Abstract
A field experiment was designed to investigate the effects of cadmium and lead on biomass production, sugar and vitamin C content of dietary vegetables. At seedling stage the reduction magnitudes in biomass production with added cadmium up to 50 mg kg−1 soils were 49–51 % in fenugreek root and 32.2–39.6 % in spinach leaf; while at 40 days after sowing (vegetative growth stage); they were observed as 36.1–47.2 % in spinach root and 17.8–20 % in coriander leaf. Cadmium caused maximum reduction in sugar content (62 %) in radish leaf and vitamin C content (56.4 %) in coriander leaf at seedling stage; whereas, maximum reduction in sugar content (66.7 %) and vitamin C content (59.4 %) was observed in spinach leaf and coriander leaf, respectively, under the combined treatment (Cd 50 mg kg−1 + Pb 500 mg kg−1) at vegetative growth stage. The dietary vegetables enhance their antioxidant activity against metal stress when applied below the critical level, however, dosage of Cd higher than critical level (≥25 mg kg−1 soil in treatments II, III and IX) drastically alters plant growth (stunted), reduced yield as well as dietary contents (sugar and vitamin C) of these important vegetables especially it’s antioxidant content; and the hazardous effect was more visible at higher bioaccumulation of heavy metals during vegetative growth stage. It is concluded that dietary vegetables should be utilized for human consumption before the vegetative growth stage especially in the soils polluted with cadmium and lead in order to minimize the intake of pollutants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The purpose of the present study is to develop the understanding of behaviour of heavy metals in the soil, their bioaccumulation during growth stages and their impact on dietary contents of some important dietary vegetables. Vegetables supply minerals, vitamins, certain hormone precursors as well as proteins and energy [1, 2]; and they help the human beings to maintain healthy diet. Vitamin C (ascorbic acid) is a water soluble antioxidant found in variable quantities in different vegetables [3, 4] which is required for normal metabolic function of our body.
Mostly heavy metals accumulate in the top soil and in the long term, increase their concentration resulting into increase in their absorption and accumulation in plant. The discharge of heavy metals as by-product of various human activities has been accompanied by large scale soil pollution and bioaccumulation, so that contamination levels in these soil–plant systems were either more than normal level, or expected soon to reach those levels. The anthropogenic use of heavy metals either through sewage-sludge or through fertilizers has received much attention due to enrichment of heavy metals in soil which affects human health causing social problems [5–7]. Among toxic heavy metals, cadmium is of great environmental concern because of its higher bioavailability and toxicity to human and livestock by readily getting in the food chain [8]. Cadmium (Cd) toxicity in plants causes leaf roll, chlorosis and reduced growth of both root and stem. The levels of Cd, Chromium (Cr), Nickel (Ni), and lead (Pb) have been observed above the critical toxic level in plant leaves in both dry and wet seasons. In general, the concentration of Cd in plant decreases in the order: Root > fruit > seed. The pH-induced Cd immobilization in soil is attributed to various reasons such as increase in adsorption due to increase in surface negative charge, greater affinity of hydroxyl species (Cd OH+) for adsorption sites [9, 10] than Cd++ and precipitation of Cd as Cd(OH)2. Cadmium is also an effective inhibitor of photosynthesis [11].
Human studies have shown that chronic exposure to cadmium can lead to serious health problems including lung cancer, emphysema, other lung diseases and kidney damage. Cadmium, with normal levels of 0.06–1.10 mg kg−1 soil, is considered as one of the most important and most active heavy metals present in soil. Permissible level of consumption of Cd for human being is 70 μg/day.
The study regarding the impact of heavy metal pollution on the quality of vegetables is very limited in India. The authors therefore, carried out the present experiment to investigate the effects of Cd and Pb in isolation as well as in combination on biomass production and quality of vegetable. Keeping in view the above mentioned facts the present study has been conducted to determine (a) the effects of Cd and Pb pollution on biomass production of leafy vegetables (b) vitamin C and sugar content in Cd/Pb polluted soils and (c) bioaccumulation of Cd and Pb in vegetables in the Cd/Pb polluted soils. In order to develop the database, the values of sugar contents and vitamin C contents in common tropical dietary vegetables are hereby presented. The uses of vitamin C in diseases where it can promote healing are also discussed.
Material and Methods
Experimental Site
The experimental site is situated in northern India at 25º57′N latitude and 81º50′E longitude on south-east facing slopes of comparable inclination at altitudes between 200 and 80 m above sea level. A sandy clay loam soil, derived from sewage-sludge irrigated Indo-Gangetic alluvial soils of SDI farm situated on the confluence of Ganga and Yamuna alluvial deposit, was sampled from Allahabad city, India. The properties of the soil were: pH 8.0, EC 0.28 dSm−1, organic matter (K2Cr2O7 oxidation) 5.6 g kg−1, total N 0.08 %, total P 0.04 %, CEC 19.8 C mol (P+) kg−1, DTPA–Cd 0.38 mg kg−1 and DTPA–Pb 0.64 mg kg−1. The texture comprised of sand (>0.2 mm) 56.0 %, silt (0.002–0.2 mm) 20.0 % and clay (<0.002 mm) 24.0 %. The detailed physicochemical properties of the investigated soil have been given in the Table 1. The soil was ground to pass through a 2 mm sieve. Nitrogen, phosphorus and potash were added to all the plots @ 120, 30 and 30 kg ha−1 respectively through calcium ammonium nitrate, di-ammonium phosphate and sulphate of potash. The experiment was replicated thrice and conducted in completely randomized block design following nine treatments as follows:
(I) control (II) Cd 25 mg kg−1 (III) Cd 50 mg kg−1 (IV) Pb 250 mg kg−1 (V) Pb 500 mg kg−1 (VI) Cd 25 mg kg−1 + Pb 250 mg kg−1 (VII) Cd 25 mg kg−1 + Pb 500 mg kg−1 (VIII) Cd 50 mg kg−1 + Pb250 mg kg−1 and (IX) Cd 50 mg kg−1 + Pb 500 mg kg−1. Cadmium was added as CdCl2 and Pb as Pb(CH3COO)2. After 24 h of the treatment seeds were sown. There were 27 plots, each plots having (1 × 1 m2). Soil moisture was maintained by irrigating the crops at interval of 5–6 days. Some samples were taken at seedling stage or at 20 days after sowing (DAS) for fresh weight, bioaccumulation analysis as well as quality analysis (sugar content and vitamin C). Vegetables were harvested at 40 DAS for the aforesaid analyses. Other samples were oven-dried at 70–80 °C for further mineral analysis.
Soil Analysis
Soil Sampling
For surface soil sampling, the larger fields were divided into suitable and uniform parts, and each of these uniform parts was considered a separate sampling unit. In each sampling unit, soil samples were drawn from several spots in a zigzag pattern, leaving about 2 m area along the field margins. Silt and clay were separated by Pipette method and fine sand by decantation.
Extraction for Total Heavy Metals Content in Soil
One gram of soil in 5 ml concentration HNO3 and 5 ml HClO4 (Perchloric acid 60 %) were added and the contents were heated up to dryness. The hot distilled water was added. The contents filtrated and volume was made up to 50 ml.
Preparation of DTPA Solution
Di-ethyl-triamine-penta-acetic acid (DTPA) solution [1.97 g (0.05 M) DTPA powder, 13.3 ml (0.1 M) Tri-ethanol amine and 1.47 g (0.01 M) CaCl2 were dissolved in distilled water to make up 1 litre after adjusting the pH to 7.3] was prepared [12] to extract the available heavy metals in soil samples.
Available Heavy Metals in Soil
Five gram soil and 20 ml DTPA solution was added and the contents were shaken for 2 h and then filtered through Whatman filter paper No. 42. The clean filtrate was used for the estimation of heavy metals (Cd and Pb) by AAS.
Soil pH
Soil pH was measured with 1:2.5 soil water ratio using ELICO pH meter (Model LI 127) at the Laboratory of Sheila Dhar Institute of Soil Science, University of Allahabad, Allahabad-211002, Uttar Pradesh, India. Double distilled water was used for the preparation of all solutions.
Organic Carbon
One gram soil (0.5 mm) was taken in a 500 ml conical flask. Then 10 ml of 1 N potassium dichromate (K2Cr2 O7) solution and 20 ml of concentrated sulphuric acid (H2SO4) were added. The solution was shaken well for two minutes and kept for half an hour and then diluted with 200 ml of distilled water. Then 10 ml of phosphoric acid and 1 ml of diphenylamine indicator were added in solution. The solution becomes deep violet in colour and further it was titrated against N/2 ferrous ammonium sulphate solution, till the violet colour changed to purple and finally to green. Organic carbon was determined by chromic acid digestion method [13].
Cation Exchange Capacity
CEC was determined by using neutral 1 N ammonium acetate solution. A known weight of soil (5 g) was shaken with 25 ml of neutral ammonium acetate solution for 5 min and filtered through Whatman filter paper No. 42 [14, 15].
Total Nitrogen
A known weighed soil (1 g) was taken in a 150 ml conical flask and treated with 10 ml of digestion mixture containing sulphuric acid and selenium dioxide. Salicylic acid was also added to include the nitrates and nitrites. Digestion was carried out till the soil colour changed to white. The N in the digest was estimated by using micro-Kjeldahl method [14, 15].
Total Phosphorus
Two gram soil was taken with 4 ml HClO4 (70 %) in a 50 ml beaker covered with watch glass and put on a hot plate and digestion was carried out till the soil colour changes to white. Ten ml HNO3 was added to the filtrate solution. Ammonia was added to saturate the solution. Then 30 ml standard ammonium molybdate solution was added in the solution to extract the total phosphorus content from soil [14, 15].
Plant Analysis
Processing Plant Samples
The fresh samples first washed with tap water followed by 0.2 % detergent solution, 0.1 N HCl and then with a plenty of water. Final rinsing was done with distilled water. The samples were rinsed with double distilled water. Samples so cleaned and freed from dirt or other adhered contaminants were then soaked with tissue paper, and air-dried for 2–3 days in a dust- and contamination-free environment. Thereafter the samples were placed in clean paper envelopes and dried in a hot-air oven at a temperature of 65 °C.
Preparation of Plant Extract
One gram of ground plant material was taken in a 100 ml beaker and 15 ml of tri-acid mixture (HNO3, conc. H2SO4 and HClO4 in 5:1:1 ratio) was added. The content was heated on hot plate at low heat (60 °C) for 30 min and the volume was reduced to about 5 ml until a transparent solution was obtained. After cooling, 20 ml distilled water was added to the beaker and the content was filtered through Whitman number 42 into a 100 ml volumetric flask and the volume was made up with distilled water [15, 16].
Biochemical Analysis
Fresh leaves of spinach, radish, coriander and fenugreek were sampled for the determination of vitamin C and sugar contents. Vitamin C was measured with 2, 4-dinitrophenylhydrazine (DNP) method [17] using Spectrophotometer (Model SP 1700). DNP method involved the following steps:-
(a) Homogenization of 10 g sample of vegetable with 50 ml of freshly prepared 6 % (6.0 g/dL) metaphosphoric acid, centrifuged at 6,000 rpm for 10 min at 4 °C and the volume preparation up to 100 ml with standard metaphosphoric acid. Filtration of the solution through Whatman No. 1 filter paper followed by further filtration using 0.45 μm or 0.2 μm filter paper for the final working solution, (b) Preparation of 25 mL standard ascorbic acid solution of each of the concentrations: 0.10, 0.40, 0.80, 1.20, 2.0, 3.0 and 4.0 mg/dL using 6 % metaphosphoric acid, (c) Mixing of triplicate samples of 1.2 mL of both the clear supernatant extract and the working calibrators into 13 × 100 mm Teflon-lined screw–cap test tubes. Then 1.2 mL of the 6 % metaphosphoric acid was placed into two separate tubes for use as blanks, (d) Addition of 0.4 mL of di-nitrophenyl-hydrazine-thio-urea-copper sulfate (DTCS) reagent to all tubes, (e) Removal of the tubes from the water bath and chilling for 10 min in an ice bath, (f) Addition of 2.0 mL cold sulfuric acid (12 mol/L) to all tubes followed by capping and mixing in a vortex mixer, (g) Adjustment of the Spectrophotometer with the blank to read zero at 520 nm.
To the filtered sample a few drops of bromine water was added until solution became coloured (oxidation of ascorbic acid to dehydroascorbic acid). Then a few drops of thio-urea were added to remove the excess of bromine and finally a cleared solution was obtained. Then 2, 4 dinitrophenylhydrazine solutions were added with all standards and also with oxidized ascorbic acid. The ascorbic acid content was determined with spectrophotometric determination. In the second part of reaction l-dehydroascorbic acid reacts with 2, 4 dinitrophenylhydrazine to produce osazone which forms red coloured solution on treatment with 85 % H2SO4 [17, 18].
Sugar was directly measured by hand sugar meter. The Delta TRAK Brix meter was used to measure sugar in juices prepared from vegetables. The readings relative to Brix scale, representing the unit in percent of dissolved sugar were recorded [19].
Heavy Metals in Plant
Heavy metals (Cd and Pb) in plants and soil were determined with inductively coupled plasma spectrometry (ICP-AES [15, 20]; Model- LABTEM, Perkin Elmer, Inc.) at Central Environment Pollution Control Lab, Indian Farmer Fertilizer Cooperative (IFFCO) Ltd., Phulpur Unit, Ghiya Nagar, Phulpur, Allahabad-212404, Uttar Pradesh, India. Statistical analysis and graphical illustrations were determined and designed using Prism 5.04 software.
Results and Discussion
Effects of Cd and Pb Pollution on Biomass Production of Leafy Vegetables
Vegetables show the immense ability to accumulate heavy metals due to the assimilation of these elements from soil by the roots of plants. These elements gradually get accumulated in different parts of the plant including leaves and roots. Plant response to increased levels of Cd in soil differs in terms of the ability of various plant species to take up and transport varied levels of Cd. Cadmium can be easily transported within plants. Table 2 shows that the yield of vegetables was reduced remarkably by the addition of Cd (25–50 mg kg−1) as compared to the control. The reduction magnitudes were 32.3–42.7 %, 26.8–32.9 %, 44.6–54.2 % and 48.9–51 % in roots and 32.2–39.6 %, 33.3–34.2 %, 23.9–32.6 % and 33.3–38.8 % in leaves of spinach, radish, coriander and fenugreek respectively, at 20 DAS, due to heavy metals addition at the aforesaid two levels. Similarly the reduction magnitudes were 36.1–47.2 %, 16.9–33.8 %, 37.5–40.6 % and 40.9–42.5 % in roots and 18.4–24.6 %, 13.6–15.9 %, 17.8–20 % and 14.9–25 % in leaves of spinach, radish, coriander and fenugreek respectively, at 40 DAS [21] after addition of the heavy metal.
On the other hand, the biomass yields of vegetables marginally decreased by the addition of Pb. Although the amount of Cd addition was rather less than that of Pb, the influence of Cd was greater than that of Pb. Cd thus leads to higher yield reduction of vegetables as compared to Pb [22, 23]. The reduction of biomass production by Cd toxicity could be the direct consequence of the inhibition of chlorophyll synthesis and photosynthesis [24].The most common effect of Cd toxicity in plants is stunted growth, leaf chlorosis and alteration in the activity of many key enzymes of various metabolic pathways [25]. The study clearly indicated that Cd application ≥25 mg kg−1 severely inhibited root growth of all the tested edible vegetables. It might be due to increase in Cd-induced Indole-3 acetic acid- peroxidase (IAA-POD) activity as well as H2O2 production in root tips [26].
Vitamin C and Sugar Content of Edible Tissues Grown in Cd/Pb Polluted Indo-Gangetic Soil
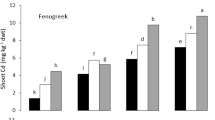
Data graphically represented in Fig. 1a–d indicates that the sugar content of radish leaf reduces remarkably by the single application of Cd. Cadmium @ 50 mg kg−1 caused the maximum reduction (61.9 %) in sugar content in leaves of radish as compared to the control at 20 DAS. However, minimum reduction (3.8 %) of sugar content was observed in coriander under the application IV (Pb 250 mg kg−1) as compared to the control at 20 DAS.
Effect of different treatment of Cd and Pb on sugar content (%) in a Spinach––sugar content reduction was maximum (66.7 %) under application III (Cd @ 50 mg kg−1) in spinach leaf; b Radish––sugar content reduction was maximum (60 %) under application IX (Cd @ 50 mg kg−1 + Pb @ 500 mg kg−1) in radish leaf; c Coriander––sugar content reduction was maximum (33.3 %) under application III in coriander leaf; d Fenugreek––sugar content reduction was maximum (44.4 %) under application III in fenugreek leaf; Data are mean values of three replications (mean ± SD)
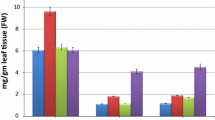
Data graphically represented in Fig. 2a–d indicates that the vitamin C content of coriander leaf reduces remarkably by the individual addition of Cd. Cadmium @ 50 mg kg−1 caused the maximum reduction (61.8 %) in vitamin C content in leaves of coriander as compared to the control at 20 DAS. However, low reduction of vitamin C content was observed in leaves of both radish (2.9 %) and coriander (13.6 %) under the application IV (Pb 250 mg kg−1) as compared to the control at 20 DAS [27].
Effect of different treatment of Cd and Pb on vitamin C content (%) in a Spinach––vitamin C content reduction was maximum (56 %) under application IX (Cd @ 50 mg kg−1 + Pb @ 500 mg kg−1) in spinach leaf; b Radish––vitamin C content reduction was maximum (28.6 %) under application IX in radish leaf; c Coriander––vitamin C content reduction was maximum (59.9 %) under application IX in coriander leaf; d Fenugreek––vitamin C content reduction was maximum (44 %) under application IX in fenugreek leaf; Data are mean values of three replications (mean ± SD)
Cadmium @ 50 mg kg−1 caused the maximum reduction (66.7 %) in sugar content in leaves of spinach as compared to the control at 40 DAS [28]. However, low reduction of sugar content was observed in leaves of both radish (4 %) and coriander (2.7 %) under the application IV (Pb 250 mg kg−1) as compared to the control.
The comparative study of data graphically represented in Fig. 2a–d indicates maximum reduction of vitamin C up to 59.4 % in coriander leaf [29, 30] in combinatorial application (Cd 50 mg kg−1 + Pb 500 mg kg−1). The minimum reduction of vitamin C was observed up to 16.7 % in leaf of radish under the combinatorial application (Cd 50 mg kg−1 + Pb 250 mg kg−1) as compared to the control.
The sugar and vitamin C content in leaves of Raphanus sativus L. (Figs. 1b, 2b) were affected by the application of soil pollutants and the reduction was observed up to 28 % in sugar and 28.6 % in vitamin C in the Cd @ 50 mg kg−1 and combinatorial application (Cd 50 mg kg−1 + Pb 500 mg kg−1), respectively. These results demonstrated that Cd/Pb treatment in isolation or in combination resulted in physiological changes in vegetables and induced quality deterioration of edible plant tissues.
Ogunlesi et al. [30] also found higher content of vitamins in leafy vegetables, spinach, lettuce, cabbage etc. In order to maintain vitamin C content in the dietary vegetables in Cd and Pb contaminated soil, these heavy metals must be monitored/checked and their contamination be kept at least below their critical levels. Vitamin C, a water soluble antioxidant, has been found to prevent tissue damage, identified to prevent sperm agglutination with resultant improvement in male fertility and healing touch to human health against several ailments and diseases including cold, cough, influenza, sores, wounds, gingivitis, skin diseases, diarrhea, malaria and bacterial infections. It has been reported to be protective and therapeutic in cardiovascular diseases, cancer as well as in eye diseases, associated with reduced risk of ischemic stroke (high blood pressure).
Bioaccumulation of Cd and Pb in Vegetables Grown in Cd/Pb Polluted Soil
The results presented in the Table 3 indicate that high dosage of combined application IX (Cd 50 mg kg−1 + Pb 500 mg kg−1 soil) enriched the content of Cd in spinach up to 45.4–52 folds, in radish 6.2–35 folds, in coriander 38–41 folds and in fenugreek 7.2–18.8 folds over control at 20 DAS [31, 32]. However, combined application VIII (Cd 50 mg kg−1 + Pb 250 mg kg−1 soil) enriched Cd in spinach up to 52.8–54.8 folds, in radish 7.6–38 folds, in coriander 43.9–45 folds and in fenugreek 11.9–25.2 folds over control at 40 DAS (Table 3) [21, 33]. There were no distinct differences among treatments Nos. I, IV and V which revealed lesser toxicity of Pb in vegetables as compared to Cd. These findings suggest that Cd is transferable to the leaves of vegetable crops. On the basis of Cd concentration in the leaves of the vegetable crops the phytoaccumulation potential were established in the descending order of magnitude as spinach > coriander > fenugreek ≥ radish at both growth stages of the dietary vegetables (Table 3) [34]. The higher accumulation of heavy metals in plant led to reduced photosynthesis rate and chlorophyll pigments, disturbed photochemical light quenching, increased lipid peroxidation/proline content/protein content, stunted growth and lowering of yield [35].
The results presented in the Table-4 indicate that application VII (Cd 25 mg kg−1 + Pb 500 mg kg−1) increased the Pb content in spinach up to 3.1–4.5 folds, in radish 1.2–1.3 folds, in coriander 1.8–3.5 folds and in fenugreek 1.2–1.6 folds over the control at 20 DAS [21]. The Pb content also increased in spinach up to 3.8–5.3 folds, in radish 1.7–1.9 folds, in coriander 3.4–5.1 folds and in fenugreek 2.3–2.4 folds over the control at 40 DAS (Table 4). The Pb concentration in the leaves of the vegetable crops was observed in descending order of magnitude as spinach > coriander > fenugreek > radish at 20 DAS (Table 4). Similarly Pb concentration in the leaves of spinach and coriander was observed higher in magnitude than the other two tested vegetables. The maximum Pb content was found in spinach leaves in treatments VII followed by treatment V and lower ones in leaves due to treatments I, II and III. The uptake of metals from soil to plant depends on different factors such as their soluble salt content, soil pH, plant growth stages, types of species, fertilizer and soil [36, 37].
It is evident from Fig. 1a–d that dosage of Pb (treatment IV and V) and combined treatments (VI, VII) slightly favoured the sugar content in the dietary vegetables over the control. Similarly dosage of Pb (treatment IV and V) did not alter antioxidant content (vitamin C) in the four tested dietary vegetables (Fig. 2). The study reveals that plant physiology plays significant role in bioaccumulation of heavy metals in plants as well as it determines the dietary contents during the growth stages of plants. The dietary vegetables enhance their specific phytochemical against the heavy metal accumulation (below the critical level) in order to adapt the stress condition and protect the plants. Similarly the dietary vegetables may enhance their antioxidant activity against Cd stress when applied below to 25 mg kg−1 soil, whose levels were not tested in this study.
On the other hand it is also evident from Figs (1 and 2) that dosage of Cd higher than critical levels (≥25 mg kg−1 soil in treatments II, III, IX) drastically alters plant growth, biomass and dietary contents (sugar and vitamin C) of these important vegetables. The bio-molecules such as carbohydrates, amino acids, vitamins and hormones etc. are essential for plant growth, development, stress adaptation and defense. Besides the importance for the plant itself, these bio-molecules determine the nutritional and other qualities such as color, taste, smell, antioxidative, anticarcinogenic, antihypertension, anti-inflammatory, antimicrobial, immune-stimulating, and cholesteremic properties of the food [38]. The enrichment of heavy metals in the edibles through sewage-sludge, beyond a critical level degrades the quality of dietary vegetables in terms of reduced sugar and vitamin C as is evident from present study, which may induce several metabolic disorders pertaining to health of human beings. The toxicity may lead to the simultaneous existence of chronic inflammatory mechanism and downregulation of antioxidant defense mechanism of cells, which are emerging as major causes of neoplastic transformation and the progression of many solid cancers [39]. Higher intake of cadmium chloride through dietary vegetables may cause renal toxicity to human beings. Al-Madani et al. [40] reported renal toxicity of mercuric chloride at different time intervals in rats.
In toxic condition, vegetable growth requires correction with calcium through soil plant rhizospheric process [41, 42] to mitigate the adverse effects of metals toxicity. Mixing of heavy metal polluted soils with Ca, Zn and organic matter after each harvest has been shown to be useful for a better re-growth and quality of the plants [43]. This treatment also helps to minimize the heavy metal accumulation in plants and to depict the phenomena of soil–plant relationship in the process of bioaccumulation.
Suitable nutrition of plants plays a very important role in the production of yield and in the quality of agricultural products. The soil fertility and high degree of weathering of soil also plays an important role on the cycles of micronutrients in soils affecting sugar and vitamin contents in plants.
Replacement of the nutrient cation and anions by each other may have important effects on the nutritional value of plants and in economy of their biomass production. Quantitatively, Ca is much more important element to animals, and it is much less expensive, and this suggests substituting Ca for trace metals in the investigated soil. The reduction in quality of vegetables in term of decrease in sugar and antioxidant content in the combinatorial treatment might be ascribed due to the substitution of Ca ions by Cd ions. Plants that are high in mineral cations tend to be high in protein and organic acids. The mineral and protein content of vegetable tends to decrease by the substitution of nutrient minerals by trace elements in the rhizosphere of plants. The study enlarges the database for Cd and Pb bioaccumulation, which alters the sugar and vitamin C content of the dietary vegetables during their various growth stages. The results presented in this study will provide a suitable guide to the population in their choice of vegetables with high content of antioxidants (vitamin C). Adequate consumption of fresh vegetables at their earlier growth stage will result in improved health thereby reducing cardiovascular diseases, diabetes, eye diseases, infertility and cancers that are prevalent in India and many other developing tropical countries.
Conclusion
The present findings indicate that the individual effect of Cd was most hazardous, the combined application of Cd and Pb was also harmful while the individual effect of Pb was found least harmful in relation to biomass production, bioaccumulation of heavy metals, sugar content and vitamin C content in the dietary vegetables.
The study shows that all the four vegetables analyzed at 20 DAS posses lower risk of heavy metals contamination. It is concluded that dietary vegetables may be utilized for human consumption before the vegetative growth stage especially in the soils polluted with cadmium and lead; and the aforesaid dietary vegetables harvested at maturity stage must be restricted for human consumption. Bioaccumulation of cadmium in leaves of spinach was maximum (mean 3.79 mg kg−1) among tested vegetables, which revealed that leafy vegetables are more prone to heavy metal contamination, therefore, selection of crop species is very important in agricultural practices in heavy metal polluted soil.
Cadmium and lead contamination reduces the contents of sugar (up to 66.7 %) in spinach leaves and vitamin C (up to 59.4 %) in coriander leaves as compared to the control. The contents of Cd and Pb in different tissues of vegetables changed with various ways of treatments. The entry of heavy metals to the food chain through soil–plant rhizosphere process and the possible influence to human health needs to be explored. Being an important part of the human diet, vegetable consumption by human beings has got an increasing trend. Metal accumulation in vegetables therefore may pose a direct threat to the human health. The bio-molecules in the form of organic matter may be applied to improve the nutritional quality of vegetable especially to improve the antioxidant contents in plants.
The daily intake of these metals at present is much less than concentrations that affect health; this situation could however change in the future depending on the dietary pattern of the community and the volume of contaminants being added to the ecosystems. Caution is required for growing the leafy vegetables, e.g. spinach in heavy metal polluted soils. Choosing crop species or varieties with low metal transfer factors is one effective approach. Cd moved more easily onto the aerial parts of the plants such as leaves whereas Pb accumulated primarily in the roots.
References
Ifron ET, Basir O (1979) Nutritive value of some Nigerian leafy vegetables part 3. Food Chem 5:231–235
Gockowski J, Mbazo’o G, Mbah TF (2003) African traditional leafy vegetables and the urban and peri-urban poor. Food Policy 28:221–235
Szeto YT, Tomlinson B, Benzie IF (2002) Total antioxidant and ascorbic acid content of fresh fruits and vegetables: implications for dietary planning and food preservation. Br J Nutr 87(1):55–59
Igbal K, Khan A, Khattak MAK (2004) Biological significance of ascorbic acid (vitamin C) in human health-a review. Pakistan J Nutri 3(1):5–13
Gholamabbas S, Majid A, Sayed FM, Karim CA, Brian KR, Rainer S (2010) Transport of Cd, Cu, Pb and Zn in a calcareous soil under wheat and safflower cultivation––a column study. Geoderma 154:311–320
Angelova A, Ivanova K, Ivanova R (2004) Effect of chemical forms of lead, cadmium, and zinc in polluted soils on their uptake by tobacco. J Plant Nutri 27(5):757–773
Yusuf AA, Arowolo TA, Bamgbose O (2003) Cadmium, copper and nickel levels in vegetables from industrial and residential areas of Lagos City, Nigeria. Food Chem Toxicol 41:375–378
Gupta UC, Gupta SC (1998) Trace element toxicity relationships to crop production and livestock and human health: implications for management. Commun Soil Sci Plant Anal 29:1491–1522
Bolan NS, Duraisamy VP (2003) Role of inorganic and organic soil amendments on immobilization and phyto-availability of heavy metals: a review involving specific case studies. Aust J Soil Res 41:533–555
Carlos A, Constantin L, Garcia FP, Razo LMD, Vazquez RR, Varaldo HMP (2005) Chemical fraction of heavy metals in soils irrigated with waste water in central Mexico. Agric Eco Environ 108:57–71
Vassilev A, Perez SA, Semane B, Carleer R, Vangronsveld J (2005) Cadmium accumulation and tolerance of two salix genotypes hydrophonically grown in presence of cadmium. J Plant Nutri 28:2159–2177
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese and copper. Soil Sci Soc Am J 42:421–428
Walkley A, Black AI (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modification of chromic acid titration method. Soil Sci 37:29–38
Chopra SL, Kanwar JS (1999) Analytical agricultural chemistry. Kalyani Publication, New Delhi
Kumar C, Mani D (2010) Enrichment and management of heavy metals in sewage-irrigated soil. Lap LAMBERT Acad Publishing, Dudweiler (Germany)
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology. Blackwell Scientific Publication, London, pp 285–344
Al-Ani M, Opara LU, Al-Bahri D, Al-Rahbi N (2007) Spectrophotometric quantification of ascorbic acid contents of fruit and vegetables using the 2,4-dinitrophenylhydrazine method. J Food Agric Environ 5(3 &4):165–168
Kharn MMR, Rahman MM, Islam MS, Begum SA (2006) A simple UV-spectrophotometric method for the determination of vitamin c content in various fruits and vegetables at Sylhate area in Bangladesh. J Biol Sci 6(2):388–392
Qingling Fu, Hongqing Hu, Li Jiajia, Li Huang, Haizheng Y, Lv Yi (2009) Effects of soil polluted by cadmium and lead on production and quality of pepper (Capsicum annuum L.) and radish (Raphanus sativus L.). J Food Agric Environ 7(2):698–702
Li X, Coles BJ, Ramsey MH, Thornton I (1995) Sequential extraction of soils for multi-element analysis by ICP-AES. Chem Geol 124:109–123
Dang YP, Chhabra R, Verma KS (1990) Effect of Cd, Ni, Pb and Zn on growth and composition of onion and fenugreek. Commun Soil Sci Plant Anal 21(9/10):717–735
Pandey N, Sharma CP (2002) Effect of heavy metals Co2+, Ni2+ and Cd2+ on growth and metabolism of cabbage. Plant Sci 163:753–758
Muhammad A, Zahida P, Muhammad I, Riazuddin Iqbal S, Ahmed M, Bhutto R (2010) Monitoring of toxic metals (cadmium, lead, arsenic and mercury) in vegetables of Sindh Pakistan. Kathmandu Univ J Sci Eng Technol 6(2):60–65
Padmaja K, Prasad DDK, Prasad ARK (1990) Inhibition of chlorophyll synthesis in Phaseolus vulgaris seedling by cadmium acetate. Photosynthetica 24:399–405
Arduini I, Godbold DL, Onnis A (1996) Cadmium and copper uptake and distribution in Mediterranean tree seedlings. Physiol Plant 97:111–117
Haluskov L, Valentovicova K, Huttova J, Mistrik I, Tamas L (2010) Elevated indole-3-acetic acid peroxidase activity is involved in the cadmium-induced hydrogen production in barley root tip. Plant Growth Regul 62(1):59–64
John R, Ahmad P, Gadgil K, Sharma S (2009) Heavy metal toxicity: effect on plant growth biochemical parameters and metal accumulation by Brassica juncea L. Int J Plant Prod 3(3):65–75
Fang-YI Chu, Sun JIE, Xianzhong WU, Rui Hai LIU (2002) Antioxidant and anti-proliferative activities of common vegetables. J Agric Food Chem 50:6910–6916
Rajeshwari CU, Andallu B (2011) Oxidative stress in NIDDM patients: influence of coriander (Coriandrum sativum) seeds. Res J Pharm Biol Chem Sci 2(1):31
Ogunlesi M, Okiei W, Azeez L, Obakachi V, Osunsanmi M, Nkenchor G (2010) Vitamin C contents of tropical vegetables and foods determined by voltametric and titrimetric methods and their relevance to the medicinal uses of the plants. Int J Electrochem Sci 5:105–115
Girisha ST, Ragavendra BV (2009) Accumulation of heavy metals in leafy vegetables grown in urban areas by using sewage water and its effect. Arch Phytopathol Plant Prot 42(10):956–959
Abbas M, Parveen Z, Iqbal M, Riazuddin Iqbal S, Ahmed M, Bhutto R (2010) Monitoring of toxic metals (cadmium, lead, arsenic and mercury) in vegetables of spinach, Pakistan. Kathmandu Univ J Sci Eng Technol 6(2):60–65
Jiang W, Liu D, Hou W (2000) Hyperaccumulation of lead by roots hypocotyles and shoots of Brassica juncea. Biol Plant 43(4):603–606
Bigdeli M, Seilsepour M (2008) Investigation of metals accumulation in some vegetables irrigated with waste water in Shahre Rey-Iron and toxicological implications. American-Eurasian J Agric Environ 4(1):86–92
Singh RP, Agrawal M (2007) Effect of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere 67:2229–2240
Sharma RK, Agrawal M, Marshall F (2006) Heavy metals contamination in vegetables grown in waste water irrigated areas of Varanai, India. Bull Environ Contam Toxicol 77:312–318
Ismail BS, Farihah K, Khairiah J (2005) Bioaccumulation of heavy metals in vegetables from selected agricultural areas. Bull Environ Contam Toxicol 74:320–327
Hounsome N, Hounsome B, Tomos D, Edwards JG (2008) Plant metabolites and nutritional quality of vegetables. J Food Sci 73(4):48–65
Singh RK, Sudhakar A, Lokeshwar BL (2011) From normal cells to malignancy: distinct role of pro-inflammatory factors and cellular redox mechanisms. J Cancer Sci Ther 03:70–75
Al-Madani WA, Siddiqi NJ, Alhomida AS (2009) Renal toxicity of mercuric chloride at different time intervals in rats. Biochem Insights 02:37–45
Mani D, Sharma B, Kumar C (2007) Phytoaccumulation, interaction, toxicity and remediation of cadmium from Helianthus annuus L. (sunflower). Bull Environ Contam Toxicol 79:71–79
UNCTAD (2003) Organic fruit and vegetables from the Tropic, United Nations Conference on Trade & Development (UNCTAD), Geneva 10, Switzerland, 203–210
Mani D, Kumar C, Srivastava RK (2007) Effect of calcium, zinc and organic matter on the uptake of cadmium in Brassica rapa L. (turnip). Proc Natl Acad Sci India 77(2):263–276
Acknowledgments
The research was supported by The National Academy of Sciences, India (Allahabad), the Sheila Dhar Memorial Scholarship Fund and the Indian Farmer Fertilizer Cooperative (IFFCO) Ltd., Phulpur Unit, Ghiya Nagar, Phulpur Allahabad-212404, Uttar Pradesh, India. Opinions in the paper do not constitute an approval by the funding agencies but only reflect the personal views of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mani, D., Sharma, B., Kumar, C. et al. Cadmium and Lead Bioaccumulation During Growth Stages Alters Sugar and Vitamin C Content in Dietary Vegetables. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 82, 477–488 (2012). https://doi.org/10.1007/s40011-012-0057-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-012-0057-6