Abstract
The effects of sewage water on the accumulation of heavy metals (Zn, Cu, Pb, Cd, and Ni) in soils and commonly grown plants were evaluated by monitoring the fields along the water channel running some 30 km eastward of Kolkata, West Bengal, India. The results revealed that the mean Cu, Cd, and Zn contents in sewage water were higher than the recommended level whereas mean concentration of Cd in the irrigated soil is several folds higher than the safe limit. The highest single element pollution index (SEPI) value was found for Cd which ranged from 2.93 to 6.03 with a mean of 5.32 indicating high contamination levels. The value of combined pollution index (CPI) ranged from 1.32 to 1.93 with an average of 1.67 for all the sites indicates that metal concentrations are above the hazard criteria and exhibit multi-element contamination. Furthermore, the results of enrichment factor (EF) indicated that the soils of the study area were highly enriched with metals such as Cd (452.04, extremely high enrichment) followed by Pb (17.32, significant to very high enrichment) > Zn (5.99, significant enrichment) > Cu (3.14, minimal to significant) > Ni (3.07, minimal to moderate). The concentrations of Pb, Cd, and Ni were above the permissible limit in all the crops and Colocasia and Amaranthus accumulate highest metal on the basis of overall metal uptake. The highest mean transfer coefficients (TCs) values for Zn, Cu, Pb, Cd, and Ni were found for cauliflower (0.59), Colocasia (0.67), Amaranthus (0.93), Colocasia (1.02), and Amaranthus (1.09), respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The scarcity of ground water for irrigation is an ever increasing problem, owing to which the use of sewage water in agriculture has become a common practice in three-fourth of the cities in Asia, Africa, and Latin America (Gupta et al. 2008). An estimated 38,354 million liters of sewage per day is presently generated in India (Kaur et al. 2012). Among the various options available, its application to farmland is the most economical outlet as it provides an opportunity to recycle the beneficial plant nutrients contained in it for crop production. Different risk factors have been identified in wastewater reuse. Some of them are short termed (e.g., microbial pathogens), whereas others have long-term impacts that increase with the continued use of recycled water (e.g., saline effect on soil) (Papadopoulos et al. 2009). The presence of pollutants like heavy metals in urban and industrial waste water is another problem associated with the reuse of such water. Continuous use of such wastewater results in contamination of water and soil. Household effluents, drainage water, industrial effluents, atmospheric deposition, and traffic-related emissions transported with storm water into the sewage system carry number of pollutants and enrich the urban waste water with heavy metals (Oliveira et al. 2007).
The unscientific disposal of untreated or under-treated sewage sludge, city waste, and sewage water or their continuous unlawful use in the agricultural fields, the heavy metal load of some areas of West Bengal has been raised to an alarming level.
Therefore, the characterization of soils receiving urban waste water is a necessity towards establishing the pollutional status (Jagtap et al. 2010). Metal transfers from biosolids to soil and subsequently to plants pose potential health risks, since they can enter the food chain and the environment (Jamali et al. 2007). Uptake of heavy metals in plants is of major concern as it may reach human through food web (Gupta et al. 2008). Plants can accumulate higher concentration of heavy metals if they are grown in metal polluted soils.
So, keeping these points in view, the present study was undertaken to study the heavy metal (Zn, Cu, Pb, Cd, and Ni) loads in sewage water and in soils and plants affected by its use in the peri-urban areas of Kolkata, West Bengal, India.
Materials and methods
Study area and sampling
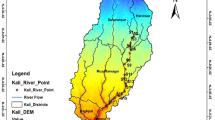
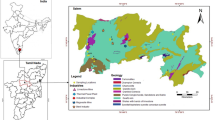
Kolkata (22.82° N-88.20° E) being the capital of West Bengal is one of the busiest metropolitan cities in India with 24,306 people per square km (Population Census, 2011). It is located in the eastern India and is situated on the bank of the river Hooghly (Fig. 1). The multiple-use wetlands popularly known as “East Kolkata Wetlands (EKWs)” lie east of the city and include a garbage dump known as Dhapa Square Mile, a mosaic of vegetable fields, a series of 300-odd fishponds connected by major and secondary canals, rice fields, wholesale markets, a few roads, and 43 villages (with 60,000 people in all). The waste water stream running about 30 km eastward and finally falls into the river Kulti-Bidyadhari which ultimately drains into the Bay of Bengal (Table 1). About 150 t of vegetables per day are harvested from small-scale plots irrigated with wastewater. Some 3500 t of municipal waste and 680 million liters of raw sewage enter the wetland system every day. Still, only 30 % of the total wastewater is used for aquaculture or irrigation, while the remaining 70 % flows directly into the Bay of Bengal (which pollutes the estuary, highlighting the need for improved efficiency of the system) (Bunting et al. 2001).
Crops were mainly irrigated through sewage water as and when required. Generally, rice-based cropping system is practiced by the farmers for growing crops on the bank of this stream where rice cultivation is generally followed by the cultivation of rabi vegetables and summer vegetables. Samples of sewage water and soils (at three depths viz. 0.0–0.2, 0.2–0.4, and 0.4–0.6 m) receiving sewage water as irrigant from the disposal system as well as plant samples grown onto the soils have been collected during summer season of 2011. In order to represent the whole areas of the selected sites, three subsites within each site were marked for soil, water, and plant sampling and one sample from each subsite is collected and then mixed to obtain a bulk sample.
Analytical procedure for analysis
The soil samples were air-drie while the plant samples were first washed with running tap water followed by washing with 0.01 N HCl and finally with double distilled water. After that, plant samples were dried in a hot air oven at 50 °C for 48 h till the constant weight was achieved. After drying, the samples were ground to fine powder by using stainless steel grinder for further analysis. The soil and water samples were analyzed for their pH (Page et al. 1982), EC (Richard 1954), and oxidizable organic carbon content (Walkley and Black method as modified by Nelson and Sommers 1982).
Heavy metals analysis in soils, water and plants
Fifty milliliters of water samples were digested with 10 mL of concentrated HNO3 and HClO4 in 9:4 ratio at 80 °C until the solution became transparent (APHA 1995). The solution was filtered through Whatman no. 42 filter paper, and the filtrate was diluted to 25 mL with distilled water. For total content of metals (Zn, Cu, Pb, Cd, and Ni) in the soil and plants, 1 g of each soil and plant samples were digested (wet acid digestion) with 10 mL of concentrated HNO3 and HCIO4 in 9:4 ratio at 80 °C until a clear solution was obtained. The digested samples of soil and plants were filtered through Whatman no. 42 filter paper, and the filtrates were diluted to 50 mL with distilled water.
Trace elements concentrations in water, soil, and in plant samples were estimated by an atomic absorption spectrometer (GBC-902, Australia).
Estimation of different parameters for quantification of soil pollution
Single pollution index
Single element pollution index (SEPI) is one of the simple and well-known index generally applied to measure environmental quality of soil which was used as evaluation methods and to identify single-element contamination resulting in increased such metal toxicity due to continuous use of sewage. The formula used to calculate SEPI is as follows:
The tolerable levels for soil suggested by Kabata and Pendias (1992)) were adopted as permissible levels, and each heavy metal was classified as low contamination (SEPI ≤1), moderate contamination (1< SEPI ≤3), or high contamination (SEPI >3) (Chen et al. 2005).
Combined pollution index
Another commonly used evaluation methods of heavy metal accumulation, i.e., combined pollution index (CPI), were also measured to identify multi-element contamination (Lee 2003). It is also generally agreed that most heavy metal contamination in the surface environment is associated with a mixture of contaminants rather than one metal contaminant (Jung 2001). The CPI is calculated by the average ratio of metal concentrations in soil to assumed permissible level and was then classified as low (CPI ≤1), medium (1< CPI ≤2), or high (CPI >2) (Chen et al. 2005). Furthermore, if CPI ≤ 1, it shows that there is no heavy metal accumulation in soil. If CPI > 1.0, it means that there is heavy metal accumulation in soil. The higher the CPI is the more serious heavy metal accumulation in soil. The permissible limit of different heavy metals for soil suggested by Kabata and Pendias (1992)) was adopted here and pollution index was calculated as:
Soil enrichment factors
Enrichment factor was assessed to determine the level of metal contamination and the possible anthropogenic impact in sewage-irrigated soils of the area. Therefore, to assess the relative degree of metal contamination, comparisons were made to background concentrations in the earth’s crust using Fe as reference element following the assumption that its content in the crust has not been disturbed by anthropogenic activity, and it has been chosen as the element of normalization because natural sources (98 %) vastly dominate its input (Tippie 1984). The earth’s crust data of Taylor 1964 were used for the calculation of enrichment factor. Deely and Fergusson (1994) suggested Fe as an acceptable normalization element to be used in the calculation of the enrichment factor since they considered the Fe distribution was not related to other heavy metals. The EFs were calculated according to the equation mentioned below (Zoller et al. 1974):
Where EFs is the enrichment factor, C/Fe (sample) is the ratio of metal and Fe concentration of the sample, and C/Fe (earth’s crust) is the ratio of metals and Fe concentration of the earth crust. Five contamination categories are recognized on the basis of the enrichment factor: EF <2 states deficiency to minimal enrichment, EF = 2–5 moderate enrichment, EF = 5–20 significant enrichment, EF = 20–40 very high enrichment, and EF > 40 extremely high enrichment (Sutherland 2000).
Transfer of metals from soil to plants
The transfer coefficients (TCs) of different heavy metals under study were calculated to indicate the mobility of metals in soils. TC actually quantifies the existing differences in the bioavailability of metal to plants (Kachenko and Singh 2006). It is calculated by dividing the concentration of a metal in a plant (dry weight) by the total soil metal concentration: TC = Mpc/Msc, where Mpc is the metal plant concentration (mg kg−1) and Msc is the soil metal concentration (mg kg−1). Higher TC depicts relatively poor retention in soil or greater efficiency of plants to absorb metal, and low TC depicts the strong sorption of metal to the soil colloid (Alloway and Ayres 1997).
Results and discussions
Physicochemical properties and heavy metal concentrations in irrigation water
The sewage water of 16 sites showed variability in chemical properties (Table 2). The pH of water was neutral to slightly alkaline which is within safe limit of 6.0 to 8.5 as suggested by United States Salinity Laboratory Staff (1954). Electrical conductivity (EC) has ranged from 1.00 to 3.20 dS m−1 which is quite high and found to exceed the toxic limit (0.7 dS m−1) as per the FAO (1985). Heavy metal concentrations (mg L−1) in sewage water at different locations ranged from 0.22 to 0.33 for Zn, 0.19–0.31 for Cu, 1.11–1.50 for Pb, 0.10–0.13 for Cd, and 0.25–0.36 for Ni. Among all the heavy metals in sewage water, concentration of Pb was highest followed by Ni, Zn, Cu, and Cd.
Except for Zn and Pb, the concentration of heavy metals (viz. Cu, Cd, and Ni) in sewage water was higher than the recommended maximum concentration suggested by FAO (1985) indicating that the transfer of metals to the soil (irrigated with sewage water) and subsequently to plants poses potential health risks, since they can enter the food chain and the environment. Except for Cd, the mean concentrations of Zn, Cu, Pb, and Ni of irrigation water in the area under study were several times lower than those reported in Titagarh (Gupta et al. 2008), Solapur (Jagtap et al. 2010), and Ahmednagar (Kharche et al. 2011), India. However, mean Zn, Cu, Pb, Cd, and Ni concentrations were higher than those reported in Delhi (Singh and Kumar 2006) and Allahabad (Yadav et al. 2013), India. On the other hand, mean Cd concentration was 200 folds lower than those reported in Solapur, India (Jagtap et al. 2010).
Physicochemical properties of soils
The results of the physicochemical properties of the soils receiving the sewage irrigation were presented in Table 3. The soils of the 16 sites at various depths were slightly alkaline. The pH of soils irrigated with sewage water was not much influenced as compared to the normal soils. The EC in sewage-irrigated soils was below the threshold limit to cause salinity hazard to the soil. The organic carbon content of the soils on the other hand was high which might be due to the addition of organic matter through long-term application of sewage water. Singh and Kumar (2006) also reported the similar type of findings.
Heavy metal concentration in soil
Among the heavy metals, Pb content was the highest followed by Zn, Ni, Cu, and lowest with Cd (Table 4). The results, therefore, revealed that there was a marked variation in the level of heavy metals content. The differences in heavy metal accumulation of soil at different locations might be due to the use of different amount of sewage water used as per crop water requirement as well as quality of sewages flowing at different seasons. This may also be related to the heavy metal contamination from highway vehicles as well as due to the use of various agrochemicals containing heavy metals (Singh and Kumar 2006). Except for Zn and Cu, total content of Pb, Cd, and Ni in the sewage-irrigated soils was above the maximum permissible limits as given by Kabata and Pendias (1992), but those values were below the permissible limits (except for Cd which is several folds higher than the safe limit) as suggested by the Department of Environment (1989) and Indian standard (Awashthi 2000).
Mean concentrations of Zn (66.56 mg kg−1) and Cu (27.81 mg kg−1) in surface soils receiving sewage water in the present investigation were lower than those reported in different cities in India viz., Titagarh, West Bengal (Gupta et al. 2008), Solapur, Maharashtra (Jagtap et al. 2010), Delhi (Singh and Kumar 2006), and Ahmednagar, Maharashtra (Kharche et al. 2011) and also in industrial areas of Dhaka, Bangladesh (Ahmad and Goni 2010). However, mean Cu concentration (27.81 mg kg−1) was higher than the values reported in sewage-irrigated vegetable growing soils of Beijing, China (Liu et al. 2005). The mean concentration of Pb (102.99 mg kg−1) in soil of the area under study was higher than the level reported in Delhi (Singh and Kumar 2006) and Allahabad (Yadav et al. 2013), India; Beijing, China (Liu et al. 2005); and Dhaka, Bangladesh (Ahmad and Goni 2010) but lower than those values reported in Titagarh, India (Gupta et al. 2008); Solapur, Maharashtra, India (Jagtap et al. 2010), and industrial areas of Baghdad, Iraq (Obaidy and Mashhadi 2013). On the other hand, the mean Cd concentration (15.95 mg kg−1) was more or less similar to the values reported in industrial areas of Dhaka, Bangladesh (Ahmed and Goni 2010) but was several folds higher than those reported in Delhi (Singh and Kumar 2006) and Ahmednagar, India (Kharche et al. 2011); Beijing, China (Liu et al. 2005); and industrial areas of Baghdad, Iraq (Obaidy and Mashhadi 2013). However, the mean Cd concentration was lower than those observed in Titagarh (Gupta et al. 2008) and Allahabad (Yadav et al. 2013), India and was several folds lower than those reported in Solapur, India (Jagtap et al. 2010). Mean Ni concentration (36.81 mg kg−1) in sewage-irrigated soils of the area was lower than those reported in Titagarh (Gupta et al. 2008), Solapur (Jagtap et al. 2010), Ahmednagar (Kharche et al. 2011), Allahabad (Yadav et al. 2013), India and industrial areas of Dhaka, Bangladesh (Ahmad and Goni 2010) and Baghdad, Iraq (Obaidy and Mashhadi 2013).
Enrichment of different heavy metals in sewage-irrigated soils was significantly influenced by their contents in sewage water (Fig. 2) as it is observed from the simple regression analysis. The relationship being found most significant for Cu (R 2 = 0.95) followed by Pb (R 2 = 0.81), Ni (R 2 = 0.69), and Zn (R 2 = 0.65), whereas least significant for Cd (R 2 = 0.53).
Quantification of soil pollution
Soil pollution index (SPI) often used to quantify the degree of pollution of sewage-treated soils with respect to background values. In this study, SPI was estimated by the procedure given below:
Single pollution index
The highest SEPI value in sewage-irrigated soils was found for Cd which ranged from 2.93 to 6.03 (Table 5) indicating moderate to high contamination level with a mean of 5.32 for all surface soil samples indicating high contamination level. The SEPI value for Ni and Pb varied from 0.65 to 1.85 and 0.39 to 1.11, respectively, suggesting low to moderate contamination with both the metals. On the other hand, the SEPI value for Zn and Cu varied from 0.53 to 0.84 and 0.22 to 0.79, respectively, which indicated low contamination with both Zn and Cu.
Combined pollution index
The value of CPI ranged from 1.32 to 1.93 with an average of 1.67 (Table 5) for surface soils of all the sites indicated that metal concentrations were above the hazard criteria and exhibit multi-element contamination by sewage irrigation and recommended for soil treatment and continuous environmental monitoring of the area.
Soil enrichment factors
It can be observed that the EF values significantly higher than 1 indicate an origin of heavy metals and may not come from the local soil background but other natural and/or anthropogenic sources in urbanized areas, including sewage irrigation, vehicle emissions, industrial discharges, and other activities (Thornton 1991).
Values of EFs are presented in Table 6. Generally, with increasing EF values, the contributions of the anthropogenic origins also increase. The results of EF indicated that the sewage-irrigated soils of the study area were highly enriched with metals such as Cd which were observed to show extremely high enrichment, and Pb exhibits significant to very high enrichment, while Zn was observed to show significant enrichment. However, Cu and Ni in the sewage-irrigated soils showed minimal to significant and minimal to moderate enrichment, respectively.
Heavy metals concentration in plants
The use of untreated sewage water in agricultural soils may give rise to the accumulation of metals to toxic levels in the top soil and hence in the crops grown on it. In order to check the flux of heavy metals, different crops grown on sewage-irrigated soils were analyzed for metals contents.
The distribution of five metals in crops collected from various sites in the peri-urban areas of Kolkata was estimated and presented in Table 7. Heavy metals content of the different crops showed significant differences.
Results showed that heavy metals were accumulated by the crops irrigated with sewage water on the bank of the stream but their accumulation was not to the same extent for all the crops. Few crops accumulated specific heavy metals at higher amount than the others. Accumulations of specific heavy metals for different crops were averaged and maximum accumulations of Zn, Cu, Pb, and Cd were reported in Colocasia which were 39.3, 21.3, 91.9, and 16.8 μg g−1 for Zn, Cu, Pb, and Cd, respectively, and maximum Ni accumulation was in Amaranthus (26.6 μg g−1) (Table 7).
Therefore, it’s appeared that Colocasia and Amaranthus accumulate highest metal on the basis of overall metal uptake. This is corroborated with the findings of the previous studies showing increased levels of heavy metals in edible food crops grown in sewage-irrigated soils (Jagtap et al. 2010; Kharche et al. 2011; Liu et al. 2005; Singh and Kumar 2006). Higher standard deviations in metal accumulation could be due to breed variety of plants as well as soil types.
The comparison of the mean heavy metals concentrations (Zn, Cu, Pb, Cd, and Ni) in different crops with the permissible limit of Prevention of Food Adulteration (PFA) Act 1954 (Awashthi 2000), FAO/WHO Standard (1984), and SEPA (2005) showed that the concentrations of heavy metals such as Pb, Cd, and Ni were above the permissible limit in all the examined crops generally grown in these areas (Table 7). Concentrations of Cu were within the safe limit of FAO/WHO and Indian Standard (PFA Act, 1954) in all the crops except for Colocasia as suggested by SEPA (2005). The mean maximum concentration of Pb was found in Colocasia (91.9 mg kg−1) followed by Amaranthus (84.8 mg/kg), which exceeded the maximum limit in India for Pb by 36.8 and 33.92 times, respectively. Mean concentration of Pb in all the crops (66.3–91.9 mg kg−1) was several times higher than the values earlier reported in Titagarh, West Bengal, India (21.59–57.63 mg kg−1) (Gupta et al. 2008) and in vegetables grown in and around industrial areas of Dhaka, Bangladesh (12.0–22.1 mg kg−1) (Ahmad and Goni 2010) but it was significantly lower than the mean concentration of Pb (409 mg kg−1) reported in vegetables from Turkey by Turkdogan et al. (2002).
The highest concentration of Cd was found in Colocasia (16.8 mg kg−1) followed by Sesame (13.5 mg kg−1) and Amaranthus (13.3 mg kg−1) which exceeded the Indian Standard (Awashthi 2000) by 11.2, 9.0, and 8.9 times, respectively. The present investigation showed that the mean Cd level (11.6–16.8 mg kg−1) measured in different crops from sewage-irrigated areas of Kolkata was more or less similar with the vegetables from waste water-irrigated areas of Titagarh, West Bengal, India (10.37–17.79 mg kg−1) (Gupta et al. 2008); Solapur, Maharashtra (4.1–24.46 mg kg−1) (Jagtap et al. 2010) and the vegetables from endemic upper gastrointestinal cancer region of Turkey (25 mg kg−1) (Turkdogan et al. 2002) but higher than the vegetables from sewage-irrigated areas of Delhi, India (1.1–7.1 mg kg−1) (Singh and Kumar 2006); Varanasi, India (0.5–4.36 mg kg−1) (Sharma et al. 2007); and significantly more than the vegetables from Egypt (0.002–0.08 mg kg−1) (Dogheim et al. 2004) and China (0.03–0.73 mg kg−1) (Liu et al. 2005).
The mean maximum Ni content in leafy vegetable Amaranthus (26.6 mg kg−1) was 17.7–1.3 and 2.7 fold higher than the recommended safe limit of PFA Act of India (Awashthi 2000), FAO/WHO standard (Codex Alimentarious Commission 1984), and of China (SEPA 2005), respectively. The mean concentrations of Ni in different crops varied from 13.4 to 26.6 mg kg−1 which were much lower than the data reported for vegetables in Titagarh, West Bengal, India (42.79 to 62.7 mg kg−1) by Gupta et al. (2008). However, it was more or less similar to the findings (8.78–21.5 mg kg−1) of Rattan et al. (2005) in Delhi, India but much higher than that of the findings (1.81–7.57 mg kg−1) of Sharma et al. (2007) in Varanasi, India. The exhibition of spatial variations in concentrations of all the heavy metals may be ascribed to the variations in their sources and the quantity of heavy metals discharged through the effluents and sewage in irrigating water of the study area (Table 8).
Order of preference to the metals for plants prepared on the basis of metal accumulation values was presented in Table 9. Among the five metals under study, Pb was the most preferred metal followed by Zn and Ni, whereas Cd and Cu were appeared to be the least preferred metals for all the plants except for Colocasia where the order of metal accumulation was as follows: Pb > Zn > Cu > Ni > Cd.
Transfer of metals from soil to plants
Table 10 gives the ranges as well as the means values of TCs of Zn, Cu, Pb, Cd, and Ni calculated for each crops. The highest mean TC values for Zn, Cu, Pb, Cd, and Ni were found for cauliflower (0.59), Colocasia (0.67), Amaranthus (0.93), Colocasia (1.02) and Amaranthus (1.09), respectively. It is also observed that the toxic heavy metals like Pb and Cd have higher TC in comparison to other metals for almost all plants. On the other hand, transfer coefficient for Zn and Cu is low compared to other elements in all the crops. Among the different crops under study, leafy vegetables (e.g., poi, Amaranthus, Colocasia, and cowpea) showed higher TCs as compared to other plants for Pb and Cd. The bioaccumulation factors for heavy metals were significantly higher for leafy than non-leafy vegetables were also found by Zhuang et al. 2009 in the food crops in the vicinity of a metal mine. The TC value of Cd was found quite high for crops like Amaranthus (1.16), radish (0.78), and cauliflower (0.19) that were observed in the agricultural land at Ruppur area of Pabna District of Bangladesh (Jolly et al. 2013).
Conclusions
Except for Zn and Cu, all the crops are severely contaminated with Pb and Cd as the level is far greater than the safe limits recommended by Prevention of Food Adulteration Act of India (1954), FAO/WHO standard and SEPA, 2005 and should not be consumed at all. However, the mean Ni accumulated value was above the permissible limit of Indian Standard (PFA Act of 1954) and SEPA, 2005 in all the crops but below the safe limit as described by FAO/WHO standard in the crops (viz. chilli, okra, jute, poi, and cauliflower). The results, therefore, revealed that the crops grown on sewage-irrigated soils were generally contaminated with toxic heavy metals, posing a potential health concern due to their entry into the food chain which can cause different diseases like brain and kidney damage and cancer in the human body. Continuous monitoring of transfer of these heavy metals from sewage to soils and plants is therefore essential to prevent food chain contamination.
So, the sewage water contaminated with toxic heavy metals should not to be directly used in the agriculture field. A prior treatment through sewage treatment plant is very essential. The treated sewage water then might be a good source of irrigation. Otherwise, long-term use of untreated sewage water might cause an entry of toxic heavy metals into the food chain.
References
Ahmad, J. U., & Goni, M. A. (2010). Heavy metal contamination in water, soil, and vegetables of the industrial areas in Dhaka, Bangladesh. Environmental Monitoring and Assessment, 166, 347–357.
Alloway, B. J., & Ayres, D. C. (1997). Chemical principles of environmental pollution (2nd ed.). London: Blackie Academic and Professional.
APHA. (1995). Standards methods for the examination of water and wastewater. American Public Health Association (19th ed.). New York: Water Pollution Control Federation.
Awashthi, S. K. (2000). Prevention of Food Adulteration Act No. 37 of 1954. Central and State rules as amended for 1999 (3rd ed.). New Delhi: Ashoka Law House.
Bunting, S.W., Kundu, N., Punch, S. and Little, D.C. (2001). “Workshop Proceedings: The East Kolkata Wetlands and Livelihoods”. Online link (http://www.dfid.stir.ac.uk/dfid/nrsp/kolkata.htm).
Chen, T. B., Zheng, Y. M., Lei, M., Huang, Z. C. H., Wu, T., Chen, H., Fan, K. K., Yu, K., Wu, X., & Tian, Q. Z. (2005). Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere, 60(4), 542–551.
Commission, C. A. (1984). Contaminants, Joint FAO/WHO Food standards Program (Vol. XVII (1st ed.). Geneva: Codex Alimentarious.
Deely, J. M., & Fergusson, J. E. (1994). Heavy metal and organic matter concentration and distributions in dated sediments of a small estuary adjacent to a small urban area. The Science of the Total Environment, 153(1–2), 97–111.
Department of Environment. (1989). The Use of Sewage Sludge in Agriculture. London: A National Code of Practice. HMSO.
Dogheim, S. M., Ashraf, E. M. M., Alla, S. A. G., Khorshid, M. A., & Fahmy, S. M. (2004). Pesticides and heavy metal levels in Egyptian leafy vegetables and some aromatic medicinal plants. Food Additives and Contaminants, 21, 323–330.
FAO. (1985). Water Quality for Agriculture. In R. S. Ayers & D. W. Westcot (Eds.), Irrigation and Drainage Paper 29 Rev. 1 (p. 174). Rome: FAO.
Gupta, N., Khan, D. K., & Santra, S. C. (2008). An assessment of heavy metal contamination in vegetables grown in wastewater-irrigated areas of Titagarh, West Bengal, India. Bulletin of Environmental Contamination and Toxicology, 80, 115–118.
Jagtap, N. M., Kulkarni, M. V., & Puranik, P. R. (2010). Flux of heavy metals in soils irrigated with urban waste waters. American-Eurasian Journal of Agricultural and Environmental Science, 8(5), 487–493.
Jamali, M. K., Kazi, T. G., Arain, M. B., Afridi, H. I., Jalbani, N., Memon, A. R., & Shah, A. (2007). Heavy metals from soil and domestic sewage sludge and their transfer to sorghum plants. Environmetal Chemistry Letter, 5, 209–218.
Jolly, Y. N., Islam, A., & Akbar, S. (2013). Transfer of metals from soil to vegetables and possible health risk assessment. SpringerPlus, 385(2), 1–8.
Jung, M. C. (2001). Heavy metal contamination of soils and waters in and around the Incheon Au-Ag Mine, Korea. Applied Geochemistry, 16(11), 1369–1375.
Kabata, P., & Pendias, H. (1992). Trace Elements in Soils and Plants. Florida: CRC Press Inc. Boca Raton. 365p.
Kachenko, A. G., & Singh, B. (2006). Heavy metal contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water, Air, and Soil Pollution, 169, 101–123.
Kaur, R., Wani, S.P., Singh, A.K. and Lal, K. (2012). Wastewater Production, Treatment and Use in India. National Report presented at the 2nd regional workshop on Safe Use of Wastewater in Agriculture, May 16-18, 2012, New Delhi, India (http://www.ais.unwater.org).
Kharche, V. K., Desai, V. N., & Pharande, A. L. (2011). Effect of sewage irrigation on soil properties, essential nutrient and pollutant element status of soil and plant in a vegetable growing area around Ahmednagar city in Maharashtra. Journal of the Indian Society of Soil Science, 59(2), 177–184.
Lee, C. H. (2003). Assessment of contamination load on water, soil and sediment affected by the Kongjujeil Mine Drai- nage, Republic of Korea. Environmental Geology, 44(5), 501–515.
Liu, W. H., Zhao, J. Z., Ouyang, Z. Y., Soderlund, L., & Liu, G. H. (2005). Impacts of sewage irrigation on heavy metals distribution and contamination. Environment International, 31, 805–812.
Nelson, D. W., & Sommers, L. E. (1982). Total carbon, organic carbon, and organic matter (p. 539–579. In: Page, A. L., Miller, R. H. and Keeney, D. R. (eds.). Methods of soil analysis. Part 2. 2nd ed. Agronomy Monograph. 9). Madison: ASA and SSSA.
Obaidy, A. H. M. J. A., & Mashhadi, A. A. M. A. (2013). Heavy metal contaminations in urban soil within Baghdad City, Iraq. Journal of Environmental Protection, 4, 72–82.
Oliveira, A. S., Bocio, A., Trevilato, T. M. B., Takayanagui, A. M. M., Domingo, J. L., & Segura-MunoZ, S. I. (2007). Heavy metals in untreated/treated urban effluent and sludge from a biological wastewater treatment plant. Environmental Science and Pollution Research, 14, 483–489.
Page, A. L., Miller, R. H., & Keeney, D. R. (Eds.). (1982). Methods of Soil Analysis, Part-2 (2nd ed.). Wisconsin: Soil Science Society of America Journal. Madison.
Papadopoulos, F., Parissopoulos, G., Papadopoulos, A., Zdragas, A., Ntanos, D., Prochaska, C., & Metaxa, I. (2009). Assessment of reclaimed municipal wastewater application on rice cultivation. Environmental Management, 43, 135–143.
Rattan, R. K., Datta, S. P., Chhonkar, P. K., Suribabu, K., & Singh, A. K. (2005). Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater—a case study. Agriculture Ecosystems and Environment, 109, 310–322.
Richard, L. A. (1954). Diagnosis and improvement of saline and alkaline soils. Handbook No. 60, Washington, D.C.
SEPA. (2005). The limits of pollutants in food. China: State Environmental Protection Administration. GB2762-2005.
Sharma, R. K., Agrawal, M., & Marshall, F. (2007). Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicology and Environmental Safety, 66, 258–266.
Singh, S., & Kumar, M. (2006). Heavy metal load of soil, water and vegetables in peri-urban Delhi. Environmental Monitoring and Assessment, 120, 79–91.
Sutherland, R. A. (2000). Bed sediment-associated trace metals in an urban stream, Oahu, Hawaii. Environmental Geology, 39(6), 611–627.
Taylor, S. R. (1964). The abundance of chemical elements in the continental crust- a new table. Geochimica Cosmochimica Acta, 28, 1273–1285.
Thornton, I. (1991). Metal Contamination of Soils in Urban Areas, In: P. Bullock and P. J. Gregory, Eds., Soils in the Urban Environment, Blackwell Publishing.
Tippie, V. K. (1984). An environmental characterization of chesapeake bay and a frame work for action. In V. Kennedy (Ed.), The Estuary as a Filter. New York: Academic.
Turkdogan, M. K., Killicel, F., Kara, K., Tuncer, I., & Uygan, I. (2002). Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environmental Toxicology and Pharmacology, 13, 175–179.
United States Salinity Laboratory Staff. (1954). Diagnosis and Improvement of Saline and Alkali Soils, Agriculture Handbook No. 60, U.S. Washington, D.C: Department of Agriculture.
Yadav, A., Yadav, P. K., & Shukla, D. N. (2013). Investigation of heavy metal status in soil and vegetables grown in urban area of Allahabad, Uttar Pradesh, India. International Journal of Scientific and Research Publications, 3(9), 1–7.
Zhuang, P., McBride, M. B., Xia, H., Li, N., & Li, Z. (2009). Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Science of the Total Environment, 407, 1551–1561.
Zoller, W. H., Gladney, E. S., & Duce, R. A. (1974). Atmospheric concentrations and sources of trace metals at the South Pole. Science, 183(4121), 198–200.
Acknowledgments
The authors wish to acknowledge the financial assistance of the All India Co-ordinated Research Project on “Micro- and Secondary Nutrients and Pollutant Elements in Soils and Plants” funded by Indian Council of Agricultural Research, Govt. of India for carrying out this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saha, S., Hazra, G.C., Saha, B. et al. Assessment of heavy metals contamination in different crops grown in long-term sewage-irrigated areas of Kolkata, West Bengal, India. Environ Monit Assess 187, 4087 (2015). https://doi.org/10.1007/s10661-014-4087-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4087-9