Abstract
Concentrations of six heavy metals (Cu, Ni, Zn, Cd, Cr, and Pb) in sediments and fine roots, thick roots, branches, and leaves of six mangrove plant species collected from the Futian mangrove forest, South China were measured. The results show that both the sediments and plants in Futian mangrove ecosystem are moderately contaminated by heavy metals, with the main contaminants being Zn and Cu. All investigated metals showed very similar distribution patterns in the sediments, implying that they had the same anthropogenic source(s). High accumulations of the heavy metals were observed in the root tissues, especially the fine roots, and much lower concentrations in the other organs. This indicates that the roots strongly immobilize the heavy metals and (hence) that mangrove plants possess mechanisms that limit the upward transport of heavy metals and exclude them from sensitive tissues. The growth performance of propagules and 6-month-old seedlings of Bruguiera gymnorhiza in the presence of contaminating Cu and Cd was also examined. The results show that this plant is not sufficiently sensitive to heavy metals after its propagule stage for its regeneration and growth to be significantly affected by heavy metal contamination in the Futian mangrove ecosystem. However, older mangrove seedlings appeared to be more metal-tolerant than the younger seedlings due to their more efficient exclusion mechanism. Thus, the effects of metal contamination on young seedlings should be assessed when evaluating the risks posed by heavy metals in an ecosystem.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove plants comprise a group of intertidal plants that dominate the coastlines of many tropical and subtropical regions. These plants are highly productive and play a vital role as the major primary producers in estuarine ecosystems (Macfarlane et al. 2007). Mangrove ecosystems provide great ecological and commercial services (e.g., Das and Vincent 2009), but are often affected by contaminants in effluent discharges, urban and agricultural runoff, and dumped solid wastes due to their frequent proximity to dense human populations and inadequate protection (Polidoro et al. 2010). Some of the most potentially serious anthropogenic pollutants in mangrove ecosystems from these sources are heavy metals. Although mangrove sediments may act as sinks or buffers that immobilize heavy metals entering aquatic ecosystems, they can also act as sources of heavy metals that may be released by changes in abiotic conditions. In addition, heavy metals may be accumulated by mangrove plants and concentrated in exported dead plant materials, which are important food sources for higher organisms in estuarine food chains (Harbison 1986).
Elevated concentrations of heavy metals have been recorded in mangrove sediments from many parts of the world (Marchand et al. 2006). Several studies have also reported high concentrations of accumulated metals in tissues of mangrove species in polluted localities (e.g., Agoramoorthy et al. 2008), and some mangrove species have been shown to have relatively high tolerance to heavy metal pollution, compared to most plants (Macfarlane et al. 2007; Yan et al. 2010). However, excessive uptake of metals by mangroves may cause reductions in biomass (Yan et al. 2010) and damage at the cellular level (see Moura et al. 2012). Furthermore, heavy metal cycling is a potentially serious problem in mangrove environments (Marchand et al. 2006; Pekey 2006) since excessive transfer of heavy metals from sediments to mangrove plants may lead to contamination of the food pathway (Ahmed et al. 2011).
In addition to the background levels of heavy metals and the plant species, the metal sensitivity of plants may also be influenced by their age. Notably, germinating seeds and young seedlings are thought to generally be more sensitive to metal pollution than mature plants because some of their defense mechanisms have not yet fully developed (Liu et al. 2005). Therefore, it is important to consider the age of the plants used in toxicity assessments. Surprisingly, however, most previous investigations of the physiological basis of heavy metal resistance have considered plant material at a single age.
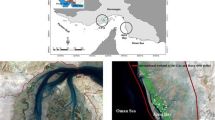
Futian mangrove forest is situated within a National Nature Reserve in Shenzhen Special Economic Zone, China, adjacent to the Mai Po Nature Reserve of Hong Kong (Fig. 1). Together with the Mai Po marshes, this area was declared a wetland of international importance under the Ramsar Convention (Tam et al. 1995). Both the mangroves and the associated mudflats are important feeding, nesting, and staging grounds for some 67,000 birds belonging to 270 species, including some rare and endangered species, such as Platalea minor, Tringa guttifer, and Larus saundersi (Young and Melville 1993). It is the only mangrove forest located in the heart of a major urban area in China having experienced a major economic and industrial boom since the early 1990s. Thus, there are serious concerns about the impact of pollution, especially metal contamination, on this Ramsar site. Levels of Cu, Mn, Zn Cd, Cr, and Pb in the sediments and leaf tissues of Kandelia candel (L.) Druce and Aegiceras corniculatum (L.) Blanco collected from this site in the 1990s have been reported (Tam et al. 1995), but there is insufficient information to determine the extent of recent heavy metal contamination.
Sketch map showing a the locality of the sampling area (the Futian Mangrove ecosystem) and b the sampling design for the field experiment (filled circles sampling point, BG B. gymnorhiza community, AI A. ilicifolius community, KC K. candel community, AM A. marina community, AC A. corniculatum community, SA S. apetala community)
Therefore, in this study, we first investigated the concentrations of six heavy metals—three essential metals (Cu, Ni, and Zn) and three nonessential metals (Cd, Cr, and Pb)—in the sediments and various parts (fine roots, thick roots, branches, and leaves) of six adult mangrove species to assess the extent of contamination by these metals and their accumulation and distribution patterns in mangrove plants. To further assess the risks posed by heavy metal contamination to this mangrove ecosystem, we then evaluated the heavy metal tolerance of propagules and 6-month-old seedlings of Bruguiera gymnorhiza (L.) Lam by observing their growth performance and patterns of metal accumulation and distribution in the presence of contaminating levels of an essential heavy metal (Cu) and a nonessential heavy metal (Cd) in the greenhouse. We hypothesized that (1) there would be high degrees of heavy metal contamination in the Futian mangrove ecosystem; (2) both propagules and seedlings of B. gymnorhiza would have relatively high heavy metal (Cu and Cd) tolerance; and (3) relatively old (6-month-old) seedlings would have higher tolerance of heavy metal exposure than the propagules.
Material and methods
Collection of sediment and plant samples
Mangrove plants are segregated in distinctive bands of species in the Futian mangrove ecosystem (Wang et al. 2010; Fig. 1), with a horizontal zonation pattern, landward to seaward of B. gymnorhiza, Acanthus ilicifolius Linn., K. candel, Avicennia marina (Forsk.) Vierh, A. corniculatum, and Sonneratia caseolaris (L.) Engler. In March 2009, samples of absorbing roots, branches, and leaves of four representative adult plants of each of these six mangrove species were collected, together with sediment samples from 0 to 5 cm and from 10 to 20 cm depths from approximately 5 × 5 cm plots close to each sampled plant. The sediment and plant samples were separately stored in clean, acid-washed plastic containers and plastic sample bags until transportation to the laboratory.
Soil property analysis
Sediment samples were air-dried at room temperature and ground with an agate pestle and mortar. Soil properties, including the organic matter content, total and available N, P, and K contents, pH, and electrical conductivity were measured based on the methods described by Wang et al. (2010).
Analysis of heavy metals
Each absorbing root sample was separated into two subsamples: fine root (consisting of the root tip and the fine secondary absorbing root within 5 cm of it) and thick root (further from the tip). All plant samples (fine root, thick root, branch, and leaf) were thoroughly washed, oven-dried at 70 °C for 48 h, and ground to pass through a 100-mesh sieve. Each sample was then digested with a 6.5-ml mixture of HNO3–H2O2 (10:3 v/v) in a microwave oven (Arao et al. 2003). After digestion, samples were allowed to cool at room temperature, diluted to a volume of 10 ml with Milli-Q water, and subjected to heavy metals analysis by flame atomic absorption spectrophotometry. The detection limits for the Cu, Ni, Zn, Cd, Cr, and Pb analyses were 3.0, 2.0, 30, 0.5, 2.0, and 5.0 μg L−1, respectively. The precision of the analytical procedures for plant material was assessed using a certified reference material (GBW-07603, provided by the National Research Center for Certified Reference Material, China), and the percentage recoveries for all the analyzed heavy metals were in the range of 94.5–105.0 %. Total concentrations of heavy metals (Cu, Ni, Zn, Cd, Cr, and Pb) and their diethylenetriaminepentaacetic acid (DTPA)-extractable fractions (which were used as a measure of the bioavailable parts) in soil were determined by flame atomic absorption spectrophotometry, following mixed-acid digestion (HNO3–HClO4–HF) and DTPA extraction, respectively (Page et al. 1982).
Greenhouse experiment
Plant materials
B. gymnorhiza was chosen for the greenhouse experiment because of its widespread distribution in China and the nearly year-round availability of its mature propagules. Mature B. gymnorhiza propagules were separately collected in May and December 2009, and healthy specimens with no emergent hypocotyls were selected for planting. Propagules collected in May were first planted in wet sand in a greenhouse environment with daily temperatures of 25–30 °C. They were watered daily with tap water, and 50 ml Hoagland solution was added biweekly per plant after germination. In December 2009, 20 newly collected mature propagules (similar in height and weight) and 20 individual 6-month-old seedlings that were similar in apparent health, height, and leaf numbers were chosen for transplantation to pots to assess their heavy metal tolerance, accumulation, and distribution patterns, as described in the succeeding sections.
Soil preparation and experimental design
The soil used in the experiment was collected from a mangrove swamp in Zhuhai, Guangdong Province, China, air-dried, and then ground with a wooden roller to pass through a 5-mm sieve. The electronic conductivity, pH, and organic matter content of this soil were 2.65 dS m−1, 7.33, and 2.38 %, respectively, and the concentrations of total N, P, and K and available N, P, and K were 1.32, 0.87, and 65 g kg−1 and 118, 11.8, and 625 mg kg−1, respectively. The total Cd and Cu concentrations were 0.26 and 34.8 mg kg−1, respectively. After analyzing the soil, portions were prepared for jointly applying two Cd treatments (designated Cd1 and Cd2; final Cd concentrations of 0.91 and 3.22 mg kg−1 dry weight soil, respectively) and two Cu treatments (Cu1 and Cu2; final Cu concentrations of 96 and 183 mg kg-1, respectively) by adding solutions of CdCl2 and Cu(NO3)2 to the ground and dried soil approximately 1 year before the experiment. The prepared soil without heavy metal additions was used as the control (CK) growth medium.
Portions (3 kg) of each kind of prepared soil were then placed into pots (height, 24 cm; diameter, 18 cm at the top and 13 cm at the bottom) in a greenhouse at Sun Yat-sen University with air temperatures ranging from 25 to 30 °C, and compound fertilizer (N/P/K = 15:15:15, 3 g pot−1) was applied as basal fertilizer and allowed to equilibrate for 2 weeks. The propagules and seedlings of B. gymnorhiza were transplanted to the pots in December 2009. They were regularly watered to maintain high soil moisture and supplemented with Hoagland’s solution biweekly at a rate of 50 ml pot−1. The experiment had a randomized complete block design with two treatment factors: metal levels (CK, Cd1Cu1, Cd1Cu2, Cd2Cu1, and Cd2Cu2) and ages of plant (propagule and 6-month-old seedling). Each treatment was applied to four pots.
Harvest and measurements
All plants were harvested after 3 months of growth and washed with deionized water. Growth parameters including seedling height (excluding propagules) and diameter at ground height were measured immediately after harvest. The second leaf pair from each seedling was taken for determinations of chlorophyll a and chlorophyll b, using the method described by Inskeep and Bloom (1985). All plants were divided into leaf, branch (stem), thick root, and fine root, then oven-dried at 70 °C for 72 h, weighed, and ground. The concentrations of Cu, Cd, P, and K in all of the plant parts were determined (excluding the P and K concentrations in the fine root because of limited biomass in this part) according to the methods described above.
Statistical analysis
The mean and standard error (SE) of the metal concentrations of all sample types were calculated. Correlation analysis was then applied to evaluate the relationships between total and DTPA-extractable fractions of the heavy metals in the sediments. Differences among metal concentrations in sediments from different locations and plant parts (fine roots, thick roots, branches, and leaves) from different mangrove species were tested using one-way analysis of variance (ANOVA). To compare the ability of the fine roots, thick roots, branches, and leaves of different mangrove species to accumulate the heavy metals (Cu, Ni, Zn, Cd, Cr, and Pb), concentration factors were calculated by dividing the concentration of each metal in each plant part by the concentration of the respective metals in the sediment (means of concentrations detected at 0–5 and 10–20 cm depths) (Alberts et al. 1990). Two-way ANOVA (using generalized linear models) was also applied to analyze the effects of Cd and Cu treatments and their interactions on the plants’ growth parameters, nutrient levels, and heavy metal accumulations when there were statistically significant differences among different treatment groups assessed by one-way ANOVA. All statistical analyses were performed using SPSS Base 16.0 (SPSS Inc., USA).
Results
Biological and chemical properties of the sediments
As shown in Online Resource 1 (Table S1), all the sediment samples had acid to neutral pH, varying from 4.78 to 7.42. There were relatively high organic matter, N, P, and K levels in the sediment, especially available N, P, and K concentrations (Table S1), presumably due to discharge of domestic sewage from surrounding regions. The salinity (electrical conductivity) of the sediment from different intertidal zones varied substantially, ranging from 2.38 to 21.74 dS m−1.
Heavy metal concentrations in the sediments
There were no significant differences in either total or DTPA-extractable fractions of any of the investigated heavy metals (Cu, Ni, Zn, Cd, Cr, and Pb) between 0–5 and 10–20 cm sediment layers (P > 0.05; Fig. 2). However, there were significant differences in concentrations of all of the heavy metals in both layers from different locations (P < 0.01), except for the total concentration of Cd in the 0–5 cm sediment layer. The mean metal concentrations were found to decrease in the order Zn > Pb ≈ Cu > Ni > Cr > Cd (Fig. 2). The correlation analyses also showed that concentrations of all the heavy metals were significantly positively correlated with each other (P < 0.01), except for Cd and Cr (P > 0.05). In addition, total concentrations and DTPA-extractable fractions of all of the heavy metals, except Cr, were positively correlated (P < 0.01) in both sediment layers (Table 1). The proportions of DTPA-extractable metals (except Cr) were relatively high and significantly related to each other in the 5–10 cm layer sediments (P < 0.05).
Concentrations of heavy metals (Cu, Zn, Ni, Pb, Cd, and Cr) in sediments, at two depths, through the vegetational zones of the Futian mangrove ecosystem (mean values of four replicates; B. g B. gymnorhiza zone, A. i A. ilicifolius zone, K. c K. candel zone, A. c A. corniculatum zone, A. m A. marina zone, S. a S. apetala zone)
Heavy metal concentrations in the sampled plant parts
The concentrations of all the heavy metals were substantially higher in the fine roots than in all of the other sampled organs (Table 2). Thus, limited amounts of the metals were transferred to the branches and leaves, especially of Ni and Zn, for both of which the concentration factors in the leaves were very low. Except for the concentration factors of Pb and Cd in the fine root and thick root parts, there were statistically significant differences in the concentration factors of all plant parts among different plant species (P < 0.05), implying that there were differences in the ability of heavy metal absorption and transportation among these mangrove species. Correlation analyses showed that the concentration factors for the six heavy metals in the fine root, thick root, branch, and leaf were mostly negatively correlated with the concentrations of the respective heavy metals in the sediment (P < 0.01).
Effects of Cu and Cd contamination on the growth parameters and nutrient status of B. gymnorhiza
For both 3-month-old seedlings (planted as propagules) and 9-month-old seedlings (transplanted as 6-month-old seedlings), the Cd treatments, Cu treatments, and their interactions all had no significant effects on the tested growth and nutrient parameters of the plants (see Online Resource 1, Figs. S1, S2, S3, S4, and S5), including leaf, branch, and root biomasses and P and K levels, plant height, and diameter at ground height (P > 0.05), except that the Cu1 treatment increased the root K level in the 3-month-old seedlings (P < 0.01). For the 9-month-old seedlings, the Cd and Cu treatments had no significant effects on the chlorophyll a, chlorophyll b, total chlorophyll contents, or the chlorophyll a/b ratio, while for the 3-month-old seedlings, although the Cd and Cu treatments had no significant effects on the chlorophyll b and total chlorophyll contents (Table 3), the high Cd treatment weakly decreased the leaf chlorophyll a content (P = 0.042) and the ratio of chlorophyll a/b (P = 0.050).
Concentrations of Cu and Cd in sampled plant parts in the greenhouse experiment
The concentrations of Cu and Cd in both the 3- and 9-month-old seedlings clearly declined in the order fine root > thick root > branch ≈ leaf (Figs. 3 and 4). There were generally no significant differences in Cu concentrations between the fine roots, thick roots, branches, and leaves of 3-month-old seedlings and the corresponding parts of 9-month-old seedlings under any of the treatments (Fig. 3). Also, there were generally no significant differences in Cd concentrations between corresponding parts of the 3- and 9-month-old seedlings under the CK treatment (Fig. 4). However, under the Cd1 and Cd2 treatments, there were distinctly higher Cd concentrations in the 3-month-old seedlings than in most corresponding parts of the 9-month-old seedlings (P < 0.01).
a–d Concentrations of Cu in sampled parts of the 3-month-old (white bars) and 9-month-old (black bars) seedlings of B. gymnorhiza (mean ± SE, n = 4; ns differences in Cu concentrations between the 3- and 9-month-old seedlings were not significant; *P = 0.05, differences in Cu concentrations between the 3- and 9-month-old seedlings were significant; **P = 0.01, differences in Cu concentrations between the 3- and 9-month-old seedlings were significant)
a–d Concentrations of Cd in sampled parts of the 3-month-old (white bars) and 9-month-old (black bars) seedlings of B. gymnorhiza (mean ± SE, n = 4; ns differences in Cd concentrations between the 3- and 9-month-old seedlings were not significant; *P = 0.05, differences in Cd concentrations between the 3- and 9-month-old seedlings were significant; **P = 0.01, differences in Cd concentrations between the 3- and 9-month-old seedlings were significant)
Discussion
Our results indicate that current levels of Cu, Zn, and Pb in sediments of the Futian mangrove ecosystem are similar to those presented approximately 20 years ago by Tam et al. (1995), but there are markedly lower levels of Cd and Cr. Since heavy metals are highly stable, the apparent reductions in Cd and Cr concentrations are presumably mainly due to absorption by mangrove plants and/or the export of these heavy metals to the sea, while the similarity of the Cu, Zn, and Pb contamination levels implies that inputs of these heavy metals into the sediments have roughly balanced the outputs. The similarity of distribution patterns of all of the investigated metals in the sediments and the relatively high proportions of the DTPA-extractable fractions imply that these heavy metals have the same anthropogenic source(s) and the wastewater discharge from the nearby electroplate factories and electronic instrument factories could be important pollution sources. This is supported by the observed outlets of wastewater from these factories ultimately leading to this mangrove ecosystem.
So far, heavy metal contaminants in the mangrove sediments have been reported in many areas (see Table 4). Comparing the results of this study with those done in other areas of China and in the world, the Futian mangrove sediments seem to have relatively low Cd and Cr, moderate Pb and Ni, and high Cu and Zn concentrations. Heavy metal contaminations in mangrove plants have also been widely investigated. Zheng et al. (1997) reported the heavy metal concentrations in different parts of Rhizophora stylosa located in Yingluo Bay, China. Compared to those in the Futian mangrove ecosystems, clearly lower Zn, Cu, and Pb and similar Cd, Cr, and Ni concentrations in all plant parts were found in the Yingluo Bay. Ong Che (1999) reported the heavy metal contamination levels in the roots of three mangrove species from the Mai Po Nature Reserve in Hong Kong. Higher Zn (44.0–324.8 mg kg−1) and Pb (20.0–60.0 mg kg−1), similar Cu (19.6–65.4 mg kg−1), Cd (0.05–0.6 mg kg−1), and Ni (4.0–32.0 mg kg−1), and lower Cr (1.6–6.4 mg kg−1) concentrations were observed in the Mai Po mangrove ecosystem in comparison to those in the Futian mangrove ecosystem. MacFarlane et al. (2007) reviewed the Cu, Zn, and Pb concentrations in the roots and leaves of 19 mangrove species, based on published literature. Comparing the results of this study with those listed in their report, there were relatively high Cu and Zn and moderate Pb levels in the plant parts from Futian mangrove ecosystem. In general, both the sediments and plants in Futian mangrove ecosystem seem to have been moderately contaminated. Thus, the first hypothesis of this study, that there would be relatively high degrees of heavy metal contamination in the Futian mangrove ecosystem, is not supported.
Depending on the plant species, metal tolerance may be based on one of two basic strategies: metal exclusion or metal accumulation (Dahmani-Muller et al. 2000). The exclusion strategy, comprising avoidance of metal uptake and restriction of metal transport to the shoot, is usually adopted by pseudometallophytes (Dahmani-Muller et al. 2000). In this study, the heavy metal distributions observed in mangrove plants in both the field investigation and greenhouse experiment clearly indicated that these mangrove species adopt this strategy. High accumulations of the heavy metals were observed in the root tissues, especially in the fine roots, and much lower levels in other plant parts, indicating that the roots strongly immobilize the metals and (hence) that mangrove plants have evolved mechanisms that limit the upward transport of heavy metals and exclude them from sensitive tissues. This is consistent with previous studies showing that restriction of upward movement is a common metal tolerance strategy among wetland plants, with clear protective value since movement of metal ions into photosynthetic tissue can induce severe stress in plants (Deng et al. 2004; Macfarlane et al. 2007; Liu et al. 2009). Mangrove species could be used in constructed wetlands for wastewater treatment (Yang et al. 2008; Zhang et al. 2010), since this low transportation trait should facilitate their use for removing heavy metals from wastewater or remediating heavy metal-polluted marine environments, as it could inhibit the release of metals from the plant to the surrounding environment.
Although the retention of metals in fine roots could facilitate the heavy metal tolerance of plants, the high concentration of heavy metals retained in those roots of mangrove plants might inhibit or even destroy microbial symbionts in the fine roots, such as arbuscular mycorrhizal fungi, which have recently been found to be ubiquitous in most investigated mangrove plants (Wang et al. 2010, 2011) and reportedly play important roles in nutrient acquisition for mangrove plants (Wang et al. 2010). From this perspective, the heavy metal contaminants might pose a potential threat to mangrove ecosystems, especially oligotrophic mangrove forests. On the other hand, some of the mangrove macrobenthos (e.g., Mictyris brevidactylus, Crassostrea corteziensis, etc.) could accumulate much higher levels of heavy metals in their tissues than those of their environment (Frías-Espericueta et al. 2008; Yeh et al. 2009), thus posing a risk to the food chain in the mangrove ecosystem. Especially as fishery activities take place in and around the Futian mangrove ecosystem, further research is needed to study the cycling of heavy metals in the food web of this habitat.
No obvious negative effects of any of the metal treatments were observed on the growth and nutrient parameters of the 3- and 9-month-old seedlings of B. gymnorhiza. These results indicate that B. gymnorhiza develops relatively high heavy metal resistance following its propagule stage, thus supporting our second hypothesis, i.e., that both propagules and seedlings of B. gymnorhiza have relatively high tolerance of the heavy metals Cu and Cd. Several previous studies have also found that propagules and seedlings of other mangrove species have relatively high heavy metal tolerance (Macfarlane and Burchett 2001; Yan et al. 2010). Although the mechanisms involved in the metal tolerance of mangrove plants are still poorly understood (Macfarlane et al. 2007), some knowledge of structural adaptive metal tolerance strategies of mangrove seedlings has been acquired and several hypotheses have been postulated (Liu et al. 2009; Cheng et al. 2010). For example, it has been proposed that heavy metals could significantly reduce the permeability and radial loss of oxygen from roots of mangrove seedlings, thus leading to lower heavy metal accumulation in their tissues and higher tolerance (Liu et al. 2009; Cheng et al. 2010). It has also been well documented that the viviparity of many mangrove species is a typical adaptive evolutionary response to the tidal flooding environment (Lugo and Snedaker 1974), and it will be interesting to ascertain how the propagules of mangrove plant evolved their heavy metal tolerance.
Inhibition of photosynthesis by heavy metals in higher plants is well documented (see Macfarlane and Burchett 2001), and reductions in levels of chlorophyll a and/or b on exposure to heavy metals have been observed in many plant species, including mangrove plants (Basak et al. 1996; Macfarlane and Burchett 2001). The substitution of the central magnesium ion by heavy metals and/or the inhibition of enzymes involved in chlorophyll biosynthesis were reported to be the mechanisms directly concerned with the reduction of photosynthetic pigments (De Filippis and Pallaghy 1994). In this study, decreased leaf chlorophyll a contents and chlorophyll a/b ratios were observed in the 3-month-old seedlings before any visible toxicity symptoms, indicating that the leaf chlorophyll a content has potential use as a sensitive indicator of metal stress in B. gymnorhiza.
To date, the ability of seedlings of different ages to accumulate heavy metals has hardly been compared. In this study, under Cd1 and Cd2 treatments, the 3-month-old seedlings accumulated markedly higher Cd concentrations than the 9-month-old seedlings in most corresponding plant parts, especially the fine root. These findings indicate that 9-month-old B. gymnorhiza seedlings have developed more effective exclusion mechanisms than 3-month-old seedlings for avoiding uptake of Cd from soil. This, together with the decreased leaf chlorophyll a contents and chlorophyll a/b ratios in the 3-month-old seedlings, but not in the 9-month-old seedlings, strongly supports the third hypothesis of this study, that younger mangrove seedlings are more vulnerable to heavy metal contamination, because some of their defense mechanisms are not yet well developed. Similar results regarding heavy metal accumulation have been observed in Dorycnium pentaphyllum, in which physiological strategies of heavy metal tolerance reportedly differ according to the age of the plants, and the exclusion processes are more efficient at the adult stage than at the young seedling stage (Lefèvre et al. 2009). The physiology of resistance to other environmental stresses has also been found to vary according to the phenological status of plants (Munns 2005). Based on the results from this study, we propose that the effects of heavy metal contamination should be tested on young seedlings when evaluating its risks to an ecosystem.
Conclusions
Both the sediments and plants in Futian mangrove ecosystem are moderately contaminated by heavy metals, with the main contaminants being Zn and Cu. The similarity of the distributions of the investigated metals in the sediments and the relatively high proportions of DTPA-extractable fractions imply that these heavy metals have the same anthropogenic source(s). The wastewater discharge from the nearby electroplate factories and electronic instrument factories could be important pollution sources. As mangrove plants have relatively high heavy metal tolerance following the propagule stage, the observed levels of metal contaminants should have limited direct effects on their regeneration and growth. However, the high concentration of heavy metals retained in those roots of mangrove plants might inhibit or even destroy microbial symbionts in the fine roots. Specially, considering that fishery activities were frequently observed in Futian mangrove ecosystem, further research is needed to study the cycling of heavy metals in the food web of this habitat. The tolerance to heavy metals differed among mangrove plants of different ages, and the younger mangrove seedlings appeared to be more vulnerable to heavy metal contamination. Thus, the effects of heavy metal contamination should be tested on young seedlings when evaluating its risks to an ecosystem.
References
Agoramoorthy, G., Chen, F., & Hsu, M. (2008). Threat of heavy metal pollution in halophytic and mangrove plants of Tamil Nadu, India. Environmental Pollution, 155, 320–326.
Ahmed, K., Mehedi, Y., Haque, R., & Mondol, P. (2011). Heavy metal concentrations in some macrobenthic fauna of the Sundarbans mangrove forest, south west coast of Bangladesh. Environmental Monitoring and Assessment, 177, 505–514.
Alberts, J. J., Price, M. Y., & Kania, M. (1990). Metal concentrations in tissues of Spartina alterniflora (Loisel) and sediments of Georgia salt marshes. Estuarine, Coastal and Shelf Science, 30, 47–58.
Arao, T., Ae, N., Sugiyama, M., & Takahashi, M. (2003). Genotypic differences in cadmium uptake and distribution in soybeans. Plant and Soil, 251, 247–253.
Basak, U. C., Das, A. B., & Das, P. (1996). Chlorophylls, carotenoids, proteins and secondary metabolites in leaves of 14 species of mangrove. Bulletin of Marine Science, 58, 654–659.
Chatterjee, M., Massolo, S., Sarkar, S. K., Bhattacharya, A. K., Bhattacharya, B. D., Satpathy, K. K., et al. (2009). An assessment of trace element contamination in intertidal sediment cores of Sunderban mangrove wetland, India for evaluating sediment quality guidelines. Environmental Monitoring and Assessment, 150, 307–322.
Cheng, H., Liu, Y., Tam, N. F. Y., Wang, X., Li, S. Y., Chen, G. Z., et al. (2010). The role of radial oxygen loss and root anatomy on zinc uptake and tolerance in mangrove seedlings. Environmental Pollution, 158, 1189–1196.
Cuong, D. T., Bayen, S., Wurl, O., Subramanian, K., Wong, K. K. S., Sivasothi, N., et al. (2005). Heavy metal contamination in mangrove habitats of Singapore. Marine Pollution Bulletin, 50, 1713–1738.
Dahmani-Muller, H., van Oort, F., Gélie, B., & Balabane, M. (2000). Strategies of heavy metal uptake by three plant species growing near a metal smelter. Environmental Pollution, 109, 231–238.
Das, S., & Vincent, J. R. (2009). Mangroves protected villages and reduced death toll during Indian super cyclone. Proceedings of the National Academy of Sciences of the United States of America, 106, 7357–7360.
De Filippis, L. F., & Pallaghy, C. K. (1994). Heavy metals: Sources and biological effects. In L. C. Rai, J. P. Caur, & C. J. Soeder (Eds.), Algae and water pollution: Advances in limnology series (pp. 32–77). Stuttgart: Schweizerbart.
Deng, H., Ye, Z. H., & Wong, M. H. (2004). Accumulation of lead, zinc, copper and cadmium by 12 wetland plant species thriving in metal-contaminated sites in China. Environmental Pollution, 132, 29–40.
Essien, J. P., Antai, S. P., & Olajire, A. A. (2009a). Distribution, seasonal variations and ecotoxicological significance of heavy metals in sediments of Cross River Estuary mangrove swamp. Water, Air, and Soil Pollution, 197, 91–105.
Essien, J. P., Essien, V., & Olajire, A. A. (2009b). Heavy metal burdens in patches of asphyxiated swamp areas within the Qua Iboe estuary mangrove ecosystem. Environmental Research, 109, 690–696.
Frías-Espericueta, M. G., Osuna-López, J. I., López-López, G., Izaguirre-Fierro, G., & Muy-Rangel, M. D. (2008). The metal content of bivalve molluscs of a coastal lagoon of NW Mexico. Bulletin of Environmental Contamination and Toxicology, 80, 90–92.
Harbison, P. (1986). Mangrove muds: A sink and source for trace metals. Marine Pollution Bulletin, 17, 246–250.
Inskeep, W. P., & Bloom, P. R. (1985). Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80 % acetone. Plant Physiology, 77, 483–485.
Lefèvre, I., Marchal, G., Corréal, E., Zanuzzi, A., & Lutts, S. (2009). Variation in response to heavy metals during vegetative growth in Dorycnium pentaphyllum Scop. Plant Growth Regulation, 59, 1–11.
Liu, X. L., Zhang, S. Z., Shan, X. Q., & Zhu, Y. G. (2005). Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere, 61, 293–301.
Liu, Y., Tam, N. F. Y., Yang, J. X., Pi, N., Wong, M. H., & Ye, Z. H. (2009). Mixed heavy metals tolerance and radial oxygen loss in mangrove seedlings. Marine Pollution Bulletin, 58, 1843–1849.
Lugo, A., & Snedaker, S. C. (1974). The ecology of mangroves. Annual Review of Ecology and Systematics, 5, 39–64.
Macfarlane, G. R., & Burchett, M. D. (2001). Photosynthetic pigments and peroxidase activity as indicators of heavy metal stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Marine Pollution Bulletin, 42, 233–240.
Macfarlane, G. R., Koller, C. E., & Blomberg, S. P. (2007). Accumulation and partitioning of heavy metals in mangroves: A synthesis of field-based studies. Chemosphere, 69, 1454–1464.
Marchand, C., Lallier-Vergès, E., Baltzer, F., Albéric, P., Cossa, D., & Baillif, P. (2006). Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana. Marine Chemistry, 98, 1–17.
Marchand, C., Allenbach, M., & Lallier-Vergès, E. (2011). Relationships between heavy metals distribution and organic matter cycling in mangrove sediments (Conception Bay, New Caledonia). Geoderma, 160, 444–456.
Moura, D. J., Péres, V. F., Jacques, R. A., & Saffi, J. (2012). Heavy metal toxicity: Oxidative stress parameters and DNA repair. In D. K. Gupta & L. M. Sandalio (Eds.), Metal toxicity in plants: Perception, signaling and remediation (pp. 187–205). Berlin: Springer.
Munns, R. (2005). Genes and salt tolerance: Bringing them together. New Phytologist, 167, 645–663.
Nobi, E. P., Dilipan, E., Thangaradjou, T., Sivakumar, K., & Kannan, L. (2010). Geochemical and geo-statistical assessment of heavy metal concentration in the sediments of different coastal ecosystems of Andaman Islands, India. Estuarine, Coastal and Shelf Science, 87, 253–264.
Ong Che, R. G. (1999). Concentration of 7 heavy metals in sediments and mangrove root samples from Mai Po, Hong Kong. Marine Pollution Bulletin, 39, 269–279.
Page, A. L., Miller, R. H., & Keeney, D. R. (Eds.), (1982). Methods of Soil Analysis. Madison, Wisconsin: ASA and SSSA.
Parvaresh, H., Abedi, Z., Farshchi, P., Karami, M., Khorasani, N., & Karbassi, A. (2011). Bioavailability and concentration of heavy metals in the sediments and leaves of grey mangrove, Avicennia marina (Forsk.) Vierh, in Sirik Azini creek, Iran. Biological Trace Element Research, 143, 1121–1130.
Pekey, H. (2006). The distribution and sources of heavy metals in Izmit Bay surface sediments affected by a polluted stream. Marine Pollution Bulletin, 52, 1197–1208.
Polidoro, B. A., Carpenter, K. E., Collins, L., Duke, N. C., Ellison, A. M., Ellison, J. C., et al. (2010). The loss of species: Mangrove extinction risk and geographic areas of global concern. PLoS One, 5, e10095.
Ray, A. K., Tripathy, S. C., Patra, S., & Sarma, V. V. (2006). Assessment of Godavari estuarine mangrove ecosystem through trace metal studies. Environment International, 32, 219–223.
Shriadah, M. M. A. (1999). Heavy metals in mangrove sediments of the United Arab Emirates shoreline (Arabian Gulf). Water, Air, and Soil Pollution, 116, 523–534.
Tam, N. F. Y., & Wong, Y. S. (2000). Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environmental Pollution, 110, 195–205.
Tam, N. F. Y., Li, S. H., Lan, C. Y., Chen, G. Z., Li, M. S., & Wong, Y. S. (1995). Nutrients and heavy metal contamination of plants and sediments in Futian mangrove forest. Hydrobiologia, 295, 149–158.
Wang, Y. T., Qiu, Q., Yang, Z. Y., Hu, Z. J., Tam, N. F. Y., & Xin, G. R. (2010). Arbuscular mycorrhizal fungi in two mangroves in South China. Plant and Soil, 331, 181–191.
Wang, Y. T., Huang, Y. L., Qiu, Q., Xin, G. R., Yang, Z. Y., & Shi, S. H. (2011). Flooding greatly affects the diversity of arbuscular mycorrhizal fungi communities in the roots of wetland plants. PLoS One, 6, e24512.
Yan, Z. Z., Ke, L., & Tam, N. F. Y. (2010). Lead stress in seedlings of Avicennia marina, a common mangrove species in South China, with and without cotyledons. Aquatic Botany, 92, 112–118.
Yang, Q., Tam, N. F. Y., Wong, Y. S., Luan, T. G., Su, W. S., Lan, C. Y., et al. (2008). Potential use of mangrove as constructed wetland for municipal sewage treatment in Futian, Shenzhen, China. Marine Pollution Bulletin, 57, 735–743.
Yeh, H. C., Chen, I. M., Chen, P., & Wang, W. H. (2009). Heavy metal concentrations of the soldier crab (Mictyris brevidactylus) along the inshore area of Changhua, Taiwan. Environmental Monitoring and Assessment, 153, 103–109.
Young, L., & Melville, D. S. (1993). Conservation of the Deep Bay environment. In B. Morton (Ed.), The marine biology of the South China Sea (pp. 211–231). Hong Kong: Hong Kong University Press.
Zhang, J., Liu, J., Ouyang, Y., Liao, B., & Zhao, B. (2010). Removal of nutrients and heavy metals from wastewater with mangrove Sonneratia apetala Buch-Ham. Ecological Engineering, 36, 807–812.
Zheng, W. J., Chen, X. Y., & Lin, P. (1997). Accumulation and biological cycling of heavy metal elements in Rhizophora stylosa mangroves in Yingluo Bay, China. Marine Ecology Progress Series, 159, 293–301.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (30871475 and 31071357), the Project of Science and Technology of Guangdong Province (2010B020311005), and the Reserve Key Project of Sun Yat-sen University, Foundation for Distinguished Young Talents in Higher Education of Guangdong, China (C10438), and Foundation from Key Laboratory of Biodiversity Dynamics and Conservation of Guangdong Higher Education Institutes, Sun Yat-sen University (KLB11006), and the Open Research Fund Program from the Guangdong Key Laboratory of Plant Resources (plant01k15). We thank Li Meng-ying and Wang Xin-ya (Sun Yat-sen University, Guangzhou, China) for their generous help in sampling and analyzing heavy metals. We also thank Prof. Lars Olof Björn (Lund University, Sweden) and the anonymous reviewers for their thoughtful advice regarding our manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Qiu, Q., Xin, G. et al. Heavy metal contamination in a vulnerable mangrove swamp in South China. Environ Monit Assess 185, 5775–5787 (2013). https://doi.org/10.1007/s10661-012-2983-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-012-2983-4