Abstract
Scalp and calvarial tuberculosis (TB) is infrequent even in endemic regions. While the former is rarely encountered even by dermatologists, the incidence of the latter may increase with increase in the incidence of TB and in number of patients with immunosuppressed states. The lack of awareness of this pathology, nonspecific imaging findings, and indolent history often result in delayed diagnosis. Painless scalp swelling with a discharging sinus is the commonest presentation of calvarial TB. Definitive diagnosis can be made either by demonstration of bacilli on staining, its growth on culture, characteristic granulomas on histology, or PCR tests. Early intervention is required to prevent intracranial extension. Anti-TB medications must be given for an extended period as it is a type of CNS TB. The role of surgery is in cases of diagnostic dilemma and where mass effect is a consideration.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Tuberculosis (TB) is a disease of antiquity [1]. Mention of this disease has been found as far back as the Rig Veda, and evidence of the disease has been found in Egyptian mummies dating to 3000 BC [2]. Though it can affect any organ system of the body, central nervous system (CNS) TB is a particularly dangerous form with high mortality and morbidity [2]. Though the commonly encountered variants of CNS TB are tubercular meningitis (TBM), intracranial tuberculomas or abscesses, and spinal TB, occasionally patients may present with TB affliction of the scalp and skull bones as well.
2 Scalp Tuberculosis
Cutaneous affliction by TB can be of three types – lupus vulgaris, TB verrucosa cutis, and scrofuloderma. However, the scalp is an uncommon site of occurrence of all three. Lupus is the Latin word for wolf and is a generic name for diseases causing erosive, erythematous ulceration of the face ravaging it like the bites of a wolf [3]. The suffix vulgaris was used to denote its common occurrence. It was first described by Erasmus Wilson in 1865 [4]. Manifesting as reddish brown papules that later coalesce into elevated plaques, it is caused by hematogenous dissemination of TB bacilli from a primary site of infection [3]. It usually occurs in the face. It has an indolent course and rarely may progress to squamous cell carcinoma. “Apple-jelly” appearance of the papules is found on diascopy [3]. It is diagnosed by the clinical features and skin biopsy. It responds well to anti-TB drugs.
TB verrucosa cutis (also called warty TB or prosector’s wart) is a paucibacillary TB infection of the superficial layers of the skin in an individual who has a high degree of immunity [3,4,5]. It was first described by Laennec in 1826, based on his own disease that he contracted in the autopsy room [4]. As it was thought to be contracted from corpses, it was termed verruca necrogenica by Wilks and Poland in 1862 [4]. It commonly occurs at the distal extremities, dorsal aspects of joints, and buttocks after trivial penetrating injury [3, 6]. These warts tend to enlarge centrifugally with central healing. Ulceration is not a prominent feature. Diagnosis is made by positive tuberculin skin test and histology. They regress with anti-TB drugs.
The last variant, scrofuloderma, is the commonest type of cutaneous TB. It usually occurs following secondary involvement of the skin following liquefactive necrosis of the afflicted lymph nodes lying underneath [3]. The neck is the commonest site of involvement. Painless fistula formation with pus discharge is the main clinical feature – a finding in most cases of calvarial TB too. Diagnosis is made on clinical grounds as well as by detection of the organism on staining, culture, or polymerase chain reaction (PCR) testing. Under treatment the fistula heals with scarring [3].
3 Calvarial Tuberculosis
3.1 Introduction
Calvarial TB is a rare disease [7,8,9], infrequently encountered, and not always considered as a first differential diagnosis. It was first reported by Reid in 1842 from Germany [9, 10]. Though recent years have seen a spurt of publications on calvarial TB, these tend to be in the nature of single case reports rather than large series.
3.2 Epidemiology
The majority of cases of calvarial TB are reported from developing countries where systemic TB is endemic [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. While 1% of cases of TB occur in the bone [23], calvarial involvement accounts for only 0.2–1.3% of these [7, 23], i.e., approximately 1 in 10,000 cases of TB. Literature states that the frontal and parietal bones having greater amount of diploic space (and hence receiving more blood flow) are more commonly affected than bones like occipital or temporal which are less vascularized [7, 8, 23]. Conversely, it has also been stated that muscular attachments to the temporal and occipital bones render them vascular with higher flow rates where a TB nidus cannot gain a foothold unlike the frontal and parietal bones which have a slow flowing diploic emissary circulation that allows deposition of mycobacteria and growth of the TB focus [12].
The disease is rare in infants [23], as the skull is poor in cancellous bone [10]. Despite this, it has been described in children [24] – the youngest described being only 10 months old [19]. This condition is predominantly encountered in younger individuals, and 75–90% cases have been reported to occur in patients less than 20 years and 50% in children less than 10 years old [23]. It affects both sexes equally though certain series show a predilection toward females or males [13,14,15].
3.3 Pathogenesis
The disease follows hematogenous seeding of TB bacilli in the marrow spaces [7, 8, 13]. It can occur rarely by lymphatic or contiguous local spread from the face, paranasal sinuses, orbit, or cervical lymph nodes [10, 13]. Infrequency of calvarial TB is due to paucity of cancellous component in the flat bones of the skull and also due to lack of lymphatics in them [7, 12]. Mukherjee et al. have used the term primary calvarial TB when there is no evidence of TB detected anywhere else in the body [12].
In the setting of depressed host immunity, deposition of the bacilli in the diploic spaces of the skull is followed by proliferation of bacteria, capillary obliteration, replacement of the bone by granulation tissue and finally abscess formation with destruction of the cortex. Destruction of the outer table occurs and subgaleal collection of pus and granulation tissue results in a boggy swelling without increased temperature, tenderness, induration, or systemic symptoms like fever. On occasion this may erode through the scalp and present as a non-healing sinus. The inner table is relatively resistant but when eroded results in the development of an epidural collection [9]. The dura is usually a resistant barrier to further intracranial spread [8, 22]. However, sutures do not prevent spread of the disease [8, 23]. It is unclear if any of the tables is more likely to get eroded than the other. While Strauss held that the inner table was more likely to be initially involved [25], others [9, 13] believe that the outer table is more likely to be destroyed first, and still others [26] found both tables to be equally affected. In the series of Raut et al. [18], 85% of patients had bony destruction, and epidural collections were seen in 52%.
3.4 Role of Trauma and Immune Suppression
Several case reports of calvarial TB report a past history of trauma [17] to the involved bone. It has been speculated that these areas have focal immunosuppression with increased vascularity that predisposes to the genesis of TB here at a later date [12, 13]. Inflammatory cells that are attracted to the site of trauma are also held to act as vectors aiding transmission [15]. Several authorities, however, hold that trauma is probably coincidental rather than causal [26, 27]. Immune-suppressed states like human immunodeficiency virus (HIV) infection are also implicated in its causation [13, 20] as extrapulmonary TB has a higher incidence in such patients (70% in HIV-infected versus 15% in noninfected patients).
3.5 Presentation
The common presenting complaints are painless, boggy swelling of the scalp and which may occasionally spontaneously erode the skin (Fig. 5.1) and form a discharging sinus [13, 17, 19, 28]. The underlying bone may or may not be visible. Inflammatory signs are markedly absent even in the presence of pus. Skin discoloration (Fig. 5.2) is a late feature [13]. Unlike Pott’s puffy tumor, it may have a firmly attached base, and deficient outer table of the skull may be palpable. Rarely, headache may be present and is usually localized to the site of infection [13]. Concomitant intracranial pathologies (like epidural or cerebral abscess, tuberculoma, or meningitis) may occur due to which occasional patients have been reported with drowsiness, seizures, hemiparesis, and even increased intracranial pressure (IICP) [13, 15, 21, 29]. There is a case report of occlusion of sigmoid sinus following TBC mastoiditis with IICP and formation of an encephalocele [30] as well as one of occlusion of the superior sagittal sinus [17]. Systemic manifestations like weight loss, evening rise of temperature, decreasing appetite, etc. are rare.
(a) Plain radiograph showing erosion of the right frontal bone in a 34-year-old male that had been twice unsuccessfully operated and treated with broad-spectrum antibiotics assuming swelling was due to an infected sebaceous cyst and (b) a discharging sinus showing skin changes overlying sequestered bone
3.6 Investigations
Raised erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values and also positive Mantoux test may give a clue for diagnosis of skull TB. Mantoux test may not be positive in 10% of calvarial TB patients [23] and is of doubtful value when positive in countries having a vaccination program with attenuated TB bacilli. Likewise, ESR and CRP levels are not always raised and also are not specific for TB. Routine culture of the discharge is not regularly reported in literature and is probably not useful to clinch the diagnosis. Some series have reported sterile or polymicrobial cultures [12], and on occasion, it may even mislead the clinician into assuming the case to be one of pyogenic osteomyelitis [12].
Plain radiographs of skull are helpful in finding the bony changes which commonly manifest as punched-out lesions [23]. Computed tomography (CT) scans are useful in identifying the extent of damage to the skull bone, involvement and breach of dura, size of the swelling and associated intracranial pathologies [18]. Skull defects are usually single, but on occasion multiple punched-out lesions have been described [16, 29].
Radiologically, three variants are described – circumscribed sclerotic, lytic, and spreading [18]. The lytic variant (Fig. 5.3), also called “perforating TB of the skull” by Volkmann [13, 18], is the most common. In these areas of rarefaction are seen initially that later developing into punched-out defects. There is no periosteal reaction. Rarely these may have a central sequestrum (“button sequestrum” or “bone sand”) within [12]. The second variant is a defect having a margin of sclerotic bone. Its presence may indicate secondary infection [12] or may represent evidence of healing and is caused by new bone formation on the edges. The third variant is the “spreading type” where there is widespread destruction of the diploic spaces with abundant granulation tissue. This was called “diffuse TB of the cranium” by Konig [18]. This classification has been disputed by some authors who feel that the types are not separable [12]. It must be remembered that the radiological picture is nonspecific [18], and usual differential diagnoses include skull metastasis, myeloma, hemangioma, aneurysmal bone cyst, pyogenic osteomyelitis, and Langerhans cell histiocytosis [12, 13].
(a) 3D reconstructed CT scan showing a punched-out lesion involving both tables of the skull in a 40-year-old lady (lytic tuberculosis) almost near the midline, (b, c) coronal and axial CT images with contrast showing skull defect, and an epidural collection and (d) intraoperative picture showing a punched-out lesion with serous fluid mixed with pus coming out of it. The surrounding bone margins are hyperemic
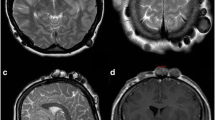
Finally, magnetic resonance imaging (MRI) is a sensitive tool in bringing out any associated intracranial pathology (changes in meninges, ventricular walls, or parenchymal foci of infection) [18], epidural abscess, or venous sinus involvement (Fig. 5.4). The extent of bone involvement beyond the margins of the punched-out lesion can also be appreciated as bone edema on MRI sequences and may help to determine the extent of craniotomy.
(a) T2 axial imaging showing an hyperintense collection below the bone pushing dura inward, (b, c) T1 axial and sagittal imaging showing isointense collection showing perforation of the skull but there is as yet no scalp collection and (d) coronal contrast imaging showing that there is enhancing soft tissue representing granulation tissue. There is as yet no intradural involvement
The gold standard for diagnosis is the demonstration of acid-fast bacilli (AFB) on microscopy and growth on culture, but this may not be always possible [7, 8]. The presence of characteristic granuloma (epitheloid cells, plasma cells, and Langhans-type giant cells with central caseous necrosis) on histology with response to empirical anti-TB drugs is then considered to be proof that the infection is TB [8, 11]. Usually, this tissue is obtained by craniotomy of the involved bone or by curettage and debridement. Some authors have advocated fine needle aspiration cytology (FNAC) to obtain tissue as a means of avoiding surgery [7, 12]. They have, however, cautioned that FNAC may not yield the correct diagnosis in the presence of secondary infection [12]. In situations with low bacillary load where detection on conventional staining and culture are difficult, automated nested PCR test using a platform for rapid nucleic acid amplification is a valid investigation to detect both mycobacterial DNA and rifampicin (RIF) resistance and can clinch the diagnosis [11].
3.7 Treatment
The mainstay of treatment is anti-TB drugs [22]. This consists of an intensive phase of therapy with four drugs – RIF, isoniazid, ethambutol, and pyrazinamide – and a continuation phase with two drugs. While the World Health Organization recommendation is to give the former for 2 months and the latter for 4 months in most cases of extrapulmonary TB, they have suggested continuing the latter for longer periods in special situations like CNS TB. Our institute protocol is to give the same for 18 months in calvarial TB – a practice followed by other centers as well [9, 14, 15, 28]. Some authorities have advocated giving anti-TB drugs for up to 24 months as well [18]. Steroids are not indicated unless there is evidence of concomitant meningitis as well. Likewise, anticonvulsants are also not required unless there is a history of seizures [18].
Surgery is indicated when diagnosis is uncertain [23], there is presence of epidural abscess, there are lesions with mass effect and to remove sequestrated bone [10, 28]. Craniotomy may be done to ensure removal of the diseased bone beyond the boundaries of the defect, but it is unclear what margins would be considered acceptable. We would think that freshening margins with a nibbler till there is bleeding from the raw edges to be acceptable. The fibrosed walls and mouth of any sinus can be dealt with at the same sitting [13]. Tension of the suture line is to be avoided to prevent necrosis of skin margins. The question of cranioplasty to repair the craniectomy defect has not been discussed in published literature [12] – particularly with respect to timing. Some authors advocate waiting for microbiological cure before cranioplasty [28]. As it is often unclear if the lesion is TB or pyogenic at the time of surgery, we too would advocate interval cranioplasty rather than primary repair.
Conclusion
The calvarium and scalp are uncommon locations of TB. These patients present with an indolent history and protean features. The clinician must bear this possibility in the back of his mind so that time is not wasted on futile therapies and further CNS involvement is arrested. Calvarial TB is a kind of CNS TB, and extended duration of anti-TB drugs is mandated.
Abbreviations
- AFB:
-
Acid-fast bacilli
- CNS:
-
Central nervous system
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- DNA:
-
Deoxyribonucleic acid
- ESR:
-
Erythrocyte sedimentation rate
- FNAC:
-
Fine needle aspiration cytology
- HIV:
-
Human immunodeficiency virus
- IICP:
-
Increased intracranial pressure
- MRI:
-
Magnetic resonance imaging
- PCR:
-
Polymerase chain reaction
- RIF:
-
Rifampicin
- TB:
-
Tuberculosis
- TBM:
-
Tubercular meningitis
References
Daniel TM (2006) The history of tuberculosis. Respir Med 100:1862–1870
Tandon PN, Pande A (2012) Tuberculosis of the central nervous system. In: Tandon PN, Ramamurthy R (eds) Textbook of neurosurgery, 1st edn. Jaypee Brothers Medical Publishers (P) Ltd., New Delhi, pp 725–741
Shimizu H (2007) Mycobacterial infections. In: Shimizu H (ed) Shimizu’s textbook of dermatology. Nakayama Shoten Publishers, Hokkaido, pp 484–488
Padmavathy L, Lakshmana Rao L, Pari T, Ethirajan N, Krishnaswamy B (2008) Lupus vulgaris and tuberculosis verrucosa cutis (TBVC) – a clinical, pathological and epidemiological study of 71 cases. Indian J Tuberc 55:203–209
Aliağaoğlu C, Atasoy M, Güleç AI, Özdemir S, Erdem T, Engin RI (2009) Tuberculosis verrucosa cutis. Eur J Gen Med 6:268–273
Rajan J, Mathai AT, Prasad PVS, Kaviarasan PK (2011) Multifocal tuberculosis verrucosa cutis. Indian J Dermatol 56:332–334
Khare P, Gupta R, Chand P, Agarwal S (2015) Calvarial tuberculosis presenting as cystic lesion: an unusual presentation in two patients. J Cytol 32:176–180
Rajmohan BP, Anto D, Alappat JP (2004) Calvarial tuberculosis. Neurol India 52:278–279
Nair AP, Mehrotra A, Das KK, Kumar B, Srivastav AK, Sahu RN, Kumar R (2015) Calvarial tuberculosis of the parietal bone: a rare complication after dental extraction. Asian J Neurosurg 10:219–221
Zaki S, Dadge D, Shanbag P (2010) Calvarial tuberculosis. Int J Infect Dis 14:e86–e87
Krishnan P, Sanyal S, Gupta D (2015) Primary lytic calvarial tuberculosis: a report of two cases. Acta Neurochir 157:2223–2225
Mukherjee KK, Kaushik R, Nada R, Khosla VK, Khandelwal N, Kak VK (2002) Calvarial tuberculosis. Surg Neurol 57:195–202
Diyora B, Kumar R, Modgi R, Sharma A (2009) Calvarial tuberculosis: a report of eleven patients. Neurol India 57:607–612
Ramdurg SR, Gupta DK, Suri A, Sharma BS, Mahapatra AK (2010) Calvarial tuberculosis: uncommon manifestation of common disease – a series of 21 cases. Br J Neurosurg 24:572–577
Jadhav RN, Palande DA (1999) Calvarial tuberculosis. Neurosurgery 45:1345–1349
Samson SK, Kulkarni V, Chacko AG (2002) An unusual presentation of calvarial tuberculosis. Postgrad Med J 78:184
Sundaram PK, Sayed F (2007) Superior sagittal sinus thrombosis caused by calvarial tuberculosis: case report. Neurosurgery 60:E776
Raut AA, Nagar AM, Muzumdar D, Chawla AJ, Narlawar RS, Fattepurkar S, Bhatgadde VL (2004) Imaging features of calvarial tuberculosis: a study of 42 cases. AJNR Am J Neuroradiol 25:409–414
Dawar P, Gupta DK, Sharma BS, Jyakumar A, Gamanagatti S (2013) Extensive calvarial tuberculosis presenting as exophytic ulcerated growth on scalp in an infant: an interesting case report with review of literature. Childs Nerv Syst 29:1215–1218
Rajesh A, Purohit AK, Lakshmi V (2009) Calvarial tubercular osteomyelitic abscess. Indian J Med Microbiol 27:380–381
Patankar T, Varma R, Krishnan A, Prasad S, Desai K, Castillo M (2000) Radiographic findings in tuberculosis of the calvarium. Neuroradiology 42:518–521
Dawar P, Satyarthee GD, Sharma BS (2015) Total resolution of large scalp swelling due to calvarial tuberculosis with medical management only: case report and review of the literature. Turk Neurosurg 25:313–316
Rosli FJ, Haron R (2016) Tuberculosis of the skull mimicking a bony tumor. Asian J Neurosurg 11:68
Stones DK, Schoeman CJ (2004) Calvarial tuberculosis. J Trop Pediatr 50:361–364
Strauss DC (1933) Tuberculosis of the flat bones of the vault of skull. Surg Gynaecol Obstet 57:384–398
Meng CM, Wu YK (1942) Tuberculosis of the flat bones of the vault of the skull. J Bone Joint Surg 34:341–353
Barton CJ (1961) Tuberculosis of the vault of skull. Br J Radiol 34:286–290
de Lima Fde M, Pessoa LA, da Silva JC, Jungman P, Maranhão S (2003) Calvarial tuberculosis: case report. Arq Neuropsiquiatr 61:144–145
García-García C, Ibarra V, Azcona-Gutiérrez JM, Oteo JA (2013) Calvarial tuberculosis with parenchymal involvement. Travel Med Infect Dis 11:329–331
Vitali AM (2008) Acquired encephalocele attributable to tuberculous osteitis: case report. Neurosurgery 62:E976
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Krishnan, P. (2017). Scalp and the Calvarium. In: Turgut, M., Akhaddar, A., Turgut, A., Garg, R. (eds) Tuberculosis of the Central Nervous System. Springer, Cham. https://doi.org/10.1007/978-3-319-50712-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-50712-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50711-8
Online ISBN: 978-3-319-50712-5
eBook Packages: MedicineMedicine (R0)