Abstract
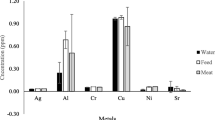
The concentrations of Cr, Cd, Pb, Hg, and As in water, poultry meat, fish, vegetables, and rice plants obtained from the area adjacent to the Hazaribag tannery campus, Dhaka, Bangladesh, were estimated and compared with permissible levels established by the WHO and FAO and levels reported previously by other authors. The metal contents were in the following order according to the concentration in contaminated irrigation water: Cr > Pb > As > Hg > Cd. Mean concentrations of Cr, Pb, Hg, and As in irrigated water were above the permissible levels, whereas the results were below the permissible levels for Cd. The metal concentrations in poultry meat, fish, rice, and vegetables were in the following orders: Pb > Cr > Cd > Hg > As, Pb > Cr > Cd > As > Hg, Pb > As, and Cr > Pb > Cd > As > Hg, respectively. The mean concentrations of metals in poultry meat, fish, rice, and vegetables were much higher than the permissible levels. The trends of weekly intake of heavy metals (WIMs) from poultry meat, fish, rice, and vegetables were as follows: Pb > Cr > Cd > Hg > As, Pb > Cr > Cd > As > Hg, Pb > As, and Cr > Pb > Cd > As > Hg, respectively. WIMs for all the metals were lower than the provisional maximum weekly intake recommended by WHO/FAO and USNAS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Unsafe food consumption is a severe problem because of heavy metal contamination, which is caused by direct or indirect activities of industries. Tannery effluent is ranked as the primary pollutant among all industrial wastes (Shen 1999). Approximately 40 heavy metals and acids are used for processing raw hides (UNIDO 2005). Only 20 % of the total chemicals used during tanning are absorbed by leather, whereas the rest is released as waste (BIWWPP 2010). Hazaribag, which is the largest tannery region in Bangladesh, consists of more than 200 tanneries that generate 7.7 million L of liquid waste and 88 million tons of solid waste daily (Azom et al. 2012). These wastes, which are directly discharged to low-lying areas, natural canals, lagoons, and other water bodies such as the Buriganga River, contain high concentrations of Cr, Cd, Pb, and As (Bhuiyan et al. 2010); these water bodies supply water to the agricultural, livestock, and fishing industries in the adjacent area (Azom et al. 2012). The contamination of rivers and lagoons results in the accumulation of these pollutants by biomagnification in common fish species, which are consumed as local food (Azom et al. 2012). Long-term use of wastewater in irrigation can affect food quality and safety (Muchuweti et al. 2006; Sharma et al. 2007). Increased amounts of heavy metals are detected in plants growing near industrial zones; thus, these plants serve as biomonitors of pollution load (Mingorance et al. 2007). Vegetables take up heavy metals during cultivation in soil polluted with toxic heavy metals from tanning activities. These heavy metals accumulate in edible and non-edible parts of plants. Solid wastes generated by the tannery industry contain appreciable amounts of toxic metals, which are converted to protein concentrate and used as poultry feed (Hossain et al. 2007). Chickens consume contaminated feeds, and the heavy metal contaminants are passed from these chickens to the body tissues of boilers and humans (Okoye et al. 2011). Food chain contamination is the major pathway of heavy metal exposure for humans (Khan et al. 2008). More than 0.2 million people live adjacent to the tannery campus of Hazaribag, and 20,000 people are directly exposed to the hazards of pollutants from the tannery complex (Asaduzzaman et al. 2002). The rest of the population is affected by these pollutants through dietary intake, which is the main route of exposure to heavy metals for most people, but inhalation of pollutants can also occur in extremely contaminated sites (Tripathi et al. 1997). Thus, information about heavy metal concentrations in food products and dietary intake of these pollutants is highly important in the assessment of risks to human health (Zhuang et al. 2009).
This study aimed to (a) assess the contamination status of heavy metals in vegetables, fish, meat, rice, and water in an area adjacent to the tannery campus and embankment of the Buriganga River, Dhaka; (b) compare the concentrations of heavy metals in vegetables, fish, and meat with the established safe limits; and (c) assess the health risks of heavy metal contamination through the dietary intake of selected foods, such as vegetables, fish, rice, and poultry meat, based on average daily consumption.
Material and methods
Study area
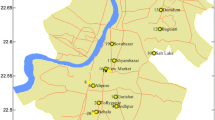
The preliminary phase of this study was conducted in the cluster of tannery industries in the Hazaribag area of southwestern Dhaka (Fig. 1), which lies within longitude 90° 22′–90° 22′ 48′′ E and latitude 23° 43′ 34′′–23° 43′ 49′′ N. This area is situated adjacent to the Buriganga River. The tannery of Hazaribag was constructed in 1960. Hazaribag has numerous industries, most of which discharge untreated effluents in the canal, lagoon, and Buriganga River, thereby leading to the contamination of water, vegetables, and fish. The solid wastes of tanneries are used to produce poultry feed as protein sources. Different kinds of vegetables and cereal crops, such as brinjal or eggplants, chili, stem amaranth, radish, pinch, and rice, are cultivated in the neighborhood surrounding the tannery. Different fish species, such as chapila, baola, tatkini, taki, tengra, batash, and tilapia, are cultured in ponds and rivers. Poultry farming is also practiced in this zone throughout the year for domestic consumption and selling.
Collection, pre-treatment, and analysis of samples
Water, stem amaranth (Amaranthus lividus), radish (Raphanus sativus), spinach (Spinacia oleracea), tatkini (Cirrhinus reba), spotted snakehead (Channa punctata), Mozambique tilapia (Oreochromis mossambicus), rice (Oryza sativa), and poultry samples were randomly collected in triplicate from different locations in the study area from 28 February 2013 to 15 April 2013. The samples were tagged and processed in the laboratory. Samples were cut into small pieces using stainless steel scissors/knife. Samples were thoroughly washed with tap water and deionized water. The samples were dried in an oven at 60 °C until a constant weight was obtained. The dried samples were finely ground with a carbide mortar and pestle, transformed into pellets by a hydraulic pressure of 10 tons, and preserved in polyethylene bags in desiccators until subsequent analysis.
PANalytical Epsilon 5 energy-dispersive X-ray fluorescence was used as a major analytical technique for elemental analysis of the samples. For irradiation of the sample with an X-ray beam, 2 g of each powdered material was pressed into a 25-mm-diameter pellet using a Specac pellet maker and pellets were loaded into an X-ray excitation chamber using an automatic sample changer system. Sample irradiation was performed by assigning a time-based program that was controlled by a software package provided with the system. The analysis was based on direct comparison. Thus, the standards of similar matrices were used for the construction of the calibration curve to avoid the matrix effect. Three laboratory-synthesized, cellulose-based multi-element standards, five plant standards (Apple Leaf/NIST 1516, Spinach/NIST 1570a, Orchard Leaf/NIST 1571, Tomato Leaf/NIST 1573a, and Peach Leaf/NIST 1574), and three fish standards (Tuna-1, Tuna-2, and Tuna-3) were used to construct calibration curves for the elemental analysis of river water and plant and fish samples, respectively. The calibration curve for each element was constructed based on the K X-ray and L X-ray intensities calculated for the respective elements in standard samples. The curves were constructed by plotting the sensitivities of the elements as a function of their atomic number. The calibration curves constructed for elements in the standards were validated by analyzing three standard reference materials. The results obtained for the elements of interest and certified values for corresponding elements are shown in Table 1. The X-ray intensities of the elements in a sample spectrum were calculated using the system software by integration of the area of the respective X-ray peaks using peak fitting deconvolution software. To assess the contamination level of heavy metals, the mean, median, minimum, maximum, and standard deviations of the concentrations in water, fish, poultry meat, rice, and vegetable samples were obtained using Microsoft Excel (version 2007).
Daily intake of metal
Daily intake of metal (DIM) was calculated to estimate the average daily metal loading of a consumer at a specified body weight and reveal the relative phytoavailability of metals. DIM does not consider the possible metabolic ejection of the metals, but information on the possible ingestion rate of a particular metal is obtained. DIM was calculated based on the equation of Singh et al. (2010) as follows: DIM = (C metal × D food intake/B average weight)
where
Results and discussion
Heavy metal concentrations (mg/L) in the Buriganga River, lagoon, and canal water
Among the five elements (Cr, Cd, Pb, Hg, and As) examined in effluent-contaminated water used for irrigation, fish cultivation, and other activities in areas adjacent to the tannery industrial area, the concentration of Cr was the highest (51.142 mg/L) and ranged from 60.092 to 40.888 mg/L, which was higher than those in wastewater (18.56 to 4.8 mg/L) and irrigation water (10.26 to 5.08 mg/L) (Mousavi et al. 2013). The maximum Pb concentration ranged from 22.44 to 15.31 mg/L, which was higher than the ranges (0.30 to 0.14 and 0.0002 to 0.0012 mg/L) reported by Ahmed and Goni (2010)) and Ideriah et al. (2012), respectively. Hg concentrations ranged from 0.07 to 0.053 mg/L, which was lower than those in irrigation water (1.42 to 0.62 mg/L) and wastewater (9.17 to 4.11 mg/L) (Mousavi et al. 2013). As concentrations ranged from 7.08 to 4.27 mg/L, which was higher than the concentration range of 0.005 to 0.003 mg/L reported by Adedeji et al. (2011). The mean concentrations of five heavy metals (i.e., Cr, Cd, Pb, Hg, and As) in water samples were 51.14, 0, 18.1, 0.06, and 5.71 mg/L, respectively (Table 2). Cr, Pb, and As concentrations in the collected samples were higher than the mean concentrations of the same metals in irrigation water and wastewater used for agriculture in Mashhad, northeastern Iran (Mousavi et al. 2013), and in water and sediments along the Abonnema shoreline, Nigeria (Ideriah et al. 2012). The standard deviation observed for Cr, Pb, and As was higher than that for Hg and Cd in wastewater. Cr, Pb, and As were not uniformly distributed in the study area, which coincided with the data obtained by Sharma et al. (2006). The data on heavy metal concentrations in water from the present study varied from the data obtained by other authors (Khan et al. 1998; Al-Nakshabandi et al. 1997). This variation might be due to the various industries discharging their treated and/or untreated wastewater into the Buriganga River, lagoon, and canal, which supply the water used for irrigation, fish cultivation, and other purposes. The concentrations of heavy metals in effluents of tannery industries also depend on the chemicals used during soaking, tanning, and post-tanning of hides and skins (Huq 1998) as well as other product manufacturing processes and raw materials (Sharma et al. 2006).
Compared with the standard guideline of irrigation water (Pescod 1992) and water quality for agriculture (FAO 1985), the mean concentrations of Cr, Pb, and As were 514-, 36-, and 57-fold higher than the recommended permissible levels, respectively, whereas Cd concentration in water samples was within the recommended permissible level. Leather, chemical, and metal-based industries, such as tanning, fabric printing, footwear, and glue manufacturing, in and around the Hazaribag area contribute to the increase in heavy metal concentrations in the Buriganga River and surrounding canals because toxic substances mainly come from coloring agents, pigments, and additives.
Heavy metal concentration in vegetables
The average per capita consumption of leafy and non-leafy vegetables is 130 g/person/day (Alam et al. 2003). This value is considerably less than the recommended amount of 200 g/person/day from a nutritional perspective (Hassan and Ahmed 2000). The heavy metal concentrations in edible parts of vegetables grown around the Hazaribag tannery industrial region, Dhaka, Bangladesh, are shown in Fig. 1. The range and mean concentrations of heavy metals (μg/g dry weight) in leafy and non-leafy vegetables are presented in Table 3. Cr concentrations varied from 9.08 to 3.44 μg/g, and they were detected from three different vegetables in the following order: stem amaranth > spinach > radish. The mean level of Cr in amaranth was significantly higher than those reported in Nigeria (1.03 to 0.77 μg/g) (Adefemi et al. 2012) and Salamn, Tanzania (6.58 to 5.68 μg/g) (Charles et al. 2011). The mean Cr concentration in radish was higher than those reported in Turkey (1.47 to 1.40 μg/g) (Saglam 2013). Cr concentrations in stem amaranth, radish, and spinach were 181-, 65-, and 146-fold higher than the safe limit established by WHO but below the safe limit of the Indian standard. Cd accumulation was highest in radish (0.55 μg/g) followed by spinach (0.17 μg/g) and stem amaranth (0.08 μg/g). In radish, Cd concentration exceeded the WHO safe limits by 5-fold. Cd concentrations in vegetables were below the recommended concentrations of the Indian and Brunei standards. This study revealed that the mean Cd level in stem amaranth was higher than those reported in China (0.053 to 0.13 μg/g) (Xiaoming et al. 2013) and Salaam, Tanzania (1.45 to 0.55 μg/g) (Charles et al. 2011), but similar to that reported in Nigeria (0.23 to 0.17 μg/g) (Adefemi et al. 2012). In spinach, Cd concentration was higher than those reported in China (0.06 to 0.09 μg/g) (Xiaoming et al. 2013), India (0.03 μg/g) (Surchi 2011), Nigeria (0.10 to 0.13 μg/g) (Rapheal and Adebayo 2011a, b), and Misurata, Libya (0.30 to 0.24 μg/g) (Elbagermi et al. 2012), but lower than those reported in India (1.98 to 0.14 μg/g) (Parashar and Prasad 2013) and Australia (0.74 to 0.14 μg/g) (Kachenko and Sing 2006). In radish, Cd concentration was higher than those reported in China (0.05 to 0.09 μg/g) (Xiaoming et al. 2013) and Turkey (0.34 to 0.33 μg/g) (Saglam 2013) but lower than those reported in India (1.52 to 0.19 μg/g) (Parashar and Prasad 2013).
The maximum Pb concentration was detected in spinach (2.52 μg/g) followed by stem amaranth (2.06 μg/g) and radish (1.05 μg/g), which exceeded the acceptable tolerance level of WHO for Pb by eight, six, and three times, respectively. Pb concentrations in the edible parts of all the vegetables in this study were higher than the permissible levels established by WHO but similar to the permissible level of the Indian standard (Awashthi 2000) and lower than the level of the Brunei standard. The mean Pb concentration in amaranth was higher than those reported in Nigeria (0.25 to 0.15 μg/g) (Adefemi et al. 2012) but lower than those reported in Salaam, Tanzania (12.45 to 10.67 μg/g) (Charles et al. 2011). In spinach, Pb concentration was higher than those reported in India (0.56 μg/g) (Surchi 2011), Nigeria (0.26 to 0.18 μg/g) (Rapheal and Adebayo 2011a, b), and Misurata, Libya (0.34 to 0.30 μg/g) (Elbagermi et al. 2012), but lower than those reported by other studies in India (36.98 to 25.7 μg/g) (Parashar and Prasad 2013) and Australia (11.6 to 1.55 μg/g) (Kachenko and Sing 2006). In radish, Pb concentration was higher than those reported in Turkey (0.70 to 0.70 μg/g) (Saglam 2013) but lower than that reported in India (28.99 μg/g) (Parashar and Prasad 2013). Among the vegetables, radish showed the maximum Hg concentration (0.05 μg/g). The mean Hg concentration in stem amaranth was lower than those reported in the USA (1.93 to 0.16 μg/g) (Dunagan et al. 2007) and Slovak Republic (0.20 μg/g) (Ruzbarsky et al. 2013). The WHO and Indian standards currently do not have a permissible limit for Hg. As concentrations ranged from 0.16 to 0.06 μg/g, and the order of vegetables according to As level was as follows: spinach > stem amaranth > radish. The mean As level in this study in stem amaranth was higher than those reported in Salaam, Tanzania (0.17 to 0.16 μg/g) (Charles et al. 2011), but lower than that reported in the Slovak Republic (2.50 μg/g) (Ruzbarsky et al. 2013). As concentrations in spinach and stem amaranth exceeded the WHO standard but were lower than the Brunei standard. All heavy metals showed spatial and temporal variations in their concentrations, and these variations may be related to the different heavy metal sources and quantities of heavy metals discharged with effluents and sewage into the Buriganga River, lagoon, and canal waters as well as the vegetables growing in adjacent areas.
Heavy metal contamination in fish
The heavy metal concentrations in fish collected from the adjacent area of Hazaribag, Dhaka, Bangladesh are shown in Fig. 2. The ranges and mean concentrations of heavy metals (μg/g dry weight) in fish are presented in Table 4. Cr concentrations varied from 12.89 to 10.40 μg/g and appeared in the following order: tatkini > spotted snakehead > Mozambique tilapia. The mean Cr concentration was significantly higher than those reported in Tirana, Albania (0.03 to 0.01 μg/g) (Ozuni et al. 2011), and Newcastle upon Tyne, UK (0.05 to 0.03 μg/g) (Jabeen and Chaudhry 2013). Cr concentrations in fish were 257-, 247-, and 207-fold higher than the safe limits set by WHO. Bioaccumulation of Cd was highest in Mozambique tilapia (6.04 μg/g) followed by tatkini (6.03 μg/g) and spotted snakehead (6.02 μg/g). The mean Cd concentration was higher than those reported in Tirana, Albania (0.004 to 0 μg/g) (Ozuni et al. 2011), and Egypt (1.01 to 0.94 μg/g) (Essa and Rateb 2011) but lower than that reported in Sari, Iran (9.50 to 1.70 μg/g) (Salaramoli et al. 2012). The maximum Pb concentration was detected in spotted snakehead (16.18 μg/g) followed by tatkini (15.76 μg/g) and Mozambique tilapia (14.53 μg/g), and the concentrations in these fish exceeded the acceptable tolerance level of Brunei for Pb by eight times in spotted snakehead and seven times in Mozambique tilapia. Pb concentrations detected in fish were above the permissible levels of the WHO and Brunei standards. The mean Pb concentrations in fish were higher than those reported in Sari, Iran (0.15 to 0.16 μg/g) (Salaramoli et al. 2012); Tirana, Albania (0.03 to 0.003 μg/g) (Ozuni et al. 2011); Egypt (0.10 to 0.15 μg/g) (Essa and Rateb 2011); and Newcastle upon Tyne, UK (0.20 to 0.16 μg/g) (Jabeen and Chaudhry 2013). Hg concentrations varied from 2.24 to 1.67 μg/g and appeared in the following order: spotted snakehead > tatkini > Mozambique tilapia. Hg concentrations in fish were 4-, 3-, and 2-fold higher than the acceptable tolerance level of WHO. Hg concentrations in fish examined in this study were above the permissible levels established by the WHO and Brunei standards. The mean Hg concentration in fish was higher than those reported in Newcastle upon Tyne, UK (0.03 to 0.01 μg/g) (Jabeen and Chaudhry 2013); Sari, Iran (0.03 to 0.02 μg/g) (Salaramoli et al. 2012); and Tirana, Albania (0.32 to 0.05 μg/g) (Ozuni et al. 2011), but lower than those reported in Egypt (4.94 to 4.81 μg/g) (Essa and Rateb 2011). As concentrations varied from 3.39 to 3.10 μg/g, and the affected fish species appeared in the following order: spotted snakehead > Mozambique tilapia > tatkini. Maximum and minimum As concentrations were obtained from spotted snakehead (3.39 μg/g) and tatkini (3.09 μg/g), respectively. As concentrations in fish exceeded the Brunei standard limit by approximately 3-fold. The spatial and temporal variations in all the heavy metal concentrations might be due to the variations in heavy metal sources and quantity of heavy metals discharged through the effluents and sewage in the Buriganga River, lagoon, and canal. Metal concentrations in the fish body depend on the uptake capacity, accumulation, age of the fish, water pH, and other factors.
Heavy metal contamination in rice
Cr concentrations in rice were lower than those reported in China (8.12 to 4.12 μg/g) (Fu et al. 2008), India (2.93 to 2.14 μg/g) (Singh et al. 2011), Bangladesh (0.83 to 0.65 μg/g) (Rahman et al. 2010), and Malaysia (1.58 to 1.10 μg/g) (Yap et al. 2009). Cd concentrations in the present study were below the recommended permissible level established by WHO (Table 5). Cd concentrations in rice were lower than those reported in China (1.29 to 1.09 μg/g) (Fu et al. 2008), India (7.79 to 6.16 μg/g) (Singh et al. 2011), and Malaysia (0.21 to 0.15 μg/g) (Yap et al. 2009). Pb concentrations in rice were higher than the safe limit established by WHO. The maximum Pb concentration in rice was 2.37 μg/g and ranged from 2.37 to 2.01 μg/g, which was lower than those reported in India (7.09 to 6.14 μg/g) (Singh et al. 2011). Hg concentrations in rice in the present study were lower than those reported in China (2.53 to 1.81 μg/g) (Fu et al. 2008) and India (0.14 to 0.10 μg/g) (Singh et al. 2011). The maximum As concentration in rice was 1.42 and ranged from 1.42 to 0 μg/g, which was lower than those reported in China (5.77 to 2.37 μg/g) (Fu et al. 2008) and India (22.77 to 20.56 μg/g) (Singh et al. 2011). Rice was not contaminated with Cr, Cd, and Hg despite the appreciable amounts of these metals (except Cd) in irrigation water because plants differed in their ability to accumulate and concentrate metals in their edible parts, which was well supported by studies carried out by Sharma et al. (2006). The variations in heavy metal concentrations in rice were due to variations in their absorption and accumulation tendency. Hence, soil properties, such as pH, organic matter, cation exchange capacity (CEC), redox potential, soil texture, and clay content, usually affect heavy metal uptake (Overesch et al. 2007) by plants. Furthermore, the interaction between different heavy metals occurs at the root surface and within the plant, which ultimately affects the uptake and translocation of heavy metals (Sharma et al. 2006). Heavy metal accumulation also depends on plant age and plant parts (Liu et al. 2007).
Heavy metal concentration in poultry meat
Among the five elements examined in poultry meat collected from the poultry farm, Pb concentration was the highest (15.44 μg/g) and ranged from 15.05 to 15.83 μg/g (Table 5), which was higher than those reported in Nigeria (0.23 to 0.20 μg/g) (Oforka et al. 2012) and Giza, Egypt (3.26 to 0 μg/g) (Hala and Shireen 2009). The mean Cd concentration in poultry meat was 6.04 μg/g and ranged from 6.034 to 6.05 μg/g, which was higher than those reported in Romania (0.01 to 0.06 μg/g) (Ghimpeeanu et al. 2012) and Giza, Egypt (0.39 to 0 μg/g) (Hala and Shireen 2009). Cr concentrations in poultry meat ranged from 16.39 to 13.29 μg/g, which was higher than those reported in Romania (1.22 to 4.71 μg/g) (Ghimpeeanu et al. 2012). The mean Hg concentration in poultry meat was 3.32 μg/g and ranged from 3.40 to 3.23 μg/g, which was higher than that reported in Romania (0.04 to 2.77 μg/g) (Ghimpeeanu et al. 2012). The mean As concentrations in poultry meat was 3.02 μg/g and ranged from 3.06 to 3.00 μg/g, which was lower than the safe limit of the Egyptian standard (1993). All heavy metals showed spatial and temporal variations in concentration possibly because of the variations in heavy metal concentration of poultry feeds. Among the routes of heavy metal eco-toxicity in the study area, feeds and fertilizer production from tanned skin-cut waste (SCW) is the most direct route leading to food chain contamination. In Hazaribag (southwestern part of Dhaka City), about 90 % of tannery industries of Bangladesh are located in a tannery area occupying 25 ha of land. The tannery industries of Hazaribag process some 220 metric tons of hide a day with an associated release of 600 to 1,000 kg of tanned SCW resulting from the production of each ton of processed rawhide or skin (Zahid et al. 2004). Being of protein origin, these SCWs are converted to protein concentrate to be used as poultry feed and fish feed and in the production of organic fertilizers with treatment. According to Hossain et al. (2007), one of the major concerns of these activities is heavy metal contamination, especially chromium, in the tanning processes.
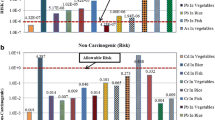
Dietary intake of heavy metals from vegetables, fish, rice, and poultry meat in Bangladesh
A food consumption survey conducted by Alam et al. (2003) and Kennedy et al. (2001) showed that the average daily diet of Bangladeshi people per person comprises the following: vegetables, 130 g; fish, 24 g; poultry meat, 7 g; and rice, 441.2 g. Weekly metal intake through the consumption of contaminated vegetables, rice, poultry meat, and fish was calculated based on the average heavy metal content and weekly intake of these foods. The weekly intake values of Cr, Cd, Pb, Hg, and As from different foods are presented in Table 6. Table 7 shows the provisional tolerable weekly intake of Cr, Cd, Pb, Hg, and As recommended by WHO/FAO and USNAS (NAS 1980; WHO 1989; WHO 1987; WHO/FAO 1999). Weekly intake of all metals through the consumption of examined samples was below the provisional maximum weekly intake recommended by WHO/FAO and USNAS.
Conclusion
The mean concentrations of heavy metals, except Cd, in water, vegetables, poultry meat, fish, and rice were higher than the safe limits suggested by WHO, Brunei standard (2001), FAO, USEPA, Indian standard, and other authors. The metals could be arranged in the following order according to the detected concentrations: in water, Cr > Pb > As > Hg > Cd; in vegetables, Cr > Pb > Cd > As > Hg; in fish, Pb > Cr > Cd > As > Hg; and in poultry meat, Pb > Cr > Cd > Hg > As. In each case, Cr concentration was high compared with the concentrations of other elements, which may be due to the discharge of untreated effluent from the tannery industries to the environment. In spite of this situation, the general population is exposed to chromium by the consumption of Cr-rich rice, fish, and vegetables. A recent study showed that poultry meat from fowl cultured in a poultry farm, which was usually fed with small pieces of leather containing Cr(VI) during the chrome tanning process of the hide, has high concentrations of Cr(VI) (Mahmud et al. 2011).
This study presents data on heavy metal pollution in and around Hazaribag and associated risk assessment for consumer exposure to heavy metals. The consumption of contaminated food results in long-term low-level accumulation of heavy metals in the human body. The detrimental effects on humans, including thalassemia, dermatitis, brain and kidney damage, and cancer, may be observed after several years of exposure. Thus, regular monitoring should be performed to investigate toxic heavy metal contamination in sewage, effluents, and main sources of food, such as vegetables, rice, fish, and meat, in Bangladesh, and prevent the excessive buildup of these toxic compounds in the food chain.
References
Adedeji, O. B., Adeyemo, O. K., & Oyedele, M. O. (2011). Heavy metals in snail and water samples from Alaro river in Oluyole industrial area of Ibadan southwestern Nigeria. Journal of Applied Sciences in Environmental Sanitation, 6(2), 115–121.
Adefemi, O. S., Ibigbami, O. A., & Awokunmi, E. E. (2012). Level of heavy metals in some edible plants collected from selected dumpsites in Ekiti State, Nigeria. Journal of Environmental Science and Technology, 1(5), 132–136.
Ahmed, J. U., & Goni, M. A. (2010). Heavy metal contamination in water, soil and vegetables of industrial areas in Dhaka, Bangladesh. Environmental Monitoring and Assessment, 166, 347–357. doi:10.1007/s10661-009-1006-6. Epub 2009 Jun 12.
Alam, M. G. M., Snow, E. T., & Tanaka, A. (2003). Arsenic and heavy metal concentration of vegetables grown in Samta village, Bangladesh. Science of The Total Environment, 308, 83–96.
Al-Nakshabandi, G. A., Saqqar, M. M., Shatanawi, M. R., Fayyad, M., & Al-Horni, H. (1997). Some environmental problems associated with the use of treated wastewater for irrigation in Jordan. Agricultural Water Management, 34(1), 81–94. doi:10.1016/S03783774 (96)01287-5.
Asaduzzaman, A. T. M., Nury, S. N., Hoque, S., & Sultana, S. (2002). Water and soil contamination from tannery waste: potential impact on public health in Hazaribag and surroundings, Dhaka, Bangladesh. Atlas of Urban Geology, 14, 415–443.
Awashthi, S. K. (2000). Prevention of Food Adulteration Act No. 37 of 1954. Center and sate rules as amended for 1999 (3rd ed.). New Delhi: Ashoka Law House.
Azom, M. R., Mahmud, K., Yahya, S. M., Sontu, A., & Himon, S. B. (2012). Environmental impact assessment of tanneries: a case study of Hazaribag in Bangladesh. International Journal of Environmental Science and Development, 3(2), 152–156.
Bhuiyan, M. A., Suruvi, N. I., Dampare, S. B., Islam, M. A., Quraishi, S. B., Ganyaglo, S., et al. (2010). Investigation of the possible sources of heavy metal contamination in lagoon and canal water in the tannery industrial area in Dhaka, Bangladesh. Environmental Monitoring and Assessment, 175(1–4), 633–649.
BIWWPP (2010). Blacksmith Institute’s world’s worst pollution problems report. Top six toxic threats: six pollutants that jeopardize the health of tens of millions of people. Produced in collaboration with Green Cross, Switzerland
CCREM. (1997). Canadian water quality guidelines. Ottawa: CCRM. Prepared by the Task Force on Water Quality Guidelines of the Canadian Council of Resource and Environment Ministers.
Charles, K., William, J. S., Mwegoha, Riziki, S., & Shemdoe. (2011). Heavy metal concentration in vegetables grow in the vicinity of the closed dumpsite. International Journal of Environmental Sciences, 2(2), 889–895.
Codex Alimentarius Commission [FAO/WHO]. (2001). Food additives and contaminants. Joint FAO/WHO Food Standards Program. Alinorm, 01(12A), 1–289.
Dunagan, S. C., Gilmore, M. S., & Varekamp, J. C. (2007). Effects of mercury on visible/near-infrared reflectance spectra of mustard spinach plants (Brassica rapa P.). Environmental Pollution, 148(1), 301–311.
Egyptian Organization for Standardization and Quality Control (EQSQC) (1993) Maximum levels for heavy metal contaminations in food. Egyptian standard N. 2360
Elbagermi, M. A., Edwards, H. G. M., & Alajtal, A. I. (2012). Monitoring of heavy metal content in fruits and vegetables collected from production and market sites in the Misurata area of Libya (pp. 1–5). Analytical Chemistry: International Scholarly Research Network.
Essa, H. H., & Rateb, H. Z. (2011). Residues of some heavy metals in fresh water fish (Oreochromis niloticus and Labeo niloticus) in Assiut City markets. Assiut University Bulletin Environmental Research, 14(1), 31–39.
FAO (1985) Water quality for agriculture. Irrigation and Drainage Paper No. 29, Rev.1.
FAO/WHO (2002) Codex Alimentarius general standards for contaminants and toxins in food. Schedule1. Maximum and guideline levels for contaminants and toxins in food, Joint FAO/WHO Food Standards Programme. Codex Committee, Rotterdam. Reference CX/FAC 02/16.
FEPA. (1991). Proposed national water quality standards. Nigeria: Federal Environmental Protection Agency.
Fu, J., Zhou, Q., Liu, J., Liu, W., Wang, T., Zhang, Q., et al. (2008). High levels of heavy metals in rice (Oryza sativa L.) from a typical E-waste recycling area in southeast China and its potential risk to human health. Chemosphere, 71, 1269–1275.
Ghimpeeanu, M. O., Das, K., Militaru, M., & Scippo, M. L. (2012). Assessment of heavy metals and mineral nutrients in poultry liver using inductively coupled plasma-mass spectrometer (ICP-MS) and direct mercury analyzer (DMA). Bulletin UASMV, Veterinary Medicine, 69(12), 258–266.
Hala, S. H. M., & Shireen, M. N. (2009). Estimation of some heavy metals in chicken shawarma. Egypt Journal of Comparative Pathology and Clinical Pathology, 22(3), 24–36.
Hassan N, Ahmed K (2000) Intra-familial distribution of food in rural Bangladesh. Institute of Nutrition and Food Science, University of Dhaka, Bangladesh. Internet pages available from http://www.unu.edu/ food/8F064e/8F064E05.htm, 11/9/01
Hossain, A. M. M. M., Monir, T., Haque, A. M. R. U., Kazi, A. L. M., Islam, S. M., & Elahi, S. F. (2007). Heavy metal concentration in tannery solid wastes used as poultry feed and ecotoxicological consequences. Bangladesh Journal og Scientific and Industrial Research, 42(4), 397–416.
Huq ISM (1998) Critical environmental issues relating to tanning industries in Bangladesh. Department of Soil Science, University of Dhaka, 1000, Bangladesh. In: Naidu R, Willett IR, Mahimarajah R, Kookana R, Ramasamy K (2000) Towards better management of soils contaminated with tannery waste. Proceeding of a workshop held at the Tamil Nadu Agricultural University, Coimbatore, India
Ideriah, T. J. K., David-Omiema, S., & Ogbonna, D. N. (2012). Distribution of heavy metals in water and sediment along Abonnema shoreline, Nigeria. Resources and Environment, 2(1), 33–40. doi:10.5923/j.re.20120201.05.
Jabeen, F., & Chaudhry, A. S. (2013). Metal uptake and histological changes in gill and liver of Oreochromis mossambicus inhabiting Indus River. Pakistan Journal of Zooloogyl, 45(1), 9–18.
Kachenko, A. G., & Sing, B. (2006). Heavy metals contamination in vegetables grown in urban and metal smelter contaminated sites in Australia. Water, Air, and Soil Pollution, 169, 101–123.
Kennedy, G., Burlingame, B., & Nguyen, V. N. (2001). Nutritional contribution of rice and impact of biotechnology and biodiversity in rice-consuming countries. Rome, Italy: Agricultural Officer Crop and Grassland Service FAO.
Khan, Y. S. A., Hossain, M. S., Hossain, S. M. G. M. A., & Halimuzzaman, A. H. M. (1998). An environment of trace metals in the GMB estuary. Journal of Remote Sensing and Environment, 2, 103–113.
Khan, S., Cao, Q., Zheng, Y. M., Huang, Y. Z., & Zhu, Y. G. (2008). Health risks of heavy contaminated soils and food crops irrigated with wastewater in Beijing, China. Environmental Pollution, 152(3), 686–692. doi:10.1016/j.envpol.2007.06.056.
Laws of Brunei (2001) Public health (food) regulations.
Liu, W. X., Shen, L. F., Liu, J. W., & Wang, Y. W. (2007). Uptake of toxic heavy metals by rice (Oryza sativa L.) cultivated in the agricultural soil near Zhengzhou City, People’s Republic of China. Bulletin of Environmental Contamination and Toxicology, 79, 209–213. doi:10.1007/s00128-007-9164-0.
Mahmud, T., Rehman, R., Ali, S., Anwar, J., Abbas, A., Farooq, M., et al. (2011). Estimation of chromium (VI) in various body parts of local chicken. Journal Chemista Soc Pak, 33(3), 339–342.
Mingorance, M. D., Valdes, B., & Oliver, R. S. (2007). Strategies of heavy metal uptake by plants growing under industrial emissions. Environment International, 33(4), 514–520. doi:10.1016/j.envint.2007.01.005.
Mousavi, S. R., Balali-Mood, M., Riahi-Zanjani, B., Yousefzadeh, H., & Sadeghi, M. (2013). Concentrations of mercury, lead, chromium, cadmium, arsenic and aluminum in irrigation water wells and wastewater used for agriculture in Mashhad, northeastern Iran. International Journal Occupational Medicine and Environmental, 4(2), 80–86.
Muchuweti, M., Birkeet, J. W., Chinyanga, E., Zvauya, R., Scrimshaw, M. D., & Lester, J. N. (2006). Heavy metal content of vegetables irrigated with mixture of wastewater and sewage sludge in Zimbabwe: implications for human health. Agriculture Ecosystem & Environment, 112(1), 41–48. doi:10.1016/j.agee.2005.04.028.
National Academy of Science. (1980). Recommended dietary allowances (9th ed.). NAS: Washington.
Oforka, N. C., Leo, C., Osuji, U. I., & Onwuachu. (2012). Assessment of heavy metal pollution in muscles and internal organs of chickens raised in rivers state, Nigeria. Journal of Emerging Trends in Engineering and Applied Sciences, 3(3), 406–411.
Okoye, C. O. B., Ibeto, C. N., & Ihedioha, J. N. (2011). Assessment of heavy metals in chicken feeds sold in south eastern, Nigeria. Advances in Applied Science Research, 2(3), 63–68.
Overesch, M., Rinklebe, J., Broll, G., & Neue, H. U. (2007). Metal and arsenic in soils and corresponding vegetation at central Elbe River flood plains (Germany). Environmental Pollution, 145(3), 800–812. doi:10.1016/j.envpol.2006.05.016.
Ozuni, E., Dhaskali, L., Abeshi, J., Fatgzim, L., Zogaj, M., Haziri, I., et al. (2011). Heavy metal in fish for public consumption and consumer protection. Nature Montenegrina, Podgorica, 9(3), 843–851.
Parashar, P., & Prasad, F. M. (2013). Study of heavy metal accumulation in sewage irrigated vegetables in different regions of Agra District, India. Open Journal of Soil Science, 3(1), 1–8.
Pescod MB (1992) Wastewater treatment and use in agriculture. FAO Irrigation and Drainage Paper 47. Rome: Food and Agriculture Organization of the United Nations.
Rahman, A. K. M. R., Hossain, S. M., & Akramuzzaman, M. M. (2010). Distribution of heavy metals in rice plant cultivated in industrial effluent receiving soil. Environment Asia, 3(2), 15–19.
Rapheal, O., & Adebayo, K. S. (2011). Assessment of trace heavy metal contaminations of some selected vegetables irrigated with water from river Benue within Makurdi Metropolis, Benue state Nigeria. Advances in Applied Science Research, 2(5), 590–601.
Ruzbarsky, J., Duranik, T., & Ulicn, A. (2013). Technology solution of decontamination process of soil. Engineering for rural development, Slovak Republic. Jelgava, 12, 107–110.
Saglam, C. (2013). Heavy metal accumulation in the edible parts of some cultivated plants and media samples from a volcanic region in southern Turkey. Ekoloji, 22(86), 1–8.
Salaramoli, J., Salamat, N., Razavilar, V., Najafpour, S. H., & Aliesfahani, T. (2012). A quantitative analysis of lead, mercury and cadmium intake by three commercial aquatics, Hypophthalmichthys molitrix, Onchorhynchus mykiss (Walbaum) and Fenneropenaeus indicus. World Applied Sciences Journal, 16(4), 583–588.
Sharma, R. K., Agrawal, M., & Marshall, F. (2006). Heavy metal contamination in vegetables grown in wastewater irrigated areas of Varanasi, India. Bulletin of Environmental Contamination and Toxicology, 77, 312–318. doi:10.1007/s00128-006-1065-0.
Sharma, R. K., Agrawal, M., & Marshall, F. (2007). Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicology and Environmental Safety, 66, 258–266. doi:10.1016/j.ecoenv.2005.11.007.
Shen, T. T. (1999). Industrial pollution prevention (2nd ed., p. 429). Verlag: Springer.
Singh, A., Sharma, R. K., Agrawal, M., & Marshall, F. M. (2010). Risk assessment of heavy metal toxicity through contaminated vegetables from wastewater irrigated area of Varanasi, India. Tropical Ecology, 51(2S), 375–387. doi:10.1016/j.fct.2009.11.041. Epub 2009 Nov 24.
Singh, J., Upadhyay, S. K., Pathak, R. K., & Gupta. (2011). Accumulation of heavy metals in soil and paddy crop (Oryza sativa), irrigated with water of Ramgarh Lake, Gorakhur, UP, India. Toxicological & Environmental Chemistry, 93(3), 462–473. doi:10.1080/02772248.2010.546559.
Surchi, K. P. (2011). Assessment of heavy metal contamination in different vegetables grown in and around urban areas. Research Journal of Environmental Toxicity, 5(3), 162–179.
Tripathi, R. M., Raghunath, R., & Krishnamoorthy, T. M. (1997). Dietary intake of heavy metals in Bombay City, India. Science of the Total Environment, 208(3), 149–159.
UNIDO. (2005). United Nations Industrial Development Organization, cost of tanned waste treatment, 15th session of the leather and leather products industry panel. Mexicom: Leon.
WHO. (1987). Principles for the safety assessment of food additives and contaminants in food. Environmental Health Criteria. No. 70. Geneva: WHO.
WHO. (1989). Evaluation of certain food additives and contaminants. Thirty-third report of the joint FAO/WHO expert committee on food additives. WHO technical series, No. 807. Geneva: WHO.
WHO (World Health Organization) (2004) Permissible level of metal in fish for human consumption.
WHO/FAO (1999) Maximum levels for lead in codex commodity standards. CX/FAC00/24. Rome
Xiaoming, H., Weibin, J., Wenjuan, L., Shuiyuan Cheng, S., & Yanyan, J. (2013). Investigation and evaluation on heavy metal copper and cadmium contaminations of vegetables grown in Huanggang city of China. Advance Journal of Food Science and Technology, 5(2), 106–109.
Yap, D. W., Adezrian, J., Khairiah, J., Ismail, B. S., & Ahmed-Mahir, R. (2009). The uptake of heavy metals by paddy plants (Oryza sativa) in Kota Marudu, Sabah, Malaysia. American Eurasian Journal of Agriculture & Environmental Science, 6(1), 16–19.
Zahid A, Balke KD, Hassan MQ, Flegr M (2004) Distribution of heavy metals in tannery effluent and their influence on sediments of Hazaribagh leather processing zone, Dhaka. In M. Q. Hassan (ed.) Water resources management and development in Dhaka City. (Goethe-Institut Dhaka)
Zhuang, P., Mcbride, B., Hanping, X., Ningyu, L. I., & Zhian, L. I. (2009). Health risk from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. Science of Total Environment, 407(5), 1551–1561. doi:10.1016/j.scitotenv.2008.10.061.
Acknowledgments
The authors would like to thank the Shahjalal University of Science and Technology Research Centre for the financial support extended to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, G.M.R., Khan, F.E., Hoque, M.M. et al. Consumption of unsafe food in the adjacent area of Hazaribag tannery campus and Buriganga River embankments of Bangladesh: heavy metal contamination. Environ Monit Assess 186, 7233–7244 (2014). https://doi.org/10.1007/s10661-014-3923-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3923-2