Abstract
The diagnosis of intrahepatic cholangiocarcinoma is based on pathology. Nevertheless, imaging plays a central role for tumor staging as well as for the assessment of locoregional and distant extension. Tumor spread is very different from that in other liver tumors, especially hepatocellular carcinoma. Parenchymal, vascular, perineural, nodal, and distant extension must be accurately assessed because it has significant prognostic value. Computerized tomography (CT) and magnetic resonance imaging (MRI) provide essential information, while positron emission tomography (PET) can be useful in selected cases.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
4.1 Overview
4.1.1 Epidemiology
Intrahepatic cholangiocarcinoma (ICC) is a primary neoplasm that develops from epithelial cells of the intrahepatic bile ducts. It represents 10–15% of all primary liver cancers and is the second most common primary hepatic cancer after hepatocellular carcinoma [1]. It is associated with a very poor prognosis because most patients with ICC present with advanced disease, characterized by large multifocal tumors, vascular invasion, and in some cases extrahepatic spread.
4.1.2 Risk Factors
Cholangiocarcinogenesis is a multifactorial process. Several risks factors have been identified, but in the vast majority of cases, no risk factor is present. Long-established risk factors are hepatobiliary flukes (Opisthorchis viverrini and Clonorchis), primary sclerosing cholangitis (PSC), biliary inherited anomalies (choledochal cystic diseases, Caroli’s disease), hepatolithiasis, and toxins. More recently, cirrhosis has been shown to be a risk factor.
4.1.3 Pathology
According to the Japanese Liver Cancer Group, different patterns of tumor growth can be described for ICC: mass-forming (exophytic), periductal (infiltrating), intraductal (polypoid), or mixed (mass-forming and periductal) pattern. Mass forming is the most frequent pattern in intrahepatic cholangiocarcinoma [2].
The pathological diagnosis of ICC is based on the WHO classification of biliary tract cancer. ICC is an adenocarcinoma or mucinous carcinoma with frequent marked fibrous stroma. Tumor spread is characterized by local intrahepatic metastases, vascular and perivascular invasion, lymphatic involvement, and perineural invasion, explaining both local and distant tumor spread patterns.
Local spread is driven by peritumoral intrahepatic metastases and vascular involvement. Intrahepatic local metastases are often referred to as “daughter” or “satellite” nodules and correspond to the growth of small tumors surrounding the main lesion. Local spread is different from that observed in hepatocellular carcinoma (HCC) and is more frequently macroscopic. Moreover, and unlike HCC, micro- and macrovascular spread are not endoluminal but perivascular and result in progressive vessel encasement.
Locoregional and more distant spread rely mostly on invasion and diffusion through the deep lymphatic system of the liver as well as on perineural invasion. The lymphatic system of the liver is divided into deep and superficial networks (◘ Fig. 4.1) [3]. The deep system follows the ramifications of the portal triads and hepatic veins, while the superficial system is in the connective tissue of convex and inferior surfaces of the liver. In the deep system, the periportal lymphatic tract is the most important, responsible for 80% of hepatic lymph drainage. Small lymphatics progressively merge into larger lymph vessels along the portal tract transporting lymph in the same direction as the bile, toward the hepatic hilum. The rich plexus of periportal tract lymphatics converges to form 12–15 lymph vessels that drain into the hepatic lymph nodes of the portal hilum. This explains the frequent hilar nodal extension of ICC. These hepatic lymph nodes are located along the hepatic vessels in the lesser omentum. The outgoing efferent lymphatic vessels from the perihilar lymph nodes reach the celiac lymph nodes, which drain into the cisterna chili (Pecquet cisterna) which is the dilated origin of the thoracic duct. The other part of the deep system contains lymphatics that follow the hepatic veins and merge into five to six large lymphatic vessels that pass through the diaphragm along with the inferior vena cava toward the posterior mediastinal lymph nodes [3]. This explains the possible presence of lung metastases as well as vascular invasion.
Schematic representation of the liver lymphatic system. a Deep lymphatic system of the liver—portal tract showing the course of the deep periportal lymphatic system (red arrows) to lymph nodes of the hepatic hilum and then to the celiac lymph nodes (yellow dots). b Deep lymphatic system of the liver—hepatic veins tract showing the course of the deep ascending lymphatic system (purple arrows) following hepatic veins to mediastinal lymph nodes (yellow dots). c Superficial lymphatic system of the liver. Right set (orange arrow): lymph vessels go by on the abdominal surface of the diaphragm to reach the celiac lymph nodes. Middle set (red arrows): through the inferior vena cava foramen to the mediastinal lymph nodes. Left set (purple arrows): through the esophageal hiatus to the superior gastric lymph nodes. Few central vessels (pink arrows) drain along the falciform ligament downward to the abdominal wall or upward to the parasternal lymph nodes. d Superficial lymphatic system of the liver—inferior surface. The majority of lymphatics (red, purple and pink arrows) converge toward the hepatic lymph nodes of porta hepatis. Few lymphatics of the posterior part of caudate and right lobes (orange and pink arrows) accompany the inferior vena cava through the diaphragm to mediastinal lymph nodes
Perineural invasion is found at histopathology in up to 80% of patients. It is a known marker of highly aggressive disease and is associated with a poor prognosis [4,5,6]. The process of perineural invasion is different from metastases via the bloodstream or the lymphatic system, with distinctive histologic features, underlying cellular mechanisms, and molecular mediators [6].
4.1.4 Diagnosis
Imaging plays an important role in the diagnostic process as it allows assessment of locoregional and distant tumor spread. Imaging features of ICC have been well described [1, 7]. The goal of imaging of ICC is threefold: (a) to characterize ICC by identifying suggestive imaging features, (b) to confirm the diagnosis with imaging-guided biopsy (c) to perform pre-therapeutic staging, and to assess surgical resectability. Several complementary imaging techniques are used to this purpose. Abdominal ultrasound (US) is often the first-line examination to identify tumor location and visualize upstream bile duct dilatation, when present. In experienced hands, US is a highly accurate diagnostic exam. However, a differential diagnosis and locoregional tumor extension cannot be determined with this technique. Multiphase contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) are therefore performed to reach a positive and differential diagnosis of the lesion for tumor staging. Although CT accurately evaluates vascular encasement and provides whole-body imaging, MRI is the best technique to assess intrahepatic spread and biliary extension.
On unenhanced CT, ICC is usually seen as a hypoattenuating focal liver mass with irregular margins. Occasionally, calcifications may be seen. Multiphase contrast-enhanced CT acquisitions show peripheral rim enhancement on arterial phase images with progressive uptake on portal venous and delayed phases due to the presence of marked fibrous stroma [8]. CT may also frequently show biliary obstruction, capsular retraction, and/or ipsilateral parenchymal atrophy. On MR imaging, ICC is hypointense on T1-weighted and moderately hyperintense on T2-weighted images [9]. T2-weighted images may also show central hypointensity corresponding to areas of fibrosis. After extracellular contrast agent administration, dynamic images show peripheral enhancement on arterial phase images followed by progressive and concentric enhancement, somewhat similar to that observed with CT. If MR cholangiopancreatography is performed, it can help visualize the level of biliary obstruction, but the value of this technique is more limited than for hilar cholangiocarcinoma. Several enhancement patterns have been described on hepatospecific gadolinium chelate-enhanced MR imaging [10]. Most lesions (60%) show a thin peripheral rim with centripetal or progressive enhancement on portal venous phase images and marked hypointensity on hepatobiliary phase images (96%) [10].
The use of 18-fluorodeoxyglucose positron emission tomography (FDG-PET) to detect cholangiocarcinomas is a subject of debate. ICCs are usually considered to be FDG-avid lesions, and tumors as small as 1 cm can be detected with a reported sensitivity of 85–95%. However, FDG-PET is less accurate for identifying infiltrating tumors [11, 12]. Thus the clinical value of PET/CT for the diagnosis of ICC is limited when CT and/or MR imaging can identify typical features [13].
4.1.5 Staging
Optimal treatment of cholangiocarcinoma is based on complete tumor resection, which mainly depends on locoregional and distant tumor extension. The goal of surgery is to obtain complete tumor resection with safe margins (R0) while preserving a sufficient and functional future liver remnant, i.e., with good vascular in- and outflow and by restoring bile flow.
Unlike hilar cholangiocarcinoma, the literature on the staging of ICC is poor. However, several staging systems have been proposed, including two based on data from Japan [8, 9]. Okabayashi et al. [8] have proposed a staging system based on several independent factors associated with poor long-term survival, including the presence of vascular invasion, symptomatic disease, regional lymph node metastases, and multiple tumors (► Box 4.1). Yamasaki et al. [9] include tumor size (<2 cm or >2 cm), solitary vs. multiple tumors, the presence/absence of vascular or peritoneal invasion, distant metastases, and the presence/absence of regional lymph node metastases in their staging system (◘ Table 4.1). Recent studies have shown that prediction of the long-term prognosis was poor with this staging system and have proposed a new one (► Box 4.2) [10]. In 2010 the 7th edition of the AJCC/UICCA staging manual was published, including a specific staging system for ICC (► Box 4.3) [11]. With the AJCC/UICCA system, tumor size is not considered a prognostic factor, while T-classification is based on the number of lesions, on the presence/absence of vascular invasion, intrahepatic metastasis, and invasion of adjacent structures. The AJCC/UICCA staging system also includes both “N” and “M” subclassifications. Regional lymph node metastases in the hilar, periduodenal, and peripancreatic nodes are considered N1 disease. This staging system has been independently validated [12] and endorsed by the European Association for the Study of the Liver (EASL) as the preferred staging system for resected ICC (recommendation level B1) [1]. Nevertheless, EASL acknowledges that future studies should focus on stratifying nonsurgical patients for clinical studies using a clinical rather than a surgical staging process [1]. Recent reports suggest that the presence of metastatic lymph node or the analysis of lymph node ratio has better prognostic value than the AJCC 7th edition staging system [13]. These reports also stress that tumor size and biliary invasion should be reintroduced [14] (◘ Table 4.2), and that tumor growth types are essential [16].
4.1.6 Treatment and Prognosis
Although surgery is the best-known treatment, only 20–40% of patients with ICC are eligible for potential curative resection at diagnosis [1]. Adjuvant chemotherapy and/or radiotherapy has failed to improve survival in most patients. The 5-year survival in patients following curative surgery is 30–35%, while the median overall survival is approximately 28 months [1]. ◘ Table 4.3 lists the main factors affecting survival in five different surgical series. The most important prognostic factors are age at diagnosis, tumor size (>2–3 cm), lymph node metastases, multiple tumors or intrahepatic metastases, vascular invasion [14, 16], and type of enhancement on contrast-enhanced CT [20]. Serosal invasion is not considered to be a prognostic factor in all studies [17, 21]. Multivariate analyses have shown that lymph node metastases, multiple tumors at presentation, and vascular invasion are the most important independent factors associated with a poor postoperative outcome [17, 18].
4.2 Patterns of Local Spread
4.2.1 Parenchymal Dissemination
According to Okabayashi et al., approximately one third of patients with a preoperative diagnosis of a solitary tumor have multiple satellite lesions in the resected specimen (◘ Fig. 4.2) [8]; when larger than 1 or 2 cm, approximately two thirds of satellite nodules are detected by imaging [20]. Historical studies have shown that CT and conventional MR imaging have similar performances for the detection of satellite lesions [17]. With both imaging modalities, nodules are appreciated because they enhance in parallel with the primary tumor. With the introduction of diffusion-weighted imaging (DWI) (◘ Fig. 4.3) and of hepatobiliary MR contrast agents, satellite nodules can be visualized better by MRI. Kang et al. reported additional daughter nodules (i.e., located around the main tumor) in 10% of the patients and intrahepatic metastases (i.e., distant from the main tumor) in 2%, using gadoxetic acid-enhanced MRI during the hepatobiliary phase [21]. CT or MR can be used to estimate the volume of the future liver remnant of potential surgical candidates. In patients with a healthy liver parenchyma, 25% of the total liver volume should be preserved to minimize morbidity from resection, whereas 40% of the total liver volume is required in patents with extensive fibrosis or cirrhosis. In patients with an insufficient future liver remnant, portal vein embolization (PVE) may be indicated to increase volume of remnant liver [18, 19]. Patients traditionally undergo sequential treatment with preoperative biliary drainage when necessary followed by PVE before resection.
Example of satellite nodules in a 63-year-old male with intrahepatic cholangiocarcinoma located in the right liver. Contrast-enhanced CT on portal venous a, b and delayed phase images c, d show a large heterogeneous mass with progressive enhancement (from a to c) and capsular retraction due to the presence of abundant fibrous stroma. Several smaller lesions (arrows in b and d) are depicted around the main tumor, with similar aspect. These lesions correspond to satellite nodules
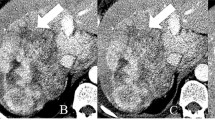
Added value of diffusion-weighted images for the detection of satellite nodules in a 47-year-old male with intrahepatic cholangiocarcinoma located in the right liver. Contrast-enhanced CT on portal venous phase a, b shows a large heterogeneous mass in the right lobe (arrow in a) and several smaller lesions (arrows in b) located in the liver dome. On diffusion-weighted images c, d with high b values, the main tumor is more conspicuous (arrow in c). Images show significantly more satellite nodules in the liver dome (dashed circle in d) and a contralateral nodule (arrow in d)
4.2.2 Vascular Invasion
Assessment of vascular extension is important because it determines the therapeutic options and is predictive of oncological outcome. Unlike hepatocellular carcinoma, which spreads to the vascular lumen, progression of ICC results in vascular encasement. Imaging features suggestive for vascular involvement include close contact between the tumor and the vessel, vascular deformation, and stenosis or irregularities with nearly complete occlusion. Vascular encasement is present in approximately 50% of cases, and it involves the portal branches (◘ Fig. 4.4) more often than the hepatic veins (◘ Fig. 4.5) [20, 22]. The presence of segmental or lobar atrophy is strongly associated with ipsilateral portal vein encasement [23]. Anatomical variants (e.g., accessory right hepatic vein, low insertion of the right posterior portal vein, etc.) should be reported because it can change the treatment strategy.
Portal vein encasement in a 52-year-old male with intrahepatic cholangiocarcinoma of the right liver lobe. Contrast-enhanced CT with maximum intensity projection shows the heterogeneous mass in the right liver and the absence of right portal vein (arrow), due to the tumoral encasement. The main portal vein and the left branch were not involved
Hepatic vein encasement in a 57-year-old female with intrahepatic cholangiocarcinoma. Contrast-enhanced CT (portal venous phase) shows a heterogeneous mass located in the central part of the liver, in close contact with the inferior vena cava. The left hepatic vein is completely encased in the tumor and shows no contrast enhancement (black arrow). The right hepatic vein (white arrow) and the portal veins (dashed arrow) are not occluded
Doppler ultrasound effectively identifies vascular invasion, encasement, or occlusion of both the portal veins and the hepatic arteries. In one study, preoperative US correctly identified 13/16 cases of liver tumors involving the hepatic vein yielding a sensitivity, specificity, and a PPV of 81%, 97%, and 87%, respectively [15]. In a second historical study, preoperative US identified 38/41 patients (sensitivity = 93%) with portal vein involvement at surgery [24]. The above reported results were comparable to those of CT arterial portography. Nevertheless, in clinical practice the reference imaging modalities for vascular invasion are contrast-enhanced CT and MR imaging preferably with 3D and multiplanar reconstructions [25]. Although the two examinations are comparable, CT is considered more reliable in the assessment of vascular involvement due to its high spatial resolution.
4.3 Regional and Distal Spread
4.3.1 Lymph Node Involvement
It is important to accurately assess lymph nodal involvement as the presence of metastatic disease within the regional lymph nodes is a strong predictor of a poor long-term outcome following curative intent resection of intrahepatic cholangiocarcinoma [26]. Regional lymph nodes are divided into N1 nodes (hilum and around the common bile duct, periportal, peripancreatic, periduodenal) and N2 nodes (superior mesenteric and celiac). In published series, the overall accuracy of CT for detection of metastatic lymph nodes is 77%, and the most common error in preoperative imaging is underestimation of nodal involvement [20]. At CT neoplastic lymph nodes are round or enlarged and with a heterogeneous enhancement (◘ Figs. 4.6 and 4.7). Unfortunately, this pattern is uncommon in the early stage of lymph node involvement. Indeed, as previously reported, the size of the nodes is poorly correlated with tumor status since small nodes may be metastatic and large lymph nodes may be benign. For instance, Adachi et al. reported a 50% sensitivity for enlarged nodal size for the detection of tumor invasion [27]. Lymph nodes around the cardiac portion of the stomach and along the lesser gastric curvature should be examined in addition to nodes in the hepatoduodenal ligament in ICC of the left lobe [28].
Metastatic lymph nodules in a 51-year-old female with intrahepatic cholangiocarcinoma. Contrast-enhanced CT (portal venous phase) shows a heterogeneous mass located in segment 8 (arrow in a). Several enlarged and heterogeneous lymph nodes are depicted around along the liver vascular pedicle, around the hepatic and splenic arteries, and the celiac axis (arrows in b)
Retroperitoneal metastatic lymph nodules in a 68-year-old male with intrahepatic cholangiocarcinoma. Contrast-enhanced CT (portal venous phase) shows numerous bilobar hypodense tumors corresponding to disseminated intrahepatic cholangiocarcinoma a. Multiple enlarged and heterogeneous lymph nodes are depicted in the retroperitoneum (arrows in b and c)
Since at diffusion-weighted imaging, lymph nodes are hyperintense regardless of the tumoral status; there is no added value from this additional sequence. Conversely, the use of hepatobiliary MR contrast agents could indirectly help in identifying nodal invasion. Indeed, Kang et al. have shown that the extent of relative enhancement of the main tumor on hepatobiliary phase images following Gd-EOB-DTPA administration was significantly higher in patients without than in those with lymph node metastases [21]. Finally, the diagnostic value of FDG-PET has been shown to be disappointing for the detection of regional lymph node metastases, even though its positive predictive value is higher than that of CT [29]. Thus, the contribution of preoperative imaging to determine malignant lymph nodes is low.
4.3.2 Perineural Invasion
The liver is innervated by both afferent and efferent autonomic nerves, which are associated with the portal vein, hepatic artery, and bile ducts within the liver hilum. The sympathetic innervation is postganglionic and originates in the celiac and superior mesenteric ganglia. The parasympathetic nerves branch off from the vagus nerve. The anterior plexus originates from the left portion of the celiac plexus and from the right abdominal branch of the vagus and forms a network of nerves surrounding the hepatic artery. The posterior plexus is derived from the right portion of the celiac plexus and is located around the portal vein with occasional innervations accompanying the hepatic vein (◘ Fig. 4.8a).
a Gross anatomy of the hepatic nervous system. The anterior plexus forms around the common hepatic artery, and the posterior plexus forms around the portal vein. These plexuses follow these structures to enter the liver hilum with the accompanying portal structures and carry afferent and efferent fibers of both sympathetic and parasympathetic origin. b Anatomy of the intrinsic sympathetic and parasympathetic nerve fibers. Sympathetic and parasympathetic fibers surround the portal area, and sympathetic fibers course into liver sinusoids
Perineural invasion is a common histological finding in biliary malignancies. It is a local diffusion mode for tumors, and it plays a critical role in prognosis [5]. Tumor perineural invasion is not correlated with patient’s age or gender and with the presence of distant metastases (including liver or abdominal cavity or peritoneum metastases). However, it is highly correlated with tumor volume, location, depth of invasion, angiogenesis, and lymph node involvement [30]. The biliary system lies close to both the peripheral nervous plexus and the coeliac plexus, and this proximity may facilitate peripheral nerve invasion by biliary tumors. Since the biliary system is rich of autonomic nerves, perineural invasion may also be facilitated (◘ Fig. 4.8b) [31]. In the past it was thought that tumors invaded the nerves through the lymphatic pathway within the nerve or perineurium [30]. However, more recently it has been shown that not all patients presenting with perineural invasion have lymphatic metastasis [30]. By performing three-dimensional reconstruction of extrahepatic bile duct pathological specimens, Maxwell et al. [32] have shown that tumor perineural invasion is actually a type of local tumor growth pattern. Indeed, the perineural interspace invasion was the fifth dependent metastasis pathway to be discovered (aside from tumor direct invasion metastasis, implantation metastasis, lymphatic, and blood route metastasis). Farges et al. [26] have shown that in patients without lymph node invasion, a larger resection margin was associated with better survival, suggesting an important role of peritumoral perineural invasion.
Unfortunately there are few studies reporting imaging features of perineural invasion. However, Raghavan et al. demonstrated that soft-tissue infiltration along the celiac plexus on CT is a sign of perineural invasion [33]. This is surgically relevant because perineural invasion adjacent to the main tumor or within the gastrohepatic or the hepatoduodenal ligaments is potentially resectable with negative surgical margins. Conversely, celiac lymph node metastases or perineural invasion within the celiac plexus is generally a negative prognostic factor that contraindicates major hepatic resection with curative intent.
4.3.3 Distant Metastases
Extrahepatic and distant metastases of intrahepatic cholangiocarcinoma are rare at presentation, the most common sites being the lungs, peritoneum, and bones. At diagnosis, peritoneal carcinomatosis is rare, while it is more frequently observed as recurrence after resection. Whole-body CT and FDG-PET/CT are interesting complementary techniques to detect distant metastases. The main advantage of FDG-PET consists in the detection of otherwise unsuspected metastases, and its findings change the surgical management strategy in up to 30% of patients [33,34,36]. Thus, before surgical resection, PET/CT could be performed to help rule out occult metastatic disease.
Baheti et al. have shown that the features of the main tumor on CT affect the distribution pattern of distant metastases [37]. The authors identified three tumor types: type I, solitary dominant mass; type II, dominant mass with satellite nodules in the same segment; and type III, multiple scattered hepatic lesions. Solitary dominant masses were smaller and had the lowest incidence of metastases at presentation (26%) and the best overall survival. Pulmonary metastases were more common in patients with multiple scattered hepatic lesions, and bone metastases were less common in those with solitary dominant masses compared to the other groups. Finally, the time to first metastasis decreased from type I to type III [37].
4.4 Conclusions
Cholangiocarcinoma is the most common primary tumor of the bile ducts. Imaging plays an important role in the diagnosis of intrahepatic tumors which typically present as focal heterogeneous progressively enhancing mass, frequently associated with capsular retraction and upstream biliary dilatation. Definition of parenchymal, vascular, perineural, and nodal involvement as well as the identification of distant metastases with imaging is most important in order to select patients for curative resection. However, while imaging is accurate in detecting parenchymal invasion and vascular encasement, it has limitations in the evaluation of the extent of nodal and perineural invasion.
References
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T et al (2014) Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 60(6):1268–1289
The general rules for the clinical and pathological study of primary liver cancer. Liver cancer study group of Japan. Jpn J Surg 1989;19(1):98–129
Pupulim LF, Vilgrain V, Ronot M, Becker CD, Breguet R, Terraz S (2015) Hepatic lymphatics: anatomy and related diseases. Abdom Imaging 40(6):1997–2011
Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM (2014) Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg 149(6):565–574
Shirai K, Ebata T, Oda K, Nishio H, Nagasaka T, Nimura Y et al (2008) Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg 32(11):2395–2402
Liebig C, Ayala G, Wilks JA, Berger DH, Albo D (2009) Perineural invasion in cancer: a review of the literature. Cancer 115(15):3379–3391
Kurzawinski TR, Deery A, Dooley JS, Dick R, Hobbs KE, Davidson BR (1993) A prospective study of biliary cytology in 100 patients with bile duct strictures. Hepatology 18(6):1399–1403
Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T et al (2001) A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer 92(9):2374–2383
Yamasaki S (2003) Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepato-Biliary-Pancreat Surg 10(4):288–291
Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD et al (2009) A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol 16(1):14–22
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474
Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P et al (2011) AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC-IHCC-2009 Study Group. Cancer 117(10):2170–2177
Kim Y, Spolverato G, Amini N, Margonis GA, Gupta R, Ejaz A et al (2015) Surgical management of intrahepatic cholangiocarcinoma: defining an optimal prognostic lymph node stratification schema. Ann Surg Oncol 22(8):2772–2778
Sakamoto Y, Kokudo N, Matsuyama Y, Sakamoto M, Izumi N, Kadoya M et al (2016) Proposal of a new staging system for intrahepatic cholangiocarcinoma: analysis of surgical patients from a nationwide survey of the Liver Cancer Study Group of Japan. Cancer 122(1):61–70
Hann LE, Schwartz LH, Panicek DM, Bach AM, Fong Y, Blumgart LH (1998) Tumor involvement in hepatic veins: comparison of MR imaging and US for preoperative assessment. Radiology 206(3):651–656
Hwang S, Lee YJ, Song GW, Park KM, Kim KH, Ahn CS et al (2015) Prognostic impact of tumor growth type on 7th AJCC staging system for intrahepatic cholangiocarcinoma: a single-center experience of 659 cases. J Gastrointest Surg 19(7):1291–1304
Zhang Y, Uchida M, Abe T, Nishimura H, Hayabuchi N, Nakashima Y (1999) Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI. J Comput Assist Tomogr 23(5):670–677
Madoff DC, Hicks ME, Abdalla EK, Morris JS, Vauthey JN (2003) Portal vein embolization with polyvinyl alcohol particles and coils in preparation for major liver resection for hepatobiliary malignancy: safety and effectiveness—study in 26 patients. Radiology 227(1):251–260
Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K et al (1997) Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology 26(5):1176–1181
Vilgrain V (2008) Staging cholangiocarcinoma by imaging studies. HPB (Oxford) 10(2):106–109
Kang Y, Lee JM, Kim SH, Han JK, Choi BI (2012) Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 264(3):751–760
Vilgrain V, Van Beers BE, Flejou JF, Belghiti J, Delos M, Gautier AL et al (1997) Intrahepatic cholangiocarcinoma: MRI and pathologic correlation in 14 patients. J Comput Assist Tomogr 21(1):59–65
Feydy A, Vilgrain V, Denys A, Sibert A, Belghiti J, Vullierme MP et al (1999) Helical CT assessment in hilar cholangiocarcinoma: correlation with surgical and pathologic findings. AJR Am J Roentgenol 172(1):73–77
Bach AM, Hann LE, Brown KT, Getrajdman GI, Herman SK, Fong Y et al (1996) Portal vein evaluation with US: comparison to angiography combined with CT arterial portography. Radiology 201(1):149–154
Uchida M, Ishibashi M, Tomita N, Shinagawa M, Hayabuchi N, Okuda K (2005) Hilar and suprapancreatic cholangiocarcinoma: value of 3D angiography and multiphase fusion images using MDCT. AJR Am J Roentgenol 184(5):1572–1577
Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A et al (2011) Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg 254(5):824–829, discussion 30
Adachi T, Eguchi S, Beppu T, Ueno S, Shiraishi M, Okuda K et al (2015) Prognostic impact of preoperative lymph node enlargement in intrahepatic cholangiocarcinoma: a multi-institutional study by the Kyushu Study Group of Liver Surgery. Ann Surg Oncol 22(7):2269–2278
Okami J, Dono K, Sakon M, Tsujie M, Hayashi N, Fujiwara Y et al (2003) Patterns of regional lymph node involvement in intrahepatic cholangiocarcinoma of the left lobe. J Gastrointest Surg 7(7):850–856
Lee SW, Kim HJ, Park JH, Park DI, Cho YK, Sohn CI et al (2010) Clinical usefulness of 18F-FDG PET-CT for patients with gallbladder cancer and cholangiocarcinoma. J Gastroenterol 45(5):560–566
Shen FZ, Zhang BY, Feng YJ, Jia ZX, An B, Liu CC et al (2010) Current research in perineural invasion of cholangiocarcinoma. J Exp Clin Cancer Res 29:24
Murakawa K, Tada M, Takada M, Tamoto E, Shindoh G, Teramoto K et al (2004) Prediction of lymph node metastasis and perineural invasion of biliary tract cancer by selected features from cDNA array data. J Surg Res 122(2):184–194
Maxwell P, Hamilton PW, Sloan JM (1996) Three-dimensional reconstruction of perineural invasion in carcinoma of the extrahepatic bile ducts. J Pathol 180(2):142–145
Raghavan K, Jeffrey RB, Patel BN, DiMaio MA, Willmann JK, Olcott EW (2015) MDCT diagnosis of perineural invasion involving the celiac plexus in intrahepatic cholangiocarcinoma: preliminary observations and clinical implications. AJR Am J Roentgenol 205(6):W578–W584
Anderson CD, Rice MH, Pinson CW, Chapman WC, Chari RS, Delbeke D (2004) Fluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinoma. J Gastrointest Surg 8(1):90–97
Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D’Angelica M, Fong Y et al (2008) 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. J Am Coll Surg 206(1):57–65
Kim YJ, Yun M, Lee WJ, Kim KS, Lee JD (2003) Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur J Nucl Med Mol Imaging 30(11):1467–1472
Baheti AD, Tirumani SH, Shinagare AB, Rosenthal MH, Hornick JL, Ramaiya NH et al (2014) Correlation of CT patterns of primary intrahepatic cholangiocarcinoma at the time of presentation with the metastatic spread and clinical outcomes: retrospective study of 92 patients. Abdom Imaging 39(6):1193–1201
Conflict of Interests
None of the authors have any conflict of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Ronot, M., Vilgrain, V. (2018). Intrahepatic Cholangiocarcinoma. In: Regge, D., Zamboni, G. (eds) Hepatobiliary and Pancreatic Cancer. Cancer Dissemination Pathways. Springer, Cham. https://doi.org/10.1007/978-3-319-50296-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-50296-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50294-6
Online ISBN: 978-3-319-50296-0
eBook Packages: MedicineMedicine (R0)