Abstract
To this date, it is a major oncological challenge to optimally diagnose, stage, and manage intrahepatic cholangiocarcinoma (ICC). Imaging can not only diagnose and stage ICC, but it can also guide management. Hence, imaging is indispensable in the management of ICC. In this article, we review the pathology, epidemiology, genetics, clinical presentation, staging, pathology, radiology, and treatment of ICC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrahepatic cholangiocarcinoma (ICC) and hepatocellular carcinoma (HCC) are the most common primary liver cancers [1]. In 2019, it is estimated that 42,030 individuals will be newly affected by primary liver tumors in the USA alone [2]. Additionally, over the past three decades, liver cancer death rates have doubled to an estimated value of 31,780 annually [2, 3]. This article will review the pathology, epidemiology, genetics, clinical presentation, staging, pathology, radiology, and treatment of ICC.
Epidemiology
ICC is less common than HCC, but its incidence rates have been rising at a quicker rate [4]. Despite rising incidence rates, ICC screening only targets specific populations that are at risk of developing this disease; screening indications include patients affected by trematodes such as Clonorchis sinensis (the most common cause of cholangiocarcinoma) and primary sclerosing cholangitis [5]. These risk factors, as well as others, promote malignant transformation of premalignant pathology such as biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct into ICC [6]. Unfortunately, ICC may develop idiopathically without any identifiable risk factors. Although chronic liver disease and cirrhosis have been found to be associated with ICC, ICC most commonly arises in the noncirrhotic liver, unlike HCC which usually arises in cirrhotic livers [7,8,9].
Pathology
ICC’s comprise 10% of all cholangiocarcinoma [10]. The remaining 90% of cholangiocarcinoma are located at the hepatic duct bifurcation and in the distal common bile duct. There are multiple histopathological subtypes of ICC, the most common being well-differentiated adenocarcinoma (> 95%) [10]. Other variants comprise less than 5% of cases and include mucoepidermoid carcinoma, sarcomatous ICC, clear cell carcinoma, lymphoepithelioma-like carcinoma, squamous and adenosquamous carcinomas, and signet-ring cell carcinoma [11].
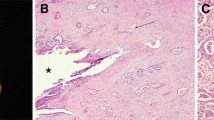
Additionally, ICCs can be classified based on macroscopic growth features. Most commonly ICC’s are mass forming (78%) with central fibrotic changes (Fig. 1) [12]. These tumors invade surrounding vessels which results in multicentricity [13]. The second most common form of ICCs are tumors with periductal infiltration, and these tumors comprise 16% of ICC’s (Fig. 2). Periductal ICC results in thickening of bile duct walls leading to stenosis and dilatation of the proximal biliary system [12]. The intraductal growing type is the least common (Fig. 3), comprising approximately 6% of cases [14].
Genetics/pathogenesis
The reported pathogenesis of ICC is complex with multiple signaling pathways involved (Fig. 4). Chronic injury and inflammation of the bile ducts induces transcription of nitric oxide synthase secondary to cytokine production. Nitric oxide (NO) produced by this enzyme induces expression of cyclooxygenase-2 which activates multiple growth factors leading to cellular growth [15]. Additionally NO can directly oxidize and damage DNA, leading to mutagenesis [16]. Another signaling pathway includes aberrant regulation of the epidermal growth factor receptor (EGFR) pathways. There is an increased expression of the erythroblastic leukemia viral oncogene homolog 2 (ERBB2) tyrosine kinase receptor secondary to gene amplification in up to 73% of tumors [17].
TP-53 is a tumor suppressor gene that codes for a protein that can arrest the cell cycle at the G1 check point as well as cause apoptosis and is prevalent in 28% of ICCs; [18, 19]. Epigenetic modifications may also play a role in pathogenesis. For example, the hyper methylation of stress associated endoplasmic reticulum protein 1 (SERP1), a modulator of Wnt signaling, has been reported in about 85% of ICCs [20]. Additionally, mutations in isocitrate dehydrogenase (IDH) 1 and 2 are identified in about 18% of ICCs, and unlike other mutations, this mutation is more specific to intrahepatic forms of cholangiocarcinoma [21]. Certain mutations have been reported to be associated with hepatitis B surface antigen (HBsAg). Zou et al. showed that TP-53 is commonly seen in HBsAg-seropositive patients. KRAS can be seen in up to 16.7% patients, with a preference to HBsAg-seronegative patients [22].
Clinical presentation and evaluation
ICC causes nonspecific symptoms resulting in delayed diagnosis. Most commonly, ICCs cause abdominal pain, and unlike extrahepatic cholangiocarcinoma, they rarely cause jaundice [4, 23]. Additionally, asymptomatic tumors are not uncommon, accounting for up to 30% of tumors reported in some studies [24]. The screening of cirrhotic patients suspected of HCC and the evaluation of lesions may result in the incidental diagnosis of small (< 3 cm) asymptomatic ICC [25, 26]. Secondary to their vague symptomatology, diagnosis of ICC may be delayed, and up to 54% of tumors may be unresectable at diagnosis [4]. Initial evaluation may include imaging and laboratory tests, although the latter are rarely beneficial. ICC may rarely cause elevation in alkaline phosphatase, aspartate transaminase, alanine aminotransferase, as well as obstructive jaundice [27]. Additionally, the tumor marker, CA 19-9 may be expressed by ICCs. A CA 19-9 value of 100 U/mL may diagnose an intrahepatic lesion as ICC with a specificity and sensitivity of 86% and 89% [28]. However, CA 19-9 can also be expressed by extrahepatic tumors [29].
Staging
The American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) staging system is the mostly commonly used staging system for intrahepatic cholangiocarcinoma (Table 1). Prior to the 7th edition of the staging system, a unique staging system adapted to ICCs was lacking; rather, cancers were staged based on a system extrapolated from an analysis of patients with HCC [30]. Although multiple studies found that the HCC based model was reliable [31, 32], some studies such as the multi-center study by Farges et al. found that the 7th edition of the AJCC staging system was more prognostically predictable than the 6th edition [33]. Since its introduction, multiple cohorts have validated the 7th edition of the staging system [33,34,35]. However, some modifications were still being proposed; for example, metastasis to the gastrohepatic lymph node were proposed to be classified as distant metastasis rather than regional lymph node metastasis [35].Therefore, the 8th edition was introduced with some modifications to the 7th edition (Fig. 5).
8th edition of the AJCC/UICC staging system for ICC illustrated. a Stage Ia disease (T1a N0 M0) is a single tumor measuring ≤ 5 cm without distant metastasis or vascular invasion. b Stage Ib disease (T1b N0 M0) is a single tumor measuring > 5 cm without vascular distant metastasis or vascular invasion. c Stage II disease (T2 N0 M0) could be a single lesion with vascular invasion intrahepatically, as illustrated. Alternatively, it may present as multiple tumors with or without vascular invasion. d Stage IIIA disease (T3 N0 M0) involves tumors that penetrate the visceral peritoneum. e Stage IIIB disease (T4 N0/1 M0) that invades extrahepatic structures locally by direct extension. f Stage IIIB disease (T4 N0/1 M0) with lymph node metastasis but without distant metastasis. g Stage IVB disease (any T and N, M1) involves tumors that have metastasized distantly
The 8th edition by AJCC, like the 7th edition, stages ICC as a separate entity from HCC or extrahepatic cholangiocarcinoma. The T1 tumors are defined as solitary tumors that do not invade vasculature. T1 is now divided into T1a (≤ 5 cm) (Fig. 6) and T1b (> 5 cm) (Fig. 7) to reflect the prognostic value of tumor size [36]. Tumor size > 5 cm is associated with a greater risk of recurrence (hazard ratio = 1.37) and lower survival rates (hazard ratio = 1.36) relative to tumors ≤ 5 cm [37].
T2 was modified into a single category since multiple tumors and single tumor with vascular invasion have similar prognostic effects (Fig. 8) [38]. Once a tumor invades the visceral peritoneum it becomes T3 (Fig. 9). The T4 category is now based on whether or not the tumor locally invades extrahepatic structures (Fig. 10).
The N category corresponds to regional lymph node metastasis, and patients are considered N1 if metastasis has occurred to the periduodenal, hepatoduodenal (hilar), and/or peripancreatic nodes [40]. Patients with metastasis beyond the regional nodes are considered M1.
Stage I disease is now separated into stage IA (T1a N0 M0) and IB (T1b N0 M0) to accommodate the change in the T1 category. Stage II disease (T2 N0 M0) includes T2 tumors without any lymph node or distant metastasis. Stage III disease now includes two sub stages, stage IIIA (T3 N0 M0) and IIIB (T4 N0/1 M0) (Fig. 11) [39]. Stage IV disease now only includes patients with metastatic disease (Fig. 12).
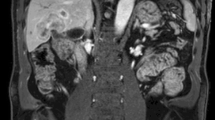
49-year-old female with metastatic intrahepatic cholangiocarcinoma. a Axial late arterial phase of contrast-enhanced CT images show a heterogeneous tumor (asterisk) centered in the left lobe of the liver. b–e Axial lung window of CT chest show pulmonary metastases (arrow). f, g Axial bone window CT and h, i 18F-FDG-PET/CT images show FDG avid mixed sclerotic and lytic metastases in the thoracic vertebra and sacrum (arrows)
Role of imaging modalities
Ultrasound (US)
ICC’s are usually diagnosed incidentally as they are commonly asymptomatic [23]. However, since patients may present with abdominal pain, weakness, fatigue, jaundice, and/or cholangitis, ICCs may be first evaluated by US [23]. On ultrasound, ICCs can have two morphological variations. Some tumors may present as a solitary nodular mass which may be hypoechoic or hyperechoic if the tumor is < 3 cm and > 3 cm, respectively [41, 42]. Infiltrative tumors may present with diffuse abnormal liver echogenicity [41] (Figs. 13 and 14). On ultrasound, ICCs may mimic HCC, but unlike HCC, ICC does not commonly cause tumor thrombi in portal branches [42, 43]. Contrast-enhanced US (CEUS) may assist in differentiating ICC from HCC. In the arterial phase, ICC most commonly expresses heterogenous hyperenhancement [44,45,46]. ICCs in patients with cirrhotic livers or chronic hepatitis are more likely to express heterogeneous hyperenhancement than ICCs in patients with healthy livers [45]. However, ICC may express homogenous hyperenhancement, hypoenhancement, peripheral hyperenhancement, or isoenhancement. The degree of enhancement then decreases in the portal venous and late phases with most tumors showing early washout before sixty seconds. In several studies, ICC’s most commonly showed a hyperenhanced rim and a hypoenhanced center during the portal venous phase and enhancement continued to decrease in the late phase whereby most lesions exhibited diffuse hypoenhancement [44,45,46,47,48]. On the other hand, HCC usually exhibits homogeneous hyperenhancement on the arterial phase [49,50,51]. For smaller tumors, ICC in cirrhotic patients may display a similar vascular pattern to HCC on CEUS [52].
Computed tomography (CT)
Multi detector CT is the most common imaging modality for the detection, diagnosis and staging of ICC. Table 2 describes our institution’s CT protocol for ICC. Contrast-enhanced CT has a reported accuracy of 70% in diagnosing ICC with a sensitivity of 78% and specificity of 80% [53, 54]. Typical imaging features for ICC on contrast-enhanced CT include irregular margins and capsular retraction. ICCs more commonly exhibit early rim enhancement and delayed central enhancement in the portal venous and delayed phase of contrast administration [55].
There are multiple reports that have attempted to identify characteristics that will help differentiate ICCs from HCCs [54, 55]. These studies have shown that a tumor with a lobulated shape is more likely to be an ICC than HCCs. This finding is likely due to the tumor’s frequent invasion of surrounding portal vein branches. As satellite nodules grow, the tumor invades the portal system and satellite tumors fuse with the primary tumor, giving rise to a single mass with an irregular border [54]. Additionally, the presence of an intratumoral artery, favors ICC, since ICC infiltrates the surrounding liver parenchyma causing the vessels to be retained inside the tumor. Meanwhile, HCC exhibits compressive growth which pushes the vessels externally [54, 56]. Most commonly, HCC exhibits nonperipheral washout on the delayed phase of contrast-enhanced studies, while ICC most commonly does not [54, 57]. ICC most commonly exhibit peripheral washout, which is highly diagnostic of ICC [58].”
A study by Zhang et al. found that CT and MRI performed equally well in detecting vascular invasion. However, the relationship between the tumor and the surrounding vessels and organs was better visualized on CT due to CT’s inherent superior spatial resolution [59]. Therefore, CT is used in surgical planning as it can assess resectability with greater accuracy than MRI, with negative predictive values that range between 85 and 100% [60].
Regarding prognosis, Ariizumi et al. showed that mass forming hypervascular ICCs on the arterial phase of CT had a more favorable prognosis, with a 5-year recurrence-free survival rate of 58%, as opposed to 28% for hypovascular ICCs [61]. Similarly, Asayama et al., demonstrated that hypovascular ICCs showed significantly poorer disease-free survival than patients with rim-enhancing lesions and hypervascular ICCs [62].
The accuracy of CT in the evaluation of lymph node involvement is reported as 77% [63]. A study by Yamamoto et al. attempted to create a predictive model for lymph node metastasis of ICC based on CT findings (LMIC score). The LMIC score was based on the presence of two variables on CT scans, namely periductal infiltration and hypervascularity. They found that patients with one or both variables had a 35% and 58% risk of metastatic adenopathy, respectively, whereas patients without any of the variables had a 0% risk of lymph node metastasis. The authors proposed that lymph node dissection only be conducted in patients with one or two of the mentioned variables on CT [64].
CT scans can also detect distant metastasis, which most commonly affect the peritoneum, the lungs, and the bones [65]. Peritoneal metastasis presents as thickening of the peritoneum with nodular implants and ascites. Lung metastasis appears as discrete nodules in the lung parenchyma. Bone metastases commonly present as lytic lesions, but sclerotic and mixed lesions may be seen [65].
Magnetic resonance imaging (MRI)
ICCs appear as hypointense and hyperintense lesions on the T1WI and T2WI, respectively, and similar to CT most commonly exhibit peripheral arterial enhancement and gradually enhance centripetally [10, 66] (Fig. 15). However, different enhancement patterns exist, and a study by Min et al. demonstrated that different enhancement patterns carried different prognoses [67]. Patients with tumors that hyperenhance diffusely had a lower risk of vascular invasion, tumor necrosis, and death than patients with peripheral rim enhancement or diffuse hypoenhancement [67]. Table 3 describes our institution’s MRI protocol for ICC. A systematic review by You et al. showed that MRI has a pooled sensitivity of 77% and a pooled specificity of 96% in diagnosing ICCs ≤ 5 cm in diameter, while lesions > 5 cm can be diagnosed with a sensitivity of 53% and a specificity of 95%. The presence of arterial rim enhancement has a diagnostic sensitivity and specificity as high as 80% and 99%, respectively, for diagnosing ICC [68].
67-year-old female with intrahepatic cholangiocarcinoma. a Axial T2WI (There is a mild hyperintense mass on T2WI with central hyperintensity). b Axial precontrast T1WI, c late arterial, d PV, e excretory and f 5 min delayed phases of post-contrast T1WI images show a 6 cm lobulated early rim-enhancing tumor (arrow) in the right lobe of the liver with delayed enhancement at 5 min. The mass is seen straddling segments VII and VIII
On DWI, ICC demonstrate restricted diffusion (Fig. 16). A target appearance on DWI has a sensitivity and specificity of 80% and 99%, respectively. Other features that are less sensitive but more specific for ICC include lobulated shape (specificity 82%), capsular retraction (specificity 95%), progressive enhancement (96%), and persistent enhancement (specificity 98%) [68]. Moreover, magnetic resonance elastography (MRE) has been reported to evaluate ICC. A study by Hennedige et al. found that ICCs and HCCs have significantly higher mean stiffness than benign lesions, however, the difference in stiffness between ICCs and HCCs was not statistically significant [69].
60-year-old female with intrahepatic cholangiocarcinoma. a Axial T2WI shows a slightly hyperintense mass, b Axial precontrast T1WI shows a hypointense mass, c late arterial, d portal venous, e excretory and f 5 min delayed phases of post-contrast T1WI (the mass shows early peripheral enhancement with mild delayed enhancement. g DWI (B value = 800 s/mm2) and h corresponding ADC map show restricted diffusion
Similar to other malignant liver tumors, ICC exhibits lower ADC values than the surrounding liver tissue. However, ADC values reported in the literature have been variable due to different modes of image acquisition. Therefore, a range of values specific for ICCs cannot yet be determined [70]. However, the current literature suggests that tumors with higher grade have lower nADC values/mean ADC values, and a worse prognosis [71, 72]. Interestingly, Lee et al. showed that hypervascular ICC had a higher degree of diffusion restriction and lower ADC values than hypovascular ICC, and this correlated with a better prognosis. [73]. Lee et al. reported that restricted diffusion in less than one third of the tumor volume was associated with more frequent lymph node metastasis and lymphatic invasion, as well as more advanced stages and desmoplasia. Tumors that demonstrated lower diffusion restriction carried a worse prognosis with a lower 3-year survival rate (26% versus 67%) [73].
Recently, hepatocyte specific contrast agents such as Gadoxetic acid have gained increasing attention. Images enhanced with Gadoxetic acid were shown to exhibit similar enhancing properties to gadolinium-enhanced studies in the arterial and portal venous phases, such as peripheral rim enhancement in the arterial phase and persistent enhancement in the transitional phase and the portal venous phase [74]. In a study by Kim et al., the use of gadoxetic acid allowed for the diagnosis of ICC with a sensitivity of 94% in Child–Pugh class A cirrhotic livers[74]. The target sign was once again more common in ICC than HCC and was an independent predictor of ICC.
The 2018 version of the liver imaging reporting and data system (LI-RADS) includes imaging features for ICC tumors in cirrhotic livers [75].The imaging features that favor ICC over HCC based on LI-RADS are rim or peripheral arterial phase hyperenhancement, portal venous and delayed phase central enhancement, progressive concentric enhancement, peripheral washout appearance, liver surface retraction, biliary obstruction disproportionate to that expected based on size of mas, target appearance at DWI or in hepatobiliary phase and vascular encasement without invasion. In contrast to HCC, ICC would have elevated CA19-9, CEA.
MRI has been used to evaluate lymph node metastasis. Park et al. demonstrated that MRI had a sensitivity of up to 74% in the detection of metastatic lymph nodes [76]. However, MRI is limited in the detection of diseased lymph nodes as it diagnoses metastatic lymph nodes based on size. Micrometastasis may not cause significant change in lymph node size, limiting MRI’s sensitivity.
MRI may also be used to determine treatment response. Several studies have suggested that tumors responsive to treatment may not express a decrease in tumor size, however, continuous ADC increase on serial MRI may suggest response [77]. In a study by Eccles et al., changes in ADC values were used to evaluate response of primary liver tumors to radiotherapy. Tumor response was directly correlated with increasing ADC values, while tumor volume changes and RECIST were not. Additionally, earlier changes in ADC values were correlated with higher radiation doses and sustained treatment response [77]. In a study by Halappa et al., patients who were successfully treated with TACE displayed increased mean volumetric ADC values following treatment without significant changes in tumor volume or mean RECIST diameter. Additionally, in their study, tumors that expressed ADC values higher than 1.60 × 10–3 mm2/s for larger proportions of their volume had a better prognosis. Patients with increased ADC values for ≥ 60% of tumor volume had the highest median overall survival of 42 months as compared to 19 months (≥ 45%) and 8 months (< 45%) [78]. These findings were recently corroborated by Pandey et al. whereby patients with unresectable ICC that responded to TACE had increasing volumetric ADC values and decreasing viable tumor volume and burden following treatment. Patients that demonstrated greater than 25% increase in volumetric ADC values had significantly higher median survival [31 months] relative to patients that did not [11 months].On the other hand, tumor volume did not change significantly, and RECIST diameter only decreased slightly (− 2.6%) [79].
Positron emission tomography/computed tomography (PET/CT)
Unlike other imaging modalities, PET/CT has the additional advantage of assessing metabolic activity of tumors. The most common role of PET/CT in patients with ICC is to detect suspected distant metastasis of tumors rather than the detection of primary tumor. However, a recent meta-analysis has shown that PET/CT can accurately detect ICCs with a pooled sensitivity and specificity of 95% and 93%, respectively [80].
A meta-analysis by Hu et al. showed that PET/CT findings have a high specificity (91.4%) but moderate sensitivity (51.6%) for intrahepatic and extrahepatic cholangiocarcinoma lymph node metastasis [81]. However, Hu et al. did not perform a subgroup analysis for intrahepatic and extrahepatic cholangiocarcinoma lymph node metastasis. Park et al. showed that PET/CT can detect metastatic lymph nodes with a sensitivity and specificity of 80% and 92%, respectively [82]. Studies have shown that PET/CT is more accurate than CT in the detection of distant metastasis (88.3% versus 78.7%) and can alter management for patients with ICC in up to 25% of cases [83, 84]. Routine lymphadenectomy is not performed for ICC but may be considered in patients with PET avid lymph nodes to improve staging and recurrence-free survival [34, 85].
Similar to MRI, PET/CT findings may have a prognostic benefit for ICC. SUVmax has been suggested to correlate with the risk of recurrence and overall survival. Wing Ma et al. determined a cut-off value of 8 for SUVmax and they found that patients with values greater than 8 had worse 3-year disease-free survival (21.2% versus 63.2%) and overall survival (29% versus 74%). They suggested that tumors with low SUVmax without metastatic disease may benefit from more aggressive surgery due to their favorable prognosis [86]. Additionally, patients with high SUVmax values may benefit from neoadjuvant treatment, although the survival benefit of neoadjuvant treatment is not yet determined for ICC [86, 87]. A study by Ikeno et al. showed that higher metabolic tumor volume (MTV) and total lesion glycolysis (TLG) values correlated with KRAS mutations within the tumor. MTV levels ≥ 38 predicted a lower 5-year survival (13.1% versus 36.7%), and KRAS somatic mutation with a total accuracy of 68%, specificity of 67.9%, and sensitivity of 77.8% [88]. Not only does this allow for noninvasive assessment of genetic composition, but it also allows for disease prognostication as tumors with KRAS mutation are known to have a poor prognosis [21, 22, 88].
Recently, PET/MRI has been gaining increasing attention regarding its value in the diagnosis and staging of tumors. Although PET/MRI has not yet been studied extensively for ICC, a study by Kirchner et al. showed that the use of the liver-specific contrast agent gadobenate dimeglumine on PET/MRI improved the differentiation between malignant and benign lesions. PET/MRI could accurately assess lesions as malignant or benign with an accuracy of 98% and 100%, respectively [89]. However, studies specific to ICC are necessary to determine the role of PET/MRI in the diagnosis and staging of ICC.
Treatment
Treatment of ICC aims at improving quality of life and progression free survival. Some patients may develop biliary obstruction, which could be managed with biliary drainage to improve liver function [90].
Surgery is the only treatment modality that may cure ICC, but the success rate is suboptimal (Figs. 17 and 18) [91]. The low success rate is due to the difficulty in obtaining negative margins due to periductal and intraductal invasion [27]. A contraindication to resection is presence of distant metastasis or lymph node metastasis distant to the regional basin [92]. Multifocal disease, such as metastasis to other lobes of the liver, is considered a contraindication to resection [93]. Based on the degree of the involved liver an extended hepatectomy can be performed, however, an adequate liver remnant is required to decrease morbidity and mortality. At least two adjacent segments with proper circulation and biliary drainage is required [4, 91, 93]. In most situations up to 25% of patients may additionally require reconstruction of the biliary system [94]. Morbidity and mortality rates following surgery may be as high as 21.4% and 6%, respectively [4, 34, 95].
Resectable intrahepatic cholangiocarcinoma in 2 different patients. a An axial post-contrast-enhanced CT image shows a solitary tumor (arrow) in segment IV of the liver. b The patient underwent left hepatectomy with resection of the tumor. c An axial contrast-enhanced CT image shows the tumor (arrow) in the right lobe of the liver. d The patient underwent extended right hepatectomy with resection of the tumor
Unresectable intrahepatic cholangiocarcinoma. a Axial and b coronal contrast-enhanced CT images show a large infiltrative mass (white arrows) involving both the lobes of the liver, surrounding the main portal vein and infiltrating the right portal vein. The mass was deemed unresectable and managed conservatively
For patients who require a large portion of their liver to be resected, volumetric analysis of the liver is performed, which may help in identifying the future liver remnant volume (FLRV) and whether it will be adequate for survival. If patients are not surgical candidates due to low FLRV, portal vein embolization may be considered to induce hypertrophy of the future liver remnant so that surgical resection may be reconsidered (Table 4) [96].
Although multiple imaging modalities such as MRI and US have been used to assess FLRV preoperatively, clinicians most commonly use CT volumetric analysis. In a study by Kishi et al., standardized future liver volume (sFLRV) was assessed in noncirrhotic livers and they found that post resection mortality was highest in patients with sFLRV less than 20%. In their study, chemotherapy did not increase the risk of liver insufficiency [97].
A safe cut-off value for sFLRV is expected to be higher in patients with cirrhotic livers. A study by Kim et al. showed that a sFLRV cut-off value of 30% could predict post resection liver failure risk with a sensitivity and specificity of 66.7% and 81.3%. However, in cirrhotic livers quantitative assessment of liver functionality is a better marker of residual liver functionality than liver volume [98].
Although surgery is the only curative treatment, only a minority of patients (12%) have localized disease at presentation [99]. Patients that do not undergo surgery may receive chemotherapy. The ABC-02 trial, a phase III trial, found gemcitabine and cisplatin may improve overall survival by up to 4 months in patients with biliary cancer [100]. Additionally, a combined subgroup analysis of ICC patients in the ABC-02 trial and the BT22 trial found that gemcitabine and cisplatin had a 0.54 hazard ratio [101]. Larger scale trials are still required to further clarify the role of systemic chemotherapy in ICC management.
Other modes of treatment for unresectable ICC include local and regional therapy, which include tumor/liver directed therapy. Liver directed therapy include TACE, Y-90, Ablation and external beam radiation. TACE refers to the direct infusion of chemotherapy agents via the hepatic artery. This allows for higher chemotherapy concentrations being delivered to the tumor with less systemic toxicity [94]. Based on the current literature, patients with ICC treated with hepatic arterial infusion have a median survival of 12.5 to 31.1 months [102, 103]. Despite the absence of a standardized treatment protocol, for a patient to be considered for TACE, the following must be assessed: absence of metastatic disease, tumor vascularity, tumor size, liver function, absence of constitutional symptoms, absence of tumor thrombus in the portal vein, and performance status of the patient [104]. In some patients, radioembolization with Y-90 may convert the unresectable status of tumors to resectable [105]. Based on a pooled analysis of the current literature, the median weighted overall survival of patients treated with Y-90 is 15.5 months [106]. Though results from current studies post Y-90 embolization seem promising, randomized clinical trials are yet to be conducted to determine the efficacy and safety of these treatment strategies for ICC [94].
Other modes of treatment include modalities that rely on thermal ablation. Radiofrequency ablation (RFA) and microwave ablation have been shown to be effective for small tumors, but less so for larger tumors [107]. A systematic review and meta-analysis by Han et al. showed that RFA led to a 1-year survival rate of 82%, 3-year survival rate of 47%, and 5-year survival rate of 24% [108]. However, thermal techniques have major complications such as a bile leak [109].
Radiotherapy may also be used. A retrospective study by Tao et al. found that giving high doses in a fractionated method may be used to ablate large tumors. In their study, higher radiation doses led to higher survival rates and better tumor control whereby patients treated with a biologically effective dose (BED) greater than 80.5 had a 3-year overall survival of 73% without significant treatment toxicity, as opposed to 38% for patients treated with lower radiation doses [110].
Since there are no randomized trials evaluating radiation treatment and survival, Koay et al. developed an algorithm based on the available trials to decide which treatment modality is best on a per patient basis (Fig. 19). In their algorithm, percutaneous ablation methods such as RFA are reserved for patients that have small tumors, which they defined as less than 4 cm in diameter. Additionally, they categorized tumors as anatomically favorable or unfavorable. Favorable tumors are those that are not adjacent to major vessels or structures such as the common bile duct, the large bowel, the stomach, most of the normal liver tissue, duodenum, and kidneys. Patients with hypovascular (hypoenhancing on imaging) anatomically favorable tumors may be treated with external beam radiation or percutaneous ablation methods due to the safe distance of critical structures. They recommend that hypervascular (hyperenhancing on imaging) anatomically favorable tumors be treated with external beam radiation, Y-90 radioembolization, TACE, or percutaneous ablation[109].Conversely, based on the algorithm, patients with anatomically unfavorable hypo or hypervascular tumor should be treated with external beam radiation due to its better safety profile when compared to other treatment modalities [110].
Recurrence
Recurrence is a frequent complication of ICC following treatment. In one study, 53.5% of cases treated surgically had recurrent disease (Fig. 20). The imaging features of recurrent disease mimic the pre-treatment presentation. Variables that increase the risk of early recurrence include tumor multiplicity, vascular invasion, lymph node metastasis, perineural invasion, and tumor size ≥ 5 cm [93]. Most commonly, recurrence of ICC occurs at the surgical bed and involves adjacent tissues such as the bowel and vasculature [111]. The treatment for recurrence is not yet accurately established, however, surgical resection appears to have the best outcome [111].
Following treatment, CT is usually the imaging modality used to detect recurrence. When patients are treated surgically, normal post-operative findings include pneumobilia and biliopathy. Although recurrent disease will usually appear as a lesion that has the same imaging characteristics as the initial tumor, differentiating between recurrent disease and post-operative changes on CT may be difficult [112, 113]. In such cases, DWI and PET/CT may be beneficial in detecting recurrence. A low ADC value on DWI indicates tumor recurrence, while post-operative changes express a high ADC value [114].
In patients that have been treated with embolization or ablation, an MRI study that exhibits linear enhancement circumferentially suggest post treatment changes, while eccentric nodular enhancement focally suggests recurrence [115].
Several studies have evaluated the use of PET/CT for tumor recurrence, and they have shown that PET/CT may detect recurrence with a sensitivity and specificity of up to 94% and 100%, respectively, as opposed to CT scans that had a sensitivity of 82% and a specificity of 43% only [83, 116, 117]. Corvera et al. demonstrated that PET/CT identified two additional cases of recurrence that were not identified on other forms of imaging [83]. PET/CT can also be used to assess tumor response in patients treated non-surgically with chemoradiotherapy or chemotherapy [112].
Conclusion
ICC is one of the most common primary liver malignancy in the world. The diagnosis, staging and management of ICC is major challenge in oncology. Imaging is at the cornerstone for diagnosing and staging ICC and allows treatment planning and surveillance. We have presented an imaging centered review of the clinical presentation, pathology, staging, treatment of patients with ICC. Knowledge of imaging features of ICC will help radiologists provide appropriate image interpretation and guide clinicians to develop an optimal treatment plan for the patients.
References
Burkhart RA, Pawlik TM. Staging and prognostic models for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Control. 2017;24(3):1073274817729235.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69(1):7–34.
Kim Y, Ejaz A, Tayal A, Spolverato G, Bridges JF, Anders RA, et al. Temporal trends in population‐based death rates associated with chronic liver disease and liver cancer in the United States over the last 30 years. Cancer. 2014;120(19):3058-65.
Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Annals of surgery. 2008;248(1):84-96.
Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66(3):167-79.
Nakanuma Y, Tsutsui A, Ren XS, Harada K, Sato Y, Sasaki M. What are the precursor and early lesions of peripheral intrahepatic cholangiocarcinoma? International journal of hepatology. 2014;2014.
London W, McGlynn K. Liver cancer. Cancer epidemiology and prevention. 2006;3:763-86.
Okuda K, Nakanuma Y, Miyazaki M. Cholangiocarcinoma: recent progress. Part 1: epidemiology and etiology. Journal of gastroenterology and hepatology. 2002;17(10):1049–55.
Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128(3):620-6.
Esnaola NF, Meyer JE, Karachristos A, Maranki JL, Camp ER, Denlinger CS. Evaluation and management of intrahepatic and extrahepatic cholangiocarcinoma. Cancer. 2016;122(9):1349-69.
Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system: World Health Organization; 2010.
Aishima S, Oda Y. Pathogenesis and classification of intrahepatic cholangiocarcinoma: different characters of perihilar large duct type versus peripheral small duct type. Journal of Hepato‐Biliary‐Pancreatic Sciences. 2015;22(2):94-100.
Sasaki A, Aramaki M, Kawano K, Morii Y, Nakashima K, Yoshida T, et al. Intrahepatic peripheral cholangiocarcinoma: mode of spread and choice of surgical treatment. British journal of surgery. 1998;85(9):1206-9.
Nakanuma Y, Kakuda Y. Pathologic classification of cholangiocarcinoma: new concepts. Best Practice & Research Clinical Gastroenterology. 2015;29(2):277-93.
Han C, Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. Journal of Biological Chemistry. 2005;280(25):24053-63.
Wink DA, Grisham MB, Mitchell JB, Ford PC. Direct and indirect effects of nitric oxide in chemical reactions relevant to biology. Methods in enzymology. 268: Elsevier; 1996. p. 12–31.
Sirica AE, Lai G-H, Endo K, Zhang Z, Yoon B-I, editors. Cyclooxygenase-2 and ERBB-2 in cholangiocarcinoma: potential therapeutic targets. Seminars in liver disease; 2002: Copyright© 2002 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New ….
Shaw PH. The role of p53 in cell cycle regulation. Pathology-Research and Practice. 1996;192(7):669-75.
Roos E, Soer E, Klompmaker S, Meijer L, Besselink M, Giovannetti E, et al. Crossing borders: a systematic review with quantitative analysis of genetic mutations of carcinomas of the biliary tract. Critical reviews in oncology/hematology. 2019.
Goeppert B, Konermann C, Schmidt CR, Bogatyrova O, Geiselhart L, Ernst C, et al. Global alterations of DNA methylation in cholangiocarcinoma target the Wnt signaling pathway. Hepatology. 2014;59(2):544-54.
Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, et al. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PloS one. 2014;9(12):e115383.
Zou S, Li J, Zhou H, Frech C, Jiang X, Chu JS, et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nature communications. 2014;5:5696.
Paik KY, Jung JC, Heo JS, Choi SH, Choi DW, Kim YI. What prognostic factors are important for resected intrahepatic cholangiocarcinoma? Journal of gastroenterology and hepatology. 2008;23(5):766-70.
Dhanasekaran R, Hemming AW, Zendejas I, George T, Nelson DR, Soldevila-Pico C, et al. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncology reports. 2013;29(4):1259-67.
Ariizumi S-i, Yamamoto M. Intrahepatic cholangiocarcinoma and cholangiolocellular carcinoma in cirrhosis and chronic viral hepatitis. Surgery today. 2015;45(6):682–7.
Shin SK, Choi DJ, Kim JH, Kim YS, Kwon OS. Characteristics of contrast-enhanced ultrasound in distinguishing small (≤ 3 cm) hepatocellular carcinoma from intrahepatic cholangiocarcinoma. Medicine. 2018;97(41).
DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Annals of surgery. 2007;245(5):755.
NICHOLS JC, GORES GJ, LARUSSO NF, WIESNER RH, NAGORNEY DM, RITTS JR RE, editors. Diagnostic role of serum CA 19–9 for cholangiocarcinoma in patients with primary sclerosing cholangitis. Mayo Clinic Proceedings; 1993: Elsevier.
Chen C-Y, Shiesh S-C, Tsao H-C, Lin X-Z. The assessment of biliary CA 125, CA 19-9 and CEA in diagnosing cholangiocarcinoma--the influence of sampling time and hepatolithiasis. Hepato-gastroenterology. 2002;49(45):616-20.
Vauthey J-N, Lauwers GY, Esnaola NF, Do K-A, Belghiti J, Mirza N, et al. Simplified staging for hepatocellular carcinoma. Journal of clinical oncology. 2002;20(6):1527-36.
Jonas S, Thelen A, Benckert C, Biskup W, Neumann U, Rudolph B, et al. Extended liver resection for intrahepatic cholangiocarcinoma: a comparison of the prognostic accuracy of the fifth and sixth editions of the TNM classification. Annals of surgery. 2009;249(2):303-9.
Nathan H, Aloia TA, Vauthey J-N, Abdalla EK, Zhu AX, Schulick RD, et al. A proposed staging system for intrahepatic cholangiocarcinoma. Annals of surgical oncology. 2009;16(1):14-22.
Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC‐IHCC‐2009 study group. Cancer. 2011;117(10):2170–7.
De Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. Journal of Clinical Oncology. 2011;29(23):3140-5.
Igami T, Ebata T, Yokoyama Y, Sugawara G, Takahashi Y, Nagino M. Staging of peripheral-type intrahepatic cholangiocarcinoma: appraisal of the new TNM classification and its modifications. World journal of surgery. 2011;35(11):2501.
Doussot A, Gonen M, Wiggers JK, Groot-Koerkamp B, DeMatteo RP, Fuks D, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. Journal of the American College of Surgeons. 2016;223(3):493–505. e2.
Hwang S, Lee Y-J, Song G-W, Park K-M, Kim K-H, Ahn C-S, et al. Prognostic impact of tumor growth type on 7th AJCC staging system for intrahepatic cholangiocarcinoma: a single-center experience of 659 cases. Journal of Gastrointestinal Surgery. 2015;19(7):1291-304.
Spolverato G, Bagante F, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Comparative performances of the 7th and the 8th editions of the American Joint Committee on Cancer staging systems for intrahepatic cholangiocarcinoma. Journal of surgical oncology. 2017;115(6):696-703.
Lee AJ, Chun YS. Intrahepatic cholangiocarcinoma: the AJCC/UICC 8 th edition updates. Chinese clinical oncology. 2018;7(5).
Nathan H, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Current opinion in gastroenterology. 2010;26(3):269-73.
Bloom CM, Langer B, Wilson SR. Role of US in the detection, characterization, and staging of cholangiocarcinoma. Radiographics. 1999;19(5):1199-218.
Wibulpolprasert B, Dhiensiri T. Peripheral cholangiocarcinoma: sonographic evaluation. Journal of clinical ultrasound. 1992;20(5):303-14.
Soyer P, Bluemke DA, Reichle R, Calhoun PS, Bliss DF, Scherrer A, et al. Imaging of intrahepatic cholangiocarcinoma: 1. Peripheral cholangiocarcinoma. AJR American journal of roentgenology. 1995;165(6):1427–31.
Li C, Wang W, Ding H, Huang B, Cao J, Mao F, et al. Value of contrast‐enhanced sonography in the diagnosis of peripheral intrahepatic cholangiocarcinoma. Journal of Clinical Ultrasound. 2011;39(8):447-53.
Li R, Zhang X, Ma K-S, Li X-W, Xia F, Zhong H, et al. Dynamic enhancing vascular pattern of intrahepatic peripheral cholangiocarcinoma on contrast-enhanced ultrasound: the influence of chronic hepatitis and cirrhosis. Abdominal imaging. 2013;38(1):112-9.
Chen T, Chang X, Lv K, Wang Y, Fu X, Tan L, et al. contrast-enhanced Ultrasound features of intrahepatic cholangiocarcinoma: A new perspective. Scientific Reports. 2019;9(1):1-8.
Kong W-T, Wang W-P, Zhang W-W, Qiu Y-D, Ding H, Huang B-J. Contribution of Contrast–Enhanced Sonography in the Detection of Intrahepatic Cholangiocarcinoma. Journal of Ultrasound in Medicine. 2014;33(2):215-20.
Xu H, Chen L, Liu L, Zhang Y, Guo L, Liu C. Contrast-enhanced ultrasound of intrahepatic cholangiocarcinoma: correlation with pathological examination. The British journal of radiology. 2012;85(1016):1029-37.
Chen L-D, Xu H-X, Xie X-Y, Xie X-H, Xu Z-F, Liu G-J, et al. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. European radiology. 2010;20(3):743-53.
Seitz K, Strobel D, Bernatik T, Blank W, Friedrich-Rust M, von Herbay A, et al. Contrast-Enhanced Ultrasound (CEUS) for the Characterization of Focal Liver Lesions–Prospective Comparison in Clinical Practice: CEUS vs. CT (DEGUM Multicenter Trial) Parts of this Manuscript were presented at the Ultrasound Dreiländertreffen 2008, Davos. Ultraschall in der Medizin-European Journal of Ultrasound. 2009;30(04):383–9.
Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsøe CP, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver–update 2012. Ultraschall in der Medizin-European Journal of Ultrasound. 2013;34(01):11-29.
Vilana R, Forner A, Bianchi L, García‐Criado Á, Rimola J, Rodríguez de Lope C, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast‐enhanced ultrasound. Hepatology. 2010;51(6):2020–9.
Petrowsky H, Wildbrett P, Husarik DB, Hany TF, Tam S, Jochum W, et al. Impact of integrated positron emission tomography and computed tomography on staging and management of gallbladder cancer and cholangiocarcinoma. Journal of hepatology. 2006;45(1):43-50.
Tsunematsu S, Chuma M, Kamiyama T, Miyamoto N, Yabusaki S, Hatanaka K, et al. Intratumoral artery on contrast-enhanced computed tomography imaging: differentiating intrahepatic cholangiocarcinoma from poorly differentiated hepatocellular carcinoma. Abdominal imaging. 2015;40(6):1492-9.
Slattery JM, Sahani DV. What is the current state-of-the-art imaging for detection and staging of cholangiocarcinoma? The oncologist. 2006;11(8):913-22.
Ueda K, Terada T, Nakanuma Y, Matsui O. Vascular supply in adenomatous hyperplasia of the liver and hepatocellular carcinoma: a morphometric study. Human pathology. 1992;23(6):619-26.
Kim Y-Y, Kim M-J, Kim EH, Roh YH, An C. Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: performance of LI-RADS version 2018. Radiology. 2019;291(1):72-80.
Choi SH, Lee SS, Kim SY, Park SH, Park SH, Kim KM, et al. Intrahepatic cholangiocarcinoma in patients with cirrhosis: differentiation from hepatocellular carcinoma by using gadoxetic acid–enhanced mr imaging and dynamic CT. Radiology. 2017;282(3):771-81.
Zhang Y, Uchida M, Abe T, Nishimura H, Hayabuchi N, Nakashima Y. Intrahepatic peripheral cholangiocarcinoma: comparison of dynamic CT and dynamic MRI. Journal of computer assisted tomography. 1999;23(5):670-7.
Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB. 2008;10(2):106-9.
Ariizumi SI, Kotera Y, Takahashi Y, Katagiri S, Chen IP, Ota T, et al. Mass‐forming intrahepatic cholangiocarcinoma with marked enhancement on arterial‐phase computed tomography reflects favorable surgical outcomes. Journal of surgical oncology. 2011;104(2):130-9.
Fujita N, Asayama Y, Nishie A, Ishigami K, Ushijima Y, Takayama Y, et al. Mass-forming intrahepatic cholangiocarcinoma: Enhancement patterns in the arterial phase of dynamic hepatic CT-Correlation with clinicopathological findings. European radiology. 2017;27(2):498-506.
Okami J, Dono K, Sakon M, Tsujie M, Hayashi N, Fujiwara Y, et al. Patterns of regional lymph node involvement in intrahepatic cholangiocarcinoma of the left lobe. Journal of gastrointestinal surgery. 2003;7(7):850-6.
Yamamoto Y, Türkoğlu MA, Aramaki T, Sugiura T, Okamura Y, Ito T, et al. Vascularity of intrahepatic cholangiocarcinoma on computed tomography is predictive of lymph node metastasis. Annals of surgical oncology. 2016;23(4):485-93.
Baheti AD, Tirumani SH, Shinagare AB, Rosenthal MH, Hornick JL, Ramaiya NH, et al. Correlation of CT patterns of primary intrahepatic cholangiocarcinoma at the time of presentation with the metastatic spread and clinical outcomes: retrospective study of 92 patients. Abdominal imaging. 2014;39(6):1193-201.
Seo N, Kim DY, Choi J-Y. Cross-sectional imaging of intrahepatic cholangiocarcinoma: development, growth, spread, and prognosis. American Journal of Roentgenology. 2017;209(2):W64-W75.
Min JH, Kim YK, Choi S-Y, Kang TW, Lee SJ, Kim JM, et al. Intrahepatic Mass-forming Cholangiocarcinoma: Arterial Enhancement Patterns at MRI and Prognosis. Radiology. 2019;290(3):691-9.
You M-W, Yun S. Differentiating between hepatocellular carcinoma and intrahepatic cholangiocarcinoma using contrast-enhanced MRI features: a systematic review and meta-analysis. Clinical radiology. 2019;74(5):406. e9-. e18.
ennedige TP, Hallinan JT, Leung FP, Teo LL, Iyer S, Wang G, et al. Comparison of magnetic resonance elastography and diffusion-weighted imaging for differentiating benign and malignant liver lesions. European radiology. 2016;26(2):398-406.
Fábrega-Foster K, Ghasabeh MA, Pawlik TM, Kamel IR. Multimodality imaging of intrahepatic cholangiocarcinoma. Hepatobiliary surgery and nutrition. 2017;6(2):67.
Lewis S, Besa C, Wagner M, Jhaveri K, Kihira S, Zhu H, et al. Prediction of the histopathologic findings of intrahepatic cholangiocarcinoma: qualitative and quantitative assessment of diffusion-weighted imaging. European radiology. 2018;28(5):2047-57.
Cui X-Y, Chen H-W, Cai S, Bao J, Tang Q-F, Wu L-Y, et al. Diffusion-weighted MR imaging for detection of extrahepatic cholangiocarcinoma. European journal of radiology. 2012;81(11):2961-5.
ee J, Kim SH, Kang TW, Song KD, Choi D, Jang KT. Mass-forming intrahepatic cholangiocarcinoma: diffusion-weighted imaging as a preoperative prognostic marker. Radiology. 2016;281(1):119-28.
Choi SH, Lee SS, Kim SY, Park SH, Park SH, Kim KM, et al. Intrahepatic cholangiocarcinoma in patients with cirrhosis: differentiation from hepatocellular carcinoma by using gadoxetic acid–enhanced mr imaging and dynamic CT. Radiology. 2016;282(3):771-81.
Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology. 2018;289(3):816-30.
Park HS, Lee JM, Choi J-Y, Lee MW, Kim HJ, Han JK, et al. Preoperative evaluation of bile duct cancer: MRI combined with MR cholangiopancreatography versus MDCT with direct cholangiography. American Journal of Roentgenology. 2008;190(2):396-405.
Eccles CL, Haider EA, Haider MA, Fung S, Lockwood G, Dawson LA. Change in diffusion weighted MRI during liver cancer radiotherapy: preliminary observations. Acta oncologica. 2009;48(7):1034-43.
Halappa VG, Bonekamp S, Corona-Villalobos CP, Li Z, Mensa M, Reyes D, et al. Intrahepatic cholangiocarcinoma treated with local-regional therapy: quantitative volumetric apparent diffusion coefficient maps for assessment of tumor response. Radiology. 2012;264(1):285-94.
Pandey A, Pandey P, Aliyari Ghasabeh M, Najmi Varzaneh F, Shao N, Khoshpouri P, et al. Unresectable intrahepatic cholangiocarcinoma: multiparametric MR imaging to predict patient survival. Radiology. 2018;288(1):109-17.
Annunziata S, Caldarella C, Pizzuto DA, Galiandro F, Sadeghi R, Giovanella L, et al. Diagnostic accuracy of fluorine-18-fluorodeoxyglucose positron emission tomography in the evaluation of the primary tumor in patients with cholangiocarcinoma: a meta-analysis. BioMed research international. 2014;2014.
Hu J-H, Tang J-h, Lin C-H, Chu Y-Y, Liu N-J. Preoperative staging of cholangiocarcinoma and biliary carcinoma using 18F-fluorodeoxyglucose positron emission tomography: a meta-analysis. Journal of Investigative Medicine. 2018;66(1):52–61.
Park TG, Yu Y-D, Park BJ, Cheon GJ, Oh SY, Kim D-S, et al. Implication of lymph node metastasis detected on 18F-FDG PET/CT for surgical planning in patients with peripheral intrahepatic cholangiocarcinoma. Clinical nuclear medicine. 2014;39(1):1-7.
Corvera CU, Blumgart LH, Akhurst T, DeMatteo RP, D’Angelica M, Fong Y, et al. 18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancer. Journal of the American College of Surgeons. 2008;206(1):57-65.
Moon CM, Bang S, Chung JB, Park SW, Song SY, Yun M, et al. Usefulness of 18F‐fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. Journal of gastroenterology and hepatology. 2008;23(5):759-65.
Nguyen KT, Steel J, Vanounou T, Tsung A, Marsh JW, Geller DA, et al. Initial presentation and management of hilar and peripheral cholangiocarcinoma: is a node-positive status or potential margin-positive result a contraindication to resection? Annals of surgical oncology. 2009;16(12):3308-15.
Ma KW, Cheung TT, She WH, Chok KSH, Chan ACY, Dai WC, et al. Diagnostic and prognostic role of 18-FDG PET/CT in the management of resectable biliary tract cancer. World journal of surgery. 2018;42(3):823-34.
Nelson JW, Ghafoori AP, Willett CG, Tyler DS, Pappas TN, Clary BM, et al. Concurrent chemoradiotherapy in resected extrahepatic cholangiocarcinoma. International Journal of Radiation Oncology Biology Physics. 2009;73(1):148-53.
Ikeno Y, Seo S, Iwaisako K, Yoh T, Nakamoto Y, Fuji H, et al. Preoperative metabolic tumor volume of intrahepatic cholangiocarcinoma measured by 18 F-FDG-PET is associated with the KRAS mutation status and prognosis. Journal of translational medicine. 2018;16(1):95.
Kirchner J, Sawicki LM, Deuschl C, Grüneisen J, Beiderwellen K, Lauenstein TC, et al. 18 F-FDG PET/MR imaging in patients with suspected liver lesions: Value of liver-specific contrast agent Gadobenate dimeglumine. PloS one. 2017;12(7):e0180349.
Van der Gaag N, Kloek J, de Castro S, Busch O, Van Gulik T, Gouma D. Preoperative biliary drainage in patients with obstructive jaundice: history and current status. Journal of Gastrointestinal Surgery. 2009;13(4):814-20.
Spolverato G, Vitale A, Cucchetti A, Popescu I, Marques HP, Aldrighetti L, et al. Can hepatic resection provide a long‐term cure for patients with intrahepatic cholangiocarcinoma? Cancer. 2015;121(22):3998-4006.
Edge SB. AJCC cancer staging manual. Springer. 2010;7:97-100.
Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153(6):811-8.
Buettner S, van Vugt JL, IJzermans JN, Koerkamp BG. Intrahepatic cholangiocarcinoma: current perspectives. OncoTargets and therapy. 2017;10:1131.
Giuliante F, Gauzolino R, Vellone M, Ardito F, Murazio M, Nuzzo G. Liver resection for intrahepatic cholangiocarcinoma. Tumori. 2005;91(6):487.
Pulitano C, Crawford M, Joseph D, Aldrighetti L, Sandroussi C. Preoperative assessment of postoperative liver function: the importance of residual liver volume. Journal of surgical oncology. 2014;110(4):445-50.
Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Annals of surgery. 2009;250(4):540-8.
Kim HJ, Kim CY, Park EK, Hur YH, Koh YS, Kim HJ, et al. Volumetric analysis and indocyanine green retention rate at 15 min as predictors of post‐hepatectomy liver failure. HPB. 2015;17(2):159-67.
Tan JC, Coburn NG, Baxter NN, Kiss A, Law CH. Surgical management of intrahepatic cholangiocarcinoma-a population-based study. Annals of surgical oncology. 2008;15(2):600-8.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. New England Journal of Medicine. 2010;362(14):1273-81.
Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Annals of oncology. 2013;25(2):391-8.
Smith GW, Bukowski R, Hewlett JS, Groppe CW. Hepatic artery infusion of 5‐fluorouracil and mitomycin C in cholangiocarcinoma and gallbladder carcinoma. Cancer. 1984;54(8):1513-6.
Kemeny NE, Schwartz L, Gönen M, Yopp A, Gultekin D, D’Angelica MI, et al. Treating primary liver cancer with hepatic arterial infusion of floxuridine and dexamethasone: does the addition of systemic bevacizumab improve results. Oncology. 2011;80(3-4):153-9.
Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. Journal of hepatology. 2015;62(5):1187-95.
Mouli S, Memon K, Baker T, Benson III AB, Mulcahy MF, Gupta R, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. Journal of Vascular and Interventional Radiology. 2013;24(8):1227-34.
Al-Adra D, Gill R, Axford S, Shi X, Kneteman N, Liau S-S. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. European Journal of Surgical Oncology (EJSO). 2015;41(1):120-7.
Kim JH, Won HJ, Shin YM, Kim PN, Lee S-G, Hwang S. Radiofrequency ablation for recurrent intrahepatic cholangiocarcinoma after curative resection. European journal of radiology. 2011;80(3):e221-e5.
Han K, Ko HK, Kim KW, Won HJ, Shin YM, Kim PN. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. Journal of Vascular and Interventional Radiology. 2015;26(7):943-8.
Koay EJ, Odisio BC, Javle M, Vauthey J-N, Crane CH. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments? Hepatobiliary surgery and nutrition. 2017;6(2):105.
Tao R, Krishnan S, Bhosale PR, Javle MM, Aloia TA, Shroff RT, et al. Ablative radiotherapy doses lead to a substantial prolongation of survival in patients with inoperable intrahepatic cholangiocarcinoma: a retrospective dose response analysis. Journal of Clinical Oncology. 2016;34(3):219.
Yamamoto M, Takasaki K, Otsubo T, Katsuragawa H, Katagiri S. Recurrence after surgical resection of intrahepatic cholangiocarcinoma. Journal of Hepato‐Biliary‐Pancreatic Surgery. 2001;8(2):154-7.
Oliveira IS, Kilcoyne A, Everett JM, Mino-Kenudson M, Harisinghani MG, Ganesan K. Cholangiocarcinoma: classification, diagnosis, staging, imaging features, and management. Abdominal radiology. 2017;42(6):1637-49.
Ringe KI, Wacker F. Radiological diagnosis in cholangiocarcinoma: application of computed tomography, magnetic resonance imaging, and positron emission tomography. Best Practice & Research Clinical Gastroenterology. 2015;29(2):253-65.
Mar WA, Shon AM, Lu Y, Jonathan HY, Berggruen SM, Guzman G, et al. Imaging spectrum of cholangiocarcinoma: role in diagnosis, staging, and posttreatment evaluation. Abdominal Radiology. 2016;41(3):553-67.
Solomon SB, Silverman SG. Imaging in interventional oncology. Radiology. 2010;257(3):624-40.
Jadvar H, Henderson RW, Conti PS. [F-18] fluorodeoxyglucose positron emission tomography and positron emission tomography: computed tomography in recurrent and metastatic cholangiocarcinoma. Journal of computer assisted tomography. 2007;31(2):223-8.
Cameron K, Golan S, Simpson W, Peti S, Roayaie S, Labow D, et al. Recurrent pancreatic carcinoma and cholangiocarcinoma: 18 F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT). Abdominal imaging. 2011;36(4):463-71.
Ribero D, Chun YS, Vauthey J-N, editors. Standardized liver volumetry for portal vein embolization. Seminars in interventional radiology; 2008: © Thieme Medical Publishers.
Acknowledgement
We would like to thank Kelly Kage, a medical illustrator at MD Anderson Cancer Center.
Funding
No financial support.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to this paper with conception and design of the study, literature review and analysis, drafting and critical revision and editing, and final approval of the final version.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
CME activity : This article has been selected as the CME activity for the current month. Please visithttps://ce.mayo.edu/node/99239 and follow the instructions to complete this CME activity.
Rights and permissions
About this article
Cite this article
Saleh, M., Virarkar, M., Bura, V. et al. Intrahepatic cholangiocarcinoma: pathogenesis, current staging, and radiological findings. Abdom Radiol 45, 3662–3680 (2020). https://doi.org/10.1007/s00261-020-02559-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02559-7