of stomatal openings. Metal (loid) bio-accessibility was significantly higher for vegetables due to chemical speciation changes (Xiong et al. 2014).
8.4.2 Effect of Metals on Physiological Processes of Vegetables Grown in Metal-Stressed Soil
Heavy metals have strong influence on nutritional quality of vegetables when grown in metal-contaminated soil. Therefore, the consumption of such vegetables may lead to nutritional deficiency in developing countries which are already facing malnutrition problems. In a study, effects of four different levels of Cd, Pb and mixture of Cd and Pb on different nutrients of three vegetables, potato, tomato and lettuce, grown in pots containing soil contaminated with Cd, Pb and Cd-Pb mixture were evaluated. The edible portions of each plant were analysed for Cd, Pb and different macro- and micronutrients including protein, vitamin C, N, P, K, Fe, Mn, Ca and Mg. Results revealed a significant variation in elemental concentrations in all the three vegetables. The projected daily dietary intake values of selected metals were significant for Fe, Mn, Ca and Mg but it was not significant for protein, vitamin C, N and P. The elemental contribution to recommended dietary allowance (RDA) was significant for Mn. Similarly, RDA for Fe and Mg was higher while Ca, N, P, K, protein and vitamin C showed the minimal contribution for different age groups. This study suggests that there can be substantial negative effects on nutritional composition when such vegetables cultivated in soil poisoned with Cd and Pb are consumed (Khan et al. 2015a). However, dosage of Cd higher than critical level (≥25 mg/kg soil in treatments) drastically alters plant growth (stunted), reduced yield as well as dietary contents (sugar and vitamin C) of these important vegetables especially its antioxidant content, and the hazardous effect was more visible at higher bioaccumulation of heavy metals during vegetative growth stage (Mani et al. 2012). Heavy metals also adversely affect the mineral uptake and metabolic processes in plants when present in excess. In a recent study, the accumulation of Cr in various plant tissues and its relation to the antioxidant activity and root exudation was evaluated (Uddin et al. 2015). The results revealed that 1 mM of Cr enhanced the weight of shoots and roots of Solanum nigrum, whereas weight of shoots and roots of Parthenium hysterophorus decreased when compared with lower levels of Cr (0.5 mM) or control plants. In both plants, the concentrations of Cr and Cl were increased while Ca, Mg and K contents in root, shoot and root exudates were declined with increasing levels of Cr. The higher levels of Cr augmented SOD and POD activities and proline content in foliage of S. nigrum, while Cr at lower levels had stimulatory effects in P. hysterophorus. Citric acid concentration in root exudates increased with increasing rates of Cr by 35% and 44% in S. nigrum, while it was 20 and 76% in P. hysterophorus. Generally, P. hysterophorus exuded maximum amounts of organic acids. Moreover, the increasing amounts of Cd showed a differential impact on the content and translocation of micro- and macronutrients in tomato (Bertoli et al. 2012). Among different organs, the aerial part had 2.25 g/kg, 2.80 g/kg, 18.93 mg/kg and 14.15 mg/kg of K, Ca, Mn and Zn, respectively, compared to the control.
Apart from these effects, heavy metals in some studies have also been found to adversely affect the water and iron content in some vegetables. For example, 100 and 400 μM Cr had an obvious effect on iron metabolism and water relations of spinach (Gopal et al. 2009). Visual symptoms and increased accumulation of Cr were observed in roots than in leaves, when spinach was exposed to Cr. Moreover, the concentration of chlorophylls and the activities of heme enzymes, catalase and peroxidase decreased following exposure to excess Cr suggesting the intervention of Cr in iron metabolism of plants. These changes coupled with reduction in Fe concentration in Cr-exposed plants further indicate that by declining Fe absorption, Cr disrupts the chlorophyll-forming process and heme biosynthesis. Additionally, the transpiration rate along with proline accumulation was found to decrease in the leaves of Cr-treated plants which indicated water stress. In contrast, heavy metal has also been found to improve growth of celery more than lettuce and spinach, when irrigated with sludge containing heavy metals (Haghighi 2011). The stimulatory effect of sludge on growth rate of all three vegetables occurred via photosynthesis. It was, therefore, concluded from this study that the increasing element uptake induces photosynthesis and concurrently enhances the growth of leafy vegetable. In yet similar experiment, the impact of mixing native soil with municipal sewage sludge (MSS) or yard waste (YW) mixed with MSS (YW + MSS) was assayed to determine (1) yield and quality of sweet potato; (2) concentration of Cd, Cr, Mo, Cu, Zn, Pb and Ni in different organs of sweet potato (edible roots, leaves, stem and feeder roots); and (3) concentrations of ascorbic acid, total phenols, free sugars and β-carotene in edible roots at harvest (Antonious et al. 2011). The results revealed that even though the total concentrations of Pb, Ni and Cr were greater in plants grown with MSS and YW, applied together, compared to control plants, the mixture of MSS and YW increased yield, ascorbic acid, soluble sugars and phenols in edible roots of sweet potato by 53, 28, 27 and 48%, respectively, compared to plants grown in native soil. β-Carotene was greater (157.5 μg/g fresh weight) in the roots of plants grown in MSS compared to roots of plants grown in MSS + YW treatments (99.9 μg/g fresh weight). In a follow-up study, the concentrations of capsaicin, dihydro-capsaicin, β-carotene, ascorbic acid, phenols and soluble sugars in the fruits of Capsicum annuum L. (cv. Xcatic) grown under four soil management practices including YW, SS, chicken manure (CM) and no-much (NM) bare soil were determined. The total marketable pepper yield was increased by 34% and 15%, when it was grown in SS- and CM-treated soil, respectively, compared to NM bare soil. However, the number of culls (fruits that fail to meet the requirements of foregoing grades) was lower in YW-treated soils compared to SS- and CM-treated soils (Antonious 2012).
Elevated levels of heavy metals also affects the plants at the cellular and at the whole-plant level (Burzynski and Klobus 2004; Shaw et al. 2004). For instance, Cd and Cu have been reported to modify plasma membrane H+-ATPase activity (Janicka-Russak et al. 2012). Also, an increased level of heat-shock proteins (hsp) in the tissues was observed as an adaptive process to survive under adverse conditions, and increased PM H+-ATPase activity could further enhance the repair processes in heavy metal-stressed plants. In other investigations, metal ions have been found to inhibit root elongation, photosynthesis and enzyme activity and cause oxidative damage to membranes (Hernandez and Cooke 1997; Shaw et al. 2004; Sheoran et al. 1990). In a similar study, the inhibitory impact of metals on physiological, biochemical and morphological characteristics of spinach grown at 20 and 40% sewage sludge-amended soil is reported (Singh and Agrawal 2007). At 40% sewage sludge application, a substantial decrease in length of root and shoot and leaf area of spinach was observed. Among the biochemical parameters, photosynthetic rate was reduced by 23.6 and 28.8% in palak at 20 and 40% sewage sludge amendment, respectively. As compared to untreated soil, foliar thiol content decreased at 20 and 40% sewage sludge amendment. There was an increase in lipid peroxidation at different concentrations of sewage sludge used, and this is attributed to the formation of reactive oxygen species (ROS) and free radicals induced by Cd, Ni and Pb leading to disorganization of membrane structures of cells. In addition to these, chlorophyll content, fluorescence ratio (Fv/Fm) and protein content were also decreased, but peroxidase activity increased with increasing sewage sludge amendment ratio. These destructive effects in turn make plants more susceptible to additional stresses such as drought which reduces water uptake capacity and water use efficiency of the smaller root system and possibly blocks aquaporins (Yang et al. 2004; Ionenko et al. 2006 and Ryser and Emerson 2007). Heavy metal toxicity and drought stresses are likely to occur simultaneously, as metal-contaminated soils tend to have poor water-holding capacity (Derome and Nieminen 1998), and evaporation rates are high due to sparse vegetation cover (Johnson et al. 1994).
8.5 Bioremediation Strategies Adopted for Heavy Metal Removal
8.5.1 Phytoremediation
Remediation of metal-contaminated soils is indeed a major challenge before the scientists working in different countries. The conventional technologies employed to remove heavy metals from soils often involve stringent physicochemical agents (Neilson et al. 2003), which can destruct soil fertility and also negatively affect the agroecosystem. Despite these, numerous methods including chlorination, use of chelating agents and acid treatments have been proposed to remove metals from contaminated sites. However, such methods are considered ineffective due to operational difficulties, high cost and low metal leaching efficiency. Due to these problems, there is an urgent need to find some viable alternative. In this regard, bioremediation which is the process of cleaning up of hazardous wastes involving the use of microorganisms or plants is considered the safest method of clearing polluted soil (Dixit et al. 2015). Among various bioremediation strategies, phytoremediation, also called as botanoremediation, green remediation and agro remediation, has been found inexpensive and more practicable method for minimizing/clearing metals from soil and water (Lasat 2000). Also, during phytoremediation practices, no hazardous product is generated. Broadly, this remediation system is plant based which is a solar-driven biological system (Santiago and Bolan 2010). Plants involved in phytoremediation have been categorized as:
-
1.
Excluders: plants that survive through restriction mechanisms and are sensitive to metals over a wide range of soil. Members of the grass family, for example, sudan grass, bromegrass, fescue, etc., belong to this group.
-
2.
Indicators: plants that show poor control over metal uptake and transport processes and correspondingly respond to metal concentrations in soils. This group includes the grain and cereal crops like corn, soybean, wheat, oats, etc.
-
3.
Accumulators: plants which do not prevent metals from entering the roots, but they have evolved specific mechanisms for detoxifying high metal levels that accumulated in the cells (Baker 1981). Tobacco, mustard and Compositae families (e.g. lettuce, spinach, etc.) fall within this category.
Apart from these three categories, extreme accumulators, often called hyperaccumulators, form a fourth category which includes plants with exceptional metal-accumulating capacity. This property of accumulating excessive metal concentration, allows plants to survive and even thrive in heavily contaminated soils (or near ore deposits). To date, about 400 plant species (Table 8.5) have been identified as metal hyperaccumulators, representing <0.2% of all angiosperms (Brooks 1998).
Phytoremediation involves many steps and techniques to clean up the contaminants from the polluted sites (Santiago and Bolan 2010). Some of the most commonly practised phytoremediation strategies are:
-
1.
Phytoextraction: contaminants are taken up by roots and translocated within the plants and are removed by harvesting the plants. In this process, toxic metals from contaminated soils, sediment and sludge are absorbed, concentrated and precipitated into the above-ground biomass such as shoots, leaves, etc. (Singh et al. 2012).
-
2.
Phytodegradation: involves the breakdown of organics, taken up by the plant to simpler molecules that are incorporated into the plant tissues (Dermentzis 2009).
-
3.
Rhizofiltration: is primarily used to remediate extracted groundwater, surface water and wastewater with low contaminant concentrations. Rhizofiltration can be used for Pb, Cd, Cu, Ni, Zn and Cr, which are primarily retained within the roots.
-
4.
Phytostabilization: primarily used for the remediation of soil, sediment and sludges. It involves the use of plant roots to limit contaminant mobility and bioavailability in the soil. Phytostabilization can occur through the sorption, precipitation, complexation or metal valence reduction. It is useful for the removal of Pb and As, Cd, Cr, Cu and Zn (Flora et al. 2008).
-
5.
Phytovolatilization: involves the use of plants to take up contaminants from the soil, transforming them into volatile forms and releasing them into the atmosphere. Phytovolatilization occurs as growing trees and other plants take up water and the organic and inorganic contaminants. Some of these contaminants can pass through the plants to the leaves and volatilize into the atmosphere at comparatively low concentrations. Phytovolatilization has been primarily used for the removal of mercury (Durnibe et al. 2007).
-
6.
Phytostimulation: using plants to stimulate bacteria and fungi to mineralize pollutant using exudates and root sloughing. Some plants can release as much as 10–20% of their photosynthates in the form of root sloughing and exudates (Pilon-Smits 2005).
Considering the importance of phytoremediation technology in metal clean up from the contaminated soils, several vegetables have also been explored for their phytoremediation ability in order to detoxify or reduce the heavy metal contamination in vegetable growing fields. For example, the growth response, metal tolerance and phytoaccumulation properties of water spinach and okra were assessed under different contaminated spiked metals by Ng et al. (2016) using control, 50 mg Pb/kg soil, 50 mg Zn/kg soil and 50 mg Cu/kg soil. Of the two vegetables, okra accumulated highest concentrations of Pb (80.20 mg/kg) in its root followed by Zn in roots (35.70 mg/kg) and shoots (34.80 mg/kg) of water spinach, respectively. Moreover, the accumulation of Pb, Zn and Cu in both water spinach and okra differed considerably. Though the accumulation of Pb in the shoots of water spinach and okra exceeded the maximum permissible limits of the National Malaysian Food Act 1983 and Food Regulations 1985 and the International Codex Alimentarius Commission, both crops were found as good Pb and Zn phytoremediators. Generally, leafy vegetables have a higher tendency for uptake and accumulation of heavy metals; these can be used as indicator and also for removal of toxic heavy metals from polluted agricultural field. In yet other study, Ipomoea aquatica Forsk., an aquatic macrophyte, was assessed for its ability to accumulate Pb by exposing it to graded concentrations of this metal. Accumulation of Pb was the highest in root followed by stem and leaf. Furthermore, Pb at 20 mg/l induced colour changes in the basal portion of stem which had significantly higher Pb concentration than in the unaffected apical part. This resulted in sequestration of excess metal in affected stem tissue, which could take up Pb by the process of caulofiltration or shoot filtration and served as a secondary reservoir of Pb in addition to the root. The ability of the plant to store Pb in its root and lower part of stem coupled with its ability to propagate by fragmentation through production of adventitious roots and lateral branches from nodes raises the possibility of utilizing I. aquatica for Pb phytoremediation (Chanu and Gupta 2016). Even among different varieties of vegetables, difference in the bioaccumulation property that can be exploited for remediation of polluted soils is reported. The high accumulator genotypes may be useful for phytoremediation, while the low accumulator varieties might be appropriate selections for growing on metal-contaminated soils to prevent potential human exposure to heavy metals and health hazards through the food chain. To categorize the pepper accessions of Capsicum chinense Jacq, collected from eight different countries, grown in a silty-loam soil under field conditions as low or high heavy metal accumulators, Antonious et al. (2010) collected mature fruits and analyse Cd, Cr, Ni, Pb, Zn, Cu and Mo. Fruits accumulated significant concentrations of Cd (0.47 μg/g dry fruit), Pb (2.12 μg/g dry fruit) and Ni (17.2 μg/g).
8.5.2 Microbe-Assisted Remediation
Numerous microbial communities belonging to different genera have evolved certain mechanisms to tolerate and detoxify metals from contaminated environment (Mosa et al. 2016). Interestingly, the high surface to volume ratio of microorganisms and their ability to circumvent metal toxicity makes such organisms a viable and inexpensive alternative to chemical methods of metal remediation (Kapoor et al. 1999; Magyarosy et al. 2002). Biological mechanisms involved in microbial survival under metal-stressed environment include complexation, biosorption to cell wall and pigments, extracellular precipitation and crystallization, transformation of metals, decreased transport or impermeability, efflux, intracellular compartmentation and sequestration (Kang et al. 2016). One or many of these strategies may be adopted by microbiota to overcome metal problems. For example, synthesis of metallothioneins or γ-glutamyl peptides is a mechanism of Cu resistance in Saccharomyces cerevisiae, but Cu binding or precipitation around the cell wall and intracellular transport are also components of the total cellular response (Gadd and White 1989).
Considering the importance of microbes in metal detoxification/removal, identification of metal-tolerant microorganisms has become important to remediate the metal-polluted soils so that larger area can be used for vegetable cultivation. In this regard, the effect of two strains of Trichoderma (T. harzianum strain T22 and T. atroviride strain P1) on the growth of lettuce plants irrigated with As-contaminated water was assessed (Caporale et al. 2014). The results revealed the accumulation of this element mainly into the root system which subsequently reduced both biomass development and net photosynthesis rate (while altering the plant P status). However, both species of plant growth-promoting fungi (PGPF) Trichoderma alleviated, at least in part, the phytotoxicity of and eventually decreased As accumulation in tissues and concurrently enhanced plant growth, P status and net photosynthesis rate (Caporale et al. 2014). In a similar experiment, heavy metal-resistant strain J62 of Burkholderia sp. has been reported to promote the growth of tomato and maize (Jiang et al. 2008). In a follow-up study, the biological properties such as dry weights of fruit, roots, stem, leaf and whole tomato plants were increased by single or combined remediation of ryegrass and arbuscular mycorrhiza, while MDA contents and antioxidant enzyme activities of foliage and roots were declined in two varieties of tomato when exposed to Cd (20 mg/kg). Cadmium accumulation in tomato followed the order leaf > stem > fruit > root. However, the Cd concentrations in leaf, stem, root and fruit of both varieties were decreased by single or combined application of ryegrass and AM-fungi (Jiang et al. 2014).
Adequate nutrients are required for proper growth and development of a plant (Anil et al. 2003). Also, it is essentially required for maintaining normal metabolic reactions of plants. In contrast, the metal-contaminated soil is generally deficient in plant nutrients, and the plants that remain under constant stress fail to take up sufficient amounts of nutrients from soil. To overcome these problems, several metal-tolerant microbes possessing one or many plant growth-promoting activities such as ability to solubilize insoluble phosphate (Kim et al. 2013), phytohormone production (Franco-Hernández et al. 2010) or by some indirect mechanisms such as biocontrol activity (Khan and Bano 2016) involving siderophore production (Rajkumar et al. 2010) have been used. Besides these, microbes also aide in the phytoremediation process (Ullah et al. 2015), and as a result, the plants grow better in metal-stressed soils. As an example, two Cd- and Ni-resistant plant growth-promoting bacteria, Pseudomonas sp. ASSP 5 and ASSP 29, were isolated from fly ash-contaminated sites, and their plant growth promotion ability was tested by inoculating Lycopersicon esculentum plants grown in fly ash-amended soil (Kumar and Patra 2013). In most cases, strain ASSP 29 of Pseudomonas sp. produced more pronounced effect on biological (plant height and wet and dry weights) and chemical (protein and chlorophyll content in leaves) characteristics of plants and accumulation of metals in root and shoot of plants. Both the strains ASSP 5 and ASSP 29 showed a remarkable ability to protect the plants against the inhibitory effect of Ni and Cd besides promoting the growth of plants through production of IAA and siderophore and solubilization of P. Similarly, Dourado et al. (2014) evaluated Cd–Burkholderia–tomato interaction studies by inoculating a Cd-tolerant Burkholderia strain SCMS54 that exhibited a higher metabolic diversity and plasticity. Inoculated tomato plants in the presence of Cd grew well compared to non-inoculated plants indicating that the strain SCMS54 abated the toxicity of Cd and consequently enhanced tomato production grown under Cd stress. Based on this study, it was suggested that the bacterial strain isolated from Cd-contaminated soil could be used for tomato cultivation in soils even contaminated with Cd.
An endophytic bacterium Serratia sp. RSC-14 isolated from the roots of S. nigrum RSC-14 was used as an inoculant against S. nigrum plants grown in metal-stressed soils. In this study, the toxic effect of Cd-induced stress was relieved, and there were some significant improvements in root/shoot growth, biomass production and chlorophyll content, while MDA and electrolyte contents were found to decrease considerably. Besides the ability to tolerate Cd concentration up to 4 mM, the strain RSC-14 exhibited P solubilizing activity and secreted plant growth-promoting phytohormones such as IAA (54 μg/ml). The regulation of metal-induced oxidative stress enzymes such as catalase, peroxidase and polyphenol peroxidase had ameliorative effects on host growth. Activities of these enzymes were significantly reduced in RSC-14-inoculated plants as compared to control plants under Cd treatments. The current findings thus supported the hypothesis that Serratia sp. RSC-14 endowed with improved phytoextraction abilities could be used as metal-tolerant microbial inoculants to enhance the overall performance of S. nigrum plants when grown intentionally or inadvertently in Cd-contaminated soil (Khan et al. 2015b). Similarly, Luo et al. (2011) isolated endophytic bacterium Serratia sp. LRE07 from Cd-hyperaccumulator S. nigrum plants which, besides expressing the ability to promote plant growth, had high metal removal efficiencies also. Cadmium tolerant endophytic fungal community associated with S. nigrum has also been isolated and characterized for host plant growth modulation under Cd contamination (Khan et al. 2016). Owing to the levels of Cd tolerance detected, in order to simulate a tripartite plant–microbe–metal interaction, S. nigrum plants were inoculated with Glomerella truncata PDL-1 and Phomopsis fukushii PDL-10 under Cd spiking of 0, 5, 15, and 25 mg/kg. The results indicated that PDL-10-inoculated plants had significantly higher Cd content in shoots and in roots than those observed in the PDL-1-inoculated plants. Additionally, irrespective of Cd stress, PDL-1 and PDL-10 inoculation significantly improved plant growth attributes. He et al.(2009) reported that two Cd-resistant strains Pseudomonas sp. RJ10 and Bacillus sp. RJ16 increased plant growth through Cd and lead (Pb) solubilization and by secreting IAA, siderophore and 1-aminocyclopropane-1-carboxylate deaminase (ACC deaminase) besides enhancing Cd and Pb uptake ability of a Cd-hyperaccumulator tomato. Significant increase in Cd and Pb contents of above-ground tissues varied from 92 to 113% and from 73 to 79% respectively in inoculated plants grown in heavy metal-contaminated soil compared to the uninoculated control. These results show that the bacteria could be exploited for bacteria enhanced-phytoextraction of Cd- and Pb-polluted soils. Also, the effects of metal-resistant microorganisms and metal chelators on the ability of plants to accumulate heavy metals have been investigated. Though the application of Cd- or Pb-resistant microorganisms improved the ability of S. nigrum to accumulate heavy metals and increased plant yield, but the effects of microorganisms on phytoextraction were smaller than the effects of citric acid (CA). When plants were grown in the presence of Cd contamination, the co-application of CA and metal-resistant strains enhanced biomass by 30–50% and increased Cd accumulation by 25–35%. In the presence of CA and the metal-resistant microorganisms, the plants were able to acquire 15–25% more Cd and 10–15% more Pb than control plants. It was therefore suggested from this study that the synergistic combination of plants, microorganisms and chelators can enhance phytoremediation efficiency in the presence of multiple metal contaminants (Gao et al. 2012).
Conclusion
Heavy metals are one of the major toxic pollutants whose removal from contaminated areas is urgently required in order to reduce their impacts on various food chains and ultimately human health. Among food commodities, vegetables are one of the main components of human dietary system because they provide essential micro and macronutrients, proteins, antioxidants and vitamins to the human body. All vegetables are often grown in suburban areas experiencing high concentrations of heavy metals both through aerial deposition and contamination accumulators through soil and irrigation water. Among vegetables, leafy vegetables have more potential to accumulate heavy metals from a contaminated environment due to their higher capacity of absorption both from contaminated soil and aerial deposits. The advantage of high biomass production and easy disposal also makes vegetables useful to remediate heavy metals from a contaminated environment, but the excessive intake and consequent accumulation in human beings through long-term consumption of contaminated food may result in negative effect on human health. Remediation and safe consumption of vegetables are, therefore, the two opposite concerns of heavy metal impact on the environment. Stringent enforcement of standards should therefore be followed for maximum allowable intake of heavy metals to avoid risk to human health. Heavy metals besides contaminating food also reduce the nutritional value of vegetables affecting other biochemical and physiological processes reducing the yield and quality of the crops. Thus regular monitoring of heavy metal contamination in the vegetables grown at wastewater irrigated area is necessary, and consumption of contaminated vegetables should be avoided in order to reduce the health risk caused by taking the contaminate vegetables. Furthermore, a safe and inexpensive metal-removing strategy like the use of plants and microbes both in isolation and association should be promoted to grow fresh and contaminant-free vegetables.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (1989) Toxicological profile for cadmium. Agency for Toxic Substances and Disease Registry, Atlanta, Georgia. Publication No. ATSDR/TP-88/08 PB89-194476
Agrawal SB, Singh A, Sharma RK, Agrawal M (2007) Bioaccumulation of heavy metals in vegetables: a threat to human health. Terres Aqua Environ Toxicol 1:13–23
Alloway BJ (1990) Heavy metal in soils. J Environ Sci 3:97–99
Anil KG, Yunus M, Pandey PK (2003) Bioremediation: ecotechnology for the present century. Int Soc Environ Botanists 9(2):9–19
Antonious GF, Dennis SO, Unrine JM et al (2011) Ascorbic acid, β-carotene, sugars, phenols, and heavy metals in sweet potatoes grown in soil fertilized with municipal sewage sludge. J Environ Sci Health, Part B 46:112–121
Antonious GF, Kochhar TS, Coolong T (2012) Yield, quality, and concentration of seven heavy metals in cabbage and broccoli grown in sewage sludge and chicken manure amended soil. J Environ Sci Health Part A: Toxic/Hazard Subs Environ Eng 476:1955–1965
Antonious GF, Snyder JC, Berke T et al (2010) Screening Capsicum chinense fruits for heavy metals bioaccumulation. J Environ Sci Health B 45:562–567
Ashfaq A, Khan ZI, Bibi Z et al (2015) Heavy metals uptake by Cucurbita maxima grown in soil contaminated with sewage water and its human health implications in peri-urban areas of Sargodha city. Pak J Zool 47:1051–1058
Awashthi SK (2000) Prevention of food adulteration, Act no. 37 of 1954. Central and state rules as amended for 1999. Ashoka Law House, New Delhi
Baker AJM (1981) Accumulators and excluders—strategies in the response of plants to heavy metals. J Plant Nutr 3:643–654
Balkhair KS, Ashraf MA (2016) Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J Biol Sci 23:32–44
Banerjee D, Kuila P, Ganguli A et al (2011) Heavy metal contamination in vegetables collected from market sites of Kolkata. Indian J Elec Environ Agric Food Chem 10:2160–2165
Barrachina AC, Carbonell FB, Beneyto JM (1995) Arsenic uptake, distribution, and accumulation in tomato plants: effect of arsenite on plant growth and yield. J Plant Nutr 18:1237–1250
Bertoli AC, Cannata MG, Carvalho R et al (2012) Lycopersicon esculentum submitted to Cd-stressful conditions in nutrition solution: nutrient contents and translocation. Ecotoxicol Environ Saf 86:176–181
Bhatti SM, Anderson CWN, Stewart RB et al (2013) Risk assessment of vegetables irrigated with arsenic-contaminated water. Environ Sci Process Impacts 15:1866–1875
Biego GH, Joyeux M, Hartemann P et al (1998) Daily intake of essential minerals and metallic micro pollutants from foods in France. J Anal Sci 20:85–88
Brooks RR (1998) Geobotany and hyperaccumulators. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals. CAB International, Wallingford, UK, pp 55–94
Budavari SE (2001) The Merck index, 11th edn. Merck and Co, Inc, Rahway, pp 432–435
Burzynski M, Klobus G (2004) Changes of photosynthetic parameters in cucumber leaves under Cu, Cd and Pb stress. Photosynthetica 42:505–510
Cai L, Xu Z, Ren M, Guo Q et al (2012) Source identification of eight hazardous heavy metals in agricultural soils of Huizhou, Guangdong Province, China. Ecotoxicol Environ Saf 78:2–8
Caporale AG, Sommella A, Lorito M et al (2014) Trichoderma spp. alleviate phytotoxicity in lettuce plants (Lactuca sativa L.) irrigated with arsenic-contaminated water. J Plant Physiol 171:1378–1384
Castro A (1998) Chromium in a series of Portuguese plants used in the herbal treatment of diabetes. J Biol Trace Elem Res 62:101–106
Chandra S, Saha R, Pal P (2016) Arsenic uptake and accumulation in okra (Abelmoschus esculentus) as affected by different arsenical speciation. Bull Environ Contam Toxicol 96:395–400
Chanu LB, Gupta A (2016) Phytoremediation of lead using Ipomoea aquatica Forsk. in hydroponic solution. Chemosphere 156:407–411
Dafelseed M (2007) Heavy metals and pesticides residues in selected fresh vegetables. B.Sc., University of Khartoum, pp 45–49
Demirezen D, Aksoy A (2006) Heavy metal levels in vegetables in Turkey are within safe limits for Cu, Zn, Ni and exceeded for Cd and Pb. J Food Qual 29:252–265
Dermentzis K (2009) Copper removal from industrial wastewaters by means of electrostatic shielding driven electrodeionization. J Eng Sci Tech Rev 2:131–136
Derome J, Nieminen T (1998) Metal and macronutrient fluxes in heavy-metal polluted Scots pine ecosystems in SW Finland. Environ Poll 103:219–228
Ding C, Zhang T, Wang X, Zhou F, Yang Y, Yin Y (2014) Effects of soil type and genotype on cadmium accumulation by rootstalk crops: implications for phytomanagement. Int J Phytoremediation 16:1018–1030
Dinis MDL, Fiúza A (2011) Exposure assessment to heavy metals in the environment: measures to eliminate or reduce the exposure to critical receptors. In: Simeonov LI et al (eds) Environmental heavy metal pollution and effects on child mental development. Springer, Netherlands, pp 27–50
Dixit R, Malaviya D, Pandiyan K et al (2015) Bioremediation of heavy metals from soil and aquatic environment: an overview of principles and criteria of fundamental processes. Sustainability 7:2189–2212
Dogheim SM, El-Ashraf MM, Alla SG et al (2004) Pesticides and heavy metals levels in Egyptian leafy vegetables and some aromatic medicinal plants. J Food Addit Contam 21:323–330
Dourado MN, Souza LA, Martins PF et al (2014) Burkholderia sp. SCMS54 triggers a global stress defense in tomato enhancing cadmium tolerance. Water Air Soil Pollut 225:1–16
Drechsel P, Keraita B (eds) (2014) Irrigated urban vegetable production in Ghana: characteristics, benefits and risk mitigation, 2nd edn. International Water Management Institute (IWMI), Colombo, Sri Lanka
Ebdon L (2001) Trace element speciation for environment, food and health. Royal Society of Chemistry, Cambridge
European Union (EU) (2002) Heavy metals in wastes. European Commission on Environment. http://ec.europa.eu/environment/waste/studies/elv/heavymetals_report.pdf
EU (2006) Commission regulation (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L364:5–24
FAO (1985) Water quality for agriculture. Paper no. 29 (Rev. 1) UNESCO, Publication, Rome
FAO/WHO (2001) Food additives and contaminants. Joint Codex Alimentarius Commission, FAO/WHO Food Standards Programme 34:745–750
Ferri R, Hashim D, Smith DR et al (2015) Metal contamination of home garden soils and cultivated vegetables in the province of Brescia, Italy: implications for human exposure. Sci Total Environ 518:507–517
Fiona M, Ravi A, Rana PB (2003) Executive summary of technical report. Heavy metal contamination of vegetables in Delhi, Imperial College, London, pp 248–256
Flora SJS, Mittal M, Mehta A (2008) Heavy metal induced oxidative stress and it’s possible reversal by chelation therapy. Division of Pharmacology and Toxicology, Defence Research and Development Establishment, Gwalior, India. Indian J Med Res 128:501–502
Franco-Hernández, MO, Montes-Villafàn, S, Ramírez-Melo M et al (2010) Comparative analysis of two phytohormone and siderophores rhizobacteria producers isolated from heavy metal contaminated soil and their effect on Lens esculenta growth and tolerance to heavy metals. In: Mendez-Vilas A (ed) Current research technology and education topics in applied microbiology and microbial biotechnology, Formatex 2010, Spain, pp 74–80
Gadd GM, White C (1989) Havy metal and redionuclide accumulation and toxicity in fungi and yeast. In: Poole RK, Gadd GM (eds) Metal-microb interactions. IRL Press, Oxford, pp 19–38
Gao Y, Miao C, Wang Y et al (2012) Metal-resistant microorganisms and metal chelators synergistically enhance the phytoremediation efficiency of Solanum nigrum L. in Cd- and Pb-contaminated soil. Environ Technol 33:1383–1389
Garcia E, Cabrera C, Lorenzo ML et al (2001) Daily dietary intake of chromium in southern Spain measured with duplicate diet sampling. J Nutr 86:391–396
Ghani A (2011) Effect of chromium toxicity on growth, chlorophyll and some mineral nutrients of Brassica juncea L. Egypt Acad J Biol Sci 2:9–15
Ghosh AK, Bhatt MA, Agrawal HP (2012) Effect of long-term application of treated sewage water on heavy metal accumulation in vegetables grown in northern India. Environ Monit Assess 184:1025–1036
Gopal R, Rizvi AH, Nautiyal N (2009) Chromium alters iron nutrition and water relations of spinach. J Plant Nutr 32:1551–1559
Grant CA (2011) Influence of phosphate fertilizer on cadmium in agricultural soils and crops. Pedologist 54:143–155
Haghighi M (2011) Sewage sludge application in soil improved leafy vegetable growth. J Biol Environ Sci 5(15)
He LY, Chen ZJ, Ren GD et al (2009) Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria. Ecotoxicol Environ Saf 72:1343–1348
Hernandez LE, Cooke DT (1997) Modification of the root plasma membrane lipid composition of cadmium-treated Pisum sativum. J Exp Bot 48:1375–1381
Hughes MF, Beck BD, Chen Y et al (2011) Arsenic exposure and toxicology: a historical perspective. Toxicol Sci 123:305–332
Hussain MM, Saeed A, Khan AA et al (2015) Differential responses of one hundred tomato genotypes grown under cadmium stress. Genet Mol Res 14:13162–13171
Intawongse M, Dean JR (2006) Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit Contam 23:36–48
Ionenko IF, Anisimov AV, Karimova FG (2006) Water transport in maize roots under the influence of mercuric chloride and water stress: a role of water channels. Biol Plant 50:74–80
IPCS (1992) International Programme on Chemical Safety (IPCS). Environmental health criteria 134, 135: cadmium
Islam MS, Hoque MF (2014) Concentrations of heavy metals in vegetables around the industrial area of Dhaka city, Bangladesh and health risk assessment. Int Food Res J 21:2121–2126
Islam GBR, Khan FE, Hoque MM et al (2014) Consumption of unsafe food in the adjacent area of Hazaribag tannery campus and Buriganga River embankments of Bangladesh: heavy metal contamination. Environ Monit Assess 186:7233–7244
Itanna F (2002) Metals in leafy vegetables grown in Addis Ababa and toxicological implications. Ethiop J Health Dev 16:295–302
IWMI (2006) Recycling realities: managing health risks to make wastewater an asset. Water Policy Brief 17:432–434
Jamali MK, Kazi TG, Arain MB et al (2007) Heavy metal contents of vegetables grown in soil, irrigated with mixtures of wastewater and sewage 53 sludge in Pakistan, using ultrasonic-assisted pseudo-digestion. J Agron Crop Sci 193:218–228
Janicka-Russak M, Kabała K, Burzyński M (2012) Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J Exp Bot 63:4133–4142
Jassir MS, Shaker A, Khaliq MA (2005) Deposition of heavy metals on green leafy vegetables sold on roadsides of Riyadh City, Saudi Arabia. J Environ Contam Toxicol 75:1020–1027
Jiang CY, Sheng XF, Qian M et al (2008) Isolation and characterization of a heavy metal-resistant Burkholderia sp. from heavy metal-contaminated paddy field soil and its potential in promoting plant growth and heavy metal accumulation in metal polluted soil. Chemosphere 72:157–164
Jiang L, Yang Y, Xu WH et al (2014) Effects of ryegrass and arbuscular mycorrhiza on activities of antioxidant enzymes, accumulation and chemical forms of cadmium in different varieties of tomato. Huan Jing Ke Xue 35:2349–2357
Jing J, Logan T (1992) Measurement of levels of heavy metal contamination in vegetables grown and sold in selected areas in Lebanon. J Environ Qual 21:1–8
John HD (2002) Heavy metals: a meaningless term (IUPAC Technical Report). Pure Appl Chem 74:793–807
Johnson MS, Cooke JA, Stevenson JKW (1994) Revegetation of metalliferous wastes and land after metal mining. In: Hester RE, Harrison RM (eds) Mining and its environmental impact, issues in environmental science and technology. Royal Society of Chemistry, London, pp 31–48
Kabata-Pendias A, Pendias H (2001) Trace elements in soils, 3rd edn. CRC Press, Boca Raton and London
Kachenko AG, Singh B (2006) Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water Air Soil Pollut 169:101–123
Kang CH, Kwon YJ, So JS (2016) Bioremediation of heavy metals by using bacterial mixtures. Ecol Eng 89:64–69
Kapoor A, Viraraghavan T, Roy Cullimore D (1999) Removal of heavy metals using the fungus Aspergillus niger. Bioresour Technol 70:95–104
Karigar C, Rao SS (2011) Role of microbial enzymes in the bioremediation of pollutants: a review. Enz Res 2011:11
Khan N, Bano A (2016) Role of plant growth promoting rhizobacteria and Ag-nano particle in the bioremediation of heavy metals and maize growth under municipal wastewater irrigation. Int J Phytoremediation 18:211–221
Khan A, Khan S, Khan MA et al (2015a) The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res Int 22:13772–13799
Khan AR, Ullah I, Khan AL et al (2015b) Improvement in phytoremediation potential of Solanum nigrum under cadmium contamination through endophytic-assisted Serratia sp. RSC-14 inoculation. Environ Sci Poll Res 22:14032–14042
Khan AR, Waqas M, Ullah I et al (2016) Culturable endophytic fungal diversity in the cadmium hyperaccumulator Solanum nigrum L. and their role in enhancing phytoremediation. Environ Exp Bot. doi:10.1016/j.envexpbot.2016.03.005
Kim JO, Lee YW, Chung J (2013) The role of organic acids in the mobilization of heavy metals from soil. KSCE J Civil Eng 17:1596–1602
Kumar KV, Patra DD (2013) Influence of nickel and cadmium resistant PGPB on metal accumulation and growth responses of Lycopersicon esculentum plants grown in fly ash amended soil. Water Air Soil Pollut 224:1–10
Kumar M, Rahman MM, Ramanathan AL et al (2016) Arsenic and other elements in drinking water and dietary components from the middle Gangetic plain of Bihar, India: health risk index. Sci Total Environ 539:125–134
Lasat MM (2000) Phytoextraction of metals from contaminated soil: a review of plant, soil, metal interaction and assessment of pertinent agronomic issues. J Hazard Subs Res 2:1–25
Lenntech (2006) Water treatment and air purification. http://www.lenntech.com/heavymetals.htm. Accessed 23 Jan 2006
Li F, Zeng XY, Wu CH, Duan ZP et al (2013) Ecological risks assessment and pollution source identification of trace elements in contaminated sediments from the pearl river delta, China. Biol Trace Elem Res 155:301–313
Liu M, Huang B, Bi X, Ren Z et al (2013) Heavy metals and organic compounds contamination in soil from an e-waste region in South China. Environ Sci Process Impacts 15:919–929
Liu X, Zhang S, Shan X et al (2005) Toxicity of arsenate and arsenite on germination seedling growth and amylolytic activity of wheat. Chemosphere 61:293–301
Lokeshwari H, Chandrappa GT (2006) Impact of heavy metal contamination of Bellandur lake on soil and cultivated vegetation. Curr Sci 91:622–627
Lui WX, Li HH, Li SR et al (2006) Heavy metal accumulation of edible vegetables cultivated in agricultural soil in the suburb of Zhengzhou City, People’s Republic of China. Bull Environ Contam Toxicol 76:163–170
Luo S, Wan Y, Xiao X et al (2011) Isolation and characterization of endophytic bacterium LRE07 from cadmium hyperaccumulator Solanum nigrum L. and its potential for remediation. Appl Microbiol Biotechnol 89:1637–1644
Magyarosy A, Laidlaw RD, Kilaas R et al (2002) Nickel accumulation and nickel oxalate precipitation by Aspergillus niger. Appl Microbiol Biotechnol 59:382–388
Mani D, Sharma B, Kumar C, Balak S (2012) Cadmium and lead bioaccumulation during growth stages alters sugar and vitamin C content in dietary vegetables. Proc Natl Acad Sci India Sect B: Biol Sci 82:477–488
Marin AR, Pezeshki SR, Masschelen PH et al (1993) Effect of dimethylarsenic acid (DMAA) on growth, tissue arsenic, and photosynthesis of rice plants. J Plant Nutr 16:865–880
Mello JP (2003) Food safety: contaminants and toxins. CABI Publishing, Wallingford, Oxon, UK; Cambridge, MA, p 480
Michio X (2005) Bioaccumulation of organochlorines in crows from an Indian open waste dumping site: evidence for direct transfer of dioxin-like congeners from the contaminated soil. J Environ Sci Technol 39:4421–4430
Midio Y, Satake M (2003) Chemicals and toxic metals in the environment. Discovery Publishing House, New Delhi, pp 45–68
Mohamed AE, Rashed MN, Mofty A (2003) Assessment of essential and toxic elements in some kinds of vegetables. Ecotoxicol Environ Saf 55:251–260
Mosa KA, Saadoun I, Kumar K et al (2016) Potential biotechnological strategies for the cleanup of heavy metals and metalloids. Front Plant Sci 7:303. PMCID: PMC4791364
Muchuweti M, Birkett JW, Chinyanga E et al (2006) Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: implications for human health. Agric Ecosyst Environ 112:41–48
Musilova J, Bystricka J, Lachman J et al (2016) Potatoes—a crop resistant against input of heavy metals from the metallicaly contaminated soil. Int J Phytoremediation 18:547–552
Nacke H, Gonçalves A Jr, Schwantes D et al (2013) Availability of heavy metals (Cd, Pb, and Cr) in agriculture from commercial fertilizers. Arch Environ Contam Toxicol 64:537–544
Naz T, Khan MD, Ahmed I et al (2015) Biosorption of heavy metals by Pseudomonas species isolated from sugar industry. Toxicol Ind Health 32:1619–1627
Neilson JW, Artiola JF, Maier M (2003) Characterization of lead removal from contaminated soils by non-toxic soil washing agents. J Environ Qual 32:899–908
Ng CC, Rahman MM, Boyc AM, Abbas MR (2016) Heavy metals phyto assessment in commonly grown vegetables: water spinach (I. aquatica) and okra (A. esculentus). Springer plus 5:469
Oluwole SO, Olubunmi Makinde SC, Yusuf KA et al (2013) Determination of heavy metals contaminants in leafy vegetables cultivated by the road side. Int J Eng Res Dev 7:01–05
Osaili TM, Al Jamali AF, Makhadmeh IM et al (2016) Heavy metals in vegetables sold in the local market in Jordan. Food Addit Contamin Part B 9:223–229
Parashar P, Prasad FM (2013) Study of heavy metal accumulation in sewage irrigated vegetables in different regions of Agra District, India. J Soil Sci 3:1
Pederno NJI, Gomez R, Moral G et al (1997) Heavy metals and plant nutrition and development. Rec Res Dev Phytochem 1:173–179
Pell A, Márquez A, López-Sánchez JF et al (2013) Occurrence of arsenic species in algae and freshwater plants of an extreme arid region in northern Chile, the Loa River Basin. Chemosphere 90:556–564
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39
Pizarro I, Gomez M, Roman D et al (2016) Bioavailability, bioaccesibility of heavy metal elements and speciation of as in contaminated areas of Chile. J Environ Anal Chem 3:175
Prabu PC (2009) Impact of heavy metal contamination of Akaki river of Ethiopia on soil and metal toxicity on cultivated vegetable crops. Elec J Environ Agric Food Chem 8:818–827
Quaghebeur M, Rengel Z (2003) The distribution of arsenate and arsenite in shoots and roots of Holcus lanatus is influenced by arsenic tolerance and arsenate and phosphate supply. Plant Physiol 132:1600–1609
Radwan MA, Salama AK (2006) Market basket survey for some heavy metals in Egyptian fruits and vegetables. J Food Chem Toxicol 44:1273–1278
Rahman MM, Ng JC, Naidu R (2009) Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ Geochem Health 31:189–200
Rajkumar M, Ae N, Prasad MNV et al (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149
Rehman ZU, Khan S, Qin K et al (2016) Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in southern selected districts of Pakistan. Sci Total Environ 550:321–329
Ryser P, Emerson P (2007) Growth, root and leaf structure, and biomass allocation in Leucanthemum vulgare Lam. (Asteraceae) as influenced by heavy-metal-containing slag. Plant Soil 301:315–324
Saha S, Hazra GC, Saha B et al (2015) Assessment of heavy metals contamination in different crops grown in long-term sewage-irrigated areas of Kolkata, West Bengal, India. Environ Monit Assess 187:1–12
Santiago M, Bolan NS (2010) Phytoremediation of arsenic contaminated soil and water. In: Proceedings of 19th world congress of soil science. Soil Solutions for a Changing World, Brisbane, Australia
Santra SC, Samal AC, Bhattacharya P et al (2013) Arsenic in food chain and community health risk: a study in Gangetic West Bengal. Procedia Environ Sci 18:2–13
Sharma RK, Agrawal M (2006) Single and combined effects of cadmium and zinc on carrots: uptake and bioaccumulation. J Plant Nutr 29:1791–1804
Sharma RK, Agrawal M, Marshall F (2006) Heavy metals contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bull Environ Contam Toxicol 77:312–318
Sharma RK, Agrawal M, Marshall F (2007) Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol Environ Saf 66:258–226
Sharma RK, Agrawal M, Marshall FM (2009) Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food Chem Toxicol 47:583–591
Shaw BP, Sahu SK, Mishra RK (2004) Heavy metal induced oxidative damage in terrestrial plants. In: Prasad MNV (ed) Heavy metal stress in plants. Springer, Berlin Heidelberg, pp 84–126
Sheoran IS, Singal HR, Singh R (1990) Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeonpea (Cajanus cajan L.) Photosynth Res 23:345–351
Singh VP (2006) Metal toxicity and tolerance in plants and animals. Sarup and Sons, New Delhi, India, p 238
Singh RP, Agrawal M (2007) Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 67:2229–2240
Singh S, Kumar M (2006) Heavy metal load of soil, water and vegetables in peri-urban Delhi. Environ Monit Assess 120:79–91
Singh R, Gautam N, Mishra A et al (2011) Heavy metals and living systems: an overview. Indian J Pharm 43:246–253
Singh A, Sharma RK, Agrawal M et al (2010) Risk assessment of heavy metal toxicity through contaminated vegetables from waste water irrigated area of Varanasi, India. Trop Ecol 51:375–387
Singh D, Tiwari A, Gupta R (2012) Phytoremediation of lead from wastewater using aquatic plants. J Agric Tech 8:1–11
Sinha S, Gupta AK, Bhatt K et al (2006) Distribution of metals in the edible plants grown at Jajmau, Kanpur (India) receiving treated tannery wastewater: relation with physico-chemical properties of the soil. Environ Monit Assess 115:1–22
Sinha S, Pandey K, Gupta AK et al (2005) Accumulation of metals in vegetables and crops grown in the area irrigated with river water. Bull Environ Contam Toxicol 74:210–218
Skeffington RA, Shewry R, Peterson PJ (1996) Chromium uptake and transport in barley seedlings (Hordeum vulgare L.) J Int Environ 132:209–214
Stalikas CD, Mantalovas AC, Pilidis GA (1997) Multielement concentrations in vegetable species grown in two typical agricultural areas of Greece. Sci Total Environ 206:17–24
Tiwari KK, Singh NK, Patel MP et al (2011) Metal contamination of soil and translocation in vegetables growing under industrial wastewater irrigated agricultural field of Vadodara, Gujarat, India. Ecotoxicol Environ Saf 74:1670–1677
Turer DG, Maynard BJ (2003) Heavy metal contamination in highway soils. Comparison of Corpus Christi, Texas and Cincinnati, Ohio shows organic matter is key to mobility. Clean Technol Environ Policy 4:235–245
UdDin I, Bano A, Masood S (2015) Chromium toxicity tolerance of Solanum nigrum L. and Parthenium hysterophorus L. plants with reference to ion pattern, antioxidation activity and root exudation. Ecotoxicol Environ Saf 113:271–278
Ullah A, Heng S, Munis MFH et al (2015) Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: a review. Environ Exp Bot 117:28–40
Uzu G, Schreck E, Xiong T et al (2014) Urban market gardening in Africa: foliar uptake of metal (loid) s and their bioaccessibility in vegetables; implications in terms of health risks. Water Air Soil Pollut 225:1–13
Wang Y, Qiu Q, Xin G, Yang Z et al (2013) Heavy metal contamination in a vulnerable mangrove swamp in South China. Environ Monit Assess 185:5775–5787
WHO (1992) Environmental health criteria 134: cadmium. Geneva, p 156
WHO/FAO (2007) Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission 13th session. Report of the thirty eight session of the Codex Committee on Food Hygiene, Houston, USA, ALINORM 07/30/13
Woimant F, Trocello JM (2014) Disorders of heavy metals. Handb Clin Neurol 120:851–864
Wu Z, Ren H, McGrath SP et al (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157:498–508
Xiong T, Dumat C, Pierart A et al (2016) Measurement of metal bioaccessibility in vegetables to improve human exposure assessments: field study of soil–plant–atmosphere transfers in urban areas, South China. Environ Geochem Health 38:1–19
Xiong TT, Leveque T, Austruy A et al (2014) Foliar uptake and metal (loid) bioaccessibility in vegetables exposed to particulate matter. Environ Geochem Health 36:897–909
Xiong J, Wu L, Tu S et al (2010) Microbial communities and functional genes associated with soil arsenic contamination and the rhizosphere of the arsenic-hyperaccumulating plant Pteris vittata L. Appl Environ Microbiol 76:7277–7284
Yang HM, Zhang XY, Wang GX (2004) Effects of heavy metals on stomatal movements in broad bean leaves. Russ J Plant Physiol 51:464–468
Yu HY, Li FB, Yu WM, Li YT et al (2013) Assessment of organochlorine pesticide contamination in relation to soil properties in the Pearl River Delta, China. Sci Total Environ 447:160–168
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Zhao H, Xia B, Fan C, Zhao P et al (2012) Human health risk from soil heavy metal contamination under different land uses near Dabaoshan mine, southern China. Sci Total Environ 417:45–54
Zhou H, Yang WT, Zhou X et al (2016) Accumulation of heavy metals in vegetable species planted in contaminated soils and the health risk assessment. Int J Environ Res Public Health 13:289
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Saif, S., Khan, M.S., Zaidi, A., Rizvi, A., Shahid, M. (2017). Metal Toxicity to Certain Vegetables and Bioremediation of Metal-Polluted Soils. In: Zaidi, A., Khan, M. (eds) Microbial Strategies for Vegetable Production. Springer, Cham. https://doi.org/10.1007/978-3-319-54401-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-54401-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54400-7
Online ISBN: 978-3-319-54401-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)